Abstract
Health inequities are a common problem for all countries and are the result of not only adverse social conditions but also poor public policies. Today chronic diseases represent the most relevant threats and are a current challenge. Parasitic infections, a leading cause of child morbidity affecting low-income populations, can be transmitted because of an unhealthy environment. Notwithstanding, scarce data have been published on the epidemiological profile of intestinal parasitoses in asymptomatic children living in shantytowns. Vulnerable populations settled in slums are growing in Argentina, particularly in Buenos Aires city. Consequently, this work intended to screen healthy carriers of enteric parasites and determine the epidemiologic profile in asymptomatic children residing in one of those communities, to explore risk factors associated with the transmission of parasites, and to initiate a basic health education campaign to promote healthy behavior in the community.
Fecal samples (n = 138) were analyzed by conventional parasitological methods and a survey gathered data on symptoms, family composition, and environmental and hygiene-related variables. High prevalence of feco-orally-transmitted parasitoses (83.3%) and polyparasitism were remarkable findings. The main environmental health determinants were those related to excreta disposal and water provision. Health promotion actions were performed through the diffusion of a set of posters with iconic images and brief messages for health education.
Results suggest the need for an environmental sanitation policy to complement health promotion actions. It is essential to spread the results of investigations that address inequities and social determinants of health in order to integrate data with local political processes and alert on acceptable actions for developing appropriate interventions.
Keywords: Intestinal parasitoses, Asymptomatic children, Vulnerable populations, Urban health, Environmental and public health, Social and environmental health determinants, Shantytown, Risk assessment
Introduction
The environment is one of the major determinants of human health; consequently any unplanned urban expansion can affect the health status. Usually the environmental components can be considered both as resources (water, food, cooking facilities, housing, health-care) and as hazards (domestic and industrial wastes, vectors). Issues involved in each component are dissimilar: for resources, the realistic problem affects availability, access, and cost; for risks, issues entail contact with people, prevention, suppression, or even amelioration. Eventually, another aspect of concern is the relationship with other people in the neighborhood.1
Infectious diseases are a leading cause of child morbidity affecting low-income populations, and easily transmitted through the environment. Among them, water- and excreta-related diseases2 include various intestinal parasitoses, which are very frequent in populations residing in poor sanitary environments, although a more complex synergism with social risk factors cannot be disregarded.1 From this perspective, lack of education, high numbers of siblings, deficient hygiene practices, unsatisfactory water supply, inadequate garbage handling, and a poor household sanitation level have been associated with parasitoses.3–6 They usually tend to chronicity and can remain asymptomatic, thus undiagnosed for long periods.7 By far the most common effect on health is a subtle constraint on normal physical and cognitive development.8,9 Furthermore, infant and childhood mortality differentials between areas within a city have been attributed mainly to infectious and parasitic diseases.1
National epidemiological morbidity data are unavailable in Argentina because intestinal parasite infections are not communicable diseases; consequently, scarce reports sometimes show alarming information.10 On the other hand, as a consequence of the deep economic crisis which affected the country during 2001–2003, gaps in health-related social inequalities became widened and populations in shantytowns experienced disproportionate growth.11 This was the situation in Villa 21–24, whose population rose 115% during 2002–2006.
In the aforementioned context, this project was intended to screen healthy carriers of enteric-parasites and determine the epidemiological profile of children living in Villa 21–24 as well as to explore risk factors associated with the transmission of parasites and, finally, to initiate a basic health education campaign to promote healthy behavior in the community.
Methods
Study area and population
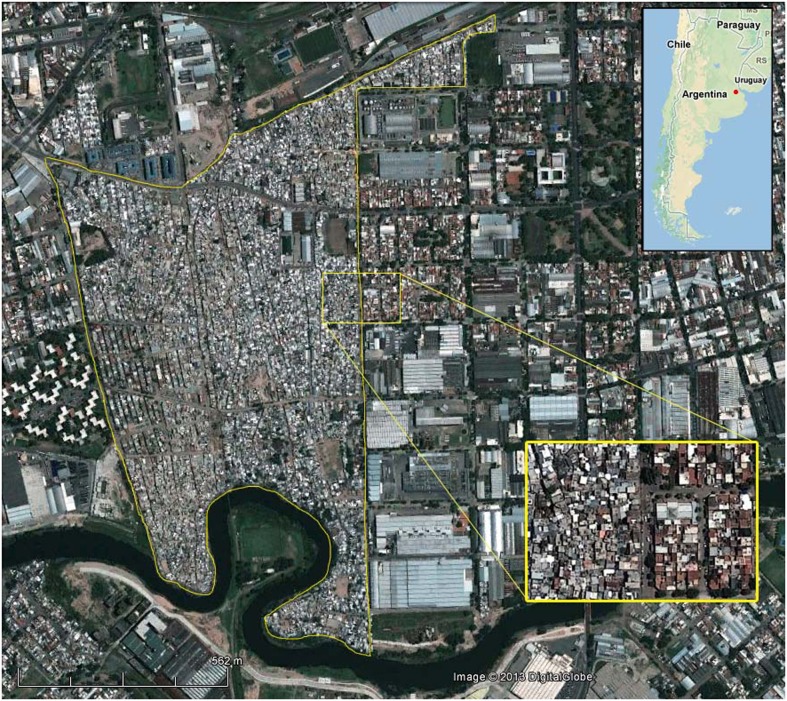
The study area was Villa 21–24, a shantytown (villa miseria) with approximately 30 000 inhabitants, which at the time the survey was performed, represented nearly 1% of the whole population of Buenos Aires. Unofficial records estimate 6000 children live in this area. Villa 21–24 is built on about 65 ha at the south of the city (34°39'S, 58°22'W) in an industrial area bordered on the Riachuelo (Fig. 1), the most polluted river in Argentina, where the main contaminants are untreated industrial effluents and sewage. Therefore, the population density is estimated to be close to 46 800 inhabitants per km2, almost four times the population density of Buenos Aires city.12 Precarious houses are made without planning along the narrow and wiggly passageways, which demarcate 30 irregular blocks. Dwellings are identified by both block and house numbers.
