Abstract
Cell-mediated immunity depends in part on appropriate migration and localization of cytotoxic T lymphocytes (CTL), a process regulated by chemokines and adhesion molecules. Many viruses, including human immunodeficiency virus type 1 (HIV-1), encode chemotactically active proteins, suggesting that dysregulation of immune cell trafficking may be a strategy for immune evasion. HIV-1 gp120, a retroviral envelope protein, has been shown to act as a T-cell chemoattractant via binding to the chemokine receptor and HIV-1 coreceptor CXCR4. We have previously shown that T cells move away from the chemokine stromal cell-derived factor 1 (SDF-1) in a concentration-dependent and CXCR4 receptor-mediated manner. Here, we demonstrate that CXCR4-binding HIV-1 X4 gp120 causes the movement of T cells, including HIV-specific CTL, away from high concentrations of the viral protein. This migratory response is CD4 independent and inhibited by anti-CXCR4 antibodies and pertussis toxin. Additionally, the expression of X4 gp120 by target cells reduces CTL efficacy in an in vitro system designed to account for the effect of cell migration on the ability of CTL to kill their target cells. Recombinant X4 gp120 also significantly reduced antigen-specific T-cell infiltration at a site of antigen challenge in vivo. The repellant activity of HIV-1 gp120 on immune cells in vitro and in vivo was shown to be dependent on the V2 and V3 loops of HIV-1 gp120. These data suggest that the active movement of T cells away from CXCR4-binding HIV-1 gp120, which we previously termed fugetaxis, may provide a novel mechanism by which HIV-1 evades challenge by immune effector cells in vivo.
An effective host immune response requires the active movement of leukocytes (8, 39). After migrating to a site of infection, effector cells including cytotoxic T lymphocytes (CTL) must make contact with appropriate target cells, recognize them, and induce lysis. Chemokines, a superfamily of 8- to 10-kDa proteins, are intimately involved in the orchestration of this complex process (20, 39). Many intracellular pathogens, including the human immunodeficiency virus type 1 (HIV-1), elaborate proteins and chemokine receptor homologues that interfere with cell movement (3, 4, 72). The HIV-1 proteins Nef and Tat and the envelope protein gp120 have been shown to influence T-cell and dendritic cell migration (2, 29, 62). The HIV-1 envelope also binds the chemokine receptors CXCR4 and CCR5 in order to gain entry into host CD4+ T cells. The effects of gp120 binding to chemokine receptors on CD4-negative cells such as CTL are incompletely understood (24).
Increasing evidence points to the central role that CTL play in the control of HIV-1 infection (13, 30, 34, 54). Most HIV-infected persons, in the absence of treatment, control viral replication transiently before progression to AIDS despite the presence of robust CTL responses (1, 21, 49). The question of why HIV-specific CTL are necessary but not sufficient to prevent disease progression remains unanswered and is the focus of intense study. Furthermore, virus-specific CTL responses documented in vitro do not always correlate with effective effector responses in vivo for many viruses that establish chronic infection in humans (23, 40, 46, 66). HIV-1 employs numerous mechanisms in order to evade the cell-mediated arm of the host immune response. These mechanisms include infection and elimination of HIV-specific CD4+-T-helper cells, viral mutational escape from immunodominant CTL epitopes, and downregulation of class I major histocompatibility complex molecules by Nef (12, 31, 37, 38, 42).
Dysregulation of virus-specific CTL colocalization with infected cells has been described for HIV and simian immunodeficiency virus (SIV) infection. In SIV infection, the total numbers and proliferative capacity of virus-specific CTL are decreased in lymphoid tissue compared to blood (35, 41). Additionally, SIV-infected monkeys have demonstrated a significant accumulation of virus-specific CTL in the liver without a concomitant focus of viral replication (55). In primary, untreated HIV-1 infection, a significant number of HIV-specific CTL rapidly disappear while viral load persists at high levels (48). The remaining HIV-specific CTL preferentially accumulate in the blood, whereas HIV-infected cells predominantly localize to the lymph nodes (47). The failure of HIV-specific CTL to migrate to areas where HIV-1 proliferation is high (such as the lymph nodes) may be due to an as-yet-undefined mechanism.
The HIV-1 envelope protein gp120 initiates virus entry into T cells through attachment to the CD4 molecule and subsequent binding to a chemokine coreceptor, CXCR4 or CCR5, depending on viral tropism (10, 54). HIV-1 gp120 has also been shown to elicit T-cell chemotaxis in a CD4-independent, concentration-dependent manner via binding CXCR4 or CCR5 (10, 30, 44). The role of gp120 in the modulation of HIV-specific CTL migration and effector function remains largely undefined. We hypothesize that high local levels of HIV-1 gp120 disrupt normal CTL trafficking, thereby leading to the dysregulation of immune effector cell localization. It was previously demonstrated that the potent T-cell chemokine stromal cell-derived factor 1 (SDF-1) can repel T cells in vitro and in vivo through binding its cognate receptor, CXCR4, attracting resting T cells at a concentration of 100 ng of SDF-1 per ml but causing T cells to move away from SDF-1 at a peak concentration of 1 μg per ml (52). Subsequent work demonstrated that this novel mechanism, which we term fugetaxis, plays a physiological role in thymic emigration (51). Given that HIV-1 gp120 binds CXCR4 and can mediate T-cell chemotaxis, we examined whether gp120 might also repel T cells and influence HIV-specific CTL migration. We also investigated how the effects of CXCR4-binding gp120 (X4 gp120) on CTL migration might alter killing efficacy. We found that HIV-specific CTL move away from recombinant HIV-1 gp120 in a receptor-mediated, concentration-dependent manner. Furthermore, the expression of X4 gp120 by target cells reduces CTL efficacy in vitro by dysregulating T-cell migration. We also show that high-dose X4 gp120 inhibits antigen-specific CD8+-T-cell infiltration into a site of antigen challenge in vivo. These data suggest that X4 gp120 may employ a novel mechanism of viral immune evasion that results in the dysregulation of virus-specific CTL localization.
MATERIALS AND METHODS
CTL clones from HIV-1-infected individuals.
HIV-1-specific CTL clones were obtained by cloning stimulated peripheral blood mononuclear cells from HIV-1-infected individuals at limiting dilution and were characterized for specificity and HLA restriction as previously described (71, 73). CTL clones designated DMD, ND-25, and ASB-C11 were all HLA B8-restricted CTL clones isolated from different donors, specific for the HIV-1 Nef epitope FL8 (amino acids [aa] 90 to 97; FLKEKGGL). The CTL major histocompatibility complex B60-restricted clone 161JD27 recognized a Gag epitope IL10 (aa 92 to 101; IEIKDTKEAL). Amino acids are numbered according to the most recent clade B consensus sequence. All cells were free of mycoplasma as determined by testing with a mycoplasma tissue culture RNA detection kit (Jen-Probe, San Diego, Calif.).
