Abstract
Glycosmis pentaphylla, Tridax procumbens, and Mangifera indica are well-known plants available throughout India and they are commonly used for the treatment of various diseases including diabetes mellitus. The antidiabetic activity of the individual plant parts is well known, but the synergistic or combined effects are unclear. The concept of polyherbalism has been highlighted in Sharangdhar Samhita, an Ayurvedic literature dating back to 1300 AD. Polyherbal formulations enhance the therapeutic action and reduce the concentrations of single herbs, thereby reducing adverse events. The aim of the present study is to formulate a polyherbal formulation and evaluate its antidiabetic potential in animals. The polyherbal formulation was formulated using the ethanol extracts of the stem bark of G. pentaphylla, whole plant of T. procumbens, and leaves of M. indica. The polyherbal formulation contains the ethanol extracts of G. pentaphylla, T. procumbens, and M. indica in the ratio of 2:2:1. The quality of the finished product was evaluated as per the World Health Organization's guidelines for the quality control of herbal materials. The quality testing parameters of the polyherbal formulation were within the limits. Fingerprint analysis of the polyherbal formulation showed effective separation at 366 nm, and it revealed that the active compound present in the polyherbal formulation and the active compounds present in all the three extracts were the same. The acute toxicity studies of the polyherbal formulation did not show any toxic symptoms in doses up to 2000 mg/kg over 14 days. The oral antidiabetic activity of the polyherbal formulation (250 and 500 mg/kg) was screened against streptozotocin (50 mg/kg; i.p.) + nicotinamide (120 mg/kg; i.p.) induced diabetes mellitus in rats. The investigational drug was administered for 21 consecutive days, and the effect of the polyherbal formulation on blood glucose levels was studied at regular intervals. At the end of the study, the blood samples were collected from all the animals for biochemical estimation, and the animals were sacrificed and the liver and pancreatic tissues were collected for histopathologic analysis. Polyherbal formulation showed significant antidiabetic activity at 250 and 500 mg/kg, respectively, and this effect was comparable with that of glibenclamide. The antidiabetic activity of polyherbal formulation is supported by biochemical and histopathologic analysis.
Keywords: Glycosmis pentaphylla, High Performance Thin Layer Chromatography fingerprint analysis, Mangifera indica, Polyherbal formulation, Tridax procumbens
INTRODUCTION
Plants are very useful to mankind. Many of them are used exclusively for medicinal purposes. According to the World Health Organization (WHO), “a medicinal plant is a plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes, or which are precursors for chemo-pharmaceutical semi-synthesis.” Such plants are in great demand by pharmaceutical companies for their active ingredients.[1,2]
Diabetes mellitus is one of the most common disorders affecting almost 6% of the world population and the dynamics of the diabetes are changing rapidly in low- to middle-income countries.[3] According to International Diabetes Federation's (IDF) estimates, 80% of the world diabetic population will be from low- and middle-income countries in 2030. As per IDF 2011 report, China, India, and the United States of America have a diabetic population of 90.0, 61.3, and 23.7 million, which may be increased up to 129.7, 101.2, and 29.3 million, respectively, in 2030.[4] Globally, diabetes is one of the six major causes of death and also causing various systemic complications. Diabetes mellitus is treated by hormone therapy (insulin) or by administering glucose-lowering agents such as alpha-glucosidase inhibitors, sulfonylureas, biguanides, and thiazolidinediones. Development of an adverse event is one of the complications in the treatment of any systemic disorder; hence, many of the research institutes and pharmaceutical companies are involved in drug development to find the molecules with good therapeutic potential and less adverse events.[5] In the USA, 10-25% of patients experience an adverse drug reaction and these adverse drug reactions are responsible for 3.4-7.0% of hospital admissions.[6]
In traditional systems of medicine, many plants have been documented to be useful for the treatment of various systemic disorders. Many of the traditional/indigenous systems of medicine are effective than the modern system of medicine, but they suffer from lack of complete standardization which is one of the important challenges faced by the traditional system of medicine. The concept of polyhedral formulation is well documented in the ancient literature. Compared to the single herb, the polyherbal formulation has better and extended therapeutic potential. Hence, the present study was planned to formulate and standardize a polyherbal formulation using a plant having known antidiabetic activity and evaluate its therapeutic effects in rodents.
MATERIALS AND METHODS
Collection of the plant
Taxonomically identified stem bark of Glycosmis pentaphylla (Rutaceae), whole plant of Tridax procumbens (Asteraceae), and leaves of Mangifera indica (Anacardiaceae) were collected from the Alagar kovil region, Madurai district. The collected plants were authenticated at the Department of Botany, American college, Madurai, Tamil Nadu. The voucher specimen of the plant was deposited in the Department of Pharmacology, Ultra College of Pharmacy, Madurai, India for further reference.
Animals
Adult Wistar rats (180 ± 10 g) of either sex were obtained from Sainath Enterprises, Hyderabad, India. The animals were housed in large, spacious polyacrylic cages at an ambient room temperature with 12-h light/12-h dark cycle. Rats had free access to water and rodent pellets diet (Hindustan Lever Ltd, Bangalore, India). The study was approved by the Institute Animal Ethics Committee of the Ultra College of Pharmacy and all the animal experiments were carried out according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, Ministry of Environment and Forests, Government of India.
Preparation of ethanolic extract of G. pentaphylla, T. procumbens, and M. indica plants
The shadow-, air-dried stem bark of G. pentaphylla, whole plant of T. procumbens, and leaves of M. indica were powdered and extracted with 80% absolute ethanol using Soxhlet apparatus for 6 h. The extracts were evaporated to dryness (resinous material) under reduced pressure at 60°C and stored at 4°C until use.
Phytochemical analysis
One gram of each of the ethanolic extracts of G. pentaphylla, T. procumbens and M. indica was dissolved in 100 ml of its own mother solvent to obtain a stock of concentration 1% w/v and tested for the presence of carbohydrates, proteins, sterols, alkaloids, tannins, glycosides, flavonoids, phenolic compounds, and saponins.[7]
Preparation of polyherbal formulation
The polyherbal formulation (capsules) contained the ethanolic extracts of G. pentaphylla, T. procumbens, and M. indica in the ratio of 2:2:1. The quality of the polyherbal formulation was tested as per the WHO guidelines for the quality control of herbal materials.[8] As per the guidelines, specific tests such as sampling, ash content, extractable matter, foaming index, loss on drying, tannin content, foreign matters, and specific powder characteristic tests such as angle of repose and bulk density were undertaken and significant results were recorded.
