SUMMARY
Leucine-rich repeat (LRR) proteins have recently been identified as important regulators of synapse development and function, but for many LRR proteins the ligand-receptor interactions are not known. Here we identify the heparan sulfate (HS) proteoglycan glypican as a novel receptor for LRRTM4 using an unbiased proteomics-based approach. Glypican binds LRRTM4, but not LRRTM2, in a HS-dependent manner. Glypican 4 (GPC4) and LRRTM4 localize to the pre- and postsynaptic membranes of excitatory synapses, respectively. Consistent with a trans-synaptic interaction, LRRTM4 triggers GPC4 clustering in contacting axons and GPC4 induces clustering of LRRTM4 in contacting dendrites in a HS-dependent manner. LRRTM4 positively regulates excitatory synapse development in cultured neurons and in vivo, and the synaptogenic activity of LRRTM4 requires the presence of HS on the neuronal surface. Our results identify glypican as a novel LRRTM4 receptor and indicate that a trans-synaptic glypican-LRRTM4 interaction regulates excitatory synapse development.
INTRODUCTION
Precise synaptic connectivity is essential for the proper functioning of neural circuits. Establishing functional synapses between pre- and postsynaptic neurons requires target cell recognition, transformation of initial cell-cell contacts into specialized synaptic junctions, and their differentiation and maturation into distinct synapse types (Shen and Scheiffele, 2010; Waites et al., 2005; Williams et al., 2010). Cell surface interactions likely play key roles at each of these steps, but the identity of the surface molecules involved is only now beginning to be uncovered.
Synaptic adhesion molecules are a key class of cell surface molecules that orchestrate synaptic connectivity. Besides physically linking and stabilizing pre- and postsynaptic membranes, synaptic adhesion molecules mediate target recognition, drive pre- and postsynaptic specialization, and may contribute to the diversity and plasticity of synapses (Dalva et al., 2007; Yamagata et al., 2003). Recent work has identified a wide variety of trans-synaptic adhesion complexes with partially overlapping but distinct roles in organizing synapse development. These include the neuroligins and their binding partners neurexins (Ichtchenko et al., 1995; Scheiffele et al., 2000), SynCAMs (Biederer et al., 2002), NGLs and Netrin-Gs/LAR (Kim et al., 2006; Woo et al., 2009), Slitrks and PTPδ (Takahashi et al., 2012), and LRRTMs (de Wit et al., 2009; Linhoff et al., 2009). The LRRTMs (leucine-rich repeat transmembrane neuronal proteins) are of particular interest because LRRTM isoforms are differentially expressed by neuronal populations in the CNS (Lauren et al., 2003), suggesting that they may contribute to the development of specific synaptic connections. LRRTM1 and LRRTM2 regulate excitatory synapse development by trans-synaptically interacting with presynaptic neurexins (de Wit et al., 2009; Ko et al., 2009a; Siddiqui et al., 2010). Whether all LRRTMs function through the same presynaptic receptor, or whether there is diversity in LRRTM-receptor interactions is unknown.
Another class of cell surface molecules with a critical role in organizing neuronal connectivity is the heparan sulfate proteoglycans (HSPGs). Proteoglycans are cell surface and extracellular matrix constituents made up of a core protein and covalently attached glycosaminoglycan (GAG) chains composed of repeating disaccharide units. The GAG chains are enzymatically modified to contain highly sulfated domains that are negatively charged and serve as protein binding sites (Bernfield et al., 1999). The role of proteoglycans in the development of neuronal connectivity is best described for axon pathfinding, where HSPGs modulate axon guidance cue distribution, availability and function (de Wit and Verhaagen, 2007; Van Vactor et al., 2006). Less is known about their role in synapse development, especially in the CNS. Neuromuscular synapse development is regulated by the secreted HSPG agrin (Nitkin et al., 1987; reviewed in Sanes and Lichtman, 2001) and by the Drosophila glypican Dallylike, a GPI-anchored HSPG (Johnson et al., 2006). In the CNS, overexpression of the transmembrane HSPG syndecan-2 accelerates dendritic spine morphogenesis (Ethell and Yamaguchi, 1999). Secreted forms of glypican 4 and 6 promote glutamate receptor clustering and excitatory synapse formation in retinal ganglion cells (Allen et al., 2012), suggesting that glypican may have synaptic organizing activity. However, the molecular interactions that mediate the effects of glypican have not been identified.
Here we used a mass spectrometric approach to compare LRRTM2 and LRRTM4 binding partners and find that these proteins have distinct receptor preferences: whereas LRRTM2 primarily binds to neurexins, the preferential binding partners for LRRTM4 are glypicans. We find that the glypican-LRRTM4 interaction requires HS and can occur in trans. LRRTM4 regulates excitatory synapse development in cultured neurons and in vivo, and the synaptogenic activity of LRRTM4, but not of LRRTM2, requires HS. Our data identify glypican as a novel receptor for LRRTM4 and indicate that a trans-synaptic glypican-LRRTM4 interaction regulates excitatory synapse development.
RESULTS
Unbiased, proteomics-based identification of glypican as a novel receptor for LRRTM4
The LRRTM genes are expressed in specific and partially non-overlapping expression patterns during synaptogenesis and in the adult brain (de Wit et al., 2009; Lauren et al., 2003). During the synaptogenic period from postnatal day (P) 7 to P14, LRRTM2 and LRRTM4 show complementary expression patterns in cortex, with LRRTM2 expression restricted to layer 6 and LRRTM4 mainly expressed in layer 2/3 and layer 5 (Figure 1A, B). In the hippocampus, LRRTM2 and LRRTM4 are co-expressed in dentate gyrus (DG) granule cells (Figure 1A, B, arrowheads). Whether different LRRTM family members expressed in the same neuron signal through the same presynaptic receptor, or whether various LRRTMs have different mechanisms of action is unknown. Phylogenetic analysis indicates that human LRRTM2 and LRRTM4 share only 43% amino acid identity and that LRRTM4 and LRRTM3 are more closely related to each other than to other LRRTMs (Lauren et al., 2003). These observations raised the possibility that LRRTM4 may have a different presynaptic receptor than LRRTM2.
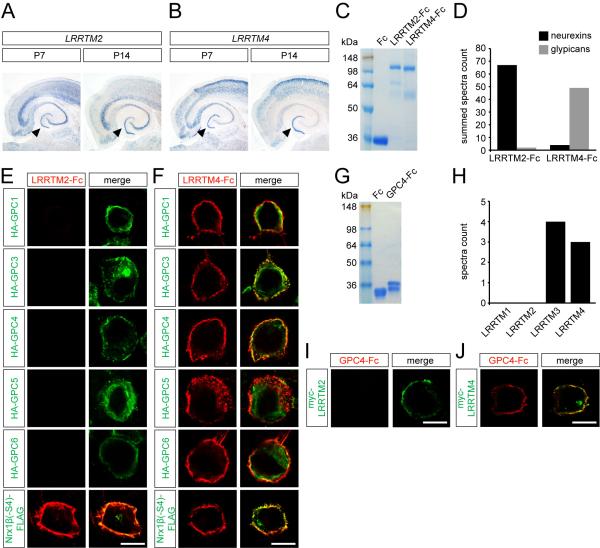
Figure 1. Identification of glypican as a novel receptor for LRRTM4.
(A and B) In situ hybridizations showing expression of LRRTM2 (A) and LRRTM4 (B) in horizontal rat brain sections (arrowheads indicate DG). (C) Coomassie-stained SDS-PAGE gel of purified recombinant LRRTM2- and LRRTM4-ecto-Fc proteins. (D) Identification of glypicans as candidate LRRTM4 interactors by tandem mass spectrometry. LRRTM2-Fc and LRRTM4-Fc proteins were used as bait and Triton X-100-solubilized whole rat brain homogenate was used as prey. Neurexins (black bars) are the major surface protein identified by LRRTM2-Fc; glypicans (grey bars) are the major surface proteins identified by LRRTM4-Fc. Bar graph shows total number of spectra in which the identified proteins were found. (E and F) Cell surface binding assays. LRRTM2-Fc (red) shows no detectable binding to HEK293T cells expressing HA-tagged glypicans (green) (E). LRRTM4-Fc strongly binds to glypicans (F). Both bind FLAG-Nrx1ß(−S4). (G) Coomassie-stained SDS-PAGE gel of purified recombinant GPC4-Fc protein. (H) Identification of LRRTM3 and LRRTM4 in GPC4-Fc affinity purification of Triton X-100-solubilized crude synaptosomes. (I and J) Binding assays. GPC4-Fc (red) does not bind to myc-LRRTM2 (green) (I), but does bind to myc-LRRTM4 (J). See also Figure S1. Scale bar in (E, F, I, J) 10 μm.