Figure 1.
Geographical location of Villa 21–24. Villa 21–24 is located at the south of the city of Buenos Aires, in a zone which is dominated by industries and also includes small residential areas. The yellow line is used to demarcate the boundaries in the original satellite image. The Riachuelo River can be distinguished on the southern edge and railroad tracks on the northern one. A section of the border zone (yellow box, below right) is expanded to illustrate the difference in the density of dwellings with surrounding neighborhoods (3 inches = 200 m). The approximate location of Villa 21–24 is identified with a red dot on the map of Argentina (top right).
Study sample and survey
The entire program was part of an investigation requested by the Health and Human Rights Committee of Villa 21–24, which is composed of members of the community and professionals from diverse disciplines (psychologists, anthropologists, sociologists, social workers, nurses, health promoters, and physicians). The research team was composed of chemists, biochemists, biologists, mathematicians, and students of biology, health professions, and social sciences.
The intended sample for the present study included 200 children, drawn as a systematic random subsample. Specifically, one out of every eight households was invited to take part in the study. To avoid potential sources of bias only one of their children was selected randomly using a lottery method. To be eligible the children had to: (i) reside in Villa 21–24 and (ii) be between six months and 13 years old. Children who had been treated for intestinal parasites in the previous two months were excluded from the study. When a family refused, the next house was chosen. At the beginning of the dialog, the scope of the study was explained and consent from the parents or the primary caretaker of the child was obtained and recorded.
All interviews were conducted on weekends by non-medical, trained field workers or members of the Health Committee of the Villa 21–24, who visited the area in groups of two accompanied by a dweller who knew the vicinity.
The survey gathered data on family composition, access to health-care services, characteristics of dwelling and toilet facilities, type of drinking water supply, sanitation, and other hygiene-related variables to assemble socio-demographic and epidemiological information. Additionally, a structured questionnaire for recognizing parasitoses-related symptoms was filled for each child. Symptoms covered by the questionnaire were: nasal and anal itching, diarrhea or bloody stools, nausea and vomiting, abdominal discomfort, fatigue, weight loss, a worm in vomit or stool, bruxism, sleep disturbance, dry cough, and allergies.
The procedure for stool sample collection (repeated over seven consecutive days) was explained verbally to all children and their parents, who were provided with labeled containers with 15 ml sodium acetate–acetic acid–formalin. Seven glass slides with a cellophane adhesive tape for Graham’s test were given to parents, and the methodology for collection was also described. To facilitate communication with dwellers and their comprehension of stool collection procedures, an auxiliary tool previously created to overcome illiteracy was employed.12 Two sets of validated instructions, each consisting of six drawings in a comic strip format, were used to describe the stool collection for the diagnosis of enteric parasite infection and to illustrate the transparent adhesive tape test for enterobiosis detection.12
The local Health-Care Center was closely involved with the study, assuming the responsibility to provide the appropriate antiparasitic treatment. Results of the parasitological analysis were sent to the pediatrician who cited families for prescribing pharmacotherapy through the network of social workers and health promoters. Mothers attending scheduled meetings received information about parasites and ways of preventing infections.
As a consequence of the actions undertaken for sharing the results, there was a late incorporation of families who knew the program but did not participate in the survey sample. Their children were examined for parasitological infections and the results were kept outside the statistical analysis.
Resources for the graphical communication strategy
Spreading the results of the entire program required the creation of graphical material formatted as bulletins, leaflets, and posters. Many graphical aspects were accounted for achieving the best way to get the message across the community, few of those to be mentioned are: adequate contrast between the ink and the paper to facilitate reading; utilization of large font size to avoid the need for glasses for reading; the use of simple language and style, appropriate to the educational level of the population; the use of vocabulary reflecting the language commonly used among the dwellers; simple drawings to create a center of attention.12
Laboratory procedures
Graham samples were examined directly by light microscopy for Enterobius vermicularis diagnosis. Fecal samples were processed by the flotation technique in NaCl (saturated solution) and by a centrifugation method. In order to recognize and diagnose protozoan trophozoites, the conventional centrifugation procedure was modified in our laboratory. Briefly, washing was done always using saline solution and the lipid extraction step was avoided. At least two fresh preparations (one of them iodine-stained) were examined by light microscopy (400× magnification). The finding of cysts morphologically compatible with Entamoeba histolytica and/or Entamoeba dispar was corroborated in fixed specimens stained with trichrome reagent and examined by light microscopy (1000× magnification). Only those samples exhibiting trophozoites with nuclear features of E. histolytica and/or E. dispar were considered positive and diagnosed as E. histolytica/dispar.13 Cryptosporidium sp. was diagnosed by indirect immunofluorescence assay employing mouse-anti-Cryptosporidium parvum oocyst monoclonal antibody (anti-Cry-moAb; Chemicon International Inc., Temecula, CA, USA) diluted to 1:600 as the primary antibody, and fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse IgG (FITC-anti-mouse IgG; Chemicon International Inc., Temecula, CA, USA) as the secondary antibody (1:600 dilution). A 5 μl volume of concentrated stool sample was dropped individually onto 12-well multispot slides for microscopy, left to dry (37°C, 10 minutes) and fixed with absolute methanol (5 μl, room temperature, 5 minutes). Oocysts were incubated with anti-Cry-moAb (37°C, 60 minutes, humidified atmosphere). After careful washing with phosphate buffered solution (PBS), samples were labeled by the FITC-anti-mouse IgG (37°C, 30 minutes, humidified atmosphere), washed with PBS, and mounted with anti-fade medium containing 2% 1,4-diazabicyclo-[2,2,2]-octane (DABCO, Sigma-Aldrich, Saint Louis, MO, USA) in PBS:glycerol (40:60). Slides were examined by epifluorescence microscopy under 400–600× magnification.