Preparation of formulation by wet granulation method
The formulation preparation began with trials by adding a different ratio of binders and selecting the quantity of lubricants and preservatives, and finally the procedure was optimized. G. pentaphylla, T. procumbens, and M. indica extracts were finely powdered (sieve 40), and mixed in the ratio of 2:2:1 and taken for the preparation of capsules by wet granulation technique using 20% lactose solution as a binder. The wet mass was passed through sieve number 22 to obtain granules. The granules were dried at 45°C in a tray dryer. The granules were lubricated with 1% magnesium stearate. Diluents and preservatives were added. The optimized formulation showed very good flow properties. After this, the granules from the optimized batch (20% lactose) were filled in capsules colored yellow-red of size “0” in a capsule filling machine. The capsules were then deducted and transferred into poly bags, labeled, and the samples were evaluated as per the testing requirements. Each 750 mg of herbal capsule contained the extracts of G. pentaphylla (100 mg), T. procumbens (100 mg), M. indica (50 mg), and lactose and excipients– quantity sufficient (q.s.).
Preformulation studies
Preformulation parameters such as bulk density, tap density, Carr's index, Hausner's ratio, and angle of repose were determined for the laboratory granules.[9,10]
Standardization of formulation
Physicochemical parameters of raw materials were determined as per the guidelines of the WHO, which includes moisture content, total ash value, water-soluble ash, acid-insoluble ash, heavy metals, water-soluble extractive, alcohol-soluble extractive, and acidity (pH).[11,12,13]
pH value: pH of 1% solution was determined by using a digital pH meter.
Ash value and extractive value: Total ash, water soluble ash, acid soluble ash and extractive values were determined as the procedure described elsewhere.[11,12]
Limit test for heavy metals: Qualitative estimation of heavy metals was done for the detection of arsenic and lead as per the Ayurvedic pharmacopoeial procedures.
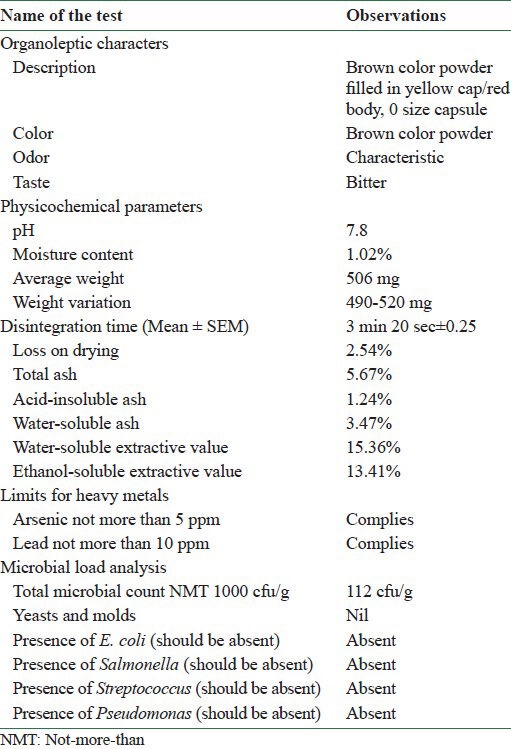
Capsule evaluation: The polyherbal capsules were evaluated for their description, microbial load, uniformity of dosage units, weight variation, disintegration time, and moisture content, and compared with Indian pharmacopoeial standards.
Microbial load analysis: For the safe use of the polyherbal capsules, microbial count was done and it was checked whether the total aerobic viable count, yeasts and molds were within the prescribed limits and the microorganisms, Escherichia coli, Clostridia, Salmonellae, Shigella, Pseudomonas, and Staphylococcus, were absent in the final formulation.
Weight variation: Twenty capsules were individually weighed and the average weight of the capsule was calculated. The individual weights of the each capsule should be within the limits of 90% and 110% of the average weight.
Moisture content: Moisture content was determined by using automatic Karl Fischer titration apparatus.
Disintegration time: Disintegration test was performed using the digital microprocessor based disintegration test apparatus (Electro lab, Mumbai, India). One capsule was introduced into each tube and a disk was added to each tube. The assembly was suspended in water in a 1000 ml beaker. The volume of water at its highest point was at least 25 mm below the surface of the water and at its lowest point was at least 25 mm above the bottom of the beaker. The apparatus was operated and maintained at a temperature of 37 ± 2°C.
High-performance thin layer chromatography (HPTLC) fingerprint analysis: Randomly few capsules were selected. They were opened and the contents were collected. The capsule content was dissolved in a minimum volume of mobile phase and used for the fingerprint analysis. Twenty milligram either individual herbal extract or polyherbal formulation was reconstituted in 1 ml of ethanol and filtered through a 0.45 μm membrane filter. The filtrate was used for the HPTLC analysis. The butanol:glacial acetic acid:water in the ratio 4:1:1, and toluene:ethyl acetate:methanol in the ratio 7:2:1 was used as mobile phase for the development of chromatogram. The chromatogram developed using mobile phase consisting of toluene:ethyl acetate:methanol (7:2:1) has showed good separation.
Linomat IV automated TLC applicator CAMAG (Switzerland), densitometer–CAMAG TLC scanner 3, and CAMAG CATS 4 software were used for fingerprint analysis. A 2 μl sample (8 mm band) was spotted (Track 1: G. pentaphylla, Track 2: M. indica, Track 3: T. procumbens, and Track 4: Polyherbal formulation) and the chromatogram was developed in 20 × 10 cm twin trough glass chamber saturated with toluene: ethyl acetate: methanol (7:2:1) solvent system. The plates were exposed to the mobile phase for 20 min and the air-dried plates were viewed in Ultraviolet light to mid-day light. The chromatograms were scanned by densitometer at 254, 366, and 520 nm. The Rf values, peak areas, and fingerprint data were recorded by winCATS 1.4.3 software. The Rf values were calculated using the formula: “distance traveled by the solute from the origin/distance traveled by the solvent from the origin.”
Development of quality control standards for the polyherbal capsule
The physiochemical (moisture content, limit test for heavy metals, monographic analysis of plant material, pH, and disintegration time) and microbial load analyses were performed for the herbal capsules to ensure the content uniformity and quality.[12]
Acute oral toxicity
Acute oral toxicity of the polyherbal formulation was carried out as per the guidelines set by the Organization for Economic Co-operation and Development (OECD), revised draft guidelines 423. The principle involves a stepwise procedure with the use of a minimum number of animals per step to obtain sufficient information on the acute toxicity of the test substance to enable its classification. Healthy Wistar rats (3 animals/dose) of either sex were used for the experiment. Overnight fasted rats were orally fed with the plant extracts and polyherbal formulation in increasing dose levels of 5, 50, 300, and 2000 mg/kg body weight, respectively. The animals were observed for their behavioral (alertness, restlessness, irritability, and fearfulness), neurological (spontaneous activity, reactivity, touch response, pain response, and gait), and autonomic (defecation and urination) profiles continuously for 24 h. After a period of 24 h, the animals were observed for 14 days for mortality.[14]
Antidiabetic effect of herbal formulation in streptozotocin- and nicotimanide-induced diabetic rats
The male Wistar rats were divided into five different groups of six animals each as follows.
