Abstract
Multidrug resistance (MDR) is the main cause of failure in the chemotherapy of cancer patients. The present study aimed to evaluate the effects of sesquiterpene coumarins of Ferula gummosa fruits on P-glycoprotein (P-gp)–mediated MDR. Drimane-type sesquiterpene coumarins from the fruits of F. gummosa were extracted with dichloromethane and subjected to column chromatography. The effects of the isolated compounds on P-gp–mediated MDR were evaluated in the breast cancer cell line MCF-7 which shows high resistance to doxoribicin (MCF-7/Dox). Phytochemical investigation of dichloromethane extract of F. gummosa fruits resulted in three sesquiterpene coumarins including conferone (1), mogoltacin (2), and feselol (3). The structures of these compounds were confirmed by 1D and 2D Nuclear Magnetic Resonance (NMR) spectroscopy. Exposure of cells to conferone, mogoltacin, feselol, and verapamil (positive control) enhanced doxorubicin uptake by MCF-7/Dox cells. This effect was dose dependent, but varied with the structure of the chemical. At 25 μM, all the tested sesquiterpene coumarins restored at least 50% of the reference uptake (uptake by sensitive cells); but at 10 μM, their potency varied where conferone showed the highest potency and feselol showed the lowest potency. Conferone, mogoltacin, and feselol from F. gummosa suppress P-gp–mediated drug efflux in highly resistant human breast cancer cells.
Keywords: Breast cancer, Ferula gummosa, P-glycoprotein inhibitor, Sesquiterpene coumarins
INTRODUCTION
Multidrug resistance (MDR) is the main cause of failure in chemotherapy of cancer patients. Although MDR is a multifactorial phenomenon, its predominant cause is the overexpression of membrane transporter proteins belonging to the ATP-binding cassette superfamily, namely P-glycoprotein (P-gp; ABCB1) and multidrug resistance protein (MRP1 or ABCC1).[1,2]
The observation that many compounds of natural origin are capable of modulating MDR proteins has called increasing attention to these substances. Recent reports have demonstrated that sesquiterpene coumarins, which contain drimane-type structure in the sesquiterpene moiety, reverse P-gp–mediated resistance considerably.[3,4] This class of natural compounds has been especially reported from the genus Ferula (Apiaceae).[5] Many bioactive compounds have been reported from Ferula species,[6,7,8,9] but the biological activities of drimane-type sesquiterpene coumarins have poorly been documented.[3,4,10]
Objectives
In the present work, we isolated the sesquiterpene coumarins of Ferula gummosa fruits and evaluated the effects of three of them (conferone, mogoltacin, and feselol) on P-gp–mediated MDR in the breast cancer cell line MCF-7 which shows high resistance to doxorubicin (MCF-7/Dox). The tested compounds belong to drimane-type sesquiterpene coumarins.
MATERIALS AND METHODS
Plant material
Fruits of F. gummosa were collected from Hezar Masjed Mountains, northeast of Iran, in August 2005 and identified by Mohammadreza Joharchi at the Ferdowsi University of Mashhad Herbarium (FUMH). A voucher specimen (1002) was deposited at the herbarium of the Department of Pharmacognosy, School of Pharmacy, Mashhad University of Medical Sciences.
General experimental procedures
The 1H, gradient COrrelation SpectroscopY (gCOSY), Rotating-frame Overhauser Effect SpectroscopY (ROESY), gradient Heteronuclear Single Quantum Coherence (gHSQC), and gradient Heteronuclear Multiple Bond Coherence (gHMBC) Nuclear Magnetic Resonance (NMR) experiments were run under standard conditions on Bruker DRX-500 and DRX-600 spectrometers at 300 K. NMR samples were prepared by dissolving each sample in CDCl3(99.8% D) (Carlo Erba, Italy). The spectra were calibrated using the solvent signal as the internal standard (1H, δ: 7.27 ppm; 13C, δ: 77.0 ppm). The ROESY spectra were obtained with a mixing time of 400 ms. The NMR data were processed on a Silicon Graphic Indigo2 Workstation using UXNMR software. Column chromatography was conducted with silica gel 230-400 mesh (Merck, Germany).
