Abstract
Epstein-Barr virus (EBV) is a lymphotropic herpesvirus. However, access to B lymphocytes during primary infection may be facilitated by replication in mucosal epithelial cells. Attachment and penetration of EBV into these two cell types are fundamentally different. Both the distribution of receptors and the cellular origin of the virus impact the efficiency of infection. Epithelial cells potentially offer a wide range of receptors with which virus can interact. We report here on analyses of epithelial cells expressing different combinations of receptors. We find that the stoichiometry of the virus glycoprotein complex that includes gHgL and gp42 affects the use of gHgL not just for entry into epithelial cells but also for attachment. Penetration can be mediated efficiently with either a coreceptor for gp42 or gHgL, but the use of gHgL for attachment as well as penetration greatly compromises its ability to mediate entry.
Epstein-Barr virus (EBV) is predominantly a lymphotropic herpesvirus. It is the etiologic agent of most cases of infectious mononucleosis and has been implicated in development of immunoblastic lymphoma, endemic Burkitt's lymphoma, and certain types of Hodgkin's disease. However, the virus also has tropism for epithelial cells. It causes oral hairy leukoplakia, a wart-like lesion of the oral cavity, and is associated with development of nasopharyngeal and gastric carcinomas (28). Initiation of infection of these two key targets, B lymphocytes and epithelial cells, is substantially different. It probably involves different routes (18) and certainly involves different envelope glycoproteins and cell receptors.
B-cell infection is initiated by attachment of glycoprotein gp350/220 to the complement receptor type 2 (CR2) (6, 21, 22, 34). Entry requires fusion of virus with the B-cell membrane, which is mediated by glycoprotein gB (8), and a noncovalently linked complex of three glycoproteins, gH, gL, and gp42 (9, 20, 37). Glycoprotein gL serves as a chaperone for gH (40), and a recombinant virus with gH deleted also lacks gL (20). Thus, with few exceptions, the functions of gH and gL cannot be mapped to either one of the two components. However, the third protein, gp42, plays no known role in gHgL maturation and is unique among human herpesviruses. It interacts with HLA class II (32), which functions as an essential coreceptor for B-cell infection (7, 14). A monoclonal antibody (MAb) to gp42 that blocks the interaction with HLA class II inhibits virus cell fusion (15, 19), and a MAb to HLA class II that blocks gp42 binding neutralizes virus infection. In further support of a critical role for gp42 in B-cell infection, a virus that lacks gp42 fails to infect B cells unless cells and bound virus are fused with polyethylene glycol (37) or a soluble form of gp42 which lacks a transmembrane domain but retains the ability to bind to gH and gL is added in trans (38).
In contrast, not only is gp42 completely dispensable for entry into epithelial cells that do not constitutively express HLA class II, its presence is also inhibitory. Stoichiometric analysis of virus demonstrated the presence of much larger amounts of gHgL than gp42 in the virion, implying that some complexes naturally lack or are low in gp42. Saturation of the complexes by addition of soluble gp42 in trans blocked epithelial infection (38). In addition, infection of epithelial cells, but not B cells, could be blocked by antibodies that interacted with gH or gHgL alone. These findings were interpreted to mean that there is a coreceptor on epithelial cells, which we refer to as gHgLR, that can substitute for HLA class II and with which gHgL interacts in the absence of gp42. The absolute requirement for gp42 in B-cell infection coupled with the inhibitory effect it had on epithelial cell infection suggested that gp42 could function as a molecular switch of virus tropism.
Two sets of observations support these hypotheses. The first provided evidence for a direct interaction between gHgL and the epithelial surface. Some epithelial cell lines express at least low levels of CR2, and recent studies have found that EBV may use the BMRF-2 protein and α5/β1 integrins for attachment to polarized epithelial cells (35). However, binding to epithelial lines lacking CR2, such as the gastric carcinoma cell line AGS, is dependent on gHgL. gHgL null virus failed to bind to AGS cells (20, 25), and a MAb to gHgL that blocked entry into but not binding to an epithelial cell engineered to express CR2 (15) also inhibited this gHgL-dependent virus binding, suggesting, although not definitively proving, that the epithelial coreceptor was also used for attachment on a cell lacking CR2 (20).
The second set of observations provides evidence that levels of virion-associated gp42 have an effect on virus tropism (4). Virus made in HLA class II-negative epithelial cells was found to be up to 100-fold-more infectious for B lymphocytes than was the same amount of virus produced by an HLA class II-positive B cell. Virus originating from either cell type bound equally well to CR2 on the B-cell surface, but virus made in the B cell entered less efficiently. This reflected the fact that in an HLA class II-positive virus-producing cell, some complexes containing gp42 interacted with class II during biosynthesis and were targeted to the HLA class II trafficking pathway, where they were vulnerable to degradation. The resulting loss of three-part complexes from virus reduced the efficiency of HLA class II-dependent entry. Such a loss did not occur in an HLA class II-negative epithelial cell, where virus showed a relative increase in gp42 and an increased efficiency for HLA class II-dependent entry. However, constitutive expression of HLA class II in an epithelial cell as a result of transfection of the class II transactivator gene reversed the phenotype.
