Abstract
Objective
Natural immunity is emerging as an important mediator of protection from atherogenesis. Natural IgM antibodies that recognize oxidation-specific epitopes on low-density lipoprotein or phospholipids and the B-1a B cells that produce them attenuate atherosclerosis. We previously demonstrated that Apoe−/− mice globally deficient in the helix-loop-helix protein inhibitor of differentiation 3 (Id3) develop early diet-induced atherosclerosis. Furthermore, B cell–mediated attenuation of atherosclerosis in B cell–deficient mice was dependent on Id3. Here, we sought to determine whether Id3 regulates B-1a B cells and the natural antibodies that they produce and identify mechanisms mediating these effects.
Approach and Results
Mice lacking Id3 had significantly fewer B-1a B cells in the spleen and peritoneal cavity and reduced serum levels of the natural antibody E06. B cell–specific deletion of Id3 revealed that this effect was not because of the loss of Id3 in B cells. Interleukin (IL)-33 induced abundant, Id3-dependent IL-5 production in the recently identified innate lymphoid cell, the natural helper (NH) cell, but not Th2 or mast cells. In addition, delivery of IL-5 to Id3-deficient mice restored B-1a B cell proliferation. B-1a B cells were present in aortic samples also containing NH cells. Aortic NH cells produced IL-5, a B-1a B cell mitogen in response to IL-33 stimulation.
Conclusions
These studies are the first to identify NH and B-1a B cells in the aorta and provide evidence that Id3 is a key regulator of NH cell IL-5 production and B-1a B cell homeostasis.
Keywords: arteriosclerosis, basic helix-loop-helix transcription factors, immune system
Atherosclerosis is a chronic inflammatory disease of the blood vessel wall that can lead to heart attacks and stroke. Despite current therapy targeting traditional risk factors, atherosclerosis remains the leading cause of death in Westernized countries.1 Substantial work during the past several decades clearly establishes a key role for the immune system in the atherosclerosis development and progression.2,3 As such, immune modulation holds promise as an effective addition to current prevention approaches. Thus, a deeper understanding of the contributions of various immune cells to atherogenesis is of key importance. Although abundant evidence implicates macrophages and some T cell subsets in promoting inflammation in the vessel wall,3–6 B cells have emerged as another important immune cell that can modulate atherogenesis. Characterization of atheroprotective antibodies, passive and active immunization studies,7–14 and adoptive transfer studies of splenic B cells from Apoe−/− mice15,16 support an atheroprotective role for B cells in mice. Yet, recent studies suggest that the role of B cells is subset dependent, where B-2 B cells may be atherogenic and B-1a B cells protective.17
A subset of B cells from the B-1 lineage, the B-1a B cell,18,19 has been reported to rescue enhanced atherosclerosis caused by splenectomy. Atheroprotection was demonstrated to be dependent on the ability of the B-1a B cells to secrete IgM natural antibodies (NAbs).20 B-1a B cells are considered part of the innate immune system, develop from fetal tissues, have a high capacity to undergo homeostatic proliferation,18,19,21–24 and spontaneously secrete IgM NAbs.14,20,25 IgM NAbs are present before pathogen exposure, recognize self-antigens for housekeeping functions,26,27 and are thought to be the product of natural selection. A representative IgM NAb, E06, has been reported to be atheroprotective through recognition of oxidation-specific epitopes on oxidized low-density lipoprotein and apoptotic cells, blocking the uptake of oxidized low-density lipoprotein by macrophage scavenger receptors and mediating apoptotic cell clearance.25,26,28–31 Although B-1a B cells are important because of the IgM NAbs they produce, the factors that regulate B-1a B cells in atherosclerosis are poorly understood. Interleukin (IL)-5 is an important atheroprotective cytokine known to promote B-1a B cell proliferation and E06 production.14,32–34 Many cell types, including Th2 T cells and the recently discovered natural helper (NH) cells, produce IL-5. Moreover, NH cells produce large amounts of IL-5 relative to other IL-5–producing cells.35
NH cells belong to an emerging arm of the innate lymphoid cell family, the group 2 innate lymphoid cell helper subset.36–40 NH cells are organized into fat-associated lymphoid clusters in the mesenteric adipose depot. No specific markers have been identified that define NH cells, and they are therefore defined by lineage negative (lin−). They are positive for Sca1, CD117 (c-kit), CD44, CD90, and the IL-33R (T1/ST2).35,41 NH cells produce mainly Th2-associated cytokines in response to a variety of stimuli, including IL-33. Direct evidence with coculture experiments demonstrates that NH cells support B-1 B cell proliferation, similar to IL-5 alone.35
Inhibitor of differentiation 3 (Id3), a helix-loop-helix protein, is a widely expressed dominant-negative regulator of gene transcription that acts through interaction with DNA binding basic helix-loop-helix proteins, such as E-proteins.42 These helix-loop-helix proteins are part of a complex gene-regulatory network of lineage-specific transcription factors that orchestrate lymphocyte development and activation.43–52 Studies suggest that Id3 is also important in human and murine atherosclerosis. In humans, the ID3 gene contains a single nucleotide polymorphism (SNP) at rs11574. This non-synonymous SNP results in an amino acid substitution in the C terminus of the ID3 protein, which attenuates ID3 antagonism of the E-protein, E12. Notably, this SNP is associated with increased carotid intimal media thickness in humans,53 suggesting that loss of Id3 function may promote vascular disease. In murine models, global deletion of Id3 results in increased atherosclerosis in both Ldlr−/− and Apoe−/− mice.53–55 Moreover, Id3 was necessary for B cell–mediated attenuation of atherosclerosis in B cell–deficient/Apoe−/− mice.54,55 Id3 has also been reported to modulate B cell homing and vessel wall adhesion molecule expression,54,55 yet Id3 may also regulate factors involved in innate immunity as loss of Id3 in Apoe−/− mice resulted in early onset of diet-induced atherosclerosis.54 These results raise the interesting hypothesis that Id3 may be an important regulator of B-1a B cells and natural immunity.
