Abstract
We have shown that the protective HIV-1 antibody, 2F5, avidly reacts with a conserved mammalian self-antigen, kynureninase, and that the development of B cells specific for the 2F5 epitope is constrained by immunological tolerance. These observations suggest that the capacity to mount antibody responses to the 2F5 epitope is mitigated by tolerance, but such capacity may be latent in the pre-tolerance and/or anergic B cell pools. Here, we use B cell tetramer reagents to track the frequencies of B cells that recognize the HIV-1 2F5 epitope (SP62): in BL/6 mice, SP62-binding transitional B cells that bind this epitope are readily identified in bone marrow but are lost during subsequent development. Unsurprisingly then, immunization with SP62 immunogen does not elicit significant humoral responses in normal BL/6 mice. Reconstitution of Rag1null mice with normal congenic B cells that have matured in vitro, restores the capacity to mount significant serum Ab and germinal center responses to this HIV-1 epitope. These B cell cultures are permissive for the development of autoreactive B cells and support the development of SP62-specific B cell compartments normally lost in 2F5 antibody knockin mice. The recovery of humoral responses to the 2F5/SP62 epitope of HIV-1 by reconstitution with B cells containing forbidden, autoreactive clones provides direct evidence that normal BL/6 mice latently possess the capacity to generate humoral responses to a conserved, neutralizing HIV-1 epitope.
Introduction
Serum antibody (Ab) responses to HIV-1 envelope antigens are elicited in infected individuals, but the initial Ab response is non-neutralizing and focused on epitopes that are poorly conserved among independent HIV-1 isolates [reviewed in (1)]. Neutralizing, strain-specific Ab responses to HIV-1 do emerge in a significant fraction of patients within months of infection (2, 3) and these select for resistant HIV-1 mutants (4). In contrast, serum Ab that effectively binds to neutralizing epitopes conserved on most or all HIV-1 clades are quite rare and arise in only a minority of infected individuals and after several years of chronic exposure to virus (5). These broadly neutralizing Ab (bnAb) are protective in animal challenge experiments (6-9) but to date, no HIV-1 vaccine has been capable of inducing bnAb responses. A major goal of HIV-1 vaccine study is to understand how these rare bnAb responses are elicited.
Among the conserved, neutralizing epitopes of HIV-1 is the membrane proximal external region (MPER) of gp41, a structure critical for viral fusion with target cell membranes (10). A series of neutralizing and broadly protective human Abs, 2F5, 4E10, Z13, and 10E8, react with linear epitopes of the HIV-1 MPER (11-14) and yet Ab specific for these MPER epitopes are rarely elicited (3). Indeed, despite significant effort, no vaccine or immunization strategy has been found to induce robust MPER bnAb responses (15-17).
A variety of hypotheses have been proposed to explain ineffective MPER Ab responses to HIV-1 infection and vaccines [reviewed in (4)]. We observed that the 2F5 and 4E10 bnAb react with self-antigens (18), and proposed that this structural mimicry would result in the loss MPER-reactive B cells by immunological tolerance mechanisms (19); such mimicry would specifically impair Ab responses to HIV-1 epitopes that mimic host-antigens (19). This hypothesis has been supported by studies of 2F5 and 4E10 VDJ “knock-in” mice that exhibit a potent blockade in B cell development at the transition of small pre-B to immature B cells (20-23); this developmental blockade is characteristic of transgenic/knockin mice expressing BCR for known autoantigens(24-26).
We subsequently demonstrated that the enzyme kynureninase (KYNU) contains an epitope that is closely mimicked by the HIV-1 2F5 epitope and avidly bound by the 2F5 bnAb and its unmutated ancestor (27). As this KYNU epitope is conserved in most mammalian species, the normal processes of immunological tolerance that remove KYNU specific B cells should purge equally B cells that are specific for the 2F5 MPER epitope of HIV-1. Likewise, the 4E10 bnAb was shown to react with many recombinant human proteins in microarrays and, under stringent conditions, to the RNA splice factor 3B subunit 3 (SF3B3) (27).
Both KYNU and SF3B3 are highly conserved and the expected consequence of this epitopic mimicry is tolerization of 2F5 and 4E10-like B cells and impaired MPER Ab responses in most mammalian species, including mice and humans (19, 27). Indeed, laboratory opossums, which lack the cross-reactive KYNU epitope but retain SF3B3 mimicry, respond to immunization with HIV-1 envelope protein (gp140) with extraordinarily high titers of serum IgG to the 2F5 HIV-1 epitope but not to the adjacent 4E10 determinant (27). These observations strongly suggest that B cells specifically reactive with the KYNU/2F5 and SF3B3/4E10 HIV-1 epitopes are present before the first tolerance checkpoint but are then purged (28-30).
During their development, self-reactive, immature B cells are tolerized by apoptosis, receptor editing, or anergy (24, 25, 31-35). Self-reactive B cells that are not purged in the bone marrow (BM) remain susceptible to mechanisms of peripheral tolerance that limit their capacity to respond productively to antigen ligands (36). These activities rely in part on the cellular environment and we have demonstrated that immunological tolerance can be bypassed in a stromal-cell independent culture system that supports B lymphopoiesis (37). The culture-derived (CD) B-lineage cells that develop in vitro are phenotypically and functionally similar to their in vivo counterparts (37). In the absence of the normal BM environment (38, 39), however, CD B cells are enriched for autoreactivity, including high-affinity, autoreactive VDJ rearrangements that are normally deleted at the first tolerance checkpoint; this biased repertoire in retained even after transfer to RAG1 deficient hosts (37).
The generation of mature, functional CD B cells that mature in the absence of central B cell tolerance allows us to test directly whether the weak immunogenicity of the conserved, neutralizing 2F5 epitope of the HIV-1 MPER is intrinsic or the consequence of immune tolerance. The answer to this question is crucial to HIV vaccine design: do HIV-1 vaccines fail to elicit bnAb because vaccine immunogens are structurally imperfect or because the most fit responder B cells have been tolerized?
Here, we use B cell tetramers to identify B cells specific for the 2F5 nominal epitope and demonstrate that the frequency of 2F5 epitope-binding cells is highest in the BM immature and T1 compartments and then declines with increasing cellular maturity. In contrast, the frequency of CD B cells that bind the 2F5 MPER epitope remains stable through in vitro development and RAG1 deficient BL/6 mice reconstituted with CD B and T cells rescue germinal center (GC) and serum IgG Ab responses to a MPER HIV-1 peptide immunogen containing the 2F5 epitope. Indeed, reconstituted mice mount GC and serum IgG responses to the 2F5 immunogen that are 20- to 40-fold greater than BL/6 controls despite their significantly reduced ability to respond to NP-chicken γ globulin. The provision of mature, 2F5 epitope reactive B cells rescues the virtual unresponsiveness of BL/6 mice to immunization with a simple HIV-1 MPER immunogen, further strengthening the hypothesis that at least some of the conserved neutralizing epitopes of HIV-1 mimic self-antigens and thereby evade effective immune control.
Materials and Methods
Mice
C57BL/6 (BL/6) and congenic RAG-1−/− (B6.129S7-Rag1tm1Mom/J) mice were obtained from Jackson Laboratory (Bar Harbor, ME). 2F5 VH-KI mice (BL/6 genetic background) were provided by L. Verkoczy. CD-RAG and LN-RAG mice were generated by tail vein injection of 2 × 107 CD or LN cells into Rag1−/− mice as described (37). CD- and LN-RAG mice were used in no earlier than 4 weeks after reconstitution and paired with age-matched BL/6 controls; in all other experiments, mice were used at 6-8 wk of age. All mice were housed in a pathogen-free barrier facility in sterile bedding and fed ad libitum. These studies were approved by the Duke University Animal Care and Use Committee.
Antibodies and flow cytometry
To identify, characterize, and isolate lymphocytes, mAbs included: B220-PacificBlue (RA3-6B2), CD23-biotin (B3B4), CD93-APC (AA4.1), GL7-FITC (GL7) and APC-Alexa750-conjugated streptavidin were purchased from BD Pharmingen (San Diego, CA); and anti-mouse IgMPEcy7 (eB121-15F9), anti-mouse IgD-FITC (11-26), CD21-PE (eBio8D9) and TCRβ-APC (H57-597) were purchased from eBioscience (San Diego, CA). Single cell suspension of dissociated tissues and cultured cells were counted on a hemacytometer using Trypan Blue exclusion to determine total cell numbers. 106 cells were suspended in FACS buffer and labeled with mAbs described above. FACS buffer contained 1xPBS (pH7.2) with 3% FBS (Sigma) and 0.01% sodium azide. Propidium iodide (PI) was used to exclude dead cells from our samples. All FACS analysis was performed using a BD LSRII or Canto cytometer and presented with FlowJo software. Cell sorting was performed on a BD FACSVantage cytometer.
Peptides and tetramers
All peptides were synthesized by SynPep Corporation (Dublin, CA). All tetramers were created as previously described (40). Peptides used for immunization, ELISA, tetramer and ELISpot analysis include: DP178-Q16L -YTSLIHSLIEESQNQLEKNEQELLELDKWASLWNWF, SP62 (MPER) - GGGQQEKNEQELLELDKWASLWN, R4A - GGGGGDWEYSVWLSN. All tetramer reagents used for FACS were labeled with APC to track antigen-specific B cell populations. The empty-tetramer reagent was biotin-saturated streptavidin-APC that did not contain peptide. All tetramer reagents were used at ~10 nM concentration (~125 ng per 106 cells) to label cells for 30 mins on ice. Cells were then washed and labeled with mAbs (described above) to determine specific B cell populations.