Statistical methods
Continuous variables like age were made categorical by constructing intervals. Children were assigned to five age groups according to familial, social, and cultural habits. The first group included children up to the age of two (n = 16), who spend most of the time with their parents. Second age group: children aged 2–5 years (n = 44) who are sent to the nursery school or child-care centers. At the age of five, children would be included in the system of compulsory public education, attending kindergarten and later primary school. For this reason the next groups were split into three-year age periods (5–8 years, n = 34; 8–11 years, n = 27; 11–14 years, n = 17). Age ranges were constructed to include the lower limit and exclude the upper limit.
Data analysis was performed employing statistical softwares Infostat14 and Epidat v.3.1. To assess association analyses among environmental, social, and parasitological variables only surveys with no missing data and complete parasitological diagnosis were considered. Variable associations were studied using the Pearson chi-square test of independence with a significance of α = 0.05 and the Odds Ratio (OR) was used to explore the strength of the relationship. To detect confounder factors data were assigned to strata defined by the potential confounding variable. Care was taken in verifying whether stratification variables were related to the disease or exposure, therefore the Mantel–Haenszel’s OR was calculated.
Prevalence of the disease and the corresponding ratio were estimated in exposed and non-exposed children. To describe the likelihood of developing a parasitic disease from exposure to a given risk factor, the prevalence of exposure was calculated in parasitized and non-parasitized children. The ratio of exposure was obtained as a ratio between the percentage of parasitized exposed children and the percentage of non-parasitized exposed children.
Results
Survey analysis
The analysis of socio-demographic characteristics from the family surveys showed that 46% of the interviewed household heads came from neighboring countries (Paraguay, 42%; other countries 4%), 30% were from various Argentine provinces, and 24% from the Buenos Aires city.
The employment situation was precarious and most of the people lived below the poverty line: 21% of individuals were unemployed or received government aid or social welfare benefits, while 65% received low remuneration for unskilled labor. Moreover, 40% of the main family breadwinner was a woman. In addition, to meet food needs 63.5% of families sent their children to soup kitchens or to the school refectory at least once a day.
The illiteracy rate was high, as 16% of the population had not completed their elementary education.
Parasitological analysis
Surveys were fulfilled by 200 families and complete parasitological data were obtained from 138 children (63 girls; 75 boys), resulting in a compliance of 69.9%. Among participants, only 23 children (16.7%) were found not infected with intestinal parasites. Almost one-third of the infected children showed a single-parasite infection (29.7%) and nearly 54% of them harbored two or more parasite species concurrently (29.0, 13.0, 8.7, and 2.9% for two, three, four, or five parasites, respectively). The overall prevalence of intestinal parasitoses was 83.3% (95% CI: 77.1–89.6%; n = 115 out of 138).
The most ubiquitous parasites were those that were direct life cycle transmitted by the feco-oral route (80.0%, n = 110). Among them, whole water-borne parasitoses (including hymenolepiosis and protozoal infections) showed the highest dominance (71.7%, n = 99), and enterobiosis accounted for the highest frequency (41.7%, n = 55). In the latter, only 2 out of the 55 cases detected by Graham’s test could also be detected by the stool sample examination. The only soil-transmitted helminth diagnosed was Ascaris lumbricoides, and the only parasite with indirect life cycle was Fasciola hepatica. Table 1 displays the more frequently recorded parasites.
Table 1. Prevalence of enteroparasitoses.
| Parasite | Positive casesa | Prevalence (%)b | 95% CIc |
| Endolimax nana | 49 | 35.5 | 27.4–43.6 |
| Blastocystis hominis | 41 | 29.7 | 22.0–37.4 |
| Giardia lamblia | 38 | 27.5 | 20.1–35.1 |
| Entamoeba coli | 22 | 15.9 | 9.8–22.1 |
| Entamoeba histolytica/dispar | 17 | 12.3 | 6.8–17.9 |
| Cryptosporidium sp. | 5 | 3.6 | 4.7–6.8 |
| Chilomastix mesnili | 1 | 0.7 | 0.01–2.2 |
| Iodamoeba bütschlii | 1 | 0.7 | 0.01–2.2 |
| Hymenolepis nana | 2 | 1.5 | 0.0–3.4 |
| Enterobius vermicularis | 55d | 41.7 | 33.2–50.2 |
| Ascaris lumbricoides | 11 | 8.0 | 3.4–12.5 |
| Fasciola hepatica | 1 | 0.7 | 0.01–2.2 |
a Total number of fecal samples N = 138.
b Calculated as percentage of infected children with respect to the total sample.
c CI: confidence interval.
d Number of Graham′s test examined for E. vermicularis N = 132.
Analysis of clinical symptoms
Nearly all children were asymptomatic and, even though any symptom was stated, none was statistically associated either with single parasites or with polyparasitism.