As might be expected, the levels of gp42 in virus also impacted infection of epithelial cells via the HLA class II-independent pathway. B-cell virus was on average fivefold better at infecting epithelial cells than was epithelial virus. However, the AGS cells used for these experiments were those that used the gHgL complex for attachment. In addition, epithelial lines that express at least low levels of CR2 sustain infection via a CR2-dependent, HLA class II-independent pathway (5). Since HLA class II is an inducible molecule, this suggests that EBV might encounter cells in vivo with several different combinations of receptors and coreceptors. We explore here the effect that this might have on the efficiency of infection by virus. Our results indicate that the stoichiometry of the gHgLgp42 complex significantly influences the use of gHgL not just for entry into epithelial cells but also for attachment and that the use of the same protein(s) for attachment and penetration compromises the efficiency of virus infection.
MATERIALS AND METHODS
Cells.
Akata, a CR2-positive Burkitt lymphoma-derived cell line that carries and can be induced to make EBV (33), and EBV-negative Akata cells that have lost the EBV genome, were grown in RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco/BRL, Grand Island, N.Y.). Medium for Akata cells carrying EBV in which the thymidine kinase gene was interrupted with a double cassette expressing neomycin resistance and the green fluorescence protein (GFP) (EBV-TKdel) (20) was supplemented with 500-μg/ml active G418 (Mediatech, Herndon, Va.). AGS, a CR2-negative gastric carcinoma-derived cell line (American Type Culture Collection), was grown in Ham's F-12 nutrient mixture (Gibco/BRL) supplemented with 10% heat-inactivated fetal bovine serum. Medium for AGS cells carrying EBV-TKdel was supplemented with 500-μg/ml active G418. Medium for AGS cells which expressed HLA class II proteins as a result of stable transfection with the human class II transactivator (AGS-class II) (4) was supplemented with 0.4-μg/ml puromycin (BD Biosciences Clontech, Palo Alto, Calif.). SVKCR2, a keratinocyte cell line stably transfected with a plasmid expressing CR2 (16), was grown in Joklik's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and 10-ng/ml cholera toxin (Sigma). Medium for SVKCR2 cells expressing HLA class II as a result of stable transfection with CIITA (SVKCR2-class II) was supplemented with 0.2-μg/ml puromycin. Sf9 insect cells were grown in CCM3 medium (HyClone, Logan, Utah).
Virus.
B-cell virus (B-EBV) was collected from clarified culture medium of EBV-carrying Akata cells after induction of the lytic cycle with antihuman immunoglobulin (Ig) (20). Epithelial cell virus (E-EBV) was harvested from AGS cells after induction with 12-O-tetradecanoylphorbol-13-acetate (Sigma) and 2.5 mM sodium butyrate (Calbiochem, La Jolla, Calif.). Yield from B cells was consistently higher than that from epithelial cells, but virus from both sources was concentrated by centrifugation as previously described (20) to ensure that all stocks had been treated in an identical manner. Previous experiments have shown that the method of induction does not influence the behavior of the virus (4).
Antibodies.
The following MAbs were used: CL59 reacting with gH (20); E1D1 reacting with gHgL (15); 72A1 reacting with gp350/220 (10); HB5 reacting with CR2 (39); anti-HLA-DR recognizing HLA class II, which was conjugated to phycoerythrin (anti-HLA-DR PE; BD Immunochemistry Systems, San Jose, Calif.); and 1969, which recognizes the α5/β1 integrin complex (Chemicon International, Temacula, Calif.). 72A1, E1D1, CL59, and HB5 were purified by chromatography on protein A (Sigma) coupled to Affigel-15 (Bio-Rad, Richmond, Calif.).
Assay of total virus and virus binding.
Virus concentrations were equilibrated for the amount of encapsidated EBV DNA by hybridization with the BamHI W fragment of EBV DNA labeled with 32P as previously described (38) and quantification with a Molecular Dynamics Storm PhosphorImager. Virus binding was measured by incubating virus with 2 × 106 cells for 2 h on ice, after which cells were washed, pelleted, and resuspended for electrophoresis in a Gardella gel as described previously (20). DNA was transferred to neutral nylon membranes for hybridization with the BamHI W fragment and quantified by scanning with a Molecular Dynamics Storm PhosphorImager.
Infection of cell lines.
SVKCR2, SVKCR2-class II, AGS, and AGS-class II cells were plated onto four-chamber Falcon slides (BD Biosciences Clontech) at a dilution that produced a 30% confluent monolayer 24 h later. Cells were incubated at 37°C for 4 h with 300 μl of virus. Growth medium (1 ml) was added, and the cells were reincubated and 72 h later directly examined for GFP expression by fluorescence microscopy.
Soluble forms of glycoproteins.