Definition of B cell subsets
We have defined developmental B cell subsets by their surface phenotypes following the definitions of R.R. Hardy (41) and our prior work (42). Briefly, B cell subsets were defined as: pro-/pre-B cells (B220loIgM IgD−CD21−CD23−CD93hi); immature B cells (B220loIgM+IgD−CD21−CD23−CD93hi); T1 B cells (B220loIgMhiIgDloCD21−CD23loCD93hi); T2 B cells (B220hiIgMhiIgDhiCD21loCD23hiCD93int); MZ B cells (B220hiIgMhiIgDloCD21hiCD23loCD93−); and mature B cells (B220hiIgMintIgDhiCD21loCD23hiCD93−). Characteristic flow cytometry gatings are illustrated in Supplemental Fig. 2. The B cell tetramers used to identify Ag-specific B cells in different B cell compartments replaced CD93; we therefore relied on expression patterns of B220, IgM, IgD, CD21, and CD23 to characterize the developmental state of MPER-binding B cells. CD cells were analyzed as described (37). Germinal center (GC) B cells were identified as B220hiGL-7hiIgD B cells (43).
Cell lines
P3 and 13H11 cell lines were grown and maintained in DMEM media (Gibco) containing 10% FCS, 10−4 M 2-ME and penicillin/streptomyacin (P/S) antibiotics. The R4A cell line was grown and maintained in DMEM media (Gibco) containing 20% FCS, 1% MEM non-essential amino acids, 10−4 M 2-ME and P/S antibiotics as described (44).
B cell culture system
BM cells were cultured to generate CD B cells as described (37). Briefly, mice were killed by cervical dislocation and BM was collected from tibiae and femurs by flushing with cold, serum-containing media. BM was plated for 5-10 mins in a humidified CO2 incubator at 37°C to remove adherent cell populations. Non-adherent cells were collected and centrifuged at ~400 g at 4°C for 5 mins. RBCs were lysed using 1xACK buffer. Cells were washed and the number of live cells was determined by Trypan Blue exclusion. BM cells were plated at 7.5×105 cells/ml (25mls) in T-75 flasks for 4 d in IMDM (Gibco) containing 10−4 M 2-ME, 10% HyClone Serum (Defined) and P/S antibiotics. Recombinant cytokines were added at 10 ng/ml IL-7 or 20 ng/ml BAFF from R&D Systems (Minneapolis, MN).
ELISA
ELISA plates (BD Falcon) were coated (overnight, 4°C) with 2-5 μg/ml (50μl/well) of capture reagent (NIP-BSA or DP178-Q16L) in carbonate buffer (0.1M; pH9.5). Coated plates were washed with 1xPBS (pH7.4) containing 0.1% Tween-20 and 0.5% BSA (USB Corporation). Wells were incubated (2hrs; 25°C) with blocking buffer (PBS (pH7.4), 0.5% BSA, 0.1% Tween-20). Serum samples were initially diluted from 1:5 to 1:50; followed by serial, 3-fold dilutions. Purified mouse IgG (H33Lγ1 and 13H11) mAbs were used as a standard (10-30 μg/ml to 1.5-5 ng/ml) to determine serum Ab concentrations. HRP-conjugated goat anti-mouse IgG was used to detect bound antibody (Southern Biotechnology Associates, Birmingham, AL). Only samples that fell within the linear portion of our standard curve were used for analysis.
ELISpot assays
ELISpot plates (Millipore) were coated with 2 μg/ml (50 μl/well) of goat anti-mouse Ig(H+L) in 0.1M carbonate buffer (pH9.5) overnight at 4°C. Washing/blocking buffer contained 1xPBS (pH7.4), 0.1% Tween-20 and 0.5% BSA (USB Corporation).
Antigen-specific AFC
Activated B cells (5 μg/ml LPS and 20 ng/ml BAFF; 72h) were washed and plated at 1.5-2×103 cells/well in triplicate on ELISpot plates coated with goat anti-mouse Ig(H+L) (2 μg/ml) to capture all secreted Ig types. Activated cells were incubated at 37°C in a humidified CO2 incubator for 4h with IMDM media, and then washed and re-blocked for 1-2 d using wash/blocking buffer. To identify MPER-specific AFC, membranes were subsequently incubated with 20 μM biotin-DP178-Q16L or biotin-R4A peptide for 2 h at room temperature. Streptavidin-AP (Southern Biotech) and SIGMA FAST BCIP/NBT (Sigma) were then used to enumerate MPER- or R4A-specific AFC. This method identifies all MPER AFC regardless of H- or L-chain type. ELISpots were photographed using a Canon EOS 20D digital camera with an EFS60mm lens.
Total AFC
LPS-activated B cells were washed and plated at 2.5-5×102 cells/well in triplicate. Plates were washed and re-blocked as described above. Membranes were probed with goat-anti-mouse IgM-AP and IgG-AP detection Ab. SIGMA FAST BCIP/NBT (Sigma) was used to develop spots.
Immunizations
NP-CGG immunizations
6-8 wk old BL/6 mice were immunized (i.p.) with NP13-CGG (5 μg) precipitated in alum and suspended in 200 μl PBS. CD-RAG mice were immunized with equivalent amounts of antigen 3.5 wk after CD B cell transfer. Mice were bled before and 12d after immunizations to determine antigen-specific serum Ab levels.
MPER immunizations
6-8 wk old BL/6 mice were immunized (i.p.) 1-2 times with DP178-Q16L peptide (10 μg) precipitated in alum and suspended in 200μl PBS. CD-RAG mice were immunized (i.p.) 1-2 times with DP178-Q16L peptide (10 μg) precipitated in alum and suspended in 200μl PBS 3.5-4 wk after CD B cell transfer. Secondary immunizations came 28 d after the primary immunization. Mice were bled 16 d after each immunization as indicated to determine antigen-specific serum Ab levels. Spleen and MLN were harvested 16 d post-immunization and analyzed via FACS and immunofluorescent labeling of tissue sections.
Immunofluorescence assays
Histology
A portion of the spleen and individual MLN from naïve and immunized mice were embedded in OCT compound, snap frozen using N2- chilled 2-methylbutane, and stored at −80°C. 5 μm sections were prepared using a cryostat and poly-lysine coated slides. Sections were fixed with 1:1 acetone:methanol for 10 min at −20°C and labeled with B220-biotin, TCRβ-PE (red) and GL-7-FITC (green) mAb. FITC signal was amplified using anti-FITCAF488 mAb (Invitrogen). Streptavidin-AlexaFluor350 (Invitrogen) was used to amplify B220-biotin signal (blue). Images were acquired using a Zeiss Axiovert 200M confocal immunofluorescent microscope. Slides bearing fixed Crithidia luciliae (Scimedx Corporation, Denville, NJ) were rehydrated (PBS (pH7.4); 30 min; 25°C). Samples were blocked (2 hr; 25°C) using PBS (pH7.2) containing rat anti-mouse CD16/CD32 (1%), purified rat IgG (5%) and Tween-20 (0.1%). Samples were washed (1 min) in PBS (pH7.2) containing BSA (1%) and Tween-20 (0.1%). Samples were labeled with serum (1:160) (2hrs; 25°C) followed by extensive washing (2x 250mls; 10min each; 1x 250mls; overnight). Ab was detected using goat anti-mouse IgG-FITC Ab (2hrs; 25°C) followed by extensive washing (3x 150mls; 10min each). Coverslips were mounted to slides using Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL). Images were acquired using a Zeiss Axiovert 200M confocal immunofluorescent microscope (400x magnification, 300 ms exposure).
Statistical Analysis
Student’s t-test (two-tailed) was used to determine the significance of any differences between matched data sets; significance was defined by p< 0.05.
Results
MPER tetramer binding to mouse B cells is specific
The 2F5 epitope is present in self-antigen kynureninase of both human and mouse (27). We hypothesized that the 2F5 epitope-binding B cell population in C57BL/6 mice is subject to regulation by tolerance mechanisms. In order to identify antigen-specific B cells, we prepared B cell tetramer reagents consisting of linear HIV-1 Env peptides synthesized with biotin and subsequently tetramerized with streptavidin-allophycocyanin (SA-APC) (40). Similar tetramer reagents have been used to identify and isolate B lymphocytes for HIV-1 Env antigens including the V3 loop of gp120 and the immunodominant region of gp41 (45, 46); their specificity has been demonstrated by surface plasmon resonance (SPR) and competitive inhibition (40, 47, 48).
Biotinylated polypeptides encompassing the 2F5 epitope were tetramerized with SA-APC. This MPER tetramer was bound only by human and mouse mAb specific for the 2F5 epitope of gp41 as determined by SPR and reactivity to mAb-coated beads [Fig. S1; (40)]. Matched tetramers bearing a scrambled MPER peptide were not bound by the same mAb and irrelevant mAbs did not bind to the MPER-tetramer [Fig. S1; (40)].