To identify candidate LRRTM4 interactors, we took an unbiased, discovery-based approach. We purified recombinant ecto-Fc proteins for LRRTM2 and LRRTM4 (Figure 1C) and used these in a side-by-side comparison to identify interacting proteins in detergent-solubilized whole brain homogenate from 3-4 week old rats by affinity chromatography. Bound proteins were analyzed by tandem mass spectrometry. In agreement with previous results (de Wit et al., 2009; Ko et al., 2009a), the most abundant proteins bound to LRRTM2-Fc were neurexins (Figure 1D). Surprisingly few neurexin spectra counts were detected in the mass spectrometry analysis of the LRRTM4-Fc bound proteins. Instead, the major surface protein identified was glypican, a GPI-anchored HSPG (Figure 1D). Few glypican spectra counts were detected in the LRRTM2-Fc sample, suggesting that glypican may preferentially interact with LRRTM4.
To validate the mass spectrometry results, we carried out cell surface binding assays to test binding of LRRTM2 and LRRTM4 to glypicans. There are six glypican genes in mammals (GPC1-6) (Bernfield et al., 1999; Filmus et al., 2008), five of which were detected in our LRRTM4-Fc sample (GPC1, GPC3-6; Figure S1A). We expressed HA-tagged mouse cDNAs for these glypicans in HEK293T cells and applied LRRTM2-Fc and LRRTM4-Fc proteins to assess LRRTM binding. LRRTM2-Fc showed no detectable binding to glypicans, but bound to neurexin 1ß lacking splice site 4 (Nrx1ß(−S4)) (Figure 1E). In contrast to LRRTM2-Fc, LRRTM4-Fc strongly bound to all glypican isofoms tested (Figure 1F), demonstrating that glypican preferentially interacts with LRRTM4.
Glypicans have been implicated in synapse development. The Drosophila glypican Dally-like regulates neuromuscular synapse development (Johnson et al., 2006), and GPC4 and GPC6 promote excitatory synapse formation in retinal ganglion cells (RGCs) (Allen et al., 2012). Since GPC4 is a Dallylike ortholog (De Cat and David, 2001; Filmus et al., 2008), and GPC4 (but not GPC6) is strongly expressed in developing cortex and hippocampus (Fig. S1B), we decided to focus our experiments on GPC4.
To identify the endogenous binding partners of GPC4, we generated and purified a recombinant GPC4-Fc protein (Figure 1G), which lacks the GPI anchor and was confirmed to contain HS by HS disaccharide analysis (data not shown). Affinity chromatography with GPC4-Fc on detergent-solubilized crude synaptosomes followed by mass spectrometry resulted in the identification of LRRTM3 and LRRTM4, but not of LRRTM1 or LRRTM2 (Figure 1H). The identification of GPC4 and LRRTM4 in reciprocal affinity chromatography experiments using LRRTM4-Fc or GPC4-Fc, respectively, strongly suggests that glypican is an endogenous binding partner of LRRTM4. To verify binding of GPC4 to LRRTMs, we added soluble GPC4-Fc to myc-LRRTM-expressing 293T cells. GPC4-Fc bound to myc-LRRTM4, but showed no detectable binding to myc-LRRTM2 (Figure 1I, J), confirming that glypican preferentially interacts with LRRTM4.
In complementary experiments we examined binding of LRRTM2 and LRRTM4 to neurexins. As previously reported (Ko et al., 2009a; Siddiqui et al., 2010), LRRTM2-Fc strongly bound to Nrx1ß(−S4), but not to Nrx1ß(+S4) expressed in 293T cells (Figure S1C). LRRTM4-Fc bound to Nrx1ß with or without S4, but did not bind to LPHN3, the receptor for the LRR protein FLRT3 (O’Sullivan et al., 2012) (Figure S1D). Fc alone showed no detectable binding to Nrx1ß (Figure S1E). These results show that, unlike LRRTM2, LRRTM4 interacts with neurexins in an S4-independent manner, suggesting that LRRTM4 can bind to a broader array of neurexin isoforms than LRRTM2.
To further explore the interaction of LRRTM proteins with glypicans and neurexins, we performed Fc pulldown assays in 293T cells. LRRTM4-Fc pulled down HA-GPC4 from cell lysate, whereas Fc, Nrx1ß(−S4)-Fc or Nrx1ß(+S4)-Fc did not interact with HA-GPC4 (Figure S1F). In reciprocal experiments, GPC4-Fc pulled down myc-LRRTM4 from cell lysate, but did not bind to FLRT3-myc (Figure S1G), confirming that GPC4 and LRRTM4 can bind each other. Under these conditions, LRRTM2 displayed a weak interaction with GPC4 (Figure S1F, G), which we did not detect in cell surface binding assays (Figure 1E, F, I, J). This suggests that LRRTM2 may have a low affinity for glypican, which would agree with the minor presence of glypican in the LRRTM2-Fc pulldown (2 spectral counts; Figure 1D). Together, our results indicate that LRRTM4 has two binding partners: neurexin and glypican. Whereas neurexins interact with both LRRTM2 and LRRTM4, glypican is a preferential binding partner of LRRTM4.
Characterization of the GPC4-LRRTM4 interaction
To determine whether LRRTM4 and GPC4 can interact directly, we performed cell-free binding assays in which we mixed recombinant His-tagged LRRTM4 ectodomain with purified Fc proteins. Fc proteins were precipitated with protein A/G agarose beads and bound proteins were analyzed by Western blot. His-LRRTM4 co-precipitated with GPC4-Fc and Nrx1ß(−S4)-Fc, but not with Fc or LPHN3-Fc (Figure 2A), confirming a direct interaction between the LRRTM4 ectodomain and GPC4.
Figure 2. The interaction of LRRTM4 with GPC4 is direct and requires HS.
(A) Direct interaction of recombinant His-tagged ecto-LRRTM4 with GPC4-Fc. Fc, GPC4-Fc, Nrx1ß(−S4)-Fc or LPHN3-Fc were mixed with His-LRRTM4, precipitated and analyzed by Western blot. His-LRRTM4 binds to GPC4-Fc and Nrx1ß(−S4)-Fc, but not to Fc or LPHN3-Fc. (B) LRRTM4 bound to Nrx1ß(−S4) cannot simultaneously bind to GPC4. Recombinant HA-GPC4, Nrx1ß(−S4)-Fc and His-LRRTM4 or His-FLRT3 were mixed and precipitated with protein A/G agarose. Proteins bound to Nrx1ß(−S4)-Fc were analyzed with His and HA antibodies. Blot shows full-length GPC4. (C) LRRTM4 bound to GPC4 cannot simultaneously bind to Nrx1ß(−S4). HA-GPC4, Nrx1ß(−S4)-Fc and His-LRRTM4 or His-FLRT3 were mixed and precipitated with HA affinity matrix. Proteins bound to HA-GPC4 were analyzed with His and Fc antibodies. (D) Cell surface binding assays. LRRTM4-Fc (red) binding to HA-GPC4 (green) expressing HEK293T cells is strongly reduced in the presence of 0.5 mg/ml HS or following treatment with 1 U/ml heparinase (hep) III. HS and hepIII abolish background binding of LRRTM4-Fc to cells expressing vector alone. (E) Quantification of assays in (D). Bar graph shows mean ± SEM; a.u., arbitrary units. Only LRRTM4-Fc binding to HA-GPC4 + vehicle was significantly above the pDisplay + vehicle control condition (***p<0.001, post-hoc analysis using Bonferroni Multiple Comparisons test; all other comparisons p>0.05; n=11-29 cells per condition). (F) Schematic representation of HA-GPC4 glycosylation mutants. Green dots indicate proteolytic cleavage site; serine residues serving as HS GAG attachment sites (red dots) were mutated to alanine (blue dots). Mutation of all three GAG attachment sites strongly reduces binding of LRRTM4-Fc (red) to HA-GPC4 (green). See also Figure S2. Scale bar in (D, F) 10 μm.
We next analyzed whether LRRTM4 can simultaneously bind to its two binding partners, neurexin and glypican. We purified recombinant HA-GPC4 from HEK293T conditioned media by affinity chromatography using HA antibodies (Figure S4A), and mixed HA-GPC4 with Nrx1ß(−S4)-Fc and His-LRRTM4 or His-FLRT3. We then precipitated neurexin with protein A/G agarose to test whether pulldown of LRRTM4 bound to neurexin would also bring down glypican. Nrx1ß(−S4)-Fc precipitated His-LRRTM4, but not His-FLRT3. HA-GPC4 did not come down with neurexin-bound LRRTM4 (Figure 2B). In the reciprocal experiment, HA-GPC4 was precipitated with HA antibody-coupled beads to test whether pulldown of LRRTM4 bound to glypican can bring down neurexin. We found that HA-GPC4 precipitated His-LRRTM4, but not His-FLRT3. Nrx1ß(−S4)-Fc did not co-precipitate with glypican-bound LRRTM4 (Figure 2C). In separate experiments, we further established that Nrx1ß(−S4)-Fc or Nrx1ß(+S4)-Fc do not bind to glypican (Figure S1F, S2A, B). These data suggest that LRRTM4 forms separate complexes with neurexin and glypican, and argue against the existence of a tripartite complex.
