Abstract
The therapeutic efficacy of direct drug infusion into the brain, the target organ of transmissible spongiform encephalopathies, was assessed in transgenic mice intracerebrally infected with 263K scrapie agent. Pentosan polysulfate (PPS) gave the most dramatic prolongation of the incubation period, and amphotericin B had intermediate effects, but antimalarial drugs such as quinacrine gave no significant prolongation. Treatment with the highest dose of PPS at an early or late stage of the infection prolonged the incubation time by 2.4 or 1.7 times that of the control mice, respectively. PPS infusion decreased not only abnormal prion protein deposition but also neurodegenerative changes and infectivity. These alterations were observed within the brain hemisphere fitted with an intraventricular infusion cannula but not within the contralateral hemisphere, even at the terminal disease stage long after the infusion had ended. Therapeutic effects of PPS were also demonstrated in mice infected with either RML agent or Fukuoka-1 agent. However, at doses higher than that providing the maximal effects, intraventricular PPS infusion caused adverse effects such as hematoma formation in the experimental animals. These findings indicate that intraventricular PPS infusion might be useful for the treatment of transmissible spongiform encephalopathies in humans, providing that the therapeutic dosage is carefully evaluated.
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are fatal neurodegenerative disorders that include Creuzfeldt-Jakob disease (CJD) and Gerstmann-Sträussler-Scheinker disease in humans, in addition to scrapie and bovine spongiform encephalopathy in animals. These disorders are characterized by deposition in the brain of a protease-resistant isoform of prion protein (PrP), which is thought to be the main pathogenic component responsible for the pathogenesis (18). Outbreaks of acquired forms of human TSEs, such as variant CJD (21) and iatrogenic CJD with cadaveric growth hormone or dura grafts (3) in younger people, are prompting the development of prophylactic and therapeutic interventions.
There are some chemicals that are effective in inhibiting the accumulation or conformational change of PrP molecules in vitro and/or in prolonging the incubation period when they are administered around the time of infection in TSE animal models (2, 17). However, no chemicals, except for amphotericin B and one of its derivatives, have been reported to improve prognosis when they are administered late in the disease course or after the infectious agent has already invaded the brain (8). This implies that it may be difficult to improve the prognosis in human patients by using chemicals, because patients first come to medical attention after the onset of neurological symptoms.
Most of the previously reported chemicals have been large and hydrophilic, and whether their ineffectiveness was actually due to their poor accessibility to the brain has not been evaluated. We have developed a more sensitive drug evaluation system, which does not depend on drug accessibility to the brain, by implanting a continuous intraventricular drug infusion device into an intracerebrally TSE-infected animal model. Using this model, we have examined clinically applicable chemicals that have previously been reported to be effective either in vitro or in vivo, including antimalarial drugs such as quinacrine and chloroquine, the E-64d cysteine protease inhibitor, amphotericin B, and pentosan polysulfate (PPS). We report that intraventricular administration of PPS through the infusion device inhibited not only abnormal PrP accumulation but also neurodegenerative changes and infectivity in the brain, thereby giving rise to a dramatic prolongation of the life spans of intracerebrally infected animals.
MATERIALS AND METHODS
Experimental animals and in vivo evaluation.
Tg7 mice, which are derived from Tg10 mice (19) and express hamster PrP but not endogenous mouse PrP, were inoculated with 20 μl of 1% 263K agent hamster homogenate in the right parietal portion of the brain. An Alzet osmotic pump (Durect, Cupertino, Calif.) filled with a chemical was placed in a subcutaneous area of the back. An intraventricular infusion cannula connected to the osmotic pump through a catheter was implanted in the left frontal portion of the brain in order to place the cannula tip into the left ventricle at either day 10 or 35 postinoculation or at another designated time. The pump worked stably from 40 h after implantation and continuously for 4 weeks. Five to 10 male mice per group, each weighing about 35 g, were used. Each mouse was kept in an individual cage under the same feeding and watering conditions in an air-conditioned, light time-controlled, specific-pathogen-free room. The mice began to exhibit ambiguous signs of reduced activity about 2 days prior to death, followed by obvious signs of ruffled fur and bradykinesia on the day before or the day of death. The incubation period during which the animals were observed every day lasted from the time of intracerebral infection to the time of an obvious clinical stage.
Tga20 mice (14), which overexpress mouse PrP, were similarly inoculated with 1% RML agent mouse homogenate or 1% Fukuoka-1 agent mouse homogenate, and 4-week continuous intraventricular infusion of a chemical was started at day 14 or 49 postinoculation as described above.
Chemicals.
The E-64d cysteine protease inhibitor was generously provided by S. Ishiura, Tokyo University, Tokyo, Japan. Quinacrine and chloroquine were obtained from Sigma. Amphotericin B (Fungizone) was purchased from Bristol-Myers Squibb (Tokyo, Japan). The sodium salt of PPS (Cartrophen Vet) was purchased from Biopharm (Bondi Junction, New South Wales, Australia) and used after removal of an alcohol additive by drying. E-64d was dissolved in dimethyl sulfoxide, amphotericin B was dissolved in distilled water, and all other chemicals were dissolved in phosphate-buffered saline (PBS).