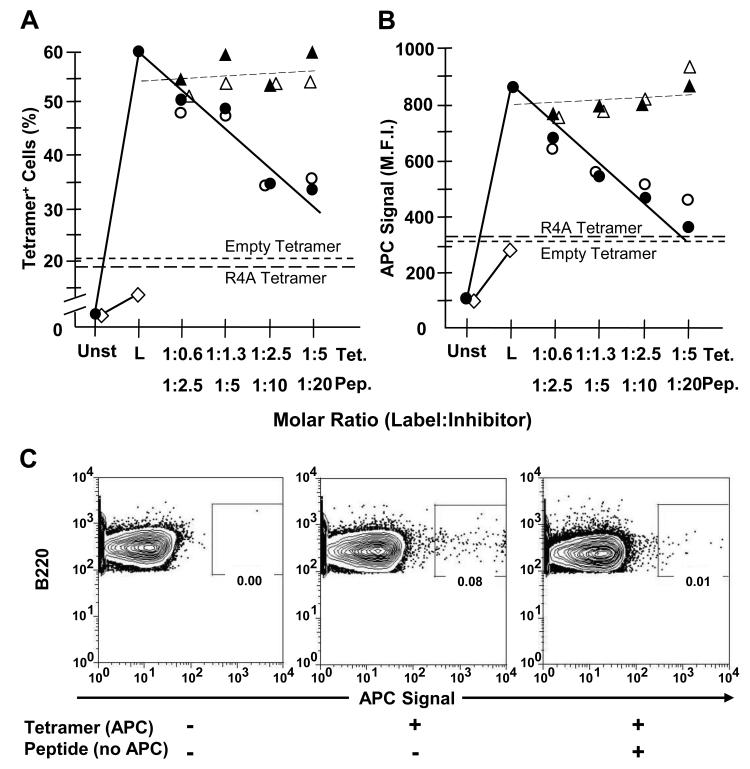
As expected, MPER-tetramer binding to 13H11 cells, a 2F5-epitope reactive hybridoma line (49), was highly specific (Fig. 1A, B). Approximately 60% of 13H11 cells were labeled by APC-conjugated MPER tetramer whereas APC-conjugated empty (no peptide) or irrelevant (R4A) tetramers labeled ≤20% of 13H11 cells (Fig. 1A, B). Labeling of the parental fusion line, P3, by MPER- or control tetramers was even lower (≈5%) (Fig. 1A). To further ensure the specificity of MPER-tetramer binding, 13H11 cells were incubated with either unlabeled homologous or irrelevant tetramer or peptide (0.6 to 20-fold molar excess) and subsequently exposed to APC-conjugated MPER-tetramer (representative histograms, Fig. S1). Homologous peptide and unlabeled tetramer comparably reduced the frequency and intensity of labeled 13H11 cells to background levels in a dose-dependent manner (Figs. 1A, B). In contrast, pre-incubation with heterologous peptide or tetramer resulted in little or no reduction of MPER-tetramer labeling (Figs. 1A, B).
Figure 1. Labeling by MPER-tetramer is blocked by homologous (MPER) but not heterologous (R4A) reagents.
13H11 cells (1-1.3 × 106) were incubated in PBS + 3% FCS containing equivalent molar excess amounts of either unlabeled SP62 (MPER) peptide (○), unlabeled MPER-tetramer (●) or control unlabeled R4A peptide (△) and unlabeled R4A tetramer (▲) for 30 min at 0°C. Unlabeled peptide and tetramer concentrations were established to represent 0.6, 1.3, 2.5 and 5.0 M excess of labeled tetramer-associated peptide epitope. Subsequently, cells were labeled (L) with 125 ng of APC-conjugated MPER-tetramer for 30 min at 0°C. Other 13H11 cells were labeled with either APC-conjugated Empty-tetramer (short dashes) or R4A-tetramer (long dashes) as negative controls for binding. Also, P3 (◇) cells were labeled with APC-conjugated MPER-tetramer as a negative control for binding. Cells were analyzed by FACS and (A) fraction of tetramer-binding cells and (B) M.F.I. of tetramer+ cells was determined. Each data point represents the average of at least 3 independent measurements (n≥3) compiled over 2 independent experiments. (C) BL/6 BM cells (106) were incubated alone or with 10-fold molar excess of MPER peptide. Then, BM cells were incubated alone or labeled with 125 ng of APC-conjugated MPER-tetramer. All samples were washed and labeled with mAb to B220. FACS plots are pre-gated on live, single, B220+ cells. Data are representative of 2 independent experiments.
To determine whether MPER-tetramer binding to mouse lymphocytes was equally specific, we incubated BL/6 BM cells (≈2×106) in ice-cold medium or medium containing a 10-fold molar excess of unlabeled MPER peptide, washed the cells and exposed them to APC-conjugated MPER tetramer (125 ng/106 cells). Subsequently the BM cells were reacted with B220 mAb to identify B-lineage cells. Whole BM cell populations contained a small (≤0.2%), but reproducible, populations of MPER-tetramer+ B220+ cells; in those BM samples pre-incubated with soluble, homologous peptide, the frequency of MPER-tetramer+ cells were reduced by ≥80% (Fig. 1C). We conclude that the substantial majority of B cells labeled by MPER-tetramer specifically bound the MPER-peptide, and that the MPER-tetramer identifies antigen-specific B cell populations (Fig. 1C).
MPER-reactive B cells are lost after transitional B cells leave the bone marrow
B cell development is strikingly impaired in 2F5 VH-KI mice by a developmental blockade consistent with the loss of self-reactive immature and transitional B cells by tolerance mechanisms (20). To determine whether MPER-reactive B cells expressing endogenous Ig rearrangements might also be lost during development, we determined the frequencies of MPER- and control tetramer binding B cells in distinct developmental compartments of the BM and spleen (Supplemental Fig. 2) (41, 43).
Empty tetramer controls were used to determine background of tetramer labeling in B cell subsets. Because MPER-tetramer positive B cells were rare (~0.3%), a stringent cut-off was defined: >4SD of the frequency of B cells binding empty tetramers.
Pro-/pre-B cells (B220loIgM−IgD−CD21−CD23−) and immature B cells (B220loIgM+IgD−CD21−CD23−) did not exhibit significant MPER-tetramer binding, whereas low but significant (p <0.05) frequencies (~0.3%) of transitional-1 (T1; B220loIgMhiIgDloCD21−CD23lo) and T2 (B220hiIgMhiIgDhiCD21loCD23hi) BM B cells were labeled by the MPER-tetramer (Fig. 2). In contrast, frequencies of splenic T1 and T2 B cells that bound MPER-tetramer were significantly lower (p <0.05), with splenic T1 binding falling below background (Fig. 2).
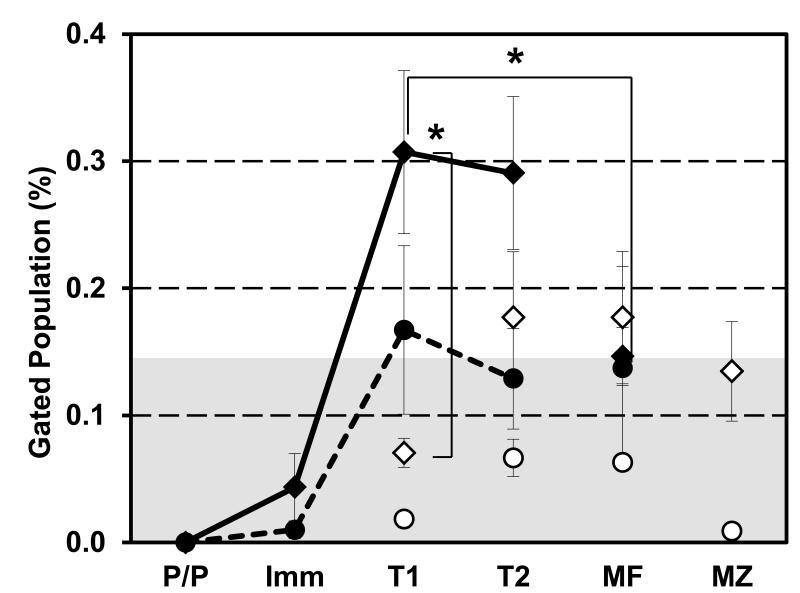
Figure 2. BM transitional compartments contain MPER-tetramer binding B cells that are absent in peripheral B cell compartments.
BL/6 BM and spleen cells were harvested for flow cytometry. Cells (106) were labeled with 125 ng of MPER-APC (◆) or R4A-APC (●) tetramer. Cells were washed and labeled with mAb to B220, IgM, IgD, CD23 and CD21. Specific B cell compartments of the BM (closed) and spleen (open) were identified (43). Unlabeled control and empty tetramer samples were acquired to determine the maximum background signal (gray background defined as mean + four times standard deviation of empty tetramer labelling). Pro/pre B (P/P), immature B (Imm), T1 B (T1), T2 B (T2), mature B (MF), and marginal zone B (MZ) cells were gated as shown in Supplemental Figure 2. Data presented as the average and S.E.M. of the percent (%) of tetramer+ cells within each B cell compartment. Each group contained multiple mice (◆; n≥10 BM & Spl, ●; n=4 BM & Spl for R4A) compiled over 2-3 independent experiment. Significant differences (*; p ≤ 0.05) between groups were determined by (two-tailed) Student’s t-test.
Reactivity with the MPER-tetramer was not a general property of BM B cells as mature, recirculating, B cells (B220hiIgMintIgDhiCD21loCD23hi) in the BM exhibited only background frequencies of MPER-binding (p <0.05 compared to BM T1 B cells; Fig. 2). Although splenic T2 and mature follicular (MF; B220hiIgMintIgDhiCD21loCD23hi) B cells were labeled by MPER-tetramer above background levels, tetramer binding in these compartments was about half that of BM T1 and T2 B cells (p <0.05). Like splenic T1 B cells, marginal zone (MZ; B220hiIgMhiIgDloCD21hiCD23lo) B cells were not labeled by MPER-tetramer above background levels (Fig. 2). Despite MPER-tetramer binding frequencies that fell above (T2 and MF) or below (T1 and MZ) background, the frequencies of MPER-binding, splenic B cells were not significantly different from each other (T1 to T2, p=0.051; T2 to MF, p=0.999; MF to MZ, p=0.497; T1 to MF, 0.052; T1 to MZ, 0.115; T2 to MZ, p=0.493).