The present study demonstrates that Id3 is important for maintenance of splenic and peritoneal cavity (PerC) B-1a B cells and serum levels of E06. However, Id3 regulation of the B-1a B cell pool is because of the loss of Id3 in a non–B cell population as B-1a B cell number were unchanged in mice with B cell–specific loss of Id3. Indeed, loss of Id3 significantly reduced NH cell production of the B-1a B cell mitogen, IL-5. In addition to the mesentery, NH cells are present and produce IL-5 in response to IL-33 stimulation in the aortic adventitia/surrounding perivascular adipose tissue (PVAT). B-1a B cells were also found in the aortic adventitia/surrounding PVAT. These results provide the first evidence that NH cells and B-1a cells are present in the aorta and implicate Id3 as a key regulator of NH cell IL-5 production and B-1a B cell homeostasis.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Id3 Regulates B-1a B Cell Number in Apoe−/− Mice
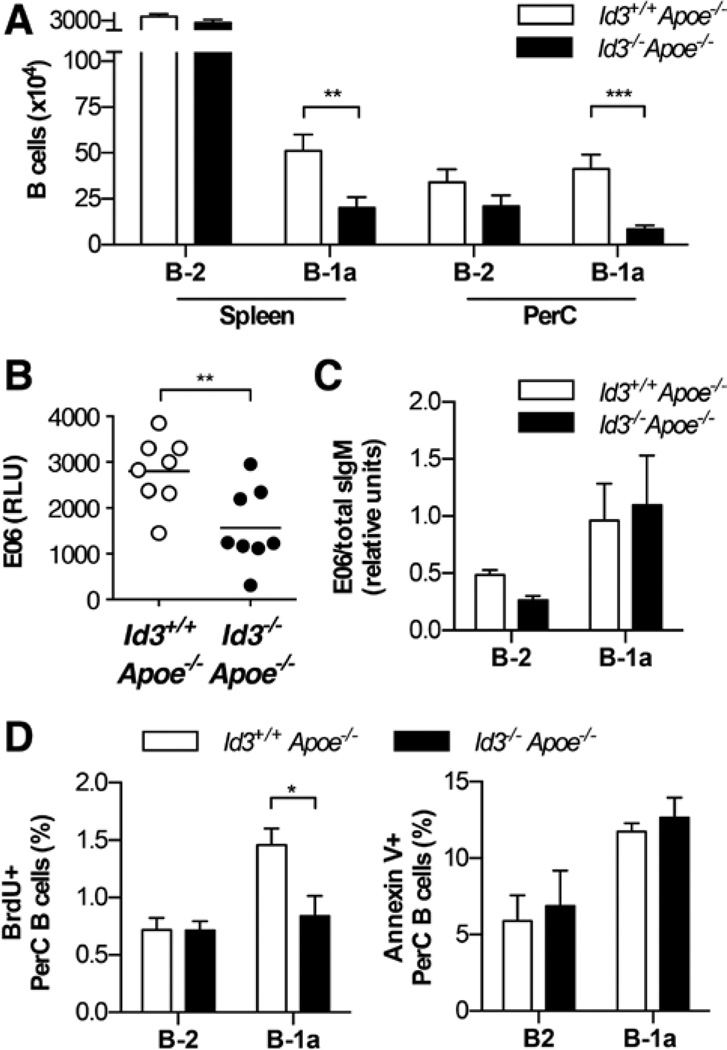
Previous studies demonstrate that B-1a B cells are atheroprotective by producing IgM NAbs.11,14,20,25,56,57 To determine whether Id3 regulates B-1a B cell number in Apoe−/− mice, B-1a B cells in Id3+/+Apoe−/− and Id3−/−Apoe−/− mice were measured by flow cytometry. The gating strategy has been previously described,58,59 and Figure IA in the online-only Data Supplement presents representative flow cytometry plots that identify B-1a B cells (CD19+B220loCD5+CD43+IgMhi) and B-2 B cells (CD19+B220hi) in both the spleen and the PerC. Results reveal significantly fewer B-1a B cells in both the spleen and the PerC in Id3−/−Apoe−/− mice compared with Id3+/+Apoe−/− mice (Figure 1A). This effect was not dependent on hypercholesterolemia, as there was also a decrease in the frequency of B-1a B cells in C57BL/6 mice lacking Id3 (Figure IB in the online-only Data Supplement). In contrast, and consistent with previous reports,49,54 the absolute number of B-2 B cells in the spleen and PerC of Id3−/−Apoe−/− mice was equivalent compared with Id3+/+Apoe−/− mice (Figure 1A). Consistent with the decreased number of B-1a B cells in Id3−/−Apoe−/− mice, we detected less E06 antibody in the serum of 8-week-old Id3−/−Apoe−/− mice compared with control mice (Figure 1B). To determine whether the lower amount of serum E06 antibody is because of reduced production of E06 on a per-cell basis, B-1a B cells were isolated from the PerC by fluorescence-activated cell sorter and analyzed for the amount of mRNA encoding the secreted form of E06 IgM. The gating strategy for fluorescence-activated cell sorter purification of B-1a and B-2 B cells is depicted in Figure II in the online-only Data Supplement. There was no difference in E06 mRNA in B-1a B cells from Id3−/−Apoe−/− compared with Id3+/+Apoe−/− mice (Figure 1C). To determine whether the loss of Id3 altered proliferation of B-1a B cells, bromodeoxyuridine (BrdU) was administered intraperitoneally to Id3+/+Apoe−/− and Id3−/−Apoe−/− mice. PerC cells were harvested 24 hours later, and BrdU incorporation in B-1a and B-2 B cells was determined by flow cytometry. There was a decrease in the proportion of B-1a B cells incorporating BrdU in Id3−/−Apoe−/− compared with Id3+/+Apoe−/− mice (Figure 1D). Consistent with no difference in B-2 B cell number with the loss of Id3, there was no difference in the proportion of B-2 B cells incorporating BrdU. In addition, there was no difference in the proportion of peritoneal B-1a or B-2 B cells that were positive for annexin V, a marker of apoptosis (Figure 1D). These results suggest that the reduced number of B-1a B cells in Id3−/−Apoe−/− mice is because of reduced B-1a B cell proliferation.
Figure 1.
Id3−/−Apoe−/− mice have fewer B-1a B cells in the spleen and peritoneal cavity (PerC) compared with Id3+/+Apoe−/− mice. A, Number of B-2 B cells (CD19+B220hi) and B-1a (CD19+B220loCD5+CD43+IgMhi) in the spleen and PerC of Id3+/+Apoe−/− (n=13) or Id3−/−Apoe−/− (n=12) mice at 8 weeks as measured by flow cytometry. B, E06 levels in the serum of Id3+/+Apoe−/− or Id3−/−Apoe−/− mice as measured by ELISA. C, E06 sIgM transcript levels in peritoneal B cell subsets, B-1a (CD19+B220lo CD5+) and B-2 (CD19+B220hi) B cells, measured by real-time polymerase chain reaction and normalized to total sIgM. D, Bromodeoxyuridine (BrdU) incorporation and annexin V staining in PerC B cell subsets of Id3+/+Apoe−/− (n=6 or 7) or Id3−/−Apoe−/− (n=6 or 7) mice from 3 independent experiments. *P<0.05, **P<0.01, and ***P<0.001.
