Abstract
We tested the ability of 20 synthetic θ defensins to protect cells from infection by type 1 and type 2 herpes simplex viruses (HSV-1 and -2, respectively). The peptides included rhesus θ defensins (RTDs) 1 to 3, originally isolated from rhesus macaque leukocytes, and three peptides (retrocyclins 1 to 3) whose sequences were inferred from human θ-defensin (DEFT) pseudogenes. We also tested 14 retrocyclin analogues, including the retro, enantio, and retroenantio forms of retrocyclin 1. Retrocyclins 1 and 2 and RTD 3 protected cervical epithelial cells from infection by both HSV serotypes, but only retrocyclin 2 did so without causing cytotoxicity or requiring preincubation with the virus. Surface plasmon resonance studies revealed that retrocyclin 2 bound to immobilized HSV-2 glycoprotein B (gB2) with high affinity (Kd, 13.3 nM) and that it did not bind to enzymatically deglycosylated gB2. Temperature shift experiments indicated that retrocyclin 2 and human α defensins human neutrophil peptide 1 (HNP 1) to HNP 3 protected human cells from HSV-2 by different mechanisms. Retrocyclin 2 blocked viral attachment, and its addition during the binding or penetration phases of HSV-2 infection markedly diminished nuclear translocation of VP16 and expression of ICP4. In contrast, HNPs 1 to 3 had little effect on binding but reduced both VP16 transport and ICP4 expression if added during the postbinding (penetration) period. We recently reported that θ defensins are miniature lectins that bind gp120 of human immunodeficiency virus type 1 (HIV-1) with high affinity and inhibit the entry of R5 and X4 isolates of HIV-1. Given its small size (18 residues), minimal cytotoxicity, lack of activity against vaginal lactobacilli, and effectiveness against both HSV-2 and HIV-1, retrocyclin 2 provides an intriguing prototype for future topical microbicide development.
The worldwide AIDS epidemic has intensified interest in identifying naturally occurring antiviral molecules (5, 29, 34, 57). Certain rabbit and human α defensins were shown to protect cells from infection by herpes simplex virus type 1 (HSV-1) and HSV-2 almost 20 years ago (17, 30), and more recent studies indicated that rabbit α defensin NP-1 blocks HSV infection at a very early stage (46). Even adenoviruses, which are nonenveloped, are susceptible to α defensins (3, 21), although the mechanism of this effect is unknown. In vitro, human α defensins human neutrophil peptide 1 (HNP 1) to HNP 3 can protect cells from infection by human immunodeficiency virus type 1 (HIV-1), and release of these defensins from the α, β CD8-positive T cells of HIV-infected subjects may (60) or may not (11) correlate with their long-term clinical stability.
Defensin peptides belonging to three subfamilies, designated α, β, and θ defensins, have been identified in leukocytes and other cells of humans or nonhuman primates. α defensins contain 29 to 35 residues and are produced as ∼100-residue prepropeptides (16). Human neutrophils (polymorphonuclear leukocytes) contain four α defensins, called HNPs 1, 2, 3, and 4. HNPs 1 and 3 are identical in 29 of their 30 residues, differing only in their N-terminal residue, which is alanine in HNP 1 and aspartic acid in HNP 3. HNP 2 contains 29 residues, and its sequence is identical to residues 2 to 30 of HNP 1 and HNP 3. Collectively, HNPs 1 to 3 constitute 5 to 7% of the total protein of human neutrophils. HNP 4 exists in much smaller amounts, and its sequence bears little resemblance to the sequences of HNPs 1 to 3. HNPs 1, 2, and 3 are also present in some human mononuclear cells, including NK and certain T cells. Human α defensins HD 5 and HD 6 are expressed in the small intestine (27, 28) and genitourinary tract (42), and HNPs 1 to 3 are found in vaginal secretions and the cervical plug (24, 53).
θ defensins are circular octadecapeptides with two antiparallel β sheets that are bridged by a tridisulfide ladder and connected by two β turns (50). Three θ-defensin peptides (or RTDs, an abbreviation for rhesus θ defensins) were purified from the leukocytes and bone marrow of rhesus macaques (31, 49, 51). In vivo, their formation entails ligation of two nonapeptides, each derived from the C-terminal domain of a prepropeptide (49) that is encoded by a θ-defensin (DEFT) gene. The human genome has at least six DEFT genes, some of which are expressed. However, because the human DEFT genes and mRNA carry a premature stop codon that aborts translation, θ-defensin peptides are not present in human polymorphonuclear leukocytes (38). Phylogenetic studies suggest that the native counterparts of the synthetic retrocyclins used in this study were lost by mutation(s) that occurred in a common ancestor of gorillas, chimpanzees, and humans (38).
It was recently reported that certain θ defensins, including retrocyclins, protect cells from infection by HIV-1 in vitro (12) and that they are miniature lectins (56) that bind gp120 and block the entry of HIV-1 (12, 36). The present experiments examined the ability of synthetic θ defensins to protect human cervical epithelial cells from infection by HSV-1 and HSV-2 and their ability to bind gB2, a glycoprotein that allows HSV-2 to attach to and enter cells.
MATERIALS AND METHODS
Peptides.
The peptides used in this study are described in Table 1. Retrocyclin 1 (RC [retrocyclin congener] 100) was synthesized by Anaspec (San Jose, Calif.) and purified, folded, and cyclized in our laboratory. The other peptides were synthesized at the University of California, Los Angeles, on a Perkin-Elmer ABI 431 A synthesizer, with prederivatized polyethylene glycol polystyrene arginine resin (PerSeptive Biosystems, Framingham, Mass.) and FastMoc chemistry, with double coupling throughout. The crude peptide was reduced at 50°C under N2 with excess dithiothreitol in 6 M guanidine HCl-0.2 M EDTA (pH 8.2). After addition of glacial acetic acid to a 5% final concentration, the reduced peptide was stored under N2 until further purification by reversed-phase high-pressure liquid chromatography (RP-HPLC). After this step, the peptides appeared homogenous and their theoretical mass agreed closely with the mass found by matrix-assisted laser desorption ionization mass spectrometry analysis.
TABLE 1.
