Abstract
Objective
To investigate the analgesic properties of different dose combinations of midazolam and dexmedetomidine administered intraperitoneally (IP) in the rat.
Study design
Prospective experimental trial.
Animals
Seventy adult male Sprague Dawley rats weighing 250–300 g.
Methods
Dexmedetomidine (D) 0.03, 0.06, 0.09, 0.12, 0.15, 0.18, 0.21 mg kg−1 and midazolam (M) 5, 10, 25, 50 mg kg−1 were administered IP, alone then in combinations ranging from 0.03 D:5 M to 0.18 D:30 M mg kg−1. Analgesia was evaluated using the tail-flick test at time 0 (before injection), 15, 30, 45, 60 and 75 minutes.
Results
Midazolam at all doses administered (5–50 mg kg−1) did not significantly change tail-flick latencies from baseline values whereas D showed clear dose-dependent increases in tail-flick latency for doses administered in the range of 0.03–0.18 mg kg−1. Tail-flick latencies in rats administered D + M combinations were significantly greater than D alone (p < 0.05).
Conclusions
A dose-related analgesic effect was demonstrated for D in the rat, which was enhanced by co-administration of M.
Clinical relevance
The combination of D + M administered IP to rats at doses of 0.12:20 and 0.09:15 mg kg−1 was shown to be a good combination to provide sedation/analgesia with a duration of action greater than 60 minutes. The onset of sedation was rapid (1–3 minutes), and onset of profound analgesia was reached within 5–10 minutes.
Introduction
Dexmedetomidine (D) is the active isomer of the analgesic medetomidine that binds to alpha-2-adrenergic receptors (alpha-2-AR) in an agonist fashion with high specificity. The alpha-2-AR agonists produce varying levels of sedation, analgesia, muscle relaxation, anxiolysis, and a decrease in the anesthetic requirements of injectable and inhalant agents (dose sparing) (Sinclair 2003). Some common side effects of D are bradycardia, decreased respiratory rate, and hypothermia. Dexmedetomidine is easily reversed with atipamezole, an alpha-2-AR antagonist.
Midazolam (M) is a benzodiazepine that has sedative-hypnotic, anxiolytic, and muscle relaxant properties. Midazolam has been shown to have equivocal effects on nociception depending on the route of administration and the method used to assess nociception. Antagonism of the antinociceptive effect of opioids when benzodiazepines including M were co-administered with opioids and non-steroidal anti-inflammatory drugs to mice has been reported (Rosland & Hole 1990a; Pakulska & Czarnecka 2001). With barbiturates (Tatsuo et al. 1997) and when benzodiazepines were given alone there was a hyperalgesic effect (Tatsuo et al. 1999). Subcutaneous injection of M decreased the analgesia associated with ketamine (Okulicz-Kozaryn et al. 2000). In contrast, M given with ketamine via the epidural route improved the relief of post-operative pain (Wang et al. 2006). Luger et al. (1995) found that M increased the antinociceptive effect when given intrathecally with morphine, however they also found that intracerebroventricular administration did not affect tail flick latencies, suggesting different mechanisms of action in the spinal cord versus the brain. As well, Wilder-Smith et al. (1999) found that the effect of M varied with the dose of thiopental and also depended on which anesthetic end-point was chosen.
Midazolam has not been reported to decrease the analgesia associated with alpha-2-AR agonists. The present study is the first to explore the analgesic properties of M combined with D in commonly used dose combinations in rats.
Benzodiazepines such as M and diazepam have been used in combination with other anesthetics such as ketamine and butorphanol to produce greater muscle relaxation and sedation (Thurmon et al. 1996). The combination of medetomidine and M to produce sedation and anesthesia has been explored for dogs (Hayashi et al. 1994; Kojima et al. 1999; Itamoto et al. 2000), sheep (Raekallio et al. 1998), swine (Nishimura et al. 1993, 1994), and Japanese macaques (Kimura et al. 2007). Salonen et al. (1992a) demonstrated a synergistic interaction between D and M, in which the combination of the two drugs for sedation of rats, reduced the individual dose of both drugs with a more rapid loss of the righting reflex of the animals than with either drug alone. The drug interaction was further explored by Salonen et al. (1992b), in which they demonstrated the synergistic effect transferred to other behaviors, namely anxiolysis, evaluated by the elevated plus-maze test in rats. They found that flumazenil blocked the hypnotic response to M, but it was not effective against D-induced loss of righting reflex. Conversely, atipamezole attenuated the hypnotic response to D, but not to M. They also noted no ‘cross-displacement’ by the agonists for the alternative receptor in a radiolabeled ligand binding study. Their data strongly support a pharmocodynamic mechanism for the synergistic interaction between M and D that is exerted at either a pre- or post-receptor locus (Salonen et al. 1992a,b). Neither study, however, evaluated the antinociceptive properties of either drug or the combination in the rat, thus leading to our study.
