Abstract
BK (large conductance calcium- and voltage-activated potassium) channels are important determinants of physiological control in the nervous, endocrine and vascular systems with channel dysfunction associated with major disorders ranging from epilepsy to hypertension and obesity. Thus the mechanisms that control channel surface expression and/or activity are important determinants of their (patho)physiological function. BK channels are S-acylated (palmitoylated) at two distinct sites within the N- and C-terminus of the pore-forming α-subunit. Palmitoylation of the N-terminus controls channel trafficking and surface expression whereas palmitoylation of the C-terminal domain determines regulation of channel activity by AGC-family protein kinases. Recent studies are beginning to reveal mechanistic insights into how palmitoylation controls channel trafficking and cross-talk with phosphorylation-dependent signalling pathways. Intriguingly, each site of palmitoylation is regulated by distinct zDHHCs (palmitoyl acyltransferases) and APTs (acyl thioesterases). This supports that different mechanisms may control substrate specificity by zDHHCs and APTs even within the same target protein. As palmitoylation is dynamically regulated, this fundamental post-translational modification represents an important determinant of BK channel physiology in health and disease.
Keywords: KCNMA1, large conductance calcium- and voltage-activated potassium (BK) channel, palmitoylation, phosphorylation, potassium channel
BK channel physiology and structure
BK (large conductance calcium- and voltage-activated potassium) channels represent the only potassium-selective channel to be activated by both membrane depolarization and elevation of intracellular-free calcium, thus providing an important determinant to couple changes in membrane excitability and changes in intracellular calcium dynamics [1]. BK channels are widely expressed in both excitable and non-excitable cells where they play an eclectic role in a range of fundamental physiological processes ranging from the control of blood flow [2,3] to neuronal excitability [4,5]. As such, dysfunction in BK channels is implicated in a wide variety of disorders including hypertension, obesity, epilepsy, incontinence and sexual dysfunction [2-9].
The pore-forming α-subunit of BK channels is encoded by a single gene (KCNMA1) that undergoes extensive alternative pre-mRNA splicing and assembles as tetramers to produce functional channels at the plasma membrane [1,10]. BK channel properties and function may also be modified by assembly with a family of regulatory β- and γ -subunits [11,12] as well as extensive post-translational modulation by a diverse array of intracellular signalling cascades including protein phosphorylation [13,14]. As for any ion channel, post-translational modification that results in changes in either the number of ion channels at the cell surface and/or the activity of the channel at the plasma membrane will have a significant impact on the physiological function of the channel. Particularly important in this regard is the large single channel conductance of BK channels, thus small changes in the number and/or activity of functional channels may have very large effects on potassium flux across cell membranes and hence changes in cellular function.
Increasing evidence suggest that protein palmitoylation is an important post-translational mechanism to control the surface expression and activity of a wide array of ion channels [15,16]. In the last few years, facilitated by the major advances in the biochemical analysis of palmitoylation [17-19] and the identification of a family of enzymes that control palmitoylation [15,20-22], evidence has begun to reveal important roles for this fundamental reversible lipid post-translational modification in controlling BK channel function. These studies not only provide new insight into the regulation and physiology of BK channels, but are also illuminating important mechanisms, properties, function and regulation of protein palmitoylation.
