Summary
Background
Staphylococcus aureus nasal carriage increases infection risk. However, few studies have investigated S. aureus acquisition/loss over >1 year, and fewer still used molecular typing.
Methods
1123 adults attending five Oxfordshire general practices had nasal swabs taken. 571 were re-swabbed after one month then every two months for median two years. All S. aureus isolates were spa-typed. Risk factors were collected from interviews and medical records.
Results
32% carried S. aureus at recruitment (<1% MRSA). Rates of spa-type acquisition were similar in participants S. aureus positive (1.4%/month) and negative (1.8%/month, P = 0.13) at recruitment. Rates were faster in those carrying clonal complex (CC)15 (adjusted (a)P = 0.03) or CC8 (including USA300) (aP = 0.001) at recruitment versus other CCs. 157/274 (57%) participants S. aureus positive at recruitment returning ≥12 swabs carried S. aureus consistently, of whom 135 carried the same spa-type. CC22 (including EMRSA-15) was more prevalent in long-term than intermittent spa-type carriers (aP = 0.03). Antibiotics transiently reduced carriage, but no other modifiable risk factors were found.
Conclusions
Both transient and longer-term carriage exist; however, the approximately constant rates of S. aureus gain and loss suggest that ‘never’ or truly ‘persistent’ carriage are rare. Long-term carriage varies by strain, offering new explanations for the success of certain S. aureus clones.
Keywords: Staphylococcus aureus, Molecular epidemiology, Colonisation, spa-typing, Carriage duration
Introduction
Staphylococcus aureus is an important cause of infections in both primary and secondary care. Carriage prevalences of ∼30% have been found consistently in studies performed over six decades,1 with the anterior nares the primary site of colonisation.1–3 Nasal carriers are at greater risk of infection than non-carriers4–7 and the carried and invasive strains are indistinguishable in ∼80% of cases.5,8 Non-carriers of S. aureus have a higher mortality following S. aureus bacteraemia suggesting recent S. aureus acquisition around the time of infection is associated with poorer subsequent outcome.5
The dynamic nature of S. aureus carriage creates complexity for cross-sectional and longitudinal studies, with people acquiring and losing all genotypes of S. aureus (the species level) and also acquiring and losing different genotypes within S. aureus.9 For example, one study found multiple genotypes were present in 7% of carriage samples.10
Rather than considering S. aureus loss and acquisition as separate events, studies have almost universally combined both these aspects and classified individuals as “persistent”, “intermittent” or “non” carriers. “Persistence” has most commonly been defined on the basis of (i) >80% positivity of 10–12 swabs taken weekly over ∼3 months (not considering strain-type)11 or (ii) two positive cultures one week apart, since this had good performance for predicting persistent carriage defined by definition (i) in one study.12 Human polymorphisms associated with “persistent” carriage using definition (ii) have been identified,13 but bacterial factors have not, to date, been associated with different carriage types. Very long-term carriage and strain switching undoubtedly occur; for example 12/17 “persistent” S. aureus carriers according to definition (i) carried S. aureus on a single swab taken eight years later, but only three carried highly similar S. aureus strains.11 However, few studies appear to have repeatedly sampled individuals over intermediate periods of >1 years,14,15 or systematically investigated carried genotypes over these timescales. The rates of acquisition and median carriage duration of newly acquired strains, and the rates of loss of individual strains present in an initial sample with unknown acquisition date, have also rarely been described outside the specific setting of methicillin-resistant strains in hospitalised patients.16–18 Longer-term follow-up might further support experimental studies which found no distinction between non- and intermittent carriers defined following definition (i) in terms of rates of loss of carriage of a nasal inoculum.19
Here we investigate S. aureus nasal carriage in individuals from primary care, swabbed bi-monthly for up to 36 months. We spa-typed all S. aureus isolates to identify acquisition and loss that would be unrecognised at the species level. Our primary objective was to describe the dynamics of S. aureus carriage (loss, gain) in the general population, and to investigate potential risk factors, in particular the contribution from particular spa-types.
Methods
Study population
Eligible participants were consecutive adults aged ≥16 years attending one of five Oxfordshire general practices (each a group of family doctors) in the Thames Valley Primary Care Research Partnership (all in the catchment area for the Oxford University Hospitals (OUH) NHS Trust). All participants provided written informed consent. 200 participants were recruited from each general practice sequentially over December 2008–December 2009, in age/sex strata approximately representing the UK population. Recruitment was completed in each practice before starting in the next. To increase numbers of younger participants, students registering at one practice were recruited during the University Freshers' week. For the first four general practices, we invited only those participants whose recruitment swab grew S. aureus to continue longitudinal follow-up. All participants from the last practice and all students were invited to continue longitudinal follow-up. Assuming 35% participants were S. aureus positive at recruitment and followed longitudinally, recruiting 1000 adults provided >80% power to detect differences of >15% in risk factors between intermittent and continuous carriers.17,20–22 The study was approved by Oxfordshire Research Ethics Committee B (08/H0605/102).
Nasal swabs were taken by all participants on recruitment under research nurse supervision. A dry cotton swab was placed in the tip of both nostrils and rotated three times. All S. aureus positive participants, all students and all participants from the last practice were posted a nasal swabbing kit one and two months after recruitment, and then every two months thereafter. The swabbing technique was demonstrated on recruitment and explained in a leaflet included with each kit. Swabs were returned by post in charcoal medium (typically <1 week), and stored at 4 °C on receipt before processing (processing took <1 week; up to two weeks in total). As the study objective was to investigate S. aureus dynamics, isolation protocols focussed on identifying all strains, even those present at low frequencies. To increase the sensitivity of culture, swabs were therefore incubated overnight at 37 °C in 5% NaCl enrichment broth (E&O Laboratories, Bonnybridge, UK). A 5 mm loop-full of broth was sub-cultured onto SASelect® chromogenic agar (Bio-Rad, Limerick, Ireland) and incubated at 37 °C overnight. Pink colonies were tested further using DNAse, catalase and Staphaurex tests following standard procedures.23 Samples positive in all three tests were presumed to be S. aureus. A selection of pink colonies from the SASelect agar were resuspended in saline from which one aliquot was stored as glycerol stock at −20 °C and another added to 10 μl 0.85% Saline (E&O Laboratories) and 50 μl TE buffer (Sigma, Dorset, UK), heated at 99.9 °C for 10 min, then centrifuged to separate the supernatant. From this, 50 μl was removed and stored at −20 °C as a crude chromosomal DNA extract.
spa-typing was performed as described,24 with DNA amplification and sequencing using the Microlab Star Liquid Handling Workstation (Hamilton Robotics Ltd, Birmingham, UK). Chromatograms for the spa gene were assembled using Ridom StaphType.24 Samples with mixed chromatograms were re-cultured and six-12 colonies separately typed. spa-types were grouped into spa-Clonal Complexes (CCs) using BURP clustering, and CCs labelled as their MLST equivalent for ease of comparison with other studies.25
Epidemiological and healthcare information was collected from a structured questionnaire at recruitment, general practice and OUH records (see Supplementary Methods). After two years follow-up, general practice and OUH records were re-reviewed to ascertain antimicrobial use and inpatient admissions throughout follow-up.
Outcome definitions
-
(1)
Loss of carriage (primary outcome)
Confirmed loss of carriage was defined as two consecutive negative swabs (or two consecutive swabs without the previous spa-type for analysis of spa-types (spa-level)). The time of confirmed loss was taken as the first of the two negatives. Single isolated negatives were ignored (given potential limited efficacy of self-swabbing). Participants with only their last swab negative were censored at the preceding positive swab. Thus loss analyses included only participants returning ≥2 swabs after the first positive to enable any loss to be confirmed. Loss rates over time were estimated using flexible parametric hazard models.26
-
(2)
Acquisition
S. aureus acquisition was defined as positive growth (or a new spa-type) after confirmed prior absence (two consecutive negatives, or absence of spa-type). Thus if the first swab after recruitment (post-recruitment swab) in individuals S. aureus negative at recruitment (recruitment-negatives) grew S. aureus (or grew a new spa-type in participants S. aureus positive at recruitment (recruitment-positives)), this was not counted as acquisition but was presumed to represent a false-negative result at recruitment. Acquisition analyses therefore also included only participants returning ≥2 swabs after the first positive. Since nasal evolution can produce small changes in repeat numbers, new spa-type acquisition was defined as having >2 differences from first positive swab25 (see Supplementary Table 1 for grouping). Results were similar allowing any spa difference to count as a new spa-type acquisition.