θ defensins used in this studya
| Number | Name | Comment | Sequence |
|---|---|---|---|
| 1 3 5 6 8 10 11 1315 1618 | |||
| RTD 1 | Net charge, +5 | GFCRC LCRRG VCRCI CTR | |
| RTD 2 | Net charge, +6 | GVCRC LCRRG VCRCI CRR | |
| RTD 3 | Net charge, +4 | GFCRC ICTRG FCRCI CTR | |
| RC 100 | Retrocyclin 1 | Net charge, +4 | GICRC ICGRG ICRCI CGR |
| RC 100b | Retrocyclin 2 | Net charge, +5 | GICRC ICGRR ICRCI CGR |
| RC 100c | Retrocyclin 3 | Net charge, +6 | RICRC ICGRR ICRCI CGR |
| RC 101 | K9-retrocyclin 1 | R→K | GICRC ICGKG ICRCI CGR |
| RC 102 | Y6-retrocyclin 1 | I→Y | GICRC YCGRG ICRCI CGR |
| RC 103 | Y15-retrocyclin 1 | I→Y | GICRC ICGRG ICRCY CGR |
| RC 104 | Y2-retrocyclin 1 | I→Y | GYCRC ICGRG ICRCI CGR |
| RC 105 | Y11-retrocyclin 1 | I→Y | GICRC ICGRG YCRCI CGR |
| RC 106 | Y4-retrocyclin 1 | R→Y | GICYC ICGRG ICRCI CGR |
| RC 107 | Y9-retrocyclin 1 | R→Y | GICIC ICGYG ICRCI CGR |
| RC 108 | Y13-retrocyclin 1 | R→Y | GICIC ICGRG ICYCI CGR |
| RC 110 | Retroenantio RC 100 | All D, 18→1 | RGCIC RCIGR GCICR CIG |
| RC 111 | Retro RC 100 | All L, 18→1 | RGCIC RCIGR GCICR CIG |
| RC 112 | Enantio RC 100 | All D, 1→18 | GICRC ICGRG ICRCI CGR |
| RC 113 | Enantio RC 101 | All D, 1→18 | GICRC ICGKG ICRCI CGR |
| RC 114 | K9, Y15-retrocyclin 1 | R→K, I→Y | GICRC ICGKG ICRCY CGR |
RC 109 (sequence not shown) was not made. Residues that differ from the corresponding residues of retrocyclin 1 (RC 100) are in boldface. Since the peptides were cyclic, the residue numbers are arbitrary. The residues in one nonapeptide domain of each RTD and retrocyclin are underlined.
Cystine disulfide bonds were formed by dissolving the reduced peptides in 0.1% acetic acid (0.1 mg of peptide/ml) and adjusting the pH to 7.4 with ammonium hydroxide. After the stirred solution was incubated for 24 h at room temperature, acetic acid was added to a 5% final concentration. The oxidized peptides were purified by another round of RP-HPLC and showed the expected 6-atomic-mass-unit decrease. To form an amide bond between their amino- and carboxy-terminal residues, the oxidized peptides were incubated for 18 h at room temperature in a 3:1 mixture of ethylenediaminecarbodiimide and anhydrous N-hydroxybenzotriazole in dimethyl sulfoxide (49). After lyophilization of the reaction products, the cyclic peptide was subjected to RP-HPLC on a C18 column with a linear gradient of acetonitrile: water in 0.1% trifluoroacetic acid, resulting in >95% purity. HNPs 1 to 3, purified from healthy human neutrophils as previously described (23), contained HNP 1, HNP 2, and HNP 3 in a ratio of 2:2:1.
Cells and viruses.
HSV strains and cell lines were from the American Type Culture Collection. They included HSV-1 M (the MacIntyre strain, ATCC VR-539), HSV-2 G (ATCC VR-734), ME-180 human cervical carcinoma cells (HTB 33), Vero African green monkey kidney cells (CCL 81), and CaSki human cervical epithelial cells (ATCC CRL 1550). The viruses were propagated on Vero cells in a conventional manner and were stored at −85°C until used.
Viral glycoprotein.
HSV-2 recombinant glycoprotein B (gB2) was generated using the Bac-to-Bac system (Gibco) and purified by heparin-affinity chromatography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining confirmed its purity (25). Anti-gB2 monoclonal antibody (1123) was purchased from the Goodwin Institute, Plantation, Fla.
Direct viral inactivation.
Viral titers were assayed by infecting ME-180 cell monolayers in 96-well plates with twofold serially diluted viral inocula and incubating the cells for 72 h at 37°C. A viral dilution that produced 75 to 80% cell death, based on MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl tetrazolium bromide] reduction, was used in the inactivation assays. The corresponding multiplicity of infection (MOI) and PFU-per-well values were approximately 0.2 and ∼6 × 103, respectively, for HSV-1 M and 0.04 and 1 × 103, respectively, for HSV-2 G. ME-180 target cells were seeded in 96-well tissue culture plates at 2.5 × 104 cells/well and incubated for 48 h at 37°C. In our initial screening assays, unless otherwise noted, the viruses were preincubated with 50 μg of peptide/ml for 2 h at 37°C before they were added to the target cells. To do so, we added the 8.8 μl of virus inoculum to 304.7 μl of RPMI with 2% fetal bovine serum and introduced 16.5 μl of the 1-mg/ml stock peptide solution. Virus-free controls (peptide only) and peptide-free controls (virus plus acidified water only) were incubated in parallel. To initiate infection, the regular medium was aspirated from the target cells and replaced with 100 μl of the peptide-treated viral inoculum/well or appropriate controls. Trays were incubated at 37°C for 72 h, and cytotoxicity was measured with the MTT kit. Calculations of activity and corrections for background cytotoxicity were as previously described (47, 48, 58).
Dose-response experiments were done on RTD 3, retrocyclin 1 (RC 100), and retrocyclin 2 (RC 100b) by incubating final peptide concentrations of 25, 20, 10, and 5 μg/ml with the virus stocks for 2 h as described above. This mixture was added directly to ME-180 cells in incubation medium containing the same peptide concentration present during preincubation. The plates were incubated at 37°C for 72 h, and cytotoxicity was measured with the MTT kit.
Confirmatory plaque reduction assays were performed with the most active peptides, retrocyclins 1 and 2 and RTD 3. In some experiments, a 2-h preincubation of virus with 5 to 50 μg of peptide/ml was followed by delivering the inoculum to ME-180 cells in wells containing the corresponding peptide concentration. In other experiments, preincubation was omitted and the viruses were delivered directly into the wells containing target cells and peptide. Plaques were counted 24 h later, after the monolayers were stained with crystal violet (43).
Time course.
Confluent monolayers of CaSki cells in six-well dishes were cooled to 4°C and inoculated with 200 to 500 PFU of HSV-2 G virus/well at 4°C for 3 h. Unbound virus was removed by washing the cells three times, and the cultures were transferred to 37°C for 1 h to permit penetration. Any nonpenetrating viruses were inactivated by washing the monolayer for 2 min with an acidic buffer (pH 3.0) that contained 50 mM sodium citrate and 4 mM KCl. Then, fresh medium was added, and 1 h later the wells were overlaid with medium containing 0.5% methylcellulose and incubated for 48 h. Plaques were counted by immunoassay (black plaque) (26, 46). HNPs 1 to 3 or retrocyclin 2 was added (i) during the 4°C binding period, (ii) when cells were shifted to 37°C, or (iii) immediately post-citrate treatment for 1 h to determine whether the defensins inhibited infection if present during these time windows.
Surface plasmon resonance.
Experiments were performed on a Biacore 2000 system (Biacore, Inc., Piscataway, N.J.). Running buffer (pH 7.4) contained 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% polysorbate 20. The HSV-2 glycoprotein, gB2, was dissolved at 20 μ g/ml in 10 mM sodium acetate, pH 5.0, and immobilized on a CM5 sensor chip by the amine coupling method. The chip was activated by mixing 400 mM N-ethyl-N-(3-dimethylaminopropyl)-carbodiimide hydrochloride and 100 mM N-hydroxysuccinimide. An immobilization level of approximately 6,000 response units was attained for the bound protein. Residual reactive groups on the chip surface were blocked using 1.0 M ethanolamine-HCl, pH 8.5.
