Abstract
Tissue macrophages are an important cellular reservoir for replication of human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus. In vitro, the ability of macrophages to support viral replication is differentiation dependent in that precursor monocytes are refractory to infection. There is, however, no consensus as to the exact point at which infection is restricted in monocytes. We have revisited this issue and have compared the efficiencies of early HIV-1 replication events in monocytes and in differentiated macrophages. Although virus entry in monocytes was comparable to that in differentiated macrophages, synthesis of full-length viral cDNAs was very inefficient. Relative to differentiated macrophages, monocytes contained low levels of dTTP due to low thymidine phosphorylase activity. Exogenous addition of d-thymidine increased dTTP levels to that in differentiated macrophages but did not correct the reverse transcription defect. These results point to a restriction in monocytes that is independent of reverse transcription precursors and suggest that differentiation-dependent cellular cofactors of reverse transcription are rate limiting in monocytes.
The presence of an extravascular reservoir of human immunodeficiency virus type 1 (HIV-1)-infected macrophages is well established (6, 7, 10, 12, 30, 31). The ability of tissue macrophages to serve as a reservoir for HIV-1 and simian immunodeficiency virus replication correlates with the ability of monocyte-derived macrophages to support productive viral infection in vitro (reviewed in reference 10). Before entering the tissues and differentiating into macrophages, monocytes briefly circulate in peripheral blood (28, 29). Several studies indicate that circulating monocytes also serve as a viral reservoir in infected individuals (15-17, 33). The infection of circulating monocytes in vivo is at odds with in vitro studies, where monocytes acquire the ability to support productive viral infection only after their differentiation to macrophages (2, 4, 5, 19, 20, 23, 25). While most studies have suggested that the viral replication cycle in monocytes is restricted at a point prior to establishment of the provirus, the exact point of restriction is a matter of debate. Chemokine receptor expression has been shown to increase during in vitro differentiation of monocytes, but this did not account fully for differences in infectibility (4, 19). Macrophages from different donors have been shown to support HIV-1 infection to highly variable degrees, but the ability to support infection was independent of receptor/coreceptor expression and was manifest at the level of late reverse transcription (5). HIV-1 pseudotyped with the envelope glycoprotein of vesicular stomatitis virus (VSV) was also restricted in monocytes (20), suggesting that the block is independent of viral attachment and receptor/coreceptor factors. Some studies have indicated that viral cDNA synthesis is extremely inefficient or completely absent in monocytes (5, 25). Other studies using an HIV-1-based vector placed the block between completion of reverse transcription and nuclear entry of viral cDNA (20). In this study we use quantitative entry and postentry assays to better define the point at which viral replication is restricted in monocytes.
Culture of fresh monocytes in the presence of monocyte colony-stimulating factor (MCSF) promotes monocyte differentiation (9). To examine the kinetics of monocyte differentiation, fresh elutriated monocytes were plated in the presence of MCSF and immunophenotyped for expression of the lipopolysaccharide (LPS) receptor (CD14), the transferrin receptor (CD71), and the hemoglobin scavenger receptor (CD163). CD14 and CD163, which are expressed on monocytes and macrophages differentiated in the presence of MCSF in vitro (14, 27), were constitutively expressed during monocyte differentiation (Fig. 1a). On the other hand, the percentage of cells expressing the macrophage-specific transferrin receptor, CD71, increased as cells underwent differentiation in culture (Fig. 1a). We examined whether fresh monocytes supported HIV-1 entry using a recently developed β-lactamase-based HIV-1 entry assay (1, 18). In this assay, the fluorescent substrate of β-lactamase (CCF2) is loaded into target cells. Cells are then infected with a wild-type virus in which a Vpr-β-lactamase fusion protein has been packaged. Upon virus binding, fusion, and uncoating, β-lactamase, which is incorporated into virions through fusion with Vpr, enzymatically cleaves the β-lactam ring in CCF2, changing its fluorescence emission from green to blue. The assay does not score for virions that have been taken up by endocytosis but depends on the liberation of the Vpr-β-lactamase fusion protein into the cytoplasm after uncoating. Single-round infection of primary cells by HIV-1 is normally inefficient. Therefore, to increase the number of infection events for analysis, fresh monocytes were infected with VSV-G-pseudotyped and nonpseudotyped HIV-1 (wild-type for Vpr). Both pseudotyped and nonpseudotyped virions were able to infect fresh monocytes, as evidenced by the presence of β-lactamase activity (Fig. 1b). Similar frequencies of β-lactamase-positive cells were observed following infection of fresh monocytes and 7-day differentiated macrophages (Fig. 1b). These results were representative of monocytes obtained from independent donors (n = 5). In comparison, quiescent lymphocytes, which do not support productive infection, also supported HIV-1 entry (Fig. 1b). VSV-G pseudotyping increased the frequency of infected cells to similar levels in monocytes and in differentiated macrophages (Fig. 1b). Therefore, events leading up to uncoating appear to progress with similar efficiencies in monocytes and in differentiated macrophages, suggesting that the block to monocyte infection is at a point in the viral replication cycle subsequent to virus binding, fusion, and uncoating.