Immunohistochemistry, immunoblotting, and infectivity assay.
Mouse brains were fixed with 10% formalin and embedded in paraffin. Sections (5 μm thick) of the coronal slice sited around one-third of the distance from the interaural line to the bregma line were dewaxed and immunostained with an anti-PrP-C antibody (1:200; Immuno-Biological Labs, Gunma, Japan) or an antibody against glial fibrillary acidic protein (GFAP) (1:1,000; Dako, Glostrup, Denmark) as previously described (11).
For the detection of protease-resistant PrP (PrPres) by immunoblotting, the whole brain hemisphere where the intraventricular cannula had been fitted was homogenized with a ninefold volume of lysis buffer (0.5% sodium deoxycholate, 0.5% Nonidet P-40, PBS), and following low-speed centrifugation, the supernatant (Sup1) was treated with 25 μg of proteinase K per ml for 30 min at 37°C. An aliquot corresponding to 2.0 mg of brain tissue was electrophoresed in a sodium dodecyl sulfate-15% polyacrylamide gel and electroblotted onto a polyvinylidene difluoride filter. The filter was incubated with an anti-PrP-2B antibody raised against a hamster PrP fragment (amino acids 89 to 103) and then with an alkaline phosphatase-conjugated secondary antibody (Promega). Signals were visualized with CDP-Star detection reagent (Amersham).
For the infectivity assay, an aliquot of the Sup1 described above was diluted with PBS to produce a 0.1% brain homogenate, and a 20-μl aliquot of this homogenate was then inoculated intracerebrally in Tg7 mice. The infectious titer was calculated from the incubation period based on standard data obtained from an inoculation study with serially diluted homogenate samples of 263K-infected, terminally diseased brain tissue.
Safety assessment of PPS.
After reference to the Japanese national guidelines for safety studies on medicinal products (rodent and nonrodent toxicity testing), continuous 2-month PPS infusions ranging from 110 to 460 μg/kg/day were performed on each of four to seven adult mongrel dogs, Wistar rats, and Tg7 mice, using an Alzet osmotic pump and a brain infusion cannula. The blood cell count, coagulation, and serum chemistry were analyzed before PPS infusion was initiated and then each month thereafter. An electroencephalogram and 48-h spontaneous activity were recorded for the rats every 2 weeks from the time PPS infusion was started. Animals were sacrificed under deep anesthesia for histological evaluation of the brain when they reached an incurable condition or after the infusion had finished. Animal handling and sacrifice were in accordance with the national prescribed guidelines, and ethical approval for the studies was granted by the Animal Experiment Committee of Kyushu University.
Statistical analysis.
Statistical significance was analyzed by repeated-measure analysis of variance followed by Scheffé's method for multiple comparisons.
RESULTS
Vehicle control.
Inoculation-related accidental death occurred within 1 week of postintracerebral inoculation and abnormal PrP deposition in the brain appeared at around day 35 postinoculation in Tg7 mice intracerebrally inoculated with 1% 263K homogenate. Accordingly, intraventricular infusion of a chemical was initiated either at an early stage (day 10) or at a late stage (day 35) of the infection in Tg7 mice. Continuous 4-week intraventricular infusion with vehicle alone from day 10 or 35 did not alter the mean incubation time (distilled water, 51.6 ± 1.8 days [n = 5] from day 10 and 51.0 ± 1.8 days [n = 5] from day 35; 25% dimethyl sulfoxide, 52.3 ± 2.1 days [n = 5] from day 10 and 51.2 ± 1.3 days [n = 5] from day 35; untreated, 51.8 ± 2.2 days [n = 8]).
Antimalarial drugs, cysteine protease inhibitor, and amphotericin B.
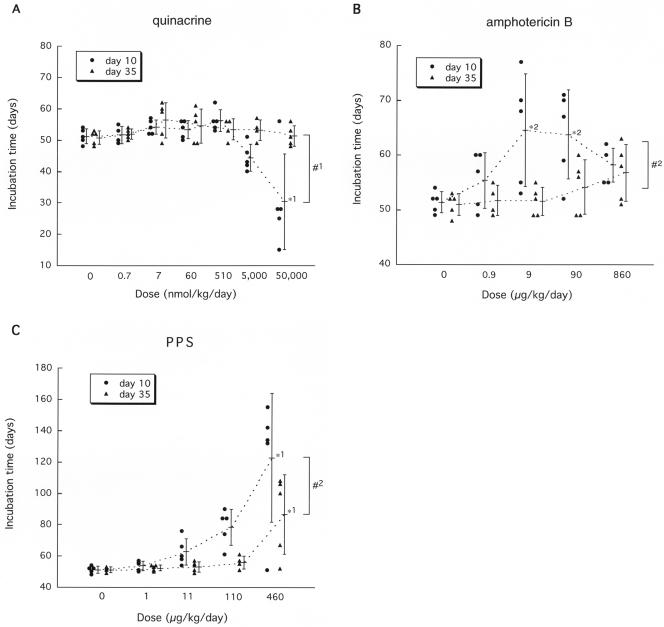
Quinacrine (Fig. 1A), chloroquine (data not shown), and the E-64d cysteine protease inhibitor (data not shown) gave no prolongation effects. Overdose of quinacrine at more than 5 μmol/kg/day from day 10, however, caused adverse effects and shortened the incubation period. Amphotericin B prolonged the incubation time at a dose of 9 or 90 μg/kg/day from day 10 and gave about 26% prolongation of the mean incubation time (from 51 to 64 days) (Fig. 1B). With infusion from day 35, however, no significant prolongation was observed.