Influence of socio-demographic variables on the occurrence of parasitoses
The distribution of parasitic infections according to sex showed no differences when analyzed all together; neither did the frequency of single-parasite species. By contrast, a rise in the prevalence of parasitic infection with age becomes evident and both variables were found associated (Pearson chi-square: 22.72; df: 4; P: 0.0001; n = 138). Particularly, E. vermicularis reached its peak value in the range of 8–11 years (Pearson chi-square: 17.61; df: 4; P: 0.0015; n = 132). It is noteworthy that the prevalence of parasitism in children older than 5 years was greater than 90% (Pearson chi square: 10.40; df: 4; P: 0.0013; n = 138) (Table 2).
Table 2. Percentage of infected children according to the age range.
| 0–2 years | 2–5 years | 5–8 years | 8–11 years | 11–13 years | ||||||
| n = 16a | n = 44a | n = 34a | n = 27a | n = 17a | ||||||
| Parasite | n | % | n | % | n | % | n | % | n | % |
| B. hominis | 0 | 0.0 | 13 | 29.5 | 12 | 35.3 | 12 | 44.4 | 4 | 23.5 |
| Cryptosporidium sp | 0 | 0.0 | 2 | 4.5 | 2 | 5.9 | 1 | 3.7 | 0 | 0.0 |
| E. histolytica/dispar | 1 | 6.3 | 5 | 11.4 | 6 | 17.6 | 3 | 11.1 | 2 | 11.8 |
| E. vermicularis | 1 | 6.3 | 13 | 29.5 | 17 | 50.0 | 18 | 66.7 | 6 | 35.3 |
| G. lamblia | 2 | 12.5 | 10 | 22.7 | 9 | 26.5 | 10 | 37.0 | 7 | 41.2 |
| Parasitism | 7 | 43.8 | 36 | 81.8 | 31 | 91.2 | 25 | 92.6 | 16 | 94.1 |
| Water-borne parasitoses | 6 | 37.5 | 29 | 65.9 | 27 | 79.4 | 23 | 85.2 | 14 | 82.4 |
| Polyparasitism | 4 | 25.0 | 22 | 50.0 | 20 | 58.8 | 17 | 63.0 | 11 | 64.7 |
Total number of children surveyed N = 138, except for E. vermicularis where N = 132.
a Total number of children in each age range.
By exploring whether the kindergarten or school attendance (which often involves overcrowding) was associated with parasitic disease, a significant association was found among the variables analyzed (Table 3). The ratio of disease indicates the occurrence of more cases of parasitism (35%), feco-oral parasitoses (54%), and polyparasitism (50%) among children attending school. Likewise, ratios of exposure imply that the proportion of infected children attending school is two times that of the healthy children when considering parasitoses (ratio 2.1) or feco-oral parasitoses (ratio 2.3) but only 30% higher for polyparasitism (ratio 1.3).
Table 3. Analysis of the association among parasitic disease indexes and school attendance.
| Odds ratio | Pearson chi2; P | Prevalence of diseasea | Prevalence of exposurec | |||||
| Enrolled (%) | Non enrolled (%) | Ratio b | Infected (%) | Non-infected (%) | Ratio d | |||
| Parasitism | 5.1 | 12.63; <<0.001 | 91.3 | 67.4 | 1.35 | 73.0 | 34.8 | 2.1 |
| Feco-oral parasitoses | 6.5 | 18.84; <<0.001 | 90.2 | 58.7 | 1.54 | 75.5 | 32.1 | 2.3 |
| Polyparasitism | 2.3 | 5.83; 0.016 | 62.0 | 41.3 | 1.50 | 75.0 | 56.4 | 1.3 |
a Prevalence of disease in enrolled and non-enrolled children measures the number of children with parasites in each group (exposed and unexposed) compared to the total population in both groups.
b Ratio compares the prevalence of disease in children attending school (exposed children) with that of those not enrolled in an educational institution and that remain in their homes (unexposed).
c Prevalence of exposure in infected and non-infected children measures the proportion of parasitized and non-parasitized children among all children in school (exposed children).
d Ratio compares the prevalence of exposure of infected children with that of non-infected among those attending school (exposed children).
Evaluating the attributable risk
Endolimax nana, the non-pathogenic protozoan most commonly diagnosed (Table 1), was deemed indicative of fecal contamination and was also positively associated with taking meals at a school refectory (chi square: 5.61, P: 0.0179). On the other side, E. histolytica/dispar was associated with attendance at a soup kitchen (chi square: 3.90, P: 0.0483) and absence of a toilet water tank (chi square: 6.68; P: 0.0098). No other parasite showed a significant association with social or environmental variables.
The source of water was not associated with feco-oral parasitoses in spite of 92.7% of families consuming water from the public network and only 7.3% using bottled or boiled water for drinking and cooking.
Table 4 summarizes the association of parasitic infection with environmental variables which account for the method of excreta disposal or are related to peculiarities of housing construction. Among the former, the absence of a water tank to flush the toilet (which involves pouring water with a bucket to remove excreta) and the drainage into a cesspool reveal a prevalence of disease consistently higher in exposed children (ratios 1.19 and 1.26). Building the house on areas that flood when it rains and the location of the bathroom on the outside determine comparable prevalence of disease (ratios 1.21 and 1.24). However, the analysis of the prevalence of exposure shows that the major risk factors are the toilets with drainage to a cesspit (ratio 6.11) or their construction outside the house (ratio 5.34).