All individuals were to be followed for two years (total 14 swabs) under the original protocol. If an individual did not return three consecutive swabs, no further swabs were sent. Following a protocol amendment, at two years further consent was sought for longer follow-up in those persistently negative or persistently positive (allowing single intermitted negatives) for S. aureus to enable longer-term rates of gain and loss to be estimated in those remaining at risk. Participants were considered formally lost to follow-up if they returned their last swab <22 months from enrolment and >4 months before the data cut-off (23 January 2012).
Statistical analysis
STATA 11.2 was used for all analyses, which included data to 23 January 2012 (minimum two years expected follow-up). Cox regression was used to identify independent predictors of loss and acquisition. Multinomial logistic regression was used to identify predictors of long-term carriage with the same spa-type versus intermittent carriage, and predictors of never observed versus intermittent carriage (see Supplementary Methods). The modal CC was defined as the most frequently observed CC per individual.
Results
S. aureus at recruitment
Of 1123 enrolled individuals, 360 (32%) were S. aureus positive at recruitment. Four hundred and eighty-three (43%) were male and the median age of all 1123 individuals was 55 (inter-quartile range (IQR)) [range] (37–67) [16–94] years. Nine individuals had MRSA (0.8%) at recruitment, all Epidemic-(E)MRSA-15 (N = 3) or EMRSA-16 (N = 6). Male sex, being in current employment, longer time since district nurse appointment, ever having been an inpatient and treatment for a skin condition within the last 30 days were associated with higher S. aureus prevalence in a multivariate model (P < 0.0001, 0.02, 0.04, 0.03, 0.03 respectively) (Supplementary Table 1).
S. aureus carriage during follow-up
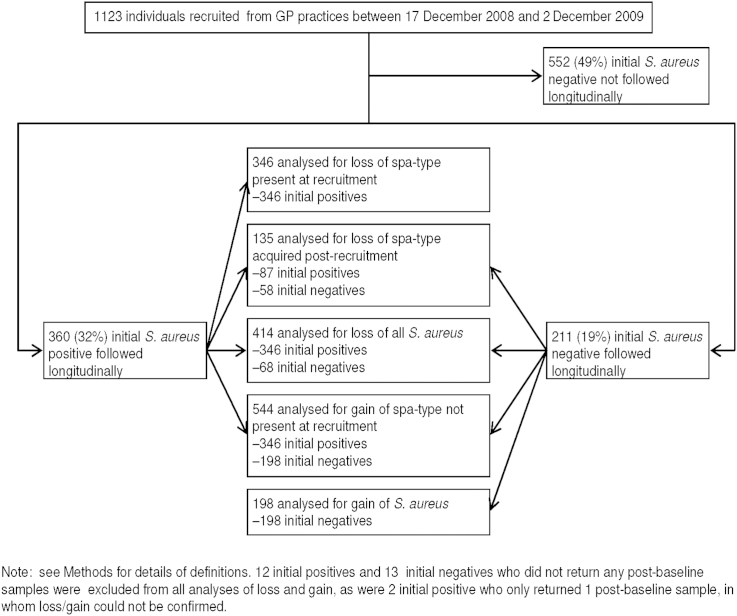
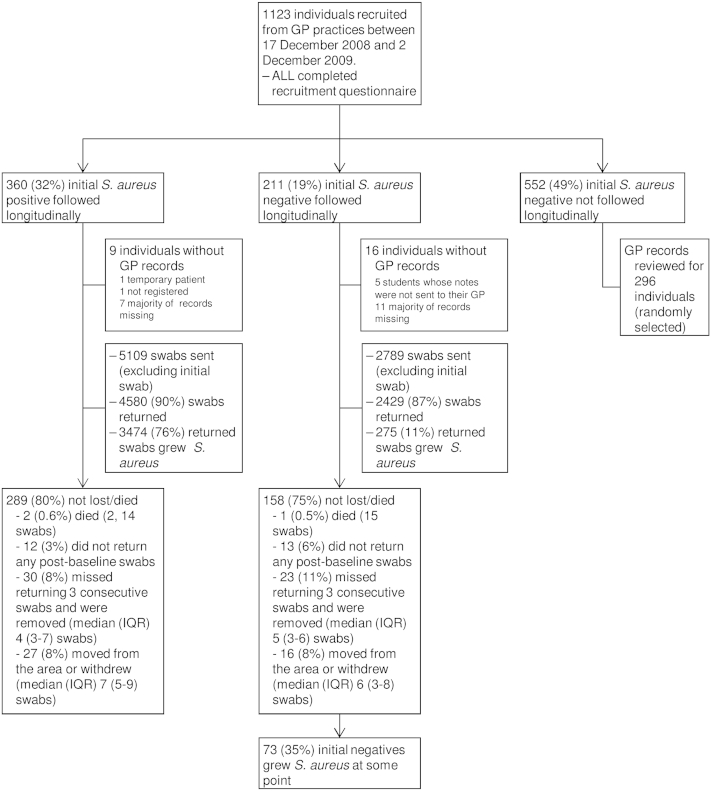
To investigate S. aureus loss and (re-)acquisition, the 360 individuals positive at recruitment (recruitment-positive) plus a further 211 S. aureus negative at recruitment (82 from the last general practice, 129 students, see Methods) were followed for a median (IQR) 2.0 (1.8–2.2) years, returning a median (IQR) 1411–15 swabs (range 1–20). Three (0.5%) individuals died and 121 (21%) were lost to follow-up (25 (4%) did not return any swabs post-baseline, 53 (9%) missed returning three consecutive swabs and were removed from follow-up and 43 (8%) moved from the area or withdrew from the study) (Fig. 1, Supplementary Fig. 1). S. aureus grew from 3749 of 7009 post-recruitment swabs returned (53%) and was subsequently recovered from 73 (35%) individuals S. aureus negative at recruitment (recruitment-negatives), ten (5%) at the first swab after recruitment. All S. aureus were spa-typed; of the 297 spa-types observed, 197 (66%) were only seen in one individual. The 297 spa-types formed 157 groups with ≤2 differences, 82 were singletons and 22 could not be grouped because they were too short (Supplementary Table 2).
Figure 1.
Study flow diagram.