The soluble form of gHgL was expressed by baculovirus (27). Sf9 cells were infected at a multiplicity of infection of 3, and 5 days later, the culture medium was clarified by low-speed centrifugation to remove cells and centrifuged at 16,000 × g for 90 min to remove virus. Polyethylene glycol 3500 was added to a final concentration of 20% (wt/vol), and the solution was stirred for 1 h at 4°C and centrifuged at 14,000 × g for 20 min. The pellet was resuspended in 1/10 original volume of phosphate-buffered saline (PBS). The precipitated gHgL was dialyzed against PBS in dialysis tubing with a 25,000 molecular weight cutoff. The soluble form of gp42 was made as previously described (32).
Flow cytometric analysis.
Virus binding to EBV-negative Akata cells was examined by incubating virus and cells on ice for 1 h, washing cells three times in medium, followed by sequential incubations and washes with MAb 72A1 and sheep anti-mouse Ig coupled to phycoerythrin (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The last wash was done with PBS without calcium or magnesium (PBS). To examine binding of gHgL to EBV-negative Akata cells, protein was incubated with cells on ice for 1 h and cells were washed three times with medium and incubated sequentially with MAb CL59 and sheep anti-mouse antibody coupled to phycoerythrin as before. The effects of E1D1 on binding were measured by preincubating protein for 1 h on ice with antibody before addition of protein to cells. Binding to adherent AGS and SVKCR2 was measured in the same way, except that cells were removed from plastic by trypsinization and allowed to recover in medium at 37°C before addition of virus or protein on ice. Effects of EDTA on binding were measured by washing cells once with PBS and twice with 20 mM EDTA in PBS before incubation with gHgL in PBS or virus in medium with 20 mM EDTA. Cells were washed twice again with 20 mM EDTA in PBS and once with PBS before incubation with antibodies as before. Cell surface expression of CR2 was examined with MAb HB5, expression of HLA class II was visualized with anti-HLA-DR PE, and expression of the α5/β1 integrin complex was examined with MAb 1969.
RESULTS
Characterization of receptor and coreceptor expression.
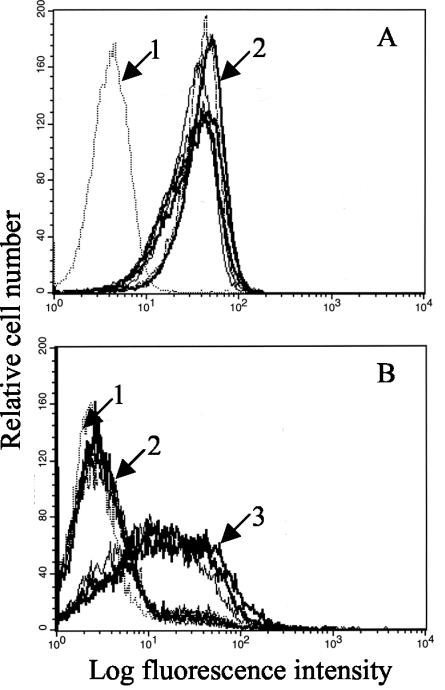
Cells used in this work had been been completely characterized for expression of CR2, which mediates virus attachment via a high-affinity interaction with gp350/220, and HLA class II, which serves as a coreceptor for gp42. However, they had not all been examined for receptors that in the absence of CR2 could mediate attachment via gHgL (gHgLR) or the BMRF2 protein (α5/β1 integrins). To determine if both the CR2-positive epithelial line SVKCR2 and the CR2-negative epithelial line AGS expressed gHgLR, cells were incubated with a soluble form of gHgL made in baculovirus and analyzed by flow cytometry using MAb CL59 to gH. Both cell lines bound gHgL (Fig. 1A and B). Specificity of binding was confirmed by demonstrating a reduction in binding by preincubation of gHgL with MAb E1D1, which recognizes the gHgL complex and partially blocks binding of virus to AGS cells (20). No gHgL bound to Akata cells (Fig. 1C), which is consistent with the failure of antibodies to gHgL (E1D1) or gH alone (CL59) to influence infection of this cell type (20, 38). Since few cell types are negative for expression of α5/β1 integrins, the extent of their expression on AGS cells was examined by comparing levels on epithelial cells and B cells. The levels of α5/β1 integrin were significantly higher on AGS cells than on Akata cells (Fig. 1D), which is consistent with the observations of Tugizov and colleagues (35). However, the failure of recombinant virus lacking gHgL to bind to AGS suggested that integrin expression could not be playing a significant role in virus attachment (20). Consistent with this observation was the fact that binding of soluble gHgL or virus to AGS cells was not significantly affected by chelation of divalent cations, which are essential for maintenance of integrin structure (Fig. 1E and F). Further work therefore focused on interaction of virus with combinations of CR2, HLA class II, and gHgLR.
FIG. 1.