The flow cell 1 (FC1) chip, which served as a control, lacked immobilized protein but was treated as described above. Signals were corrected for nonspecific binding by subtracting the FC1 signal. To regenerate the chips, bound ligands were removed with 10 mM HCl. Data were analyzed with BIAevaluation 3.1 software. Curve fitting was done assuming one-to-one binding. In some experiments, immobilized gB2 was deglycosylated for 3 h at 37°C with recombinant enzymes—peptide:N-glycosidase F (PNGase F) (5 U), sialidase A (0.005 U), and/or endo-O-glycosidase (0.00125 U)—from the GlycoPro deglycosylation kit (ProZyme, San Leandro, Calif.).
Binding studies.
To determine if the defensins inhibited binding of gB2 to cells, we exposed CaSki cells to recombinant gB2 for 1 h at 37°C in the presence or absence of HNPs 1 to 3 or retrocylin 2. After this, unbound gB2 and defensins were removed by washing the cells extensively. Cell-bound glycoprotein was analyzed by Western blotting, with the use of cell lysates and an anti-gB2 monoclonal antibody. Blots were subsequently incubated with goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase and developed with the ECL chemiluminescence kit (DuPont, Boston, Mass.). Western blots were scanned, and bound gB2 was analyzed using the GELDOC 2000 Bio-Rad system linked to an IBM PC computer.
VP16 and ICP4.
To delineate the steps in HSV infection inhibited by α and θ defensins, we conducted synchronized studies and monitored infection by examining transport of the tegument protein VP16 to the nucleus or expression of immediate-early gene product ICP4 as previously described (9). Synchronized infectivity assays were done as described above except that the MOI was equivalent to 1 PFU/cell and the penetration time (i.e., the time from temperature shift to citrate treatment) was reduced to 15 min. Defensins or control agents were added for 1 h during the 4°C binding period, at the time at which the cells were transferred to 37°C (penetration), or immediately after the citrate treatment (postpenetration). Thereafter, the cells were overlaid with medium and viral infection was monitored by examining transport of VP16 or expression of ICP4. Nuclear extracts were prepared 3 h postinfection to examine transport of VP16 to the nucleus; alternatively cell lysates were prepared 5 h postinfection to examine ICP4 expression
Cytotoxicity.
Assays were done with the MTT Cell Proliferation Kit I (Boehringer Mannheim/Roche) according to the manufacturer's instructions. Briefly, ME-180 cells grown to confluency in RPMI 1640 with 10% fetal bovine serum, 2 mM l-glutamine, and 50 μg of gentamicin/ml were harvested with trypsin-EDTA, washed, and diluted in the above medium to 5 × 104 cells/ml. Cells (100 μl) were dispensed into 96-well tissue culture plates and allowed to adhere for 5 h at 37°C in an atmosphere of room air plus 5% CO2. Then, 10 μl of peptide (various concentrations) or its vehicle (0.01% acetic acid) was added, and the incubation was continued for 20 h. The cells were solubilized overnight, and the optical density was measured at 600 and 650 nm, on a SpectraMax 250 microplate spectrophotometer (Molecular Devices, Sunnyvale, Calif.).
Statistics.
Unpaired t tests were performed with the SigmaStat 2.0 statistical package (Jandel Scientific, San Rafael, Calif.).
RESULTS
Activity against HSV-1.
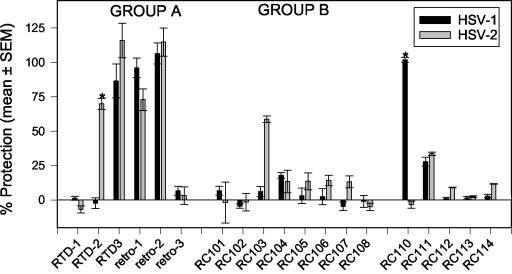
Screening studies were done in 96-well microplates, with a recently described MTT reduction assay. Peptides were tested at a single concentration (50 μg/ml), and the viral inoculum was preincubated for 2 h with each peptide. Under these conditions (Fig. 1), RTD 3 and retrocyclins 1 and 2 were effective, but RTDs 1 and 2 and retrocyclin 3 were not. The most effective peptides, RTD 3 and retrocyclins 1 and 2, constitute group A in Fig. 1. Except for RC 110 (retroenantioretrocyclin 1), none of the retrocyclin 1 analogues had much activity against HSV-1. All of the peptides in group B (RCs 101 to 108) were significantly less active against HSV-1 than were those in group A (P < 0.001). RCs 102 to 108 were peptides in which a single β-sheet residue had been replaced by a tyrosine. RC 101 contained a single arginine-to-lysine replacement in one of the β turns. Although RC 111, the retroanalogue of retrocyclin 1, retained some activity, RC 112 (enantioretrocyclin 1) was inactive.
FIG. 1.
Protection of ME-180 cells from infection by HSV-1 and HSV-2 (MTT assay). Peptides (50 μg/ml) and viruses were coincubated for 2 h, before being added to target cell monolayers that also contained 50 μg of peptide/ml. After incubation at 37°C for 72 h, cytotoxicity was measured with a kit. The bars (black, HSV-1; gray, HSV-2) represent means ± standard errors of the means of two to five experiments with each peptide. Asterisks identify the peptides whose activities against HSV-1 and HSV-2 differed significantly (P < 0.001). Group A and group B peptides are further described in the text.
Activity against HSV-2.
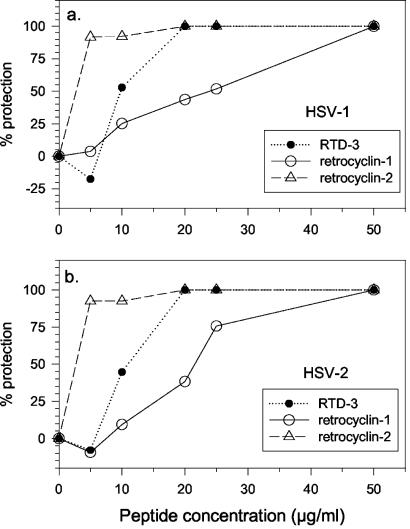
Similar studies were performed with HSV-2 (Fig. 1). RTD 3 and retrocyclin 2 showed the best activity, followed by RTD 2 and retrocyclin 1. RC 103 was moderately effective against HSV-2, but RTD 1 and RC 110 (retroenantioretrocyclin 1) lacked activity against HSV-2 despite their effectiveness against HSV-1 (P < 0.001). Only three peptides (RTD 3 and retrocyclins 1 and 2) were active against both HSV-1 and HSV-2. To confirm the activity and assess relative potency, we tested these peptides in a plaque reduction assay (Fig. 2). In descending order of potency, the peptides ranked as follows: retrocyclin 2 > RTD 3 > retrocyclin 1.
FIG. 2.
Protection against HSV-1 and HSV-2. The most active peptides against HSV-1 and HSV-2 were RTD 3, retrocyclin 1 (RC 100), and retrocyclin 2 (RC 100b). Each peptide (various concentrations) was incubated with HSV-1 or HSV-2 for 2 h and added to ME-180 cell monolayers with the same concentration of peptide. After a 24-h incubation, aliquots were harvested for plaque counting.
Comparative activity against herpesviruses and HIV-1.
