Abstract
There is substantial interest in small molecules that can be used to detect or kill the hypoxic (low oxygen) cells found in solid tumors. Nitroaryl moieties are useful components in the design of hypoxia-selective imaging agents and prodrugs because one-electron reductases can convert the nitroaryl group to nitroso, hydroxylamino, and amino metabolites selectively under low oxygen conditions. Here we describe the in vitro, cell free metabolism of a pro-fluorescent substrate, 6-nitroquinoline (1) under both aerobic and hypoxic conditions. Both LC-MS and fluorescence spectroscopic analysis provided evidence that the one-electron reducing enzyme system, xanthine/xanthine oxidase, converted the nonfluorescent parent compound 1 to the known fluorophore 6-aminoquinoline (2) selectively under hypoxic conditions. The presumed intermediate in this reduction process, 6-hydroxylaminoquinoline (6) is fluorescent and can be efficiently converted by xanthine/xanthine oxidase to 2 only under hypoxic conditions. This finding provides evidence for multiple oxygen-sensitive steps in the enzymatic conversion of nitroaryl compounds to the corresponding amino derivatives. In a side reaction that is separate from the bioreductive metabolism of 1, xanthine oxidase converted 1 to 6-nitroquinolin-2(1H)-one (5). These studies may enable the use of 1 as a fluorescent substrate for the detection and profiling of one-electron reductases in cell culture or biopsy samples. In addition, the compound may find use as a fluorogenic probe for detection of hypoxia in tumor models. The occurrence of side products such as 5 in the enzymatic bioreduction of 1 underscores the importance of metabolite identification in the characterization of hypoxia-selective probes and drugs that employ nitroaryl units as oxygen sensors.
Introduction
More than 70 years ago, it was suggested that poor vascularization and high cell densities combine to create a hypoxic (low oxygen) environment in tumors.1,2 It has now been well established that many tumors contain substantial regions of hypoxia and that these hypoxic cells play a significant role in cancer biology.3–7 For example, the hypoxic environment may select for cells that are incapable of undergoing apoptosis.6,8 In addition, there is evidence that the cancer stem cells thought to be responsible for metastases are harbored in the hypoxic niche of tumors.9–14 Therefore, it is not surprising that tumor hypoxia correlates with poor patient prognosis.3,4,15 Accordingly, there is substantial interest in agents that detect or kill the hypoxic cells found in tumor tissue.
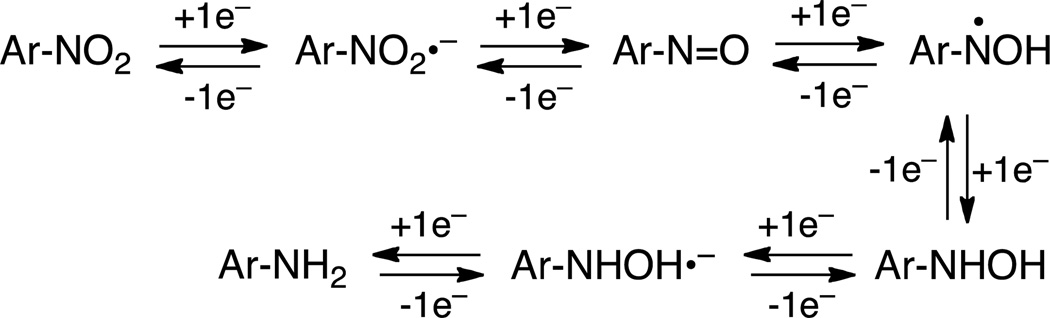
Nitroaryl moieties are useful components in the design of hypoxia-selective imaging agents and prodrugs because one-electron reductases can convert nitroaryl compounds to nitroso, hydroxylamino, and amino metabolites selectively under low oxygen conditions (Scheme 1).16–25 In normal tissue, molecular oxygen inhibits the enzymatic generation of reduced metabolites via back-oxidation of radical anion intermediates (reverse reactions, Scheme 1).21 It is generally assumed that the initially-generated nitro radical anion is the key oxygen-sensitive intermediate that confers hypoxia selectivity to these metabolic processes.19–21 Importantly, hypoxia-selective conversion of the electron-withdrawing nitro group to the electron-donating hydroxylamino or amino substituents constitutes an “electronic switch” that has been exploited for the development of anticancer drug candidates,26,27 radiochemical imaging agents,28 and immunohistochemical stains of hypoxic tissue.29
Scheme 1.
Enzymatic Reduction of Nitroaryl Compounds.
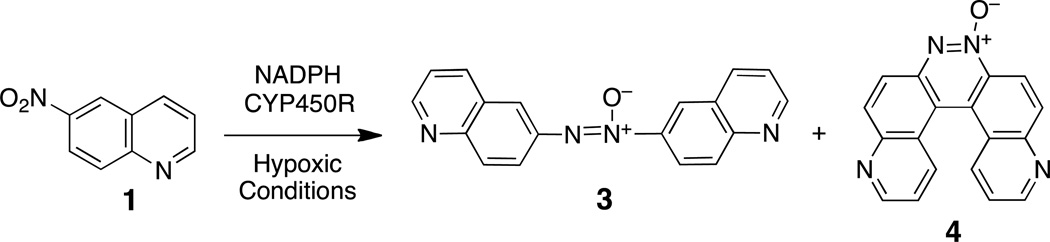
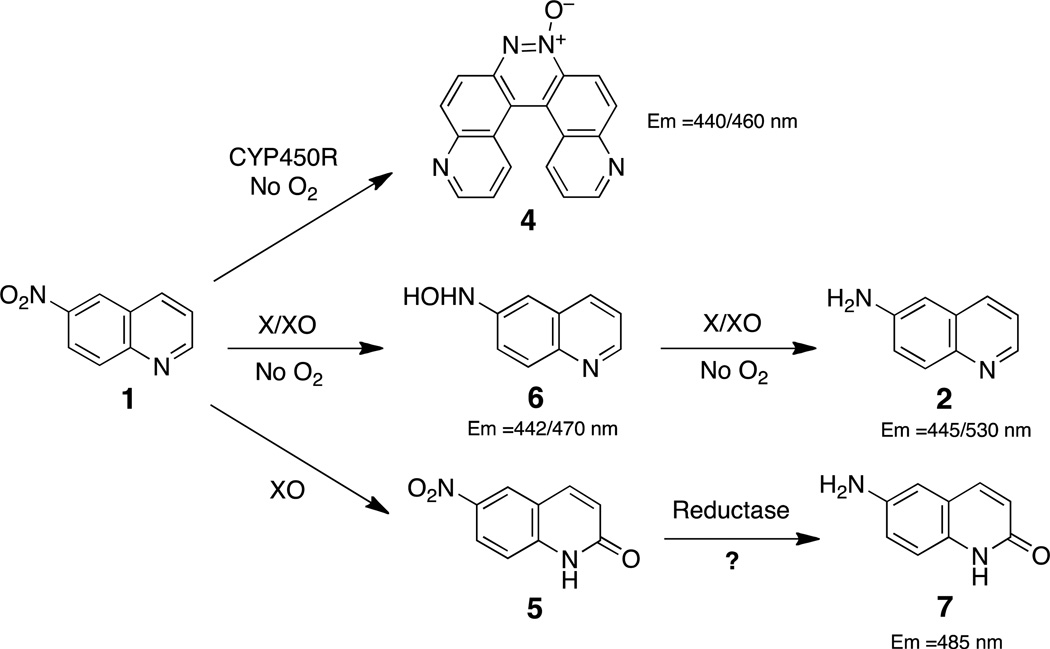
Fluorescent probes for the direct detection of cellular hypoxia would provide a useful complement to existing radiochemical and immunohistochemical imaging methods. One strategy for the development of fluorogenic probes of cellular hypoxia involves identification of nonfluorescent nitroaromatic compounds that can be metabolized to a fluorescent amine derivative.30–34 Molecules of this type could also serve as fluorescent substrates for the detection and profiling of the one-electron reductases involved in bioactivation of hypoxia-selective drugs. Indeed, recent work suggests that bioreductively-activated, hypoxia-selective drugs will benefit from the development of matching probe molecules capable of offering surety that the necessary reductase enzymes are expressed in the target tumor tissue.35,36 Therefore, we set out to characterize the hypoxic metabolism of a pro-fluorescent nitroaryl substrate, 6-nitroquinoline (1). Enzymatic metabolism of this compound has the potential to generate the known fluorophore, 6-aminoquinoline (2, Scheme 2). Compound 2 displays fluorescence emission at 530 nm and a remarkable 205 nm Stokes shift37 and has found use as a fluorescent reporter in a number of applications.38–42 We examined the hypoxic, cell-free, in vitro metabolism of 1 by the xanthine/xanthine oxidase enzyme system. Xanthine oxidase is important in human drug metabolism43,44 and has been employed for the in vitro one-electron reduction of various hypoxia-selective compounds.45–53 Here we describe hypoxia-selective conversion of 1 to the fluorescent product 2 by xanthine/xanthine oxidase enzyme system. These results stand in contrast to the previous finding that the hypoxic metabolism of 1 by NADPH:cytochrome P450 reductase produced the unexpected helicene compound, pyrido[3,2-f]quinolino[6,5-c]cinnoline 3-oxide 4 (Scheme 3).54
Scheme 2.