Flow cytometric analysis of gHgLR and α5/β1 integrin expression and effects of chelation on binding. (A) Binding of gHgL to AGS in the presence (arrow 2) or absence (arrow 3) of MAb E1D1. (B) Binding of gHgL to SVKCR2 cells (arrow 2). (C) Binding of gHgL to EBV-negative Akata cells (arrow 2). (D) Binding of MAb 1969 reacting with α5/β1 integrins to EBV-negative Akata cells (arrow 3) or AGS cells (arrow 4). (E) Binding of gHgL to AGS cells in the absence (arrow 3) or presence (arrow 4) of 20 mM EDTA. (F) Binding of B-EBV to AGS cells in the absence (arrow 3) or presence (arrow 4) of 20 mM EDTA. In panels A, B, and C, arrow 1 indicates cells incubated with CL59 in the absence of gHgL. In panel D, arrows 1 and 2 indicate Akata cells (arrow 1) or AGS cells (arrow 2) incubated with second antibody alone. In panels E and F, arrow 1 indicates untreated cells incubated with second antibody alone and arrow 2 indicates EDTA-treated cells incubated with second antibody alone.
Infection of cells expressing only gHgLR is inefficient and is enhanced by coexpression of HLA class II.
AGS epithelial cells expressing gHgLR in the absence of CR2 or HLA class II were infected with equal amounts of B-EBV or E-EBV. Levels of infection were low, but as previously noted (4), B-EBV, which has a lower ratio of gp42 to gH than E-EBV, infected about five times better than E-EBV (Table 1). Stable expression of the B-cell coreceptor HLA class II in the same cells, now referred to as AGS-class II, increased levels of infection by virus derived from both cell types, but had the most marked effect on infectivity of E-EBV, which now infected about five times better than B-EBV.
TABLE 1.
Percentage of epithelial cells that express GFP after infection with B-EBV or E-EBV
| Cells | % of cells expressing GFP in expta:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| B-EBV
|
E-EBV
|
|||||||
| 1 | 2 | 3 | Avg | 1 | 2 | 3 | Avg | |
| AGS | 0.22 | 0.53 | 0.22 | 0.32 | 0.02 | 0.01 | 0.17 | 0.07 |
| AGS-class II | 0.27 | 2.9 | 0.70 | 1.29 | 4.60 | 8.60 | 4.10 | 5.77 |
| SVKCR2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Shown are the percentages of epithelial cells carrying gHgLR (AGS), gHgLR and HLA class II (AGS-class II), or gHgLR and CR2 (SVKCR2) that express GFP after infection with B-EBV or E-EBV.
Coexpression of CR2 and gHgLR has a much greater effect on infection than coexpression of HLA class II and gHgLR; infection is only modestly increased by expression of all three receptors/coreceptors.
Expression of CR2 in AGS cells dramatically increased levels of infection with both E-EBV and B-EBV, but levels of CR2 fluctuated on these cells over time. Stable levels of expression of CR2 could, however, be maintained together with expression of gHgLR on the SVKCR2 line. SVKCR2 cells were therefore used for all subsequent work. The same amounts of E-EBV and B-EBV that induced only the modest levels of infection of AGS and AGS-class II cells described above infected 100% of SVKCR2 cells (Table 1). To obtain a better estimate of the increase in the rate of infection, experiments were repeated with higher dilutions of virus (Fig. 2A). Infection was still detectable when virus was diluted by more than 2 logs (1/256-fold, or 0.0039) from the concentration used to infect AGS cells expressing only gHgLR. Next we examined the impact that expression of HLA class II in addition to CR2 and gHgLR has on infection. Infection rates of both B-EBV and E-EBV for SVKCR2-class II cells expressing gHgLR, CR2, and HLA class II were higher than for SVKCR2 cells expressing only gHgLR and CR2. The effect on E-EBV was more pronounced (Fig. 2B). Effects were, however, much more modest than the effect of addition of CR2. In seven different experiments with B-EBV and four experiments with E-EBV, using different concentration ranges and infecting between 6 and 30% of SVKCR2 cells, the average increases were 1.4-fold ± 1.1-fold for B-EBV and 7.8-fold ± 4.7-fold for E-EBV.
FIG. 2.
Infection of SVKCR2 cells (A) or SVKCR2-class II cells (B) with equal amounts of B-EBV (solid circles) or E-EBV (open circles). Infection is measured as the percentage of cells that express GFP at 72 h.
Fusion of virus bound to B cells via CR2 requires endocytosis as judged by its sensitivity to chlorpromazine (18). In contrast, entry into epithelial cells is not affected by chlorpromazine treatment. To determine if the striking effect of CR2 on the efficiency of infection of epithelial cells was related to a concomitant switch to entry via endocytosis, the effects of chlorpromazine on infection of SVKCR2 cells and Akata cells were measured. In the presence of 20 mM chlorpromazine, the number of Akata cells positive for GFP expression dropped from 41% to 18%, whereas infection of SVKCR2 cells was not affected, with 60% cells infected in the absence of drug and 68% infected in its presence.
B-EBV, but not E-EBV, binds almost as efficiently to gHgLR as to CR2.