Cytotoxicity assays.
HLA-matched B-lymphoblastoid cell lines (BLCL) were pulsed with the appropriate peptide, incubated with 51Cr, washed, and distributed in either round- or flat-bottom 96-well plates at various cell concentrations (70). HIV-1-specific CTL were used as effectors in triplicate wells at effector-to-target (E:T) ratios from 1:1 to 10:1. Cells were incubated for 4 h at 37°C, at which point 30 μl of supernatant was harvested. Twelve hours later, γ counts were measured on a Microplate reader (Packard Instrument Company, Downers Grove, Ill.). The percentage of specific cytotoxicity was calculated as follows: percent specific lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. The average spontaneous release of 51Cr from target cells was always <20% of maximum release.
Mononuclear cell preparation and sorting of subpopulations of T cells.
Peripheral blood was obtained from healthy adult donors according to a protocol approved by the Institutional Review Board. Ficoll-Hypaque (Pharmacia Biotech Inc., Piscataway, N.J.) density gradient centrifugation was used to isolate peripheral blood mononuclear cells. Cells were then stained with saturating amounts of phycoerythrin-conjugated anti-CD4 or -CD8 monoclonal antibodies and fluorescein isothiocyanate-conjugated anti-CD45RA or -CD45RO (Becton Dickinson, San Jose, Calif.). The desired subpopulations of peripheral blood cells were sorted by using a fluorescence-activated Vantage sorter (Becton Dickinson) and cultured overnight in Iscove's modified medium containing 0.5% fetal calf serum (Life Technologies, Carlsbad, Calif.) before their use in transmigration assays. The purity of each T-cell subpopulation was determined to be greater than 99% by immunophenotyping.
Transmigration assays.
Transmigration assays were performed in a transwell system with a polycarbonate membrane of 6.5-mm diameter with a 5-μm pore size (Corning, Corning, N.Y.) as described previously (33, 52). Purified T-cell subpopulations (5 × 104 cells) were added to the upper chamber of each well in a total volume of 150 μl of Iscove's modified medium. SDF-1α (PeproTech, Rocky Hill, N.J.) or recombinant HIV-1 gp120 (Immunodiagnostics, Woburn, Mass., and AIDS Research and Reference Reagent Program, National Institutes of Health, or R. Wyatt, Dana-Farber Cancer Institute, respectively) was used at concentrations ranging from 2 ng/ml to 2 μg/ml in the lower, upper, or both lower and upper chambers of the transwell to generate a standard “checkerboard” analysis matrix of positive, negative, and absent gradients. Recombinant variable loop deletion mutants of HIV-1IIIB gp120, including ΔV1, ΔV1/V2, and ΔV1/V2/V3, were also used in these assays. Transwells were incubated for 3 h at 37°C, after which cells were collected from the lower chamber and counted.
Transduction of target cell lines.
Recombinant adeno-associated virus (rAAV) vectors were used to deliver HIV-1HXB2 env, or a control gene, red fluorescent protein (RFP), into target cells. Mock transduction was performed as an additional control. BLCL were washed in RPMI medium, and 106 cells per well were aliquoted in minimal volume in 24-well tissue culture plates. Cells were incubated for 90 min with 50 μl of rAAV (multiplicity of infection of 2 to 4), after which 0.5 ml of RPMI medium with 20% fetal calf serum was added to each well. Successful transduction was confirmed after 48 h with indirect cytofluorometry for cell surface expression of envelope glycoproteins in the case of env or fluorescent microscopy in the case of RFP, and cells were used immediately as targets in cytotoxicity assays. Secretion of HIV-1 gp120 was confirmed by performing an enzyme-linked immunosorbent assay (Immunodiagnostics) on culture supernatants from adeno-associated virus-transduced cells.
Immunization and challenge of mice.
C57BL/6 and OT-1 mice (Jackson Laboratories, Bar Harbor, Maine) were immunized subcutaneously against chicken ovalbumin (OVA; Sigma) and subsequently challenged with a second intraperitoneal (IP) injection of OVA as previously described (52). Twenty-four hours after IP OVA challenge, experimental mice received a second IP injection containing low-dose (20 ng/ml) or high-dose (200 ng/ml) HIV-1IIIB gp120. Recombinant HIV-1IIIB gp120 containing deletions of the V1/V2 and V1/V2/V3 loops were also tested at high and low doses. Control mice were exposed to IP injections of N-saline or boiled gp120. Mice were euthanized 3 and 24 h after the second IP injection, and peritoneal lavage with 5 ml of phosphate-buffered saline was performed. The total number of viable nucleated cells per milliliter of peritoneal fluid was determined with a hemocytometer and by trypan blue exclusion. Peritoneal fluid obtained in this way contained less than 0.1% red blood cells. Flow cytometry was performed on peritoneal fluid cells by using fluorochrome-conjugated antibodies against mouse T cells (phycoerythrin-anti-CD3, biotin-anti-CD8, and allophycocyanin-anti-CD4; Caltag Laboratories). Second-step staining of biotin-conjugated antibodies used streptavidin-peridinin chlorophyll protein (Becton Dickinson). The proportion of T cells of each subpopulation was determined as a percentage of the total nucleated cell fraction in the peritoneal fluid.
Statistical analysis.
All experiments were performed at least in triplicate, with the data shown representative of all results. The data were analyzed for statistical significance by using the Wilcoxon signed rank exact test or a two-tailed Student's paired t test.
RESULTS
HIV-1 gp120 repels CD8+ T cells in vitro.
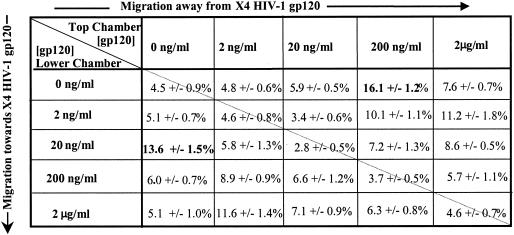
SDF-1, the natural ligand of CXCR4, serves as a bidirectional cue for T cells, attracting at one concentration and repelling at a higher concentration via a CXCR4-dependent and pertussis toxin-sensitive mechanism (52). We postulated that we would observe a similar finding for X4 gp120. Mature resting CD8+ CD45RO+ T cells isolated from the peripheral blood of healthy volunteers were used in transmigration assays to quantitate their migratory responses to positive, negative, and absent gradients of recombinant HIV-1IIIB gp120. Standard checkerboard analyses of human T-cell migration demonstrated that gp120 could serve as a bidirectional cue for subpopulations of human resting peripheral blood CD8+ T cells (Fig. 1). At a concentration of 20 ng/ml, HIV-1IIIB gp120 elicited maximal chemotaxis (13.6% ± 1.5%), or movement towards the recombinant protein. In contrast, higher concentrations of HIV-1IIIB gp120 (200 ng/ml) caused maximal migration (16.1% ± 1.2%) of T cells away from the HIV-1 protein, or fugetaxis. Minimal random movement of T cells, or chemokinesis, was seen in response to HIV-1IIIB gp120 presented in the absence of a gradient. Transmigration experiments were repeated with three different sources of recombinant X4 gp120, and similar T-cell migratory responses from mature T-cell subpopulations were observed (data not shown). We therefore concluded that X4 gp120 served as a bidirectional cue for T cells in vitro and that movement towards or away from the HIV-1 protein was concentration dependent.