Scheme 3.
Experimental
Materials and Methods
Materials were from the following sources: sodium phosphate, DMF, Raney nickel, silica gel plates for thin layer chromatography, and silica gel (0.04-0.063 mm pore size) for column chromatography, xanthine oxidase from bovine milk: CAS: 9002-17-9, Sigma X4500-25UN, 24.9 mg protein/mL, 1.3 units/mg protein, 18O-water (H2 18O): CAS: 14314-42-2, Sigma 329878, 97 atom % 18O, were obtained from Sigma-Aldrich (St. Louis, MO), 6-aminoquinoline (2), 6-nitroquinoline (1), and hydrazine hydrate from Alfa-Aesar (Ward Hill, MA), deuterated NMR solvents from Cambridge Isotope Laboratories (Andover, MA), and ethyl acetate, dichloromethane, methanol, hexane, ethanol, HPLC grade water and acetonitrile from Fischer. The non-fluorescent electron acceptor, 1,2,4-benzotriazine-1,4-di-N-oxide, was prepared using literature methods.55 The azoxy compound 3 was prepared as described previously.54
Synthesis of 6-hydroxylaminoquinoline (6)
To a stirred solution of 6-nitroquinoline (1, 0.5 g, 2.87 mmol) in EtOH/CH2Cl2 (1:1, 20 mL) at 0 °C was added a slurry of Raney nickel (0.5 mL). To this mixture under an atmosphere of nitrogen gas, hydrazine hydrate (10 equiv. based on 1) was added dropwise with stirring over the course of 1 h. The solid was removed by filtration, the resulting solution diluted with water (2 mL), and extracted with ethyl acetate (2×10 mL). The combined organic extracts were washed with brine, dried over sodium sulfate, and concentrated by rotary evaporation. Column chromatography on silica gel eluted with ethyl acetate followed by MeOH/CH2Cl2 gave 6 as a yellow solid (100 mg, 25% yield, Rf = 0.1 in MeOH/CH2Cl2 4:96). This compound is unstable upon standing in organic solvents. 1H NMR (CD3OD, 300 MHz) δ 8.53 (d, J = 5.0 Hz, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.82 (m, 1H), 7.33 (m, 3H). 13C NMR (CD3OD, 75.5 MHz) δ 151.4, 147.6, 144.8, 136.7, 131.1, 129.2, 122.5, 121.0, 107.7; HRMS (ESI, M+H+) m/z calcd for C9H9N2O 160.0715, found 160.0707. The structure of this compound was confirmed by single crystal X-ray crystallographic analysis (Rajapakse, Barnes, and Gates, manuscript in preparation).
Enzymatic synthesis of 6-nitroquinolin-2(1H)-one (5)
The compound 6-nitroquinoline (1, 250 mg, 1.32 mmol) was dissolved in warm DMF (1 mL) and sprayed into warm water (300 mL at 70 °C) with vigorous stirring. To this mixture, warm sodium phosphate (100 mL, pH 7.4, 500 mM, 70 °C) was added with stirring. After cooling to ~40 °C, xanthine oxidase (100 µL, 0.005 U/mL) was added every 12 h over the course of 3 d. The mixture was extracted with ethyl acetate (3×30 mL), the combined organic layers extracted with brine (3×30 mL), dried over sodium sulfate, and concentrated by rotary evaporation. Column chromatography on silica gel eluted with ethyl acetate gave 5 as a yellow solid (4 mg, 2% yield, Rf = 0.55, 1% methanol/ethyl acetate). X-ray quality crystals were obtained by dissolving the pure compound in a minimum amount of warm ethyl acetate followed by slow evaporation over the course of 3 d. 1H NMR (CD3OD, 500 MHz) δ 8.64 (d, J = 2.5 Hz, 1H), 8.36 (dd, J = 9.0 Hz, J = 2.5 Hz, 1H), 8.09 (d, J = 9.0 Hz, 1H), 7.46 (d, J = 9.0 Hz, 1H), 6.73 (d, J = 9.0 Hz, 1H); 13C NMR (CD3OD, 125.77 MHz) δ 165.0, 144.1, 144.1, 142.3, 126.3, 125.4, 124.3, 120.6, 117.4; HRMS (ESI, M+H+) m/z calcd for C9H7N2O3 191.0457, found 191.0456. Our crystal structure of this compound was deposited at the Cambridge Crystallographic Data Centre (CCDC 913304).
General procedure for in vitro hypoxic metabolism
For anaerobic reactions, all reagents except xanthine oxidase were degassed by three freeze-pump-thaw cycles in Pyrex tubes. The glass tubes were torch sealed and then opened inside an argon-purged glove bag and bubbled with argon for five minutes. In a typical enzymatic reaction, 1 (4 µL of a 50 mM solution in DMF, final concentration 0.8 mM) or the non fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-di-N-oxide, (24 µL from 50 mM in 15% DMF/water, final concentration 6.4 mM), was mixed with xanthine (20–160 µL of 10 mM, final concentrations 0.8–6.4 mM), xanthine oxidase (20 µL of a 20 U/mL stock solution, final concentration 2.4 U/mL), sodium phosphate buffer (6 µL from a 50 mM stock solution, final concentration 12 mM, pH 7.4) and HPLC grade water to obtain the final solution (0.25 mL final volume, with all reactions containing less than 2% DMF by volume). After mixing, the containers were covered with aluminum foil to prevent exposure to light and incubations were carried out at room temperature (24 °C) inside the inert atmosphere glove bag. Following incubation, anaerobic reactions were opened to air and after approximately 1 h diluted to 1 mL with aerobic sodium phosphate buffer (50 mM, pH 7.4) and the fluorescence measurements were then carried out in a cuvette open to air.
Incorporation of 18O-labeled water in to 6-nitroquinolin-2(1H)-one (5) during xanthine oxidase-mediated oxidation of 6-nitroquinoline (1)
Xanthine oxidase from bovine milk was diluted 4:1 with 18O-labeled water and was incubated at 37 °C in borosilicate glass test tubes under ambient oxygenation for 1 h. Protein was precipitated by the addition of two volumes of MeCN with subsequent centrifugation (3000 rcf, 10 min). The supernatant was dried under a stream of nitrogen and reconstituted in 85:15 (v/v) ammonium formate (10 mM, pH 4.1):MeCN in preparation for LC-MS analysis.
LC-MS analysis
In vitro enzymatic metabolism of 1 and 6 was carried out as described above. The reaction mixtures were extracted with ethyl acetate, the organic layer extracted with brine, and the solvent removed by rotary evaporation. The resulting solid was redissolved in methanol and analyzed by LC-MS in the positive ion mode. LC-MS analyses were carried out using two different methods. In the analysis of the in vitro metabolism of 6 shown in Figure 5, the separation of metabolites was carried using a C18 reverse phase Phenomenex Luna column (5 µm particle size, 100 Å pore size, 150 mm length, 2.00 mm i.d.) and a ThermoSeparations liquid chromatograph (TSP4000) and the metabolites were detected by their UV-absorbance at 254 nm. The elution started with a gradient of A, 99% HPLC water (0.1% acetic acid) and B acetonitrile (0.1% formic acid) followed by a linear increase to 90 % B over the course of 30 min. The elution was continued at 90% B for 3 min and decreased to 1% over the next 8 min. A flow rate of 0.25 mL/min was used. Products were monitored at both 214 and 240 nm. The LC-ESIMS analyses were carried out in the positive ion mode on a Finnigan TSQ 7000 triple quadrupole instrument using a 4.5 kV needle voltage and at a capillary temperature of 250 °C. The analyses shown in Figures 2–4 were carried out using an Agilent 1100 HPLC system was coupled to a Supelco Discovery C18 column (5 µm, 2.1×150 mm; Sigma-Aldrich Chemical Company, St. Louis, MO). Solvent A was 10 mM (pH 4.1) ammonium formate and solvent B was MeCN. The initial mobile phase was 85:15 A:B (v/v) and by linear gradient transitioned to 20:80 A:B over 20 min. The flow rate was 0.400 mL/min. The HPLC eluent was first introduced into an Agilent 1100 DAD (single wavelength selected, 254 nm) followed by electrospray ionization-assisted introduction into a Finnigan LCQ™ Deca XPPLUS ion trap mass spectrometer (Thermo Scientific Corp., San Jose, CA) operated in negative ionization mode. Ionization was assisted with sheath and auxiliary gas (ultra pure nitrogen) set at 60 and 40 psi, respectively. The electrospray voltage was set at 5 kV with the heated ion transfer capillary set at 300 °C and 30 V. Relative collision energies of 25–35% were used when the ion trap mass spectrometer was operated in the MS/MS or MSn mode.