The R4A peptide is a DNA mimetope and labels autoreactive B cells subject to tolerance control (44, 50). We observed a developmental kinetics for R4A-reactive B cells that was similar to that for the MPER-tetramer, with peak binding frequencies in the BM T1 and T2 compartments followed by substantial declines in all splenic compartments (Fig. 2, dashed and solid line). R4A-binding cells were less frequent, however, than those binding the MPER-tetramer and most of these values fell below background. Indeed, only BM T1 B cells exhibited a mean R4A-binding frequency above background (p >0.05) (Fig. 2). Nonetheless, the similar patterns of MPER and R4A-tetramer binding suggest that similar processes control the development of MPER- and DNA-reactive B cells.
Taken together, these data are consistent with the generation of HIV-1 MPER-reactive B cells and their subsequent loss following the T1 and T2 stages of B cell development in the BM. This period is known to be a major checkpoint of central B cell tolerance (51) and our experiments constitute the first demonstration of developmentally regulated reductions in the numbers of MPER-specific B cells in normal mice.
In vitro B cell culture supports the development of 2F5 VDJ-KI immature and transitional B cells
B cell development in 2F5 VDJ-KI mice is blocked resulting in significantly reduced numbers of immature, transitional and mature B cells (20). Earlier, we developed a stromal cell-independent, B cell culture method that generates substantial numbers of IgM+ B cells from pro-B and large pre-B cell precursors, including those normally lost to immunological tolerance (e.g., DNA-specific 3H9 HC-KI); autoreactive, CD B cells persist after adoptive transfer into congenic, RAG1 knockout recipients (37).
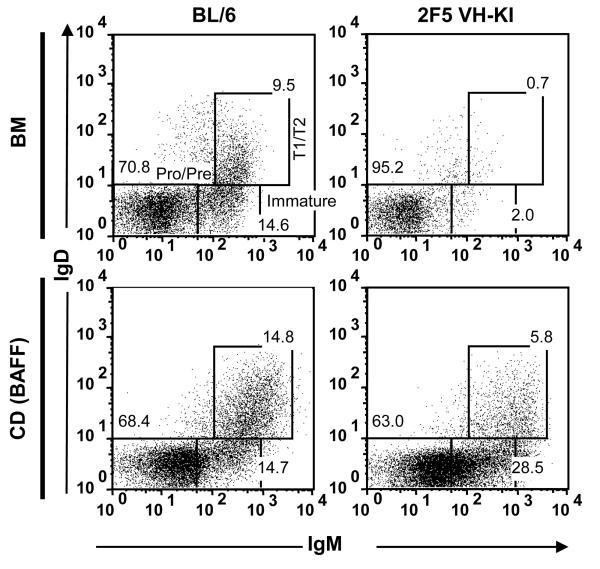
To determine whether this in vitro recovery might also rescue the development of immature and transitional B cells specific for the 2F5 epitope, we cultured non-adherent BM cells from 2F5 VDJ-KI knockin mice (20) and characterized the B cells that developed in vitro. Though the BM of 2F5 VDJ-KI mice contains significantly lower numbers (≤10% of BL/6; p<0.01) of immature and T1/T2 B cells [(Fig. 3) (20)], cultures of 2F5 VDJ-KI BM (37) contained substantially higher numbers (≈40% of BL/6 controls; p = 0.34) of immature (B220loIgM+IgDCD93hi) and T1/T2 (B220loIgMhiIgD+CD93+) 2F5 VDJ-KI B cells (Fig. 3). The increased frequencies of immature and transitional 2F5 VDJ-KI CD B cells suggests cultures are permissive for the development of 2F5-like B cells that are normally tolerized in vivo (20, 21, 27).
Figure 3. In vitro culture of 2F5 VH-KI BM rescues development of transitional B cells that were absent in vivo.
Non-adherent BL/6 (left) and 2F5 VH-KI (right) BM cells (top) were cultured with 10 ng/ml IL-7 followed by culture with 20ng/ml BAFF (bottom). Cells were labeled with mAbs to B220, IgM, IgD, and CD93 to identify B cell subsets. FACS diagrams were pre-gated on live, single B220+CD93+ cells using FlowJo software. FACS plots are representative of multiple (n=3) independent experiments.
Culture of BL/6 BM supports development of 2F5-like B cells
Mouse B lymphocytes that generate Ab specific for the 2F5 MPER epitope are rare [Fig. 2 and (40)]. As CD B cell populations contain clones that do not normally mature in vivo (38, 39), we tested whether these cultures support the development of 2F5-like B cells from normal BL/6 BM. After expansion and differentiation (37), CD cells were incubated with control (empty), R4A- or MPER-tetramers (Fig. 1 A-B) along with mAb reagents specific for B220, IgM, and IgD (37) to identify R4A- and MPER-tetramer binding B cells (Fig. 4A).
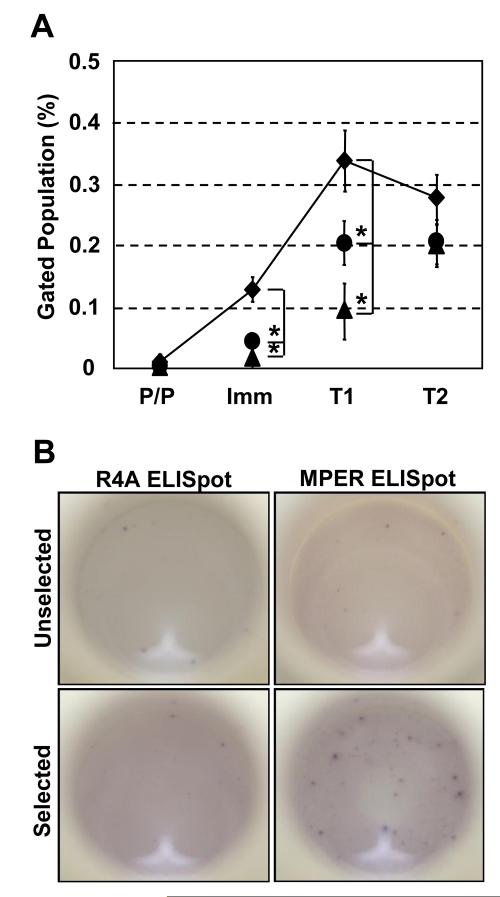
Figure 4. In vitro culture of BL/6 BM generates MPER-reactive B cells.
BM cells were cultured using a standard method to generate Ig+ B cells (37). (A) Cells were labeled with 125 ng of MPER-APC (◆), R4A-APC (●) or Empty-APC (▲) tetramer. Cells were washed and labeled with mAb to B220, IgM, and IgD. Tetramer binding was assessed on each population using a BD LSRII flow cytometer. Plots are pre-gated on live, single B220+ cells. Analysis of specific B cell subsets was performed using surface staining criteria described previously (37). Pro/pre B (P/P), immature B (Imm), T1 B (T1), T2 B (T2). Each data point represents the average and S.E.M. of multiple independent measurements (◆; n=14, ●; n=10, ▲; n=7) for each population compiled over 3-4 independent experiments. Significant differences (*; p < 0.05) between groups were determined by (two-tailed) Student’s t-test. (B) BM cells were cultured to generate Ig+ B cells (37). Live MPER-tetramer+B220+ cells were sorted using BD FACSVantage flow cytometer. biotin-MPER or biotin-R4A peptide were used to determine the frequency and enrichment of MPER peptide-specific AFC (all H-and L-chain types) using antigen-specific reverse ELISpot assay described in Materials and Methods. ELISpot images are representative of 2 independent experiments each performed in duplicate.
Whereas pro-/pre-B (B220loIgM−IgD−) CD cells did not exhibit (empty, R4A, or MPER) tetramer binding, MPER tetramer labeled significantly higher frequencies (p <0.05, compared to matched, empty tetramer controls) of immature (B220loIgMloIgD−) and T1 (B220loIgMhiIgDlo) CD B cells (Fig. 4A). Increased frequencies of R4A-tetramer binding cells were observed in the CD T1 compartment while empty tetramer labeled no or few immature or T1 CD B cells (≤ 0.1%) (Fig. 4A). For T2 (B220hiIgMhiIgDhi) CD B cells, the frequencies of MPER-binding cells remained elevated and similar to that observed in vivo (p = 0.40; Fig. 2) but were not significantly (p > 0.05) higher than the frequencies of cells labeled with empty- or R4A tetramers (Fig. 4A).
To confirm the presence of MPER-specific CD B cells, we induced CD B cells to differentiate into antibody forming cells (AFC) in the presence of BAFF and LPS (37) and enumerated R4A- and MPER-specific AFC using reverse ELISpot assays (40). The frequencies of R4A- and MPER-specific AFC (all isotypes/types) (~0.1-0.4%) in LPS/BAFF-activated CD B cells (Fig. 4B, unselected) were congruent with the frequencies of R4A- or MPER-specific B cells determined by tetramer labeling (Fig. 4A); prior enrichment of MPER-tetramer+ CD B cells by flow cytometry dramatically and specifically increased (~12-fold) the frequency of MPER-reactive AFC (Fig. 4B, selected). By comparison, selection of MPER-tetramer+ CD B cells showed no significant change (p > 0.05) in the frequency of R4A-reactive AFC (Fig. 4B). We conclude that in vitro, MPER-reactive CD B cells are generated from BL/6 BM and that tetramer labeling of B cells can be used to enumerate and enrich for antigen-specific cells (52).