FIG. 1.
Checkerboard transmigration analysis of CD8+ CD45RO+ T cells in response to recombinant HIV-1IIIB gp120. T cells (5 × 104) were placed in the upper chamber, and recombinant gp120 was added to the upper and/or lower chamber at the indicated concentrations, creating a negative gradient (above the diagonal line), a positive gradient (below the diagonal line), or equal concentrations in both chambers (along the diagonal line). After 3 h of incubation, cells were counted in the lower chamber. Results represent the mean percentage of cells placed in the top chamber that migrated to the lower chamber ± the standard error of the mean from six independent experiments.
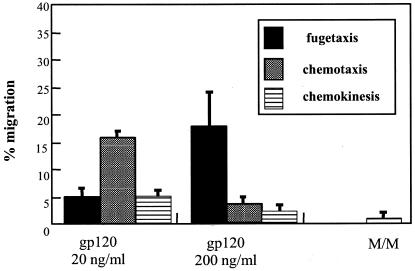
In order to determine whether X4 gp120 might also serve as a bidirectional cue for HIV antigen-specific CTL migration, we examined the effect of the recombinant HIV gp120 on the migration of CTL clones. Once again, chemotaxis occurred at a peak concentration of 20 ng/ml, and maximal migration away from X4 gp120, or fugetaxis, occurred at the higher concentration of 200 ng/ml (Fig. 2). We conclude from these results that both primary CD8+ CD45RO+ cells and HIV-specific CD8+ CTL demonstrate concentration-dependent movement toward and away from the HIV-1 protein X4 gp120 in vitro.
FIG. 2.
Transmigration responses of a representative HIV-specific CTL clone (161JD27) with recombinant HIV-1IIIB gp120 used at concentrations of 20 and 200 ng/ml. Media alone (M/M) was used as a control. Movement away (fugetaxis), movement toward (chemotaxis), and random movement (chemokinesis) were quantitated in a standard transmigration assay and are expressed along the y axis as the percentage of cells placed in the upper chamber of the transwell that migrated to the lower chamber after 3 h of incubation. Results represent the mean and standard error from three independent experiments.
Active movement towards and away from X4 gp120 is mediated by binding and signaling through CXCR4.
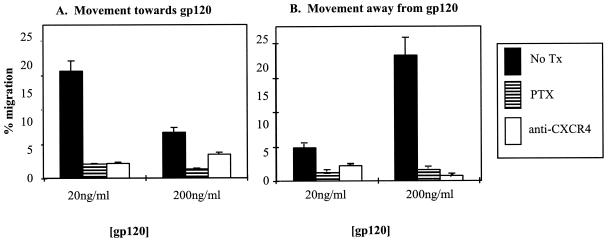
Specific components of the Gαi protein-coupled receptor signaling pathway for SDF-1 can be blocked by a number of different chemical inhibitors (51, 52, 59, 68). We examined the inhibitor profile for movement of resting T-cell subpopulations towards and away from recombinant X4 gp120 in transmigration assays. CD8+- CD45RO+-T-cell migration towards and away from HIV-1 gp120 was significantly inhibited by the Gαi protein inhibitor pertussis toxin (P = 0.0013) and CXCR4-binding antibody 12G5 (P = 0.008), suggesting that T cells migrate both towards and away from HIV-1 X4 gp120 in a manner similar to that of SDF-1 (Fig. 3).
FIG. 3.
Pertussis toxin and anti-CXCR4 antibodies inhibit active movement towards (A) and away from (B) X4 gp120. CD8+ CD45RO+ T cells were incubated with pertussis toxin (PTX; striped bars) or 12G5 (anti-CXCR4; open bars) prior to their addition to the transmigration assay. Control cells (No Tx; filled bars) received no pretreatment. The percentage of cells that had migrated to the lower transwell is shown for gp120 concentrations of 20 and 200 ng/ml. Results represent the mean and standard error from three independent experiments.
Structural alterations in HIV-1 gp120 affect its ability to induce T-cell migration.
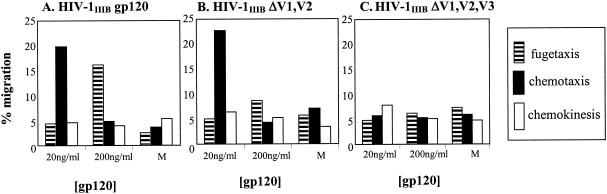
The precise binding site of gp120 to CXCR4 is not yet mapped. However, it has been demonstrated that the V3 loop plays a significant role in this interaction (53, 67). Guided by previous studies of the receptor-ligand interaction between HIV-1 gp120 and CXCR4 (9), we used specific deletion mutations of HIV-1IIIB gp120 in order to investigate which structural components might play a role in the observed migratory response of CD8+ T cells. The migratory responses of resting T-cell subpopulations in response to mutants of HIV-1IIIB gp120 containing V1/V2 or V1/V2/V3 loop deletions at concentrations of 20 or 200 ng/ml were assessed (Fig. 4). We found that the deletion of the V1 and V2 loops of HIV-1IIIB gp120 led to the exclusive movement of T cells toward gp120 (15% ± 1.1%) and a complete loss of the signal to move away from gp120. The deletion of the V1, V2, and V3 loops led to an inhibition of movement of resting T cells both towards and away from gp120. These results suggest that the V3 loop of X4 gp120 may play a significant role in signaling CD8+-T-cell migration.
FIG. 4.