Group I: Normal control
Group II: Diabetic control
Group III: Diabetic rats treated with polyherbal preparation (250 mg/kg)
Group IV: Diabetic rats treated with polyherbal preparation (500 mg/kg)
Group V: Diabetic rats treated with glibenclamide (0.25 mg/kg).
Diabetes was induced in overnight-fasted rats by administering single intraperitoneal (i.p.) injection of freshly prepared streptozotocin (STZ) 50 mg/kg b.w. followed by 120 mg/kg of nicotimanide (NIC) in 0.1 M citrate buffer (pH 4.5) in a volume of 0.5 ml/kg b.wt.[15] Diabetes was confirmed in the STZ + NIC treated rats by measuring fasting blood glucose levels after 48 h of induction. After 24 h of STZ + NIC injection, the rats were given 5% w/v of glucose solution (2 ml/kg b.w.) to prevent hypoglycemic mortality. Rats with fasting blood glucose of more than 200 mg/dl were considered as diabetics and they were divided randomly into four different groups. The standard (glibenclamide) and herbal formulation were suspended in 1% w/v carboxymethyl cellulose (CMC) and administered once daily through oral gavage for 21 consecutive days. The blood samples were collected on 1st, 7th, 14th, and 21st days of the treatment, through the tail vein of rats by pricking and were immediately used for the estimation of blood glucose with a glucometer. Weekly body weight variations were monitored for all the experimental animals.
At the end of the experiment, the blood sample was withdrawn from all the experimental animals through retro-orbital plexus puncture/posterior vena cava in plain and sodium ethylenediaminetetraacetic acid (EDTA) tubes for biochemical analysis.[16] Finally the animals were sacrificed by diethyl ether anesthesia, and liver and pancreatic tissues were excised and used for biochemical and pathological analysis. Part of the tissue sample was preserved in an ice-cold container for biochemical analysis and the remaining was stored in 10% formalin solution for histopathologic analysis.
Biochemical analysis
The whole blood sample was used for the estimation of glucose (One-Touch Horizon glucometer; Ortho-Clinical Diagnostics, Johnson and Johnson Company, USA), hemoglobin, and glycosylated hemoglobin (HbA1c). The plasma sample was used for the estimation of insulin (radioimmunoassay kit; Diasorin, Italy). The serum was used for the estimation of biochemical markers such as creatinine, urea, protein, liver glycogen, total serum cholesterol, serum triglyceride, high density lipoprotein (HDL)-cholesterol, serum glutamate oxaloacetate transaminase (SGOT), and serum glutamate pyruvate transaminase (SGPT). The biochemical markers were measured using a Prietest Easylab - Biochemistry Analyser (Robonik [India] Private Limited) and the LAB-KITS enzymatic kits. The liver tissue homogenate was used for the estimation of protein and glycogen.
Estimation of glucose: A drop of the whole blood sample was used for measuring glucose using One-Touch H orizon glucometer, with gluco strips.
Estimation of hemoglobin: Hemoglobin in the blood was estimated by the method of Drabkin and Austin (1932). To 0.02 ml of blood, 5.0 ml of Drabkin's reagent was added, mixed well, and allowed to stand for 10 min. The solution was read at 540 nm together with the standard.[17]
Estimation of HbA1c: Erythrocytes were washed with normal saline, lysed with 5 ml of water, and incubated at 37°C for 15 min. The contents were centrifuged, the supernatant was discarded, and 0.5 ml of saline was added. To 2 ml of aliquot, 4 ml of oxalate hydrochloride solution was added and the contents were heated at 100°C for 4 h, cooled, and precipitated with 2 ml of 40% trichloroacetic acid (TCA). The mixture was centrifuged, and to 0.5 ml of supernatant, 0.5 ml of 80% phenol and 3 ml of concentrated sulfuric acid were added. The developed color was read at 480 nm after 30 min.
Working standards (10-50 μg) were prepared using 1% fructose solution. Then, 0.5 ml of 80% phenol and 3 ml of concentrated sulfuric acid were added to the working standards and read at 480 nm after 30 min. The concentration of HbA1c was expressed as mg/g of hemoglobin.
Estimation of protein: The liver tissue was homogenized in 20 mM Tris-HCl and used for the estimation of protein. To 0.5 ml of tissue homogenate, 0.5 ml of 10% TCA was added and centrifuged for 10 min. The precipitate was dissolved in 1.0 ml of 0.1 N NaOH. To this, an aliquot of 4.5 ml of alkaline copper reagent was added and allowed to stand at room temperature for 10 min. To this reaction mixture, 0.5 ml of Folin's phenol reagent was added and the blue color developed was read after 20 min at 640 nm. A standard curve was obtained with standard bovine albumin and was used to assay the tissue protein level of enzyme activity. Values were expressed as mg/g of tissue.[18]
Estimation of liver glycogen: The alkali extract of the tissue was prepared by digesting 50 mg of fresh tissue with 3 ml of 30% potassium hydroxide solution in a boiling water bath for 15 min. The tubes were cooled and a drop of 1 M ammonium acetate was added to precipitate glycogen and kept in a freezer overnight for complete precipitation. Glycogen was collected by centrifuging at 300 rpm for 20 min. Then the precipitate was dissolved by heating and again the glycogen was re-precipitated by adding alcohol and 1 M ammonium acetate and centrifuged. The final precipitate was dissolved in saturated ammonium chloride solution by heating in a boiling water bath for 5 min. Aliquots of glycogen solution were taken up for suitable dilution and 4 ml of anthrone reagent was added by cooling the tubes in an ice bath. The tubes were shaken well, covered with marble caps, and heated in a boiling water bath for 20 min. After cooling, the absorbance was read at 640 nm against water blank treated in a similar manner. The standard glucose solution was also treated similarly. The glycogen content was calculated from the amount of glucose present in the sample by multiplying with the factor 0.91, and expressed as mg/100 g of tissue.