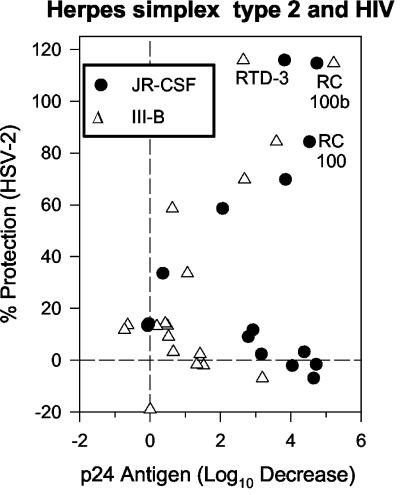
It was recently reported that retrocyclin 1 and certain RTDs can protect human peripheral blood mononuclear cells from infection by T- and M-tropic strains of HIV-1 (56). Because of these studies with HIV-1, each peptide had already been tested against HIV-1 strains IIIB and JR-CSF. In Fig. 3, the y axis shows activity against HSV-2 and the x axis shows previously published (12) activity of 20 μg of peptide/ml against HIV-1 strains JR-CSF (solid circles) and IIIB (open triangles). A 4-log10 decrease (i.e., 99.99%) in the concentration of p24 antigen on day 9 of culture, relative to the p24 concentration in the absence of peptide, represents complete protection from HIV-1 infection. With respect to activity against HSV-1, HSV-2, and the HIV-1 strains the peptides ranked as follows: retrocyclin 2 > RTD 3 > retrocyclin 1. The structures of these peptides and that of RTD 1 are shown in Fig. 4.
FIG. 3.
Comparative antiviral activity. Each peptide is represented by a closed circle, and the most active peptides are labeled with their identifier in Table 1. The x axis shows activity against HIV-1, and the y axis shows activity against HSV-2. The JR-CSF strain (R5) of HIV-1 is represented by closed circles, and the IIIB strain (X4) is represented by open triangles. The most active peptides, retrocyclin 2 (RC 100b), RTD 3, and retrocyclin 1 (RC 100) are labeled.
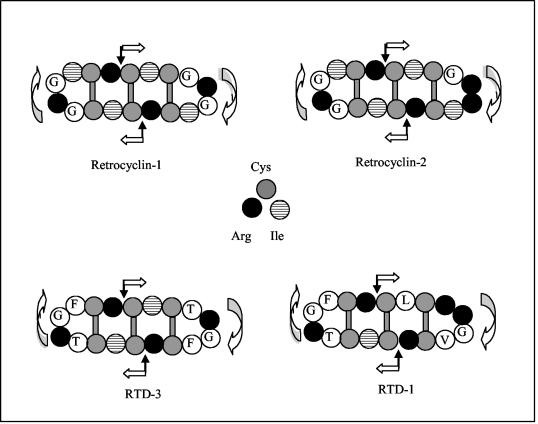
FIG. 4.
Structures of selected θ defensins. Four θ defensins are diagrammed. θ defensins are circular octadecapeptides with two antiparallel β sheets that are linked by β turns and bridged by an evenly spaced tridisulfide ladder. Their 18 residues represent two 9-residue peptides contributed by a truncated, α-defensin-like prepropeptide. Black arrows identify the sites where the individual nonapeptide elements would be spliced together in vivo. The white arrows show the direction of the peptide backbone and point from the N terminus toward the C terminus.
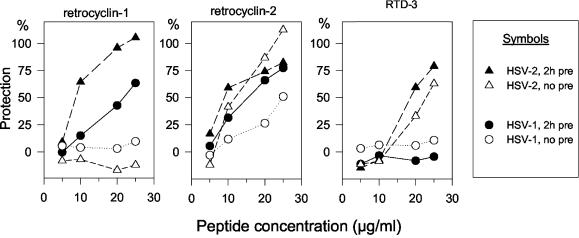
To this point, all results with HSV-1 and -2 were obtained after preincubating the viruses and peptides together for 2 h before adding them to target cells. To see if a preincubation step was necessary, we turned to plaque-forming assays (Fig. 5). These revealed that retrocyclin 2 showed substantial activity without any preincubation step and that retrocyclin 1 and RTD 3 had little or no activity unless they were preincubated with HSV-2. Figure 5 also shows that retrocyclin 1 and RTD 3 had much greater activity against HSV-2 than against HSV-1.
FIG. 5.
Effect of preincubation on activity. HSV-1 and HSV-2 were exposed to various concentrations (5, 10, 20, and 25 μg/ml) of retrocyclins 1 to 3. Some viruses were preincubated with peptide for 2 h before being added to the ME-180 target cells (“2h pre”). In other assays, the peptide and virus were added to the target cells without preincubation (“no pre”). Viral replication was assessed by plaque counting, and percent protection was equated with the percent reduction in PFU. Results are means of two separate experiments with very similar results.
Retrocyclin 2, but not HNPs 1 to 3, blocks HSV-2 attachment.
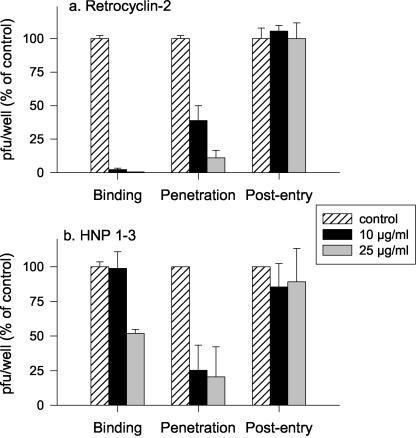
Since retrocyclin 2 was the most protective peptide, we further explored the mechanism of its antiviral activity by examining which steps in viral invasion were blocked by retrocyclin 2. For comparison, we tested HNPs 1 to 3. We have previously shown that rabbit α defensin NP-1 blocks HSV-2 infection at a step postbinding, but similar studies with human α defensins have not been reported. To define the step(s) in HSV invasion that was blocked by retrocyclin 2 and HNPs 1 to 3 (25), kinetic assays were performed as previously described. CaSki cells were cooled to 4°C and exposed to virus for 3 h to allow viral attachment. Then, unbound virus was removed by washing, and cells were shifted to 37°C to allow penetration. After 1 h, the cells were washed with a low-pH citrate buffer to inactivate nonpenetrant virus and fresh medium was added. After 1 additional h of incubation, the cells were again washed and overlaid with medium containing methylcellulose. Retrocyclin 2, HNPs 1 to 3, or control buffer (0.01% acetic acid in water) was added during the 4°C binding period (time A), at the time of temperature shift (penetration) (time B), or immediately post-citrate treatment for 1 h (postentry) (time C). Plaques were counted 48 h later. Results are depicted in Fig. 6. At 25 μg/ml, retrocyclin 2 inhibited HSV-2 infection by 99% if added at time A (during binding). An antiviral activity persisted, albeit a reduced one, if the peptide was added at time B (temperature shift) but not if added at time C (postentry). In contrast HNPs 1 to 3 primarily inhibited viral infection if added at time B (temperature shift), during the postbinding period of viral penetration.
FIG. 6.
Time signature of the antiviral effect. Cells were cooled to 4°C and inoculated with HSV-2 G in the presence of peptide-free control buffer, retrocyclin 2, or HNPs 1 to 3. Peptides were added at different times, as described in Materials and Methods. The bars, which show PFU formed in the presence of defensin as a percentage of PFU formed in the control wells, represent the means ± standard deviations of two independent experiments, each performed in duplicate.
Other effects of retrocyclin.