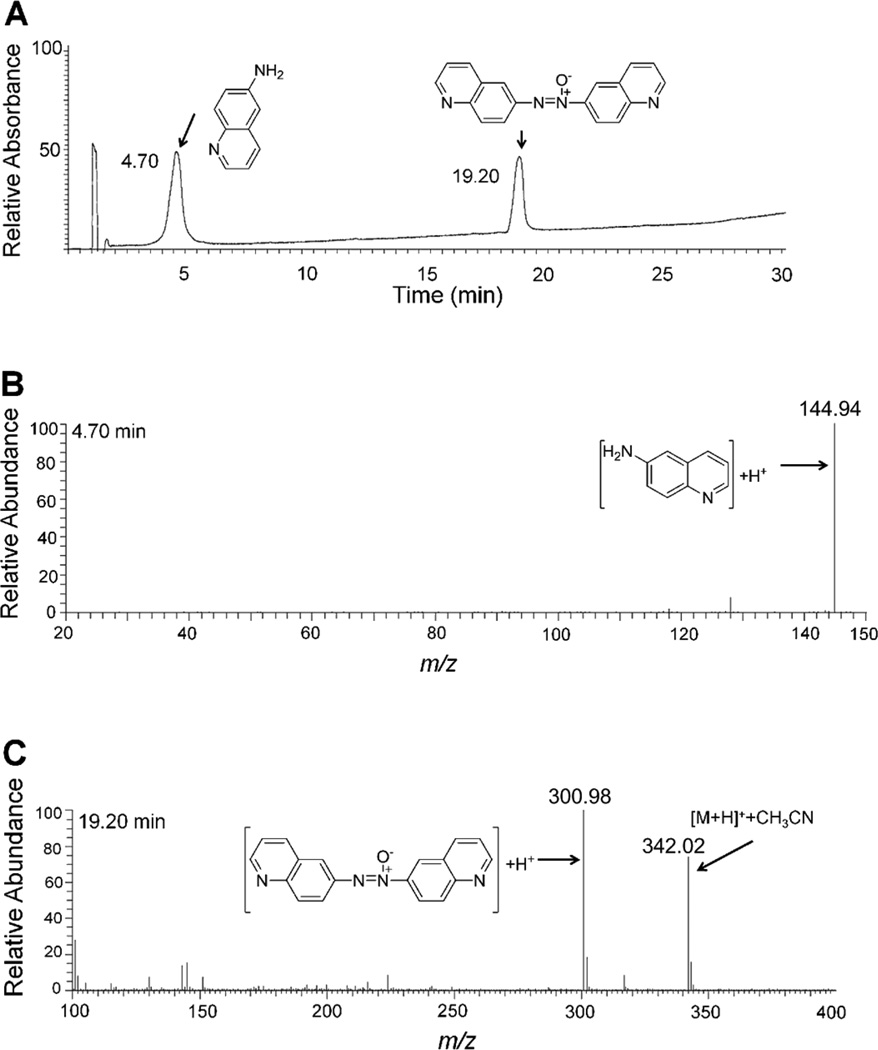
Figure 5.
LC-MS analysis of the reaction mixture generated by anaerobic metabolism of 6 (0.8 mM) by xanthine oxidase (2.4 U/mL) and xanthine (6.4 mM). The enzymatic reduction of 1 was carried out as described in the Experimental Section. Panel A: HPLC trace of the reaction mixture monitoring absorbance at 254 nm, Panel B: LC-MS spectrum of the product eluting at 4.70 min, Panel C: LC-MS spectrum of the product eluting at 19.20 min.
Results and Discussion
Conversion of 1 into fluorescent metabolites by the xanthine/xanthine oxidase enzyme system under hypoxic conditions
In these studies, we used the xanthine/xanthine oxidase enzyme system to carry out reductive metabolism of 1.43–53 For reactions carried out under anaerobic conditions, molecular oxygen was removed from the solutions by three cycles of freeze-pump-thaw degassing and assay mixtures assembled and incubated in an inert atmosphere glove bag.
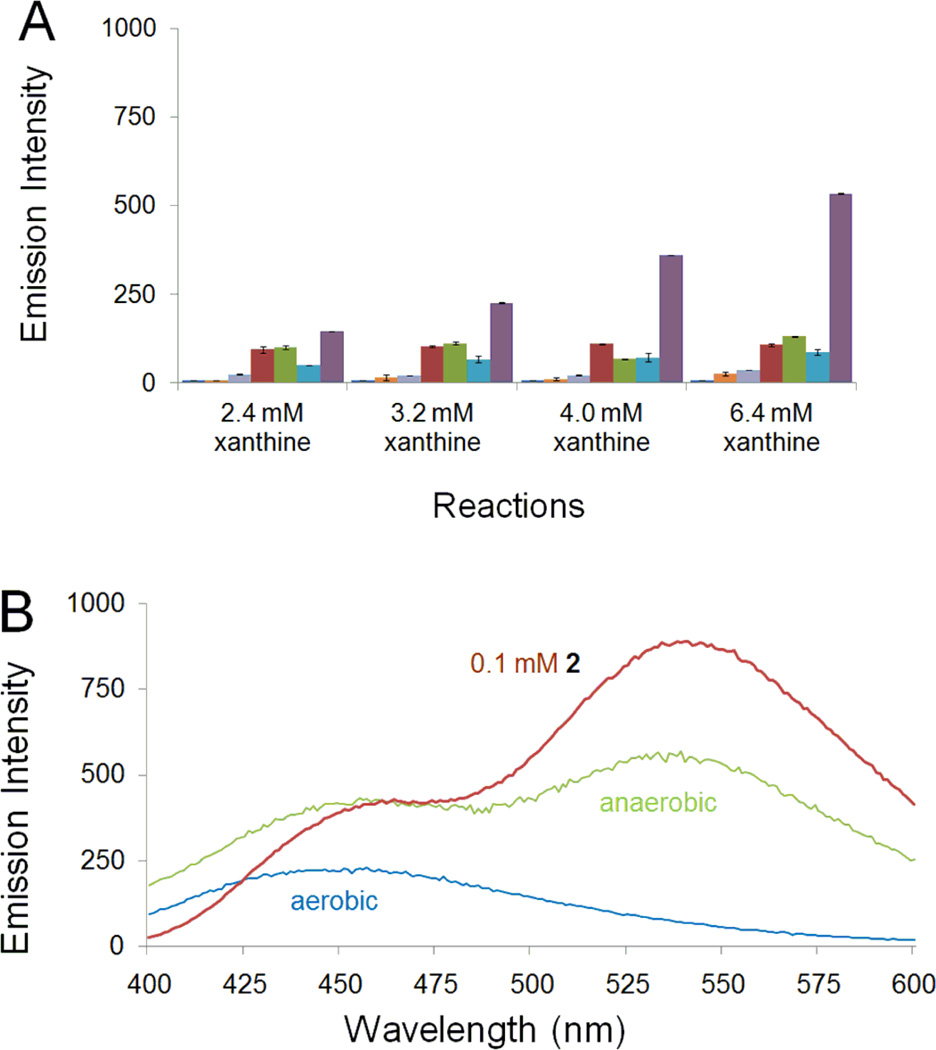
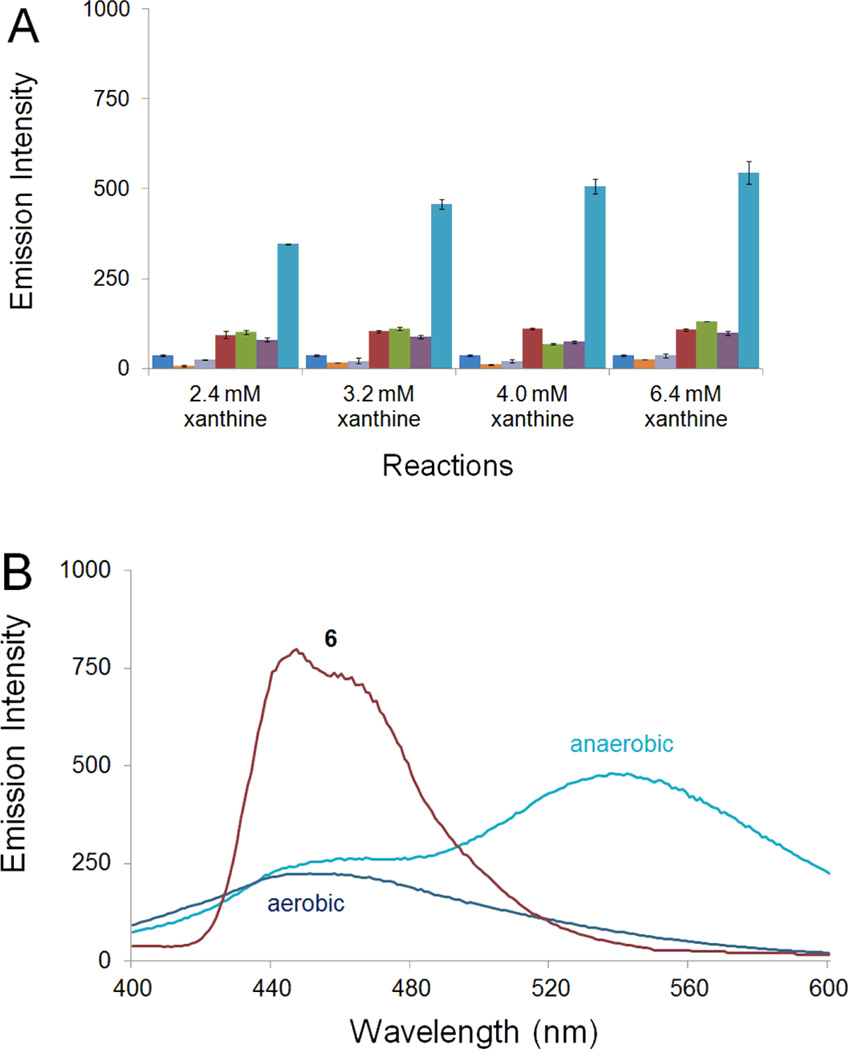
The compound 6-nitroquinoline (1) is not fluorescent in aqueous solution.54 However, incubation of 1 with xanthine (6.4 mM)/xanthine oxidase under hypoxic conditions led to a 12-fold increase in fluorescence emission at 530 nm (versus anaerobic xanthine (6.4 mM)/xanthine oxidase control, Figure 1A). The fluorescence yield increased with increasing concentration of the enzyme substrate xanthine and the fluorescence spectrum of the reaction mixture was clearly distinct from that of the fluorescent helicene, pyrido[3,2-f]quinolino[6,5-c]cinnoline 3-oxide 4 (Scheme 3) observed previously in the hypoxic metabolism of 1 by NADPH:cytochrome P450 reductase.54 Specifically, compound 4 displayed emission maxima at 440 and 460 nm54 while, the hypoxic metabolism of 1 by the xanthine/xanthine oxidase described here generated fluorescence with a broad emission maximum at 530 nm and a shoulder at 445 nm. The emission spectrum resembled that of an authentic standards of 6-aminoquinoline (2) synthesized by catalytic hydrogenation of 1 or obtained from commercial sources (Figure 1B). Importantly, relatively little fluorescence increase at 530 nm was observed when 1 was incubated with xanthine/xanthine oxidase under aerobic conditions (Figure 1B). Control assays containing only xanthine/xanthine oxidase under aerobic or anaerobic conditions, or xanthine/xanthine oxidase with a non-fluorescent electron acceptor in place of 1, similarly displayed little fluorescence at 530 nm (Figure 1A). The modest increase in background fluorescence centered near 450 nm in the control assays containing only xanthine and xanthine oxidase may be due to a previously reported byproduct resulting from decomposition of the enzyme’s pterin cofactor.56 Overall the results suggest that 1 is converted selectively under hypoxic conditions to a product(s) with a fluorescence spectrum matching that of authentic 2.