The purpose of the present study was to further investigate and characterize the properties of the combination of D and M in the rat and to explore the nature of the drug:drug interactions with respect to sedation/analgesia.
Materials and methods
Animals
Seventy male 250–300 g Sprague Dawley rats (Harlan, IN, USA) were utilized in this study. Rats that were used in the portion of the study investigating M effects were given a 2-week washout period prior to being dosed with D. Drug-naïve rats were used to study the effects of D:M combinations. Rats were pair-housed in a temperature (21 ± 0.5 °C) and light (12 hours light:12 hours dark with no twilight) controlled environment. Food (2018 Global Rodent Diet; Harlan Teklad) and water were provided ad lib throughout the study. This work was approved by the Institutional Animal Care and Use Committee.
Drugs
The drugs utilized in this investigation were dexmedetomidine HCl (Dexdomitor; Pfizer Animal Health, NY, USA) and midazolam HCl (Midazolam HCl Injectable, USP; Hospira, Inc., IL, USA). Dexmedetomidine was diluted with sterile water for injection to the appropriate concentrations so manageable volumes were administered. All drugs were administered by IP injection into the right caudal abdominal quadrant.
Tail-flick test
Analgesia was assessed by the tail-flick test. This test provided an objective, quantifiable and repeatable measure. Tail-flick latencies were determined using an analgesiometer (Model 37360; Ugo Basile, Italy). An intensity setting of 50 on a scale of 1–99 and a cut-off time of 10 seconds were used throughout the study. These settings were selected based on the methods of Dawson et al. (2004) and determined from the results of preliminary studies to yield baseline tail-flick latencies between 3–5 seconds and did not produce immediate or latent burns of the tail in rats exposed to the maximal 10-second stimulus.
Antinociceptive tests were conducted in a room separate from the animal colony with racks containing cages transported into the room on the day of testing. During the test period, rats were kept in the same groups and cages as they were housed. Rats were restrained by hand with a small hand towel. Each rat was acclimated to this method of restraint and the tail flick device on three different days before the experiment commenced. During training periods, rats were restrained for 20–30 seconds with no stimulation.
Rats were weighed, and using a permanent marker, their tails were marked with a line in 1 cm increments starting at the tip, for a total of six increments. This ensured that the same area of the tail was not reused, and the tissue did not become sensitized to the stimulus (Wilson et al. 1992). Baseline tail-flick latencies were measured at the first position on the tail (1 cm).
Rats were injected with drug, as described above, 30 minutes after the baseline measurement. Tail-flick latencies were measured 15, 30, 45, 60, and 75 minutes after injection; each measurement was conducted at a different position on the tail, commencing with a position 2 cm from the tip. The 15–75 minute post-injection time interval permitted evaluation of antinociception in a period of time considered suitable for most surgical procedures. Rats that lost their righting reflex were maintained in right lateral recumbency on a 37 °C circulating water blanket (Micro-Temp Pump; Cincinnati Sub-Zero, OH, USA).
Data analysis
Antinociceptive data are expressed as the mean maximum possible effect (MPE%) using the following formula:
Differences among treatments on tail-flick latencies at 15, 30, 45, 60 and 75 minutes after drug administration were analyzed by two-way analysis of variance (ANOVA) for repeated measures with time and treatment as the main factors using GRAPH PAD PRISM version 5.0 (Graph Pad Software Inc., CA, USA). When an overall treatment effect was detected, subsequent planned comparisons were analyzed using Bonferroni multiple comparison tests to determine what treatments at which time points differed. Statistical significance was established at p < 0.05.
The analysis of drug–drug interactions was made at the time of presumed steady state, 60 minutes post-injection. The effect metric (in seconds) was the calculated difference between the test score and the baseline score. Analysis of the effect of D alone compared to D combined with M was performed using a two sample t test for paired data. Statistical significance was set at p < 0.05.