Migration of CD8+ T cells in response to HIV-1IIIB gp120 containing variable loop deletions. Freshly isolated peripheral blood CD8+ T cells were exposed to concentrations of gp120 at 20 and 200 ng/ml in the lower or upper chambers of a transwell assay (A), HIV-1IIIB ΔV1,V2 (B), and HIV-1IIIB ΔV1,V2,V3 (C). The percentage of cells moving away from (fugetaxis; striped bars), moving towards (chemotaxis; filled bars), and moving in the absence of (chemokinesis; open bars) a gradient of the different recombinant gp120 molecules was quantitated by using the standard transmigration assay. Cell migration in medium alone (M) served as a control. Results represent the mean of three independent experiments.
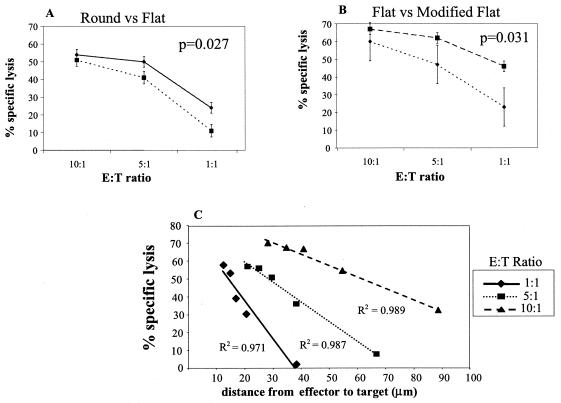
A modified chromium release assay demonstrates that CTL killing efficacy depends on the distance an effector cell must migrate to reach a target cell.
In order to test the hypothesis that migration plays a direct role in CTL efficacy, the 51Cr release assay (57) was modified in two ways. First, the cytotoxicity of HIV-specific CTL was quantitated by the standard technique in a round-bottom 96-well plate and compared to results of assays performed in a flat-bottom 96-well plate (Fig. 5A). A demonstration of significantly decreased lysis (P = 0.027) when effectors and targets were incubated in the flat-bottom wells (as opposed to being pelleted together in the round-bottom wells) supports the view that cell movement plays a role in determining CTL efficacy. Video microscopy demonstrated that effector cells incubated with targets in a flat-bottom well moved from one target cell to another, inducing lysis, whereas cells incubated in the round-bottom well did not exhibit discernible migration during the incubation period (data not shown). Secondly, we further modified the assay to delineate between the percent specific lysis due to the E:T ratio and the percent specific lysis attributable to the total number of cells placed in the flat-bottom well. In this modified flat 51Cr release assay, the total number of cells per well was kept constant at each E:T ratio compared to the standard assay, where both the E:T ratio and total number of cells per well decreased. As expected, at the E:T ratio of 10:1, conditions were identical for the standard and modified flat-bottom assays (110,000 cells/well), and no differences in the percent specific lysis were seen. At the E:T ratios of 5:1 and 1:1, however, CTL killing efficacy differed significantly (P = 0.031) between the two conditions (Fig. 5B). These data suggest that the total number of cells per well is an important variable when the 51Cr release assay is performed in a flat-bottom well.
FIG. 5.
Modifications to the standard 51Cr release assay demonstrate that CTL migration influences killing efficacy. (A) CTL killing in the standard 51Cr assay in round-bottom 96-well plates (⧫) was compared to experiments done in a flat-bottom plate (▪). (B) Standard assay in the flat-bottom well plate (▪) performed in parallel with a modified 51Cr assay in which the total number of cells was kept constant at 110,000 cells per well and only the E:T ratio was changed (⧫). Results represent the mean and standard error from three independent experiments with a representative Nef-specific CTL clone. (C) Correlation of the mathematical model to experimental data. The modified 51Cr release assay was performed in flat-bottom wells with decreasing numbers of cells per well (cell density). The percent specific lysis observed at each cell density is plotted against the calculated mean distance from effector to target cell obtained from the mathematical model for three E:T ratios (equation 1). Results represent the mean from three independent experiments with a representative HIV Nef-specific clone. R2 values represent correlation coefficients and are highly significant at all three E:T ratios.
Based on the above findings, we used probability theory to mathematically model the spatial relationship between target and effector cells in a flat-bottom well and calculate the distance a CTL has to migrate to reach a target cell for a given number of cells per well (60, 61). The model assumes a random distribution of both effector and target cells on the surface of the flat-bottom well and that the statistics governing the position of one cell type are not influenced by the other. Under these assumptions, the expected distance (D) between a CTL and a target cell equals a universal, dimensionless constant (κ) divided by the square root of the density of the target cells in the flat bottom well (λt) and it is a theorem in probability theory that κ = (equation 1). The density of target cells equals the number of targets placed in the well (n) divided by the area of the well. Our well is a circle, hence equation 2. Experimentally, we found a highly significant positive correlation between observed CTL lysis and calculated distance required to reach their targets at all E:T ratios tested (Fig. 5C). These data support the concept of a relationship between CTL efficacy and their ability to actively migrate to target cells and also provide a model system in which to examine the impact of molecules which affect cell migration on CTL efficacy.
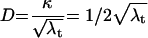 |
(1) |
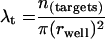 |
(2) |
Expression of X4 gp120 by target cells reduces CTL killing.
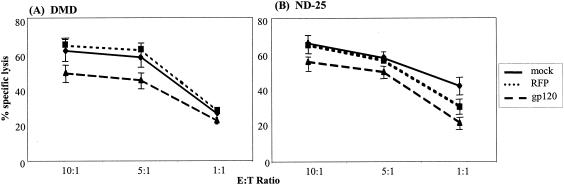
Using our modified 51Cr release assay described above, we investigated the effect of the expression of X4 gp120 by the target cell on CTL efficacy. Autologous BLCL were transduced with a rAAV vector encoding HIV-1HXBc2 env. The control rAAV vector expressed RFP. Transduced cells were used as targets in the modified 51Cr release assay 48 h after infection with viral constructs. Mock transduced BLCL provided an additional control. Surface expression and secretion of gp120 by target cells were confirmed by indirect immunofluorescence and supernatant gp120 enzyme-linked immunosorbent assay, respectively. The target cells transduced with env demonstrated a significantly lower percentage of specific lysis by two Nef-specific clones than the targets transduced with RFP (P = 0.008 for DMD, P = 0.0002 for ND-25) or to the mock-transduced cells (P = 0.02 for DMD, P = 0.0004 for ND-25) (Fig. 6). HIV-1 gp120 has been previously reported to mediate CD4+- and CD8+-T-cell apoptosis through its interaction with the CXCR4 receptor (69). We performed mock 51Cr release assays without radioisotope labeling, and after 4 h, we labeled effector and target cells with allophycocyanin-anti-CD8 (Caltag) and 7-amino-actinomycin D (Sigma). Levels of apoptosis were similar between CTL incubated with target cells expressing gp120 and the controls (data not shown). These data support the view that the reduction in CTL efficacy seen when target cells expressed X4 gp120 was not due to increased CTL death. In this way, we demonstrated that X4 gp120 expression by target cells reduced lysis by CTL.