FIG. 1.
Efficacy of quinacrine (A), amphotericin B (B), and PPS (C) in intracerebrally infected mice. Intraventricular infusion of a chemical was initiated at an early infection stage (day 10 postinoculation) or at a late stage (day 35) in 263K-infected Tg7 mice and continued for 4 weeks. Each circle or triangle represents an individual mouse. Bars represent the means and standard deviations of the incubation times for each group. *1, P < 0.05 versus the neighboring group and P < 0.01 versus the other groups; *2, P < 0.05 versus the vehicle control; #1, P < 0.05, #2, P < 0.01.
PPS.
PPS showed the greatest beneficial effects among all of the chemicals examined in this study (Fig. 1C). Infusion at 460 μg/kg/day from day 10 gave 141% prolongation (from 51 to 123 days), and even from the late stage (day 35), it gave 71% prolongation (from 51 days to 87 days).
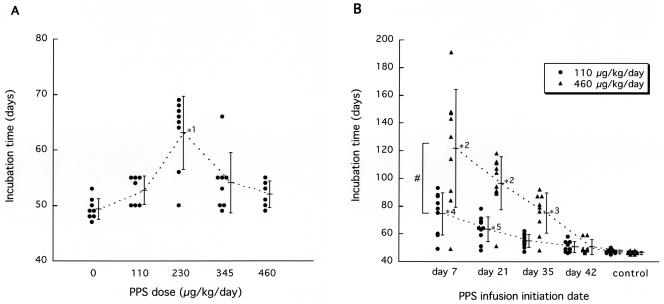
The dose response of PPS was further examined at a later stage of the infection, day 42 (Fig. 2A). The dose response was in a bell-shaped distribution, and the maximal effect was observed at a dose of 230 μg/kg/day, when the mean incubation time was prolonged by 29% (from 49 to 63 days).
FIG. 2.
Dose response (A) and administration timing (B) in PPS efficacy. Intraventricular infusion at the indicated concentrations (A) or at 110 or 460 μg/kg/day (B) was initiated at day 42 postinoculation (A) or at the indicated times (B) in intracerebrally 263K-infected Tg7 mice. *1, P < 0.01 versus the other groups; *2, P < 0.01 versus the other groups except the neighboring group(s); *3, P < 0.05 versus the control; *4, P < 0.05 versus the neighboring group and P < 0.01 versus the other groups; *5, P < 0.01 versus the control; #, P < 0.01.
The influence of infusion laterality on the outcome was examined by either ipsilateral or contralateral PPS administration to the inoculation site from day 10 or 35 postinoculation, but no significant difference according to the side of the infusion was observed (data not shown).
The relationship between the infusion initiation time and the outcome was analyzed (Fig. 2B). The effects of PPS were inversely correlated with time after inoculation. High-dose PPS infusion (460 μg/kg/day) at day 7 postinoculation prolonged the mean incubation period by 160% (from 47 to 122 days), while that at day 21 postinoculation prolonged it by 106% (from 47 to 97 days). Low-dose PPS infusion (110 μg/kg/day) showed a similar pattern but was less effective.
As a supplementary experiment to compare the efficacy of intraventricular administration with that of peripheral administration, 4-week continuous PPS infusion at 0.2, 2 or 20 mg/kg/day into a subcutaneous area of the back was performed with an osmotic pump from day 10 or 35 postinoculation. None of these treatments yielded any statistically significant effectiveness in prolonging the incubation time (51.0 ± 1.8 days [n = 4], 52.1 ± 2.1 days [n = 4], 52.3 ± 1.6 days [n = 5], and 23.8 ± 16.5 days [n = 5] in the mice receiving 0, 0.2, 2, and 20 mg/kg/day from day 10, respectively; 51.8 ± 2.0 days [n = 4], 51.0 ± 1.8 days [n = 4], 50.0 ± 1.3 days [n = 5], and 40.4 ± 4.3 days [n = 5] in the mice receiving 0, 0.2, 2, and 20 mg/kg/day from day 35, respectively). However, treatment with the highest dose showed adverse effects, such as hemorrhage in the subcutaneous area surrounding the osmotic pump in 80% of the mice examined.
PrP deposition and pathology.