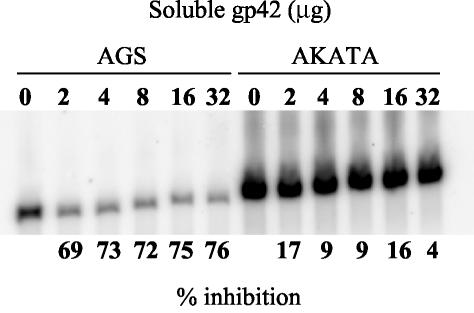
B-EBV and E-EBV bind to cells expressing CR2 with the same efficiency (4), but the relative efficiency of binding to CR2-negative gHgLR-positive epithelial cells had not been tested. Since AGS cells support only low levels of infection with EBV, it had been assumed that EBV binding to these cells would also be low. To compare binding to cells that offered gHgLR alone or both gHgLR and CR2 for attachment, equal amounts of E-EBV and B-EBV were bound to AGS or SVKCR2 cells. As expected, binding of E-EBV to AGS cells via gHgLR was very low and binding to SVKCR2 on which both gHgLR and CR2 were available was several orders of magnitude higher. However, B-EBV bound almost as well to AGS cells expressing only gHgLR as it did to SVKCR2 cells expressing both gHgLR and CR2 (Table 2). The experiments were repeated by using flow cytometry to compare binding of equal amounts of four E-EBV and three B-EBV preparations to gHgLR on AGS cells in the absence of CR2, or to CR2 on Akata B cells in the absence of gHgLR (Fig. 3). Virus from both origins bound equally well to CR2-positive Akata cells, but only B-EBV bound well to gHgLR-positive AGS cells. To determine if the stoichiometry of the gHgLgp42 complex was relevant to these observations, the relative ability of B-EBV to bind to gHgLR-positive AGS cells and CR2-positive Akata B cells was measured after increasing its gp42 content by addition of soluble gp42 in trans. Increasing amounts of soluble gp42 decreased the ability of virus to bind to gHgLR but had no effect on its ability to bind to CR2 (Fig. 4).
TABLE 2.
Binding of B-EBV and E-EBV to epithelial cells expressing gHgLR (AGS) or gHgLR plus CR2 (SVKCR2)
| Expt | Binding (relative units)a
|
|||||
|---|---|---|---|---|---|---|
| B-EBV
|
E-EBV
|
|||||
| SVKCR2 | AGS | SVKCR2/ AGS ratio | SVKCR2 | AGS | SVKCR2/ AGS ratio | |
| 1 | 513 | 255 | 2.4 | 8,540 | NDb | ∼854 |
| 2 | 1,494 | 801 | 1.9 | 18,800 | 9 | 208.8 |
| 3 | 8,020 | 1,609 | 5.0 | 4,973 | 45 | 110.5 |
| 4 | 2,914 | 868 | 3.4 | 1,837 | 11 | 167.0 |
Binding was detected by Southern blot analysis of bound EBV DNA and quantified by scanning with a Storm PhosphorImager.
ND, not determined.
FIG. 3.
Flow cytometric analysis of equal amounts of four different preparations of E-EBV and three different preparations of B-EBV bound to EBV-negative Akata cells (A) or AGS cells (B) and visualized with MAb 72A1 to gp350/220. In panel A, arrow 2 indicates binding of both B-EBV and E-EBV. In panel B, arrow 2 indicates binding of E-EBV and arrow 3 indicates binding of B-EBV. In both panels, arrow 1 indicates binding of MAb 72A1 in the absence of virus.
FIG. 4.
Southern blot of Gardella gel analysis of the amounts of B-EBV bound to gHgLR-positive AGS cells or CR2-positive, gHgLR-negative, EBV-negative Akata cells after preincubation with increasing amounts of soluble gp42. Numbers above the lanes indicate the amount (micrograms) of soluble gp42 used, and numbers below the panel indicate the percent reduction in binding determined by scanning with a Storm PhosphorImager.
Infectivity of B-EBV bound to gHgLR can be increased by treatment with polyethylene glycol.
The large differences in binding to gHgLR displayed by B-EBV and E-EBV did not translate into large differences in infection rates. For the virus preparations tested above by flow cytometry, the rates of infection for gHgLR-positive AGS cells were 0.30 ± 0.10 for B-EBV and 0.10 ± 0.02 for E-EBV, indicating that an increase in binding of B-EBV by almost 100-fold resulted in an increase in infection of only 5-fold. This dichotomy between binding and infection suggested that much of the extra B-EBV that was bound to gHgLR was unable to penetrate. To test this possibility, equal amounts of B-EBV and E-EBV were allowed to bind to AGS cells and then cells and bound virus were treated with the exogenous fusogen polyethylene glycol. Herpesvirus fusion mediated by polyethylene glycol is known to be inefficient (17), but in three separate experiments, polyethylene glycol treatment increased the number of cells infected with E-EBV by only ×(1.5 ± 0.5), whereas it increased the number of cells infected with B-EBV by ×(3.6 ± 0.4). These results are consistent with the hypothesis that less of the bound B-EBV than the bound E-EBV is able to penetrate the cells to which it is attached.
Low levels of gp42 in B-EBV target virus to use gHgL for entry even when HLA class II is available for interaction with gHgLgp42.