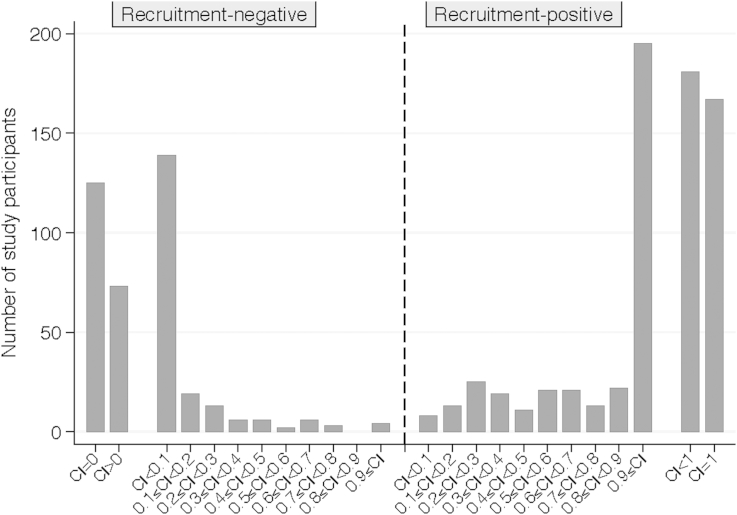
Carrier index
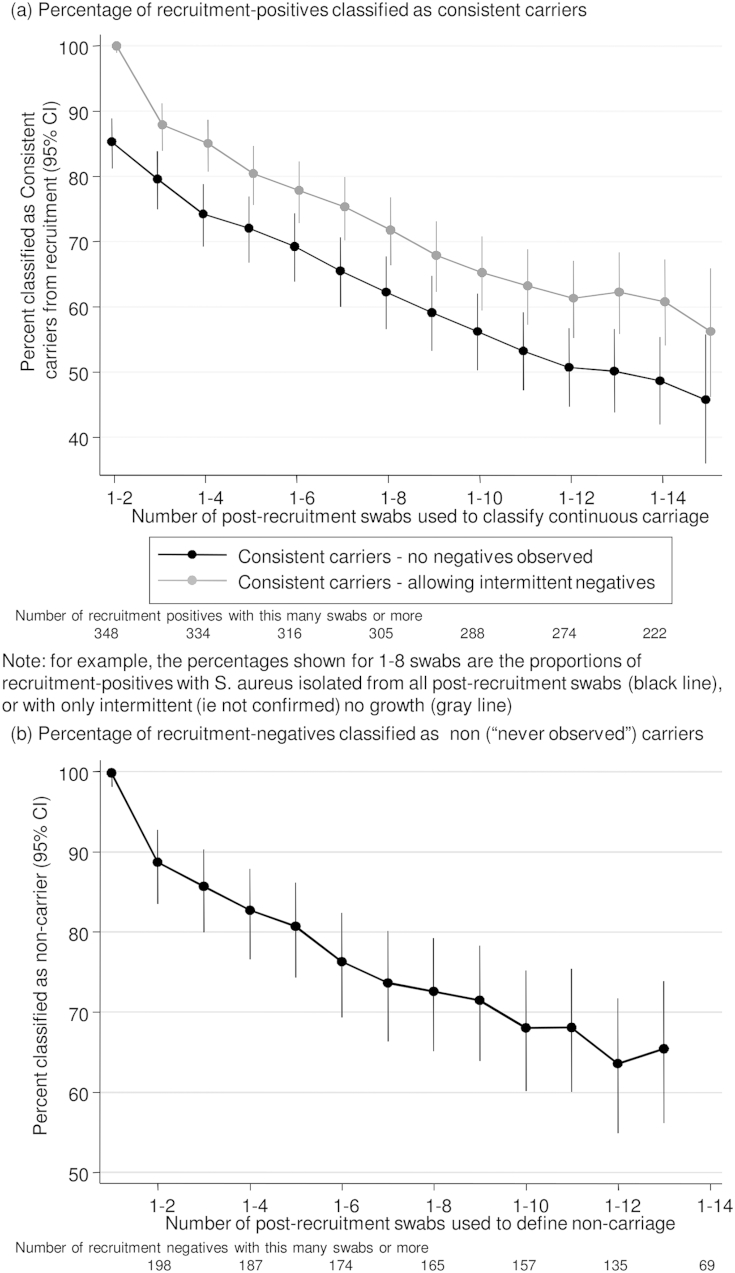
Based on the carrier index (proportion of S. aureus positive swabs/swabs returned), just under half of the recruitment-positives carried S. aureus consistently throughout the study, and just over 60% of recruitment-negatives never carried S. aureus (Fig. 2). However, most of those with intermediate carrier indices had distinct phases of carriage of specific spa-types and phases of non-carriage. In particular, recruitment-positives lost carriage at similar rates throughout the study, leading to approximately equal numbers with carrier indices below one. We therefore estimated the time course over which recruitment-negatives became positive and recruitment-positives gained a new spa-type (“gain”, Fig. 3), and over which recruitment-positives became negative and recruitment-negatives who had become positive then lost carriage (“loss”, Fig. 4).
Figure 2.
Carrier index.
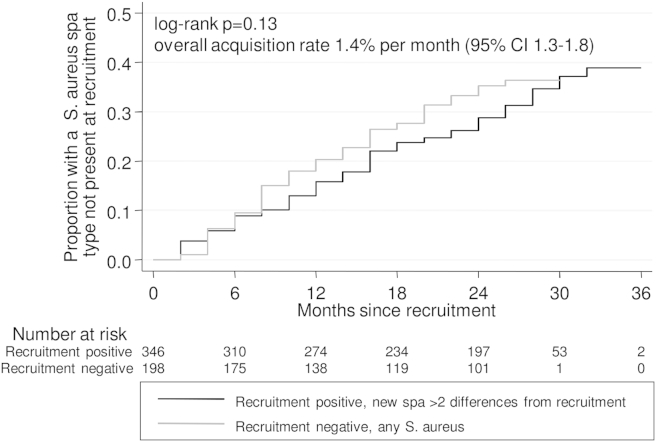
Figure 3.
Months to acquisition of a new S. aureus spa-type in recruitment-positives and negatives. Note: not counting a spa-type first observed at the first post-recruitment swab as an acquisition, see Methods.
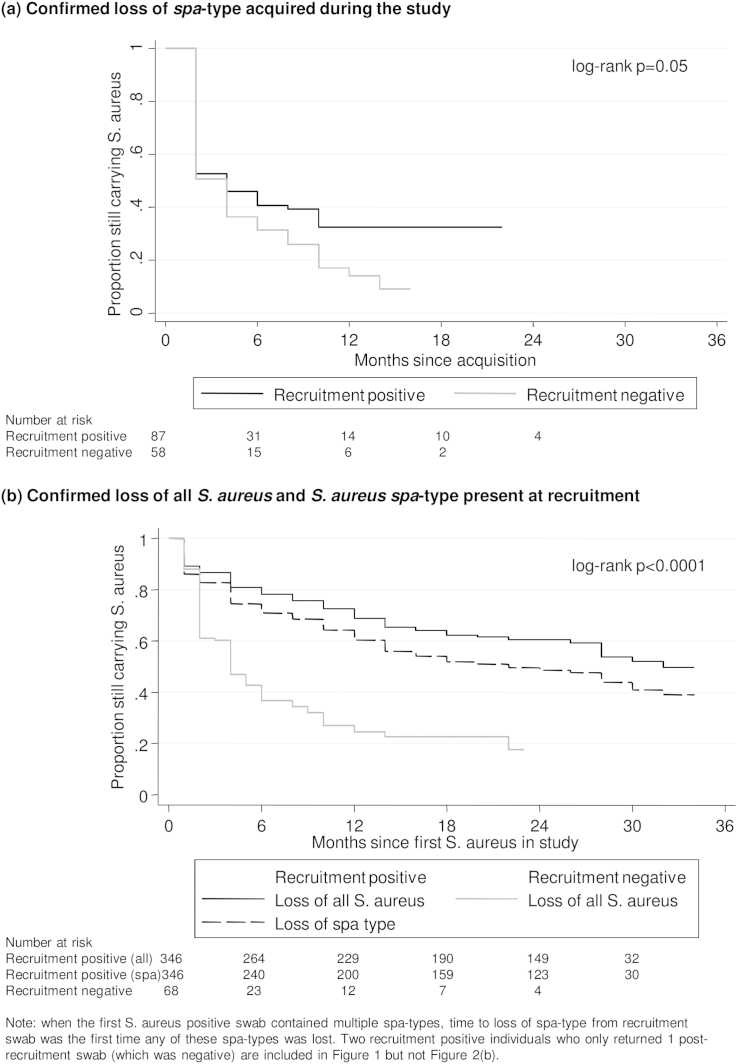
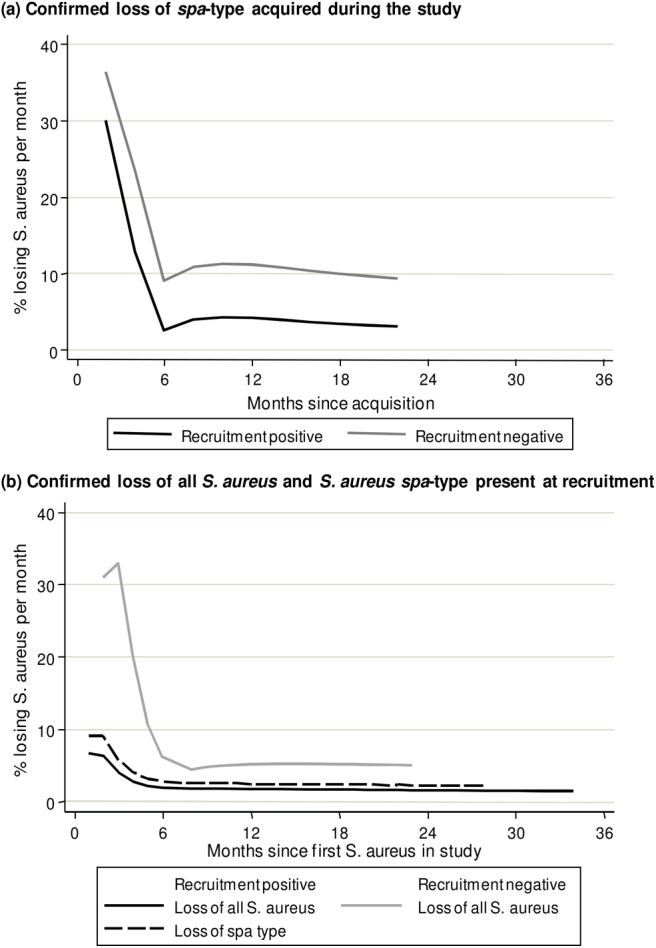
Figure 4.