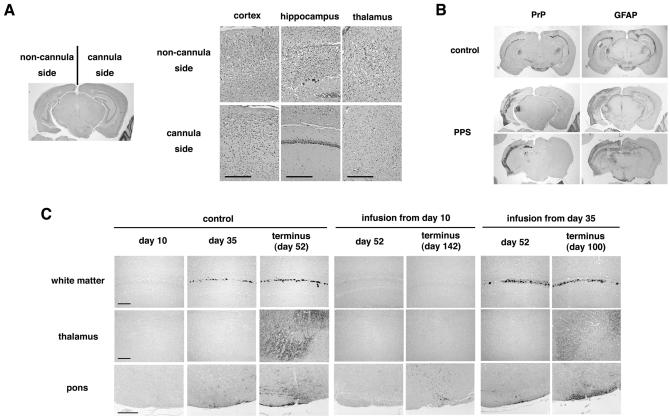
Modification of abnormal PrP deposition and pathology in the brains of PPS-treated mice were analyzed (Fig. 3A and B). In the PPS-treated mice with an incubation period of 142 days, the brain hemisphere implanted with the PPS infusion cannula showed very faint or mild pathological changes, whereas the contralateral brain hemisphere was microscopically atrophied and accompanied by prominent spongiform degeneration and neuronal cell loss, especially in the cerebral cortex, hippocampus, and thalamus (Fig. 3A). Similarly, abnormal PrP deposition was prominently reduced within the brain hemisphere implanted with the infusion cannula, while abnormal PrP deposition was much enhanced within the contralateral hemisphere, compared to the control mice with an incubation period of 52 days (Fig. 3B). GFAP immunoreactivity, indicating glial reaction, was similar to the PrP deposition.
FIG. 3.
Histopathology of the brain with or without PPS treatment. (A) Histology of the brain from an intracerebrally 263K-infected, long-surviving mouse treated with PPS at 460 μg/kg/day from day 10 postinoculation. Noncannula side and cannula side represent the sides of pathogen inoculation and intraventricular cannula implantation, respectively. Hematoxylin-eosin staining was used. (B) Immunohistochemical detection of abnormal PrP deposition (PrP) and of neurodegenerative changes by means of glial reaction (GFAP) in the same brain as for panel A (PPS) or in the brain from a nontreated mouse (control). The orientation of the sides of pathogen inoculation and cannula implantation is the same as in panel A. A coronal section sited around one-third of the distance from the interaural line to the bregma line is shown for the control mouse, and either a coronal section sited around one-third of the distance or a coronal section sited around two-thirds of the distance from the interaural line to the bregma line is shown for the PPS-treated mouse. Astrocytic glial reaction is demonstrated by immunohistochemistry for GFAP. (C) Sequential analysis of abnormal PrP deposition in intracerebrally 263K-infected, nontreated mice (control) or mice treated with PPS (infusion from day 10 or 35). Each panel shows a representative finding from three mice sacrificed at a designated time. In PPS-treated mice, the brain tissue examined was obtained from the hemisphere implanted with the intraventricular cannula, which was the hemisphere opposite to that of the inoculation site. PPS was infused at 460 μg/kg/day from day 10 or 35 postinoculation. Each image of the hippocampus or the thalamus (posterior nuclei) is from coronal sections sited around one-third of the distance from the interaural line to the bregma line. Each image of the pons (ventral area) is from a coronal section which is parallel to the interaural line and contains the culmen portion of the cerebellum. Bar, 160 μm.
In a sequential analysis of the control mice, cerebral PrP deposition had not appeared at day 10, first appeared in the cerebral white matter adjacent to the hippocampus at around day 35 in a coarse granular deposition pattern, and finally appeared in the thalamus, hypothalamus, and brain stem at terminus day 52 in a punctate pattern (Fig. 3C). Mice treated with PPS from day 10 did not show any PrP deposition within the hemisphere implanted with the cannula at day 52, and only punctate PrP deposition was observed in the brain stem at terminus day 142. Mice treated with PPS from day 35 demonstrated coarse granular PrP deposits in the cerebral white matter at day 52 within the cannula-implanted hemisphere, but there was no apparent PrP deposition in the thalamus at this stage. This PrP deposition pattern was similar to that in the control mice at day 35. Punctate PrP deposition was finally clearly visible in both the thalamus and the brain stem at terminus day 100.
PrPres and infectivity.
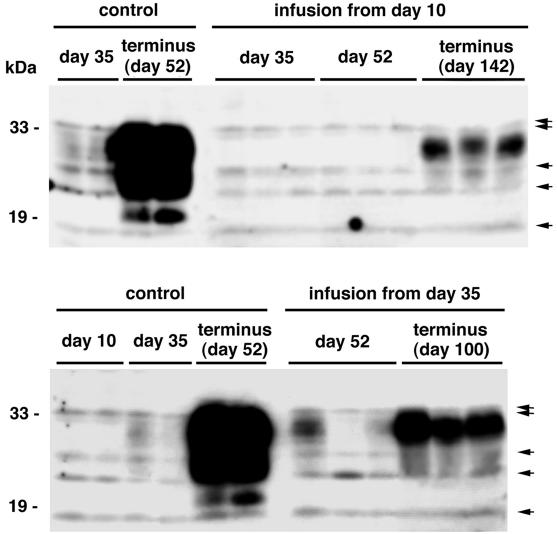
PrPres was analyzed within the brain hemisphere implanted with the infusion cannula (Fig. 4). PrPres signals in the control mice were very faint at day 35 but ultimately were very strong at terminus day 52. In mice treated with PPS from day 10, PrPres signals were not detected in the brain at day 35 or 52 but were detected at terminus day 142. However, the PrPres signals at day 142 did not reach the high level seen in the control mice at day 52. Similarly, in mice treated with PPS from day 35, the PrPres signals were very weak at day 52 and then increased at terminus day 100, but they still remained at a lower level than in the control mice.