Results
Tail-flick latency
The baseline tail-flick latency was 3.7 ± 1.1 seconds (mean ± SD) (n = 68; two animals were discarded as their baselines were greater than the mean plus two standard deviations). Tail-flick scores in sedated or anesthetized rats were analyzed at specified times following varying doses of either M, D, or a fixed 1:167 ratio of D:M on a mg kg−1 basis, administered IP. Dexmedetomidine showed a clear dose-dependency over the range 0.03–0.18 mg kg−1 whereas M was devoid of activity over the range 5.0–50 mg kg−1. Our choice of combinations to be tested was guided by the above values, i.e. we used combinations that retained the individual D doses along with corresponding M components that also spanned the range of doses used when acting alone (instead of using it in a fixed amount). Thus, combinations started with 5.0 mg kg−1 M and 0.03 mg kg−1 D and higher quantities that retained this ratio were employed.
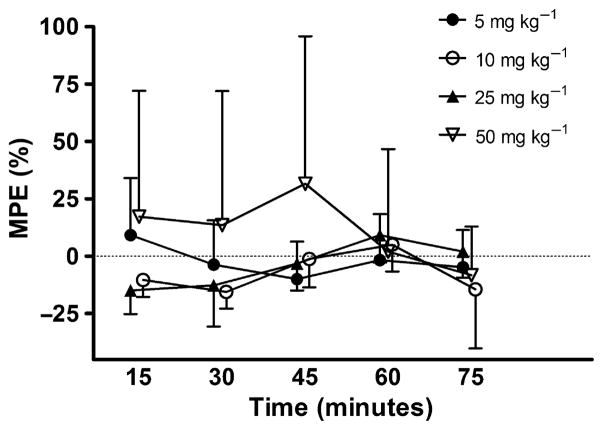
No dose of M produced a significant change in tail-flick latency from baseline over the 15–75 minute trial, p = 0.06 (Fig. 1). Although not statistically significant, animals receiving three doses of M, 5, 10, and 25 mg kg−1 had tail-flick latencies below their corresponding baseline values. In contrast, D showed a clear dose-dependent increase in tail-flick latency for doses 0.03–0.18 mg kg−1 p = 0.04; however, drug–time interactions were not statistically significant, p = 0.05 (Fig. 2). As well, tail-flick latencies produced by doses of 0.03 and 0.06 mg kg−1 peaked at 15 minutes and then showed a continuing decline over time. It should be noted, data points without error bars indicate that all the animals in the group reached 100% MPE.
Figure 1.
Effects of IP administration of M (mg kg−1) on tail-flick latency in rats. Each bar represents the mean ± SD of group (n = 5) tail-flick latency expressed as percent maximum possible effect (MPE%).
Figure 2.
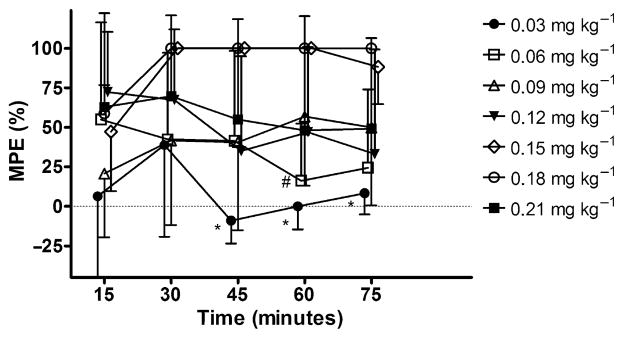
Effects of IP administration of D (mg kg−1) on tail-flick latency in rats. Each bar represents the mean ± SD of group (n = 5) tail-flick latency expressed as percent maximum possible effect (MPE%). * and #, significantly different (p < 0.05) from corresponding higher doses of drug at specified time points.