Histopathologic analysis
Part of the liver and pancreas tissue were preserved in 10% formalin for 2 days. The liver and pancreas were dehydrated with alcohol (subsequently with 70, 80, 90%, and absolute alcohol) for 12 h each. Again the tissues were cleaned by using xylene for 15-20 min and they were subjected to paraffin infiltration in automatic tissue processing unit. The tissue blocks were prepared and the blocks were cut using microtome to get sections of thickness 5 μm. The sections were taken on a microscopic slide on which egg albumin (sticky substance) was applied and allowed for drying. Finally, the sections were stained with eosin (acidic stain) and hemotoxylin (basic stain).
Statistical analysis
All the data were expressed as mean ± SEM. Statistical significance between the groups were tested using one-way analysis of variance (ANOVA) followed by Dunnett's t-test post-hoc test. A P less than 0.5 was considered significant.
RESULTS
Photochemical analysis
The yield of ethanolic extracts of G. pentaphylla, T. procumbens, and M. indica was 25.6%, 20.6%, and 5.34% w/w (dry weight basis), respectively. The phytochemical analysis of the ethanolic extract of G. pentaphylla showed the presence of alkaloids, tannins, flavonoids, and saponins, of T. procumbens showed the presence of alkaloids, flavonoids, saponins, and phenolic compounds, and of M. indica showed the presence of alkaloids, tannins, flavonoids, and phenolic compounds.
Preformulation studies
Preformulation parameters such as bulk density, tap density, Carr's index, Hausner's ratio, and angle of repose were obtained for the laboratory granules. The granules showed excellent flow property. The results are presented in Table 1.
Table 1.
Preformulation studies and results of flow properties

Development of quality control standards for polyherbal capsule
Final batch samples of polyherbal capsules were analyzed for its organoleptic characters, physico chemical parameters, limits for heavy metals and microbial load [Table 2]. The study on capsules revealed that they were uniform in content and weight. Further, the moisture content, microbial load, limit test for heavy metals, monographic analysis of plant material, pH, and disintegration time were calculated and these values were found to be within normal limits.
Table 2.
Standardization of polyherbal capsule
Fingerprint analysis of polyherbal formulation
Fingerprint analysis at 366 nm showed good and effective compound separation than the analysis at 254 and 520 nm. The obtained Rf values and peak areas in densitometry TLC Scanner 3 at 366 nm are shown in Table 3. The fingerprint of 3D display at 366 nm and in densitometry is shown in Figure 1, and visualization comparison spots of all the three extracts with polyherbal formulation at 254, 366, and 520 nm are shown in Figure 2. The fingerprint reports of all the four tracks revealed that the active compound present in the polyherbal formulation and the active compounds present in all the three extracts were the same.
Table 3.
Rf values and peak areas at 366 nm
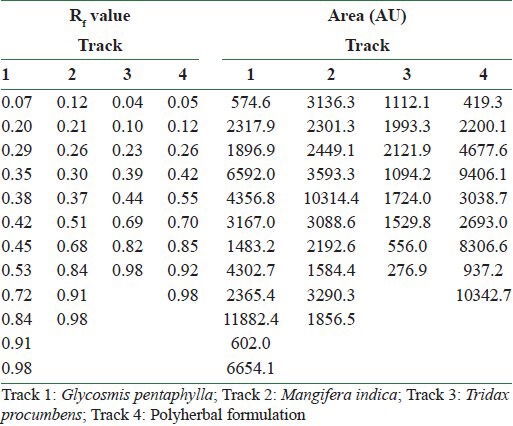
Figure 1.
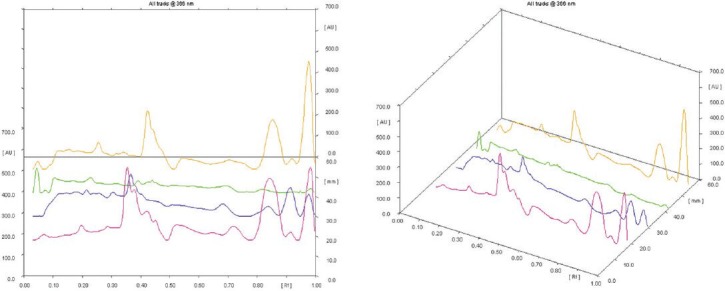
Fingerprinting of 3D display at 366 nm in densitometry detection (pink color: Glycosmis pentaphylla, blue color: Mangifera indica, green color: Tridax procumbens, brown color: polyherbal formulation)
Figure 2.
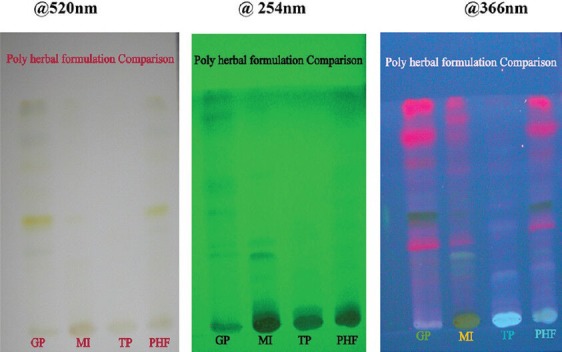
Visualization comparison spots of all the three extracts with polyherbal formulation (GP: Glycosmis pentaphylla, MI: Mangifera indica, TP: Tridax procumbens, PHF: polyherbal formulation)
The same Rf values and peak areas were obtained in the formulation when compared with the individual Rf values and peak areas of herbal drugs. This shows that the marker compound present in individual herbals extracts and the marker compounds present in the formulation are the same. The visualization comparison spots of all the three extracts with polyherbal formulation at 366 nm are shown in Figure 3.
Figure 3.