FIG. 4.
PrPres in the brain with or without PPS treatment. Each lane represents an aliquot corresponding to 2.0 mg of brain tissue homogenate from an individual mouse sacrificed at a designated time. The brain tissue homogenate examined was prepared from the whole hemisphere contralateral to that of the inoculation site in either nontreated mice (control) or PPS-treated mice (infusion from day 10 or 35). In PPS-treated mice, the brain hemisphere examined was the side implanted with the intraventricular cannula. PPS was infused at 460 μg/kg/day from day 10 or 35 postinoculation. Nonspecific signals that were observed without the primary antibody are shown by arrows.
Modification of the infectivity within the brain hemisphere implanted with the infusion cannula was clearly correlated with the level of PrPres (Tables 1 and 2). The infectious titer (log 50% lethal dose [LD50]/g of tissue) within the cannula-implanted brain hemisphere from terminally diseased mice was significantly decreased in the PPS-treated mice.
TABLE 1.
Infectious titer and incubation time in the 263K-Tg7 model
| Dilution | Mean incubation timea (days) ± SD | No. of diseased mice/total | Infectious titerb (log LD50/20 μl of tissue) | Corrected titer (log LD50/g of tissue) |
|---|---|---|---|---|
| 101 | 43.8 ± 2.5 | 9/9 | 6.3 | 8.0 |
| 102 | 48.4 ± 3.1 | 10/10 | 5.3 | 7.0 |
| 103 | 55.6 ± 5.3 | 9/9 | 4.3 | 6.0 |
| 104 | 65.1 ± 2.9 | 8/8 | 3.3 | 5.0 |
| 105 | 76.0 ± 5.3 | 8/8 | 2.3 | 4.0 |
| 106 | 93.9 ± 17.0 | 10/10 | 1.3 | 3.0 |
| 107 | 158, 177, 422, 443 | 4/8 | 0.3 | 2.0 |
| 108 | 338, 555 | 2/7 | ||
| 109 | 0/9 |
A 20-μl aliquot of serially diluted homogenate samples of the whole brain from a 263K-infected, terminally diseased Tg7 mouse was inoculated intracerebrally into Tg7 mice, and the mice were observed for up to 730 days postinoculation.
The infectious titer was calculated by the Behrens-Kärber method.
TABLE 2.
Infectious titer in the brain with PPS treatment
| Inoculuma | Mouse no. | Mean incubation timeb (days) ± SD | Infectious titerc (log LD50/g of tissue) | Mean infectious titer ± SDd |
|---|---|---|---|---|
| Control | 1 | 56.4 ± 0.5 | 8.9 | 8.87 ± 0.06* |
| 2 | 56.4 ± 1.6 | 8.9 | ||
| 3 | 57.2 ± 1.9 | 8.8 | ||
| PPS from: | ||||
| Day 35 postinoculation | 1 | 62.4 ± 1.2 | 8.3 | 8.20 ± 0.10* |
| 2 | 63.4 ± 0.8 | 8.2 | ||
| 3 | 64.2 ± 5.6 | 8.1 | ||
| Day 10 postinoculation | 1 | 69.8 ± 1.6 | 7.6 | 7.50 ± 0.14* |
| 2 | 71.4 ± 1.5 | 7.4 |
A 20-μl aliquot of a 0.1% homogenate from the infusion cannula-implanted brain hemisphere of each terminally diseased mouse was used for intracerebral inoculation into Tg7 mice.
The data were collected from five inoculated mice in each group.
The infectious titer was calculated from the incubation period based on the curve obtained from the data in Table 1 and corrected for the dilution power.
*, P < 0.01 versus the other groups.
PPS effects in other strains.
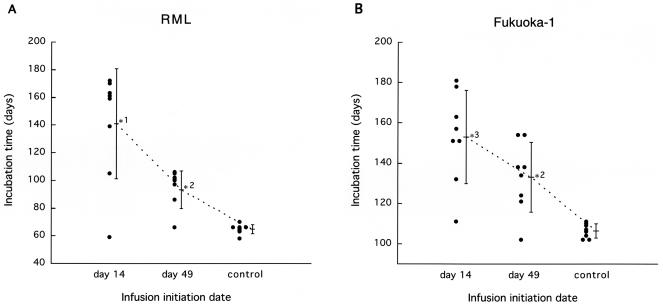
To investigate the effectiveness of PPS on pathogen strains other than 263K scrapie agent, Tga20 mice intracerebrally infected with 1% homogenates of RML scrapie agent or Fukuoka-1 Gerstmann-Sträussler-Scheinker disease agent were treated with a dose of 230 μg/kg/day for 4 weeks (Fig. 5). RML-infected mice treated with vehicle alone had a mean incubation period of 65 days, while those treated with PPS from day 14 or 49 postinoculation had a mean incubation period of 141 days (117% prolongation) or 95 days (46%), respectively. Similarly, Fukuoka-1-infected mice had a mean incubation period of 106 days, while those treated with PPS from day 14 or 49 postinoculation had a mean incubation period of 153 days (44% prolongation) or 133 days (25%), respectively. These data imply that intraventricular PPS infusion can be effective irrespective of the pathogen strain.