The net effect of lower levels of gp42 in B-EBV consistently translated into about a fivefold increase over E-EBV for infection of AGS cells expressing only gHgLR. However, this slight advantage was reversed for infection of SVKCR2 cells expressing both gHgLR and CR2 (Fig. 2A). This suggested the possibility that low levels of gp42 in B-EBV might continue to predispose to a nonproductive interaction with gHgLR even though CR2 was available for attachment. As an approach to testing this possibility, we examined the effect of MAb CL59 to gH on infection of SVKCR2-class II cells which offered CR2 for attachment and allowed either gHgL or gHgLgp42 to be used for penetration. CL59 blocks use of gHgL but not gHgLgp42. The antibody was unable to neutralize infection by E-EBV, which presumably used gHgLgp42 and HLA class II for penetration, but it effectively neutralized infection by B-EBV, indicating that even when HLA class II is available, B-EBV continues to use gHgL for penetration (Table 3).
TABLE 3.
Effect of anti-gH MAb CL59 on infection of SVKCR2 cells by B-EBV or E-EBV
| Expt | % Infection in presence of MAb CL59a
|
|
|---|---|---|
| B-EBV | E-EBV | |
| 1 | 0.9 | 108.7 |
| 2 | 0.0 | 81.2 |
| 3 | 0.8 | 135.2 |
| 4 | 1.6 | 108.3 |
| 5 | 2.7 | 90.3 |
Infection was measured by expression of GFP and calculated as a percentage of infection in the absence of antibody.
DISCUSSION
Infection of epithelial cells by EBV has been a controversial issue for many years, principally because attempts to infect cultured cells in vitro have been difficult and efforts to detect infected cells in vivo in normal healthy carriers or patients with acute mononucleosis have met with mixed results (1, 13, 23, 24, 30, 36). However, nasopharyngeal and gastric carcinomas that carry EBV episomes and oral hairy leukoplakia, which is an epithelial lesion driven by lytic EBV replication (28), clearly establish that infection is not only possible but also has important consequences for virus pathogenesis. Several somewhat different mechanisms by which virus might initiate epithelial infection have been proposed. Early work suggested that it occurred as a result of fusion between B cells and adjacent epithelial cells (2), but little progress has been made toward refuting or confirming this hypothesis. Other reports have indicated that at least low-level expression of CR2 occurs on some but not all epithelial cell lines (5, 11), although cross-reactivity between anti-CR2 antibodies and an unrelated molecule on epithelial tissues has confounded determination of its expression in vivo (41). Certainly efficient infection can be mediated in vitro by engineered expression of CR2 (16; this report). Cells that carry the polymorphic IgA receptor can be infected with virus that is coated with IgA specific for gp350/220 (31), which may principally be relevant to reinfection of cells in an immune host from the basolateral surface. The same may be true for the most recently described productive interaction between the BMRF-2 protein and α5/β1 integrins, which are predominantly expressed on the basolateral surfaces of polarized cells (35). Results presented here focus on the involvement of glycoproteins gHgL in infection of epithelial cells and suggest that although these proteins may function both in attachment (20, 25) and penetration (38) of this cell type, the efficiency of infection is significantly compromised if they are involved in both.
The identity of the molecule to which gHgL binds on the epithelial surface is not yet known. All available evidence is consistent with, though does not prove, that it is the same molecule as the coreceptor thought to be required for penetration. We show here that soluble gHgL bound both to AGS cells, where the complex is used for attachment, and to SVKCR2 cells, where it is used only for penetration. The MAb E1D1, which blocks penetration of SVKCR2 cells (38), also blocked attachment of virus or gHgL to AGS cells. Finally, soluble gp42, which can bind to gHgL in trans and block penetration of but not attachment to SVKCR2 cells (38), also impeded attachment to AGS cells via gHgL. Expression of the human B-cell coreceptor HLA class II on murine B cells to which gp350/220 cannot bind does not facilitate attachment of EBV (S. M. Turk and L. M. Hutt-Fletcher, unpublished data), but there is precedence for a virus using a coreceptor for attachment. For example, primary isolates of human immunodeficiency virus type 1 have been adapted to bind to chemokine coreceptors in the absence of CD4, although, in parallel with EBV, infection of cells expressing the primary receptor is more efficient (12).
Irrespective of whether or not the epithelial receptor and coreceptor are identical or closely associated molecules, the stoichiometry of the gHgLgp42 complex clearly affects its use for attachment or penetration. Expression of HLA class II consistently increased infectivity of both B-EBV and E-EBV and, as previously described for AGS cells alone (4), had a more marked effect on E-EBV than B-EBV. The increases were, however, not very striking, relative to the effects of expression of CR2. HLA class II and gHgLR (or an associated molecule) do not appear to be very different in the efficiency with which they function as coreceptors. The finding that B-cell virus, low in gp42, could bind so effectively to gHgLR was surprising given the low level at which cells expressing only gHgLR were infected. E-EBV, high in gp42, which bound very poorly to gHgLR, infected almost as well. CR2 increased binding of B-EBV only three- to fourfold but increased infection levels by more than 2 orders of magnitude, and the continued resistance of infection of SVKCR2 cells to the effects of chlorpromazine suggests that this cannot simply be attributed to targeting of EBV to the endocytic pathway that it uses to enter B cells. Attachment via CR2 also appears to play no essential role in triggering the fusion machinery, since efficient infection of polarized cells can be mediated in its absence (35). The possibility that CR2-mediated cell signaling influences infection levels cannot be ruled out (3, 29), but the above observations and the larger effects of polyethylene glycol on penetration of B-EBV than on penetration of E-EBV are consistent with the hypothesis that engagement of a substantial number of gHgL complexes by gHgLR is not compatible with use of the complexes for efficient fusion. The fact that infection of SVKCR2-class II cells by B-EBV, but not E-EBV, continued to be neutralized by antibody to gH might then be explained if the low levels of gp42 in this type of virus continued to be predisposed to interaction with gHgLR. A tendency to use gHgLR might also explain why addition of CR2 to a class II-negative, gHgLR-expressing cell favored E-EBV more than B-EBV. The apparent failure of virus to use α5/β1 integrins for attachment is curious but perhaps reflects the state of activation or association of these molecules on the nonpolarized AGS cells.