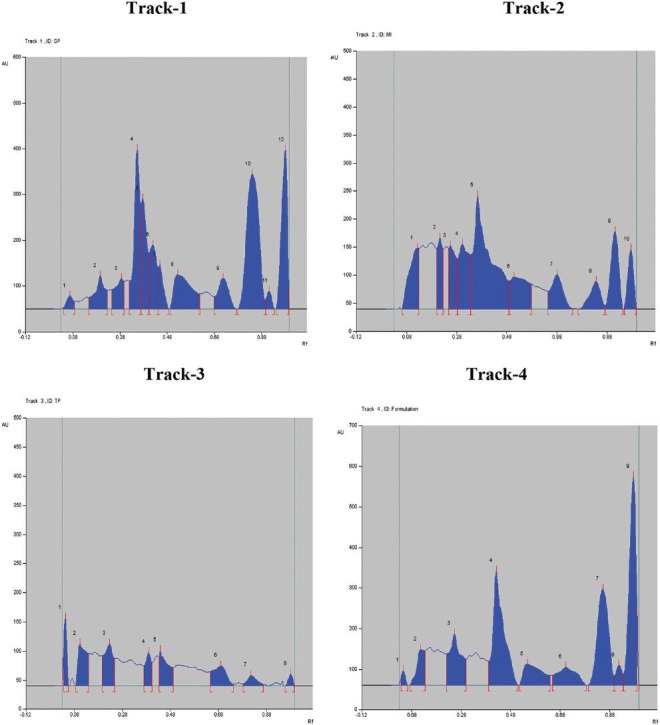
The chromatograms of all the four tracks at 366 nm (Track 1: Glycosmis pentaphylla, Track 2: Mangifera indica, Track 3: Tridax procumbens, Track 4: polyherbal formulation)
Toxicity study of polyherbal formulation
Acute toxicity studies did not show any mortality up to 2000 mg/kg given as single oral administration. Hence, the study was carried out at the dose levels of 250 and 500 mg/kg.
Antidiabetic activity of the polyherbal formulation
Throughout the study, the diabetic animals showed significant reduction in body weight when compared to the control animals. However, the polyherbal formulation and glibenclamide inhibited the diabetes-induced body weight reduction [Figure 4].
Figure 4.
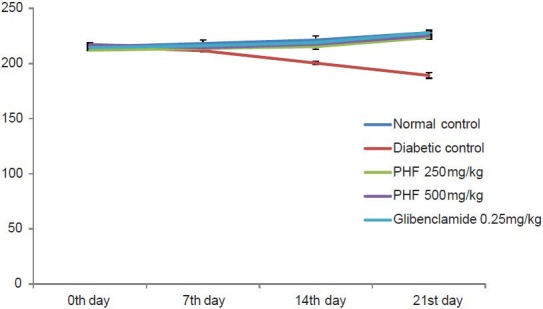
Effect of polyherbal formulation on body weight in STZ-and NIC-induced diabetic rats [values are expressed as the mean ± SEM (n= 6); PHF: Polyherbal formulation; ***P< 0.001 compared to control (one-way ANOVA followed by a Dunnett's t-test)]
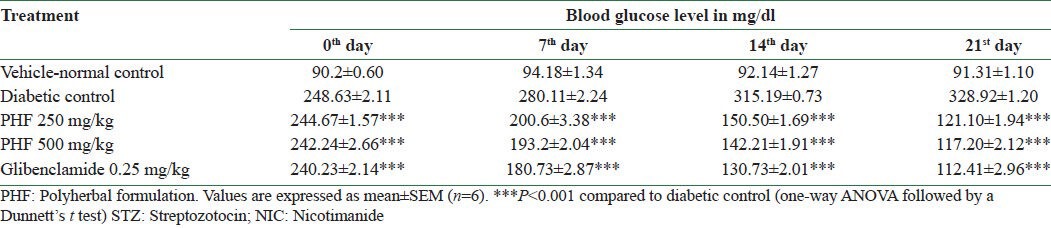
Diabetic control animals showed severe hyperglycemia compared to normal animals. The mean blood glucose level in the diabetic control group on day 0 was 248.63 ± 2.11 mg/dl and on day 21 was 328.92 ± 1.20 mg/dl. It was observed that the standard drug glibenclamide lowered the blood glucose level significantly, bringing it back to near normal level, whereas the polyherbal capsule at 250 mg/kg and 500 mg/kg significantly (P < 0.001) decreased the fasting blood serum glucose level in the diabetic rats on 7th, 14th, and 21st days, as compared to the diabetic control group. The results are presented in Table 4.
Table 4.
The effect of polyherbal formulation on fasting blood glucose levels (mg/dl) in STZ-and NIC-induced diabetic rats
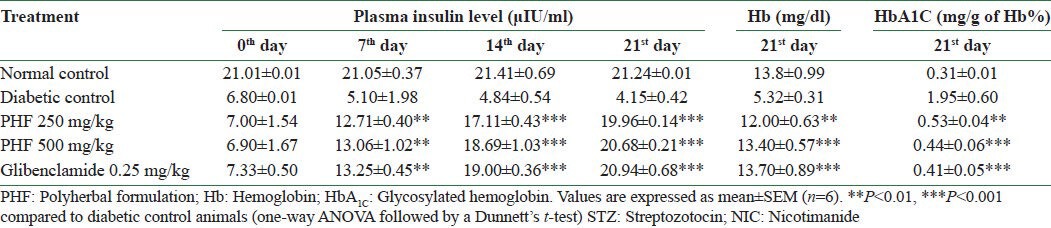
Diabetic animals showed significant decrease in plasma insulin, hemoglobin, and HbA1c levels when compared with control animals. Herbal formulation and glibenclamide reversed the insulin depletion in diabetic condition and also brought back the hemoglobin and HbA1c levels to normal [Table 5].
Table 5.
Effect of polyherbal formulation on plasma insulin level in STZ-and NIC-induced diabetic rats
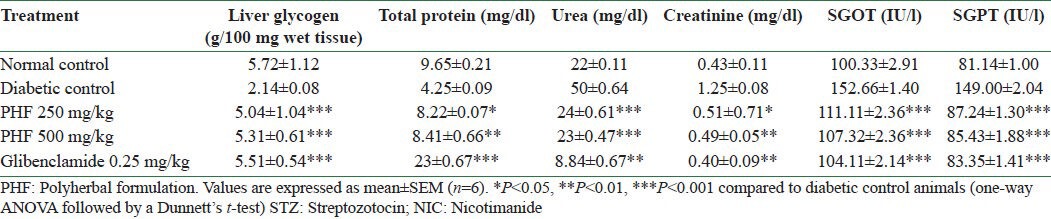
Diabetic animals showed significant reduction in liver glycogen and total protein levels when compared to the control animals, whereas herbal formulation and glibenclamide treated animals showed normal liver glycogen and total protein levels. The prevention of depletion of glycogen in the liver tissue was possibly due to stimulation of insulin release from the β cells that activates the glycogen synthase system. Effects of herbal formulation and glibenclamide on the liver and renal markers of diabetic animals are presented in Table 6.
Table 6.