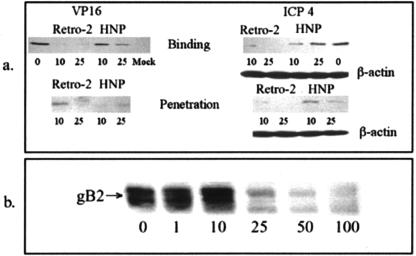
The earliest detectable measures of HSV-2 entry are translocation of VP16, a viral tegument protein, to the nucleus and expression of ICP4, the immediate-early gene product. To assess the effects of retrocyclin 2 on these indicator responses, we conducted synchronized infectivity assays as described above, except that the MOI was 1 PFU/cell and the time from temperature shift to citrate treatment (penetration period) was reduced to 15 min. Representative results are shown in Fig. 7. Nuclear VP16 and expression of ICP4 were markedly diminished when retrocyclin 2 was added during either binding or penetration. HNP 1 had little effect if added during binding, but it reduced both VP16 transport and ICP4 expression if added during the penetration period, suggesting that the α defensins prevent an early postbinding step. In contrast, retrocyclin 2 inhibits viral entry when present during either the binding or the penetration period.
FIG. 7.
VP16 transport and IC4 expression. (a) These assays examined the effects of retrocyclin 2 or HNPs 1 to 3 on VP16 transport to the nucleus and ICP4 expression after infection with HSV-2(G). Peptides were added during the 4°C binding period or at the temperature shift. After 4 h, nuclear extracts were prepared for VP16, and after 5 h, cell lysates were prepared for ICP4 expression. Lanes contained extracts or lysates from equivalent cell numbers, and VP16, ICP4, and β-actin (loading control) were detected by Western blotting. (b) To examine the effect on binding, cells were exposed to recombinant gB-2 (10−5 μg/cell) for 1 h at 37°C ± the indicated concentration of retrocyclin 2. Bound gB-2 was estimated by probing Western blots of cell lysates with anti-gB monoclonal antibody. Results are representative of three independent experiments.
To more specifically examine the effects of retrocyclin 2 on binding of gB2 to target cell heparan sulfate, binding studies were conducted with recombinant gB2. Figure 7 shows that 25 μg of retrocyclin 2/ml completely inhibited the binding of recombinant gB2 to CaSki cells. These results differ from those previously published for the rabbit α defensin NP-1, which failed to inhibit binding of recombinant gB2 to CaSki cells. Similarly, HNPs 1 to 3 also showed little or no inhibitory effects on binding of recombinant gB2 to cells (data not shown).
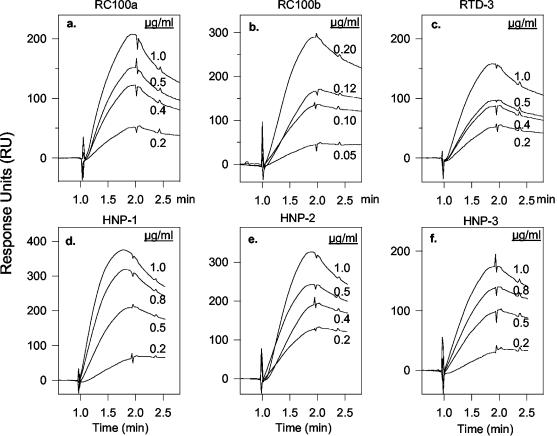
It was recently reported that θ defensins inhibit the cellular entry of HIV-1 (12, 36) and that they bind with high affinity to gp120 (56), a viral envelope glycoprotein whose interactions with CD4 and other coreceptors on the target cell surface are required for penetration. This suggested that θ defensins might protect cells from HSV-2 infection by binding gB2, an envelope glycoprotein whose presence is essential for target cell binding and penetration by HSV-2 (10). Figure 8a to c shows that 0.12 μg of retrocyclin 2/ml, 0.5 μg of retrocyclin 1/ml, and 1.0 μg of RTD 3/ml all bound to immobilized gB2 to about the same extent. Judging from its lower “off rate,” the gB2-retrocyclin 2 complex was more stable than the complexes formed between gB2 and either retrocyclin 1 or RTD 3 were. Quantitative analysis of these binding isotherms (Table 2) indicated that gB2 binds retrocyclin 2 with much higher affinity (Kd, 13.3 nM) than it bound retrocyclin 1 (Kd, 142 nM) or RTD 3 (Kd, 174 nM). The 10-fold-greater affinity of retrocyclin 2 for gB2 derives in approximately equal measure from its higher Kon (on rate) and lower Koff (off rate).
FIG. 8.
Binding of θ defensins to gB2. Surface plasmon resonance binding curves (isotherms) of binding to immobilized gB2 are shown for retrocyclin 1 (RC 100a) (a), retrocyclin 2 (RC 100b) (b), RTD 3 (c), HNP 1 (d), HNP 2 (e), and HNP 3 (f). The concentration of peptide in each run is shown adjacent to its curve. The peptides were introduced into the flow cell at the 1-min mark, and 60 s later, at the 2-min mark, the sensor chips were perfused with peptide-free buffer to allow the off rate to be measured. Rate and affinity constants can be found in Tables 2 and 3.
TABLE 2.
Correlation between the binding of θ defensins to immobilized gB2 and protective activity against HSV-2
| θ defensin peptide | Net charge | Kon (104 M−1 s−1) | Koff (10−3 s−1) | Kd (nM) | Activity vs:
|
|
|---|---|---|---|---|---|---|
| HSV-2 | HSV-1 | |||||
| RTD 1 | +5 | 0.196 | 8.33 | 4,240.0 | None | None |
| RTD 2 | +6 | 5.25 | 7.06 | 134.0 | Moderate | None |
| RTD 3 | +4 | 5.36 | 9.33 | 174.0 | High | High |
| Retrocyclin 1 | +4 | 6.76 | 9.58 | 142.0 | Moderate | High |
| Retrocyclin 2 | +5 | 22.9 | 3.05 | 13.3 | High | High |
| Retrocyclin 3 | +6 | 3.47 | 10.2 | 295.0 | None | None |
The DEFT genes that encode θ defensins are mutated α-defensin genes (38, 49). Because we have previously shown that rabbit and human α defensins protect cells from infections by HSV-1 and -2 and because α defensins are homologous to θ defensins, we performed surface plasmon resonance experiments to determine if α defensins also bind gB2. Our results with HNPs 1 to 4 are summarized in Table 3 and illustrated for selected α defensins in Fig. 8d to f. Table 3 shows that HNP 2 (net charge, +3) bound gB2 with an affinity (Kd, 30.3 nM) that was higher than that of every θ defensin shown in Table 2, except for retrocyclin 2, whose Kd was 13.3 nM. HNPs 1 and 3 bound gB2 with somewhat reduced affinity (Kd of 50 to 60 nM). The sequences of HNPs 1 to 3 are identical at 29 of their 30 residues (96.7%), differing only at their N-terminal residue (Table 3). HNP 4, which is more cationic (net charge, +4) than HNPs 1 to 3 (net charge, +2 to +3), has a 50- to 100-fold-lower affinity for gB2 (Kd, 2,880 nM) than they do. HNP 4 is identical to HNPs 1 to 3 in only 11 of 34 (32.4%) positions, and at least 8 of the 11 identical residues (six cysteines, one arginine, and one glycine) have well-established structural roles. Thus, as we found for the θ defensins, the ability of α defensins to bind gB2 is primarily determined by their sequence, rather than by their net cationic charge.