Figure 1.
Enzymatic conversion of 1 into a fluorescent product selectively under hypoxic conditions. A. Fluorescence emission at 530 nm (λex 340 nm). Each set of assays depicted in the bar graph consists of (from left to right): a control sample of compound 1 alone (0.8 mM)  , a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under aerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under aerobic conditions  , a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under anaerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under anaerobic conditions  , a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide,52 (6.4 mM) under aerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide,52 (6.4 mM) under aerobic conditions  , a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide, (6.4 mM) under anaerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide, (6.4 mM) under anaerobic conditions  , a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 1 under aerobic conditions
, a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 1 under aerobic conditions  , a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 1 under anaerobic conditions
, a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 1 under anaerobic conditions  . Reactions were incubated for 18 h in sodium phosphate buffer at (12 mM, pH 7.4) at 24 °C, diluted with aerobic sodium phosphate buffer (12 mM, pH 7.4), and the fluorescence measured (λex 340 nm, λem 530 nm). B. Fluorescence spectra of reaction mixtures generated in the aerobic and anaerobic metabolism of 1 by xanthine oxidase (2.4 U/mL) and xanthine (6.4 mM) carried out as described in the Experimental Section and fluorescence spectrum of 6-aminoquinoline (2, 0.1 mM, λex 340 nm, in sodium phosphate buffer, 10 mM, pH 7.4).
. Reactions were incubated for 18 h in sodium phosphate buffer at (12 mM, pH 7.4) at 24 °C, diluted with aerobic sodium phosphate buffer (12 mM, pH 7.4), and the fluorescence measured (λex 340 nm, λem 530 nm). B. Fluorescence spectra of reaction mixtures generated in the aerobic and anaerobic metabolism of 1 by xanthine oxidase (2.4 U/mL) and xanthine (6.4 mM) carried out as described in the Experimental Section and fluorescence spectrum of 6-aminoquinoline (2, 0.1 mM, λex 340 nm, in sodium phosphate buffer, 10 mM, pH 7.4).
LC-MS analysis of the products generated by hypoxic metabolism of 1 by xanthine/xanthine oxidase
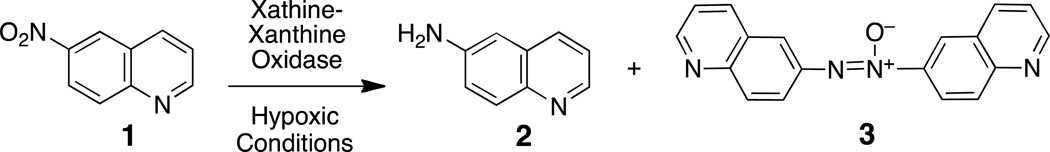
To better understand the molecular origin of the fluorescence generated in the hypoxic metabolism of 1 by the xanthine/xanthine oxidase enzyme system, we employed LC-MS to examine the reaction mixtures. We first characterized the mixture generated by incubation of 1 under hypoxic conditions in the presence of five equivalents of xanthine (Figure 2A). A major product eluting at 2.5 min in the HPLC chromatogram displayed the m/z of 145 expected for the [M+H+] ion of 2. As described above, compound 2 adequately accounts for the fluorescence emission at 530 nm generated by the hypoxic metabolism of 1 by xanthine/xanthine oxidase. A second, relatively minor product eluting around 13 min produced an [M+H]+ at m/z 301 consistent with the compound 6,6’-azoxyquinoline (3, Scheme 3). This compound can be envisioned to arise from the condensation of 6-nitroso and 6-hydroxylamino intermediates generated in the reductive metabolism of 1.57,58 Indeed, LC-MS of an authentic sample of 3 prepared by reduction of 1 with hydrazine hydrate in the presence of Raney nickel54,59,60 matched the 13 min metabolite generated in the hypoxic metabolism of 1.59,60 Compound 3 is not fluorescent in aqueous buffered solution54 indicating that this product did not contribute to the fluorescence observed in Figure 1. We note that the azoxy compound 3 was observed using thin-layer chromatography prior to concentration of the reaction mixtures. Thus, the generation of this dimerization product is not dependent upon evaporation of the samples prior to LC-MS (though we cannot rule out the possibility that concentration of the samples alters the yield of 3).
Figure 2.
LC-MS analysis of the reaction mixture generated by anaerobic metabolism of 1 (0.8 mM) by xanthine oxidase (2.4 U/mL) and xanthine. The reaction shown in panel A contained 4 mM xanthine and that shown in panel B contained 0.8 mM xanthine. Compound 1: retention time (RT) 12.3 min, [M+H]+ 175, fragments 145, 129, 117; 2: RT 2.2 min, [M+H]+ 145, fragments 128, 103; 3: 13.2 min, [M+H]+ 301, fragments 283, 273, 146, 128, 117; 5: RT 11.3 min, [M+H]+ 191, fragments 174, 161, 145, 133.