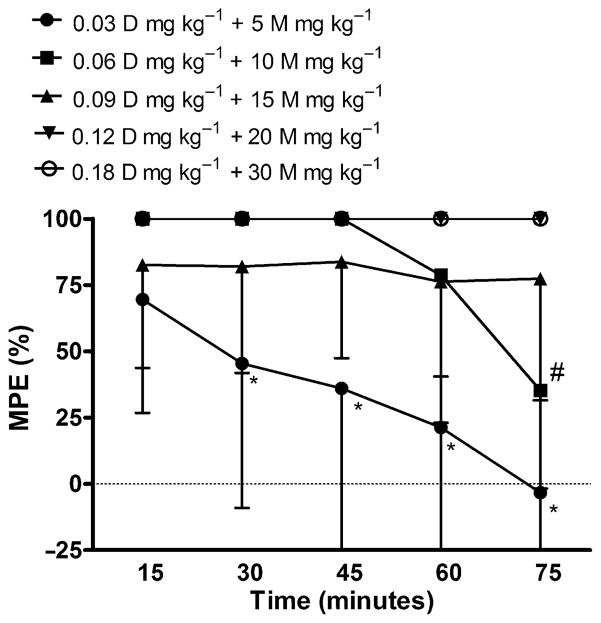
The combination of D and M increased tail-flick latencies two to fourfold over D alone (Fig. 3) and shifted the dose–response curve to the left (Fig. 4). The highest dose groups, 0.12 mg kg−1 D + 20 mg kg−1 M and 0.18 mg kg−1 D + 30 mg kg−1 M produced maximal response in all animals at all time points following injection. Statistical significance of p < 0.05 was found for pairwise comparisons between 0.03 mg kg−1 D + 5 mg kg−1 M and the higher dose combinations at 30–75 minutes post-injection and between 0.06 mg kg−1 D + 10 mg kg−1 M and the corresponding higher dose groups at 75 minutes post-injection. As with the D alone, the tail-flick latencies of animals in the lower dose combinations (0.03:5 and 0.06:10) peaked at 15 minutes and steadily declined over the 75 minute time interval.
Figure 3.
Effects of IP co-administration of D (mg kg−1) and M (mg kg−1) on tail-flick latency in rats. Each bar represents the mean ± SD of group (n = 5) tail-flick latency expressed as percent maximum possible effect (MPE%). * and #, significantly different (p < 0.05)from corresponding higher doses of drug at specified time points.
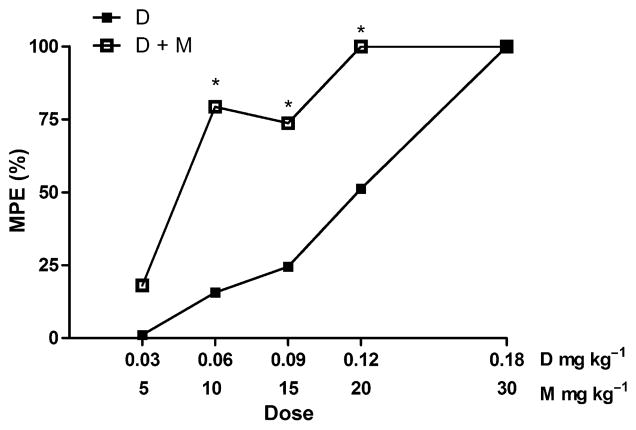
Figure 4.
Dose–response (tail-flick latency) for D versus D + M at steady state (60 minutes post-injection). All doses expressed as mg kg−1. *Significantly different (p < 0.05) from the same dose of dexmedetomidine alone.
To investigate drug–drug interaction, the effect of the drug combination on tail-flick latency was examined at 60 minutes post-injection. This time point was assumed to correspond to steady-state. Because M showed no efficacy in the tail-flick test, a simple additive interaction of the combination should produce latencies that do not differ from those of D alone. However, the tail-flick latencies resulting from administration of D + M were significantly (p = 0.021) greater than those of D alone and maximal effect was detected at lower doses of D when M was given in combination (Fig. 4), thereby indicating a synergistic interaction between the two drugs with respect to analgesia. Four dose combinations of D with M; 0.03 + 5, 0.06 + 10, 0.09 + 15 and 0.12 + 20 mg kg−1, increased tail-flick latencies 2- to 14-fold over the corresponding latency produced by D alone. Because of the cut-off time established for the tail-flick test, it was not possible to determine if the addition of 30 mg kg−1 M to 0.18 mg kg−1 D had any additive or synergistic effect.