BK channel α-subunits are palmitoylated at two different intracellular domains via distinct palmitoyl transferases (zDHHCs)
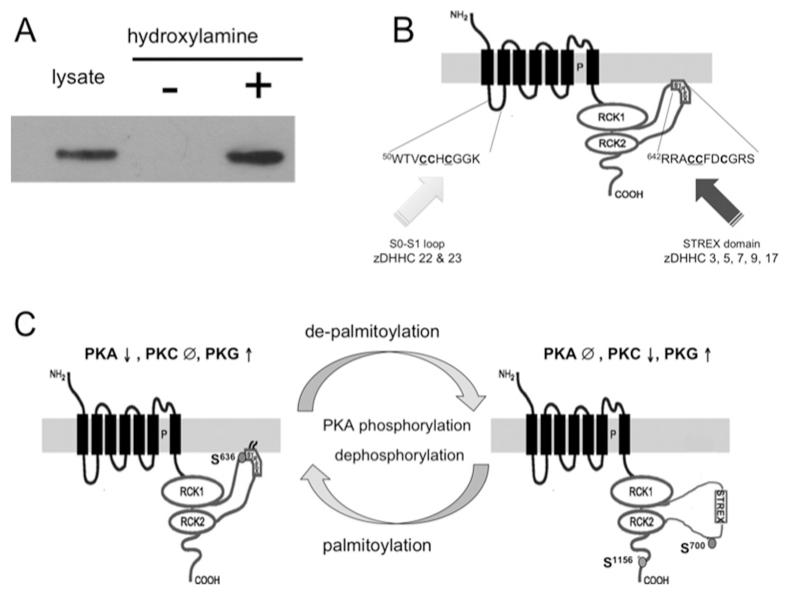
BK channel α-subunits are palmitoylated in native tissues as revealed using the acyl-RAC (resin-assisted capture) approach that allows identification of proteins that contain an hydroxylamine-sensitive thioester linkage between palmitate and the reactive cysteine residue (Figure 1A). To identify potential sites on BK channels that may be palmitoylated we took several approaches. First, using the CSS-Palm palmitoylation site prediction algorithm (http://csspalm.biocuckoo.org) [23], cysteine residues were predicted within both the intracellular loop between transmembrane domains S0 and S1 (S0–S1 loop) and the alternatively spliced STREX (stress-regulated exon) domain in the intracellular linker between the two RCK (regulator of potassium conductance) domains in the C-terminus of the channel (Figure 1B). To address whether these sites are in fact palmitoylated, we exploited a site-directed mutagenic approach together with expression of recombinant channel expression in HEK (human embryonic kidney)-293 cells. Full-length BK channels (ZERO splice variant that does not contain the STREX domain) that only include the predicted S0–S1 loop palmitoylated cysteine residues are robustly endogenously palmitoylated in HEK-293 cells using either [3H]palmitate incorporation assays or acyl-RAC [24,25]. Site-directed mutagenesis of three cysteine residues (Cys53, Cys54 and Cys56) in the loop abolished endogenous palmitoylation. As a first screen to identify potential cysteine residues in the C-terminal STREX domain, we expressed the STREX or ZERO C-terminus, in the absence of the transmembrane domain [26]. The STREX, but not ZERO, C-terminal fusion protein was robustly palmitoylated in HEK-293 cells and site-directed mutagenesis of a dicysteine cluster in STREX (the 12th and 13th amino acids in the STREX insert, Cys645 and Cys646) abolished palmitoylation. To confirm that the STREX site was palmitoylated in the full-length STREX variant, we assayed palmitoylation STREX channels (STREX*) in which the S0–S1 loop palmitoylation site was mutated [27]. Clearly a challenge for the future will be to adapt the acyl-RAC approach, for example by including on-bead tryptic digestion followed by elution of palmitoylated peptides for analysis by mass spectrometry, to allow palmitoylation of these specific sites in native tissues to be assayed in different physiological challenges.
Figure 1. Palmitoylation and regulation of BK channel pore-forming α-subunits.
(A) Acyl-RAC assay revealing endogenous S-palmitoylation of the BK channel α-subunit in mouse brain. Cleavage of the thioester bond, linking palmitate to the cognate cysteine residues in the channel, by hydroxylamine creates reactive cysteine residues that can be detected by the thio-reactive resin. (B) Site-directed mutagenesis reveals BK channels are palmitoylated at two distinct sites by distinct acyltransferases (zDHHCs): the intracellular S0–S1 loop and the alternatively spliced STREX insert in the C-terminal linker between the two RCK domains. Palmitoylation of the S0–S1 loop predominantly controls surface trafficking of the channel, whereas palmitoylation of STREX controls channel activity and regulation by AGC family kinases. (C) Regulation of STREX channels by palmitoylation and AGC family kinases. In channels where the STREX domain is palmitoylated channels are inhibited by PKA-dependent phosphorylation of Ser636 in STREX. PKC is ineffective when STREX is palmitoylated. Depalmitoylation of STREX, or PKA-mediated dissociation of the STREX domain from the plasma membrane, exposes a PKC site (Ser700) that, in conjunction with the C-terminal PKC site Ser1156, allows PKC-dependent inhibition of STREX channels. PKG-mediated activation of STREX channels, dependent on phosphorylation of other C-terminal serine residues, is not controlled by STREX domain palmitoylation.