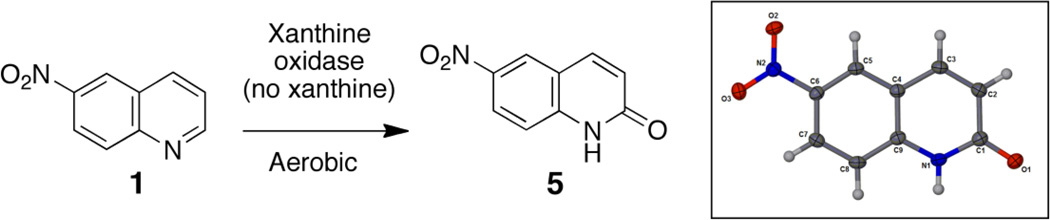
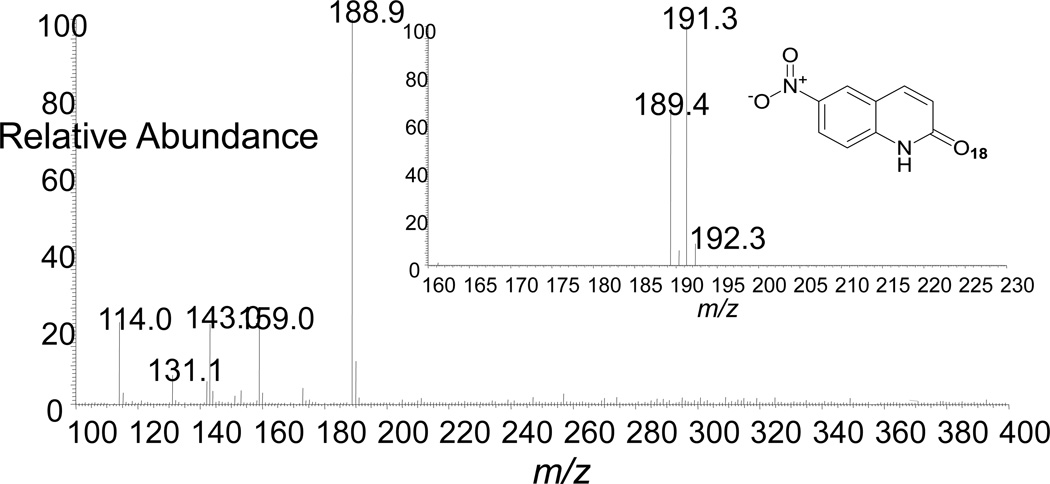
We observed an additional product eluting at about 11.5 min, slightly ahead of the parent probe 1. This compound produced an [M+H]+ at m/z 191, an increase of 16 Da relative to 1. Xanthine oxidase is able to oxidize a number of nitrogen heterocycles – usually at a position adjacent to an endocyclic nitrogen atom44,50,53,61–63 – leading us to suspect that the 11.5 min product might be 6-nitroquinolin-2(1H)-one 5 (Scheme 4). In order to generate quantities of the putative product 5 sufficient for NMR analysis, we incubated 1 with xanthine oxidase (no xanthine substrate) under aerobic conditions (Scheme 4). Spectroscopic analyses of the resulting product were consistent with the 6-nitroquinolin-2(1H)-one structure proposed for the metabolite 5. Specifically, the 1HNMR spectrum of this product was lacking a resonance for the proton adjacent to the endocyclic nitrogen in the parent quinoline heterocycle and the 13C-spectrum showed a new resonance at 165 ppm diagnostic for the carbonyl carbon of the quinolin-2(1H)-one system. Ultimately, X-ray crystallographic analysis confirmed the of structure 5 (Scheme 4).64 With an authentic standard of 5 in hand, we were able to confirm that this product was indeed generated in the metabolism of 1 by xanthine/xanthine oxidase. We further used LC-MS analysis to show that incubation of 1 with xanthine oxidase in H2 18O led to formation of an isotopomer of 5 increased by two Da (Figure 3), consistent with the incorporation of an oxygen atom from water as expected for the oxidation of nitrogen heterocycles by xanthine oxidase.43,50,53,61–63
Scheme 4.
Figure 3.
Incubation of 1 with xanthine oxidase in H2 18O yields 18O-5. LC-ESI(−)-MS of 5 generated by incubation of 1 with xanthine oxidase in H2O. The inset shows LC-ESI(−)-MS of 5 generated by incubation of 1 with xanthine oxidase in a buffered solution containing 80% H2 18O (97% isotopic purity) and 20% H2O.
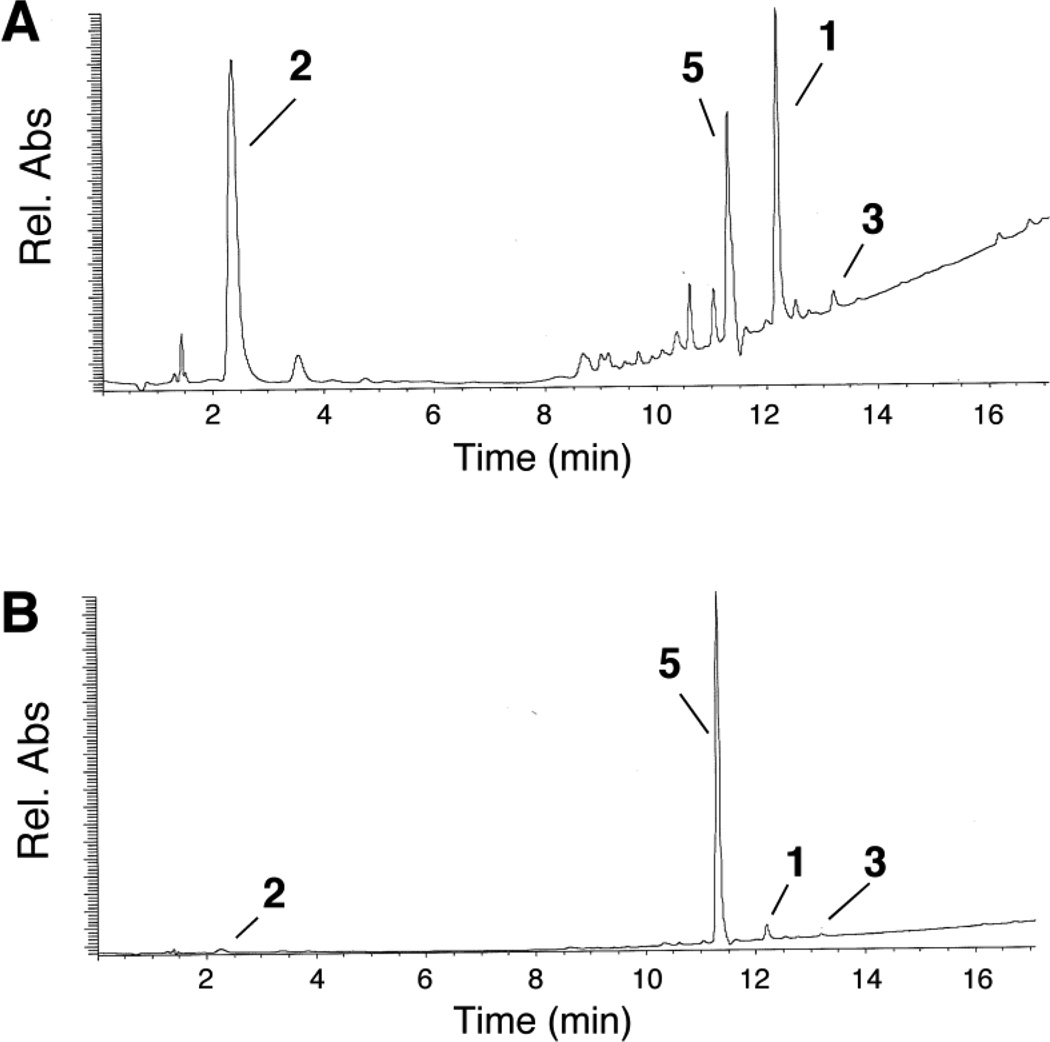
LC-MS analysis revealed that, at lower ratios of xanthine:1 under anaerobic conditions, the same products were generated but in different relative amounts. For example, when only 1 equiv of xanthine was present, the yield of the amino derivative 2 was low, while larger relative amounts of the starting probe 1, the azoxy compound 3, and the 6-nitroquinolin-2(1H)-one 5 were observed (Figure 2B).
Under aerobic conditions at either 1 or 5 equiv of xanthine, the major products were the starting probe 1 and the 6-nitroquinolin-2(1H)-one 5, alongside small amounts of the azoxy derivative 3 (Figure 4). Our LC-MS analyses did not detect 2 in the aerobic incubations of 1 with xanthine/xanthine oxidase, in line with our finding that the incubation of 1 with the enzyme system did not produce fluorescent products under aerobic conditions (Figure 1).
Figure 4.
LC-MS analysis of the reaction mixture generated by aerobic metabolism of 1 (0.8 mM) by xanthine oxidase (2.4 U/mL) and xanthine. The reaction shown in panel A contained 4 mM xanthine and that in panel B contained 0.8 mM xanthine.
Hypoxia-selective conversion of 6-hydroxylaminoquinoline (6) to 6-aminoquinoline (2)
The generation of arylamines via hypoxia-selective metabolism of nitroaryl compounds proceeds via nitroso and hydroxylamino intermediates (Scheme 1).16–18,20,22,23 Indeed, the azoxy compound 3 observed in our reactions likely arises via the condensation of the nitroso and hydroxylamino intermediates.57,58 Under physiological conditions, nitroso intermediates can undergo rapid thiol-mediated reduction to the corresponding hydroxylamino compound,65 meaning that the enzymatic reduction of nitroso compounds to hydroxylamino derivatives may not be important in vivo. In contrast, the enzymatic transformation of arylhydroxylamines to arylamines is a key step in the hypoxic conversion of nitroaryl compounds to arylamines, but this process has not been well studied. To better understand the overall bioreduction of 1 leading to the fluorescent compound 2, we set out to examine the biotransformation of 6-hydroxylaminoquinoline (6) by xanthine/xanthine oxidase under both aerobic and hypoxic conditions.