Reconstitution with CD, but not LN cells, results in serum autoantibody
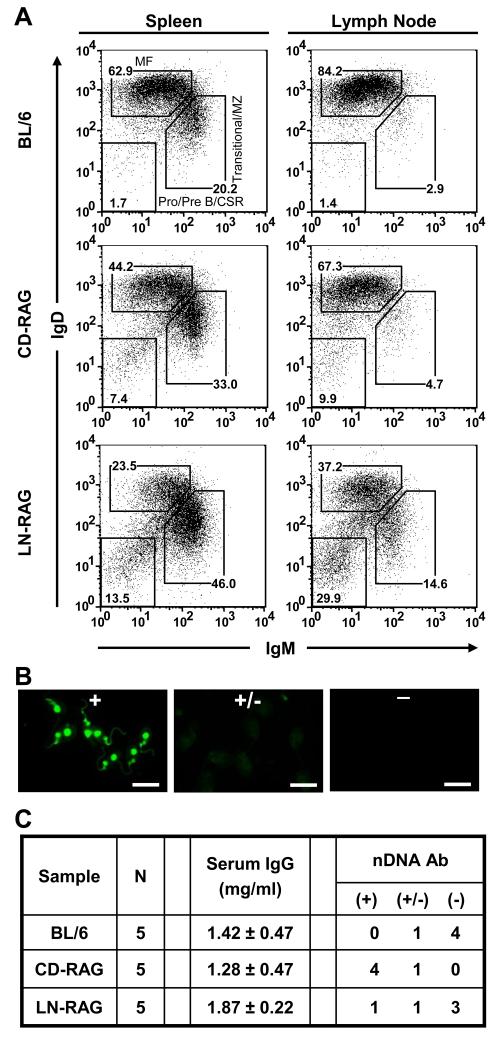
To generate CD- and LN-RAG mice, congenic RAG-1−/− mice were reconstituted with lymphocytes by i.v. transfer of (2 × 107) CD or LN cells (37). Both CD and LN cells effectively reconstituted RAG-1−/− mice as evidenced by comparable populations of B220+IgM+IgDhi cells present in spleen and LN four weeks after transfer (Fig. 5A). In addition, we observed substantial populations of B220hiIgM IgD− B cells in the spleen and LN of both CD- and LN-RAG mice, a phenotype consistent with B cells that have undergone class-switch recombination (CSR) (Fig. 5A). Interestingly, in both CD-RAG and LN-RAG animals, the frequency of splenic B cells with a transitional/MZ-like phenotype (B220hiIgMhiIgDlo) was elevated (33% and 46%, respectively) compared to BL/6 controls (20%; Fig. 5A). CD-RAG mice contained comparable frequencies of mature follicular (B220hiIgMintIgDhi) and transitional/MZ-like B cells to that of LN-RAG mice; however, LN-RAG mice contained elevated (~3 to 5-fold) frequencies of transitional/MZ-like B cells in the lymph node compared to either BL/6 or CDRAG mice (Fig. 5A). These data indicated that LN or CD cells were equivalent in their capacity to reconstitute RAG-1−/− mice.
Figure 5. RAG-1−/− mice reconstituted with CD B cells, but not LN B cells, results in serum autoantibody.
Non-adherent BL/6 BM cells were cultured to generate CD B cells for injection into B6.RAG-1−/− mice as previously described (37). BL/6 LN cells were isolated for transfer into B6.RAG-1−/− mice (LN-RAG). (A) At 6 wk post-transfer, spleen and LN cells from BL/6 (top), CD-RAG (middle) or LN-RAG (bottom) mice were labeled with mAbs to B220, IgM, and IgD. Flow diagrams were pre-gated on live, single, B220+ cells and B220+ cells were subdivided into IgM−IgD− pro-/pre-B/CSR B, IgMhiIgDlo transitional/MZ B, and IgMintIgDhi MF B cell compartments. Data are representative of each mouse analyzed (n=5 per group). Sera from each experimental group were collected via retro-orbital eye bleeding at 6 wk post-transfer. (B) Sera samples were diluted (1:160) and used to labeled C. luciliae substrate slides. After overnight washing, Ab bound to cells was detected using rat anti-mouse IgG-FITC Ab. All images were acquired using a Zeiss Axiovert 200M confocal immunofluorescent microscope with an exposure time of 300ms at 400x magnification. Representative examples of strong (++), weak (+) and no (−) nDNA binding activity are presented. Scale bar equals 20μm for all images. (C) Concentrations of serum IgG were determined using anti-mouse IgG-specific ELISAs including standard curves. Each sera sample (1:160 dil) was screened for reactivity to nDNA by immunofluorescent microscopy with a fixed exposure time (300ms) at 400x magnification. Each group contained multiple mice (n=5) that were screened independently.
We detected DNA autoantibody in the serum (1:160 dilution) of BL/6, CD- and LN-RAG mice using Crithidia luciliae direct immunofluorescence assay (53) by rating the observed binding as either strong (+), weak (+/−), or no (−) reactivity (representative images , Fig. 5B). BL/6 sera samples contained only weak (1/5) or no (4/5) reactivity to DNA (Fig. 5C). In contrast, CD-RAG sera samples contained mostly (4/5) strong reactivity to DNA (Fig. 5C), while most LN-RAG samples (3/5) showed no reactivity to DNA (Fig. 5C). To ensure that differences in serum DNA Ab were not the result of unequal IgG reconstitution, we directly measured serum IgG levels in BL/6, CD- and LN-RAG mice using ELISA (Fig. 5C). We observed that both CD- and LN-RAG mouse serum contained similar (~1.5 mg/ml) amounts of IgG to that of BL/6 controls (Fig. 5C). These data support our conclusion that the B cell repertoire formed in vitro is qualitatively different from the mature, peripheral B cell repertoire of BL/6 mice, indicating that the CD-RAG animal model can be used to study B cell populations that are normally excluded from the mature repertoire.
Robust germinal center responses in CD-RAG mice immunized with MPER antigen
CD B cells contained MPER-specific populations (Fig. 3, 4) and were able to reconstitute lymphocyte-deficient mice with a unique repertoire of B cells [(37) and Fig. 5]; we therefore immunized BL/6 and CD-RAG mice with MPER peptide precipitated in alum. The spleen and mesenteric LNs (MLN) of naive control and immunized mice (d16 post-immunization) were harvested and the frequency of germinal center (GC) B cells (B220hiGL-7hi) within the total B220+ population (Fig. 6A, B) was determined for each tissue. Additionally, the presence of GC structures was confirmed by histological analysis of spleen and MLN samples (representative examples in Fig. 6C, D). Reconstitution of RAG-1−/− mice with either CD or LN cells did not lead to hypertrophy of the spleen or MLN nor did we observe increased morbidity or mortality in reconstituted animals (not shown).
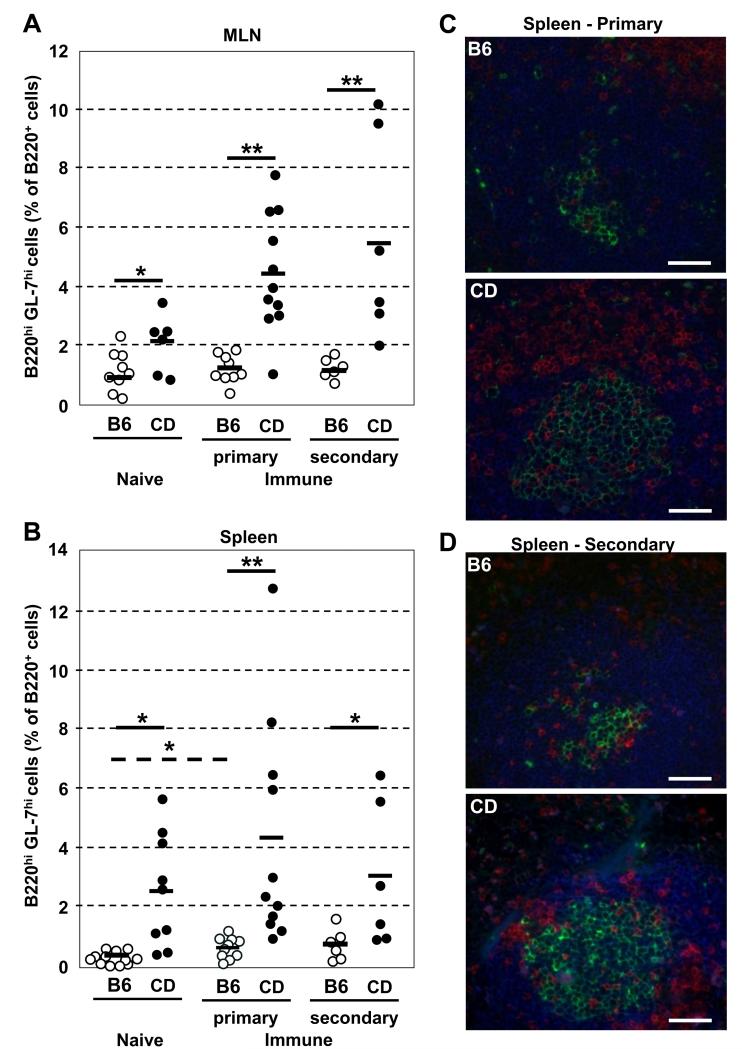
Figure 6. CD-RAG mice form robust GC responses after immunization with MPER peptide.