Extraction and isolation
The air-dried fruits (500 g) were ground into powder, defatted by petroleum ether, and extracted exhaustively by maceration with dichloromethane at room temperature. After filtration, the extract was concentrated under vacuum to yield 20 g of a brown residue.
A part of the extract (15 g) was subjected to column chromatography on silica gel (5 × 50 cm) using petroleum ether with increasing volumes of acetone in a gradient system. The fractions were compared by thin layer chromatography (TLC; silica gel using petrol–Me2CO as solvent), and those giving similar spots were combined. Five fractions were finally obtained. Fractions 1-3 gave compounds 1 (15 mg), 2 (770 mg), and 3 (48.5 mg) as white crystals, respectively.[11]
Cell line and P-gp inhibition assay
Resistant MCF-7/Dox cells were generated by one year of continuous exposure of sensitive MCF-7 cells (European Collection of Cell Cultures, Salibury, UK) to Dox. These cells have been shown to overexpress P-gp. Based on a previously described procedure,[12] cells were cultured as monolayers in 75-cm2 culture flasks in Eagle's minimum essential medium (Gibco-BRL, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS; Bio West, Nuaille’, France), vitamins, amino acids, and gentamycin (all from Gibco™, Invitrogen, Cergy-Pontoise, France). The medium was changed every 48 h and subcultured with 5X trypsin/ethylenediaminetetraacetic acid (EDTA) (Gibco). Cells were maintained at 37°C under a humidified atmosphere containing 5% CO2. The chemicals were obtained from Sigma, USA.
Cytotoxicity was assessed using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay. Briefly, the cells were seeded in 96-well tissue culture plates and incubated with increasing concentrations of each compound (10, 20, 30, 40, 50, 60, and 70 μg/ml) for 24 h. Twenty microliters of MTT in phosphate-buffered saline (PBS; 5 mg/ml) was then added to each well. Following a 4-h incubation at 37°C, the medium/MTT mixtures were removed and the blue insoluble formazan product formed by living cells was dissolved in dimethyl sulfoxide (DMSO) (200 μl/well). The optical density of the wells was then measured with a microplate reader at 570 nm. All tests were performed in triplicate and the 50% inhibitory concentrations (IC50) were calculated from concentration–effect curves by interpolation.
Cellular Dox accumulation was determined as described by Huang and Liu.[13] In brief, MCF-7/Dox cells in exponential growth were exposed to 10 μM doxorubicin in the absence or presence of conferone, mogoltacin, and feselol at 10 and 25 μM or verapamil (VPM; Sigma) at 10 μM for 3 h. Then the cells were harvested by centrifugation and rinsed with PBS. Cell pellets were suspended in 0.3 μM HCl in 50% ethanol and sonicated in an ultrasonic disintegrator for 30 s. Following centrifugation at 1500 g for 15 min, the supernatant was removed and assayed spectrofluorometrically for Dox content at excitation and emission wavelengths of 470 nm and 585 nm, respectively. The intracellular Dox content was calibrated with a standard curve of Dox and was expressed in picomoles per 106 cells.