We next investigated the aspects of glypican processing that are important for LRRTM4 binding. Glypicans consist of a core protein with a cysteine-rich globular domain and a stalk-like domain containing three HS GAG attachment sites. Many glypicans, including GPC4, contain a proteolytic cleavage site in their cysteine-rich domain (Figure 2F). Following cleavage, which is required for some glypican functions (De Cat et al., 2003), the two core protein subunits remain bound by disulfide bonds. GPC4 deletion constructs containing truncations of the core protein were retained intracellularly or lacked glycosylation, and could not be used in binding assays (data not shown). We therefore tested whether proteolytic cleavage is required for GPC4 binding to LRRTM4. We generated HA-GPC4 351-AISA, in which the protease cleavage consensus sequence R351ISR354 was mutated to A351ISA354 (Figure S2C) (De Cat et al., 2003). HA-GPC4 351-AISA was expressed on the cell surface (Figure S2C), and proteolytic processing of HA-GPC4 351-AISA was abolished as determined by the absence of the 40 kDa N-terminal proteolytic GPC4 fragment (Figure S2D). Lack of cleavage did not affect LRRTM4-Fc binding to HA-GPC4 351-AISA (Figure S2E), suggesting that GPC4 processing is not required for the interaction with LRRTM4.
To determine the role of GPC4’s HS chains in LRRTM4 binding, we first tested whether excess HS could block the interaction of LRRTM4 and GPC4. In the presence of HS (0.5 mg/ml), binding of LRRTM4-Fc to HA-GPC4-expressing 293T cells was blocked, and background binding to cells expressing the vector alone was abolished (Figure 2D, E). We next determined whether enzymatic removal of HS would affect LRRTM4 binding to GPC4. 293T cells were treated with heparinase III (hepIII; 2 hrs, 1 U/ml) before applying LRRTM4-Fc. The efficiency of heparinase treatment in removing HS was verified by staining hepIII-treated cells with 3G10 antibody, which specifically recognizes the HS stubs generated by enzymatic digestion and shows no signal in vehicle-treated cells (Figure S2F). Heparinase treatment strongly reduced binding of LRRTM4-Fc to HA-GPC4 and abolished background binding (Figure 2D, E). In a complementary approach, we mutated the three serine residues serving as GAG attachment sites to alanines and evaluated binding of LRRTM4 (Figure 2F). HA-GPC4 lacking all three GAG attachment sites (HA-GPC4 AAA) showed strongly reduced glycosylation compared to HA-GPC4 (Figure S2F). All point mutants were expressed on the cell surface (Figure S2G). Binding of LRRTM4-Fc to GPC4 lacking single GAG attachment sites was reduced, and binding to HA-GPC4 AAA was abolished (Figure 2F). Together, these results demonstrate that the HS chains in GPC4 are a key determinant of the interaction with LRRTM4.
LRRTM4 and GPC4 localize to glutamatergic synapses
LRRTM1 and LRRTM2 proteins localize to the postsynaptic density of excitatory synapses (de Wit et al., 2009; Linhoff et al., 2009), but the distribution of LRRTM4 protein in the nervous system has not yet been described. To this end, we developed a monoclonal antibody against a conserved C-terminal peptide in LRRTM4, in collaboration with the UCDavis/NIH NeuroMab initiative. Acutely prepared rat hippocampal slices were mildly fixed and thinly cryosectioned, and localization of endogenous LRRTM4 was analyzed using confocal microscopy. In the hippocampus, LRRTM4 immunoreactivity was limited to the somata of granule cells and the molecular layer (Figure 3B, arrowheads). The LRRTM4 transcript is only expressed in DG granule cells in this region (Figure 3A), suggesting that LRRTM4 localizes to granule cell dendrites. An independent, polyclonal antibody against the LRRTM4 ectodomain confirmed a dendritic, punctate distribution in cultured hippocampal neurons positive for Prox1 (Figure S3A), a DG granule cell-specific nuclear marker (Williams et al., 2011). LRRTM4 puncta partially overlapped with the presynaptic excitatory marker VGlut1 (Figure 3C) and colocalized with the postsynaptic glutamate receptor subunit GluR1 (Figure 3D). Staining for the presynaptic inhibitory marker VGAT showed no colocalization of LRRTM4 and VGAT (Figure S3B). These data suggest that endogenous LRRTM4 localizes to the postsynaptic density of glutamatergic synapses.
Figure 3. Endogenous LRRTM4 and GPC4 proteins localize to excitatory synapses.
(A) In situ hybridization showing LRRTM4 expression in a sagittal section of P14 rat hippocampus. LRRTM4 expression is limited to DG granule cells. (B) LRRTM4 protein (red) localizes to granule cell bodies and the DG molecular layer in P15 hippocampus (arrowheads). The presynaptic excitatory marker VGlut1 (blue) visualizes hippocampal architecture. (C, D) Postsynaptic localization of LRRTM4 in DIV14 hippocampal neurons. (C) LRRTM4-positive puncta (red) partially overlap with VGlut1 puncta (green). (D) LRRTM4 puncta (red) colocalize with the excitatory postsynaptic marker GluR1 (green). (E) In situ hybridization showing GPC4 expression in a sagittal section of P14 hippocampus. GPC4 is strongly expressed in DG and CA1, and weakly in CA3. (F) GPC4 protein (red) localizes to DG and CA1 cell bodies and neuropil in P21 hippocampus. Arrowheads indicate strong GPC4 staining in CA3 stratum lucidum. (G) Presynaptic localization of GPC4 in DIV16 hippocampal neuron. GPC4-positive puncta colocalize with VGlut1 (red) and are juxtaposed to puncta positive for the excitatory postsynaptic marker PSD-95 (blue). See also Figure S3. Scale bar in (A, B, E, F) 200 μm; in (C, D, G) 10 μm.
The localization of GPC4 protein in the nervous system during the postnatal synaptogenic period has not been characterized. In situ hybridizations showed that GPC4 mRNA is highly expressed in DG and CA1 neurons, and to a lesser extent in CA3 neurons (Figure 3E; Figure S1B). Labeling of hippocampal cryosections with a polyclonal GPC4 antibody (Ford-Perriss et al., 2003; Siebertz et al., 1999) revealed prominent staining of DG and CA1 cell bodies and dense labeling of the neuropil (Figure 3F). The mRNA and protein expression patterns indicate that GPC4 has a much broader distribution in the CNS than LRRTM4, suggesting that GPC4 has additional roles besides those mediated by LRRTM4 interaction.
To determine whether GPC4 is a synaptic protein, we analyzed GPC4 distribution in hippocampal neurons. GPC4 localized to discrete puncta, which colocalized with VGlut1 and were juxtaposed to puncta positive for the postsynaptic excitatory marker PSD-95 (Figure 3G), suggesting a presynaptic localization of GPC4. To test whether GPC4 shows a similar distribution in vivo, we took advantage of the strong GPC4 signal in CA3 stratum lucidum (Figure 3F, arrowheads), where large GPC4-positive puncta colocalized with VGlut1 (Figure S3C). GPC4/VGlut1-positive puncta were juxtaposed to PSD-95 puncta, suggesting that GPC4 also localizes to excitatory presynaptic terminals in vivo. GPC4 showed little colocalization with the pre- and postsynaptic inhibitory markers VGAT and gephyrin (Figure S3D). Together, these results indicate that LRRTM4 and GPC4 localize to glutamatergic synapses, consistent with GPC4 being a presynaptic binding partner for postsynaptic LRRTM4.
LRRTM4 and GPC4 interact in trans
The distribution of LRRTM4 and GPC4 proteins in hippocampal neurons suggests that they localize to opposite sides of the glutamatergic synapse. To test whether GPC4 and LRRTM4 can interact in trans, we transfected HEK293T cells with myc-LRRTM4 or HA-GPC4 and determined whether they could induce clustering of their respective binding partners in cocultured hippocampal neurons. Myc-LRRTM4 expressed in 293T induced strong clustering of the presynaptic marker VGlut1 but not of VGAT (Figure 4A, B). Endogenous neuronal GPC4 also clustered on the surface of LRRTM4-expressing cells, whereas GFP-expressing cells had no such effect (Figure 4C, D). We then performed the reciprocal experiment using HA-GPC4-expressing 293T cells and analyzed clustering of synaptic markers in contacting dendrites. HAGPC4 had a small, but significant effect on PSD-95 aggregation in dendrites compared to GFP control cells, but did not induce gephyrin clustering (Figure 4E, F). Endogenous LRRTM4 clusters also accumulated opposite to HA-GPC4-expressing 293T cells (Figure 4G, H), indicating that GPC4 induces clustering of LRRTM4 in opposing membranes. Since the GPC4-LRRTM4 interaction requires HS (Figure 2D-F), expression of GPC4 lacking GAG attachment sites in 293T cells should not induce aggregation of LRRTM4 in cocultured neurons. Consistent with this prediction, the HA-GPC4 AAA mutant did not induce clustering of LRRTM4 (Figure 4G, H). These results indicate that GPC4 and LRRTM4 can interact in trans in a HS-dependent manner.