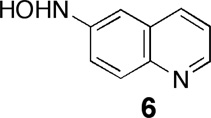
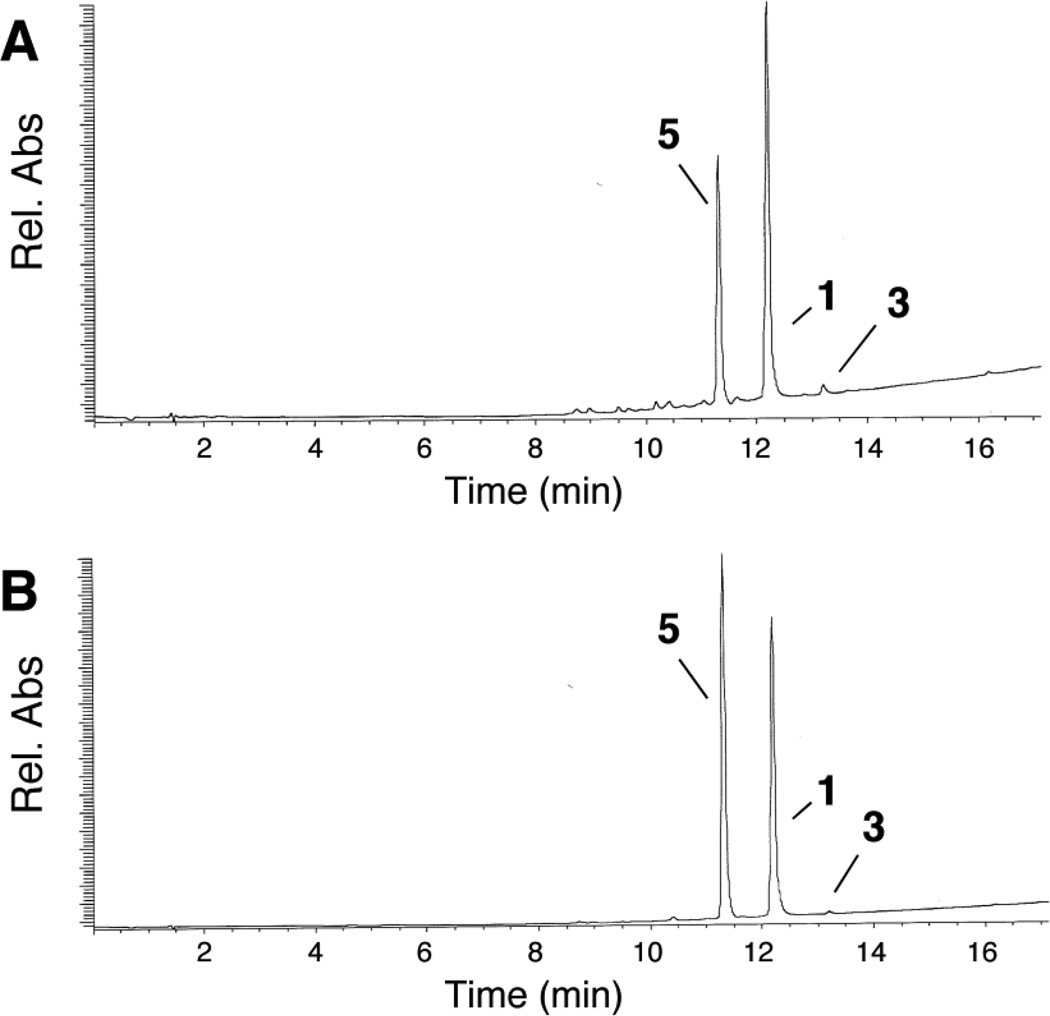
Compound 6 was prepared by the reduction of 6-nitroquinoline using hydrazine hydrate in the presence of Raney nickel.66 LC-MS analysis revealed that incubation of 6 with xanthine/xanthine oxidase under anaerobic conditions generated a mixture of 2 and 3 (Figure 5). The amino compound 2 likely arises from the expected enzymatic reduction of 6. The azoxy compound 3 was unexpected and may result from spontaneous oxidation of the arylhydroxylamine 6 to the corresponding nitroso compound during aerobic reaction workup and analysis,67 followed by condensation of the nitroso with remaining 6.57,58 Compound 6 was fluorescent, displaying emission maxima at 442 and 470 nm similar to the helicene 4,54 but clearly distinct from the emission spectrum of 6-aminoquinoline 2 (Figure 6B). In line with the LC-MS analysis, we found that anaerobic metabolism of 6 by the xanthine/xanthine oxidase enzyme system generates a strong fluorescence emission peak centered at 530 nm, consistent with generation of 2 (Figure 6). In contrast, incubation of 6 with xanthine/xanthine oxidase under aerobic conditions afforded almost no increase in emission at 530 nm. Rather, a modest increase in fluorescence at 450 nm was observed. The emission at 450 nm may represent a combination of fluorescence emission signals from xanthine oxidase degradation products56 and unreacted 6. Overall, these results provide evidence that the conversion of the hydroxylamino compound 6 to the amino derivative 2 mediated by xanthine/xanthine oxidase occurs selectively under hypoxic conditions.
Figure 6.
Enzymatic conversion of 6 into a fluorescent product selectively under hypoxic conditions. A. Fluorescence emission at 530 nm (λex 340 nm). Each set of assays depicted in the bar graph consists of (from left to right): a control sample of compound 6 alone (0.8 mM)  , a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under aerobic
, a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under aerobic  , a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under anaerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL) and xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) under anaerobic conditions  , a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide, (6.4 mM) under aerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide, (6.4 mM) under aerobic conditions  , a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide, (6.4 mM) under anaerobic conditions
, a control reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and the non-fluorescent electron acceptor, 1,2,4-benzotriazine 1,4-dioxide, (6.4 mM) under anaerobic conditions  , a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 6 under aerobic conditions
, a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 6 under aerobic conditions  , a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 6 under anaerobic conditions
, a reaction composed of xanthine oxidase (2.4 U/mL), xanthine (2.4 mM, 3.2 mM, 4.0 mM and 6.4 mM) and 6 under anaerobic conditions  . Reactions were incubated for 18 h in sodium phosphate buffer at (12 mM, pH 7.4) at 24 °C, diluted with aerobic sodium phosphate buffer (12 mM, pH 7.4), and the fluorescence measured (λex 340 nm, λem 530 nm). B. Fluorescence spectra of aerobic and anaerobic reaction mixtures containing 6 (0.8 mM), xanthine oxidase (2.4 U/mL), and xanthine (3.2 mM) and fluorescence spectrum of 6 alone (0.05 mM, λex 340 nm, in sodium phosphate buffer, 10 mM, pH 7.4).
. Reactions were incubated for 18 h in sodium phosphate buffer at (12 mM, pH 7.4) at 24 °C, diluted with aerobic sodium phosphate buffer (12 mM, pH 7.4), and the fluorescence measured (λex 340 nm, λem 530 nm). B. Fluorescence spectra of aerobic and anaerobic reaction mixtures containing 6 (0.8 mM), xanthine oxidase (2.4 U/mL), and xanthine (3.2 mM) and fluorescence spectrum of 6 alone (0.05 mM, λex 340 nm, in sodium phosphate buffer, 10 mM, pH 7.4).
Conclusions
We found that the xanthine/xanthine oxidase enzyme system converted 6-nitroquinoline (1) to 6-aminoquinoline (2) selectively under hypoxic conditions. The metabolism of nitroaryl compounds by the xanthine/xanthine oxidase enzyme system is expected to proceed via a series of one-electron reduction steps that generate nitroso and hydroxylamino intermediates, as shown in Scheme 1.16–24 Indeed, the azoxy product 3 observed in these experiments likely arises via condensation of 6-nitroso and 6-hydroxylaminoquinoline intermediates during the enzymatic reaction or subsequent analysis. Synthesis of the 6-hydroxylaminoquinoline intermediate 6 allowed us to provide evidence that xanthine/xanthine oxidase converted this aryl hydroxylamine to the fluorescent product 2 selectively under hypoxic conditions. This result is striking because the initial one-electron reduction step in the nitro-to-nitroso conversion typically is thought to be the key oxygen-sensitive step that confers hypoxia selectivity to nitroreduction processes.19–21 Our work with 6 provides evidence for an additional oxygen-sensitive step – beyond the initial one-electron reduction of the nitroaryl group – in the hypoxia-selective enzymatic conversion of some nitroaryl compounds to arylamines.
The results described here combined with our previous work54 reveal striking differences in the products generated in the hypoxic metabolism of 1 by the xanthine/xanthine oxidase and NADPH:cytochrome P450 reductase enzyme systems.54 Here we demonstrated that xanthine/xanthine oxidase converted 6-nitroquinoline (1) to 6-aminoquinoline (2) selectively under hypoxic conditions, while our earlier work showed that NADPH:cytochrome P450 reductase generates the helicene product 4 (Scheme 2).54 The mechanism by which the helicene 4 forms remains uncertain, but it seems that NADPH:cytochrome P450 reductase lacks the ability to rapidly reduce the 6-nitroso and/or 6-hydroxylamino intermediates derived from 1 (Scheme 1), thus leaving these species to combine in a manner that generates 4.