BL/6 (○) and CD-RAG (●) mice were immunized (i.p.) with 10 μg DP178-Q16L (MPER) peptide in alum. (A) MLN and (B) spleen cells were harvested at d16 after 1-2 immunizations. Cells were labeled with mAb to B220, IgM, IgD, TCRβ and GL-7. The percentage of B220hiGL-7hi B cells in B220hi cells was determined by flow cytometry. Each group contained multiple mice (n=6-12) compiled over multiple (n=2-4) independent experiments. Significant differences (*; p ≤ 0.05 and **; p ≤ 0.01) between groups were determined by (two-tailed) Student’s t-test. 5 μm sections of spleen from BL/6 and CD-RAG mice at d16 after (C) primary or (D) secondary immunizations were labeled with mAbs to B220-AF350 (blue), TCRβ-PE (red) and GL-7-FITC (green). FITC signal was amplified using anti-FITC-AF488 Ab. Scale bar equals 50μm for all images. Images were acquired using a Zeiss Axiovert 200M confocal immunofluorescent microscope at 200x magnification.
In BL/6 mice, immunization with MPER antigen did not increase (1° p=0.80; 2° p=0.52) the frequency of MLN GC B cells when compared to naïve animals (Fig. 6A). Histological analysis (Fig. 6C) confirmed that BL/6 mice did not form robust splenic GC responses after MPER antigen immunization, consistent with minimal increases (1° p=0.04; 2° p=0.10) in the frequency of GC B cells determined by FACS (Fig. 6B). The lack of robust GC responses in BL/6 mice after MPER-peptide immunization is consistent with the loss of MPER-specific mature B cells (Fig. 2).
In contrast, immunization of CD-RAG mice with MPER antigen significantly increased (1° p=0.01; 2° p=0.05) the frequency of MLN and splenic GC B cells (Fig. 6A-B). Histological analysis of spleen samples from these immunized CDRAG mice confirmed that GL-7hi B cells were organized into GC structures (Fig. 6C, D). Compared to BL/6 controls, CD-RAG mice harbored significantly elevated (MLN: 1° p<0.01; 2° p=0.01 and Spl: 1° p=0.01; 2° p=0.05) frequencies of GC B cells after each immunization with MPER antigen (Fig. 6A, B). These data demonstrate that CD-RAG mice mount robust GC responses to MPER antigen immunization and we correlate these enhanced GC responses to MPER with the recovery of MPER-reactive B cells in the in vitro culture system.
Enhanced serum IgG responses to MPER in CD-RAG mice
Historically, 2F5-like gp41 MPER-specific serum Ab is poorly elicited in mouse models after immunization with HIV-1 antigen (15-17, 54). CD B cells reconstituted peripheral lymphoid tissues, organized into follicles, and formed robust GC upon MPER antigen immunization (Fig. 5, 6). We therefore collected serum Ab of naïve and immunized CD-RAG mice, and quantified antigen-specific Ab by ELISA. As a positive control, we immunized BL/6 and CD-RAG mice with NP-CGG and compared NIP-specific IgG response to the generation of gp41 MPER-reactive Ab.
Immunization of BL/6 and CD-RAG mice with NP-CGG/alum elicited a large increase (~100- and 30-fold, respectively) in NIP-specific serum IgG Ab compared to naïve animals (Fig. 7A). NIP-specific serum IgG of CD-RAG mice was ~3-fold less than elicited in BL/6 mice (Fig. 7A), indicating that CD-RAG animals are capable of mounting a B cell response to antigen immunization that is proportional to their level of cellular reconstitution.
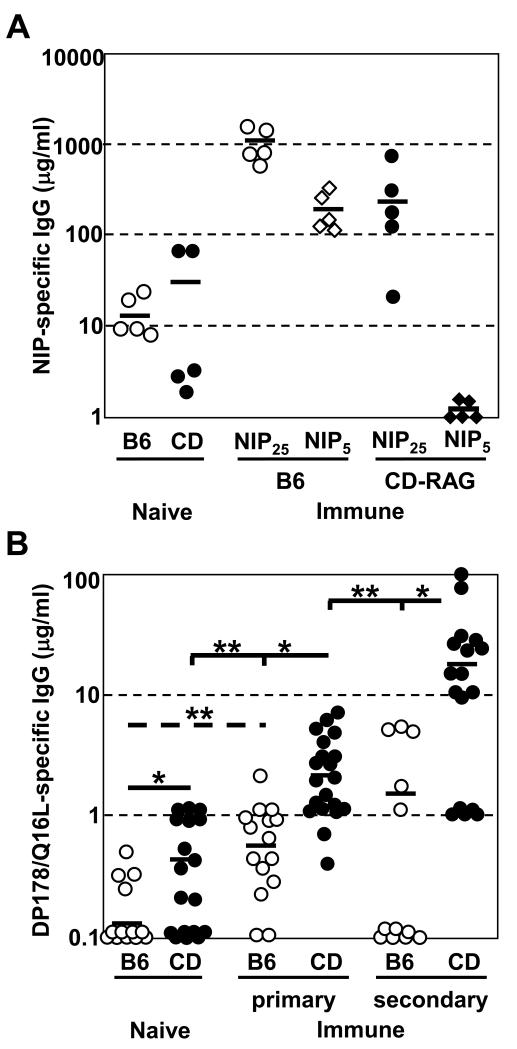
Figure 7. MPER-specific serum IgG responses are enhanced in CD-RAG mice, but not in BL/6 mice, after immunization.
(A) BL/6 (n=5) and CD-RAG (n=5) mice were immunized (i.p.) with 10 μg NP13-CGG in alum. Serum was harvested at day12 post-immunization. ELISA plates were coated with either NIP25-BSA or NIP5-BSA capture antigens. NIP-specific Ab was detected using goat anti-mouse IgG Ab. Purified H33Lγ1 (IgG) mAb was used as a standard curve to calculate antigen-specific serum Ab concentration. These results are from 2 independent experiments. (B) BL/6 (n=12-15) and CDRAG (n=17-20) mice were immunized (i.p.) 1-2 times with 10 μg MPER peptide in alum. Serum was harvested at d16 post-immunization. ELISA plates were coated with MPER-specific capture antigen and bound Ab was identified using a goat anti-mouse IgG detection reagent. Purified 13H11 mAb was used as a standard curve to calculate antigen-specific serum Ab concentration. These results are pooled from 3-4 independent experiments. Significant differences (*; p < 0.05, **; p < 0.01) between groups were determined by (two-tailed) Student’s t-test.
B cells modify their B cell receptor in the GC reaction resulting in the preferential expansion of high affinity clones while low affinity clones are eliminated, a process termed affinity maturation (55-57). To compare the extent of affinity maturation, we measured high-affinity serum IgG reactivity to NIP using NIP5-BSA-coated ELISA. Whereas in immunized BL/6 mice ~30% of NIP25-BSA-reactive serum IgG binds NIP5-BSA, NIP5-reactive IgG is essentially non-existent in the serum of immunized CD-RAG mice (Fig. 7A).
Prior to immunization, sera from most naive animals did not contain detectable amounts of MPER-reactive IgG Ab. However, 4/13 naïve BL/6 mice and 11/18 CD-RAG mice contained MPER-specific IgG (~0.5 μg/ml) that was near the limit of detection by ELISA (Fig. 7B). If such low levels of MPER Ab represent authentic binding, the enriched MPER-reactivity in naïve CD-RAG mice is consistent with higher levels of serum autoantibody (37). After primary immunization of BL/6 mice with MPER peptide, we detected a significant increase (p<0.01) in MPER-specific serum IgG; however, the average amount of Ab was low (~0.7 μg/ml) (Fig. 7B). After secondary challenge of BL/6 mice with MPER peptide, the level of antigen-specific IgG did not significantly increase (~2-fold) over primary challenge (Fig. 7B), indicating the humoral memory to this MPER peptide was not formed.
In contrast, primary immunization of CD-RAG mice resulted in significantly (p<0.01) increased MPER-specific IgG serum Ab than in immunized BL/6 mice (Fig. 7B). Indeed, the actual capacity to elicit MPER-specific IgG responses is likely underestimated in CD-RAG mice because the mature B cell compartments were not fully reconstituted (Fig. 5A) and humoral responses to a model antigen, NP-CGG, were only 30% of that mounted by BL/6 controls (Fig. 7A). The actual enhancement of MPER-specific IgG responses in CD-RAG mice may be >12-fold greater than BL/6 mice. CD-RAG mice that received secondary immunization significantly (p<0.01) increased (~10-fold) the amount of MPER-specific IgG (~25μg/ml) over primary challenge (Fig. 7B). This robust expansion of MPER-specific IgG suggests that CD-RAG mice had formed humoral memory to the MPER peptide during the initial immunization. These data demonstrate that the humoral immune response to this HIV-1 gp41 MPER peptide antigen can be restored in mice when the constraints of B cell tolerance have been relaxed.
Discussion
The inability to mount robust B cell responses to several conserved, neutralizing MPER epitopes appears to be phylogenetically conserved from rodents to humans (58, 59). Recent work has characterized the Ab response to HIV gp41 in patients whose serum contains moderate-to-high virus neutralizing activity (60). Interestingly, these mAbs were not able to compete away the binding of the 2F5 and 4E10 bnAbs for their respective MPER epitopes (60), illustrating the rarity of humoral responses to the MPER region of the gp41 envelope antigen. We previously confirmed that the 2F5 epitope of the MPER mimics the self-antigen kynureninase, and that the abundance of B cells reactive to such epitopes may be limited by tolerance mechanisms (20, 21, 23, 27).