Months to confirmed loss of S. aureus in recruitment-positives and negatives. (a) Confirmed loss of spa-type acquired during the study. (b) Confirmed loss of all S. aureus, and of S. aureus spa-type present at recruitment in recruitment-positives. Note: when the first S. aureus positive swab contained multiple spa-types, time to loss of spa-type from recruitment swab was the first time any of these spa-types was lost.
S. aureus acquisition
162 (30%) of 544 participants returning ≥2 post-recruitment swabs acquired a new spa-type (with >2 differences) during follow-up, at a rate of 1.5% (95% CI 1.3–1.8%) per month. MRSA (EMRSA-15) was acquired by one individual. Similar percentages of recruitment-positives (29%) and recruitment-negatives (32%) acquired a new spa-type, and acquisition rates were similar (1.4% (95% CI 1.2–1.7%) and 1.8% (1.4–2.3%) per month respectively; log-rank P = 0.13, Fig. 3). There was no suggestion that acquisition rates plateaued over time (Fig. 3). Age was the strongest recruitment factor associated with rate of acquisition, which was faster in younger individuals (adjusted P = 0.01) (Table 1, Supplementary Table 2). Acquisition rates also varied independently with recruitment CC (global adjusted P = 0.04); being significantly faster in those with CC8 (adjusted P = 0.001), the CC including USA300, and with CC15 (adjusted P = 0.03).
Table 1.
Recruitment factors independently associated with acquisition of a new spa-type or confirmed loss of a S. aureus spa-type.
| Factor (determined at recruitment) | (effect in Cox model) | Acquisition of a new spa-type (N = 523) |
P value | Loss of spa-type (N = 401) |
P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Acquire (N = 157) n (row %) or median (IQR) | Do not acquire (N = 366) n (row %) or median (IQR) | Hazard ratio (95% CI) from multivariable model | Number lose (N = 220) n (row %) or median (IQR) | Number do not lose (N = 181) n (row %) or median (IQR) | Hazard ratio (95% CI) from multivariable model | ||||
| S. aureus details (included by design) | |||||||||
| Recruitment-negative | No | 97 (29%) | 242 (71%) | 1.00 | 168 (50%) | 169 (50%) | 1.00 | ||
| Yes | 60 (33%) | 124 (34%) | 1.35 (0.82;2.22) | 0.23 | 52 (81%) | 12 (19%) | 3.23 (2.07;5.02) | <0.0001 | |
| CC present at recruitment | CC30 (spa-CC021) (reference category) | 23 (23%) | 76 (77%) | 1.00 | global 0.04 | 45 (45%) | 54 (55%) | 1.00 | global 0.42 |
| CC15 (spa-CC084) | 16 (38%) | 26 (62%) | 2.01 (1.06;3.83) | 0.03 | 22 (52%) | 20 (48%) | 1.31 (0.78;2.19) | 0.31 | |
| spa-CC002 | 6 (26%) | 17 (74%) | 1.04 (0.42;2.57) | 0.94 | 12 (55%) | 10 (45%) | 1.45 (0.76;2.77) | 0.26 | |
| CC22 (spa-CC005) | 7 (29%) | 17 (71%) | 1.40 (0.60;3.27) | 0.44 | 7 (29%) | 17 (71%) | 0.57 (0.26;1.27) | 0.17 | |
| spa-CC127 | 6 (27%) | 14 (73%) | 1.36 (0.55;3.37) | 0.51 | 13 (65%) | 7 (35%) | 1.46 (0.78;2.72) | 0.24 | |
| spa-CC160 | 3 (21%) | 11 (79%) | 1.07 (0.32;3.59) | 0.91 | 7 (50%) | 7 (50%) | 1.18 (0.53;2.65) | 0.68 | |
| CC8 (spa-CC024) | 7 (64%) | 4 (36%) | 4.50 (1.91;10.6) | 0.001 | 7 (64%) | 4 (36%) | 1.28 (0.57;2.87) | 0.55 | |
| Other CC | 29 (27%) | 77 (73%) | 1.34 (0.78;2.33) | 0.29 | 55 (52%) | 50 (48%) | 1.36 (0.91;2.03) | 0.13 | |
| Participant characteristics (included by design) | |||||||||
| Age (years) | (Per 10 years older) | 40 (19;60) | 54 (35;65) | 0.85 (0.75;0.97)b | 0.01 | 47 (22;62) | 56 (37;67) | 0.90 (0.81;1.00)c | 0.05 |
| Sex | Female | 84 (30%) | 192 (70%) | 1.00 | 175 (68%) | 83 (32%) | 1.00 | ||
| Male | 73 (30%) | 174 (70%) | 1.13 (0.81;1.56) | 0.47 | 98 (50%) | 98 (50%) | 0.94 (0.71;1.25) | 0.68 | |
| Student | No | 115 (27%) | 318 (73%) | 1.00 | 175 (52%) | 163 (48%) | 1.00 | ||
| Yes | 42 (47%) | 48 (53%) | 1.46 (0.86;2.47) | 0.16 | 45 (71%) | 18 (29%) | 0.94 (0.58;1.52) | 0.80 | |
| Participant behaviour | |||||||||
| Number of other household members | Lives alone | 13 (19%) | 54 (81%) | 1.00 | 24 (44%) | 31 (56%) | 1.00 | ||
| 1 household member | 55 (25%) | 162 (75%) | (trend, per category higher) | 74 (47%) | 85 (53%) | (trend, per category higher) | |||
| 2/3 household members | 67 (37%) | 116 (63%) | 1.19 (0.95;1.51) | 0.13 | 91 (62%) | 55 (38%) | 1.22 (1.02;1.47) | 0.03 | |
| 4 or more/shared | 22 (39%) | 34 (61%) | 31 (76%) | 10 (34%) | |||||
| Healthcare exposure | |||||||||
| Days since last outpatient appointmenta | (Per year longer) | 322 (97;921) | 299 (86;1106) | 0.95 (0.90;1.00) | 0.06 | 324 (106;900) | 294 (84;1174) | 0.93 (0.89;0.97) | 0.001 |
| Co-morbidities | |||||||||
| Has a long-term illness | No | 83 (33%) | 170 (67%) | 1.00 | 110 (61%) | 71 (39%) | 1.00 | ||
| Yes | 74 (27%) | 196 (73%) | 0.91 (0.63;1.30) | 0.59 | 110 (50%) | 110 (50%) | 0.77 (0.57;1.04) | 0.09 | |
| Had MSSA previously | No | 144 (29%) | 350 (71%) | 1.00 | 205 (54%) | 173 (46%) | 1.00 | ||
| Yes | 13 (45%) | 16 (55%) | 1.68 (0.95;3.00) | 0.08 | 15 (65%) | 8 (35%) | 1.32 (0.77;2.26) | 0.28 | |
Note: IQR = inter-quartile range, CI = confidence interval. Including only individuals with general practice data at recruitment. See Supplementary Methods for details of model selection; factors with P < 0.10 shown in bold. Number of household contacts included as a trend term as point estimates increased monotonically in both adjusted models. All effects in the same direction as univariable models. See Supplementary Table 2 for univariable effects of these and other factors considered but not included.