Figure 4. Trans-cellular GPC4-mediated clustering of endogenous LRRTM4 requires HS.
(A-H) Coculture assays. (A) Myc-LRRTM4 (green) expressed in HEK293T cells cocultured with DIV7-8 hippocampal neurons induces clustering of the presynaptic excitatory marker VGlut1 (red) but not of the presynaptic inhibitory marker VGAT (red). (B) Quantification of assays in (A). LRRTM4 significantly increases the fractional VGlut1 area (area occupied by VGlut1 staining per myc-labeled surface area normalized to GFP-expressing control cells) compared to GFP cells (VGlut1: GFP 1.00 ± 0.32 (n=30 cells) vs. LRRTM4 14.18 ± 2.54 (n=27); ***p<0.0001, Mann-Whitney test. VGAT: GFP 1.00 ± 0.22 (n=21 cells) vs. LRRTM4 2.07 ± 0.54 (n=23); n.s. p=0.1103 Mann-Whitney test). (C) Myc-LRRTM4 induces clustering of endogenous GPC4 (red). (D) Quantification of (C); GFP 1.00 ± 0.34 (n=19 cells) vs. LRRTM4 22.67 ± 4.52 (n=24); ***p<0.0001, Mann-Whitney test. (E) HA-GPC4 expressed in 293T cells induces clustering of the excitatory postsynaptic marker PSD-95 (red; arrowheads) but not of the inhibitory postsynaptic marker gephyrin (red). (F) Quantification of (E); PSD-95: GFP 1.00 ± 0.21 (n=18) vs. HA-GPC4 5.30 ± 0.58 (n=26); ***p<0.0001, Student’s t-test. Gephyrin: GFP 1.00 ± 0.23 (n=20) vs. HA-GPC4 1.96 ± 0.81 (n=25); n.s. p=0.7441, Student’s t-test. (G) HAGPC4-mediated clustering of endogenous LRRTM4 (red) in contacting dendrites requires GAG chains. HA-GPC4-mediated LRRTM4 clustering (arrowheads) is absent around HA-GPC4 AAA expressing cells. (H) Quantification of (G); GFP 1.00 ± 0.22 (n=53), HA-GPC4 15.06 ± 3.07 (n=78), HA-GPC4 AAA 2.17 ± 0.52 (n=53); ***p<0.001, Kruskal-Wallis test, Dunn’s multiple comparisons post-hoc test. Bar graphs show mean ± SEM. See also Figure S4. Scale bar in (A, C, E, G) 10 μm.
Upon expression in cell lines, GPC4 is constitutively released from the cell surface and secreted into the culture media (Watanabe et al., 1995). To determine whether soluble GPC4 can induce clustering of LRRTM4 and trigger postsynaptic differentiation similar to surface-expressed GPC4, we purified recombinant HA-GPC4 from 293T-conditioned media and bath-applied it to cultured hippocampal neurons. Purified HA-GPC4 (Figure S4A) directly bound the LRRTM4 ectodomain in cell-free binding assays (Figure 2C and data not shown). We applied recombinant HA-GPC4 to DIV13 neurons for 24 hours at a concentration of 10 nM, within the effective range for soluble GPC4-induced glutamate receptor clustering in RGCs (0.1-10 nM; Allen et al., 2012), and quantified density and area of LRRTM4-positive clusters per length of MAP2-positive dendrite. Since hippocampal LRRTM4 expression is limited to DG granule cells, we only included Prox1-positive neurons in our analysis. The density and area of LRRTM4 clusters did not differ between HA-GPC4- and Fc-treated neurons (Figure S4B-D). Treatment with 10 nM preclustered GPC4-Fc did not affect density and area of LRRTM4 clusters either (Figure S4E-G), suggesting that soluble GPC4 does not induce clustering of LRRTM4 on the dendritic surface.
To determine whether soluble GPC4 can induce postsynaptic differentiation, we treated hippocampal neurons with 1 or 10 nM HA-GPC4 for 6 days and quantified the density of VGlut1/PSD-95-positive puncta. In contrast to RGCs (Allen et al., 2012), 6-day treatment with soluble HA-GPC4 did not increase excitatory synapse density in DIV14 hippocampal neurons (Figure S4H, I). Since the peak of synaptogenesis may occur earlier in hippocampal neurons compared to RGCs (Xu et al., 2010), we also added recombinant HA-GPC4 from DIV5-8 to determine whether GPC4 may promote synaptogenesis in immature neurons. Treatment with 1 or 10 nM HA-GPC4 did not affect excitatory synapse density at this earlier time point (Figure S4J, K). These results indicate that soluble GPC4 does not induce LRRTM4 clustering or postsynaptic differentiation in hippocampal neurons and suggest that a local concentration of GPC4 is needed to aggregate LRRTM4. Alternatively, the amounts of soluble GPC4 in the culture media may be saturating, such that exogenous addition does not increase synapse formation.
LRRTM4 regulates functional excitatory synapse development in hippocampal neurons
The experiments described above support a ligand-receptor relationship between LRRTM4 and glypican, and a potential role of this interaction in excitatory synapse development. To directly examine whether LRRTM4 regulates excitatory synapse formation, we analyzed the consequences of overexpressing LRRTM4 in cultured hippocampal neurons. Myc-LRRTM4 overexpression from DIV7 to DIV14 significantly increased the density of excitatory synapses compared to GFP-expressing control neurons (Figure 5A, B). Quantification of VGAT/gephyrin-positive puncta in sister cultures showed no effect of LRRTM4 overexpression on inhibitory synapse density (Figure 5C, D). To test whether endogenous LRRTM4 contributes to excitatory synapse development, we designed a short hairpin RNA (shRNA) to specifically reduce LRRTM4 expression. The LRRTM4 shRNA effectively reduced mouse myc-LRRTM4 expression in HEK293T cells, whereas expression of shRNA-resistant human myc-LRRTM4 was not affected (Figure S5A). Expression of endogenous LRRTM4, but not of LRRTM2, was strongly reduced in hippocampal neurons infected with lentivirus containing shLRRTM4 (Figure S5B, C). Furthermore, LRRTM4 immunoreactivity was strongly reduced in shLRRTM4-expressing Prox1-positive neurons, but not in neighboring, non-electroporated cells (Figure S5D). Knockdown of LRRTM4 in Prox1-positive hippocampal neurons using this shRNA resulted in a 40% decrease in the density of excitatory synapses, which could be rescued by coexpressing human myc-LRRTM4 (Figure 5E, F). Expression of shLRRTM4 in Prox1-positive hippocampal neurons did not affect the density of inhibitory synapses (Figure 5G, H), indicating that endogenous LRRTM4 selectively regulates the density of excitatory synapses.
Figure 5. LRRTM4 regulates functional excitatory synapse density in hippocampal neurons.
(A) Overexpression of myc-LRRTM4 from DIV7-14 increases excitatory synapse density. (B) Quantification of the number of VGlut1/PSD-95-positive puncta per length of dendrite normalized to GFP control neurons (GFP 1.00 ± 0.06 (n=36 neurons) vs. LRRTM4 1.42 ± 0.09 (n=35); ***p<0.0001, Student’s t-test). (C) Overexpression of myc-LRRTM4 does not affect inhibitory synapse density. (D) Quantification of normalized VGAT/gephyrin-positive puncta density (GFP 1.00 ± 0.07 (n=37) vs. LRRTM4 0.99 ± 0.07 (n=36); p=0.9315, Student’s t-test). (E) LRRTM4 knockdown reduces excitatory synapse density, which is rescued by coexpression of shRNA-resistant human LRRTM4. (F) Quantification of normalized VGlut1/PSD-95 puncta density in Prox1-positive cells (control 1.00 ± 0.04 (n=34), shLRRTM4 0.63 ± 0.05 (n=35), rescue 0.91 ± 0.05 (n=35); ***p<0.001, ANOVA, Tukey-Kramer multiple comparisons post-hoc test). (G) LRRTM4 knockdown does not affect inhibitory synapse density. (H) Quantification of normalized VGAT/gephyrin puncta density in Prox1-positive neurons (control 1.00 ± 0.05 (n=27), shLRRTM4 0.97 ± 0.09 (n=28), rescue 1.04 ± 0.07 (n=27); p=0.7419, ANOVA). (I, J) Example mEPSC (Vhold −80 mV) (I) and mIPSC traces (Vhold 0 mV) (J) from neurons expressing sh-vector (control, black), shLRRTM4 (red) or shLRRTM4 + human LRRTM4 (rescue, grey). (K) LRRTM4 knockdown reduces mean mEPSC frequency (values normalized to control cells: control 1.00 ± 0.13 (n=35), shLRRTM4 0.64 ± 0.10 (n=41), rescue 0.90 ± 0.17 (n=19); **p<0.01, Kruskal-Wallis test, Dunn’s multiple comparisons test). (L) Mean mEPSC amplitude is not affected by LRRTM4 knockdown (normalized values: control 1.00 ± 0.02 (n=35), shLRRTM4 1.00 ± 0.03 (n=41), rescue 1.03 ± 0.04 (n=19); p=0.7248, ANOVA). (M, N) Neither mIPSC frequency (control 0.44 ± 0.07 Hz (n=18) vs. shLRRTM4 0.36 ± 0.07 Hz (n=18); p=0.42, Student’s t-test) nor mIPSC amplitude differs between conditions (control 18.31 ± 0.78 pA (n=18) vs. shLRRTM4 16.63 ± 0.81 pA (n=18); p=0.14, Student’s t-test). (O) DIV9-14 treatment with 10 μg/ml LRRTM2- or LRRTM4-Fc reduces excitatory synapse density in Prox1-positive cells (Fc 1.00 ± 0.06 (n=28), LRRTM2-Fc 0.50 ± 0.06 (n=20), LRRTM4-Fc 0.61 ± 0.05 (n=28); ***p<0.001, ANOVA, Tukey-Kramer multiple comparisons post-hoc test). See also Figure S5. Bar graphs show mean ± SEM. Scale bar in (A, C, E, G) 10 μm.