TABLE 3.
Binding of α defensins HNP 1 to HNP 4 to immobilized gB2
| α defensin peptide | Net charge | Kon (104 M−1 s−1) | Koff (10−3 s−1) | Kd (nM) | Sequence |
|---|---|---|---|---|---|
| HNP 1 | +3 | 12.0 | 6.30 | 52.6 | ACYCRIPACIAGERRYGTCIYQGRLWAFCC |
| HNP 2 | +3 | 22.1 | 6.68 | 30.3 | -CYCRIPACIAGERRYGTCIYQGRLWAFCC |
| HNP 3 | +3 | 9.02 | 5.36 | 59.5 | DCYCRIPACIAGERRYGTCIYOGRLWAFCC |
| HNP 4 | +4 | 1.57 | 45.2 | 2,880 | VCSCRLVFCRRTELRVGNCLIGGVSFTYCCTRVD |
α and θ defensins bind carbohydrate moieties on gB2.
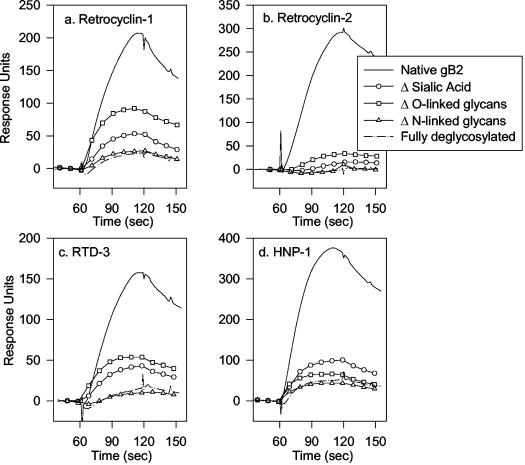
Selective removal of O-linked or N-linked glycans from immobilized gB2 greatly reduced its binding by α and θ defensins (Fig. 9). Removing O-linked glycans with endo-O-glycosidase reduced the magnitude of binding by at least 50%, and simply removing the sialic acids with sialidase A decreased binding by at least two-thirds. Removing the N-linked glycans of gB2 had an even greater effect, such that binding was nearly undetectable. Overall, these findings indicate that sialic acid and other carbohydrate moieties in the O- and N-linked glycans of gB2 are responsible for defensin binding. Since removing the O-linked glycans alone substantially reduced binding and removing the N-linked residues virtually abolished binding, we infer that the O-linked and N-linked glycans may act cooperatively in providing binding sites for defensins or that defensin binding was influenced by the local density of sugar residues.
FIG. 9.
Effect of deglycosylation on binding to gB2. Immobilized gB2 was selectively deglycosylated by incubation with sialidase A, endo-O-glycosidase, PNGase F, or a mixture of all three enzymes. Sialidase A removes nonreducing terminal and branched sialic acid residues. Endo-O-glycosidase removes O-linked glycans by cleaving Ser/Thr-linked unsubstituted Galβ(1-3)GalNAcα disaccharides. PNGase F removes N-linked glycans.
Cytotoxicity.
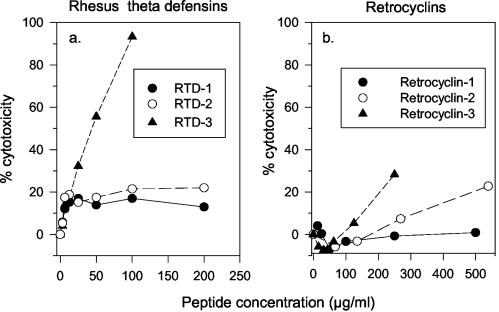
The cytotoxicity of the three RTDs, 1 to 3, for human ME-180 cervical epithelial cells is shown in Fig. 10a. Whereas RTD 3 caused substantial cytotoxicity, RTD 1 and RTD 2 were well tolerated, even at 200 μg/ml. Neither retrocyclin 1 nor retrocyclin 2 caused appreciable cytotoxicity to ME-180 cells, even at 500 μg/ml (Fig. 10b). Retrocyclin 3 was more cytotoxic than was retrocyclin 1 or 2 but less so than RTD 3. Since retrocyclin 2 (RC 100b) was the most effective θ defensin in preventing HSV-1 and HSV-2 from infecting ME-180 cells, its activity against these viruses cannot be attributable to cytotoxicity.
FIG. 10.
Lack of cytotoxicity. (a) Effects of three RTDs, 1 to 3, on human ME-180 cervical epithelial cells. (b) Effects of retrocyclins 1 to 3 on these cells. The sequences of these peptides can be found in Table 1.
Inactivity against vaginal lactobacilli.
Unlike many previously reported α and β defensins, θ defensins have shown distinctly unimpressive antibacterial properties in our experiments. Their inactivity against two strains of lactobacilli, Lactobacillus acidophilus and Lactobacillus crispatus, at pH 4.5 (a normal vaginal pH) and pH 7.0 was entirely consistent with this behavior (data not shown). Although this property would be considered an unusual attribute for an antimicrobial peptide, it may be highly advantageous for an antiviral peptide, especially if it were to be used as a topical microbicide.
DISCUSSION
The prevalence of HSV-2 infection has increased greatly over the past 20 years, and it is estimated that 22% of adult Americans are currently infected with this virus (20). A recent modeling study concluded that, without intervention, the prevalence of HSV-2 infection among individuals aged 15 to 39 years would likely increase to 39% among men and 49% among women by 2025. Unlike the sexually transmitted infections caused by HIV-1, those caused by HSV-2 are infrequently lethal. Nonetheless, HSV-2 infections cause substantial morbidity, and over the next 25 years new HSV-2 infections will cost upwards of 61 billion dollars (20).
Since it was determined that nonoxynol-9 failed to protect at-risk individuals from acquiring sexually transmitted infections (19, 29), interest has grown in developing topical microbicides able to prevent infection by HIV-1. Recent reviews mention that over 30 agents are currently in clinical or preclinical development (52, 54). They include BufferGel (22), an acidic buffer; surfactants such as C31G4 (Savvy) (4) and sodium dodecyl sulfate; various acidic polymers (37) including carrageenan (32) (Carraguard); D2S (a dextran-sulfate analogue); certain reverse transcriptase inhibitors (18); monoclonal antibodies (41, 55, 59); and the lectin cyanovirin-N (Cv-N) (35). A few of these agents also have the potential to protect against HSV-2 infection (59).
Of the above, retrocyclins and θ defensins most closely resemble Cv-N, because their carbohydrate-binding and antiviral properties are intimately related. Cv-N, an 11-kDa protein from the cyanobacterium Nostoc ellipsosporum (39), selectively binds Man (8) and Man (9) oligosaccharides with nanomolar affinity (6), including those on HIV-1 envelope glycoproteins gp120 and gp41 (7, 45). Extremely low Cv-N concentrations protect cells from infection by M- and T-tropic strains of HIV-1. As concentrations that completely inhibit HIV-1 infection do not block binding of soluble CD4 receptor to HIV-1 lysates or attachment of intact HIV-1 virions to target T-cell lines, the protective effects of Cv-N against HIV-1 may arise from interference with postbinding fusion events (33). Cv-N is active against other viruses, including Ebola virus (1) and influenza A and B viruses (40), but was inactive (50% effective concentration of >10 μg/ml) against HSV-2 and had only moderate activity (a 50% effective concentration of 0.7 μg/ml) against a strain of HSV-1. Cv-N may be much more cytotoxic than retrocyclin 2 and other θ defensins are, since its 50% inhibitory concentration (the concentration that caused 50% cytotoxicity) for Vero cells was only 2.3 μg/ml (40).