Id3 has been shown to be necessary for protection from atherosclerosis in Apoe−/− mice.53,54 To investigate whether the global loss of Id3 resulted in alterations of other immune cell populations important in atherosclerosis, flow cytometry was performed for splenic CD4+ T cells, CD4+Foxp3+ regulatory T cells, CD8+ T cells, and peritoneal CD4+ T cells, CD8+ T cells, and F4/80+ macrophages in Id3−/−Apoe−/− and Id3+/+Apoe−/− mice. No differences were observed in the proportions of these populations (data not shown). Cholesterol levels also affect atherogenesis,2 and there was no difference in the serum lipid profiles of Id3−/−Apoe−/− mice compared with control mice (Table I in the online-only Data Supplement) consistent with previous observations.54
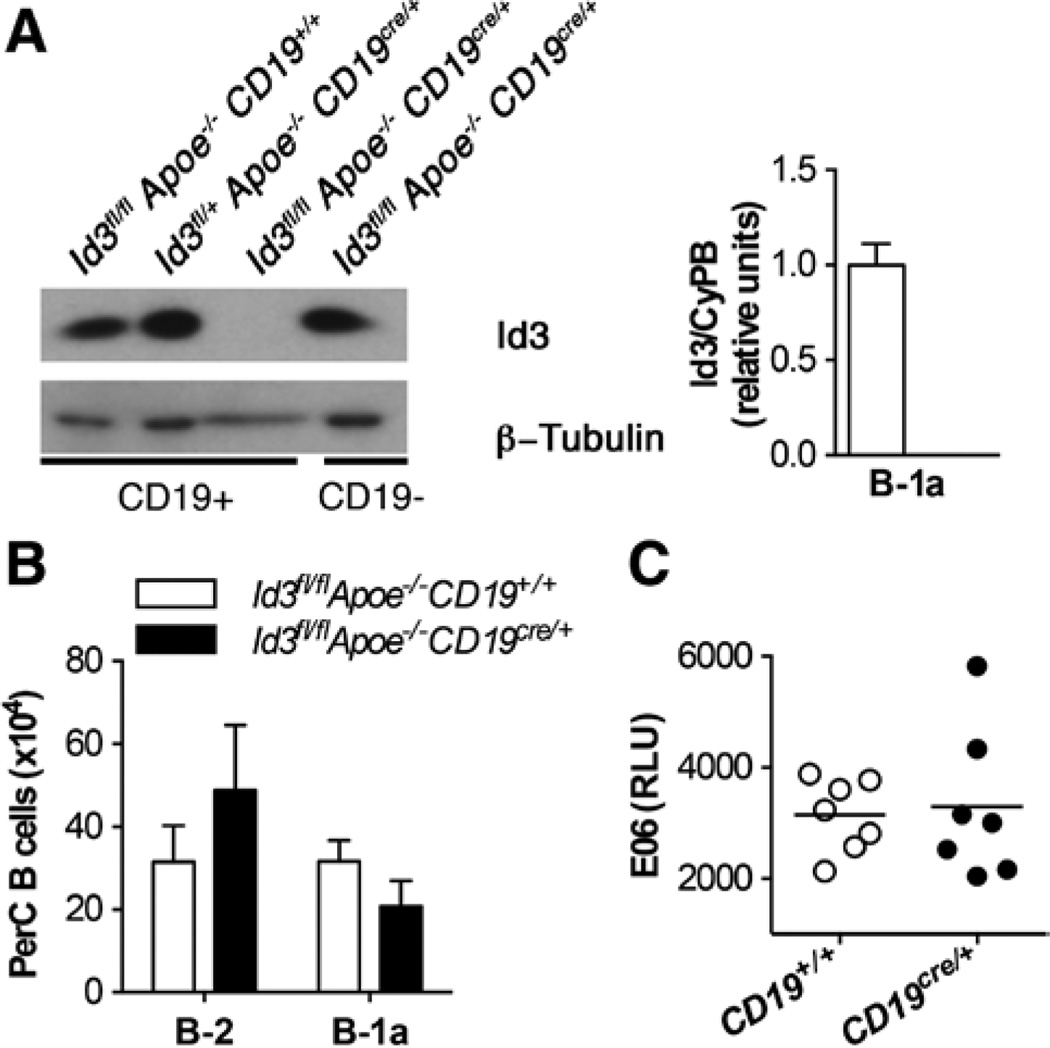
To determine whether the reduced number of B-1a B cells in Id3−/−Apoe−/− mice was because of the loss of Id3 in B cells, Id3 was specifically deleted in B cells on the Apoe−/− background. These mice were generated by first crossing a floxed Id3 allele onto the Apoe−/− background to produce Id3fl/flApoe−/− mice. These mice were then crossed with Apoe−/−CD19cre/+ mice to produce Id3fl/flApoe−/−CD19cre/+ and Id3fl/flApoe−/ −CD19+/+ control mice. Western blotting of lysates from splenic B cells demonstrated the inability to detect Id3 protein in B cells from Id3fl/flApoe−/−CD19cre/+ mice compared with controls (Figure 2A). Furthermore, transcript levels of Id3 were not detectable by real-time polymerase chain reaction in sorted B-1a B cells from Id3fl/flApoe−/−CD19cre/+ mice (Figure 2A). Consistent with results in the Id3−/−Apoe−/− mice, Id3fl/flApoe−/−CD19cre/+ mice contained an equivalent number of B-2 B cells compared with control mice (Figure 2B). However, in contrast Id3−/−Apoe−/− mice, despite the loss of Id3, the number of PerC B-1a B cells detected in Id3fl/flApoe−/−CD19cre/+ mice was equivalent to that detected in control mice. Furthermore, the amount of EO6 antibody detected in the serum was also the same (Figure 2C). These results suggest that Id3 regulates B-1a B cell number through effects in a cell type other than B cells.
Figure 2.
Generation of a B cell–specific knockout of inhibitor of differentiation 3 (Id3) reveals that the loss of Id3 in B cells does not result in altered numbers of B-1a B cells or E06 levels. A, Left, Id3 and β-tubulin protein in total splenic B cells from Id3fl/flApoe−/−CD19+/+, Id3fl/+Apoe−/−CD19Cre/+, Id3fl/flApoe−/−CD19Cre/+ and Id3fl/fl, Apoe−/−, CD19Cre/+ mice. Right, Peritoneal lavage cells were sorted from Id3fl/flApoe−/−CD19+/+ (open bar) or Id3fl/flApoe−/−CD19Cre/+ (closed bar) mice for B-1a (CD19+B220lo CD5+) cells as in Figure II in the online-only Data Supplement, and Id3 transcript was measured by real-time polymerase chain reaction and normalized to a housekeeping gene, cyclophilin b (CypB). B, Quantification of peritoneal B-2 and B-1a B cell subsets by flow cytometry in Id3fl/flApoe−/−CD19+/+ (n=10) or Id3fl/flApoe−/−CD19cre/+ (n=11) mice at 8 weeks. C, Serum levels of E06 in Id3fl/flApoe−/−CD19+/+ (open circles) or Id3fl/flApoe−/−CD19cre/+ (closed circles) mice were determined by ELISA. Values are the mean relative light units of duplicate determinations.