To determine whether the decrease in excitatory synapse density following LRRTM4 knockdown corresponds to a decrease in functional synapses, we recorded miniature excitatory and inhibitory postsynaptic currents (mEPSCs and mIPSCs, respectively) in hippocampal neurons (Figure 5I, J). LRRTM4 knockdown significantly decreased mEPSC frequency, but did not change mEPSC amplitude compared to control cells, and these effects could be rescued by coexpressing human LRRTM4 (Figure 5K, L). Frequency and amplitude of mIPSCs were not affected by knockdown of LRRTM4 (Figure 5M, N). These results are consistent with the selective reduction in excitatory synapse density following LRTM4 knockdown, as assessed by immunofluorescence.
In a complementary, shRNA-independent approach to assess the role of LRRTM4 in synapse development, we treated hippocampal neurons with excess LRRTM4-Fc to competitively disrupt the trans-synaptic interaction of LRRTM4 with presynaptic receptors. Neurons were treated for 6 days and the density of VGlut1/PSD-95-positive puncta in Prox1-positive cells was analyzed at DIV14. LRRTM4-Fc treatment reduced excitatory synapse density by 40% compared to cells treated with Fc control protein, similar to treatment with LRRTM2-Fc (Figure 5O). These results are in agreement with the effects of LRRTM4 knockdown, supporting a role of LRRTM4 in regulating excitatory synapse development.
LRRTM4’s synaptogenic activity requires heparan sulfate
LRRTM2 and LRRTM4 share a similar synaptogenic activity in hippocampal neurons, but LRRTM4 is distinct from LRRTM2 in that it has two presynaptic binding partners, neurexin and glypican. To begin assessing the role of these two LRRTM4 receptors in synapse development, we tested whether excess GPC4-Fc or Nrx1ß(−S4)-Fc could block excitatory synapse formation in Prox1-positive neurons. In agreement with previous results (Chih et al., 2006), 6-day treatment with Nrx1ß(−S4)-Fc caused a reduction in excitatory synapse density in DIV14 hippocampal neurons (Figure S6A, B). However, GPC4-Fc did not affect excitatory synapse density at this time-point, nor did 3-day treatment with GPC4-Fc in immature neurons (Fig. S6A-D). Possibly, neurexin can compensate when the glypican-LRRTM4 interaction is blocked. Alternatively, since LRRTM4-Fc decreases excitatory synapse density (Figure 5O), but GPC4-Fc does not (Figure S6), it could be that GPI-anchored glypican is only part of a functional presynaptic LRRTM4 receptor and requires a yet unidentified transmembrane signaling coreceptor. Such signaling might be required for the development of synaptic contacts between neurons.
We next analyzed whether excess HS could interfere with LRRTM4-mediated synapse formation onto heterologous cells. HEK293T cells expressing myc-LRRTM2 or myc-LRRTM4 were cocultured with DIV7 hippocampal neurons for 12 hrs in the presence of 0.5 mg/ml HS. Exogenous HS did not affect LRRTM2-mediated presynaptic differentiation, but abolished the synaptogenic activity of LRRTM4 (Figure 6A-D). To test whether LRRTM4-mediated presynaptic differentiation requires endogenous HS, we treated DIV7 neurons with heparinase III (2 hrs, 1U/ml), washed and cocultured them for an additional 8 hrs with 293T cells expressing myc-LRRTM. Staining with the 3G10 HS stub antibody confirmed the efficiency of hepIII treatment in hippocampal neurons (data not shown). Enzymatic removal of HS did not affect LRRTM2’s ability to instruct presynaptic differentiation (Figure 6E, F), but strongly reduced LRRTM4’s synaptogenic activity (Figure 6G, H). These results indicate that LRRTM4-mediated presynaptic differentiation requires the presence of HS.
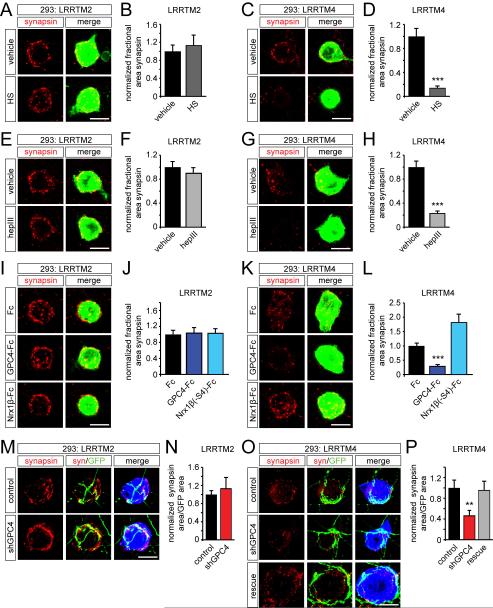
Figure 6. The synaptogenic activity of LRRTM4, but not of LRRTM2, requires HS.
(A-P) Coculture assays. (A) Myc-LRRTM2-induced clustering of synapsin puncta on the HEK293T cell surface in vehicle- or HS (0.5 mg/ml)-treated cocultures. (B) Quantification of synapsin immunoreactivity as a measure of presynaptic differentiation in assays in (A); vehicle 1.00 ± 0.14 (n=29 cells) vs. HS 1.14 ± 0.22 (n=30); p=0.9784, Mann-Whitney test. (C) HS blocks myc-LRRTM4-induced presynaptic differentiation. (D) Quantification of (C); vehicle 1.00 ± 0.13 (n=31) vs. HS 0.14 ± 0.03 (n=34); ***p<0.0001, Mann-Whitney test. (E) Myc-LRRTM2-induced presynaptic differentiation in vehicle- or hepIII (1 U/ml)-treated cultures. (F) Quantification of (E); vehicle 1.00 ± 0.09 (n=25) vs. hepIII 0.91 ± 0.09 (n=25); p=0.4676, Student’s t-test. (G) HepIII treatment strongly reduces myc-LRRTM4-induced presynaptic differentiation. (H) Quantification of (G); vehicle 1.00 ± 0.10 (n=27) vs. hepIII 0.23 ± 0.03 (n=35); ***p<0.0001, Student’s t-test. (I) Effect of Fc, GPC4-Fc and Nrx1ß(−S4)-Fc proteins (50 μg/ml) on LRRTM2-induced presynaptic differentiation. (J) Quantification of (I) (Fc 1.00 ± 0.11 (n=28), GPC4-Fc 1.05 ± 0.13 (n=27), Nrx1ß(−S4)-Fc 1.04 ± 0.10 (n=30); p=0.9511, ANOVA). (K) GPC4-Fc reduces myc-LRRTM4-induced presynaptic differentiation. (L) Quantification of (K); Fc 1.00 ± 0.10 (n=30), GPC4-Fc 0.30 ± 0.05 (n=33), Nrx1ß(−S4)-Fc 1.83 ± 0.27 (n=27); ***p<0.001; Fc vs. Nrx1ß(−S4)-Fc p>0.05; Kruskal-Wallis test, Dunn’s multiple comparison post-hoc test. (M) Neuronal GPC4 knockdown does not affect synapse formation onto LRRTM2-expressing 293T cells. Synapsin (syn), red; GFP, green; myc, blue. (N) Quantification of (M); control 1.00 ± 0.09 (n=19) vs. shGPC4 1.14 ± 0.24 (n=17); p=0.7934, Mann-Whitney test. (O) GPC4 knockdown decreases synapse formation onto LRRTM4-expressing cells. (P) Quantification of (O); control 1.00 ± 0.15 (n=24), shGPC4 0.47 ± 0.10 (n=30), rescue 0.96 ± 0.17 (n=32); **p<0.01, Kruskal-Wallis test, Dunn’s multiple comparison post-hoc test. See also Figure S6. Bar graphs show mean ± SEM. Scale bar in (A, C, E, G, I, K, M, O) 10 μm.