Discussion
Our study looked at the antinociceptive effect of D and M alone, and in combination with each other. We confirmed that M alone has no antinociceptive effect; D has a dose-dependent effect, and that when combined with M, lower doses of D produced maximal response in tail flick latency and for a longer duration than D alone. That is, M interacted synergistically with D to increase depth of and duration of sedation/analgesia, i.e. a dose sparing effect on D. The ability to use lower doses of D combined with M to achieve sedation/analgesia may be beneficial in reducing or minimizing the cardiovascular and respiratory side effects of D.
The mechanisms by which M enhanced the action of D could include the facilitation of the release and/or binding of the inhibitory neurotransmitter GABA to its receptor. Also, alpha-2-AR agonists are able to inhibit adenylate cyclase, thereby changing the activity of cAMP-dependent protein kinase, which may alter the conductance properties of the chloride channel (Salonen et al. 1992b). Midazolam’s ability to alter antinociception has been reported for other types of analgesic drugs acting at other types of receptors and by different mechanisms. Pakulska & Czarnecka (2001) reported that M, when given with morphine, metamizol, or indomethacin in mice, decreased the antinociceptive effect of all three drugs. Similarly, Rosland & Hole (1990a) reported that M induced a dose-dependent attenuation of the effect of morphine, fentanyl, and buprenorphine in the hot-plate and tail-flick tests in mice. Similar results were found when M was used with low doses of ketamine (3.0 mg kg−1) in rats, but not with higher doses (5.0 mg kg−1) (Okulicz-Kozaryn et al. 2000). However, it has also been shown that M given alone can induce hyperalgesia in rats (Tatsuo et al. 1997). Thus it is imperative that when combining M with other anesthetic and analgesic drugs, this property be explored.
In the present investigation, we observed tail-flick latencies below baseline levels in rats administered M alone at doses of 5–25 mg kg−1. Although the parameters of the tail-flick test were not set specifically to detect hyerpalgesia, our findings are consistent with Tatsuo’s report (1997) which demonstrated dose-dependent M-induced hyperalgesia. Rosland & Hole (1990b) assessed the degree of dose-dependent sensorimotor impairment of mice given diazepam and their performance on the rota-rod. This sensorimotor impairment makes it difficult to assess the effect of M and D on antinociception, as an increase in tail-flick latency may be due in part to a lack of muscle tone rather than purely an antinociceptive effect of the drug. Because many of the doses of D and the D + M combinations produced deep sedation that abolished the righting reflex, it was not possible to use a test such as the rota-rod in the present study to ascertain what contribution impaired sensorimotor control may have played in the tail-flick test. The animals receiving the highest dose of M, 50 mg kg−1, did have tail-flick latencies above baseline 15–45 minutes post-injection which we attribute primarily to impaired muscle tone.
While motor impairment may make it difficult to distinguish whether the increased tail-flick latencies in response to higher doses of D or combinations of D + M were purely antinociceptive in nature or some combination of antinociception and reduced muscle tone, the data presented demonstrate that a synergistic interaction between the two drugs occurred with respect to the degree of sedation. Although it is difficult to relate a specific increase in tail-flick latency to surgical anesthesia, we found in a pilot study (data not shown) that rats administered 0.09 mg kg−1 D + 15 mg kg−1 M or 0.12 mg kg−1 D + 20 mg kg−1 M, corresponding to 75–100% MPE, reached a level of sedation/analgesia sufficient to perform a surgical castration.
Despite the lack of analgesic effect of M alone, when combined with D, it actually potentiated the antinociceptive/sedative effect of the α2-AR agonist. This potentiation, rather than attenuation, of the antinociceptive/sedative effect may be attributed to the difference in receptors. Benzodiazepines, ethanol, and barbiturates all potentiate the actions of the neurotransmitter GABA by prolonging the open state of the chloride-associated channels (Tatsuo et al. 1997). Dexmedetomidine is a specific and selective alpha-2-AR agonist. By binding to the presynaptic alpha-2-AR, it inhibits the release of norepinephrine, and thereby decreases the propagation of pain signals. The combination of two drugs that act on different receptors showed an obvious synergistic effect on sedation/analgesia in the present study.
Bol et al. (2000) measured the synergism of the D–M combination in rats. They found that the interaction was synergistic for all stimulus-response measures, which included the whisker reflex, startle reflex to noise, tail clamp response, and corneal reflex. However, each parameter was only measured at two time points per target concentration, and they measured the drug concentrations as plasma concentrations, rather than dose concentrations. Also, the tail clamp test was a modified clipboard clamp test; latencies between 0–15 seconds were defined as positive values and latencies between 15–30 seconds were denoted as negative values, resulting in quantitative data points rather than qualitative data points.