The carbohydrate residues to which θ defensins bind are not yet defined, and there is a dearth of published information about glycans associated with HSV-2 glycoproteins. We have established that retrocyclin 2 and other θ defensins do not bind to the high-mannose oligosaccharides that are recognized by Cv-N (unpublished data), and studies to identify the glycan residues that are recognized by θ defensins are in progress.
This report showed that retrocyclin 2 bound to HSV-2 gB2 with extremely high affinity, that it prevented gB2 from associating with target cell membranes, and that it blocked the entry of HSV-2 into target cells. For initial binding to cells, both HSV-1 and -2 use heparan sulfate moieties as receptors (10). Given the critical role of gB2 in HSV-2 binding and its essential role in penetration and cell-cell spread, this glycoprotein, which contains eight potential N-glycosylation sites (8), is an important target for development of novel antiviral drugs. It remains to be determined if retrocyclin 2 can also interact with glycan moieties in the other essential glycoproteins, gD and gH-gL. Furthermore, since retrocyclin 2 has shown effectiveness against herpesviruses 1 and 2 and HIV-1, its effects on other viruses of biomedical interest merit exploration.
Although phylogenetic analysis suggests that θ defensins have been around for over 30 million years (38), they are “novel” molecules for many reasons. Whereas circular peptides have been identified in various plants (2, 13, 15, 44) and bacteria (14), θ defensins are the only known circular peptides of animal origin. They were first described in 1999 (49), but as yet only a few publications (12, 31, 49, 50, 56) describe their provenance and properties. The unusual posttranslational modifications involved in their formation by leukocytes (49) remain to be explored, as does the in vivo significance that their antiviral properties may have for the primates still able to produce them. There is remarkable parallelism between the effects of retrocyclins on HIV-1 and those on HSV. In both cases, the peptide binds with very high affinity to a viral surface glycoprotein involved in viral entry, either gp120 (56) or gB2 (this paper), and it is able to prevent cellular entry of HIV-1 (12, 36) and HSV-2 (this paper).
Retrocyclin 2 is currently the most potent antiviral θ defensin that we have tested that is active against both HIV-1 and HSV-2. Whether θ defensins will prove to be effective against other viruses remains to be established. Their inactivity against lactobacilli and their negligible cytotoxicity are desirable properties for a potential topical microbicide. While additional questions remain to be addressed, retrocyclins and other θ defensins are interesting molecules whose chemical and biological properties deserve increased attention.
Acknowledgments
This study was supported by grants from the National Institutes of Health, AI 37845 and AI 22838 to R.I.L. and HD 43733 and AI 39740 to B.C.H.
REFERENCES
- 1.Barrientos, L. G., B. R. O'Keefe, M. Bray, A. Sanchez, A. M. Gronenborn, and M. R. Boyd. 2003. Cyanovirin-N binds to the viral surface glycoprotein, GP(1,2) and inhibits infectivity of Ebola virus. Antivir. Res. 58:47-56. [DOI] [PubMed] [Google Scholar]
- 2.Barry, D. G., N. L. Daly, R. J. Clark, L. Sando, and D. J. Craik. 2003. Linearization of a naturally occurring circular protein maintains structure but eliminates hemolytic activity. Biochemistry 42:6688-6695. [DOI] [PubMed] [Google Scholar]
- 3.Bastian, A., and H. Schafer. 2001. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 101:157-161. [DOI] [PubMed] [Google Scholar]
- 4.Bax, R., K. Douville, D. McCormick, M. Rosenberg, J. Higgins, and M. Bowden. 2002. Microbicides—evaluating multiple formulations of C31G. Contraception 66:365-368. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, J. A., J. B. McMahon, T. R. Johnson, B. R. O'Keefe, R. A. Buzzell, D. Robbins, R. Gardella, J. Wilson, and M. R. Boyd. 2002. High throughput screening for cyanovirin-N mimetics binding to HIV-1 gp41. J. Biomol. Screen. 7:105-110. [DOI] [PubMed] [Google Scholar]
- 6.Bewley, C. A., S. Kiyonaka, and I. Hamachi. 2002. Site-specific discrimination by cyanovirin-N for alpha-linked trisaccharides comprising the three arms of Man(8) and Man(9). J. Mol. Biol. 322:881-889. [DOI] [PubMed] [Google Scholar]
- 7.Bolmstedt, A. J., B. R. O'Keefe, S. R. Shenoy, J. B. McMahon, and M. R. Boyd. 2001. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol. Pharmacol. 59:949-954. [DOI] [PubMed] [Google Scholar]
- 8.Bzik, D. J., C. Debroy, B. A. Fox, N. E. Pederson, and S. Person. 1986. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology 155:322-333. [DOI] [PubMed] [Google Scholar]
- 9.Cheshenko, N., B. Del Rosario, C. Woda, D. Marcellino, L. M. Satlin, and B. C. Herold. 2003. Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. 163:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheshenko, N., and B. C. Herold. 2002. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. 83:2247-2255. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, J. 2002. AIDS research. Mystery anti-HIV factor unmasked? Science 297:2188. [DOI] [PubMed] [Google Scholar]
- 12.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craik, D. J. 2001. Plant cyclotides: circular, knotted peptide toxins. Toxicon 39:1809-1813. [DOI] [PubMed] [Google Scholar]
- 14.Craik, D. J., N. L. Daly, I. Saska, M. Trabi, and K. J. Rosengren. 2003. Structures of naturally occurring circular proteins from bacteria. J. Bacteriol. 185:4011-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craik, D. J., S. Simonsen, and N. L. Daly. 2002. The cyclotides: novel macrocyclic peptides as scaffolds in drug design. Curr. Opin. Drug Discov. Dev. 5:251-260. [PubMed] [Google Scholar]
- 16.Daher, K. A., R. I. Lehrer, T. Ganz, and M. Kronenberg. 1988. Isolation and characterization of human defensin cDNA clones. Proc. Natl. Acad. Sci. USA 85:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daher, K. A., M. E. Selsted, and R. I. Lehrer. 1986. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Fabio, S., J. Van Roey, G. Giannini, M. G. Van Den, M. Spada, A. Binelli, M. F. Pirillo, E. Germinario, F. Belardelli, M. P. De Bethune, and S. Vella. 2003. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS 17:1597-1604. [DOI] [PubMed] [Google Scholar]
- 19.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 20.Fisman, D. N., M. Lipsitch, E. W. Hook III, and S. J. Goldie. 2002. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United States. Sex. Transm. Dis. 29:608-622. [DOI] [PubMed] [Google Scholar]
- 21.Gropp, R., M. Frye, T. O. Wagner, and J. Bargon. 1999. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum. Gene Ther. 10:957-964. [DOI] [PubMed] [Google Scholar]