Increasing evidence points to zDHHC selectivity for their substrates [21]; however, in most palmitoylated proteins the zDHHCs that control palmitoylation of defined sites are not known. To address whether the two distinct sites of palmitoylation on BK channels are regulated by different zDHHCs we took two approaches. First, we developed an siRNA (small interfering RNA)-based screen to allow the systematic knockdown of each endogenous zDHHC mRNA in HEK-293 cells [25,28]. One important caveat of this approach is that although all but one of the major zDHHCs are expressed in HEK-293 cells, high-quality antibodies that recognize most zDHHCs are not currently available thus knockdown efficiency is based on suppression of endogenous mRNA or exogenous transfected zDHHC. Thus as the lifetime of most zDHHCs is not known, the extent to which endogenous DHHC activity is reduced is not known in most cases. With these caveats we developed an imaging screen for both the C-terminal STREX and S0–S1 domains based on a GFP (green fluorescent protein)-fusion protein approach [25,28]. Both the isolated S0–S1 loop and C-terminus of the STREX channel associate with the plasma membrane in a palmitoylation-dependent mechanisms dependent upon the identified cysteine residues. Thus we used these constructs to assay for endogenous zDHHCs that could support membrane association of the wild-type constructs. We identified zDHHC22 and zDHHC23 as candidate zDHHCs for the S0–S1 loop and could confirm regulation of palmitoylation of the isolated S0–S1 loop and full-length channel by these zDHHCs [25]. Overexpression of zDHHC23 resulted in a significant increase in S0–S1 loop palmitoylation; however, such studies were not amenable with zDHHC22 as overexpression of this zDHHC was not well tolerated by HEK-293 cells. Using the same STREX C-terminus, or a short peptide encompassing the minimal STREX domain required for palmitoylation and plasma membrane localization approach, we identified zDHHCs 3, 5 7, 9 and 17 as potential palmitoylating enzymes [28]. Palmitoylation of the domain was again increased by overexpression of the cognate zDHHCs with zDHHC17 overexpression showing the greater selectivity for the dicysteine Cys645/Cys646 motif. Clearly a challenge for the future is to establish the tissue and cellular distribution of these zDHHCs and their regulation of BK channels in native tissues. The zDHHCs identified as regulator of BK channels are expressed at the ER (endoplasmic reticulum), Golgi or plasma membrane suggesting that the BK channel may be regulated at multiple sites in the trafficking pathways to the plasma membrane.
Palmitoylation is a reversible process, but for most proteins the APTs (acyl thioesterases) responsible for depalmitoylation are largely unknown. Increasing evidence suggests that the major cytosolic thioesterases are APT1 and APT2, which may display substrate specificity [29,30]. In addition, the lysosomal PPT1 (palmitoyl-protein thioesterase 1) probably plays a role in depalmitoylating proteins undergoing lysosomal degradation. We have not yet identified which APTs control depalmitoylation of the STREX domain. However, APT1, but not APT2, overexpression depalmitoylated BK channels at the S0–S1 loop [25]. Knockdown of either APT1 or APT2 had no significant effect on steady state BK channel palmitoylation suggesting that either the channel palmitoylation has a long half-life or depalmitoylation is not rate limiting. In addition, we identified a splice variant of another putative thioesterase, APT1-like, that also depalmitoylated the S0–S1 loop upon over expression. Although a catalytically ‘dead’ mutant of APT1-like was ineffective, the recent crystal structure of an APT1-like monomer reveals the enzyme has an active site that should preferentially cleave shorter-chain lipids [31]. This suggests that the effect of APT1-like overexpression on S0–S1 loop palmitoylation may be indirect.
Palmitoylation of S0–S1 loop controls channel cell-surface trafficking
As palmitoylation controls two distinct sites of the BK channel, and this is mediated by different subsets of zDHHCs, we hypothesized that palmitoylation may control distinct functions of the channel.