Another difference between xanthine oxidase and NADPH:cytochrome P450 reductase is highlighted by our observation that xanthine oxidase converts 1 to the 6-nitroquinolin-2(1H)-one derivative 5 under both aerobic and anaerobic conditions (Scheme 5). This reaction is not altogether unexpected given that xanthine oxidase can oxidize a wide range of nitrogen heterocycles.43,44,50,53,61–63 In principle, reductive metabolism of 5 could generate 6-aminoquinolin-2(1H)-one (7) which is a much brighter fluorophore than 2, with an emission maximum of 485 nm (data not shown); however, we observed no evidence for the formation of 7 in either our LC-MS or fluorescence experiments. Nonetheless, 5 clearly has the potential to serve as a bioreductively-activated, hypoxia-selective fluorescent probe. The relative yields of 5 were lower when greater amounts of xanthine were present in the reaction mixture to drive the reduction of 1 by xanthine oxidase suggesting that, under our anaerobic reaction conditions, the reduction of 1 by the xanthine/xanthine oxidase system was faster than the oxidation of 1 to 5 by xanthine oxidase.
Scheme 5.
Overview of the fluorescent products that can be generated in the hypoxic metabolism of 1.
Our work may enable the use of 6-nitroquinoline (1) as a fluorescent substrate for the detection and profiling of one-electron reductases in cell culture or biopsy samples. The compound also could find use as a fluorogenic probe for the visualization of hypoxia in tumor models, though information regarding the potential toxicity of 1 will be required for this application. The occurrence of side products such as 3, 4, and 5 in enzymatic bioreduction of 1 underscores the importance of metabolite identification in the characterization of hypoxia-selective probes and prodrugs that employ nitroaryl groups as oxygen-sensing units.
Acknowledgments
Funding
We thank the National Institutes of Health for partial support of this work (CA 100757).
References
- 1.Mottram JC. A factor of importance in the radio sensitivity of tumours. Br. J. Radiol. 1936;9:606–614. [Google Scholar]
- 2.Tomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clincal outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 4.Vaupel P. Hypoxia and Aggressive tumor phenotype: Implications for therapy and prognosis. Oncologist. 2008;13:21–26. doi: 10.1634/theoncologist.13-S3-21. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM. The hypoxic cell: A target for selective cancer therapy. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 6.Brown JM, Wilson WR. Exploiting tumor hypoxia in cancer treatment. Nature Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 7.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–409. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 8.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat. Rev. Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RP, Delphine TM-E, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin. Radiat. Oncol. 2009;19:106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 11.Heddleston JM, Li Z, Lathia JD, Bao s, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer. 2010;102:789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Nat. Acad. Sci. USA. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidel S, Garvalov BK, Wirta V, von Stechow L, Schanzer A, Meletis K, Wolter M, Sommerlad D, Henze A-T, Nister M, Reifenberger G, Lundeberg J, Frisén J, Acker T. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133:983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 14.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur M-H, Diebel ME, Monville F, Dutcher J, et al. Breast Cancer Cell Lines Contain Functional Cancer Stem Cells with Metastatic Capacity and a Distinct Molecular Signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fardin P, Barla A, Mosci S, Rosasco L, Verri A, Versteeg R, Caron HN, Molenaar JJ, Ora I, Eva A, Puppo M, Varesio L. A biology-driven approach identifies the hypoxia gene signature as a predictor of the outcome of neuroblastoma patients. Mol. Cancer. 2010;9:185. doi: 10.1186/1476-4598-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardman P, Dennis MF, Everett SA, Patel KB, Stratford MRL, Tracy M. Radicals from one-electron reduction of nitro compounds, aromatic N-oxides and quinones: the kinetic basis for hypoxia-selective, bioreductive drugs. Biochem. Soc. Trans. 1995;61:171–194. doi: 10.1042/bss0610171. [DOI] [PubMed] [Google Scholar]
- 17.Fitzsimmons SA, Workman PA, Grever M, Paull K, Camalier R, Lewis AD. Reductase enzyme expression across the National Cancer Institute tumor cell line panel: correlation with sensitivity to mitomycin C and E09. J. Natl. Cancer Inst. 1996;88:259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- 18.Rooseboom M, Commandeur JNM, Vermeulen NPE. Enzyme-catalyzed activation of anticancer prodrugs. Pharm. Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Hu L. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 2009;29:29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 20.Wilson WR, Anderson RF, Denny WA. Hypoxia-selective antitumor agents. 1. Relationships between structure, redox properties and hypoxia-selective cytotoxicity for 4-substituted derivatives of nitracrine. J. Med. Chem. 1989;32:23–30. doi: 10.1021/jm00121a006. [DOI] [PubMed] [Google Scholar]
- 21.Denny WA, Wilson WR. Considerations for the design of nitrophenyl mustards as agents with selective toxicity for hypoxic tumor cells. J. Med. Chem. 1986;29:879–887. doi: 10.1021/jm00156a001. [DOI] [PubMed] [Google Scholar]
- 22.Walton MI, Wolf CR, Workman P. Molecular enzymology of the reductive bioactivation of hypoxic cell cytotoxins. Int. J. Radiat. Oncol. Biol. Phys. 1989;16:983–986. doi: 10.1016/0360-3016(89)90900-0. [DOI] [PubMed] [Google Scholar]
- 23.Wen B, Coe KJ, Rademacher P, Fitch WL, Monshouwer M, Nelson SD. Comparison of in vitro bioactivation of flutamide and its cyano analogue: evidence for reductive activation by human NADPH:cytochrome P450 reductase. Chem. Res. Toxicol. 2008;21:2393–2406. doi: 10.1021/tx800281h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James AL, Perry JD, Jay C, Monget D, Rasburn JW, Gould FK. Fluorogenic substrates for the detection of microbial nitroreductases. Lett. Appl. Microbiol. 2001;33:403–408. doi: 10.1046/j.1472-765x.2001.01021.x. [DOI] [PubMed] [Google Scholar]
- 25.Helsby NA, Goldthorpe MA, Tang MHY, Atwell GJ, Smith EM, Wilson WR, Tingle MD. Influence of mustard group structure on pathways of in vitro metabolism of anticancer N-(2-hydroxyethyl)-3,5-dinitrobenzamide 2-mustard prodrugs. Drug Metab. Dispos. 2008;36:353–360. doi: 10.1124/dmd.107.018739. [DOI] [PubMed] [Google Scholar]
- 26.Duan J-X, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J, Ma H, Li X, Yang Z, Hoffman RM, Ammons WS, Hart CP, Matteucci M. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J. Med. Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 27.Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel KB, Pullen SM, Hicks KO, Syddall SP, Atwell GJ, Yang S, Denny WA, Wilson WR. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin. Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 28.Evans BJ, Doi JT, Musker WK. Fluorine-19 NMR study of the reaction of p-fluorobenzenethiol and disulfide with periodate and other selected oxidizing agents. J. Org. Chem. 1990;55:2337–2344. [Google Scholar]
- 29.Koch CJ. Measurements of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002;352:3–31. doi: 10.1016/s0076-6879(02)52003-6. [DOI] [PubMed] [Google Scholar]
- 30.Wardman P, Clarke ED, Hodgkiss RJ, Middleton RW, Parrick J, Stratford MRL. Nitroaryl compounds as potential fluorescent probes for hypoxiaI, Chemical criteria and constraints. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:1347–1351. doi: 10.1016/0360-3016(84)90346-8. [DOI] [PubMed] [Google Scholar]
- 31.Stratford MRL, Clarke ED, Hodgkiss RJ, Middleton RW, Wardman P. Nitroaryl compounds as potential fluorescent probes for hypoxia. II. Identification and properties of reductive metabolites. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:1353–1356. doi: 10.1016/0360-3016(84)90347-x. [DOI] [PubMed] [Google Scholar]