FIG. 6.
Effects of X4 gp120 expression by target cells on CTL lysis. Two HIV-1 Nef-specific CTL clones, DMD (A) and ND-25 (B), were tested for lytic activity against peptide-pulsed, autologous BLCL (⧫), BLCL transduced with an rAAV vector encoding RFP (▪), or X4 gp120 (▴). Lysis was measured by using the standard 51Cr release assay in flat-bottom 96-well plates at the indicated E:T ratios. Results represent the mean and standard error from three independent experiments.
Movement of T cells away from HIV-1 X4 gp120 in vivo.
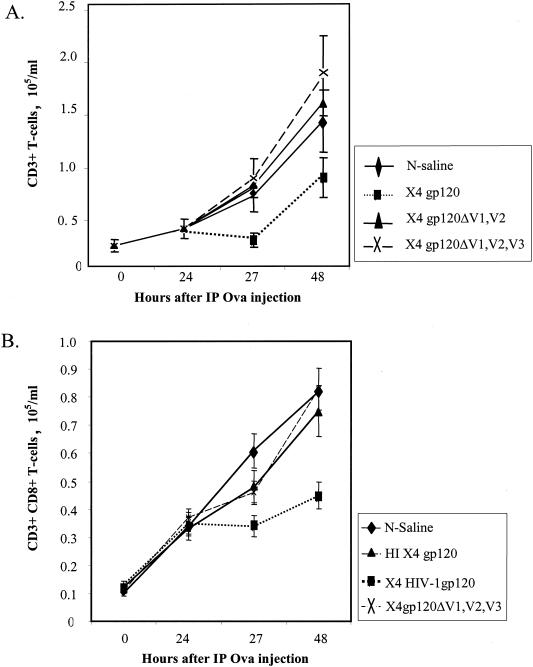
The chemokine receptor for SDF-1 and X4 gp120, CXCR4, is structurally and functionally highly conserved between humans and mice, sharing 91% amino acid sequence homology (25). As in humans, X4 gp120 elicits chemotaxis in murine T cells expressing CXCR4 in a CD4-independent manner (9a). We confirmed that migratory responses of resting murine CD8+ T cells to both human SDF-1 and recombinant X4 gp120 closely resemble those of human resting CD8+ T cells within transmigration assays (data not shown). It was previously demonstrated that a concentration of SDF-1 of 1 μg/ml can inhibit established immune responses in a mouse model (52). Using a similar protocol, we examined whether X4 gp120 might do the same. C57BL/6 mice immunized against OVA were challenged 3 days later with an IP injection of OVA (time zero). Twenty-four hours later, experimental mice received a second IP injection containing high-dose (200 ng/ml) or low-dose (20 ng/ml) X4 gp120. Recombinant loop-deleted forms of X4 gp120 were also tested at high and low doses. Control mice were exposed to IP injections of N-saline or boiled recombinant X4 gp120. High-dose X4 gp120 led to a significant reversal in T-cell infiltration into the IP cavity in response to antigen to which the mouse had been sensitized (Fig. 7A). Compared to control animals, the mice that received 200 ng of X4 gp120 per ml were found to have significantly reduced T-cell infiltration into the peritoneal cavity in response to antigen challenge (P = 0.04; Student's t test) 27 h after initial IP OVA injection (3 h after the second injection). At 48 h, the difference had lessened, but decreased CD3+ cells were still seen in the mice receiving X4 gp120 versus controls (P = 0.05). Recombinant loop mutants of X4 gp120 had no detectable effect on the infiltration of immune effector cells into the IP cavity. The chemotactic concentration of gp120 (20 ng/ml) did not augment T-cell infiltration into the peritoneal cavity beyond the robust reaction seen with antigen stimulation alone (data not shown). These data were similar to those generated with a low chemotactic concentration (100 ng/ml) of SDF-1, which did not augment T-cell infiltration into the peritoneal cavity beyond the levels induced by ovalbumin alone (52).
FIG. 7.
HIV-1 X4 gp120 inhibits T-cell infiltration into a site of antigen challenge in vivo. C57BL/6 (A) and OT-1 (B) mice were immunized with OVA subcutaneously. Three days later, mice were challenged with IP OVA (time zero). Twenty-four hours after IP OVA injection, one of several forms of recombinant HIV-1 X4 gp120, HIV-1IIIB gp120 (▪), HIV-1IIIB gp120 ΔV1/V2 (ΔV1V2) (▴ [A]), or HIV-1IIIB gp120 ΔV1/V2/V3 (ΔV1V2V3) (×), was administered. Control mice received an IP injection of normal saline (⧫) or heat-inactivated (HI) HIV-1IIIB gp120 (▴ [B]). T-cell infiltration following the IP injection of recombinant gp120 or the control was then quantitated 3 h later and after 24 h by cell counting, immunostaining, and flow cytometry. The results represent the mean and standard error of three independent experiments.
Antigen-specific CD8+-T-cell migration was examined in the context of OT-1 mice engineered to express an OVA-specific T-cell receptor. We determined the number of CD3+ CD8+ T cells migrating into the IP cavity in response to challenge with OVA as described above. Recombinant X4 gp120 led to a significant reduction of CD3+- CD8+-T-cell infiltration into the peritoneal cavity compared to control N-saline administration (P = 0.038) or administration of heat-inactivated HIV-1 gp120 (P = 0.047) or HIV-1 gp120 with the V1, V2 and V3 loops deleted (P = 0.044) at 48 h following the IP injection of OVA (Fig. 7B).
DISCUSSION
In order for CTL to successfully control HIV-1 infection, they must home efficiently to infected tissue sites, migrate within the infected tissue to the virus-infected cells, and then mediate contact-dependent target cell lysis (15, 16, 50, 74). Our present investigations demonstrate that X4 gp120 elicits bidirectional T-cell movement in a CD4-independent, concentration-dependent manner, attracting CD8+ lymphocytes and HIV-specific CTL maximally at a concentration of 20 ng/ml and repelling the same cells at a higher concentration of 200 ng/ml. This bidirectional response induced through the binding of HIV-1 gp120 to CXCR4 is similar to that seen with the receptor's natural ligand, the potent T-cell chemokine SDF-1. We have previously demonstrated that human T lymphocytes migrate away from SDF-1 at high concentrations such as those found in the bone marrow and thymus where mature T cells are notably absent (51, 52). We have termed the active movement of T cells away from a chemokinetic agent fugetaxis. Here, we provide the first description of a viral gene product causing T-cell fugetaxis. The fact that viruses other than HIV-1 encode chemokine homologues (43) suggests that this may be a conserved mechanism of host immune evasion. Interestingly, although SDF-1 is maximally active as a T-cell repellant in the 100-nM range, gp120 demonstrates maximal activity in the 10-nM range, possibly due to a higher binding affinity for CXCR4. T-cell migratory responses to X4 gp120 and SDF-1 are inhibited by anti-CXCR4 antibodies and pertussis toxin. Further investigation may reveal distinct downstream signaling pathways for chemotaxis and fugetaxis, thereby providing targets for biochemical manipulation of the immune response to HIV-1 gp120.