Loss of Id3 Markedly Attenuates IL-33–Induced IL-5 Production
IL-5 is an IL-33–induced Th2 cytokine critically important for homeostatic proliferation and survival of B-1a B cells. To determine whether Id3 is necessary for IL-33–induced production of IL-5, Id3+/+Apoe−/− and Id3−/−Apoe−/− mice were treated with PBS vehicle control or IL-33, and serum and peritoneal fluid IL-5 levels were measured by a high-sensitivity ELISA. As presented in Figure 3A, IL-33 induced abundant IL-5 in the serum and peritoneal fluid, and this effect was abolished in mice lacking Id3. IL-5 was undetectable in the serum and peritoneal fluid of PBS-treated Apoe−/− mice, consistent with previous reports35 (Figure 3A). Because Id3−/−Apoe−/− mice had a markedly attenuated IL-33–induced IL-5 response and reduced homeostatic proliferation of B-1a B cells, we next sought to determine whether the delivery of exogenous IL-5 could increase the proliferation of PerC B-1a B cells in Id3−/−Apoe−/− mice. Id3−/−Apoe−/− mice were injected with IL-5 intraperitoneally every day for 5 days, and on the last day BrdU was coinjected. Consistent with earlier findings (Figure 1D), there was a smaller percentage of BrdU+ B-1a B cells in PBS control–injected Id3−/−Apoe−/− mice compared with Id3+/+Apoe−/− mice. Moreover, exogenous IL-5 increased the proportion of BrdU+ B-1a B cells in Id3−/−Apoe−/− mice compared with PBS control (Figure 3B).
Figure 3.
Inhibitor of differentiation 3 (Id3) is necessary for interleukin (IL)-33–induced IL-5 levels. A, IL-33 or PBS vehicle control was administered intraperitoneally to Id3+/+Apoe−/− (PBS group, n=7; IL-33, n=5) or Id3−/−Apoe−/− (PBS group, n=7; IL-33, n=6) mice every 2 days 3× in 4 independent experiments. On day 7, serum and peritoneal fluid were harvested and IL-5 levels were determined by ELISA. Values were considered not detectable (n.d.) if below the sensitivity of the assay (≤1.56 pg/mL). B, Id3+/+Apoe−/− or Id3−/−Apoe−/− mice were administered PBS vehicle control and IL-5 for 5 days, including BrdU on day 5. *P<0.05, **P<0.001, and ****P<0.0001.
Id3 Regulates NH Cell Production of IL-5
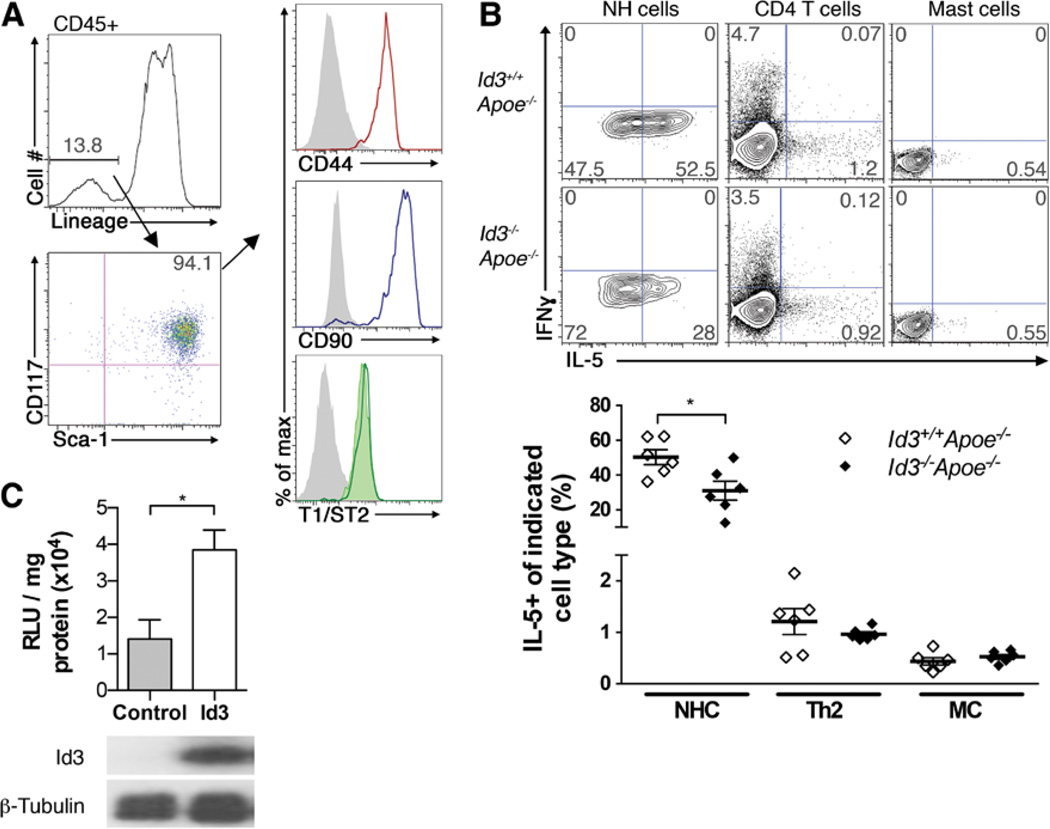
Previous studies have identified NH cells as the predominant source of IL-33–induced IL-5 production in vivo, and coculture experiments demonstrated that NH cells can directly support B-1 B cell proliferation.33,35 To determine whether Id3−/−Apoe−/− mice had lower IL-33–induced IL-5 serum and peritoneal fluid levels because of fewer total number of NH cells, flow cytometry was performed to quantify NH cells in the mesentery. NH cells were identified as CD45+, lineage negative (Lin−: CD3ε, CD4, CD5, CD8α, CD11b, CD11c, CD19, CD45R/B220, FcεR1α, Ly6G, NK1.1, TCRβ, TCRγδ, and Ter119) Sca1+ CD117+ T1/ST2+ CD90+ and CD44+ cells35 (Figure 4A). In contrast to mice that are null for Id2 and lack NH cells,35 Id3−/−Apoe−/− mice contain a similar number of NH cells compared with Id3+/+Apoe−/− mice (Figure IIIA in the online-only Data Supplement). In addition, the lower IL-33–induced IL-5 serum levels were not because of Id3 regulation of the IL-33 receptor, T1/ST2, on NH cells as Id3−/−Apoe−/− mice had similar expression of NH cell T1/ST2 compared with control mice (Figure 4A). There was also no difference in levels of IL-33 in the peritoneal fluid of Id3−/−Apoe−/− mice compared with control mice (21.78±10.01 versus 29.11±8.18 pg/mL, respectively; n=6), and serum levels were below the sensitivity of the assay (≤2.39 pg/mL). To determine whether Id3 regulates production of IL-5 by a specific Th2 cytokine–producing cell, flow cytometry with intracellular cytokine staining for IL-5 was performed on isolated cells cultured with IL-33. Consistent with previous reports,35 the percentage of NH cells producing IL-5 is markedly higher than other Th2 cytokine–producing cells, such as polarized CD4+ T cells and bone-marrow–derived mast cells (Figure 4B). Intracellular cytokine staining of IL-5 in IL-33–treated basophils or eosinophils was not detected (data not shown). Furthermore, 94.5%, on average, of IL-5–producing cells in the fat-associated lymphoid clusters were NH cells. Interestingly, there was a 50% reduction in the percentage of NH cells that produce IL-5 in response to IL-33 in Id3−/−Apoe−/− mice compared with NH cells isolated from control Id3+/+Apoe−/− mice. Consistent with previous data,35 NH cells do not produce detectable interferon-γ. Loss of Id3 did not alter IL-5 production in CD4+ T cells or bone-marrow–derived mast cells, other cell types known to produce IL-5 (Figure 4B).