Table 4. Association of parasitic infection with environmental variables.
| Risk factor | Pearson chi2; P | N | Odds ratio | Stratified by | Confounding factor | Prevalence of diseasea | Prevalence of exposurec | ||||
| Exposed (%) | Unexposed (%) | Ratiob | Infected (%) | Non-infected (%) | Ratiod | ||||||
| Feco-oral parasitoses | |||||||||||
| Absence of toilet water tank | 4.00; 0.046 | 138 | 2.33 | Toilet location | No | 85.4 | 71.4 | 1.19 | 63.6 | 42.8 | 1.48 |
| Toilet drainage | No | ||||||||||
| Toilet drainage to a cesspool | 5.01; 0.025 | 138 | 7.53 | Toilet location | No | 96.0 | 76.1 | 1.26 | 21.8 | 3.57 | 6.11 |
| Outside toilet location | 4.01; 0.045 | 138 | 6.37 | Toilet drainage | No | 95.5 | 76.7 | 1.24 | 19.1 | 3.57 | 5.34 |
| Flooded land | 4.33; 0.038 | 138 | 3.17 | Toilet drainage | No | 90.5 | 75.0 | 1.21 | 34.5 | 14.3 | 2.42 |
| Pin worm infection (E. vermicularis) | |||||||||||
| Absence of toilet water tank | 3.90; 0.048 | 132 | 2.07 | Toilet location | No | 48.7 | 31.4 | 1.55 | 69.0 | 51.9 | 1.33 |
| Toilet drainage | No | ||||||||||
| Toilet drainage to a cesspool | 4.23; 0.040 | 132 | 2.58 | Toilet location | No | 60.9 | 37.6 | 1.62 | 25.5 | 11.7 | 2.18 |
| Flooded land | 4.20; 0.040 | 132 | 2.19 | Toilet location | No | 55.0 | 35.9 | 1.53 | 40.0 | 23.4 | 1.71 |
| Toilet drainage | |||||||||||
| Soil-transmitted helminthiases (Ascaris lumbricoides) | |||||||||||
| House built on a dirt passage | 5.12; 0.024 | 138 | 4.39 | Flooded land | No | 14.3 | 3.66 | 3.91 | 72.7 | 37.8 | 1.92 |
a Prevalence of disease in exposed and unexposed children measures the number of children with parasites in each group (exposed and unexposed to the corresponding risk factor) compared to the total population in both groups.
b Ratio compares the prevalence of disease in children exposed to the risk factor with that of those not exposed.
c Prevalence of exposure in infected and non-infected children measures the proportion of parasitized and non-parasitized children among all children exposed to the corresponding risk factor.
d Ratio compares the prevalence of exposure of infected children with that of non-infected children among all those exposed to the risk factor.
From the same table it can be observed that the prevalence of A. lumbricoides infection is four times higher in children living in houses constructed in unpaved areas of the slum (ratio 3.91) and the risk of exposure for parasitized children is twice of that for uninfected ones (ratio 1.92). None of the associations presented in Table 4 were affected by confounding variables.
Table 5 displays the analysis of associations of parasitoses with social variables. Considering places where children take their meals as risk factors, children having meals at school refectories or school soup kitchens had higher prevalence for feco-oral parasitoses. Clearly, the chance of infection is increased in children receiving their major meals away from home, whether kids eat at school (ratio of exposure: 2.42) or if they do it in any of the dining rooms of the shantytown (ratio of exposure: 3.08).
Table 5. Association of parasitic infection with social variables.
| Risk factor | Pearson chi2; P | N | Odds ratio | Stratified by | Confounding factor | Prevalence of diseasea | Prevalence of exposurec | ||||
| Exposed (%) | Unexposed (%) | Ratiob | Infected (%) | Non-infected (%) | Ratiod | ||||||
| Feco-oral parasitoses | |||||||||||
| Eating at school refectory | 8.31; 0.004 | 138 | 3.94 | Meals at soup kitchens | No | 90.5 | 70.7 | 1.28 | 51.8 | 21.4 | 2.42 |
| Eating at soup kitchens | 4.73; 0.030 | 138 | 4.65 | Meals at school refectory | No | 92.2 | 75.4 | 1.22 | 26.6 | 8.62 | 3.08 |
| Pin worm infection (E. vermicularis) | |||||||||||
| Eating at school refectory | 6.43; 0.011 | 132 | 2.48 | Meals at soup kitchens | No | 53.2 | 31.4 | 1.69 | 60.0 | 37.6 | 1.59 |
| Breadwinner (mother) | 5.24; 0.022 | 132 | 2.29 | — | — | 54.7 | 33.7 | 1.62 | 51.8 | 31.1 | 1.66 |
a Prevalence of disease in exposed and unexposed children measures the number of infected children in each group (exposed and unexposed to the corresponding risk factor) compared to the total population in both groups.
b Ratio compares the prevalence of parasitosis in children exposed to the risk factor with that of those not exposed.
c Prevalence of exposure in infected and non-infected children measures the proportion of parasitized and non-parasitized children among all children exposed to the corresponding risk factor.
d Ratio compares the prevalence of exposure of infected children with that of non-infected children among all those exposed to the risk factor.
Pinworm infections proved to be associated with attendance to school refectories or with the mother as breadwinner (Table 5). Children who stayed under the care of other relatives showed the highest prevalence of E. vermicularis (Table 1). There was no association between the breadwinner level of education or even overcrowding and parasitoses or polyparasitism (P > 0.05).
Actions leading to the adoption of healthy household practices
These actions included the diffusion of results, a simple educational strategy, and the promotion of healthy practices.
The return of the results to the community stakeholders had the underlying purpose of initiating an educational process to elicit household decision making aimed at generating healthy practices. The overall project results were reported by two means: meetings held in canteens or soup kitchens and through the distribution of newsletters and leaflets delivered by hand (Fig. 2). The latter included four questions whose answers were the trigger of messages to prevent infection and promote healthy actions: ‘what are parasites?’; ‘how they come into our body?’; ‘how can they harm us?’ and ‘how to prevent their entry to our body?’
Figure 2.