There are several possible reasons why binding of significant numbers of gHgL complexes might compromise infection. For example, the conformation adopted by gHgL when attached to gHgLR might be different from the conformation required for fusion; a threshold number of complexes not adopting the binding conformation might be required for efficient fusion and entry to occur or for potential interaction with other components of the fusion machinery, notably glycoprotein gB. Complexes that are relatively rich in gHgL and low in gp42 might also allow for a higher avidity of complex binding via gHgLR that is incompatible with fusion. Recent studies of the influenza virus hemagglutinin (HA) protein, which mediates both binding and fusion, showed that mutations that increased the affinity of HA molecules for sialic acid impaired their ability deliver virus into the cell. The number of fusion pores was reduced, but in addition, a significant effect was seen on the ability of the pores to dilate (26). The hypothesis proposed to explain these results was that HA spikes cross-link receptor molecules surrounding a fusion pore and that the high-affinity binding of the mutants prevented an adjustment in binding activity that is required for pore expansion to occur. An increase in the number of gHgL complexes available to bind and cross-link gHgLR might be having a similar effect.
The difficulty in finding replicating EBV in epithelial cells in vivo has led to the general assumption that virus that is released into the saliva of persistently infected individuals is primarily of B-cell origin. We have previously proposed that such virus, low in gp42, would be better prepared than epithelial virus to initiate infection of HLA class II-negative epithelial mucosa in a new host (4). We believe that this still holds true for any mucosal cells that offer access to gHgLR, which is required for entry, but not an additional functional receptor for a distinct attachment protein, such as gp350/220 or BMRF-2. The effect of low levels of gp42 is now shown to be primarily on increased binding to gHgLR, and it comes at the expense of the ability to mediate efficient entry. However, low levels of gp42 may be important to ensure that free virus in saliva is not immediately washed unproductively across a mucosal surface. If mucosal cells do express an additional receptor capable of mediating attachment, then B-EBV loses its slight advantage over any E-EBV that might be present in saliva, but in this case, both B-EBV and E-EBV infect with high efficiency, so the biological outcome would be relatively neutral.
Adaptation to the use of HLA class II as a coreceptor on B cells that lack gHgLR has come at the expense of losing gHgLgp42 complexes to a degradation pathway and a reduction in the ability of B-EBV to infect new B cells (4). Initiation of infection in an HLA class II-negative epithelial cell overcomes this potentially serious defect in a virus that needs to establish latency in the B-cell compartment. The ability of the same virus to reinfect an epithelial cell is only slightly reduced as long as an additional attachment receptor is expressed. Replication in epithelial cells may or may not be extensive in vivo, but passage through an epithelial cell as colonization of the host occurs would not only ease passage across an intact mucosal surface but also optimize the ability of virus to move into the B-cell compartment.
Acknowledgments
This work was supported by Public Health Service grant AI20662 from the National Institute of Allergy and Infectious Diseases.
We thank Melanie K. Spriggs, Amgen Corp., Seattle, Wash., for the gift of soluble gp42.