FIG. 1.
Monocytes support HIV-1 entry. (a) Elutriated monocytes were cultured in the presence of MCSF, and, at daily intervals, cells were removed and immunophenotyped for the indicated cell surface receptors by flow cytometry. ND, not done. (b) Efficiency of HIV-1 entry in monocytes and macrophages. The extent of virus entry was examined as described elsewhere (1, 18). Briefly, monocytes and macrophages were incubated with 2 nM CCF2/AM for 2 h and then infected with wild-type or VSV-G-pseudotyped HIV-1 virions into which a Vpr-β-lactamase fusion protein had been packaged. Two to 4 h postinfection, cells were examined by fluorescence microscopy using 424/444- and 516/520-nm band-pass filters in order to discriminate infected from uninfected cells. The ability of resting lymphocytes, which are refractory to productive HIV-1 infection, to support HIV-1 entry was compared with that of monocytes and macrophages. The effect of spinoculation (21) and Polybrene (3) on the efficiency of monocyte and lymphocyte infection was also examined.
We next compared the abilities of monocytes and macrophages to support viral cDNA synthesis. Fresh monocytes were plated in medium in the presence of MCSF to promote differentiation. At daily intervals following initiation of the cultures, monocytes were infected. Early (minus-strand strong-stop cDNA) and late products of reverse transcription were measured 18, 42, and 66 h following infection by real-time PCR quantitation of late reverse transcription products as well as two-long-terminal repeat (2-LTR) circle forms of viral cDNA. The presence of viral integrants was determined by an Alu-LTR PCR method (8). In undifferentiated monocytes early (not shown) and late (Fig. 2) reverse transcripts were synthesized very inefficiently. As monocytes differentiated to macrophages, they acquired the ability to support HIV-1 reverse transcription (Fig. 2). Expression of the macrophage marker CD-71 became evident after 3 to 4 days in culture (Fig. 1a). This was also the interval at which reverse transcription, based on synthesis of late reverse transcripts and 2-LTR circles (Fig. 2a and b), became evident. These late reverse transcripts were also competent for integration since we were able to detect the presence of integrated proviruses at these intervals (Fig. 2c). This pattern of reverse transcription in monocytes and macrophages was extremely consistent in cells from multiple donors (n = 6). These results suggest the presence of a reverse transcription defect in monocytes that is alleviated upon differentiation.
FIG. 2.
Analysis of reverse transcription in infected monocytes and differentiated macrophages. At daily intervals following initiation of monocyte cultures in MCSF, cells were removed and infected with a VSV-G-pseudotyped HIV-1. At 18, 42, and 66 h postinfection, total cellular DNA was isolated and the presence of late reverse transcripts (a) and 2-LTR circle forms of viral cDNA (b) was determined by real-time PCR, while integrated proviruses (c) were visualized by Alu-LTR PCR as described elsewhere (8).
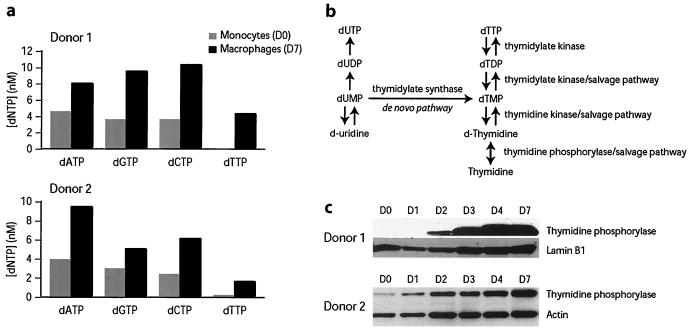
Previous studies have indicated that quiescent peripheral blood lymphocytes, which are refractory to productive infection, inefficiently support reverse transcription (32). While it was originally suggested that this was due to rate-limiting levels of deoxynucleotide triphosphates (dNTPs), increasing dNTP levels improved reverse transcription but did not overcome the infectivity block (13). Comparison of dNTP levels in fresh monocytes and in 7-day macrophages indicated only a twofold difference in dATP, dGTP, and dCTP levels between monocytes and macrophages (Fig. 3a). However, dTTP levels in fresh monocytes were low to undetectable and increased upon culture (Fig. 3a). For monocytes from four independent donors, dTTP levels in monocytes were close to background and increased over 10-fold upon macrophage differentiation. d-Thymidine, the major biosynthetic precursor of dTTP, is derived from thymine by the action of thymidine phosphorylase (Fig. 3b). In cells from independent donors (n = 3), this enzyme was present at low to undetectable levels in fresh monocytes and was induced during differentiation to macrophages (Fig. 3c). We examined whether artificially increasing dTTP levels in fresh monocytes would overcome the reverse transcription defect present in these cells. To increase dTTP levels, fresh