Effect of polyherbal formulation on serum creatinine, protein, urea, and liver glycogen levels in STZ-and NIC-induced diabetic rats
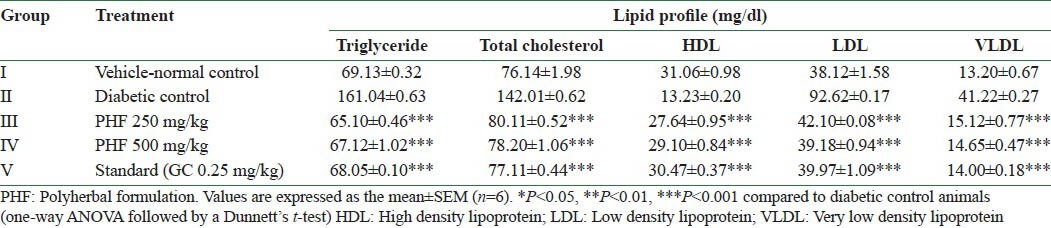
The diabetic rats showed significant (P < 0.001) increase in serum lipid profiles except HDL when compared to the control animals, whereas the levels in the treatment group remained within normal limits at the end of the study. Effects of herbal formulation and glibenclamide on the lipid profile of diabetic animals are presented in Table 7.
Table 7.
Effect of ethanolic extracts of the polyherbal formulation on serum lipids
Histopathology results
The histopathologic analysis of pancreas revealed severe congestion, huge decrease in the number of islets of Langerhans and β cells, and fibrosis and inflammatory cell infiltration into the islets of Langerhans in STZ- and NIC-induced hyperglycemic rats. While the polyherbal formulation at the dose of 250 mg/kg and 500 mg/kg showed mild congestion and mild decrease in the number of islets of Langerhans with normal β cell population, indicating significant amount of recovery. Glibenclamide treatment showed moderate congestion with moderate decrease in the number of islets of Langerhans and β cells and mild lymphocytic infiltration [Figure 5].
Figure 5.
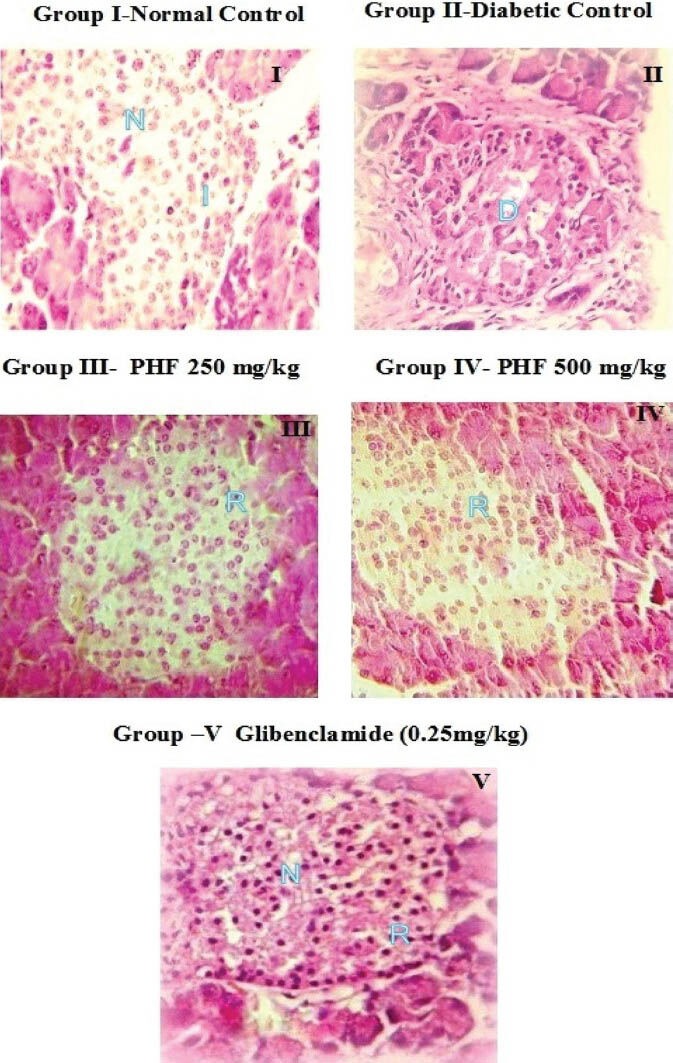
Histopathology of pancreas in rats [photomicrograph of hematoxylin and eosin (H and E) stained paraffin section from the pancreas (×400); D: Damage, I: Islet cells, N: Nuclei, R: Regeneration, PHF: Polyherbal formulation]
Histopathology of the liver [Figure 6] in normal control animals showed normal hepatic cells with well-preserved cytoplasm, nucleus, nucleolus, and central vein. In diabetic control animals, the liver sections showed that the lobular architecture was maintained, but there was also a severe fatty change, sinusoidal dilation and congestion, mild portal inflammation, fibrosis, severe feathery degeneration, and necrosis. Diabetic rats treated with herbal formulation showed hepatocytes with nearly normal appearance and minimal necrosis. The sections of glibenclamide treated animals showed normal hepatic cells and no abnormality.
Figure 6.