Figure 4.
Inhibitor of differentiation 3 (Id3) regulates interleukin (IL)-5 production in natural helper cells (NHCs). A, Representative flow cytometry for the gating strategy of fat-associated lymphoid cluster NHCs. Live, CD45+ cells were gated for lineage-negative markers, then for Sca1+ CD117+ cells. CD44, CD90, and T1/ST2 expression on Sca1+ CD117+ cells from Id3+/+Apoe−/− mice (open histograms) or T1/ ST2 from Id3−/−Apoe−/− mice (green-shaded histogram). Fluorescence-minus-one control, gray-shaded histograms. B, Top, Representative flow cytometry of NH cells, CD4+ T cells, and mast cells (MC) from Id3+/+Apoe−/− or Id3−/−Apoe−/− mice stained for intracellular interferon-γ (IFNγ) and IL-5 after IL-33 stimulation and (bottom) quantification of IL-5 production. Results are expressed as individual mice from 2 independent experiments performed in duplicate. C, Luciferase activity (top) and protein expression of Id3 and β-tubulin (bottom) in lymphoid cells cotransfected with IL-5 promoter-reporter and either empty vector (control) or Id3. Data are from 3 independent experiments performed in duplicate. *P<0.05.
Id3 is known to both activate and antagonize gene expression through the regulation of promoter elements termed E-boxes. The IL-5 promoter contains several of these E-box elements. To determine whether Id3 regulates the IL-5 promoter, an IL-5 luciferase promoter-reporter, pLS-IL5, was cotransfected with control empty vector (pEF4) or Id3 (pEF4-Id3) in a lymphoid cell line. As shown in Figure 4C, Id3 significantly increases IL-5 promoter activity above control empty vector. Western blots confirm Id3 protein overexpression. Together, results demonstrate that Id3 regulates IL-5 expression in lymphoid cells and in particular, NH cells.
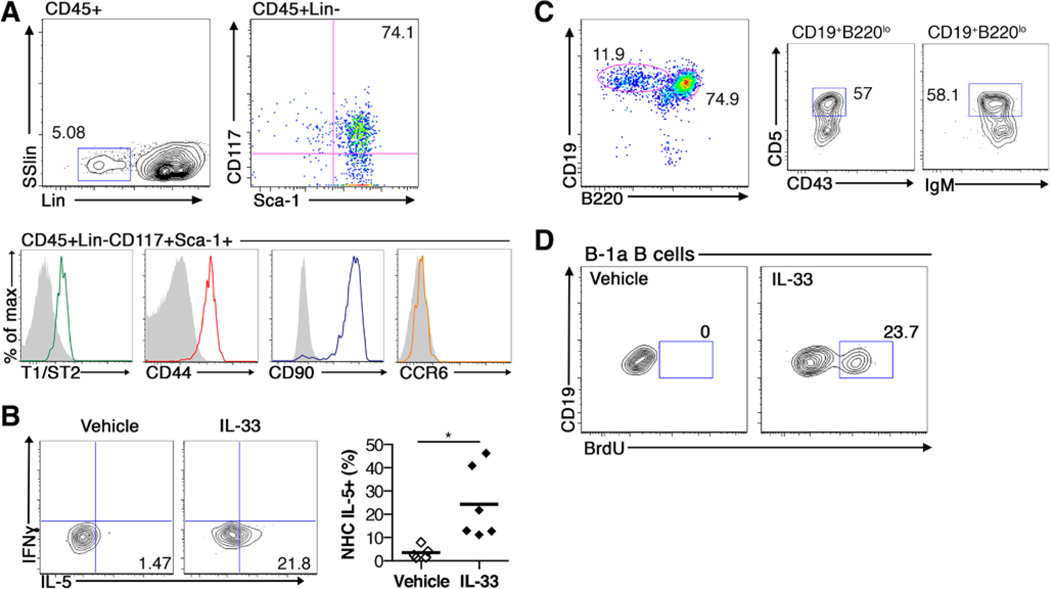
IL-5–Producing NH Cells and B-1a B Cells Are Present in the Perivascular Aortic Adipose Tissue
NH cells reside in fat-associated lymphoid clusters in the mesenteric adipose depot. The PVAT also contains immune cells, is intimately associated with the adventitial layer of the vessel wall, and has been implicated in regulating atherogenesis.5,60 B cells are known to reside in the adventitial layer of vessels and the presence of B cells in the adventitia has been associated with both atherogenesis61 and atheroprotection.54 However, whether NH cells and B-1a B cells are present in periaortic adipose tissue and adventitia is unknown. To determine whether NH cells reside in periaortic adipose tissue, flow cytometry for NH cells was performed on cells isolated from whole aortas including the adjacent adventitia and PVAT from chow-fed, 8- to 10-week-old Apoe−/− mice. Indeed, a population of NH cells, identified as CD45+ Lin− Sca1+ CD117+ cells, was present in whole aortas of Apoe−/− mice (Figure 5A). Similar to the mesentery, the number of aortic NH cells was not different with the loss of Id3 (Figure IIIB in the online-only Data Supplement). Aortic NH cells expressed markers such as CD44, CD90, and the IL-33 receptor (T1/ST2) comparable with mesenteric NH cells. Similar to NH cells in the lung, intestines, and mesentery, these NH cells did not express C-C chemokine receptor 6.41 To determine whether aortic NH cells produce IL-5 in response to IL-33 stimulation in vivo, Apoe−/− mice were treated with PBS vehicle control or IL-33 every 2 days and euthanized on day 7. Flow cytometry analysis of whole aortas revealed IL-33–induced IL-5 expression in aortic NH cells (Figure 5B). NH cells have been shown to directly support B-1 B cell proliferation.35 To determine whether B-1a B cells also reside in and around the aorta of mice before atheroma development, flow cytometry was performed on aortas including the periaortic adventitia and PVAT for B cell subsets. B-1a cells were present in the whole aorta (Figure 5C). To determine whether IL-33 can induce B-1a B cell proliferation in the aorta, Apoe−/− mice were treated with PBS vehicle control or IL-33 every 2 days with BrdU coinjected for the final treatment and euthanized on day 7. Flow cytometry analysis of whole aortas revealed IL-33–induced BrdU incorporation in aortic B-1a B cells (Figure 5D). These data are the first to identify NH cells and B-1a B cells in the aortic adventitia/PVAT and provide evidence that IL-33 can induce aortic NH cells to increase local IL-5 production and B-1a B cell proliferation.