Using quantitative cell surface expression image analysis as well as surface biotinylation assays [24,25] mutation of the S0–S1 loop cysteine to alanine residues, to prevent channel palmitoylation, resulted in a very significant (by more than 50%) inhibition of steady state cell-surface expression of the channel α-subunit irrespective of whether the C-terminal STREX site was present or not. Inhibition of cell-surface expression was recapitulated using the non-selective zDHHC inhibitor 2-BP (2-bromopalmitate), or knockdown of zDHHC 22 or 23, that control S0–S1 loop palmitoylation. In agreement with these studies, overexpression of the thioesterase AT1, but not APT2, also decreased steady state cell-surface expression by approximately half in accordance with the decrease in channel palmitoylation [25]. Channels that were depalmitoylated by APT1 were largely retained in the TGN (trans-Golgi network) although channel palmitoylation is also important for ER exit. This Golgi accumulation was associated with a reduced channel localization with recycling endosomes and suggests a major checkpoint for channel trafficking by palmitoylation is at the exit from the TGN. Clearly the real-time dynamics and spatial organization of palmitoylation-dependent trafficking through the forward, and recycling, trafficking pathways requires further investigation. Such studies should also provide important insights into the localization and function of the different zDHHCs and APTs that control BK channel trafficking.
Palmitoylation of STREX domain controls channel activity and regulation by AGC family kinases
The alternatively spliced STREX domain represents a cysteine-rich insert located in the structurally disordered intracellular linker between the RCK domains [32,33]. Insertion of STREX shifts the G/V (conductance/voltage) relationship of BK channels to the left so that the channel is active at more physiological calcium and voltage levels. Importantly, this domain also switches the response of BK channels to regulation by cAMP-dependent protein kinase (PKA; protein kinase A): channels that include STREX are inhibited whereas channels that lack the STREX insert are activated by PKA [14,33]. PKA-dependent inhibition of STREX channels is dependent on Ser636 which is located immediately upstream of the palmitoylated dicysteine residues (Cys645/Cys646) of STREX. Intriguingly, Ser636 is located within a large polybasic domain and thus introduction of negative charge upon phosphorylation would be predicted to destabilize the region [27]. As the palmitoylated STREX domain is associated with the plasma membrane [26], we hypothesized that the mechanism for PKA-mediated inhibition may involve dissociation of the palmitoylated STREX domain from the plasma membrane. In support of this idea, phosphorylation of Ser636 by PKA, or phosphomimetic (S636E or S636D) mutation resulted in loss of plasma membrane localization of the GFP fusion of the STREX C-terminus to a cytosolic/nuclear accumulation [26,27]. This effect was not recapitulated with other manipulations such as hypoxia that also control STREX function [34] nor through phosphorylation of sites out with the STREX insert [26]. Importantly, in electrophysiological assays PKA-dependent inhibition of the STREX channel was abolished in C645A/C646A palmitoylation-deficient mutant channels or in wild-type channels in which the cognate zDHHC activity was inhibited by 2-BP, or knocked down by siRNA [26,28]. This revealed an important level of cross-talk by the phosphorylation and palmitoylation signalling pathways that is emerging as an important theme in other ion channels and signalling molecules [16]. How does PKA destabilize the STREX domain from the plasma membrane? As indicated above, the Ser636 PKA site in STREX is buried within a polybasic domain. Thus phosphorylation of Ser636 would include a large negative charge and would be predicted to destabilize the polybasic domain that may act as an additional membrane-binding surface for the domain in addition to the palmitoylated dicysteine motif. Palmitoylation may be insufficient for robust association of the STREX domain with the plasma membrane. Furthermore, destabilization of the polybasic domain may make the dicysteine motif more accessible/susceptible to depalmitoylation by the cytosolic APTs. Determination of key APTs that regulate this site and their role in this process requires further investigation. Phosphorylation of Ser636 also prevents phosphorylation of STREX, probably owing to a requirement for the polybasic domain to act as an initiating membrane association domain to allow zDHHCs to palmitoylate the target cysteine residues [21,22]. In this way, palmitoylation and phosphorylation may interact and allow temporal control of STREX channel function.