and 2-day monocytes were incubated with several dTTP biosynthetic precursors. Addition of d-thymidine to fresh monocytes was sufficient to increase dTTP concentration to levels exceeding that of differentiated macrophages (Fig. 4a). d-Uridine did not influence dTTP levels, while thymine increased dTTP levels only in 2-day monocytes (Fig. 4a). This was to be expected since thymidine phosphorylase, which is required for conversion of thymine to d-thymidine, was more abundant in monocytes that had been in culture for a least 2 days (Fig. 3c). Since dTTP levels could be stimulated to levels comparable to that in fully differentiated macrophages (Fig. 3a), we examined whether this was sufficient to promote reverse transcription in fresh monocytes. Infection of monocytes in the presence or absence of exogenous d-thymidine did not have a significant impact on the extent of reverse transcription whether infections were conducted with pseudotyped or with nonpseudotyped viruses (Fig. 4b). Therefore, increasing dTTP levels to that of differentiated macrophages was not sufficient to significantly impact reverse transcription efficiency.
FIG. 3.
Analysis of dNTP levels in monocytes and differentiated macrophages. (a) dNTP levels were determined as described previously (24). (b) De novo and salvage pathways for dTTP synthesis. (c) Levels of thymidine phosphorylase in monocytes and differentiating macrophages. Levels of thymidine phosphorylase and the control proteins lamin B1 and actin were determined by Western blotting.
FIG. 4.

Normalization of dTTP levels and effect on HIV-1 reverse transcription. (a) Fresh monocytes (D0) and monocytes cultured for 2 days (D2) were incubated with d-thymidine (dT), d-uridine (dU), and thymine (T) for 2 h, and dTTP levels were measured. (b) D0 monocytes were incubated in the presence or absence of d-thymidine as for panel a and then infected with wild-type or VSV-G-pseudotyped HIV-1. Late reverse transcripts and 2-LTR circles were quantitated at the indicated time intervals postinfection. Experiments were done with monocytes from three independent donors (results from two donors are shown).
In this study we have attempted to characterize the point at which monocyte infection by HIV-1 is restricted. Collectively, our data suggest a reverse transcription defect in undifferentiated monocytes that cannot be corrected by normalization of dNTP levels. The restricted infection of fresh monocytes parallels that which has been described for quiescent T lymphocytes (13). Nuclear factor of activated T cells (NFAT) is present at low levels in quiescent lymphocytes, and it has been proposed that this accounts for the inefficient reverse transcription in these cells (11). However, NFAT was not detectable in either fresh monocytes or in differentiated macrophages, so differences in NFAT expression could not account for the observations reported in our study. It has been proposed that inefficient reverse transcription due to low dNTP levels accounts for the restriction to productive viral infection of quiescent lymphocytes. However, although exogenous stimulation of dNTP levels enhanced reverse transcription and generation of full-length transcripts, this did not overcome the block to infection. These and other studies (22, 26) support the notion that a block subsequent to reverse transcription prevents productive infection of quiescent lymphocytes. Upon infection of monocytes, reverse transcription was highly inefficient when analyzed up to 66 h postinfection and was not improved by normalization of dNTP levels (Fig. 2). This suggests a reverse transcription block in monocytes rather than a post-reverse transcription block. Therefore, for monocytes, differentiation-dependent cellular factors important for reverse transcription may be rate limiting while, for quiescent lymphocytes, activation-dependent cellular cofactors that regulate a post-reverse transcription step in viral infection may be lacking.
Acknowledgments
We thank B. Blais for assistance with flow cytometry analysis, N. Landau for the Vpr-β-lactamase expression plasmid, members of the Stevenson laboratory for scientific discussion, B. Mellor for preparation of figures, and N. Nelson for manuscript preparation.
This work was supported in part by grants RR-11589 from the National Institute of Research Resources and AI-37475 from the National Institute of Allergy and Infectious Diseases to M.S.