6% of individuals with no outpatient appointment recorded set to 10 years, and all those with last outpatient appointment >10 years ago truncated at 10 years. Median (IQR) calculated in those with previous outpatient appointment.
Effect stronger if number of household members not included in model: HR per 10 years older = 0.82 (95% CI 0.73–0.92) P = 0.001. Effects of other factors unchanged.
Effect stronger if number of household members not included in model: HR per 10 years older = 0.86 (95% CI 0.78–0.94) P = 0.002. Effects of other factors unchanged.
In time-updated models including post-recruitment factors, having S. aureus isolated from the previous swab significantly decreased the rate of acquisition of a new spa-type (adjusted for Table 1 factors hazard ratio (a)HR = 0.61 (0.40–0.91), P = 0.02). Based on the analysis of recruitment factors above, we divided carriage of pre-existing S. aureus into CC8, CC15 or another CC, and found significant variation in this effect across these clonal complex groups (P = 0.002). Compared to those without pre-existing S. aureus, acquisition of a new spa-type occurred at similar rates in those with CC15 (aHR = 1.18 (0.60–2.31) and possibly at even higher rates in those with pre-existing CC8 (aHR = 2.03 (0.79–5.20); acquisition of a new spa-type was only reduced in those with other CCs (aHR = 0.50 (0.32–0.76)). Anti-staphylococcal antibiotics (see Supplementary Methods) were taken by 158/571 (28%) participants during the study; their use in the interval between the previous and current swab did not significantly affect S. aureus acquisition (aHR = 0.97 (0.49–1.91), P = 0.93). However, having received antibiotics more than two swabs ago increased the rate of S. aureus acquisition (aHR = 1.66 (1.16–2.38), P = 0.006), suggesting that individuals who lose S. aureus due to antibiotics are likely to re-acquire. There was no evidence that current inpatient admissions significantly affected S. aureus acquisition at the species or spa-level (adjusted P > 0.3) and the effects of previous antibiotics and co-colonisation remained when adjusted for one another, that is, were independent.
S. aureus loss
We first considered loss of S. aureus spa-type in those in whom the date of acquisition was observed, that is those who acquired a new spa-type in the study and subsequently returned ≥2 swabs (n = 145; Fig. 4(a)). 98 (68%) subsequently lost this spa-type (53/87 (61%) recruitment-positives and 45/58 (78%) recruitment-negatives, log-rank P = 0.05). Median (IQR) carriage duration of acquired spa-types was two2–10 months in recruitment-negatives and two (2–>18) months in recruitment-positives. Loss rates varied substantially over time since acquisition (Supplementary Fig. 1(a)), averaging 19%/month (95% CI 15–24%) in the first four months versus 5%/month (3–8%) subsequently (3%/month (2–6%) in recruitment-positives versus 10%/month (5–18%) in recruitment-negatives) with no evidence of further slowing during the study.
We then considered loss of all S. aureus at the species level (Fig. 4(b)). 134 (39%) of 346 recruitment-positives returning ≥2 post-recruitment swabs subsequently lost all S. aureus during the study. Whilst overall loss rates were greater in recruitment-negatives subsequently observed to carry S. aureus (log-rank P < 0.0001), the difference in loss rates was largest early on (Supplementary Fig. 1(b)); after four months, losses occurred at low and more similar rates in both groups (2%/month (95% CI 1–2%) in recruitment-positives versus 6%/month (3–10%) in recruitment-negatives). First confirmed absence of any spa-type present at recruitment occurred at a slightly faster rate than loss of all S. aureus (Fig. 4(b)), indicating lost strains were often merely replaced. Age was independently associated with rate of spa-type loss, which was faster in younger individuals (adjusted P = 0.05; Table 1). More recent outpatient exposure, having more household members and being negative for S. aureus on recruitment were independent predictors of loss (adjusted P = 0.001, P = 0.03 and P < 0.0001 respectively). There was no evidence of an impact of recruitment CC on spa-type loss (adjusted global P = 0.42).
In time-updated models including post-recruitment factors, having multiple spa-types (differing by >2 repeats) in the previous swab had no significant effect on loss at the species level (adjusted for Table 1 factors aHR = 0.64 (95% CI 0.23–1.74), P = 0.38), but significantly increased loss of the original pre-existing spa-type (aHR = 3.40 (2.15–5.37), P < 0.001). Thus observations of multiple spa-types were commonly followed by replacement of the original with the new spa-type. Recent use of anti-staphylococcal antibiotics independently increased the rate of S. aureus loss at the species level (aHR = 2.51 (95% CI 1.54–4.10), P < 0.0001) (similar results for spa-type loss). There was no evidence that current inpatient admissions significantly affected S. aureus loss at the species or spa-level (adjusted P > 0.3).
Exploration of long-term carriage patterns
-
(i)
Long-term consistent carriage at the S. aureus species level
To explore whether a consistent (long-term) carriage phenotype existed in our study, we combined the carrier index (Fig. 2) and time-to-loss (Fig. 4(b)) approaches to estimate the proportion of recruitment-positives observed to have carried S. aureus consistently in their first two, three, four, five etc swabs (Fig. 5(a)). The proportion of long-term consistent carriers declined linearly at least through to the first 12 swabs (∼24 months). After 12 swabs, confidence intervals were wide, and results were compatible with the ongoing low rates of loss seen in Supplementary Fig. 1. For example, of 140 individuals who were classified as consistent long-term carriers based on their first 12 swabs and who returned ≥14 swabs, 11 (8%) subsequently lost carriage on two consecutive samples. Allowing single intermittent negative swabs increased estimates of consistent long-term carriers by ∼10%, but the relationship with number of swabs was similar (Fig. 5(a)). Of the 274 recruitment-positive participants returning ≥12 swabs, 157 (57%) never had two consecutive negative swabs, i.e. could be considered to have carried consistently long-term throughout the study. 4/61 (7%) recruitment-negatives returning ≥12 swabs with ≥1 positive could also be considered to have carried consistently long-term throughout the study (i.e. plausibly had an initial false-negative recruitment swab).
-
(ii)
Long-term carriage at the S. aureus spa-type level
Figure 5.
Impact of duration of follow-up on classification of carriage phenotypes. (a) Percentage of recruitment-positives classified as consistent carriers. Note: for example, the percentages shown for 1-8 swabs are the proportions of recruitment-positives with S. aureus isolated from all post-recruitment swabs (black line) or with only intermittent (i.e. not confirmed) no growth (grey line). (b) Percentage of recruitment-negatives classified as non (“never observed”) carriers.
Of the 161 individuals without two consecutive negative swabs (i.e. defined long-term consistent carriers at the species level), 92 (57%) carried a single spa-type throughout without any other spa-type being observed, 45 (28%) carried a single spa-type throughout as well as gaining/losing other types; and 24 (15%) did not carry one spa-type consistently. Therefore 137/335 (41%) participants ever observed to carry S. aureus were consistent long-term carriers of the same spa-type, 135/274 (49%) recruitment-positives and 2/61 (3%) recruitment-negatives. Gaining/losing other spa-types was more common in persistent carriers of CC8 (3/3,100%) and CC15 (9/14,64%) than persistent carriers of other spa-types (33/120,28%) (P = 0.001), although numbers were small so results may not be robust.
-
(iii)
“Non-carriage”
Taking a similar approach to explore a “never carriage” phenotype, the percentage of recruitment-negatives classified as non-carriers continued to decline linearly with increasing numbers of swabs. 90/151 (60%) recruitment-negatives returning ≥12 swabs never grew S. aureus during the study.
Characteristics of different carriage phenotypes in those returning ≥12 swabs
The characteristics of those carrying one spa-type consistently long-term (allowing gain/loss of other spa-types), intermittent carriers of one or multiple spa-types and non-carriers are shown in Table 2 and Supplementary Table 4. Intermittent carriers had median (IQR) carrier index 0.33 (0.16–0.57) for their most commonly observed spa-type. Consistent carriers of one spa-type long-term appeared to differ in the CC of the spa-type they carried consistently, being more likely to carry CC22 (which includes EMRSA-15) (adjusted P = 0.03) and somewhat less likely to carry CC15 (P = 0.08) than intermittent carriers. Consistent carriers of one spa-type long-term were also less likely to have received anti-staphylococcal antibiotics, had fewer other household members and longer times since their last outpatient appointment (P = 0.04, 0.02 and 0.01 respectively).