Statistical analysis
Statistical analyses were performed using the SPSS (version 11.0) software. Statistical comparison between groups was made by Student's t-test and one-way analysis of variance (ANOVA) with Tukey–Kramer, a post-hoc test. A P < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Silica gel column chromatography of the dichloromethane extract of fruits of F. gummosa followed by preparative TLC resulted in three sesquiterpene coumarins including conferone (1), mogoltacin (2), and feselol (3), as reported in an earlier study.[11] The isolated coumarins [Figure 1] were identified by comparison of their NMR, mass spectrometry (MS), and melting point data with those previously described in the literature.[5,14,15] Briefly, the structure of 1 was established from analysis of the 1H and 13C NMR spectra [Tables 1 and 2]. Compound 1 displayed 24 carbon signals, with 9 being typical of an umbelliferone skeleton and the other 15 signals were ascribable to a sesquiterpene moiety. The downfield signal at δC 161.5 was assigned to the carbonyl carbon of the coumarin moiety, whereas the downfield signal at δC 216.4 was indicative of a ketone group belonging to the sesquiterpene unit. HSQC spectrum classified the carbon signals to four aliphatic methylenes at δC 38.8 (C-1′), 34.8 (C-2′), 24.3 (C-6′), and a primary alcoholic carbon at δC 67.0 characteristic for C-11′ and nine methines, five of them for umbelliferone moiety at δC 113.4 (C-3), 143.7 (C-4), 129.2 (C-5), 113.6 (C-6), and 101.7 (C-8) and four for methyls at δC 25.6 (C-12′), 22.7 (C-13′), 25.6 (C-14′), and 14.9 (C-15′). The 1H NMR spectrum of 1 showed resonance characteristics of four methyl singlets at δH 1.75 (H-12′), 1.17 (H-13′), 1.13 (H-14′), and 1.18 (H-15′) and three olefinic resonances at δH 6.28 (H-3), 7.66 (H-4), and 5.62 (H-7′). Three aromatic protons at δH 7.39 (H-5), 6.86 (H-6), and 6.85 (H-8) suggested the presence of a 7, 9, 10-trisubstituted benzene ring, which was supported by the 13C NMR spectrum [Tables 1 and 2 and Figures 2 and 3].
Figure 1.
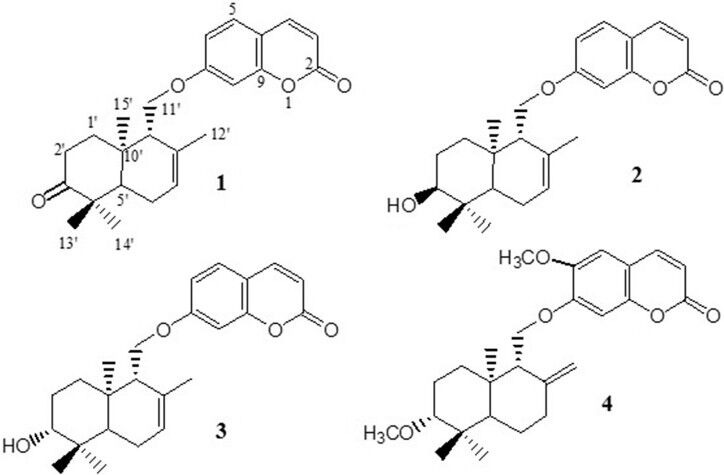
Chemical structures of the compounds isolated from Ferula gummosa fruits conferone (1), mogoltacin (2) and feselol (3) and the structure of diportlandin (4)
Table 1.
1H-NMR data obtained with compounds 1-5 (600 MHz, δ ppm)*
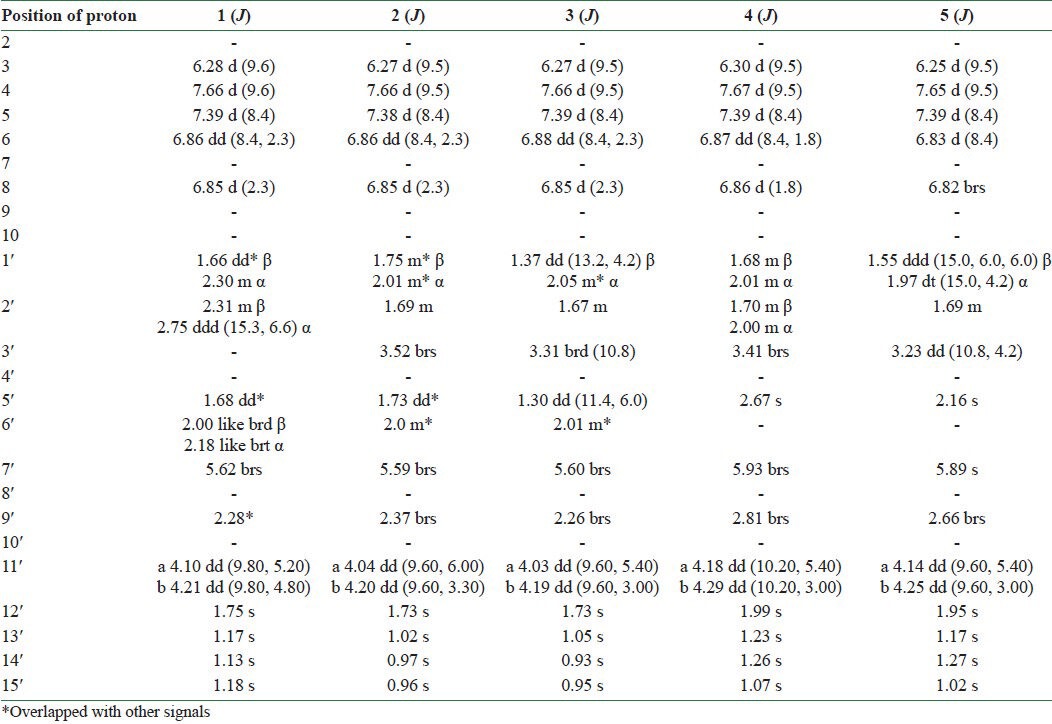
Table 2.