Bulletins to disseminate the results. The newsletters were designed as two-sided leaflets. The external and internal faces are displayed in upper and lower panels, respectively. The design of iconic images and the selection of texts for information were made by students from the University of Buenos Aires.
The cultural diversity, resulting from the high proportion of immigrants and migrants, determined the need for colloquial expressions that might be understood by all subjects. Thus, special attention was devoted to establish the semantic equivalence of the language employed to disseminate the information according to the social, economic, and cultural characteristics of the population in Villa 21–24.
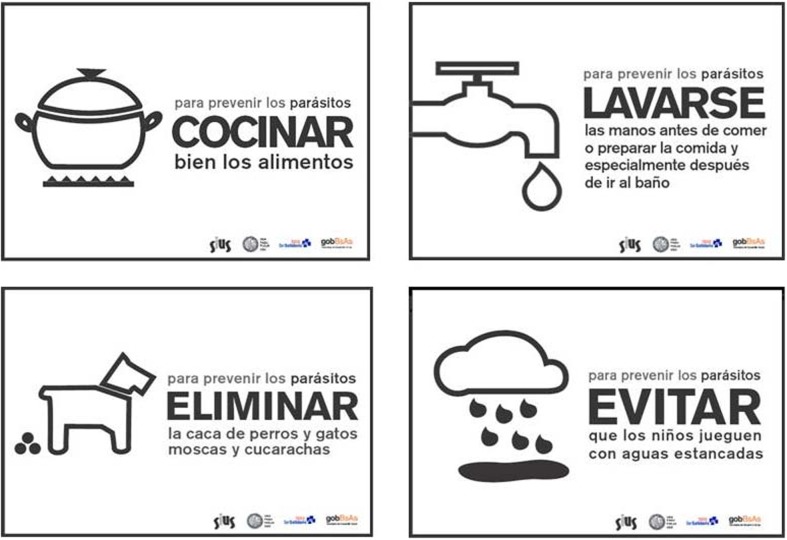
A set of A3 posters were drawn to illustrate the messages intended to encourage the adoption of healthy household practices schematically (Fig. 3). Posters were distributed on neighborhood soup kitchens and the health center as well. To reduce variations of the perceived objects and to produce a positive effect on the recipients of the message certain characteristics related to graphic expression and technological features had to be considered, which conditioned the design. Obviously, the words usually employed to represent the same object in different cultures do not always refer to the same object,15 hence texts were written trying to find a balance of synthesis without compromising on the content to be transmitted. Especially, the key words of the message of health-care and prevention of parasitic diseases were emphasized and reinforced. For the typeface a simple font for easy reading was selected. Texts were accompanied by pictures and graphs that allow a quick and easy identification. From the technological point of view, economic formats suitable for photocopying or duplication and simple printing systems were proposed. The reproduction was performed by members of a community cooperative printing.
Figure 3.
Messages for health education. The A3 size posters were prepared in a landscape format and printed in a cooperative located in the same slum. The texts mention brief educational messages to prevent the transmission of parasitoses and zoonoses. The design of the iconic images and text writing were performed by students from the University of Buenos Aires.
Discussion
Epidemiological studies on the prevalence of intestinal parasites in populations living in urban slums worldwide are scarce.5,16,17 These reports generally involve people with diarrhea, abdominal pain, nausea or vomiting.16–18 The present study provides one of the first quantitative estimations of this health problem in asymptomatic children in shantytowns of Argentina and constitutes a preliminary approach to the determinants of health having an effect on asymptomatic intestinal parasitoses.
The Villa 21–24 child population has a high prevalence rate of water- and excreta-related intestinal parasitoses, mainly those that are feco-orally-transmitted. It is noteworthy that, even when analyzing asymptomatic children, the prevalence of the disease is up to three times higher than those reported by others in children with symptoms.16–18 Polyparasitism was a remarkable finding and the predominant pathogen species were E. vermicularis, B. hominis, G. lamblia, and, E. histolytica/dispar. The low prevalence of soil-transmitted helminthiases could be ascribed to morbidity control, based on the periodical administration of anthelminthic treatments9 because many pediatricians implement deworming strategies without previous diagnosis.11 Consequently, the other parasites remain underdiagnosed.
The low enterobiosis prevalence reported in most studies, even considering heavily feco-oral-infected populations, could be attributed to the use of unsuitable diagnostic methodology.16–19 In our hands, the Graham's test proved to be highly sensitive compared to stool examination.
Health determinants refer to the variety of external conditions that affect health (e.g. the physical environment, pathogenic agents, income, and education). Unemployment, illiteracy, poor housing conditions, overcrowding, and lack of health insurance adversely have impact on the people and thus contribute to their social exclusion and that of their own family.20 In this work, social health determinants were explored in relation to water- and excreta-transmitted parasitoses. School attendance proved to be associated with the presence of feco-orally-transmitted parasites. Similar findings have been reported for a child population in two suburban communities in which the prevalence of parasites increased with age and school attendance.21 The continuous exposure to risk factors over time, both close contact with classmates and overcrowding, are frequent in public educational establishments and nurseries, increasing the probability of infection with the age of children. The lack of epidemiological surveillance contributes to widening the infection rate.
The structure and characteristics of relationships among people within a community affect health in different ways. Hence, risk factors are modified by individual, family, or community behavior (e.g. housing, food, informal health-care).22 This study and others16 fail to detect association between intestinal parasitoses and parental education level, almost certainly because environmental and sanitary conditions worsen risks among children. Notwithstanding, intensive parental care seems to be a protective factor against pinworm, as the highest prevalence was detected in children whose mothers were the breadwinners.