REFERENCES
- 1.Anagnostopoulos, I., M. Hummel, C. Kreschel, and H. Stein. 1995. Morphology, immunophenotype and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood 5:744-750. [PubMed] [Google Scholar]
- 2.Bayliss, G. J., and H. Wolf. 1980. Epstein-Barr virus induced cell fusion. Nature 287:164-165. [DOI] [PubMed] [Google Scholar]
- 3.Bohnsack, J. F., and N. R. Cooper. 1988. CR2 ligands modulate B cell activation. J. Immunol. 141:2569-2576. [PubMed] [Google Scholar]
- 4.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 5.Fingeroth, J. D., M. E. Diamond, D. R. Sage, J. Hayman, and J. L. Yates. 1999. CD-21-dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 73:2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingeroth, J. D., J. J. Weis, T. F. Tedder, J. L. Strominger, P. A. Biro, and D. T. Fearon. 1984. Epstein-Barr virus receptor of human B lymphocytes is the C3d complement CR2. Proc. Natl. Acad. Sci. USA 81:4510-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haan, K. M., W. W. Kwok, R. Longnecker, and P. Speck. 2000. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. J. Virol. 74:2451-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein gB are involved in Epstein-Barr virus induced membrane fusion. Virology 290:106-114. [DOI] [PubMed] [Google Scholar]
- 9.Haddad, R. S., and L. M. Hutt-Fletcher. 1989. Depletion of glycoprotein gp85 from virosomes made with Epstein-Barr virus proteins abolishes their ability to fuse with virus receptor-bearing cells. J. Virol. 63:4998-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman, G. J., S. G. Lazarowitz, and S. D. Hayward. 1980. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antibody. Proc. Natl. Acad. Sci. USA 77:2979-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolchinsky, P., T. Mirzabekov, M. Farzan, E. Kiprilov, M. Cayabyab, L. J. Mooney, H. Choe, and J. Sodroski. 1999. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J. Virol. 73:8120-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemon, S. M., L. M. Hutt, J. E. Shaw, J.-L. H. Li, and J. S. Pagano. 1977. Replication of EBV in epithelial cells during infectious mononucleosis. Nature 268:268-270. [DOI] [PubMed] [Google Scholar]
- 14.Li, Q., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71:4657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 17.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase seqences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, N., and L. M. Hutt-Fletcher. 1988. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J. Virol. 62:2366-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molesworth, S. J., C. M. Lake, C. M. Borza, S. M. Turk, and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J. Virol. 74:6324-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemerow, G. R., C. Mold, V. K. Schwend, V. Tollefson, and N. R. Cooper. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J. Virol. 61:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemerow, G. R., R. Wolfert, M. McNaughton, and N. R. Cooper. 1985. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J. Virol. 55:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niedobitek, G., S. Hamilton-Dutoit, H. Herbst, T. Finn, M. Vetner, G. Pallesen, and H. Stein. 1989. Identification of Epstein-Barr virus-infected cells in tonsils of acute infectious mononucleosis by in situ hybridization. Hum. Pathol. 20:796-799. [DOI] [PubMed] [Google Scholar]
- 24.Niedobitek, G., H. Herbst, L. S. Young, L. Brooks, M. G. Masucci, J. Crooker, A. Rickinson, and H. Stein. 1992. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood 79:2520-2526. [PubMed] [Google Scholar]
- 25.Oda, T., S. Imai, S. Chiba, and K. Takada. 2000. Epstein-Barr virus lacking glycoprotein gp85 cannot infect B cells and epithelial cells. Virology 276:52-58. [DOI] [PubMed] [Google Scholar]
- 26.Ohuchi, M., R. Ohuchi, T. Sakai, and A. Matsumoto. 2002. Tight binding of influenza virus hemagglutinin to its receptor interferes with fusion pore dilation. J. Virol. 76:12405-12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulford, D. J., P. Lowrey, and A. J. Morgan. 1995. Co-expression of the Epstein-Barr virus BXLF2 and BKRF2 genes with a recombinant baculovirus produces gp85 on the cell surface with antigenic similarity to the native protein. J. Gen. Virol. 76:3145-3152. [DOI] [PubMed] [Google Scholar]
- 28.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott-Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 29.Sinclair, A. J., and P. J. Farrell. 1995. Host cell requirements for efficient infection of quiescent primary B lymphocytes by Epstein-Barr virus. J. Virol. 69:5461-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sixbey, J. W., J. G. Nedrud, N. Raab-Traub, R. A. Hanes, and J. S. Pagano. 1984. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310:1225-1230. [DOI] [PubMed] [Google Scholar]
- 31.Sixbey, J. W., and Q.-Y. Yao. 1992. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science 255:1578-1580. [DOI] [PubMed] [Google Scholar]
- 32.Spriggs, M. K., R. J. Armitage, M. R. Comeau, L. Strockbine, T. Farrah, B. MacDuff, D. Ulrich, M. R. Alderson, J. Mullberg, and J. I. Cohen. 1996. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR β chain and inhibits antigen presentation. J. Virol. 70:5557-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada, K. 1984. Cross-linking of cell surface immunoglobulin induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 34.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping and endocytosis. Cell 50:203-213. [DOI] [PubMed] [Google Scholar]
- 35.Tugizov, S. M., J. W. Berline, and J. M. Palefsky. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9:307-314. [DOI] [PubMed] [Google Scholar]
- 36.Venables, P. J. W., C. G. Teo, C. Baboonian, B. E. Griffin, and R. A. Hughes. 1989. Persistence of Epstein-Barr virus in salivary gland biopsies from healthy individuals and patients with Sjogren's syndrome. Clin. Exp. Immunol. 75:359-364. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weis, J. J., T. F. Tedder, and D. T. Fearon. 1984. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc. Natl. Acad. Sci. USA 81:881-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]
- 41.Young, L. S., C. W. Dawson, K. W. Brown, and A. B. Rickinson. 1989. Identification of a human epithelial cell surface protein sharing an epitope with the C3d/Epstein-Barr virus receptor molecule of B lymphocytes. Int. J. Cancer 43:786-794. [DOI] [PubMed] [Google Scholar]