To analyze the relative contributions of glypican and neurexin to LRRTM4’s synaptogenic activity, we cocultured 293T cells expressing myc-LRRTM with DIV7 neurons for 12 hrs in the presence of excess Fc, Nrx1ß(−S4)-Fc or GPC4-Fc. GPC4-Fc did not affect LRRTM2-mediated presynaptic differentiation, but markedly reduced LRRTM4’s synaptogenic activity (Figure 6I-L), suggesting that GPC4 is a presynaptic receptor for LRRTM4-induced synapse formation. Unexpectedly, and in contrast to a previous report (Ko et al., 2009a), two independently generated batches of Nrx1ß(−S4)-Fc did not reduce LRRTM2’s synaptogenic activity in three separate experiments (Figure 6I, J). A compensatory role of α-neurexins (Ko et al., 2009b), or rapid internalization of Nrx-Fc in cocultures (Chubykin et al., 2005), might explain the lack of effect of Nrx1ß(−S4)-Fc on LRRTM2-induced presynaptic differentiation.
These experiments suggest that LRRTM4 interacts with a presynaptic HSPG to induce synapse formation. To determine whether HS is required presynaptically, we first tested a large number of shRNAs to knock down expression of EXT1 and EXT2, the two key enzymes in heparan sulfate synthesis. However, expression of two working EXT1 shRNAs in hippocampal neurons resulted in the fasciculation of neurites and retraction of neurites from the substrate; effects not seen in neurons expressing the control vector or shRNAs against other targets. We therefore determined whether glypican is required in neurons for LRRTM4-induced synapse formation. We designed an shRNA against mouse and rat GPC4 and an shRNA-resistant GPC4 rescue construct containing silent mutations in the shRNA target region (GPC4*), and confirmed knockdown and rescue of GPC4 expression in 293T cells (Figure S6E). We then electroporated hippocampal neurons with control, shGPC4, or shGPC4 and GPC4* plasmids, cocultured 293T cells expressing myc-LRRTM at DIV7, and quantified the area of synapsin clusters per GFP-positive axon area on the 293T cell surface. Neuronal knockdown of GPC4 did not affect synapse formation onto LRRTM2-expressing cells (Fig. 6M, N), but strongly reduced synapse formation onto LRRTM4-expressing HEK293T cells (Fig. 6O, P). This decrease could be rescued by coexpression of shRNA-resistant GPC4* (Fig. 6O, P). The selective effect of GPC4 knockdown on LRRTM4-, but not on LRRTM2-mediated presynaptic differentiation, and the complete rescue by GPC4* support the specificity of the shRNA used. Taken together, these data indicate that LRRTM4’s synaptogenic activity depends on presynaptic glypican.
LRRTM4 regulates synapse development in L2/3 pyramidal neurons in vivo
In the final series of experiments we examined whether loss of LRRTM4 affects synapse development in vivo. LRRTM4 is co-expressed with other LRRTM proteins in some neuronal populations, but whether LRRTM4 serves a unique or redundant role in synapse development or function is not known. Since LRRTM4 is highly expressed in DG, we initially injected lentiviral vectors expressing shLRRTM4 in rat DG at P5 and recorded from neighboring infected and non-infected granule cells in P13-16 acute slices, while stimulating their perforant path inputs. In granule cells, knockdown of LRRTM4 did not affect the strength of glutamatergic transmission (data not shown), which could be due to incomplete knockdown or the expression of other LRRTMs (Lauren et al., 2003), which may functionally compensate. We therefore decided to investigate LRRTM4’s role in synapse development in cortical layer 2/3 (L2/3) pyramidal neurons, which do not express LRRTM2 (Figure 1A). We first tested whether LRRTM4 regulates synapse formation in cultured cortical neurons and found a significant decrease in the density of dendritic spines and of PSD-95-positive spines following LRRTM4 knockdown (Fig. S7A-D). Embryonic day 15.5 mouse embryos were electroporated with control or shLRRTM4 plasmids, resulting in the transduction of L2/3 pyramidal neurons in primary somatosensory cortex (Figure 7A). We verified by in situ hybridization that LRRTM4 is expressed in mouse P15 L2/3 neurons and that GPC4 is expressed in L2/3 and L4 neurons (Figure S7E, F), indicating that GPC4 is presynaptic to the neurons we recorded from. GFP-positive electroporated L2/3 cells were scattered amongst a majority of non-electroporated cells and targeted for whole-cell recording (Figure 7B). We recorded mEPSCs from labeled cells in acute brain slices and compared mEPSCs from control, GFP-expressing neurons to shLRRTM4-electroporated neurons (Figure 7C). Knockdown of LRRTM4 did not affect the frequency of mEPSCs (Figure 7D), but significantly reduced the mean amplitude of mEPSCs (Figure 7E). These results indicate that LRRTM4 regulates the strength of glutamatergic synaptic transmission in cortical neurons in vivo, most likely by regulating AMPA receptor content at synapses.
Figure 7. LRRTM4 regulates synapse development in L2/3 pyramidal neurons in vivo.
(A) GFP epifluorescence and DIC images from somatosensory cortex slices of 2 week old mouse brain electroporated in utero at E15-16. Note GFP expression in L2/3, and barrels in L4. (B) High magnification image of a recording from an electroporated GFP-positive L2/3 pyramidal neuron surrounded by non-electroporated cells. (C) Example mEPSC traces from control (black) and shLRRTM4 (red) electroporated cells on compressed timescale (left). Right, averaged mEPSC traces on expanded timescale. Scaled, overlaid traces show normal decay kinetics for both conditions. (D) Summary of mEPSC frequencies plotted as cumulative probability distributions of inter-event intervals (IEIs) for control (black) and shLRRTM4 (red) cells. Inset: quantification of mean mEPSC IEIs. LRRTM4 knockdown does not affect mEPSC frequency (control 1006.5 ms ± 136.2 (n=16) vs. shLRRTM4 953.0 ms ± 116.4 (n=13); p=0.99, Student’s t-test). (E) Summary of mEPSC amplitudes plotted as cumulative probability distributions. Inset: quantification of mean mEPSC amplitude. LRRTM4 knockdown reduces mean mEPSC amplitude (control 12.64 ± 0.51 pA (n=16) vs. shLRRTM4 10.28 ± 0.45 pA (n=13); p=0.0023, Student’s t-test). (F) Dendrites of electroporated L2/3 cortical neurons were imaged in L2/3. (G) LRRTM4 knockdown significantly decreases the density of dendritic protrusions (control 0.66 ± 0.02 protrusions/μm (n=56 dendrites, 4 animals) vs. shLRRTM4 0.54 ± 0.01 protrusions/μm (n=78 dendrites, 4 animals); p<0.0001, Student’s t-test). See also Figure S7. Bar graphs show mean ± SEM. Scale bar in (F) 5 μm.
To determine whether LRRTM4 may regulate synapse density in vivo as it does in cultured hippocampal and cortical neurons, we analyzed the density of dendritic spines in L2/3 cortical neurons in electroporated mice at P14. LRRTM4 knockdown resulted in a significant, 18% decrease in the density of dendritic protrusions relative to control neurons (Fig. 7F, G). Together, these results indicate that endogenous LRRTM4 is required for synapse development in vivo.
DISCUSSION
Cell surface interactions play key roles in establishing functional neural circuits. Here we identify glypican as an LRRTM4 receptor in an unbiased, proteomics-based approach to find the endogenous receptors for LRRTM2 and LRRTM4. Glypican preferentially interacts with LRRTM4, and this interaction is HS dependent. GPC4 and LRRTM4 localize to opposing membranes of glutamatergic synapses. GPC4 and LRRTM4 expressed on the surface of nonneuronal cells induce clustering of their respective binding partners in cocultured neurons, supporting a trans-synaptic interaction of presynaptic glypican and postsynaptic LRRTM4. Overexpression, knockdown, and competition experiments with soluble LRRTM4 ectodomains show that LRRTM4 regulates excitatory synapse development in cultured hippocampal neurons. The synaptogenic activity of LRRTM4, but not of LRRTM2, requires HS. Knockdown of LRRTM4 in vivo decreases the strength of glutamatergic synaptic transmission and the density of dendritic spines, indicating that LRRTM4 controls synapse development in vivo. These results identify glypican as a novel receptor for LRRTM4, and highlight the diversity in ligand-receptor interactions that regulate excitatory synapse development.
LRRTM4 binds to HS GAGs
Glypican binding to LRRTM4 requires HS, and HS is required for LRRTM4 function. Binding of GAGs to LRR proteins is not unprecedented: a recent study identified chondroitin sulfate (CS) proteoglycans as ligands for the Nogo receptor family members NgR1 and NgR3 (Dickendesher et al., 2012). Interestingly, NgR1 and NgR3 showed strong selectivity toward specific CS GAG types, suggesting that differences in GAG sulfation patterns may regulate NgR binding. Synaptic transmission at the Drosophila neuromuscular junction is differentially affected by knockdown of two different enzymes that regulate HSPG sulfation (Dani et al., 2012), suggesting that HS modifications are also important for synapse development. Whether LRRTM4 displays any selectivity with regard to modifications of HS chains is unknown.