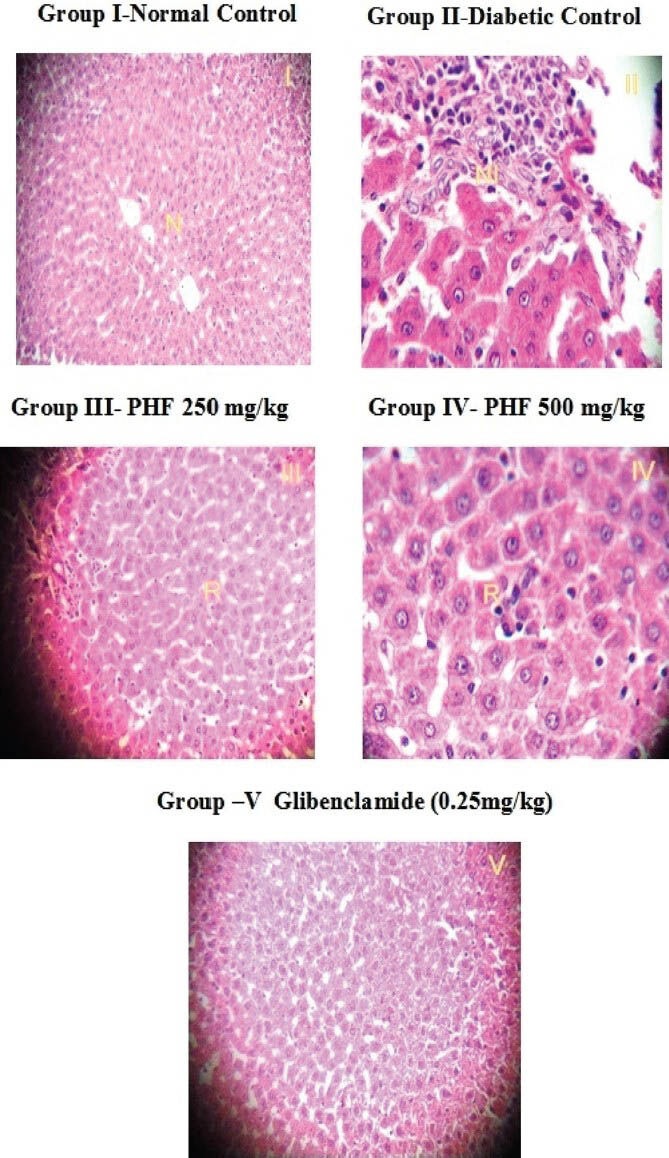
Histopathology of liver in rats [photomicrograph of hematoxylin and eosin (H and E) stained paraffin section from the liver (×400); N: normal, NI: Necrosis and inflammation, R: Regeneration, PHF: Polyherbal formulation]
DISCUSSION
The polyherbal formulation was formulated using the ethanolic extracts of the stem bark of G. pentaphylla, whole plant of T. procumbens, and leaves of M. indica, which are mixed properly in 2:2:1 ratio. The antidiabetic activity of the individual plants has been proven. The stem bark of G. pentaphylla showed significant antidiabetic and antiarthritic activities against STZ-induced diabetes and Freund's complete adjuvant induced arthritis, respectively, in rats at the dose levels of 400 and 800 mg/kg.[19] The 50% ethanol extract of the leaves of M. indica showed a significant hypoglycemic effect against STZ-induced diabetes in rats at a dose of 250 mg/kg.[20] In humans, the aqueous and alcoholic extracts of the leaves of M. indica showed a significant hypoglycemic effect at a dose level of 1 g/kg/d.[21] Methanolic extract of the whole plant of T. procumbens showed significant antidiabetic activity against alloxan-induced diabetes in rats at 250 and 500 mg/kg b.wt.[22] In some studies, the leaves of T. procumbens showed significant antidiabetic potential in rodents.[23]
In the modern era, herbal formulations have gained greater importance than ever before, mainly due to their efficacy and easy availability,[24] as well as less side effects as compared to the synthetic drugs.[25] These advantages have led the people move toward herbal preparations, for disease treatment and prevention, as they are claimed to display synergistic, potentiative, and agonistic/antagonistic actions and the mixture of species in them shows better therapeutic effect than either species on its own.[26] The concept of polyherbalism has been highlighted in Sharangdhar Samhita, an Ayurvedic literature dating back to 1300 AD.[27] Polyherbal formulations enhance the therapeutic action and reduce the concentrations of single herbs, thereby reducing the adverse events.
The HPTLC analysis of polyherbal formulation was carried out at the wavelengths of 254, 366, and 520 nm. The quality control system for the herbal preparation differs from the allopathic system of medicine. Polyherbal formulation comprises hundreds of unique or species-specific compounds and it is difficult to completely characterize these compounds. When formulating a polyhedral formulation, there are chances for chemical interaction and loss of a particular bioactive compound.[28] In the present study, single herbs, viz., G. pentaphylla, T. procumbens, M. indica, in the radio of 2:2:1 showed clear compound separation.
The toxicity studies were carried out as per the OECD guidelines. The polyherbal formulation did not show any mortality or adverse event up to 2000 mg/kg. Hence, the study was carried out at the dose levels of 250 and 500 mg/kg.
STZ is toxic glycoside obtained from Streptomyces achromogenes, a gram-positive bacterium. It accumulates in pancreatic β cells via the glucose transporter 2 (GLUT2) and reduces their expression. The alkylating properties of the STZ modify the biological macromolecules, fragment DNA, and destroy the β cells, causing insulin-dependent diabetes.[29] In the diabetic control group, severe body weight loss was observed, which may be due to increased muscle wasting and loss of tissue proteins.[30] In the present study, the treatment groups showed significant improvement in body weight, which indicates that polyherbal formulation and glibenclamide prevent the hyperglycemia-induced muscle wastage. The reduction in glucose levels may be due to increase in plasma insulin levels or enhanced transport of blood glucose in the peripheral tissue.[31] Our study gives evidence that the polyherbal formulation increases the plasma insulin levels and has promising antidiabetic activity.
Diabetic animals showed enhanced levels of HbA1c due to excessive production of glucose in blood, which further reacts with blood hemoglobin and produces HbA1c.[32]
The diabetic hyperglycemia induced by STZ and NIC causes elevation of plasma levels of SGPT, SGOT, urea, and creatinine, which are considered as significant markers of liver and renal dysfunction. The polyherbal formulation treated animals reversed the effect of STZ and NIC on the liver and renal markers. This may be due to the hepatoprotective mechanism of the individual herbs present in the polyherbal formulation.[33]
STZ diabetic rat has increased levels of lipid peroxides and reactive oxygen species, which cause hyperglycemia. Incessant generation of free radicals can lead to tissue damage through peroxidation of unsaturated fatty acids.[34] The polyherbal formulation treated animals inhibited the hyperglycemia induced by STZ, which may be due to the free radical scavenging properties of the individual herbs present in it.
Histopathology of the pancreas of STZ diabetic animals showed severe decrease in the number of islets of Langerhans and β cells, with fibrosis and inflammatory cell infiltration into the islets of Langerhans, and these findings are supported by the reports published earlier.[35] Histopathology of the liver of STZ diabetic animals showed a severe fatty change, sinusoidal dilation and congestion, mild portal inflammation, fibrosis, severe feathery degeneration, and necrosis. The hepatic changes may be due to the hypertrophy of hepatocytes by an increase in the intracytoplasmic eosinophilic granules.[36] Polyherbal formulation and glibenclamide treatment to the animals reduced the severity of the histopathologic changes caused by STZ.
CONCLUSION
Thus, our study findings demonstrate the antidiabetic effect of the polyherbal formulation at the dose levels of 250 and 500 mg/kg. The antidiabetic potential of the polyherbal formulation is comparable with that of glibenclamide, which is evidenced by decreased levels of blood glucose, HbA1c, total cholesterol, triglyceride, low density lipoprotein (LDL)-cholesterol, urea, creatinine, SGOT, and SGPT, and increase in plasma insulin, HDL-cholesterol, liver glycogen, and total protein levels.