In this work, attendance at school refectories and/or kitchen soups of the neighborhood are critical social factors involved in the acquisition of intestinal parasitoses. Furthermore, lack of administration of pharmacological therapy adds to the unhealthy living conditions. However, even considering that treatments are given, they may be ineffective if the above-mentioned social as well as environmental health determinants are not simultaneously reduced.
The environment, both built and natural, is an essential determinant of health, particularly for low-income groups that have less options to select the location to set up their homes.23 Access to water, sanitation, and hygiene are some of the environmental factors with special incidence on the health of people living in low-income urban settings.20 Health equity in urban settings may be improved through interventions dealing with drinking water, sanitation, solid waste removal, and wastewater drains, among others24 while local authorities are the crucial actors with the appropriate capacity to compensate for these inequities and improve the welfare of the population.23
Poor-quality housing conditions are often associated with many health problems in children20 and, in this work, the most relevant environmental health determinants contributing to parasitoses were those related to excreta disposal and water provision. The high relative risk of inhabiting a house with drainage to cesspool suggests that interventions at this level could reduce the prevalence of feco-oral parasitic infections. Cesspools frequently get covered by rain water and, consequently, sewage gushes to the surface. This observation is supported by the increased number of parasitic infections diagnosed in children living in flood plains. However, it should also be considered that the precarious sewage system was constructed by neighbors, employing polyvinylchloride pipes, to conduct excreta through a cistern network located in the corridors of the shantytown, which finally drain into the main sewer network outside the Villa 21–24 or to the Riachuelo River. Open drainage pipes and uncovered septic tanks frequently observed along corridors are other environmental health determinants for children because the flooding enhanced fecal dispersion. Similarly, the absence of a toilet water tank is a risk factor probably because delivering water with a bucket favors splashing and stool dispersal.
Infrastructural irregularities are limited not only to the sewer system, but also to the water supply system. Mostly, water reaches the house through a shared-connection between neighbors usually manufactured with a rubber hose in such a way that water leaks from the joints. The resulting water supply system is a messy net of pipes precariously coupled, buried a few centimeters beneath, and in some places sticking out of the floor, easily in touch with sewage leaks or flood waters. The water provision system stands as a risk factor for parasite infection in homes as well as in neighborhood soup kitchens. Consequently, consumption of bottled or boiled water does not protect people because of the simultaneous use of unsafe tap water for cleansing food and cooking utensils.
It has been suggested that actions are needed so that low-income communities can move from the condition of vulnerability which affects their health to resilience.20 In this context, we proposed an innovative approach to design an awareness campaign targeted at families with limited access to information on health-care with the aim to ensure sustainable changes in health practices. The use of iconic images representing real-world structures enables a visual discourse, which encompasses the communicative purpose of the sender and the appropriate inferential interpretation by the receiver. The intuitive knowledge that people have about the iconic images is caused by the simple analogy between the picture and the real object. Thus, the image acts as if it were the same thing; its perception is immediate and does not require specialized learning. At the semantic level, the image refers to the historical social usage of the icon in which it says something about reality.25
Conclusions
Asymptomatic children of Villa 21–24 exhibit a high prevalence of water- and excreta-related intestinal parasites. Several risk factors converge in the deterioration of the health quality. Unsafe drinking water acts as a passive vehicle for infective agents (water-borne parasitoses), but even assuming that water was safe, the inadequate or insufficient attention to personal hygiene results in a route of transmission for other parasites such as pinworms.
In conjunction with the defective water distribution network, main health determinants for Villa 21–24 dwellers are the rudimentary sewer system and the open drainage gutters in flooded areas. These difficulties are admitted by local non-government organizations, such as the Committee of Health and Human Rights, which claim to city governments. Municipal authorities are responsible for addressing sanitation and infrastructure works to reduce the impact of enteroparasitoses. Any intervention on the environmental determinants of health problems will have a clear effect on environmental diseases. There is no doubt that conflicts among the different levels of the national government, or even across local government agencies, may generate an additional impediment at the time of managing the economic resources to finance improvements in the urban infrastructure and services.23 Nevertheless, the seriousness involved in these observations makes it unrealistic to even think of mitigating risk through the accomplishment of health education and health promotion activities, since changes in personal hygiene habits will have little effect if betterment in excreta disposal and safe water provision are ignored. It is essential for the equitable development of communities that government authorities address the specific needs of vulnerable groups, socioeconomically disadvantaged, living in slums, and lacking basic public services. Depending on the completion of these actions and the degree of commitment made by public policymakers it will be possible to change the course set by environmental degradation, for a sustainable and healthy future.
Conflict of Interests
The authors declare that they have no conflict of interests.
Authors' Contributions
GG conceived and designed the study protocol, coordinated the study implementation, designed the sampling strategy, was involved in all stages of the fieldwork, performed parasitological diagnosis and statistical analysis, interpreted data, and wrote the manuscript. MPB and LG participated in sample collection and surveys, and contributed to drafting and revising the manuscript. LLA, SF, and LG carried out laboratory analysis and contributed to drafting the manuscript. LLA and SF performed the immunofluorescense assays for Cryptosporidium sp diagnosis. LLA contributed to the literature search. SF prepared the figures and tables. ASH assisted in the statistical analysis of data and surveys, and revised the manuscript. AEM and JCF were in charge of the direction and leadership of the group of students who designed the newsletters and posters with messages for health education and, contributed to drafting the manuscript. ABN is the principal of the research project for social emergency (grant X702) which gave rise to this publication, conceived and designed the study protocol, and took the responsibility for critical revision of the manuscript. NB took the responsibility for the intellectual interpretation and critical revision of the manuscript for publication. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the members of the Human Rights and Health Committee of the Villa 21–24, the interviewers, the dwellers, and the families who participated in this survey. In addition, they wish to thank all the college students who participated actively during the various stages involved in the project, providing the knowledge acquired in the subjects of different undergraduate courses and the teachers directly responsible for such work .This work was funded by Universidad de Buenos Aires [Grant: X702 to ABN] [Grant: R022 to NB] and Gobierno de la Ciudad de Buenos Aires, Secretaría de Cultura [Grant: Fondo Cultura BA to GG].