Glypicans are widely expressed throughout the body and bind many secreted and surface-bound proteins (Bernfield et al., 1999; Van Vactor et al., 2006). Based on mRNA and protein expression patterns, it appears likely that LRRTM4 is not the only endogenous binding partner of GPC4, as LRRTM4 expression is much more restricted than that of GPC4. The full complement of synaptic GPC4 interactors is not yet known. In addition to LRRTM4, our GPC4-Fc pulldown experiment also identified LRRTM3, a largely uncharacterized LRRTM family member. LRRTM3 and LRRTM4 are more closely related to each other than to LRRTM1 and LRRTM2 (Lauren et al., 2003), and this evolutionary relationship appears to be reflected in LRRTM-receptor interactions.
Our experiments suggest that GPC4 needs to aggregate on the cell surface before it can induce LRRTM4 clustering and postsynaptic differentiation. Although GPC4 released from the cell surface was able to bind LRRTM4 in solution, bath-applied soluble GPC4 did not affect LRRTM4 clustering or postsynaptic differentiation. In RGCs, soluble GPC4 induces clustering of the glutamate receptor subunit GluR1 and promotes excitatory synapse formation (Allen et al., 2012). Cultured RGCs are more reluctant to form synapses than hippocampal neurons, and soluble GPC4 may have more pronounced effects on RGC synaptogenesis. Alternatively, soluble GPC4 levels in hippocampal cultures may already be saturating or secreted GPC4 may induce GluR1 clustering through an LRRTM4-independent mechanism. It will be of interest to determine whether GPC4 exerts these effects through LRRTM4 in RGCs.
Role of the GPC4-LRRTM4 interaction in synapse development
GPC4 mRNA was expressed in neurons during synaptogenesis, GPC4 protein localized to excitatory presynaptic terminals, and GPC4 was functionally required in neurons for LRRTM4’s synaptogenic activity. The neuronal localization of GPC4 is in agreement with previous studies that showed neuronal expression and axonal localization for other glypicans (Ivins et al., 1997; Litwack et al., 1998; Litwack et al., 1994; Saunders et al., 1997; Stipp et al., 1994). Our findings do not rule out expression in astrocytes in early postnatal hippocampus (Allen et al., 2012), but we conclude that GPC4 is primarily expressed in neurons and presynaptically localized during synapse formation. Since GPC4 is a GPI-anchored HSPG, additional, yet unknown, signaling coreceptors may be required to promote LRRTM4-mediated presynaptic differentiation. Our finding that excess LRRTM4-Fc, but not GPC4-Fc, disrupted excitatory synapse development in hippocampal neurons supports the existence of a signaling coreceptor for GPC4. This result is reminiscent of a previous study on the LRR protein NGL-1 and its GPI-anchored axonal ligand Netrin-G1 (Lin et al., 2003). This study concluded that Netrin-G1 is only part of the NGL-1 receptor, since soluble NGL-1, but not soluble Netrin-G1, blocked outgrowth of thalamic neurons. The identity of the putative GPC4 coreceptor is unknown. Drosophila Dallylike binds to LAR (leukocyte common antigen related), a receptor protein tyrosine phosphatase (Johnson et al., 2006). Although LAR was not identified in our GPC4-Fc pulldown experiment (data not shown), it will be important to determine whether LAR is a functional presynaptic GPC4 receptor.
LRRTM4 regulates excitatory synapse development in vitro and in vivo. Knockdown of LRRTM4 in cultured hippocampal neurons decreased the density of functional excitatory synapses. In vivo, LRRTM4 knockdown resulted in a significant decrease in the density of dendritic spines, the predominant sites of excitatory synapses in the CNS (Bourne and Harris, 2008). Importantly, we used sparse knockdown in subsets of cells in both our in vitro and in vivo experiments. A recent study showed that transcellular differences in the relative levels of neuroligin-1 determine synapse number in vitro and in vivo (Kwon et al., 2012), suggesting that neurons with lower neuroligin-1 levels compared to their neighbors are less successful in competing for synaptic inputs. Such a mechanism may apply to LRRTMs as well. Despite the significant reduction in dendritic spine density in L2/3 cortical neurons, we did not detect a corresponding decrease in mEPSC frequency. Cortical L2/3 neurons displayed a small decrease in mEPSC amplitude following LRRTM4 knockdown, suggesting a decrease in AMPA receptor (AMPAR) content. Since spine size and AMPAR number are correlated (Matsuzaki et al., 2001; Takumi et al., 1999), it is possible that a decrease in AMPAR content following LRRTM4 knockdown results in smaller spines, which may fall below the detection threshold in our image analysis. Alternatively, LRRTM4 knockdown may predominantly affect immature spines with low AMPAR content (‘silent’ synapses), resulting in decreased spine density but no effect on mEPSC frequency. Our current image resolution was not sufficient to rigorously analyze the morphology of individual spines. Another possible explanation for the lack of decrease in mEPSC frequency following LRRTM4 knockdown might be that LRRTM4 regulates spine development in select dendritic processes, rather than globally affecting spine density. Loss of LRRTM1 affects VGlut1 clustering in select CA1 hippocampal laminae (Linhoff et al., 2009), suggesting that at least some LRRTMs may have lamina-specific effects on synapse development.
The reduction in synaptic strength following LRRTM4 knockdown in vivo could be mediated by a direct role of LRRTM4 in AMPAR trafficking. Both LRRTM4 and LRRTM3 were identified as components of AMPAR complexes (Schwenk et al., 2012; Shanks et al., 2012), and LRRTM2 binds GluR1 via its extracellular domain in heterologous cells (de Wit et al., 2009). A similar reduction in synaptic strength has been observed in GPC4 knockout mice, which was attributed to decreased recruitment of the AMPAR subunit GluR1 to synaptic sites (Allen et al., 2012). These findings suggest that a disruption of the glypican-LRRTM4 interaction may lead to reduced recruitment or stabilization of AMPARs at the synapse, resulting in a decrease in synaptic strength.
Finally, genome-wide association studies have linked GPC1 and GPC6 to ADHD, neuroticism and schizophrenia (Calboli et al., 2010; Lesch et al., 2008; Potkin et al., 2009). The association of glypicans with these nervous system disorders indicates that glypicans may be important for proper brain function. The identification of the trans-synaptic glypican-LRRTM4 interaction as a key regulator of excitatory synapse development should provide an avenue for a deeper understanding of these disorders.
EXPERIMENTAL PROCEDURES
Neuronal Cultures
Hippocampal neurons were cultured from P0 Long-Evans rats (Charles River) and plated on poly-D-lysine (Millipore), and laminin (Invitrogen) coated chamber slides (Nalge Nunc International). Neurons were maintained in Neurobasal-A medium (Invitrogen) supplemented with B27, glucose, glutamax, penicillin/streptomycin (Invitrogen) and 25 μM β-mercaptoethanol. Neurons were transfected using calcium phosphate at 7 DIV. For knockdown experiments, neurons were electroporated at time of plating using a Bio-Rad Gene Pulser Xcell. For Fc treatments of neuronal cultures, Fc proteins (10 μg/ml final concentration) were added to the culture media. For 6-day treatments, half the media was replaced after 3 days with fresh feeding media containing the same final concentration Fc protein.
Immunocytochemistry
Neurons were fixed in 4% paraformaldehyde, 4% sucrose in PBS, washed in PBS and blocked in 3% bovine serum albumin (BSA), 0.2% Triton X-100 in PBS. Primary antibodies were: goat anti-GFP (Abcam), mouse anti-PSD-95 (Thermo Scientific/Affinity BioReagents), mouse anti-Prox1 (Millipore), guinea pig anti-VGlut1 (Millipore), mouse anti-gephyrin and guinea pig anti-VGAT (Synaptic Systems, Goettingen, Germany), mouse anti-LRRTM4 (clone N205B/22, UCDavis/NIH Neuromab, CA, USA), sheep anti-LRRTM4 (R&D Systems), rabbit anti-GPC4 (aa 88-101; Immundiagnostik), rabbit anti-GluR1 (Calbiochem), rabbit anti-synapsin (Millipore), chicken anti-MAP2 (Sigma), mouse anti-myc 9E10 (Santa Cruz Biotechnology), mouse anti-HA (Covance), mouse anti-FLAG M2 (Sigma), mouse anti-heparan sulfate delta (3G10 epitope) (USBiological). Fluorophore-conjugated secondary antibodies were from Jackson ImmunoResearch or Invitrogen. Quantification of synapse density was performed blind to condition as described (de Wit et al., 2009).
Cell Surface Binding Assays
HEK293T cells were transfected with expression constructs using Fugene6 (Promega). Twenty-four hours after transfection, the cells were incubated with Fc proteins (10 μg/ml in DMEM supplemented with 20 mM HEPES pH 7.4) for 1 hour at RT. Following two brief washes with DMEM/20 mM HEPES pH 7.4, cells were fixed and immunostained as above.