REFERENCES
- 1.Huai H. Ethnomedicinal analysis of toxic plants from five ethnic groups in China. Ethnobot Res Appl. 2010;8:169–79. [Google Scholar]
- 2.Husain SZ, Malik RN, Javaid M, Bibi S. Ethonobotanical properties and uses of Medicinal plants of Morgah Biodiversity Park, Rawalpindi. Pak J Bot. 2008;40:1897–911. [Google Scholar]
- 3.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 4.Petchi RR, Parasuraman S, Vijaya C. Antidiabetic and antihyperlipidemic effects of an ethanolic extract of the whole plant of Tridax procumbens (Linn.) in streptozotocin-induced diabetic rats. J Basic Clin Pharm. 2013;4:88–92. doi: 10.4103/0976-0105.121655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parasuraman S, Kumar E, Kumar A, Emerson S. Free radical scavenging property and diuretic effect of triglize, a polyherbal formulation in experimental models. J Pharmacol Pharmacother. 2010;1:38–41. doi: 10.4103/0976-500X.64535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandavi, D’Cruz S, Sachdev A, Tiwari P. Adverse drug reactions and their risk factors among Indian ambulatory elderly patients. Indian J Med Res. 2012;136:404–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Petchi RR, Vijaya C, Parasuraman S. Anti-arthritic activity of ethanolic extract of Tridax procumbens (Linn.) in Sprague Dawley rats. Pharmacognosy Res. 2013;5:113–7. doi: 10.4103/0974-8490.110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quality control methods for herbal materials (Updated edition of Quality control methods for medicinal plant materials, 1998) [Last accessed on 2013 Oct 15]. Available from: http://apps.who.int/medicinedocs/documents/h1791e/h1791e.pdf .
- 9.United States Pharmacopoeia. 30th ed. NF-25: The Official Standard of Compendia; 2007. Powder flow; p. 1174. [Google Scholar]
- 10.30th ed. NF-25: The Official Standard of Compendia; 2007. Bulk Density and Tapped Density; p. 1186. [Google Scholar]
- 11.Kumar SS. Raw material standardization and formulation development of polyherbal capsule lactare forte. Int J Herb Med. 2013;1:8–16. [Google Scholar]
- 12.Lohar DR, Singh R. Vol. 1. Ghaziabad: Department of Ayush, Ministry of Health and Family Welfare, Pharmacopoeial Laboratory for Indian Medicine; 2008. Quality Control Manual for Ayurvedic, Siddha and Unani Medicine; pp. 21–4. [Google Scholar]
- 13.Vol. 1. Mumbai, India: Indian Drug Manufacturer Association; 2002. Indian Herbal Pharmacopeia. [Google Scholar]
- 14.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–9. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68:307–18. doi: 10.1007/s13105-011-0142-y. [DOI] [PubMed] [Google Scholar]
- 16.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James SA, Auta R, Goje DJ. In vitro study on inhibition of glycosylation of methanolic leaf extract of Hibiscus cannabinus. Sci World J. 2011;6:7–9. [Google Scholar]
- 18.Waterborg HH. The Protein Protocols Handbook. Berlin, Heidelberg: Springer; 2002. The Lowry Method for Protein Quantitation; pp. 7–9. [Google Scholar]
- 19.Petchi RR, Vijaya C. Anti-diabetic and anti-arthritic potential of Glycosmis pentaphylla stem bark in FCA induced arthritis and Streptozotocin Induced diabetic rats. Int J Pharm Bio Sci. 2012;3:328–36. [Google Scholar]
- 20.Sharma SR, Dwivedi SK, Swarup D. Hypoglycaemic potential of Mangifera indica leaves in rats. Pharm Biol. 1997;35:130–3. [Google Scholar]
- 21.Waheed A, Miana GA, Ahmad SI. Clinical investigation of hypoglycemic effect of leaves of Mangifera inidca in type-2 (NIDDM) diabetes mellitus. Pak J Pharmacol. 2006;23:13–8. [PubMed] [Google Scholar]
- 22.Pareek H, Sharma S, Khajja BS, Jain K, Jain GC. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.) BMC Complement Altern Med. 2009;9:48. doi: 10.1186/1472-6882-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhagwat DA, Killedar SG, Adnaik RS. Anti-diabetic activity of leaf extract of Tridax procumbens. Int J Green Pharm. 2008;2:126–8. [Google Scholar]
- 24.Katare YS, Bhujbal SS, Bafna AR, Shyale SS, Shelar MK, Kadam SD, et al. Evaluation of anxiolytic effect of a polyherbal for mulation in mice. Eur J Exp Biol. 2012;2:2093–8. [Google Scholar]
- 25.Sen A, Yokokura T, Kankel MW, Dimlich DN, Manent J, Sanyal S, et al. Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. J Cell Biol. 2011;192:481–95. doi: 10.1083/jcb.201004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sujatha S, Shalin JJ. Complementary therapeutic potential: A focus on polyherbal products for hyperglycemia. Asian J Sci Res. 2012;5:1–13. [Google Scholar]
- 27.Srivastava S, Lal VK, Pant KK. Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharmacology. 2012;2:1–15. [Google Scholar]
- 28.Deattu N, Suseela L, Narayanan N. Chromatographic analysis of polyherbal extract and formulation by HPTLC and GC-MS methods. J Pharm Res. 2013;6:6–10. [Google Scholar]
- 29.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:536–46. [PubMed] [Google Scholar]
- 30.Cheng D, Liang B, Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int 2013. 2013 doi: 10.1155/2013/162724. 162724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox G. Insulin and Insulin Resistance. Clin Biochem Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 32.Pari L, Saravanan R. Antidiabetic effect of diasulin, an herbal drug, on blood glucose, plasma insulin and hepatic enzymes of glucose metabolism in hyperglycaemic rats. Diabetes Obes Metab. 2004;6:286–92. doi: 10.1111/j.1462-8902.2004.0349.x. [DOI] [PubMed] [Google Scholar]
- 33.Shah SA, Patel MB, Patel RJ, Parmar PK. Mangifera Indica (Mango) Pharmacogn Rev. 2010;4:42–8. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar V, Ahmed D, Gupta PS, Anwar F, Mujeeb M. Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement Altern Med. 2013;13:222. doi: 10.1186/1472-6882-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Abdollahi M, Zuki AB, Goh YM, Rezaeizadeh A, Noordin MM. Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol Histopathol. 2011;26:13–21. doi: 10.14670/HH-26.13. [DOI] [PubMed] [Google Scholar]
- 36.Honjo K, Doi K, Doi C, Mitsuoka T. Histopathology of streptozotocin- induced diabetic DBA/2N and CD-1 mice. Lab Anim. 1986;20:298–303. doi: 10.1258/002367786780808695. [DOI] [PubMed] [Google Scholar]