Our data indicate that directional cell movement depends on the interaction of the V2 and V3 loops of the gp120 molecule with the CXCR4 receptor. The experiments in which selected loop deletions of gp120 were assessed for their ability to elicit T-cell chemotaxis or fugetaxis showed that, compared to wild-type HIV-1HXBC2 gp120, V1/V2 deletion mutants inhibited the fugetactic effect of the molecule but had a minimal effect on chemotaxis. When all three variable loops were deleted, no directional movement was seen. Others have previously noted that the V3 loop as well as the V2 loop of HIV-1 gp120 determines coreceptor usage and subsequent signal transduction events (9, 26, 28, 36) for both X4 and R5 viruses. In early infection, the majority of circulating virus in HIV-infected individuals is CCR5 binding (R5), or M-tropic (58, 75), and the emergence of X4 viruses is generally associated with a rapid decline in CD4+ cells and an increased likelihood of developing AIDS (17, 19, 56). The natural ligands for CCR5, macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES, are potent chemoattractants for monocytes and activated T cells, including CD8+ effector cells (63, 64). CCR5 has been reported to be significantly upregulated on HIV-specific CTL compared to other virus-specific CD8+ cells (19). Elucidation of the effects of both X4 and R5 gp120 on immune cell migration may shed light on the accelerated disease progression seen with X4 viruses.
The question of whether the migratory effects on CTL elicited by X4 gp120 have in vivo relevance remains unanswered. Our data demonstrate that the immune cell infiltration in response to an antigen challenge in immunized, healthy mice can be inhibited by local injection of high-dose X4 gp120. This effect was not reproducible with heat-inactivated gp120 or with gp120 containing mutations in the V1 and V2 or V1, V2, and V3 loops. A disruption in the ability of CTL to migrate or remain at sites of high-antigen burden clearly handicaps the immune response. Interestingly, the genetic transfer of virally encoded chemokine antagonists MC148 (molluscum contagiosum) and vMIP-II (human herpesvirus 8) into murine cardiac allografts has been shown to reduce CTL infiltration of the allograft and prolong graft survival (18).
Although several studies report a failure of HIV-specific CTL to colocalize with HIV-infected cells (35, 41, 47, 48, 55), others have demonstrated equivalent or higher levels of ex vivo functionally active CTL in mucosal and lymphoid tissue compared to peripheral blood (5, 32). Except in the rare cases of long-term nonprogressors who immunologically control HIV-1 and demonstrate increased levels of perforin expression and proliferative capacity of their HIV-specific CTL (40), additional analyses of markers of in vivo functional activity, such as in situ granzyme B expression and apoptosis, have revealed low levels of actual cytolysis at the interface between virus-specific CTL and HIV-infected cells (6, 14). Our data support the hypothesis that HIV-1 gp120 suppresses the ability of T cells to effectively migrate or remain at sites of high viral replication by eliciting fugetaxis at high concentrations. Current technology does not allow the measurement of the precise concentration of HIV-1 gp120 seen by a given CTL in its in vivo microenvironment. Existing data support the possibility that concentrations of gp120 reach levels high enough to induce fugetaxis in vivo. Free envelope protein concentrations in the serum of HIV-infected individuals have been measured from levels of 250 pg/ml to 2 ng/ml (22, 45). These levels are considered to be underestimations of the true level of free gp120 in the serum, however, because of blocking antibodies and serum proteins. Local concentrations of gp120 in lymph nodes are likely to be considerably higher than those found in serum because of vigorous HIV-1 replication and binding to matrix proteins (7, 11, 27, 65).
In summary, we have shown that high concentrations of the HIV-1 protein X4 gp120 cause T cells and, in particular, antigen-specific CTL to actively migrate away from the chemokinetic stimulus in vitro and in vivo and that the expression of gp120 on target cells reduces CTL efficacy. This novel mechanism of immune evasion may be more broadly applicable to other retroviruses, poxviruses, papillomaviruses, and herpesviruses, all of which have been shown to encode viral proteins which influence cell migration. Furthermore, selective manipulation of chemotactic and fugetactic signals may allow the augmentation of the host immune response, thereby providing a novel immunotherapeutic strategy and potentially enhancing vaccine efficacy in the context of HIV-1 infection.
Acknowledgments
This work was supported in part by Public Health Service grants R01 AI49757 (M.C.P.) and T32 AI07387 (D.M.B.) and the American Foundation for AIDS Research (M.C.P.).