In our study, D at the highest dose, 0.21 mg kg−1 showed less antinociception than the next three lower doses. A possible explanation for the attenuation of antinociceptive effect of D at the highest dose could be abolishment of all inhibitory pain modulating pathways, thus permitting an ‘over-expression’ of another α-adrenergic insensitive pain pathway (Mense 2000).
An interesting observation from our study was that rats given higher doses of D (0.12–0.21 mg kg−1) produced large amounts of urine, as reported previously (Horváth et al. 1996). Central stimulation of α2-AR in the hypothalamus decreases the secretion and or production of anti-diuretic hormone from the pituitary (Sinclair 2003). Alpha2-AR agonists also antagonize the renal tubular effects of ADH (Sinclair 2003). A decrease in sympathetic tone may also contribute to the increased urine volumes. The large amounts of urine are noteworthy when performing abdominal surgery so as not to inadvertently cut into a full urinary bladder. We also observed that the higher doses of M (25–50 mg kg−1) caused many of the rats to begin shredding paper towels after the M injection. Both of these phenomena occurred to a lesser degree with the lower doses and dose combinations.
The results of this study show that M does not diminish the sedative and antinociceptive properties of D when the two drugs are used in combination. Dexmedetomidine (0.06–0.12 mg kg−1) combined with M (10–20 mg kg−1) produced rapid onset of sedation/analgesia of 60–75 minutes duration.
Acknowledgments
This work was supported in part by the Department of Comparative Medicine Endowment for Excellence (Penn State Hershey). The authors gratefully acknowledge the help of Victor Ruiz-Velasco, PhD and Joy Ellwanger, BS, CVT.
References
- Bol CJ, Vogelaar JP, Tang JP, et al. Quantification of pharmacodynamic interactions between dexmedetomidine and midazolam in the rat. J Pharmacol Exp Ther. 2000;294:347–355. [PubMed] [Google Scholar]
- Dawson C, Ma D, Chow A, et al. Dexmedetomidine enhances analgesic actions of nitrous oxide. Anesthesiology. 2004;100:894–904. doi: 10.1097/00000542-200404000-00020. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Nishimura R, Yamaki A, et al. Comparison of sedative effects induced by medetomidine, medetomidine-midazolam and medetomidine-butorphanol in dogs. J Vet Med Sci. 1994;56:951–956. doi: 10.1292/jvms.56.951. [DOI] [PubMed] [Google Scholar]
- Horváth G, Morvay Z, Kovács M, et al. Drugs acting on calcium channels modulate the diuretic and micturition effects of dexmedetomidine in rats. Life Sci. 1996;59:1247–1257. doi: 10.1016/0024-3205(96)00448-1. [DOI] [PubMed] [Google Scholar]
- Itamoto K, Hikasa Y, Sakonjyu I, et al. Anaesthetic and cardiopulmonary effects of balanced anaesthesia with medetomidine-midazolam and butorphanol in dogs. J Vet Med A. 2000;47:411–420. doi: 10.1046/j.1439-0442.2000.00302.x. [DOI] [PubMed] [Google Scholar]
- Kimura T, Koike T, Matsunasu T, et al. Evaluation of a medetomidine-midazolam combination for immobilizing and sedating Japanese monkeys (Macaca fuscata) J Am Assoc Lab Anim Sci. 2007;46:33–38. [PubMed] [Google Scholar]
- Kojima K, Nishimura R, Mutoh T, et al. Comparison of sedative effects of medetomidine-midazolam, acepromazine-butorphanol and midazolam-butorphanol in dogs. J Vet Med A. 1999;46:141–148. doi: 10.1046/j.1439-0442.1999.00194.x. [DOI] [PubMed] [Google Scholar]
- Luger TJ, Hayashi T, Weiss CG, et al. The spinal potentiating effect and the supraspinal inhibitory effect of midazolam on opioid-induced analgesia in rats. Eur J Pharmacol. 1995;275:153–162. doi: 10.1016/0014-2999(94)00759-z. [DOI] [PubMed] [Google Scholar]