Mixed-Culture Assays
Mixed-culture assays were performed as described (Biederer and Scheiffele, 2007). Briefly, HEK293T cells were transfected with the appropriate plasmid using Fugene6 (Promega), trypsinized or mechanically dissociated and co-cultured with hippocampal neurons (7 or 14 DIV) for 8, 12 or 24 hours depending on the experiment. For analysis of the effect of heparinase III treatment, hippocampal neurons (7 DIV) were treated with 1 U/ml heparinase III (Sigma) or vehicle (20 mM Tris-HCl pH 7.5, 0.1 mg/ml BSA, 4 mM CaCl2) for 2 hrs at 37°C. Cells were washed twice with hippocampal feeding media and subsequently cocultured with transfected 293T cells for an additional 8 hrs. For competition experiments with heparan sulfate, hippocampal neurons (7 DIV) were cocultured with transfected 293T cells for 12 hrs in the presence of heparan sulfate (0.5 mg/ml; Sigma) or vehicle (PBS). For competition experiments with Fc proteins, Fc control, Nrx1ß(−S4)-Fc or GPC4-Fc proteins (final concentration 50 μg/ml) were added to the mixed-cultures, 45 minutes after plating the 293T cells on DIV7 neurons. After 12 hrs of coculturing, the mixed-culture assays were fixed and stained as above.
In Utero Electroporation
Cortices of 15.5 day-old (E15.5) embryos of timed pregnant CD1 mice (Charles River) were unilaterally electroporated with control or shLRRTM4 FCK0.4GW vector plasmid. Briefly, the dam was anesthetized with isoflurane and the uterus exposed. A solution of DNA and 0.01% Fast Green dye was injected into the embryonic lateral ventricle with a beveled glass micropipette. The embryo’s head was positioned between the paddles of pair of platinum tweezer-type electrodes (BTX) with the cathode lateral to the filled ventricle, and 5 75 ms, 40 V pulses were delivered at 1 Hz by a CUY21 electroporator (BEX). After electroporation, the uterus was replaced, the incision sutured closed, and the dam allowed to give birth normally.
Supplementary Material
HIGHLIGHTS.
Proteomics-based discovery of glypican as a novel receptor for LRRTM4
Glypican binds LRRTM4, but not LRRTM2, in a heparan sulfate-dependent manner
LRRTM4 regulates excitatory synapse development in vitro and in vivo
LRRTM4’s synaptogenic activity requires heparan sulfate on the neuronal surface
ACKNOWLEDGMENTS
We thank the Ghosh lab for discussion, and Laura DeNardo, Emily Sylwestrak and Guido David for critical reading of the manuscript. We thank Katie Tiglio, Christine Wu, Christopher Sanchez, Merve Oney, Joseph Antonios, Tev Stachniak and Stefanie Otto for help with in situ hybridizations, recombinant protein and virus production; and Stéphane Baudouin (Scheiffele lab, Biozentrum, University of Basel) for advice on immunohistochemistry. The LRRTM4 monoclonal antibody N205B/22 was developed with the UCDavis/NIH NeuroMab Facility. Mono- and disaccharide analysis of GPC4-Fc was performed by the UCSD Glycotechnology Core. This work was supported by a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation, an ERC Starting Grant (#311083) and FWO Odysseus Grant (JdW), National Institute on Aging NRSA Fellowship 1F32AG039127 (JNS), and NIH grants P41 GM103533, R01 MH067880 (JRY) and R01 NS064124 and NS067216 (AG).
Footnotes
See the Supplemental Experimental Procedures for more information.
REFERENCES
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Biederer T, Scheiffele P. Mixed-culture assays for analyzing neuronal synapse formation. Nat Protoc. 2007;2:670–676. doi: 10.1038/nprot.2007.92. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calboli FC, Tozzi F, Galwey NW, Antoniades A, Mooser V, Preisig M, Vollenweider P, Waterworth D, Waeber G, Johnson MR, et al. A genome-wide association study of neuroticism in a population-based sample. PLoS One. 2010;5:e11504. doi: 10.1371/journal.pone.0011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Sudhof TC. Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J Biol Chem. 2005;280:22365–22374. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani N, Nahm M, Lee S, Broadie K. A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling. PLoS Genet. 2012;8:e1003031. doi: 10.1371/journal.pgen.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cat B, David G. Developmental roles of the glypicans. Semin Cell Dev Biol. 2001;12:117–125. doi: 10.1006/scdb.2000.0240. [DOI] [PubMed] [Google Scholar]
- De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B, David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625–635. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Verhaagen J. Proteoglycans as modulators of axon guidance cue function. Adv Exp Med Biol. 2007;600:73–89. doi: 10.1007/978-0-387-70956-7_7. [DOI] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Yamaguchi Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J Cell Biol. 1999;144:575–586. doi: 10.1083/jcb.144.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Perriss M, Turner K, Guimond S, Apedaile A, Haubeck HD, Turnbull J, Murphy M. Localisation of specific heparan sulfate proteoglycans during the proliferative phase of brain development. Dev Dyn. 2003;227:170–184. doi: 10.1002/dvdy.10298. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ivins JK, Litwack ED, Kumbasar A, Stipp CS, Lander AD. Cerebroglycan, a developmentally regulated cell-surface heparan sulfate proteoglycan, is expressed on developing axons and growth cones. Dev Biol. 1997;184:320–332. doi: 10.1006/dbio.1997.8532. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009a;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, Sudhof TC. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. Embo J. 2009b;28:3244–3255. doi: 10.1038/emboj.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, Saulnier JL, Gu C, Sabatini BL. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat Neurosci. 2012;15:1667–1674. doi: 10.1038/nn.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T. A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics. 2003;81:411–421. doi: 10.1016/s0888-7543(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Roser C, Nguyen TT, Craig DW, Romanos J, Heine M, Meyer J, et al. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- Lin JC, Ho WH, Gurney A, Rosenthal A. The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat Neurosci. 2003;6:1270–1276. doi: 10.1038/nn1148. [DOI] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack ED, Ivins JK, Kumbasar A, Paine-Saunders S, Stipp CS, Lander AD. Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev Dyn. 1998;211:72–87. doi: 10.1002/(SICI)1097-0177(199801)211:1<72::AID-AJA7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Litwack ED, Stipp CS, Kumbasar A, Lander AD. Neuronal expression of glypican, a cell-surface glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan, in the adult rat nervous system. J Neurosci. 1994;14:3713–3724. doi: 10.1523/JNEUROSCI.14-06-03713.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkin RM, Smith MA, Magill C, Fallon JR, Yao YM, Wallace BG, McMahan UJ. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol. 1987;105:2471–2478. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates JR, 3rd, Ghosh A. FLRT Proteins Are Endogenous Latrophilin Ligands and Regulate Excitatory Synapse Development. Neuron. 2012;73:903–910. doi: 10.1016/j.neuron.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Guffanti G, Lakatos A, Fallon JH, Nguyen DD, Mathalon D, Ford J, Lauriello J, Macciardi F. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr Bull. 2009;35:96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Saunders S, Paine-Saunders S, Lander AD. Expression of the cell surface proteoglycan glypican-5 is developmentally regulated in kidney, limb, and brain. Dev Biol. 1997;190:78–93. doi: 10.1006/dbio.1997.8690. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Muller CS, Bildl W, Baehrens D, Huber B, Kulik A, et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Shanks NF, Savas JN, Maruo T, Cais O, Hirao A, Oe S, Ghosh A, Noda Y, Greger IH, Yates JR, 3rd, Nakagawa T. Differences in AMPA and kainate receptor interactomes facilitate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep. 2012;1:590–598. doi: 10.1016/j.celrep.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30:7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebertz B, Stocker G, Drzeniek Z, Handt S, Just U, Haubeck HD. Expression of glypican-4 in haematopoietic-progenitor and bone-marrow-stromal cells. Biochem J. 1999;344(Pt 3):937–943. [PMC free article] [PubMed] [Google Scholar]
- Stipp CS, Litwack ED, Lander AD. Cerebroglycan: an integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Biol. 1994;124:149–160. doi: 10.1083/jcb.124.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Katayama K, Sohya K, Miyamoto H, Prasad T, Matsumoto Y, Ota M, Yasuda H, Tsumoto T, Aruga J, Craig AM. Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction. Nat Neurosci. 2012;15:389–398. S381–382. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamada H, Yamaguchi Y. K-glypican: a novel GPI-anchored heparan sulfate proteoglycan that is highly expressed in developing brain and kidney. J Cell Biol. 1995;130:1207–1218. doi: 10.1083/jcb.130.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, de Wit J, Ghosh A. Molecular Mechanisms of Synaptic Specificity in Developing Neural Circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, Ripley B, Bushong EA, Ellisman MH, Klein G, Ghosh A. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron. 2011;71:640–655. doi: 10.1016/j.neuron.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.