An alternative explanation for the late onset and infrequency of MPER bnAb responses to HIV-1 infection and vaccines is that these antigens are normally evanescent or imperfectly represented in vaccine immunogens. The possibility of imperfect antigens has been rigorously addressed by Schief and colleagues who have generated immunogenic antigen “scaffolds” that stabilize and present the 2F5 epitope in its proper structural configuration (61, 62). Indeed, this scaffold immunogen elicited non-neutralizing serum Abs that otherwise closely resembled the 2F5 bnAb; even when presented in this optimized form, however, the fraction of Abs directed to the 2F5 MPER epitope constituted <0.1% of the induced IgG Ab (61). This miniscule response emphasizes the intrinsically weak immunogenicity of the 2F5 MPER epitope, even when its availability and stability are ensured. We propose that the simplest explanation for the weak immunogenicity of the 2F5 linear epitope is the removal of (most) responsive B cells by immune tolerance.
Without resorting to transgenic or “knock-in” mice, it is possible to identify antigen-specific B cells either by antigen-binding (63-65) or by anti-idotypic mAb (66, 67). The development of B cell tetramers, analogous to those routinely used to identify antigen-specific T cells (68), has enhanced the ability to identify and isolate antigen-specific B cells despite their low frequencies (45, 46, 69-71). If MPER reactive B cells react with the kynureninase self-antigen and are consequently purged by immune tolerance, the enumeration of MPER-reactive B cells before and after tolerance checkpoints should reveal stage-and antigen-specific B cells losses (28, 72, 73).
Our tetramer studies demonstrate the loss of MPER-tetramer reactive cells in the splenic T1/T2 B cell compartments, suggesting their removal by central tolerance mechanisms (e.g. deletion or receptor editing). A critical question is whether other B cell compartments that are typically enriched for self-reactivity contain elevated frequencies of HIV-1 MPER-reactive cells. In mice, the marginal zone (MZ) B cell compartment is a natural reservoir of autoreactive B cells [reviewed in (74)]. Our data demonstrate that in normal mice, the MZ B cell compartment was not enriched for MPER-tetramer+ B cells (Fig. 2), indicating that MPER-reactive B cells are efficiently deleted or edited as demonstrated in knockin animals (20, 21). In contrast, our data indicate that B cells reactive to this MPER epitope can be recovered in mice that have been reconstituted with B cells that have matured in the absence of the first tolerance checkpoint. CD B cells are enriched for autoreactive specificities [(37), and Fig. 5C] and substantially increase humoral responses to the 2F5 epitope shared by the HIV-1 MPER and KYNU [(27), Figs. 6, and 7B]. We submit that the enhanced GC and serum Ab responses of CDRAG mice are analogous to our immunizations of laboratory opossums: opossums mount robust humoral response to the 2F5 MPER epitope because their KYNU lacks the shared determinant and does not tolerize (27), whereas responses are enhanced in CD-RAG mice because their B cells have not been tolerized by self-antigens.
We have reported that C57BL/6 mice mount poor humoral responses to the MPER peptide compared to BALB/c mice and that BALB/c mice harbor significantly higher frequencies of MPER-reactive splenic B cells than do C57BL/6 and that this difference maps to the Igh locus (40). These observations indicate that genetics may play a crucial role in the efficacy of tolerization for MPER-/self-reactive B cells. Interestingly, BALB/c mice are known to be relatively more susceptible to autoimmune syndromes than are C57BL/6 animals (75-78), supporting the possibility that the abundance of MPER-specific B cells may be enriched when tolerance controls are reduced (79).
Although 2F5 contains a long, hydrophobic CDR3 and exhibits affinity for cardiolipin and phosphatidylserine (PS) (18), the 2F5 binding to self-antigen KYNU is only blocked by MPER peptide and not by cardiolipin (27). We conclude that it is the KYNU/2F5 MPER determinant and not generalized lipid-reactivity that is critical for the induction of tolerance control. Indeed, using MPER-tetramers we directly demonstrated reduction of B cells specific for the MPER peptide during B cell development, verifying that reactivity to the MPER peptide mediates the tolerizing deletion of 2F5-like B cells. This notion is supported by observations that many residual peripheral B cells that escape central clonal deletion in 2F5 VH-KI mice lose MPER reactivity but retain their capacity to bind lipids (21).
Normally, the GC reaction must balance affinity maturation with the elimination of self-reactive B cells that arise as a consequence of somatic hypermutation (80). Therefore, it is somewhat surprising that both 2F5 and 4E10 exhibit the hallmarks of affinity maturation in the GC, yet retain high affinity for phylogenetically conserved self-antigens (27). Although it is known that post-GC B cells may acquire increased autoreactivity (81), we propose that for B cells to acquire HIV-neutralizing capacity, a tortuous path of mutation and antigen-mediated selection is required to balance tolerizing clonal with increased affinity for viral epitopes that mimic host determinants (82). In mice, the mature peripheral B cell pool is purged of MPER-reactive cells that would be normally recruited to the GC reaction upon infection or immunization (Fig. 2). Our B cell transfer model may enhance Ab and GC responses to the 2F5 and 4E10 epitopes simply by increasing the initial frequencies of B cells that can initiate this difficult path to Ab production.
That immune tolerance may be a major factor in the weak immunogenicity of neutralizing MPER epitopes has been challenged by the discovery of the 10E8 bnAb (14). This bnAb recognizes an epitope that overlaps the conserved 4E10 MPER determinant and exhibits great neutralizing breadth [98% (178/181) of HIV-1 isolates tested] in vitro. The 10E8 bnAb was reported to have no affinity for phospholipids, including phosphatidyl choline-cardiolipin, did not label fixed HEp-2 human epithelial cells, nor did it bind several human autoantigens diagnostic for various autoimmune diseases (14). Nonetheless, the 10E8 bnAb is encoded by highly mutated V(D)J rearrangements that are typical of virtually all potent bnAb and indicative of complex maturation pathways (82). The extraordinary frequency of V(D)J mutations in the 10E8 bnAb is consistent with the recruitment of mutated, cross-reactive B cells into the empty niche created by the tolerization of B cells specific for an MPER epitope that mimics a self-antigen. We predict, therefore, that 10E8 may recognize some host antigen(s) that was absent in the initial screenings for autoreactivity. Indeed, in a protein microarray (27), 10E8 exhibited no polyreactivity but strong affinity for a human protein that is expressed in testis, brain, pituitary gland, and several fetal tissues. (in preparation, M. Liu, G.Y. B.F.H. and G.K.). Whether this autoreactivity is physiologically significant remains to be determined by the generation of 10E8 knockin mice (20, 21, 23).
Our experiments are likely relevant to HIV-1 vaccine design and development. MPER-specific Ab responses in normal C57BL/6 mice are virtually absent [Figs. 6, 7; (40)] but this deficit is substantially ameliorated by reducing B cell tolerance (Fig. 7). CD-RAG mice are genetically identical to their intact controls and differ only in the way the B cell repertoire develops. Significantly, CD-RAG mice do not exhibit any evidence of systemic autoimmunity; changes in the repertoire of CD B cells do not lead to evident autoimmunity but are permissive for MPER responses. We conclude, therefore, that even small differences in the repertoire of naïve B cells can result in significant differences in the intensity and scope of HIV-1 serum Ab responses. It would be interesting to determine whether differences in the repertoire of naïve human B cells might be associated with different probabilities of mounting bnAb responses. Could a modest relaxation of B cell tolerance increase the chances for bnAb induction in response to infection or immunization? If so, one might expect bnAb activity to be present inHIV-1 patients with disordered B cell tolerance. Indeed, an autoreactive mAb, CH98, recently recovered from an SLE/HIV-1 patient has been found to possess strong bnAb activity, suggesting that bnAb and SLE autoAbs may arise from the similar pools of B cells (83).
More speculatively, if the likelihood of mounting bnAb responses is increased by broadening the primary B cell repertoire, pharmacologic agents [e.g., BAFF (84, 85)] that transiently suppress B cell tolerance may be useful in conjunction with HIV-1 vaccines. We imagine that HIV-1 vaccines that normally activate few or no naïve B cells [(Figs. 6, 7) (61, 62)] might induce enhanced Ab responses in the context of a more diverse B cell repertoire.
Supplementary Material
Acknowledgements
We are grateful for the technical assistance of D. Liao, Y. Li, and L.C. Armand. This study was supported in part by NIH grants AI81579 and AI91693 (G.K.), the Center for HIV AIDS Vaccine Immunology and Immunogen Design [UMI-AI100645 (B.F.H.)], and the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (B.F.H.).