Table 2.
Impact of participant characteristics on long-term consistent carriage of a S. aureus spa-type versus intermittent carriage, and never observed carriage versus intermittent carriage, in participants returning ≥12 swabs (∼24 months).
| Factor | (effect in multinomial regression model) | S. aureus long-term consistent spa-type carriers (N = 136): n (row %) or median (IQR) | S. aureus intermittent carriers (N = 192): n (row %) or median (IQR) | Long-term consistent spa-type versus intermittent carriage |
Never observed carriers (n = 88):n (row%) or median (IQR) | Never observed versus intermittent carriage |
||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |||||
| Modal CC | CC30 (spa-CC021) (reference category) | 43 (41%) | 62 (59%) | 1.00 | global 0.13 | N/A | N/A | N/A |
| CC15 (spa-CC084) | 14 (29%) | 34 (71%) | 0.49 (0.22;1.08) | 0.08 | ||||
| spa-CC002 | 9 (33%) | 18 (67%) | 0.72 (0.28;1.88) | 0.50 | ||||
| CC22 (spa-CC005) | 16 (73%) | 6 (27%) | 3.17 (1.09;9.24) | 0.03 | ||||
| spa-CC127 | 6 (33%) | 12 (67%) | 0.58 (0.19;1.75) | 0.33 | ||||
| spa-CC160 | 5 (45%) | 6 (54%) | 0.96 (0.24;3.80) | 0.95 | ||||
| CC8 (spa-CC024) | 3 (30%) | 7 (70%) | 0.73 (0.16;3.35) | 0.69 | ||||
| Other CC (<10 people) | 40 (46%) | 47 (54%) | 1.06 (0.56;1.99) | 0.85 | ||||
| Participant characteristics at recruitment | ||||||||
| Age (years) | (Per 10 years) | 60 (47;68) | 50 (26;63) | 1.05 (0.87;1.27)b | 0.58 | 59 (41;65) | 1.27 (1.01;1.59)c | 0.04 |
| Sex | Female | 58 (27%) | 109 (50%) | 1.00 | 51 (23%) | 1.00 | ||
| Male | 78 (39%) | 83 (42%) | 1.58 (0.96;2.58) | 0.07 | 37 (19%) | 0.83 (0.48;1.43) | 0.50 | |
| Student | No | 127 (36%) | 155 (43%) | 1.00 | 75 (21%) | 1.00 | ||
| Yes | 9 (15%) | 37 (63%) | 0.41 (0.15;1.17) | 0.10 | 13 (22%) | 1.69 (0.60;4.78) | 0.32 | |
| Participant behaviour at recruitment | ||||||||
| Number of other household members | Lives alone | 25 (45%) | 22 (40%) | 1.00 | 8 (15%) | 1.00 | ||
| 1 household member | 71 (36%) | 72 (37%) | (trend, per category higher) | 53 (27%) | (trend, per category higher) | |||
| 2/3 household members | 36 (27%) | 77 (57%) | 0.64 (0.45;0.92) | 0.02 | 21 (16%) | 0.81 (0.54;1.22) | 0.32 | |
| 4 or more/shared | 4 (13%) | 21 (67%) | 6 (19%) | |||||
| Participant behaviour at recruitment | ||||||||
| Days since last outpatient appointmenta | (Per year longer) | 289 (83;1087) | 324 (105;909) | 1.11 (1.03;1.21) | 0.01 | 241 (48;1123) | 1.00 (0.91;1.10) | 0.98 |
| Co-morbidities at recruitment | ||||||||
| Has a long-term illness | No | 46 (25%) | 93 (50%) | 1.00 | 48 (27%) | 1.00 | ||
| Yes | 90 (39%) | 99 (43%) | 1.74 (0.98;3.10) | 0.06 | 40 (17%) | 0.58 (0.31;1.07) | 0.08 | |
| Had MSSA previously | No | 130 (33%) | 179 (46%) | 1.00 | 84 (21%) | 1.00 | ||
| Yes | 6 (26%) | 13 (57%) | 0.62 (0.22;1.77) | 0.37 | 4 (17%) | 0.73 (0.23;2.36) | 0.60 | |
| Antibiotic exposure | ||||||||
| Received anti-staphylococcal antibiotics during follow-up | No | 90 (35%) | 111 (44%) | 1.00 | 54 (21%) | 1.00 | ||
| Yes | 46 (29%) | 81 (50%) | 0.58 (0.35;0.97) | 0.04 | 34 (21%) | 0.87 (0.50;1.50) | 0.61 | |
Note: IQR = inter-quartile range; RR = risk ratio; CI = confidence interval. 9 individuals with missing general practice records excluded from analysis (1 persistent, 6 intermittent, 2 non-carriers). See Supplementary Methods for details of model selection; factors with P < 0.10 shown in bold. Number of household contacts included as a trend term. All effects in the same direction as univariable models. See Supplementary Table 3 for univariable effects of these and other factors considered but not included.
6% of individuals with no outpatient appointment recorded set to 10 years, and all those with last outpatient appointment >10 years ago truncated at 10 years. Median (IQR) calculated in those with previous outpatient appointment.
Effect stronger if number of household members not included in model: HR per 10 years older = 1.16 (95% CI 0.98–1.38) P = 0.09. Effects of other factors unchanged.
Effect stronger if number of household members not included in model: HR per 10 years older = 1.32 (95% CI 1.08–1.63) P = 0.007. Effects of other factors unchanged.
Discussion
In this large primary care-based study, we found 32% participants positive for S. aureus on a recruitment nasal swab, remarkably similar to S. aureus prevalence in other population studies, suggesting our results are likely generalisable.1,2,11 However, unlike the majority of other studies, our median follow-up of two years with bi-monthly swabs allowed detailed investigation of long-term carriage, and spa-typing every isolate enabled discrimination at the strain rather than the species level.
Our findings are compatible with a carriage spectrum in which the extremes are characterised by two phenotypes present at different proportions in recruitment-positives and negatives. The first is highly transient carriage, exemplified by most acquisitions in recruitment-negatives, who carried for a median of only two months. Interestingly the rate of acquisition of new spa-types was similar and approximately constant in recruitment-negatives and positives, and recruitment-positives lost newly acquired strains at only slightly slower rates than recruitment-negatives, suggesting that both are susceptible to highly transient carriage. The second carriage phenotype is long-term S. aureus carriage, exemplified by the much slower loss of recruitment spa-types in recruitment-positives, and low loss rates >4–6 months after acquisition in both recruitment-positives and negatives.
Our data could not fully support or refute the presence of a third “truly persistent” carriage phenotype as the proportion classified as consistent long-term carriers continued to decline as length of follow-up increased throughout the study. Further follow-up will be necessary to assess this definitively. Using our method of analysis, truly persistent carriage would be indicated by loss rates reducing to zero some time after 24–30 months (Supplementary Fig. 1(b)) with no further change in the proportion still carrying S. aureus (Figs. 4(b) and 5(a)). Other studies have defined “persistence” using more frequent sampling over shorter timescales,11,12 sometimes using quantitative culture27; when this study was set-up resource-constraints required a compromise between less intensive long-term versus more intensive short-term follow-up. One important study limitation is clearly the lack of a sampling point earlier than one month, e.g. at one week, which would have enabled us to investigate “persistent” carriers defined using van Nouwen's rule.12 The fact that these “persistent” carriers have been shown to differ significantly in characteristics such as clearance of a S. aureus inoculum,19 and host genetics,13 indicates that at least a subgroup form a distinct sub-population. However, we did have a sampling point at one month, and Fig. 5(a) demonstrates a clear ongoing linear decline in consistent long-term carriage even in those with two initial positive cultures, suggesting that a proportion with “persistent” carriage will not carry S. aureus long-term. In fact five of 17 “persistent” carriers (29%, 95% CI 10–56%) were not carrying S. aureus eight years later in the original study of Van den Bergh.11 Since van Nouwen et al. found that two qualitative and two qualitative + quantitative cultures had almost identical performance for classifying “persistence” in a validation set,12 it is unclear that doing quantitative cultures in our study would have materially altered this finding; we prioritised spa-typing all isolates over such quantitative culture.