- Mense S. Neurobiological concepts of fibromyalgia –the possible role of descending spinal tracts. Scand J Rheumatol Suppl. 2000;113:24–29. doi: 10.1080/030097400446599. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kim H, Matsunaga S, et al. Sedative effect induced by a combination of medetomidine and midazolam in pigs. J Vet Med Sci. 1993;55:717–722. doi: 10.1292/jvms.55.717. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kim H, Matsunga S, et al. Cardio-pulmonary effects of medetomidine-midazolam and medetomidine-midazolam-atipamezole in laboratory pigs. J Vet Med Sci. 1994;56:359–363. doi: 10.1292/jvms.56.359. [DOI] [PubMed] [Google Scholar]
- Okulicz-Kozaryn I, Kaminska E, Juczak J, et al. The effects of midazolam and morphine on analgesic and sedative activity of ketamine in rats. J Basic Clin Physiol Pharmacol. 2000;11:109–125. doi: 10.1515/jbcpp.2000.11.2.109. [DOI] [PubMed] [Google Scholar]
- Pakulska W, Czarnecka E. Effect of diazepam and midazolam on the antinociceptive effect of morphine, metamizol and indomethacin in mice. Pharmazie. 2001;56:89–91. [PubMed] [Google Scholar]
- Raekallio M, Tulamo R-M, Valtamo T. Medetomidine-midazolam sedation in sheep. Acta Vet Scand. 1998;39:127–134. doi: 10.1186/BF03547814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosland JH, Hole K. 1,4-Benzodiazepines antagonize opiate-induced antinociception in mice. Anesth Analg. 1990a;71:242–248. doi: 10.1213/00000539-199009000-00005. [DOI] [PubMed] [Google Scholar]
- Rosland JH, Hole K. Benzodiazepine-induced antagonism of opioid antinociception may be abolished by spinalization or blockade of the benzodiazepine receptor. Pharmacol Biochem Behav. 1990b;37:505–509. doi: 10.1016/0091-3057(90)90020-i. [DOI] [PubMed] [Google Scholar]
- Salonen M, Reid K, Maze M. Synergistic interaction between α2-adrenergic agonists and benzodiazepines in rats. Anesthesiology. 1992a;76:1004–1011. doi: 10.1097/00000542-199206000-00022. [DOI] [PubMed] [Google Scholar]
- Salonen M, Onaivi ES, Maze M. Dexmedetomidine synergism with midazolam in the elevated plus-maze test in rats. Psychopharmacology. 1992b;108:229–234. doi: 10.1007/BF02245313. [DOI] [PubMed] [Google Scholar]
- Sinclair MD. A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J. 2003;44:885–897. [PMC free article] [PubMed] [Google Scholar]
- Tatsuo MA, Yokoro CM, Salgado JV, et al. Hyperalgesic effect induced by barbiturates, midazolam and ethanol:pharmacological evidence for GABA-A receptor involvement. Braz J Med Biol Res. 1997;30:251–256. doi: 10.1590/s0100-879x1997000200015. [DOI] [PubMed] [Google Scholar]
- Tatsuo MA, Salgado JV, Yokoro CM, et al. Midazolam-induced hyperalgesia in rats: modulation via GABA A receptors at supraspinal level. Eur J Pharmacol. 1999;370:9–15. doi: 10.1016/s0014-2999(99)00096-5. [DOI] [PubMed] [Google Scholar]
- Thurmon JC, Tranquilli WJ, Benson GJ. Lumb and Jones’ Veterinary Anesthesia. 3. Williams & Wilkins; Baltimore, MD: 1996. [Google Scholar]
- Wang X, Xie H, Wang G. Improved postoperative analgesia with coadministration of preoperative epidural ketamine and midazolam. J Clin Anesth. 2006;18:563–569. doi: 10.1016/j.jclinane.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith OH, Ravussin PA, Decosterd LA, et al. Midazolam premedication and thiopental induction of anaesthesia: interactions at multiple end-points. Br J Anaesth. 1999;83:590–595. doi: 10.1093/bja/83.4.590. [DOI] [PubMed] [Google Scholar]
- Wilson RP, Zagon IS, Larach DR, et al. Antinociceptive properties of tiletamine-zolazepam improved by addition of xylazine or butorphanol. Pharmacol Biochem Behav. 1992;43:1129–1133. doi: 10.1016/0091-3057(92)90492-x. [DOI] [PubMed] [Google Scholar]