Previous studies have revealed that PKC (protein kinase C) inhibits BK channels, which lack the STREX insert, through phosphorylation of two sites in the C-terminus [35]. A PKC consensus site is found at the C-terminus of the channel protein as well as a site that is located just downstream of the site of splicing where STREX may be included in the RCK1–RCK2 linker. However, even though these same sites are present in STREX channels (Ser1156 and Ser700 respectively) PKC has no effect [36]. Phosphorylation of Ser700 is dependent upon previous phosphorylation of Ser1156 for PKC to exert its functional effect. This raised the question that the Ser700 site may be occluded in the presence of the palmitoylated STREX insert. In support of this hypothesis, wild-type STREX channels pre-treated with 2-BP, or palmitoylation-deficient STREX channels (C645A/C646A) were robustly inhibited by catalytic subunits of PKC in isolated membrane patches. As predicted, PKA did not inhibit depalmitoylated STREX channels. In contrast, PKG, which phosphorlyates residues at the C-terminus of the channel [37] activated both palmitoylated and palmitoylation-deficient STREX channels. However, in channels with the Ser700 phosphomimetic mutation S700E channel activity was suppressed suggesting that the lack of PKC inhibition when STREX is palmitoylated results from Ser700 being inaccessible to PKC phosphorylation. Furthermore, in native endocrine cells that express both the ZERO and the STREX variant, inhibition of channel palmitoylation resulted in a significantly enhanced PKC-mediated inhibition of BK current density. Similar enhanced inhibition of BK channels by PKC was also observed in heteromultimers of ZERO and STREX channels expressed in HEK-293 cells treated with 2-BP supporting that depalmitoylation reveals an additional PKC-dependent inhibition through phosphorylation of STREX variant channels.
Taken together, these data reveal an important functional role for STREX palmitoylation by switching channel inhibition from a PKA- to a PKC-dependent mechanism (Figure 1C). Furthermore, as PKA inhibition results in dissociation of the STREX domain from the plasma membrane it may provide a mechanism to allow a combined PKA and PKC phosphorylation cascade to more robustly inhibit STREX BK channels.
Conclusion
The work discussed in the present article implicates reversible S-palmitoylation as a major mechanism to control both the number of BK channels at the cells surface as well as their activity and regulation at the plasma membrane. Palmitoylation is dynamically regulated, including by diet and other pathways that control cysteine reactivity [38,39] or accessibility. As distinct zDHHCs control either trafficking or channel activity/regulation, palmitoylation may be an important determinant of physiological control of these channels in a diverse array of physiological processes. A major challenge for the future will be to decipher the contribution of palmitoylation in controlling BK channel function at the system level and whole animal level and understanding how palmitoylation status may be controlled and/or dysregulated in disease [40]. To date, we know relatively little regarding how the zDHHCs and APTs that control BK channels are themselves regulated. The continued development of biochemical and imaging tools to interrogate dynamic palmitoylation is essential to address these questions and represents both a major challenge as well as opportunity to the field to understand the physiological importance of protein palmitoylation.
Abbreviations used
- APT
acyl thioesterase
- BK
large conductance calcium- and voltage-activated potassium channel
- 2-BP
2-bromopalmitate
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HEK
human embryonic kidney
- PK
protein kinase
- RAC
resin-assisted capture
- RCK
regulator of potassium conductance
- siRNA
small interfering RNA
- STREX
stress-regulated exon
- TGN
trans-Golgi network
- zDHHC
DHHC family palmitoyl acyltransferase.