Our findings suggest that “persistence” as previously defined12,27 could overestimate long-term carriage at the species level, and thus that there is no quick and reliable method to identify consistent long-term S. aureus carriers. Furthermore, 15% of long-term carriers at the species level in our study did not carry the same spa-type consistently (similarly to Ref.28). Whilst colonised with S. aureus long-term, the arrival of a new strain may still have the potential to alter outcomes, similarly to acquiring S. aureus de novo,5 highlighting the importance of using strain typing to identify truly persistent carriage. Assuming those followed long enough to identify this group were representative, “spa-consistent” long-term carriers would comprise 17% of those enrolled. Interestingly, two-thirds of these “spa-consistent” long-term carriers never had any other strain identified despite the long follow-up and the fact that multi-strain colonisation was actively investigated.
We found that the rate of new acquisitions increased linearly through the study (Fig. 3) and the proportion never observed to carry correspondingly decreased linearly (Fig. 5(b)). Our data are thus compatible with van Belkum's suggestion, based on experimental inoculation studies,19 that there are no true S. aureus non-carriers, i.e. that a fourth “never carriage” group does not exist. Whilst 90 participants returning ≥12 swabs never had S. aureus isolated from any study sample, the highly transient carriage that was observed suggests it could have been found at intermediate timepoints. Extrapolating from Fig. 3, 5–10 years follow-up would be needed to distinguish a never carriage phenotype (where the cumulative new acquisition probability would plateau) from continued acquisitions (where the cumulative new acquisition probability would reach 100%). The former scenario would imply that host, rather than bacterial, genetics determines this phenotype.
Despite this being the largest longitudinal study of S. aureus carriage to date, we failed to find strong predictors of gain, loss or persistence, possibly reflecting multifactorial causes and limited power to detect modest absolute differences of around 10%, given that the study was powered to detect 15% differences. Overall effects on loss, gain, and persistence were broadly compatible, although these reflect subtly different aspects of the underlying dynamics. Host effects likely reflected potential for S. aureus exposure (household members, students), underlying host-immunity (age, previous MSSA), and complex effects of health status (long-term illness, recent outpatient appointments). Interestingly receiving anti-staphylococcal antibiotics significantly increased the likelihood of losing S. aureus in the next swab, but also increased the likelihood of later acquisition. This is consistent with antibiotics only temporarily removing S. aureus from the nares, followed by re-acquisition from other body sites/close contacts, as in one study of artificial decolonisation and re-colonisation.19 These findings question the validity of S. aureus eradication as a concept, and suggest that reducing S. aureus load around high-risk procedures (e.g. through decontamination/prophylaxis pre-surgery) is a more biologically plausible approach to reducing S. aureus infection risk.
Unexpectedly, we found large effects of spa-type on acquisition and long-term consistent carriage. Compared to other CCs, more new spa-types were gained if CC8 or CC15 were present either at recruitment or in the previous swab, and CC15 in particular was less likely to be carried consistently long-term than intermittently, similar to findings based on using only two swabs to define “persistence”.29 Whilst pre-existing colonisation did not affect S. aureus loss at the species level, co-carriage was significantly associated with loss of the original strain. This demonstrates the highly dynamic nature of carried populations, and that nasal competition from particular strains is an important factor in co-carriage in vivo. Interestingly, CC8 includes USA300, so tolerance of co-carriage might contribute to the success of this lineage if it is also more readily acquired (not evaluable here due to low numbers of acquisitions of specific clones). Only CC22 was found in significantly more long-term carriers; this CC includes EMRSA-15, again potentially explaining this clone's success. This finding was implied by analyses of loss (Table 1). However, clearly the fact that the most widely dispersed CC8 and CC22 strains contain mecA is a confounding factor.
Our study had four main limitations. Firstly, participants were swabbed only in the nares, which likely missed some carriage even at the timepoints assayed30,31; a recent study suggests that this may have particularly underestimated carriage in younger people.32 However, swabbing of the nares enabled participant self-swabbing, which was vital for our study and was recently shown to have reasonable accuracy.33 Secondly, the bi-monthly sampling interval may have missed some transient carriage. However, it seems probable that those consistently observed to carry the same spa-type for >20 months (n = 137) would have also carried this spa-type at intermediate timepoints. Thirdly, smoking status was not available, but has been associated with reduced carriage in cross-sectional studies.34–36 Finally, since we combined unrelated CCs with less than ten isolates into a single group for analysis, and since numbers of isolates even in some larger CCs were still relatively modest, our findings on CCs should be confirmed in future studies. Our choice to perform overnight culture of swabs in enrichment medium only and not use direct plating may be seen both as a limitation and a strength. A limitation is that quantitation, which can predict persistent carriage,12 becomes impossible. A strength is that enrichment increases the sensitivity of the test and since resources prevented us from identifying isolates using both methods, we opted to maximise detection.
In conclusion, two years follow-up is insufficient to identify whether truly persistent long-term or “never” carriage phenotypes exist; this will require five-ten years follow-up (continuing in this study). Long-term S. aureus carriage at the S. aureus species level is not the same as long-term carriage at the spa-type level (observed in 57% and 49% of recruitment-positives respectively). Thus to identify long-term carriage reliably requires swabs over at least two years and spa-typing, including systematic methods for identifying co-colonisation, limiting the potential for accurate identification of long-term consistent carriage phenotypes for future genome-wide association studies. However, we have conclusively demonstrated bacterial lineage-specific effect on carriage dynamics. The transient carriage of spa-types with/without underlying persistent carriage, the lack of modifiable risk factors and the strong influence of antibiotics and strain-type on carriage acquisition, loss and persistence, highlights the dynamic nature of S. aureus as a human commensal. This emphasises the importance of focussing prevention efforts on reducing universal infection risk rather than eradication of carriage in individuals.37,38
Funding
This work was supported by both the National Institute for Health Research (NIHR) under its Oxford Biomedical Research Centre Infection Theme, and the UKCRC Modernising Medical Microbiology Consortium, the latter being funded under the UKCRC Translational Infection Research Initiative supported by Medical Research Council, Biotechnology and Biological Sciences Research Council and the National Institute for Health Research on behalf of the Department of Health (Grant G0800778) and The Wellcome Trust (Grant 087646/Z/08/Z). DWC and TEAP are NIHR Senior Investigators. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health.
The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Contributions
The study was conceived and designed by RM, DWC, TEAP, ASW, KK, RB and DM, with analysis performed by RM and ASW. HG, RF, RM and AV contributed to data acquisition. RM, ASW, KK, DM, TEAP and DCW contributed to data interpretation. RM wrote the first draft which all authors commented on, and all authors approved the final version. RM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and the decision to submit for publication.
Conflict of interest
No author has a conflict of interest.
Acknowledgements
We thank all the people of Oxfordshire who took part in the study and Martin Llewelyn for his comments on an earlier draft of the manuscript.
Footnotes
Presentation at previous meetings – Presented as poster at: (1) ICAAC, Boston, 2010. (2) IDweek, San Diego, 2012.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1.
Flow diagram of participants followed up. Note: when the first S. aureus positive swab contained multiple spa-types, time to loss of spa-type from recruitment swab was the first time any of these spa-types was lost.
Supplementary Figure 2.
Rate of confirmed loss of S. aureus in recruitment-positives and negatives. (a) Confirmed loss of spa-type acquired during the study. (b) Confirmed loss of all S. aureus and S. aureus spa-type present at recruitment.