- 32.Dai M, Zhu W, Xu Y, Qian X, Liu Y, Xiao Y, You Y. Versatile nitro-fluorophore as highly effective sensor for hypoxic tumor cells: design, imaging, and evaluation. J. Fluoresc. 2008;18:591–597. doi: 10.1007/s10895-007-0303-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhu W, Dai M, Xu Y, Qian X. Novel nitroheterocyclic hypoxic markers for solid tumor: synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2008;16:3255–3260. doi: 10.1016/j.bmc.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Cui L, Zhong Y, Zhu W, Xu Y, Du Q, Wang X, Qian X, Xiao Y. A New Prodrug-Derived Ratiometric Fluorescent Probe for Hypoxia: High Selectivity of Nitroreductase and Imaging in Tumor Cell. Org. Lett. 2011;13:928–931. doi: 10.1021/ol102975t. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Foehrenbacher A, Su J, Patel R, Hay MP, Hicks KO, Wilson WR. The 2-nitroimidazole EF5 is a biomarker for oxidoreductases that activate the bioreductive prodrug CEN-209 under hypoxia. Clin. Cancer Res. 2012;18:1684–1695. doi: 10.1158/1078-0432.CCR-11-2296. [DOI] [PubMed] [Google Scholar]
- 36.Brown JM. Imaging tumor sensitivity to a bioreductive drug. Two for the price of one. Clin. Cancer Res. 2012;18:1487–1489. doi: 10.1158/1078-0432.CCR-11-3267. [DOI] [PubMed] [Google Scholar]
- 37.Molecular-Probes-Website. The Handbook: A guide to fluorescent probes and labeling technologies. 2009 http://www.invitrogen.com/site/us/en/home/References/Molecular-Probes-The-Handbook.html. [Google Scholar]
- 38.Danieli E, Shabat D. Molecular probe for enzymatic activity with dual output. Bioorg. Med. Chem. 2007;15:7318–7324. doi: 10.1016/j.bmc.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 39.Azimi NT, Lytle FE, Huber DM, Whitaker JE, Haugland RP. Multiple reagent aminopeptidase profiling of bacteria. Applied Spectros. 1990;44:400–403. [Google Scholar]
- 40.Tanaka F, Thayumanavan R, Barbas CF. Fluorescent detection of carbon-carbon bond formation. J. Am. Chem. Soc. 2003;125:8523–8528. doi: 10.1021/ja034069t. [DOI] [PubMed] [Google Scholar]
- 41.Brynes PJ, Bevilacqua P, Green A. 6-Aminoquinoline as a fluorogenic leaving group in peptide cleavage reactions: a new fluorogenic substrate for chymotrypsin. Anal. Biochem. 1981;116:408–413. doi: 10.1016/0003-2697(81)90381-x. [DOI] [PubMed] [Google Scholar]
- 42.Huang W, Hicks SN, Sondek J, Zhang Q. A fluorogenic, small molecule reporter for mammalian phospholipase C isozymes. ACS Chem Biol. 2011;6:223–228. doi: 10.1021/cb100308n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison RD, Blobaum AL, Byers FW, Santomango TS, Bridges TM, Stec D, Brewer KA, Sanchez-Ponce R, Corlew MM, Rush R, Felts AS, Manka J, Bates BS, Venable DF, Rodriguez AL, Jones CK, Niswender CM, Conn PJ, Lindsley CW, Emmitte KA, Daniels JS. The role of aldehyde oxidase and xanthine oxidase in the biotransformation of a novel negative allosteric modulator of metabotropic glutamate receptor subtype 5. Drug Metab. Disp. 2012;40:1834–1845. doi: 10.1124/dmd.112.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma R, Eng H, Walker GS, Barreiro G, Stepan AF, McClure KF, Wolford A, Bonin PD, Cornelius P, Kalgutkar AS. Oxidative metabolism of a quinoxaline derivative by xanthine oxidase in rodent plasma. Chem. Res. Toxicol. 2011;24 doi: 10.1021/tx200329k. [DOI] [PubMed] [Google Scholar]
- 45.Laderoute KL, Wardman P, Rauth M. Molecular mechanisms for the hypoxia-dependent activation of 3-amino-1,2,4-benzotriazine 1,4-dioxide (SR4233) Biochem. Pharmacol. 1988;37:1487–1495. doi: 10.1016/0006-2952(88)90010-x. [DOI] [PubMed] [Google Scholar]
- 46.Daniels JS, Gates KS. DNA Cleavage by the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (SR4233): Evidence for Involvement of Hydroxyl Radical. J. Am. Chem. Soc. 1996;118:3380–3385. [Google Scholar]
- 47.Birincioglu M, Jaruga P, Chowdhury G, Rodriguez H, Dizdaroglu M, Gates KS. DNA Base Damage by the Antitumor Agent 3-Amino-1,2,4-benzotriazine 1,4-Dioxide (Tirapazamine) J. Am. Chem. Soc. 2003;125:11607–11615. doi: 10.1021/ja0352146. [DOI] [PubMed] [Google Scholar]
- 48.Jones GDD, Weinfeld M. Dual action of tirapazamine in the induction of DNA strand breaks. Cancer Res. 1996;56:1584–1590. [PubMed] [Google Scholar]
- 49.Chowdhury G, Kotandeniya D, Barnes CL, Gates KS. Enzyme-activated, hypoxia-selective DNA damage by 3-amino-2-quinoxalinecarbonitrile 1,4-di-N-oxide. Chem. Res. Toxicol. 2004;17:1399–1405. doi: 10.1021/tx049836w. [DOI] [PubMed] [Google Scholar]
- 50.Ganley B, Chowdhury G, Bhansali J, Daniels JS, Gates KS. Redox-activated, hypoxia-selective DNA cleavage by quinoxaline 1,4-di-N-oxide. Bioorg. Med. Chem. 2001;9:2395–2401. doi: 10.1016/s0968-0896(01)00163-8. [DOI] [PubMed] [Google Scholar]
- 51.Junnotula V, Rajapakse A, Abrillaga L, Lopez de Cerain A, Solano B, Villar R, Monge A, Gates KS. DNA strand cleaving properties and hypoxia-selective cytotoxicity of 7-chloro-2-thienylcarbonyl-3-trifluoromethylquinoxaline 1,4-dioxide. Bioorg. Med. Chem. 2010;18:3125–3132. doi: 10.1016/j.bmc.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Junnotula V, Sarkar U, Sinha S, Gates KS. Initiation of DNA strand cleavage by 1,2,4-benzotriazine 1,4-dioxides: mechanistic insight from studies of 3-methyl-1,2,4-benzotriazine 1,4-dioxide. J. Am. Chem. Soc. 2009;131:1015–1024. doi: 10.1021/ja8049645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chowdhury G, Sarkar U, Pullen S, Wilson WR, Rajapakse A, Fuchs-Knotts T, Gates KS. DNA strand cleavage by the phenazine di-Noxide natural product myxin under both aerobic and anaerobic conditions. Chem. Res. Toxicol. 2012;25:197–206. doi: 10.1021/tx2004213. [DOI] [PubMed] [Google Scholar]
- 54.Rajapakse A, Gates KS. Hypoxia-selective, enzymatic conversion of 6-nitroquinoline into a fluorescent helicene: pyrido[3,2-f]quinolino[6,5-c]cinnoline 3-oxide. J. Org. Chem. 2012;77 doi: 10.1021/jo3004748. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelson AB, McNamara JP, Pandey A, Ryan KJ, Dorie MJ, McAfee PA, Menke DR, Brown JM, Tracy M. 1,2,4-Benzotriazine 1,4-dioxides. An important class of hypoxic cytotoxins with antitumor activity. Anti-Cancer Drug Design. 1998;13:575–592. [PubMed] [Google Scholar]
- 56.Claassen VP, Oltmann LF, Van't Riet J, Brinkman UAT, Stouthamer AH. Purification of molybdenum cofactor and its fluorescent oxidation products. FEBS Lett. 1982;142:133–137. doi: 10.1016/0014-5793(82)80236-6. [DOI] [PubMed] [Google Scholar]
- 57.Pizzolatti MG, Yunes RA. Azoxybenzene formation from nitrosobenzene and phenylhydroxylamine. A unified view of the catalysis and mechanisms of the reactions. J. Chem. Soc. Perkin. 1990;2:759–764. [Google Scholar]
- 58.Agrawal A, Tratnyek PG. Reduction of nitroaromatic compounds by zero-valent iron metal. Env. Sci. Technol. 1996;30:153–160. [Google Scholar]
- 59.Fletcher TL, Namkung MJ. Derivatives of fluorene. IV. Raney nickel-hydrazine hydrate reduction of various mono- and dinitrofluorene derivatives; some new 9-substituted fluorenes. J. Org. Chem. 1958;23:680–683. [Google Scholar]
- 60.Furst A, Moore RE. Reductions with hydrazine hydrate catalyzed by Raney nickel. II. Aromatic nitro compounds to intermediate products. J. Am. Chem. Soc. 1957;79:5492–5493. [Google Scholar]
- 61.Rastelli G, Costantino L, Albasini A. A model of the interaction of substrates and inhibitors with xanthine oxidase. J. Am. Chem. Soc. 1997;119:3007–3016. [Google Scholar]
- 62.McCormack JJ, Allen BA, Hodnett CN. Oxidation of quinazoline and quinoxaline by xanthine oxidase and aldehyde oxidase. J. Heterocyclic Chem. 1978;15:1249–1254. [Google Scholar]
- 63.Krenitsky TA, Neil SM, Elion GB, Hitchings GH. A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch. Biochem. Biophys. 1972;150:585–599. doi: 10.1016/0003-9861(72)90078-1. [DOI] [PubMed] [Google Scholar]
- 64.da Silva LE, Joussef AC, Foro S, Schmidt B. 6-Nitroquinolin-2(1H)-one. Acta Cryst. E. 2005;61:o2992–o2993. [Google Scholar]
- 65.Kazanis S, McClelland RA. Electrophilic intermediate in the reaction of glutathione with nitrosoarenes. J. Am. Chem. Soc. 1992;114:3052–3059. [Google Scholar]
- 66.Böge N, Kruger S, Schroder M, Meier C. A New Short and Efficient Synthetic Route to C8-N-Acetylarylamine 2'-Deoxyguanosine Phosphoramidites. Synthesis. 2007;24:3907–3914. [Google Scholar]
- 67.Becker AR, Sternson LA. Oxidation of phenylhydroxylamine in aqueous solution: A model for study of the carcinogenic effect of primary aromatic amines. Proc. Nat. Acad. Sci. USA. 1981;78:2003–2007. doi: 10.1073/pnas.78.4.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]