Abbreviations
- Alum
aluminum sulfate
- APC
allophycocyanin
- AFC
antibody forming cell
- BAFF
B cell activating factor belonging to the TNF family
- bnAb
broadly neutralizing antibody
- CD
culture-derived
- CD-RAG
RAG-deficient mice reconstituted with CD B cells
- CGG
chicken gamma-globulin
- CSR
class-switch recombination
- LN-RAG
RAG-deficient mice reconstituted with LN cells
- MF
mature follicular
- MZ
marginal zone
- MPER
membrane proximal external region
- MFI
mean fluorescence intensity
- MLN
mesenteric lymph node
- NP
4-hydroxy-3-nitrophenylacetyl hapten
- PI
propidium iodide
- PS
phosphatidylserine
- P/S
penicillin/streptomyacin
- T1
transitional-1
- T2
transitional-2
References
- 1.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS. 2009;4:373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 3.Shen X, Parks RJ, Montefiori DC, Kirchherr JL, Keele BF, Decker JM, Blattner WA, Gao F, Weinhold KJ, Hicks CB, Greenberg ML, Hahn BH, Shaw GM, Haynes BF, Tomaras GD. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J Virol. 2009;83:3617–3625. doi: 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 5.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 7.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 8.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 9.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 11.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson JD, Brunel FM, Jensen R, Crooks ET, Cardoso RM, Wang M, Hessell A, Wilson IA, Binley JM, Dawson PE, Burton DR, Zwick MB. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J Virol. 2007;81:4033–4043. doi: 10.1128/JVI.02588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derby NR, Kraft Z, Kan E, Crooks ET, Barnett SW, Srivastava IK, Binley JM, Stamatatos L. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J Virol. 2006;80:8745–8762. doi: 10.1128/JVI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coeffier E, Clement JM, Cussac V, Khodaei-Boorane N, Jehanno M, Rojas M, Dridi A, Latour M, El Habib R, Barre-Sinoussi F, Hofnung M, Leclerc C. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine. 2000;19:684–693. doi: 10.1016/s0264-410x(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 17.Eckhart L, Raffelsberger W, Ferko B, Klima A, Purtscher M, Katinger H, Ruker F. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J Gen Virol. 1996;77(Pt 9):2001–2008. doi: 10.1099/0022-1317-77-9-2001. [DOI] [PubMed] [Google Scholar]
- 18.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 19.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum Antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 20.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 Broad Neutralizing Antibody-Expressing B Cells in 2F5 VH × VL Knockin Mice Reveals Multiple Tolerance Controls. J Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle-Cooper C, Hudson KE, Cooper AB, Ota T, Skog P, Dawson PE, Zwick MB, Schief WR, Burton DR, Nemazee D. Immune Tolerance Negatively Regulates B Cells in Knock-In Mice Expressing Broadly Neutralizing HIV Antibody 4E10. J Immunol. 2013 doi: 10.4049/jimmunol.1301285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Zhang J, Hwang KK, Bouton-Verville H, Xia SM, Newman A, Ouyang YB, Haynes BF, Verkoczy L. Common Tolerance Mechanisms, but Distinct Cross-Reactivities Associated with gp41 and Lipids, Limit Production of HIV-1 Broad Neutralizing Antibodies 2F5 and 4E10. J Immunol. 2013;191:1260–1275. doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 25.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 26.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 27.Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, Kepler TB, Alam SM, Liao HX, Cain DW, Spicer L, Vandeberg JL, Haynes BF, Kelsoe G. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013;210:241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 29.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 30.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 32.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 36.Adams E, Basten A, Goodnow CC. Intrinsic B-cell hyporesponsiveness accounts for self-tolerance in lysozyme/anti-lysozyme double-transgenic mice. Proc Natl Acad Sci U S A. 1990;87:5687–5691. doi: 10.1073/pnas.87.15.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holl TM, Haynes BF, Kelsoe G. Stromal cell independent B cell development in vitro: Generation and recovery of autoreactive clones. J Immunol Methods. 2010 doi: 10.1016/j.jim.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandel PC, Gendelman M, Kelsoe G, Monroe JG. Definition of a novel cellular constituent of the bone marrow that regulates the response of immature B cells to B cell antigen receptor engagement. J Immunol. 2001;166:5935–5944. doi: 10.4049/jimmunol.166.10.5935. [DOI] [PubMed] [Google Scholar]
- 39.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10:289–299. doi: 10.1016/s1074-7613(00)80029-1. [DOI] [PubMed] [Google Scholar]
- 40.Verkoczy L, Moody MA, Holl TM, Bouton-Verville H, Scearce RM, Hutchinson J, Alam SM, Kelsoe G, Haynes BF. Functional, non-clonal IgMa-restricted B cell receptor interactions with the HIV-1 envelope gp41 membrane proximal external region. PLoS One. 2009;4:e7215. doi: 10.1371/journal.pone.0007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 42.Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray ES, Moody MA, Wibmer CK, Chen X, Marshall D, Amos J, Moore PL, Foulger A, Yu JS, Lambson B, Karim S. Abdool, Whitesides J, Tomaras GD, Haynes BF, Morris L, Liao HX. Isolation of a monoclonal antibody that targets the alpha-2 helix of gp120 and represents the initial autologous neutralizing-antibody response in an HIV-1 subtype C-infected individual. J Virol. 2011;85:7719–7729. doi: 10.1128/JVI.00563-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moody MA, Yates NL, Amos JD, Drinker MS, Eudailey JA, Gurley TC, Marshall DJ, Whitesides JF, Chen X, Foulger A, Yu JS, Zhang R, Meyerhoff RR, Parks R, Scull JC, Wang L, Vandergrift NA, Pickeral J, Pollara J, Kelsoe G, Alam SM, Ferrari G, Montefiori DC, Voss G, Liao HX, Tomaras GD, Haynes BF. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol. 2012;86:7496–7507. doi: 10.1128/JVI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, Xia SM, Montefiori DC, Tomaras GD, Weinhold KJ, Karim SA, Hicks CB, Liao HX, Robinson J, Shaw GM, Haynes BF. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beger E, Deocharan B, Edelman M, Erblich B, Gu Y, Putterman C. A peptide DNA surrogate accelerates autoimmune manifestations and nephritis in lupus-prone mice. J Immunol. 2002;168:3617–3626. doi: 10.4049/jimmunol.168.7.3617. [DOI] [PubMed] [Google Scholar]
- 51.Melchers F, Rolink AR. B cell tolerance--how to make it and how to break it. Curr Top Microbiol Immunol. 2006;305:1–23. doi: 10.1007/3-540-29714-6_1. [DOI] [PubMed] [Google Scholar]
- 52.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 53.Gilkeson GS, Pippen AM, Pisetsky DS. Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. J Clin Invest. 1995;95:1398–1402. doi: 10.1172/JCI117793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrantelli F, Ruprecht RM. Neutralizing antibodies against HIV -- back in the major leagues? Curr Opin Immunol. 2002;14:495–502. doi: 10.1016/s0952-7915(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 55.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 56.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 58.Graham BS. Clinical trials of HIV vaccines. Annu Rev Med. 2002;53:207–221. doi: 10.1146/annurev.med.53.082901.104035. [DOI] [PubMed] [Google Scholar]
- 59.Letvin NL, Barouch DH, Montefiori DC. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu Rev Immunol. 2002;20:73–99. doi: 10.1146/annurev.immunol.20.081501.094854. [DOI] [PubMed] [Google Scholar]
- 60.Pietzsch J, Scheid JF, Mouquet H, Seaman MS, Broder CC, Nussenzweig MC. Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity. J Virol. 84:5032–5042. doi: 10.1128/JVI.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, Kwong PD. Elicitation of structure-specific antibodies by epitope scaffolds. Proc Natl Acad Sci U S A. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guenaga J, Dosenovic P, Ofek G, Baker D, Schief WR, Kwong PD, Karlsson Hedestam GB, Wyatt RT. Heterologous epitope-scaffold prime:boosting immuno-focuses B cell responses to the HIV-1 gp41 2F5 neutralization determinant. PLoS One. 2011;6:e16074. doi: 10.1371/journal.pone.0016074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJ. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lalor PA, Nossal GJ, Sanderson RD, McHeyzer-Williams MG. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992;22:3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- 65.McHeyzer-Williams MG, Nossal GJ, Lalor PA. Molecular characterization of single memory B cells. Nature. 1991;350:502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- 66.Takemori T, Tesch H, Reth M, Rajewsky K. The immune response against anti-idiotope antibodies. I. Induction of idiotope-bearing antibodies and analysis of the idiotope repertoire. Eur J Immunol. 1982;12:1040–1046. doi: 10.1002/eji.1830121210. [DOI] [PubMed] [Google Scholar]
- 67.Reth M, Imanishi-Kari T, Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979;9:1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- 68.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 69.Newman J, Rice JS, Wang C, Harris SL, Diamond B. Identification of an antigen-specific B cell population. J Immunol Methods. 2003;272:177–187. doi: 10.1016/s0022-1759(02)00499-4. [DOI] [PubMed] [Google Scholar]
- 70.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O’Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S, Futatsugi-Yumikura S, Fukuoka A, Yoshimoto T, Nakanishi K, Yonehara S. Fas deficiency in mice with the Balb/c background induces blepharitis with allergic inflammation and hyper-IgE production in conjunction with severe autoimmune disease. Int Immunol. 2013;25:287–293. doi: 10.1093/intimm/dxs109. [DOI] [PubMed] [Google Scholar]
- 76.Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- 77.Lohler J, Sadlack B, Schorle H, Klebb G, Haber H, Horak I. [Generalized autoimmune diseases in BALB/c mice with a genetically dependent interleukin-2 deficiency] Verh Dtsch Ges Pathol. 1996;80:293–296. [PubMed] [Google Scholar]
- 78.Baddack U, Hartmann S, Bang H, Grobe J, Loddenkemper C, Lipp M, Muller G. A chronic model of arthritis supported by a strain-specific periarticular lymph node in BALB/c mice. Nat Commun. 2013;4:1644. doi: 10.1038/ncomms2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verkoczy L, Kelsoe G, Moody MA, Haynes BF. Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Curr Opin Immunol. 2011;23:383–390. doi: 10.1016/j.coi.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonsignori M, Wiehe K, Grimm SK, Lynch R, Yang G, Kozink DM, Perrin F, Cooper A, Hwang KK, Chen X, McKee K, Parks R, Eudailey J, Clowse M, Criscione-Schreiber LG, Moody MA, Ackerman ME, Liao HX, Tomaras GD, Gao F, Kelsoe G, Kepler TB, Montefiori D, Mascola JR, Haynes BF. An autoreactive broadly neutralizing CD4 binding site antibody from an HIV-1 controller with systemic lupus erythematosus. J Clin Invest. 2014 doi: 10.1172/JCI73441. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levine MH, Haberman AM, Sant’Angelo DB, Hannum LG, Cancro MP, Janeway CA, Jr., Shlomchik MJ. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci U S A. 2000;97:2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.