References
- 1.Bradley D, Stephens C, Harpham T, Cairncross S.A review of environmental health impacts in developing country cities.,1st edn Washington, DC: The World Bank; 1992. pxi [Google Scholar]
- 2.Mara DD, Feachem RGA. Water and excreta-related disease: unitary environment classification. J Environ Eng. 1999:334–9. [Google Scholar]
- 3.Nematian J, Nematian E, Gholamrezanezhad A, Asgari AA. Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop. 2004;92(3):179–86. doi: 10.1016/j.actatropica.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers A.Quantifying selected major risks to health In: Campanini B, Haden A, (eds.) The world health report 2002. Reducing risks, promoting healthy lifeGeneve: World Health Organization; 2002. pp47–97. [Google Scholar]
- 5.Teixeira JC, Heller L. Impact of water supply, domiciliary water reservoirs and sewage on faeco-orally transmitted parasitic diseases in children residing in poor areas in Juiz de Fora, Brazil. Epidemiol Infect. 2006;134:694–8. doi: 10.1017/S0950268805005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller L. Environmental determinants of infectious and parasitic diseases. Mem Inst Oswaldo Cruz. 1998;93(Suppl 1):7–12. doi: 10.1590/s0074-02761998000700002. [DOI] [PubMed] [Google Scholar]
- 7.Benguigui Y, Bossio JC, Fernández HR.(eds)Operations research on integrated management of childhood illness (IMCI). Series FCH/CA-AIEPI 27.IWashington, DC: Pan American Health Organization; 2005. pxviii [Google Scholar]
- 8.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30(6):1457–64. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Deworming for health and development Geneva: World Health Organization; 2005. p64 [Google Scholar]
- 10.Salomón MC, Tonelli RL, Borremans CG, Bertello D, De Jong LI, Jofré CA, et al. Prevalencia de parásitos intestinales en niños de la ciudad de Mendoza, Argentina. Parasitol Latinoam. 2007;62:49–53. [Google Scholar]
- 11.Zeballos JL.(ed.)Argentina: efectos sociosanitarios de la crisis, 2001–2003, 1st edn Buenos Aires: Organización Panamericana de la Salud; 2003. p108 [Google Scholar]
- 12.Buyayisqui MP, Bordoni N, Garbossa G. Overcoming language and cultural barriers: a graphical communication tool to perform a parasitological screening in two vulnerable populations from Argentina. J Health Commun. 2013;18:92–104. doi: 10.1080/10810730.2012.688242. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Amoebiasis. Wkly Epidemiol Rec. Geneve. 1997;Apr(14):97–9. [PubMed] [Google Scholar]
- 14.Di Rienzo J, Casanoves F, Balzarini M, Gonzalez L, Tablada M, Robledo C. 2011. InfoStat. Córdoba, Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba. [Google Scholar]
- 15.Allott R.The physical foundation of language, 1st edn Knebworth: Able; 2001. p285 [Google Scholar]
- 16.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):e3680. doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Moraes Neto AHA, Pereira APMF, Alencar MdeFL, Souza-Júnior PRB, Dias RC, Fonseca JG, et al. Prevalence of intestinal parasites versus knowledge, attitudes, and practices of inhabitants of low-income communities of Campos dos Goytacazes, Rio de Janeiro State, Brazil. Parasitol Res. 2010;107(2):295–307. doi: 10.1007/s00436-010-1861-7. [DOI] [PubMed] [Google Scholar]
- 18.Mirdha B, Samantray J. Hymenolepis nana: a common cause of paediatric diarrhoea in urban slum dwellers in India. J Trop Pediatr. 2002;48(6):331–4. doi: 10.1093/tropej/48.6.331. [DOI] [PubMed] [Google Scholar]
- 19.Matthys B, Bobieva M, Karimova G, Mengliboeva Z, Jean-Richard V, Hoimnazarova M, et al. Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors. 2011;4(1):195. doi: 10.1186/1756-3305-4-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harpham T. Urban health in developing countries: what do we know and where do we go? Health Place. 2009;15(1):107–16. doi: 10.1016/j.healthplace.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Gamboa MI, Basualdo JA, Kozubsky L, Costas E, Cueto Rua E, Lahitte H. Prevalence of intestinal parasitosis within three population groups in La Plata, Argentina. Eur J Epidemiol. 1998;14(1):55–61. doi: 10.1023/a:1007479815249. [DOI] [PubMed] [Google Scholar]
- 22.Vlahov D, Freudenberg N, Proietti F, Ompad D, Quinn A, Nandi V, et al. Urban as a determinant of health. J Urban Health. 2007;84(3 Suppl):i16–26. doi: 10.1007/s11524-007-9169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization In: Grady M, Goldblatt P, (eds.) Addressing the social determinants of health: the urban dimension and the role of local governmentCopenhagen: World Health Organization; 2012. p56 [Google Scholar]
- 24.Kjellstrom T, Mercado S, Sami M, Havemann K, Iwao S. Achieving health equity in urban settings. J Urban Health. 2007;84(1):i1–6. doi: 10.1007/s11524-007-9192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pericot J. Graphic images. From implicit meaning to inferred sense. Formats. J Audiovisual Commun. 2005;4:9. [Google Scholar]