FIG. 5.
Efficacy of PPS in mice infected with RML agent (A) or Fukuoka-1 agent (B). PPS at a dose of 230 μg/kg/day was intraventricularly infused into intracerebrally infected Tga20 mice from day 14 or 49 postinoculation for 4 weeks. *1, P < 0.01 versus the other groups; *2, P < 0.05 versus the control; *3, P < 0.01 versus the control.
Safety assessment of intraventricular PPS.
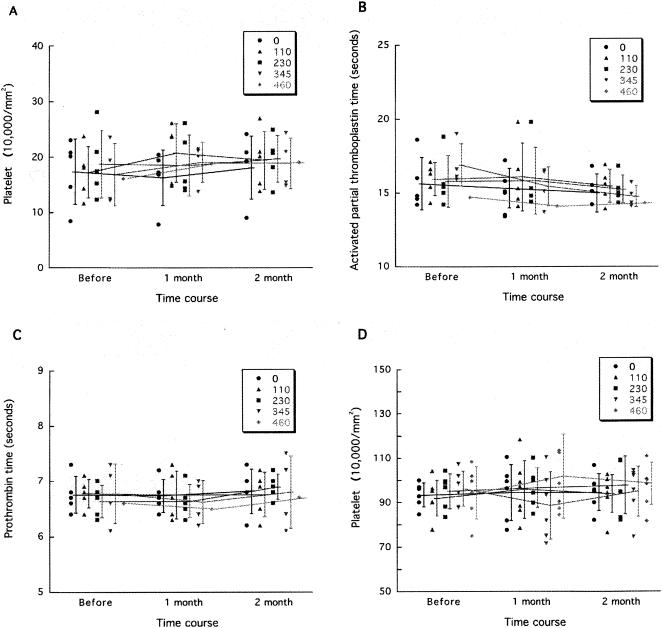
Thrombocytopenia and coagulation abnormality are known to occur occasionally with PPS, and PPS has been utilized in humans enterally, percutaneously, or intravenously but never intraventricularly. Thus, the safety of continuous intraventricular infusion should be evaluated before the possibility of application of this specific treatment to human patients is discussed. Continuous intraventricular infusion at doses ranging from 110 to 460 μg/kg/day was examined for 2 months in rodents (mice and rats) and nonrodents (dogs). Intraventricular infusion of up to 230 μg/kg/day did not influence the data for the blood cell count, coagulation (Fig. 6), or serum chemistry, nor did it influence the electroencephalogram records, spontaneous activity pattern, behavior, or histological findings for the brain. However, higher doses showed adverse effects, but only in dogs. Three of the six dogs receiving 345 μg/kg/day and all four dogs receiving 460 μg/kg/day suffered partial or generalized seizures, which began within 24 h after PPS infusion was initiated. After treatment with anticonvulsants, the seizures of one dog from each group disappeared. The remaining five dogs did not recover from the seizures. Histologically, three of the five dogs had a large hematoma in the cerebral white matter where the cannula had been placed. None of the other dogs showed any notable pathological findings except for localized tissue damage and gliosis around the cannula route (data not shown).
FIG. 6.
Representative data from PPS safety assessment. Platelet counts in dogs and rats (A and D, respectively) and coagulation data for dogs (B and C) are shown. Each circle, triangle, square, inverted triangle, or diamond represents an individual animal. The data for two of the six dogs receiving 345 μg/kg/day and for three of the four dogs receiving 460 μg/kg/day were not obtained because they suffered seizures shortly after PPS infusion had been started and were sacrificed or died. However, the data for two dogs, one receiving 345 μg/kg/day and the other receiving 460 μg/kg/day, both of which suffered seizures but recovered, are included.
DISCUSSION
Here, we report on the effectiveness of clinically applicable chemicals by using a new drug evaluation system composed of gene-manipulated mice with substantially shorter disease incubation times and a continuous intraventricular infusion device. This system enabled us to evaluate the absolute therapeutic potency of chemicals in vivo within relatively short periods, regardless of their accessibility to the brain. Our observations indicate that two of the chemicals examined were effective in prolonging the incubation periods for intracerebrally infected animals. This suggests that intraventricular drug infusion could improve the prognosis of infected humans.