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini, A., S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, M. G. Aluigi, A. E. I. Proudfoot, S. Alouani, T. N. C. Wells, G. Mariani, R. L. Rabin, J. M. Farber, and D. M. Noonan. 1998. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 95:13153-13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485-490. [DOI] [PubMed] [Google Scholar]
- 4.Alicami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3:36-50. [DOI] [PubMed] [Google Scholar]
- 5.Altfeld, M., J. van Lunzen, N. Frahm, X. G. Yu, C. Schneider, R. L. Eldridge, M. E. Feeney, D. Meyer-Olson, H.-J. Stellbrink, and B. D. Walker. 2002. Expansion of preexisting, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Investig. 109:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson, J., S. Kinloch, A. Sönnerborg, J. Nilsson, T. E. Fehniger, A.-L. Spetz, H. Behbahani, L.-E. Goh, H. McDade, B. Gazzard, H. Stellbrink, D. Cooper, and L. Perrin. 2002. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 185:1355-1358. [DOI] [PubMed] [Google Scholar]
- 7.Bacon, K. B., B. A. Premack, P. Gardner, and T. J. Schall. 1995. Activation of dual T cell signaling pathways by the chemokine RANTES. Science 269:1727-1730. [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392:565-568. [DOI] [PubMed] [Google Scholar]
- 9.Basmaciogullari, S., G. J. Babcock, D. Van Ryk, W. Wojtowicz, and J. Sodroski. 2002. Identification of conserved and variable structures in the human immunodeficiency virus gp120 glycoprotein of importance for CXCR4 binding. J. Virol. 76:10791-10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Bieniasz, P. D., R. A. Fridell, K. Anthony, and B. R. Cullen. 1997. Murine CXCR4 is a functional coreceptor for T-cell-tropic and dual-tropic strains of human immunodeficiency virus type 1. J. Virol. 71:7097-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleul, C. C., R. C. Fuhlbrigge, J. M. Casasnovas, A. Aiuti, and T. A. Springer. 1996. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 184:1101-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobardt, M. D., A. C. S. Saphire, H.-C. Hung, X. Yu, B. Van der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 12.Bobbitt, K. R., M. M. Addo, M. Altfeld, T. Filzen, A. A. Onafuwa, B. D. Walker, and K. L. Collins. 2003. Rev activity determines sensitivity of HIV-1-infected primary T cells to CTL killing. Immunity 18:289-299. [DOI] [PubMed] [Google Scholar]
- 13.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodie, S. J., D. A. Lewinsohn, B. K. Patterson, D. Jiyamapa, J. Krieger, L. Corey, P. D. Greenberg, and S. R. Riddell. 1999. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat. Med. 5:34-41. [DOI] [PubMed] [Google Scholar]
- 15.Cerwenka, A., T. M. Morgan, A. G. Harmsen, and R. W. Dutton. 1999. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J. Exp. Med. 189:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coles, R. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834-838. [DOI] [PubMed] [Google Scholar]
- 17.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBruyne, L. A., K. Li, D. K. Bishop, and J. S. Bromberg. 2000. Gene transfer of virally encoded chemokine antagonists vMIP-II and MC148 prolongs cardiac allograft survival and inhibits donor-specific immunity. Gene Ther. 7:575-582. [DOI] [PubMed] [Google Scholar]
- 19.Fukada, K., Y. Sobao, H. Tomiyama, S. Oka, and M. Takiguchi. 2002. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J. Immunol. 168:2225-2232. [DOI] [PubMed] [Google Scholar]
- 20.Gangur, V., N. P. Birmingham, and S. Thanesvorakul. 2002. Chemokines in health and disease. Vet. Immunol. Immunopathol. 86:127-136. [DOI] [PubMed] [Google Scholar]
- 21.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. Bernaldo de Quiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert, M., J. Kirihara, and J. Mills. 1991. Enzyme-linked immunoassay for human immunodeficiency virus type 1 envelope glycoprotein 120. J. Clin. Microbiol. 29:142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulder, P. J. R., Y. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. J. McKinney, K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene, W. C., and B. M. Peterlin. 2002. Charting HIV's remarkable voyage through the cell: basic science as a passport to future therapy. Nat. Med. 8:673-680. [DOI] [PubMed] [Google Scholar]
- 25.Heesen, M., M. A. Berman, J. D. Benson, C. Gerard, and M. E. Dorf. 1996. Cloning of the mouse fusin gene, homologue to a human HIV-1 co-factor. J. Immunol. 157:5455-5460. [PubMed] [Google Scholar]
- 26.Hoffman, N. G., F. Seillier-Moiseiwitsch, J. Ahn, J. M. Walker, and R. Swanstrom. 2002. Variability in the human immunodeficiency virus type 1 gp120 Env protein linked to phenotype-associated changes in the V3 loop. J. Virol. 76:3852-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogewerf, A. J., G. S. Kuschert, A. E. Proudfoot, F. Borlat, I. Clark-Lewis, C. A. Power, and T. Wells. 1997. Glycosoaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 36:13570-13578. [DOI] [PubMed] [Google Scholar]
- 28.Isaka, Y., et al. 1999. Small amino acid changes in the V3 loop of human immunodeficiency virus type 2 determines the coreceptor usage for CXCR4 and CCR5. Virology 264:237-243. [DOI] [PubMed] [Google Scholar]
- 29.Iyengar, S., D. H. Schwartz, and J. E. K. Hildreth. 1999. T cell-tropic HIV gp120 mediates CD4 and CD8 cell chemotaxis through CXCR4 independent of CD4: implications for HIV pathogenesis. J. Immunol. 162:6263-6267. [PubMed] [Google Scholar]
- 30.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, et al. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaul, R., P. Thottingal, J. Kimani, P. Kiama, C. W. Waigwa, J. J. Bwayo, F. A. Plummer, and S. L. Rowland-Jones. 2003. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS 17:1139-1144. [DOI] [PubMed] [Google Scholar]
- 33.Kim, C. H., L. M. Pelus, J. R. White, and H. E. Broxmeyer. 1998. Differential chemotactic behavior of developing T cells in response to thymic chemokines. Blood 91:4434-4443. [PubMed] [Google Scholar]
- 34.Koup, R. A., J. T. Safrit, Y. Cao, G. Andrews, W. Mcleod, C. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Comparative analysis of cytotoxic T lymphocytes in lymph nodes and peripheral blood of simian immunodeficiency virus-infected rhesus monkeys. J. Virol. 73:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, S. K., Z. Xu, J. Lieberman, and P. Shankar. 2002. The functional CD8 T cell response to HIV becomes type-specific in progressive disease. J. Clin. Investig. 110:1339-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieberman, J. 2002. Defying death—HIV mutation to evade cytotoxic T lymphocytes. N. Engl. J. Med. 347:1203-1204. [DOI] [PubMed] [Google Scholar]
- 39.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 40.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 41.Mitsuyasu, R. T., P. A. Anton, S. G. Deeks, D. T. Scadden, E. Connick, M. Downs, A. Bakker, M. R. Roberts, C. H. June, S. Jalali, A. A. Lin, R. Pennathur-Das, and K. M. Hege. 2000. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus-infected subjects. Blood 96:785-793. [PubMed] [Google Scholar]