References
- 1.Kluytmans J., van Belkum A., Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997 Jul;10(3):505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams R.E. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963 Mar;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wertheim H.F., Melles D.C., Vos M.C., van Leeuwen W., van Belkum A., Verbrugh H.A. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005 Dec;5(12):751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 4.Calia F.M., Wolinsky E., Mortimer E.A., Jr., Abrams J.S., Rammelkamp C.H., Jr. Importance of the carrier state as a source of Staphylococcus aureus in wound sepsis. J Hyg (Lond) 1969 Mar;67(1):49–57. doi: 10.1017/s0022172400041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wertheim H.F., Vos M.C., Ott A., van Belkum A., Voss A., Kluytmans J.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004 Aug 21–27;364(9435):703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 6.Jensen A.G., Wachmann C.H., Poulsen K.B., Espersen F., Scheibel J., Skinhoj P. Risk factors for hospital-acquired Staphylococcus aureus bacteremia. Arch Intern Med. 1999 Jul 12;159(13):1437–1444. doi: 10.1001/archinte.159.13.1437. [DOI] [PubMed] [Google Scholar]
- 7.Kalmeijer M.D., van Nieuwland-Bollen E., Bogaers-Hofman D., de Baere G.A. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000 May;21(5):319–323. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 8.von Eiff C., Becker K., Machka K., Stammer H., Peters G., Grp S. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001 Jan 4;344(1):11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 9.Bloemendaal A.L., Fluit A.C., Jansen W.T., Vriens M.R., Ferry T., Amorim J.M. Colonization with multiple Staphylococcus aureus strains among patients in European intensive care units. Infect Control Hosp Epidemiol. 2009 Sep;30(9):918–920. doi: 10.1086/605640. [DOI] [PubMed] [Google Scholar]
- 10.Cespedes C., Said-Salim B., Miller M., Lo S.H., Kreiswirth B.N., Gordon R.J. The clonality of Staphylococcus aureus nasal carriage. J Infect Dis. 2005 Feb 1;191(3):444–452. doi: 10.1086/427240. [DOI] [PubMed] [Google Scholar]
- 11.Van den Bergh M.F., Yzerman E.P., van Belkum A., Boelens H.A., Sijmons M., Verbrugh H.A. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J Clin Microbiol. 1999 Oct;37(10):3133–3140. doi: 10.1128/jcm.37.10.3133-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouwen J.L., Ott A., Kluytmans-Vandenbergh M.F., Boelens H.A., Hofman A., van Belkum A. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule”. Clin Infect Dis. 2004 Sep 15;39(6):806–811. doi: 10.1086/423376. [DOI] [PubMed] [Google Scholar]
- 13.van den Akker E.L., Nouwen J.L., Melles D.C., van Rossum E.F., Koper J.W., Uitterlinden A.G. Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J Infect Dis. 2006 Sep 15;194(6):814–818. doi: 10.1086/506367. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen N.H., Espersen F., Rosdahl V.T., Jensen K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol Infect. 1995 Aug;115(1):51–60. doi: 10.1017/s0950268800058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell J.G., Ford C.R., Peterson D.E., Mitchell C.R. Long-term study of nasal staphylococci among hospital personnel. Am J Surg. 1969 Dec;118(6):849–854. doi: 10.1016/0002-9610(69)90245-1. [DOI] [PubMed] [Google Scholar]
- 16.Sanford M.D., Widmer A.F., Bale M.J., Jones R.N., Wenzel R.P. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1994 Dec;19(6):1123–1128. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]
- 17.Scanvic A., Denic L., Gaillon S., Giry P., Andremont A., Lucet J.C. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001 May 15;32(10):1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 18.Vriens M.R., Blok H.E., Gigengack-Baars A.C., Mascini E.M., van der Werken C., Verhoef J. Methicillin-resistant Staphylococcus aureus carriage among patients after hospital discharge. Infect Control Hosp Epidemiol. 2005 Jul;26(7):629–633. doi: 10.1086/502592. [DOI] [PubMed] [Google Scholar]
- 19.van Belkum A., Verkaik N.J., de Vogel C.P., Boelens H.A., Verveer J., Nouwen J.L. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009 Jun 15;199(12):1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 20.Marschall J., Muhlemann K. Duration of methicillin-resistant Staphylococcus aureus carriage, according to risk factors for acquisition. Infect Control Hosp Epidemiol. 2006 Nov;27(11):1206–1212. doi: 10.1086/507917. [DOI] [PubMed] [Google Scholar]
- 21.Lucet J.C., Paoletti X., Demontpion C., Degrave M., Vanjak D., Vincent C. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009 Aug 10;169(15):1372–1378. doi: 10.1001/archinternmed.2009.217. [DOI] [PubMed] [Google Scholar]
- 22.Harbarth S., Liassine N., Dharan S., Herrault P., Auckenthaler R., Pittet D. Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2000 Dec;31(6):1380–1385. doi: 10.1086/317484. [DOI] [PubMed] [Google Scholar]
- 23.Health Protection Agency . 2007. Identification of Staphylococcus species, micrococcus species and rothia species. National Standard Methods. [SOP] 17.09.07(2.1) [Google Scholar]
- 24.Harmsen D., Claus H., Witte W., Rothganger J., Claus H., Turnwald D. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003 Dec;41(12):5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellmann A., Weniger T., Berssenbrugge C., Rothganger J., Sammeth M., Stoye J. Based Upon Repeat Pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 2007;7:98. doi: 10.1186/1471-2180-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royston P., Parmar M.K.B. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002 Aug 15;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 27.Verhoeven P.O., Grattard F., Carricajo A., Lucht F., Cazorla C., Garraud O. An algorithm based on one or two nasal samples is accurate to identify persistent nasal carriers of Staphylococcus aureus. Clin Microbiol Infect. 2011 Jun 23;18(6):551–557. doi: 10.1111/j.1469-0691.2011.03611.x. [DOI] [PubMed] [Google Scholar]
- 28.Sakwinska O., Blanc D.S., Lazor-Blanchet C., Moreillon M., Giddey M., Moreillon P. Ecological temporal stability of Staphylococcus aureus nasal carriage. J Clin Microbiol. 2010 Aug;48(8):2724–2728. doi: 10.1128/JCM.02091-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangvik M., Olsen R.S., Olsen K., Simonsen G.S., Furberg A.S., Sollid J.U. Age- and gender-associated Staphylococcus aureus spa types found among nasal carriers in a general population: the Tromso Staph and Skin Study. J Clin Microbiol. 2011 Dec;49(12):4213–4218. doi: 10.1128/JCM.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurjadi D., Lependu J., Kremsner P.G., Zanger P. Staphylococcus aureus throat carriage is associated with ABO-/secretor status. J Infect. 2012 Jun 1;65(4):310–317. doi: 10.1016/j.jinf.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Miller L.G., Eells S.J., Taylor A.R., David M.Z., Ortiz N., Zychowski D. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis. 2012 Jun;54(11):1523–1535. doi: 10.1093/cid/cis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith T.C., Forshey B.M., Hanson B.M., Wardyn S.E., Moritz E.D. Molecular and epidemiologic predictors of Staphylococcus aureus colonization site in a population with limited nosocomial exposure. Am J Infect Control. 2012 Dec;40(10):992–996. doi: 10.1016/j.ajic.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 33.van Cleef B.A., van Rijen M., Ferket M., Kluytmans J.A. Self-sampling is appropriate for detection of Staphylococcus aureus: a validation study. Antimicrob Resist Infect Control. 2012;1(1):34. doi: 10.1186/2047-2994-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu F., Cui E., Guo T., Li H., Chen S., Liu L. Nasal colonization of and clonal transmission of methicillin-susceptible Staphylococcus aureus among Chinese military volunteers. J Clin Microbiol. 2010 Jan;48(1):64–69. doi: 10.1128/JCM.01572-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herwaldt L.A., Cullen J.J., French P., Hu J., Pfaller M.A., Wenzel R.P. Preoperative risk factors for nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 2004 Jun;25(6):481–484. doi: 10.1086/502426. [DOI] [PubMed] [Google Scholar]
- 36.Olsen K., Falch B.M., Danielsen K., Johannessen M., Ericson Sollid J.U., Thune I. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromso Staph and Skin Study. Eur J Clin Microbiol Infect Dis. 2011 Aug 3;31(4):465–473. doi: 10.1007/s10096-011-1331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S.S., Septimus E., Kleinman K., Moody J., Hickok J., Avery T., editors. Randomized evaluation of decolonization vs. universal clearance to eliminate methicillin-resistant Staphylococcus aureus in ICUs (REDUCE-MRSA trial). IDweek. 2012. San Diego, CA, 17–21 October [Abstract 1234] [Google Scholar]
- 38.Bleasdale S.C., Trick W.E., Gonzalez I.M., Lyles R.D., Hayden M.K., Weinstein R.A. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007;167:2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.