Among the chemicals examined here, PPS showed the most beneficial effects, and despite a limited infusion period, mice treated with PPS survived long after the infusion ended. Effectiveness was clearly observed even for the infusion at a late stage of infection, when abnormal PrP deposition was already visible in the affected brain. PPS is known to be effective in inhibiting abnormal PrP formation in vitro (5) and/or in prolonging the disease incubation time in vivo (9, 12, 13, 16). However, its effectiveness in vivo has been restricted to administration either before or soon after peripheral infection. Thus, treatment with PPS has been thought of as being preventive only for those individuals with accidental inoculation in the periphery (7). Our observations, however, indicate that intraventricular PPS is in fact quite effective in prolonging the life spans of infected animals, even after abnormal PrP has already accumulated in the brain. The difference between previous observations and ours can be explained by poor or even no accessibility of peripherally administered PPS to the brain, and this is supported by our observation of the ineffectiveness of continuous subcutaneous PPS administration in intracerebrally infected mice.
The present studies revealed that PPS prevented not only new deposition of abnormal PrP but also the accumulation of neurodegeneration and infectivity. These findings suggest that prolongation of the life spans of infected animals by PPS could be due to its direct action on abnormal PrP generation. PPS interferes with the conversion of normal PrP molecules to abnormal ones by competitively binding to the PrP molecules (4, 5) and/or by altering the cellular localization of normal PrP molecules (20). PPS is also known to play a stimulatory role in the conversion of PrP molecules into protease-resistant ones in vitro (22). However, neither stimulatory effects on abnormal PrP accumulation nor acceleration of the disease course was observed in the present in vivo study.
From the histopathological studies, the effectiveness of PPS was clearly observed within the brain hemisphere where the intraventricular cannula was fitted, but not within the other hemisphere, in treated mice that survived long after the infusion had ended. This suggests that the infused PPS persisted around the infusion site and did not diffuse throughout the ventricular system, especially into the contralateral side of the brain. To obtain a much better outcome following PPS administration, it may be important to facilitate the diffuse distribution of PPS into every vulnerable area of the brain.
These studies also demonstrated that both the cerebral cortex and the hippocampus were apparently involved in the pathological conditions at the latest stage of the disease in the Tg7-263K model. Coarse granular PrP deposits in the cerebral white matter were the earliest abnormal finding in the affected brain, and these were followed by punctate PrP deposits in the brain stem, hypothalamus, and thalamus, but not in the cerebral cortex and hippocampus, in the nontreated mice. However, mice surviving long after intraventricular PPS infusion had devastating pathological changes in the cerebral cortex and hippocampus of the hemisphere opposite to that of the PPS infusion.
The PPS effects were quite dependent upon the timing of the infusion, and at a later disease stage or a terminal disease stage the effects on prolongation of the life span were substantially limited. We have no precise knowledge as to when the mice used began to exhibit the very initial signs or symptoms of the disease, although we do know that abnormal PrP deposition in the brain began to be visible at around 5 weeks postinoculation and that the mice started to show definite signs about 2 days before death. Thus, our data do not guarantee similar effectiveness in human patients who already have signs and symptoms of the disease. On the other hand, the effectiveness of intraventricular PPS infusion was demonstrated not only for infection with the 263K strain but also for infection with two other distinct strains. These findings suggest that this treatment may have universal validity for TSE diseases.
PPS is utilized as a clinical medicine for interstitial cystitis, thrombophlebitis, and thrombosis, and its safety by enteral, percutaneous or intravenous administration has been clearly established. Here, the safety of intraventricular PPS infusion at up to a dose yielding the maximal effectiveness in mice was demonstrated in experimental animals. However, at higher doses than this, there was a gap between small rodents and dogs, with the dogs showing adverse effects, such as seizures that were mostly caused by hematoma formation around the intraventricular cannula, although such adverse effects appeared only very early in the treatment. It is well known that smaller animals metabolize drugs much more quickly, and therefore intraventricular PPS at the same dosage was not toxic in mice and rats but was toxic in dogs. For application of this treatment to humans, it will be important to take account of the drug metabolism differences between mice and humans.
Amphotericin B and one of its derivatives are known to prolong the life spans of infected animals even with administration late in the disease course (8). In our experiments, however, amphotericin B did not cause any significant prolongation at a late-stage administration. Differences in the administration route, dose, and duration, as well as the experimental models, might account for this difference, but it remains to be elucidated.
Antimalarial chemicals, including quinacrine, had no effects in the present studies. These chemicals were previously found to be effective in inhibiting abnormal PrP formation in a scrapie-infected cell line (10, 15), and quinacrine has been used in clinical trials for TSE patients. However, together with the recent findings of two other research groups (1, 6), our data suggest that quinacrine may not improve the prognosis of the patients.
Finally, the placement of an intraventricular cannula in TSE patients may create public health issues due to fears of contamination of the operating room or safety issues with respect to the personnel involved with this procedure. For these reasons, other drug delivery systems which do not need a surgical procedure should be developed, and PPS derivatives that can be delivered into the brain after peripheral administration also need to be developed. As an immediately applicable remedy, however, continuous intraventricular PPS administration with an infusion device may be a candidate for a clinical trial, with a view to preventing the disease in those people categorized as being at extremely high risk or to improving the prognosis of diseased people with TSEs.