- 42.Moore, C. B., M. John, I. R. James, F. T. Christiansen, C. S. Witt, and S. A. Mallal. 2002. Evidence of HIV-1 adaptation to HLA-restricted immune response at a population level. Science 296:1439-1443. [DOI] [PubMed] [Google Scholar]
- 43.Murphy, P. M. 2001. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2:116-122. [DOI] [PubMed] [Google Scholar]
- 44.Oberlin, E., A. Amara, F. Bachelerie, C. Bessia, J. L. Virelizier, F. Arenzana-Seisdedos, O. Schwartz, J. M. Heard, I. Clark-Lewis, D. F. Legler, M. Loetscher, M. Baggiolini, and B. Moser. 1996. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833-835. (Erratum, 384:288.) [DOI] [PubMed]
- 45.Oh, S. K., W. W. Cruikshank, J. Raina, G. C. Blanchard, W. H. Adler, J. Walker, and H. Kornfeld. 1992. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J. Acquir. Immune Defic. Syndr. 5:251-256. [PubMed] [Google Scholar]
- 46.Ozdemir, E., L. S. St. John, G. Gillespie, S. Rowland-Jones, R. E. Champlin, J. J. Molldrem, and K. V. Komanduri. 2002. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 100:3690-3697. [DOI] [PubMed] [Google Scholar]
- 47.Pantaleo, G., H. Soudeyns, J. F. Demarest, M. Vaccarezza, C. Graziosi, S. Paolucci, M. Daucher, O. J. Cohen, F. Denis, W. E. Biddison, R. P. Sekaly, and A. S. Fauci. 1997. Accumulation of human immunodeficiency virus-specific cytotoxic T lymphocytes away from the predominant site of virus replication during primary infection. Eur. J. Immunol. 27:3166-3173. [DOI] [PubMed] [Google Scholar]
- 48.Pantaleo, G., H. Soudeyns, J. F. Demarest, M. Vaccarezza, C. Graziosi, S. Paolucci, M. Daucher, O. J. Cohen, F. Denis, W. E. Biddison, R. P. Sekaly, and A. S. Fauci. 1997. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc. Natl. Acad. Sci. USA 94:9848-9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poli, G., G. Pantaleo, and A. S. Fauci. 1993. Immunopathogenesis of human immunodeficiency virus infection. Clin. Infect. Dis. 17:S224-S229. [PubMed] [Google Scholar]
- 50.Posavad, C. M., M. L. Huang, S. Barcy, D. M. Koelle, and L. Corey. 2000. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J. Immunol. 165:1146-1152. [DOI] [PubMed] [Google Scholar]
- 51.Poznansky, M. C., I. T. Olszak, R. H. Evans, Z. Wang, R. B. Foxall, D. P. Olson, K. Weibrecht, A. D. Luster, and D. T. Scadden. 2002. Thymocyte emigration is mediated by active movement away from stroma-derived factors. J. Clin. Investig. 109:1101-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poznansky, M. C., I. T. Olszak, R. Foxall, R. H. Evans, A. D. Luster, and D. T. Scadden. 2000. Active movement of T cells away from a chemokine. Nat. Med. 6:543-548. [DOI] [PubMed] [Google Scholar]
- 53.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz, J. E., M. J. Kuroda, R. S. Veazey, A. Seth, W. M. Taylor, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J. Immunol. 164:6015-6019. [DOI] [PubMed] [Google Scholar]
- 56.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, et al. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siliciano, R. F., T. Lawton, C. Knall, R. Karr, P. Berman, T. Gregory, and E. Reinherz. 1988. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effects of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell 54:561-575. [DOI] [PubMed] [Google Scholar]
- 58.Skrabal, K., V. Trouplin, B. Labrosse, V. Obry, F. Damond, A. J. Hance, F. Clavel, and F. Mammano. 2003. Impact of antiretroviral treatment on the tropism of HIV-1 plasma virus populations. AIDS 17:809-814. [DOI] [PubMed] [Google Scholar]
- 59.Sotsios, Y., G. C. Whittaker, J. Westwick, and S. G. Ward. 1999. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J. Immunol. 163:5954-5963. [PubMed] [Google Scholar]
- 60.Stoyan, D., W. Kendall, and J. Mecke. 1995. Stochastic geometry and its applications, 2nd ed. John Wiley & Sons, New York, N.Y.
- 61.Stoyan, D., and H. Stoyan. 1994. Fractals, random shapes, and point fields. John Wiley & Sons, New York, N.Y.
- 62.Swingler, S., A. Mann, J. Jacque, B. Brichacek, V. G. Sasseville, K. Williams, A. A. Lackner, E. N. Janoff, R. Wang, D. Fisher, and M. Stevenson. 1999. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 5:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taub, D. D., K. Conlon, A. R. Lloyd, J. J. Oppenheim, and D. J. Kelvin. 1993. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science 260:355-358. [DOI] [PubMed] [Google Scholar]
- 64.Taub, D. D., A. R. Lloyd, J. M. Wang, J. J. Oppenheim, and D. J. Kelvin. 1993. The effects of human recombinant MIP-1 alpha, MIP-1 beta, and RANTES on the chemotaxis and adhesion of T cell subsets. Adv. Exp. Med. Biol. 351:139-146. [DOI] [PubMed] [Google Scholar]
- 65.Tellier, M. C., G. Greco, M. Klotman, A. Mosoian, A. Cara, W. Arap, E. Ruoslahti, R. Pasqualini, and L. M. Schnapp. 2000. Superfibronectin, a multimeric form of fibronectin, increases HIV infection of primary CD4+ T lymphocytes. J. Immunol. 164:3236-3245. [DOI] [PubMed] [Google Scholar]
- 66.Urbani, S., C. Boni, G. Missale, G. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76:12423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verrier, F., A. M. Borman, D. Brand, and M. Girard. 1999. Role of the HIV type 1 glycoprotein 120 V3 loop in determining coreceptor usage. AIDS Res. Hum. Retrovir. 15:731-743. [DOI] [PubMed] [Google Scholar]
- 68.Vicente-Manzanares, M., M. Rey, D. R. Jones, D. Sancho, M. Mellado, J. M. Rodriguez-Frade, M. A. del Pozo, M. Yanez-Mo, A. M. de Ana, A. C. Martinez, I. Merida, and F. Sanchez-Madrid. 1999. Involvement of phosphatidylinositol 3-kinase in stromal cell-derived factor-1 alpha-induced lymphocyte polarization and chemotaxis. J. Immunol. 163:4001-4012. [PubMed] [Google Scholar]
- 69.Vlahakis, S. R., A. Algeciras-Schimnich, G. Bou, C. J. Heppelmann, A. Villasis-Keever, R. G. Collman, and C. V. Paya. 2001. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Investig. 107:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker, B. D., S. Chakrabarti, B. Moss, T. J. Paradis, T. Flynn, A. G. Durno, R. S. Blumberg, J. C. Kaplan, M. S. Hirsch, and R. T. Schooley. 1987. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328:345-348. [DOI] [PubMed] [Google Scholar]
- 71.Walker, B. D., C. Flexner, L. K. Birch, L. Fisher, T. J. Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:9514-9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber, K. S., H. J. Grone, M. Rocken, C. Klier, S. Gu, R. Wank, A. E. Proudfoot, P. J. Nelson, and C. Weber. 2001. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur. J. Immunol. 31:2458-2466. [DOI] [PubMed] [Google Scholar]
- 73.Yang, O., S. A. Kalams, A. Trocha, H. Cao, A. D. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yap, K. L., G. L. Ada, and I. F. C. McKenzie. 1978. Transfer of specific cytotoxic lymphocytes protects mice inoculated with influenza virus. Nature 238:238-239. [DOI] [PubMed] [Google Scholar]
- 75.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]