References
- 1.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 2.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 3.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou X-B, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 4.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 5.Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du W, Bautista JF, Yang H, Diez-Sampedro A, You S-A, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 7.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J. Biol. Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 8.Jiao H, Arner P, Hoffstedt J, Brodin D, Dubern B, Czernichow S, van’t Hooft F, Axelsson T, Pedersen O, Hansen T, et al. Genome wide association study identifies KCNMA1 contributing to human obesity. BMC Med. Genomics. 2011;4:51. doi: 10.1186/1755-8794-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J. Physiol. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fodor A, Aldrich R. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu. Rev. Physiol. 2008;71:1–18. doi: 10.1146/annurev.physiol.010908.163124. [DOI] [PubMed] [Google Scholar]
- 11.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol. Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. U.S.A. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Olsen J, Park K, Li W, Bildl W, Schulte U, Aldrich R, Fakler B, Trimmer J. Profiling the phospho-status of the BKca channel a subunit in rat brain reveals unexpected patterns and complexity. Mol. Cell. Proteomics. 2008;7:2188–2198. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian L, Coghill LS, McClafferty H, MacDonald SH-F, Antoni FA, Ruth P, Knaus H-G, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 16.Shipston MJ. Ion channel regulation by protein palmitoylation. J. Biol. Chem. 2011;286:8709–8716. doi: 10.1074/jbc.R110.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Global profiling of dynamic protein palmitoylation. Nat. Methods. 2012;9:84–89. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. BioTechniques. 2004;36:276–285. doi: 10.2144/04362RR02. [DOI] [PubMed] [Google Scholar]
- 19.Forrester MT, Hess DT, Thompson JW, Hultman R, Moseley MA, Stamler JS, Casey PJ. Site-specific analysis of protein S-acylation by resin-assisted caption. J. Lipid Res. 2011;52:393–398. doi: 10.1194/jlr.D011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem. Sci. 2011;36:245–253. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng., Des. Sel. 2008;21:639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffries O, Geiger N, Rowe ICM, Tian L, McClafferty H, Chen L, Bi D, Knaus H-G, Ruth P, Shipston MJ. Palmitoylation of the S0–S1 linker regulates cell surface expression of voltage- and calcium-activated potassium (BK) channels. J. Biol. Chem. 2010;285:33307–33314. doi: 10.1074/jbc.M110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L, McClafferty H, Knaus H-GG, Ruth P, Shipston MJ. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium (BK) channels. J. Biol. Chem. 2012;287:14718–14725. doi: 10.1074/jbc.M111.335547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian L, Jeffries O, McClafferty H, Molyvdas A, Rowe ICM, Saleem F, Chen L, Greaves J, Chamberlain LH, Knaus H-G, et al. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc. Natl. Acad. Sci. U.S.A. 2008;105:21006–21011. doi: 10.1073/pnas.0806700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffries O, Tian L, McClafferty H, Shipston MJ. An electrostatic switch controls palmitoylation of the large conductance voltage- and calcium-activated potassium (BK) channel. J. Biol. Chem. 2011;287:1468–1477. doi: 10.1074/jbc.M111.224840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian L, McClafferty H, Jeffries O, Shipston MJ. Multiple palmitoyltransferases are required for palmitoylation-dependent regulation of large conductance calcium- and voltage-activated potassium channels. J. Biol. Chem. 2010;285:23954–23962. doi: 10.1074/jbc.M110.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeidman R, Jackson CS, Magee AI. Protein acyl thioesterases. Mol. Membr. Biol. 2009;26:32–41. doi: 10.1080/09687680802629329. [DOI] [PubMed] [Google Scholar]
- 30.Tomatis VM, Trenchi A, Gomez GA, Daniotti JL. Acyl-protein thioesterase 2 catalyzes the deacylation of peripheral membrane-associated GAP-43. PLoS ONE. 2010;5:e15045. doi: 10.1371/journal.pone.0015045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bürger M, Zimmermann TJ, Kondoh Y, Stege P, Watanabe N, Osada H, Waldmann H, Vetter IR. Crystal structure of the predicted phospholipase LYPLAL1 reveals unexpected functional plasticity despite close relationship to acyl protein thioesterases. J. Lipid Res. 2012;53:43–50. doi: 10.1194/jlr.M019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 33.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J. Biol. Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 34.McCartney CE, McClafferty H, Huibant J-M, Rowan EG, Shipston MJ, Rowe ICM. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel α-subunits. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17870–17876. doi: 10.1073/pnas.0505270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X-B, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8005–8010. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Wulfsen I, Korth M, McClafferty H, Lukowski R, Shipston MJ, Ruth P, Dobrev D, Wieland T. Palmitoylation and membrane association of STREX controls bk channel regulation by protein kinase C. J. Biol. Chem. 2012;287:32161–32171. doi: 10.1074/jbc.M112.386359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X-B. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J. Biol. Chem. 2001;276:43239–43245. doi: 10.1074/jbc.M104202200. [DOI] [PubMed] [Google Scholar]
- 38.Burgoyne JR, Haeussler DJ, Kumar V, Ji Y, Pimental DR, Zee RS, Costello CE, Lin C, McComb ME, Cohen RA, Bachschmid MM. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J. 2012;26:832–861. doi: 10.1096/fj.11-189415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho GPH, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH. S-Nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron. 2011;71:131–141. doi: 10.1016/j.neuron.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resh MD. Targeting protein lipidation in disease. Trends Mol. Med. 2012;18:206–214. doi: 10.1016/j.molmed.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]