Acknowledgments
This study was supported by grants to K.D. from the Ministry of Health, Labour and Welfare (H13-kokoro-025) and from the Ministry of Education, Culture, Sports, Science and Technology (13557118 and 14021085), Tokyo, Japan.
We thank B. Chesebro of the Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for providing the Tg7 mice and C. Weissmann of the Imperial College School of Medicine at St. Mary's, London, United Kingdom, for providing the Tga20 mice. We also thank I. Goto for the electroencephalogram analyses.
REFERENCES
- 1.Barret, A., F. Tagliavini, G. Forloni, C. Bate, M. Salmona, L. Colombo, A. De Luigi, L. Limido, S. Suardi, G. Rossi, F. Auvré, K. T. Adjou, N. Salés, A. Williams, C. Lasmézas, and J. P. Deslys. 2003. Evaluation of quinacrine treatment for prion diseases. J. Virol. 77:8462-8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, P. 2002. Drug therapy in human and experimental transmissible spongiform encephalopathy. Neurology 58:1720-1725. [DOI] [PubMed] [Google Scholar]
- 3.Brown, P., M. Preece, J. P. Brandel, T. Sato, L. McShane, I. Zerr, A. Fletcher, R. G. Will, M. Pocchiari, N. R. Cashman, J. H. d'Aignaux, L. Cervenakova, J. Fradkin, L. B. Schonberger, and S. J. Collins. 2000. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 55:1075-1081. [DOI] [PubMed] [Google Scholar]
- 4.Caughey, B., K. Brown, G. J. Raymond, G. E. Katzenstein, and W. Thresher. 1994. Binding of the protease-sensitive form of PrP (prion protein) to sulfated glycosaminoglycan and Congo red. J. Virol. 68:2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, S. J., V. Lewis, M. Brazier, A. F. Hill, A. Fletcher, and C. L. Masters. 2002. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann. Neurol. 52:503-506. [DOI] [PubMed] [Google Scholar]
- 7.Dealler, S. 1998. Post-exposure prophylaxis after accidental prion inoculation. Lancet 351:600. [DOI] [PubMed] [Google Scholar]
- 8.Demaimay, R., K. T. Adjou, V. Beringue, S. Demart, C. I. Lasmézas, J. P. Deslys, M. Seman, and D. Dormont. 1997. Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J. Virol. 71:9685-9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diringer, H., and B. Ehlers. 1991. Chemoprophylaxis of scrapie in mice. J. Gen. Virol. 72:457-460. [DOI] [PubMed] [Google Scholar]
- 10.Doh-ura, K., T. Iwaki, and B. Caughey. 2000. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74:4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doh-ura, K., E. Mekada, K. Ogomori, and T. Iwaki. 2000. Enhanced CD9 expression in the mouse and human brains infected with transmissible spongiform encephalopathies. J. Neuropathol. Exp. Neurol. 59:774-785. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers, B., and H. Diringer. 1984. Dextran sulphate 500 delays and prevents mouse scrapie by impairment of agent replication in spleen. J. Gen. Virol. 65:1325-1330. [DOI] [PubMed] [Google Scholar]
- 13.Farquhar, C., A. Dickinson, and M. Bruce. 1999. Prophylactic potential of pentosan polysulphate in transmissible spongiform encephalopathies. Lancet 353:117. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 15.Korth, C., B. C. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladogana, A., P. Casaccia, L. Ingrosso, M. Cibati, M. Salvatore, Y. G. Xi, C. Masullo, and M. Pocchiari. 1992. Sulphate polyanions prolong the incubation period of scrapie-infected hamsters. J. Gen. Virol. 73:661-665. [DOI] [PubMed] [Google Scholar]
- 17.Priola, S. A., B. Caughey, and W. S. Caughey. 1999. Novel therapeutic uses for porphyrins and phthalocyanines in the transmissible spongiform encephalopathies. Curr. Opin. Microbiol. 2:563-566. [DOI] [PubMed] [Google Scholar]
- 18.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Race, R. E., S. A. Priola, R. A. Bessen, D. Ernst, J. Dockter, G. F. Rall, L. Mucke, B. Chesebro, and M. B. Oldstone. 1995. Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron 15:1183-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shyng, S. L., S. Lehmann, K. L. Moulder, and D. A. Harris. 1995. Sulfated glycans stimulate endocytosis of the cellular isoform of the prion protein, PrPC, in cultured cells. J. Biol. Chem. 270:30221-30229. [DOI] [PubMed] [Google Scholar]
- 21.Will, R. G., J. W. Ironside, M. Zeidler, S. N. Cousens, K. Estibeiro, A. Alperovitch, S. Poser, M. Pocchiari, A. Hofman, and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 22.Wong, C., L. W. Xiong, M. Horiuchi, L. Raymond, K. Wehrly, B. Chesebro, and B. Caughey. 2001. Sulfated glycans and elevated temperature stimulate PrP(Sc)-dependent cell-free formation of protease-resistant prion protein. EMBO J. 20:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]