Figure 5.
Natural helper cells are present in the aorta, including periaortic adventitia and perivascular adipose tissue (PVAT), and produce interleukin (IL)-5. Whole aortas containing the adventitia and PVAT were isolated from Apoe−/− mice. A, Representative flow cytometry plots of natural helper (NH) cells (CD45+, lineage negative, CD117+, Sca1+). B, Left, Interferon-γ (IFNγ) and IL-5 intracellular staining in aortic NH cells and right, quantification of the percentage of NH cells expressing IL-5 (n=6 in each group) after vehicle or IL-33 treatment. Representative flow cytometry plots of (C) B cell subsets, B-2 (CD19+B220hi), and B-1a (CD19+B220loCD5+CD43+IgMhi) and (D) BrdU incorporation in aortic B-1a B cells after vehicle or IL-33 treatment. Numbers on flow cytometry plots indicate the percentage of the population of interest. *P<0.05. SSlin indicates side scatter linear.
Discussion
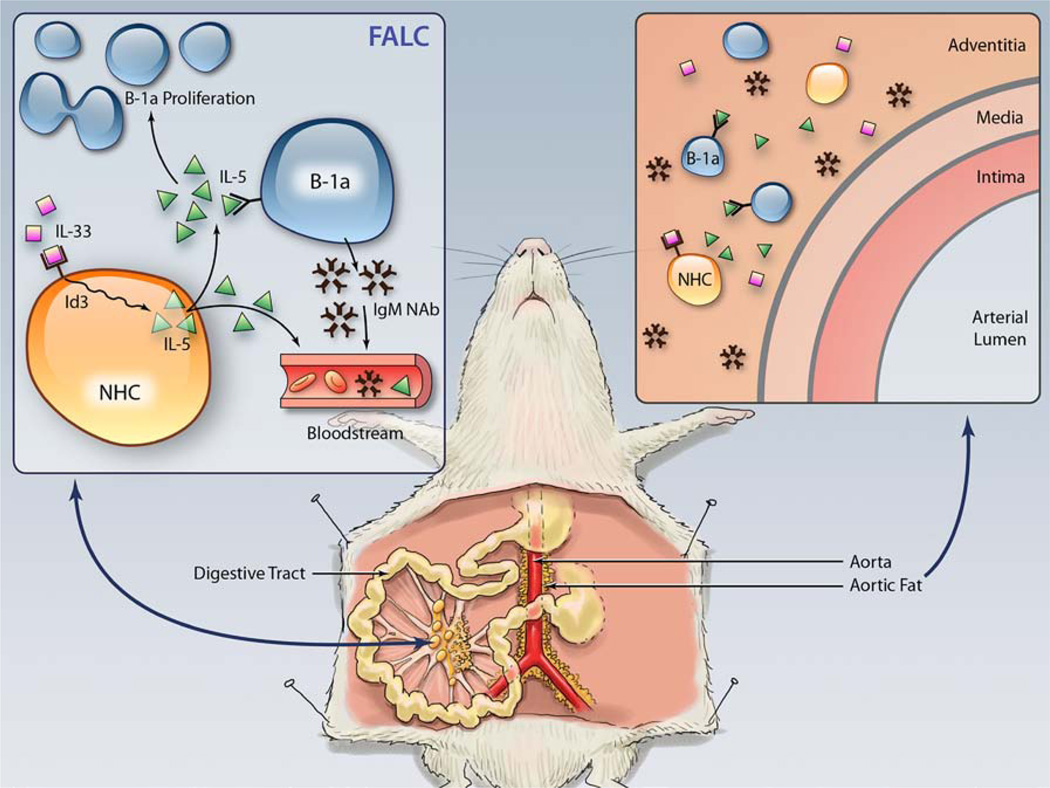
The present study provides evidence that Id3 is a key mediator of natural immunity and the IL-33/IL-5 pathway using a genetic model of early and accelerated atherosclerosis, the Apoe−/− mouse null for Id3.53,54 First, we show reduced numbers and proliferation of B-1a B cells and lower serum levels of an IgM NAb, E06, in Id3−/−Apoe−/− mice. However, when Id3 is deleted only in B cells, there is no effect on B-1a B cell number or E06 serum levels, suggesting that Id3 regulates the number of B-1a B cells by a mechanism that involves a non–B cell. Second, we show that IL-33–induced IL-5 levels are attenuated in Id3−/−Apoe−/− mice, and administration of IL-5 can rescue the B-1a B cell proliferation defect in Id3−/−Apoe−/− mice. Third, we show reduced production of IL-5 in IL-33–treated NH cells isolated from Id3−/−Apoe−/− mice. We also demonstrate by flow cytometry that the aorta, including the adventitia and PVAT, contain B-1a B cells and NH cells. Aortic NH cells can be stimulated to produce the IL-5 that may maintain B-1a B cell proliferation. Thus, our data identify Id3 as a key regulator of NH cell production of the atheroprotective cytokine IL-5 that promotes B-1a B cell proliferation (Figure 6), linking this event to natural immune protection from atherosclerosis.
Figure 6.