REFERENCES
- 1.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 2.Collman, R., N. F. Hassan, R. Walker, B. Godfrey, J. Cutilli, J. C. Hastings, H. Friedman, S. D. Douglas, and N. Nathanson. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 170:1149-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello, E., M. Munoz, E. Buetti, P. R. Meylan, H. Diggelmann, and M. Thali. 2000. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 7:596-604. [DOI] [PubMed] [Google Scholar]
- 4.Di Marzio, P., J. Tse, and N. R. Landau. 1998. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res. Hum. Retrovir. 14:129-138. [DOI] [PubMed] [Google Scholar]
- 5.Eisert, V., M. Kreutz, K. Becker, C. Konigs, U. Alex, H. Rubsamen-Waigmann, R. Andreesen, and H. von Briesen. 2001. Analysis of cellular factors influencing the replication of human immunodeficiency virus type I in human macrophages derived from blood of different healthy donors. Virology 286:31-44. [DOI] [PubMed] [Google Scholar]
- 6.Embretson, J., M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362:359-362. [DOI] [PubMed] [Google Scholar]
- 7.Gartner, S., P. Markovits, D. M. Markovitz, M. H. Kaplan, R. C. Gallo, and M. Popovic. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215-219. [DOI] [PubMed] [Google Scholar]
- 8.Jacque, J.-M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNAi. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalter, D. C., M. Nakamura, J. A. Turpin, L. M. Baca, D. L. Hoover, C. Dieffenbach, P. Ralph, H. E. Gendelman, and M. S. Meltzer. 1991. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J. Immunol. 146:298-306. [PubMed] [Google Scholar]
- 10.Kedzierska, K., and S. M. Crowe. 2002. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr. Med. Chem. 9:1893-1903. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 12.Koenig, S., H. E. Gendelman, J. M. Orenstein, M. C. DalCanto, G. H. Pezeshkpour, M. Yungbluth, F. Janotta, A. Aksamit, M. A. Martin, and A. S. Fauci. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089-1093. [DOI] [PubMed] [Google Scholar]
- 13.Korin, Y. D., and J. A. Zack. 1999. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristiansen, M., J. H. Graversen, C. Jacobsen, O. Sonne, H. J. Hoffman, S. K. Law, and S. K. Moestrup. 2001. Identification of the haemoglobin scavenger receptor. Nature 409:198-201. [DOI] [PubMed] [Google Scholar]
- 15.Lambotte, O., Y. Taoufik, M. G. de Goer, C. Wallon, C. Goujard, and J. F. Delfraissy. 2000. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 23:114-119. [DOI] [PubMed] [Google Scholar]
- 16.Lewin, S. R., J. Kirihara, S. Sonza, L. Irving, J. Mills, and S. M. Crowe. 1998. HIV-1 DNA and mRNA concentrations are similar in peripheral blood monocytes and alveolar macrophages in HIV-1-infected individuals. AIDS 12:719-727. [DOI] [PubMed] [Google Scholar]
- 17.McElrath, M. J., J. E. Pruett, and Z. A. Cohn. 1989. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc. Natl. Acad. Sci. USA 86:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naif, H. M., S. Li, M. Alali, A. Sloane, L. Wu, M. Kelly, G. Lynch, A. Lloyd, and A. L. Cunningham. 1998. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 72:830-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 75:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierson, T. C., Y. Zhou, T. L. Kieffer, C. T. Ruff, C. Buck, and R. F. Siliciano. 2002. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 76:8518-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich, E. A., I. S. Chen, J. A. Zack, M. L. Leonard, and W. A. O'Brien. 1992. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J. Clin. Investig. 89:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman, P. A., and J. A. Fyfe. 1989. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal. Biochem. 180:222-226. [DOI] [PubMed] [Google Scholar]
- 25.Sonza, S., A. Maerz, N. Deacon, J. Meanger, J. Mills, and S. Crowe. 1996. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J. Virol. 70:3863-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulahian, T. H., P. Hogger, A. E. Wahner, K. Wardwell, N. J. Goulding, C. Sorg, A. Droste, M. Stehling, P. K. Wallace, P. M. Morganelli, and P. M. Guyre. 2000. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12:1312-1321. [DOI] [PubMed] [Google Scholar]
- 28.van Furth, R. 1989. Origin and turnover of monocytes and macrophages. Curr. Top. Pathol. 79:125-150. [PubMed] [Google Scholar]
- 29.van Furth, R., and W. Sluiter. 1983. Current views on the ontogeny of macrophages and the humoral regulation of monocytopoiesis. Trans. R. Soc. Trop. Med. Hyg. 77:614-619. [DOI] [PubMed] [Google Scholar]
- 30.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 31.Wahl, S. M., T. Greenwell-Wild, G. Peng, H. Hale-Donze, and J. M. Orenstein. 1999. Co-infection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J Infect. Dis. 179(Suppl. 3):S457-S460. [DOI] [PubMed] [Google Scholar]
- 32.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 33.Zhu, T., D. Muthui, S. Holte, D. Nickle, F. Feng, S. Brodie, Y. Hwangbo, J. I. Mullins, and L. Corey. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]