- 22.Harwell, J. I., T. Moench, K. H. Mayer, S. Chapman, I. Rodriguez, and S. Cu-Uvin. 2003. A pilot study of treatment of bacterial vaginosis with a buffering vaginal microbicide. J. Women's Health 12:255-259. [DOI] [PubMed] [Google Scholar]
- 23.Harwig, S. S., A. S. Park, and R. I. Lehrer. 1992. Characterization of defensin precursors in mature human neutrophils. Blood 79:1532-1537. [PubMed] [Google Scholar]
- 24.Hein, M., E. V. Valore, R. B. Helmig, N. Uldbjerg, and T. Ganz. 2002. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 187:137-144. [DOI] [PubMed] [Google Scholar]
- 25.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, M. Dzuzelewski, F. Francois, R. Morin, V. M. Casullo, R. A. Anderson, C. Chany, D. P. Waller, L. J. Zaneveld, and M. E. Klotman. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 76:11236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, D. E., and C. L. Bevins. 1992. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267:23216-23225. [PubMed] [Google Scholar]
- 28.Jones, D. E., and C. L. Bevins. 1993. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315:187-192. [DOI] [PubMed] [Google Scholar]
- 29.Jung, M., S. Lee, and H. Kim. 2000. Recent studies on natural products as anti-HIV agents. Curr. Med. Chem. 7:649-661. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer, R. I., K. Daher, T. Ganz, and M. E. Selsted. 1985. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J. Virol. 54:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonova, L., V. N. Kokryakov, G. Aleshina, T. Hong, T. Nguyen, C. Zhao, A. J. Waring, and R. I. Lehrer. 2001. Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70:461-464. [PubMed] [Google Scholar]
- 32.Maguire, R. A., N. Bergman, and D. M. Phillips. 2001. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex. Transm. Dis. 28:259-265. [DOI] [PubMed] [Google Scholar]
- 33.Mariner, J. M., J. B. McMahon, B. R. O'Keefe, K. Nagashima, and M. R. Boyd. 1998. The HIV-inactivating protein, cyanovirin-N, does not block gp120-mediated virus-to-cell binding. Biochem. Biophys. Res. Commun. 248:841-845. [DOI] [PubMed] [Google Scholar]
- 34.Matthee, G., A. D. Wright, and G. M. Konig. 1999. HIV reverse transcriptase inhibitors of natural origin. Planta Med. 65:493-506. [DOI] [PubMed] [Google Scholar]
- 35.Mori, T., and M. R. Boyd. 2001. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. Antimicrob. Agents Chemother. 45:664-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munk, C., G. Wei, O. O. Yang, A. J. Waring, W. Wang, T. Hong, R. I. Lehrer, N. R. Landau, and A. M. Cole. 2003. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retrovir. 19:875-881. [DOI] [PubMed] [Google Scholar]
- 37.Neurath, A. R., N. Strick, and Y. Y. Li. 2002. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect. Dis. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen, T. X., A. M. Cole, and R. I. Lehrer. 2003. Evolution of primate θ -defensins: a serpentine path to a sweet tooth. Peptides 24:1647-1654. [DOI] [PubMed] [Google Scholar]
- 39.O'Keefe, B. R. 2001. Biologically active proteins from natural product extracts. J. Nat. Prod. 64:1373-1381. [DOI] [PubMed] [Google Scholar]
- 40.O'Keefe, B. R., D. F. Smee, J. A. Turpin, C. J. Saucedo, K. R. Gustafson, T. Mori, D. Blakeslee, R. Buckheit, and M. R. Boyd. 2003. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 47:2518-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quayle, A. J., E. M. Porter, A. A. Nussbaum, Y. M. Wang, C. Brabec, K. P. Yip, and S. C. Mok. 1998. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152:1247-1258. [PMC free article] [PubMed] [Google Scholar]
- 43.Rawls, W. 1992. Herpes simplex viruses: types 1 and 2, p. 443-461. In E. H. Lennette (ed.), Laboratory diagnosis of viral infections. Marcel Dekker, New York, N.Y.
- 44.Rosengren, K. J., N. L. Daly, M. R. Plan, C. Waine, and D. J. Craik. 2003. Twists, knots, and rings in proteins. Structural definition of the cyclotide framework. J. Biol. Chem. 278:8606-8616. [DOI] [PubMed] [Google Scholar]
- 45.Shenoy, S. R., B. R. O'Keefe, A. J. Bolmstedt, L. K. Cartner, and M. R. Boyd. 2001. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J. Pharmacol. Exp. Ther. 297:704-710. [PubMed] [Google Scholar]
- 46.Sinha, S., N. Cheshenko, R. I. Lehrer, and B. C. Herold. 2003. NP-1, a rabbit α-defensin, prevents the entry and intracellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 47:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sudo, K., K. Konno, T. Yokota, and S. Shigeta. 1994. A sensitive assay system screening antiviral compounds against herpes simplex virus type 1 and type 2. J. Virol. Methods 49:169-178. [DOI] [PubMed] [Google Scholar]
- 48.Sudo, K., K. Konno, T. Yokota, and S. Shigeta. 1995. A screening system for antiviral compounds against herpes simplex virus type 1 using the MTT method with L929 cells. Tohoku J. Exp. Med. 176:163-171. [DOI] [PubMed] [Google Scholar]
- 49.Tang, Y. Q., J. Yuan, G. Osapay, K. Osapay, D. Tran, C. J. Miller, A. J. Ouellette, and M. E. Selsted. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498-502. [DOI] [PubMed] [Google Scholar]
- 50.Trabi, M., H. J. Schirra, and D. J. Craik. 2001. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from rhesus macaque leukocytes. Biochemistry 40:4211-4221. [DOI] [PubMed] [Google Scholar]
- 51.Tran, D., P. A. Tran, Y. Q. Tang, J. Yuan, T. Cole, and M. E. Selsted. 2002. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277:3079-3084. [DOI] [PubMed] [Google Scholar]
- 52.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 53.Valore, E. V., C. H. Park, S. L. Igreti, and T. Ganz. 2002. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187:561-568. [DOI] [PubMed] [Google Scholar]
- 54.van de Wijgert, J., and C. Coggins. 2002. Microbicides to prevent heterosexual transmission of HIV: ten years down the road. BETA 15:23-28. [PubMed] [Google Scholar]
- 55.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]
- 56.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 170:4708-4716. [DOI] [PubMed] [Google Scholar]
- 57.Yang, S. S., G. M. Cragg, D. J. Newman, and J. P. Bader. 2001. Natural product-based anti-HIV drug discovery and development facilitated by the NCI developmental therapeutics program. J. Nat. Prod. 64:265-277. [DOI] [PubMed] [Google Scholar]
- 58.Yasin, B., M. Pang, J. S. Turner, Y. Cho, N. N. Dinh, A. J. Waring, R. I. Lehrer, and E. A. Wagar. 2000. Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 19:187-194. [DOI] [PubMed] [Google Scholar]
- 59.Zeitlin, L., and K. J. Whaley. 2002. Microbicides for preventing transmission of genital herpes. Herpes 9:4-9. [PubMed] [Google Scholar]
- 60.Zhang, L., W. Yu, T. He, J. Yu, R. E. Caffrey, E. A. Dalmasso, S. Fu, T. Pham, J. Mei, J. J. Ho, W. Zhang, P. Lopez, and D. D. Ho. 2002. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298:995-1000. [DOI] [PubMed] [Google Scholar]