Inhibitor of differentiation 3 (Id3) is a key regulator of natural helper (NH) cell interleukin (IL)-5 production and B-1a B cell proliferation, and NH cells may be important in innate protection from atherosclerosis. IL-33 stimulates fat-associated lymphoid cluster (FALC) resident NH cells to produce IL-5, which is dependent on Id3. IL-5 stimulates B-1a B cell proliferation and IgM natural antibody (NAb) production, an atheroprotective process. IL-5 and IgM NAb can enter the blood-stream. Aorta/perivascular adipose tissue resident NH cells can be stimulated by IL-33 to produce IL-5. This local process may stimulate resident atheroprotective B-1a cells.
Natural IgM antibodies, such as E06, and the B-1a B cells that produce these antibodies have been reported to attenuate diet-induced atherosclerosis.7,8,11,14,20,25,26,29,31,56,57 Kyaw et al20 demonstrated a significant reduction in the number of PerC B-1a B cells and levels of serum IgM antibodies that bind modified lipids after splenectomy, which was associated with enhanced atherosclerosis in Apoe−/− mice. Moreover, adoptive transfer of B-1a B cells was shown to rescue splenectomy-induced atherosclerosis, an effect dependent on the ability for B-1a B cells to secrete IgM.20 Here, we provide evidence that Id3 may be a key factor for maintaining normal numbers of B-1a B cells and levels of E06 in the serum, suggesting that Id3 may be important in B-1a B cell–mediated atheroprotection. However, B cell–specific deletion of Id3 did not have reduced number of B-1a B cells or levels of E06 in the serum compared with controls, suggesting that it is the loss of Id3 in a non–B cell that results in altered B-1a B cell homeostatic maintenance in Id3−/−Apoe−/− mice.
Results herein demonstrate that Id3 regulates IL-33–induced systemic levels of IL-5. Consistent with IL-5’s known ability to promote homeostatic proliferation of B-1a B cells,33 administration of exogenous IL-5 rescued B-1a B cell proliferation in Id3-deficient mice. Similar to Id3,53,54 both IL-33 and IL-5 attenuate atherosclerosis.14,62,63 Treatment with IL-33 increased serum levels of IL-5 and oxidation-specific IgM antibodies and reduced lesion size in Apoe−/− mice.62,63 In addition, Ldlr−/− mice reconstituted with Il5−/− bone marrow had decreased amounts of plasma E06 antibody and increased atherosclerosis compared with controls.14 In the present study, we provide evidence that Id3 regulates levels of IL-5, thereby mediating this important IL-33/IL-5 atheroprotective pathway.
Id3 regulates the production of IL-5 by the recently identified innate lymphoid cell, the NH cell.35 Importantly, Id3 is not required for the development of NH cells, unlike Id2.35 NH cells produce large amounts of IL-5 in response to IL-33 relative to Th2 cells, basophils, invariant natural killer T cells, mast cells, and CD43+ cells.35,64,65 Results of the present study support these findings in that IL-33 stimulated 50% of NH cells to produce IL-5, whereas <2% of Th2 or mast cells were IL-5 producing. In this study, we did not detect any basophils and eosinophils producing IL-5 in response to IL-33. Moreover, of these cell types, only NH cells showed reduced percentage of IL-33–stimulated IL-5–producing cells in Apoe−/− mice with deletion of Id3. Of note, differences in the intracellular staining for IL-5 in NH cells harvested from Id3−/−Apoe−/− mice compared with control mice were less marked than differences in the serum and peritoneal fluid, raising the interesting possibility that there may be an additional, yet unidentified, cell type producing IL-5 in an Id3-dependent manner. Although NH cell–specific deletion of Id3 would help address this question, this is not feasible as there is no lineage-specific marker for NH cells. Moreover, it is more likely that measuring intracellular levels of this secreted protein under-represents the effects of Id3 on total production of IL-5, including that which has already been secreted. Nevertheless, results clearly demonstrate that Id3 regulates NH cell production of IL-5 in response to IL-33.
Although lymphocytes have long been identified in human and murine aortic adventitia,66,67 recent studies provide evidence that adventitial lymphocyte activation, including B cells, may be important in regulating atherosclerosis.54,55,61,68–70 Data from this study suggest that adventitial/PVAT B-1a B cells and NH cells present before Western diet initiation may be part of the local IL-33/IL-5 pathway poised to protect from atherogenesis. Aortic NH cells express markers identical to fat-associated lymphoid clusters NH cells including the IL-33 receptor. IL-33 is produced by cells in the aortic adventitia,62 which may provide a local stimulus for NH cell production of IL-5. Exogenous IL-33 induced IL-5 production by NH cells and proliferation of aortic B-1a B cells in vivo, suggesting that NH cell–derived IL-5 may function to support proliferation of the resident B-1a B cells. These B-1a B cells could produce E06 or other natural IgM antibodies in the adventitia/PVAT.71 Although further study is needed to more fully understand the role of innate-like lymphocytes in the aortic adventitia and surrounding PVAT, results herein demonstrate the existence of functional aortic NH cells.
The present study is the first to demonstrate Id3 regulation of the IL-33/IL-5 pathway and natural immunity. As we have clearly shown that loss of Id3 leads to early atherosclerosis in mice,54,55 this may be an important mechanism whereby Id3 promotes innate protection from atherosclerosis. Prior studies demonstrated that loss of Id3 led to decreased B cell homing to the aorta54,55 and increased intimal adhesion molecule expression55 in atheroprone mouse models. In addition, it is interesting to speculate that Id3 may also provide atheroprotection by regulating regulatory T cells. Regulatory T cells have been shown to be atheroprotective,4 and Id3 has been implicated in the promotion of regulatory T cell development in neonates47; although consistent with previous data,47 we did not observe a difference in the frequency of regulatory T cells with the loss of Id3. There may be other, as yet unidentified, mechanisms whereby Id3 may regulate atheroprotection. The fact that a single SNP in a single gene (ID3) is associated with carotid intimal medial thickness in humans would support a role for Id3 in regulating pathways in many cell types involved in atherosclerosis. Although the SNP does not result in reduced expression of Id3 protein, as seen with a knockout mouse, it does result in decreased Id3 binding to its basic helix-loop-helix partner, E12, which markedly attenuates Id3 function.53 Therefore, identification of specific Id3-mediated atheroprotective pathways, such as the IL-33/IL-5 axis, in mice has the potential to lead to interesting hypotheses that can be tested in humans, such as whether humans with polymorphism at rs11574 have alterations in natural immunity that could be linked to premature atherosclerosis. Such findings may identify individuals amenable to novel prevention or treatment approaches.
Supplementary Material
Significance.
Natural immunity is emerging as a protective process in atherosclerosis. Natural IgM antibodies that recognize oxidative epitopes on low-density lipoprotein or phospholipids prevent atherosclerosis and are produced by the atheroprotective B cell subset, the B-1a B cell. We previously demonstrated that splenic B cells provide atheroprotection to B cell–deficient mice, an effect dependent on the helix-loop-helix transcription factor, inhibitor of differentiation 3 (Id3). Here, we identify Id3 as an important regulator of B-1a B cells and natural immunity. The loss of Id3 results in reduced B-1a B cells and serum levels of natural IgM antibodies, an effect not attributable to the loss of Id3 in B cells. We found that Id3 is necessary for the production of interleukin-5, an important B-1a B cell mitogen. Interleukin-5 can be abundantly produced by the innate lymphoid cell, the natural helper cell. Indeed, Id3 was important for natural helper cell production of interleukin-5. Results are the first to implicate Id3 as a key regulator of natural helper cell function and atheroprotective B-1a B cell proliferation.
Acknowledgments
We acknowledge Jim Garmey, Jeff Bergen, and Stacey Gorski for technical advice and assistance; Ben Smith for the artistic illustration; Dr Yuan Zhuang (Duke University) for providing Id3fl/fl mice; and Dr Loren Erickson for advice on B-1 cell biology and the UVa Flow Cytometry core for their continued support.
Sources of Funding
This work was supported by National Institutes of Health (NIH) P01 HL55798 (C.A. McNamara), NIH R01 HL096447 (C.A. McNamara), NIH R01 HL107490 (C.A. McNamara) R01 HL086559 (J.L. Witztum and S. Tsimikas), P01 HL088093 (J.L. Witztum and S. Tsimikas), NIH American Recovery and Reinvestment Act HL096447-01A1 (S. Tsimikas), American Heart Association Pre-Doctoral Fellowship (H.M. Perry), and Pharmacological Sciences Training Grant T32 GM07055-35 (H.M. Perry).
Nonstandard Abbreviations and Acronyms
- Id3
inhibitor of differentiation 3
- NAb
natural antibody
- NH cell
natural helper cell
- PerC
peritoneal cavity
- PVAT
perivascular adipose tissue
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302571/-/DC1.
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 5.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012;110:889–900. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 9.Nicoletti A, Kaveri S, Caligiuri G, Bariéty J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest. 1998;102:910–918. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 11.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 12.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Ameli S, Hultgårdh-Nilsson A, Regnström J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1996;16:1074–1079. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 14.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 17.Perry HM, Bender TP, McNamara CA. B cell subsets in atherosclerosis. Front Immunol. 2012;3:373. doi: 10.3389/fimmu.2012.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 20.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 21.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39:2383–2394. doi: 10.1002/eji.200838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci USA. 2011;108:13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 28.Bird DA, Gillotte KL, Hörkkö S, Friedman P, Dennis EA, Witztum JL, Steinberg D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci USA. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson LD, Foy TM, Waldschmidt TJ. Murine B1 B cells require IL-5 for optimal T cell-dependent activation. J Immunol. 2001;166:1531–1539. doi: 10.4049/jimmunol.166.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172:6020–6029. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Köhler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 35.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 36.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 37.Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol. 2012;24:284–289. doi: 10.1016/j.coi.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mjösberg J, Bernink J, Peters C, Spits H. Transcriptional control of innate lymphoid cells. Eur J Immunol. 2012;42:1916–1923. doi: 10.1002/eji.201242639. [DOI] [PubMed] [Google Scholar]
- 39.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 40.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 43.Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, Wang E, Schrump DS, Marincola FM, Restifo NP, Gattinoni L. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones-Mason ME, Zhao X, Kappes D, Lasorella A, Iavarone A, Zhuang Y. E protein transcription factors are required for the development of CD4(+) lineage T cells. Immunity. 2012;36:348–361. doi: 10.1016/j.immuni.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zúñiga-Pflücker JC, Wiest DL. Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Maruyama T, Zhang P, Konkel JE, Hoffman V, Zamarron B, Chen W. Mutation of inhibitory helix-loop-helix protein Id3 causes γδ T-cell lymphoma in mice. Blood. 2010;116:5615–5621. doi: 10.1182/blood-2010-03-274506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, Zhuang Y, Gutkind JS, Chen W. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, Murre C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat Immunol. 2011;12:992–1001. doi: 10.1038/ni.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19:5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D’Cruz LM, Watowich SS, Murre C, Goldrath AW. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, Ideker T, Glass CK, Murre C. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 53.Doran AC, Lehtinen AB, Meller N, Lipinski MJ, Slayton RP, Oldham SN, Skaflen MD, Yeboah J, Rich SS, Bowden DW, McNamara CA. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circ Res. 2010;106:1303–1311. doi: 10.1161/CIRCRESAHA.109.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doran AC, Lipinski MJ, Oldham SN, et al. B-cell aortic homing and atheroprotection depend on Id3. Circ Res. 2012;110:e1–e12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipinski MJ, Campbell KA, Duong SQ, Welch TJ, Garmey JC, Doran AC, Skaflen MD, Oldham SN, Kelly KA, McNamara CA. Loss of Id3 increases VCAM-1 expression, macrophage accumulation, and atherogenesis in Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2012;32:2855–2861. doi: 10.1161/ATVBAHA.112.300352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fahl SP, Crittenden RB, Allman D, Bender TP. c-Myb is required for pro-B cell differentiation. J Immunol. 2009;183:5582–5592. doi: 10.4049/jimmunol.0901187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 60.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gräbner R, Lötzer K, Döpping S, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller AM, Liew FY. The IL-33/ST2 pathway–A new therapeutic target in cardiovascular disease. Pharmacol Ther. 2011;131:179–186. doi: 10.1016/j.pharmthera.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 65.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz CJ, Mitchell JR. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- 67.Zhou X, Hansson GK. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand J Immunol. 1999;50:25–30. doi: 10.1046/j.1365-3083.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 68.Hamze M, Desmetz C, Berthe ML, Roger P, Boulle N, Brancherau P, Picard E, Guzman C, Tolza C, Guglielmi P. Characterization of resident B cells of vascular walls in human atherosclerotic patients. J Immunol. 2013;191:3006–3016. doi: 10.4049/jimmunol.1202870. [DOI] [PubMed] [Google Scholar]
- 69.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huan T, Zhang B, Wang Z, et al. Coronary ARteryDIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) Consortium, International Consortium for Blood Pressure GWAS (ICBP). A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1427–1434. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welch CL, Bretschger S, Latib N, Bezouevski M, Guo Y, Pleskac N, Liang CP, Barlow C, Dansky H, Breslow JL, Tall AR. Localization of atherosclerosis susceptibility loci to chromosomes 4 and 6 using the Ldlr knockout mouse model. Proc Natl Acad Sci USA. 2001;98:7946–7951. doi: 10.1073/pnas.141239098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.