13C-NMR data obtained with compounds 1–5 (125.7 MHz, δ ppm)
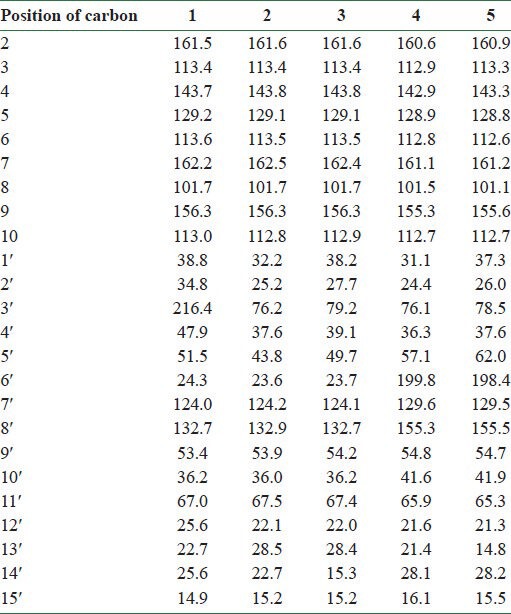
Figure 2.
The 1H-NMR spectrum of conferone (1)
Figure 3.
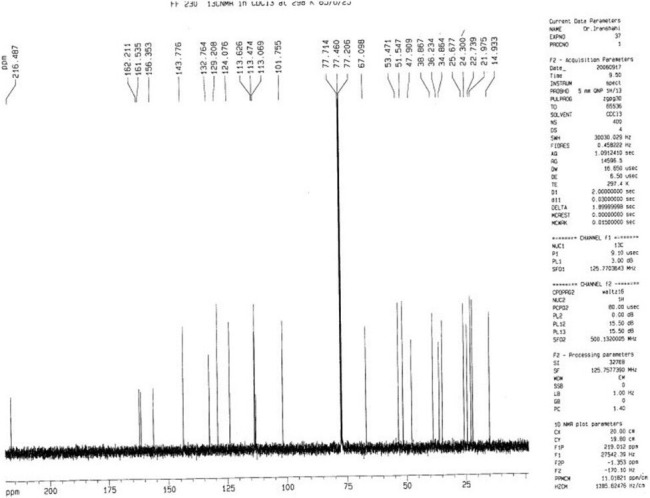
The 13C-NMR spectrum of conferone (1)
The NMR spectral data of 2 were similar to those of 1, except a slight difference in the signals assigned to the sesquiterpene unit which was due to the replacement of the ketone group at C-3′ with an oxymethine. Regarding this portion and with respect to conferone, HMBC spectrum showed the correlations of the secondary alcoholic H-3′ (δH 3.52) to δC 25.2 (C-2′), δC 37.6 (C-4′), and to δC 43.8 (C-5′). Other NMR spectral data of 2 were closely comparable to those of 1. Hence, the structure of 2 was determined as mogoltacin (or conferol) [Tables 1 and 2 and Figures 4 and 5].
Figure 4.
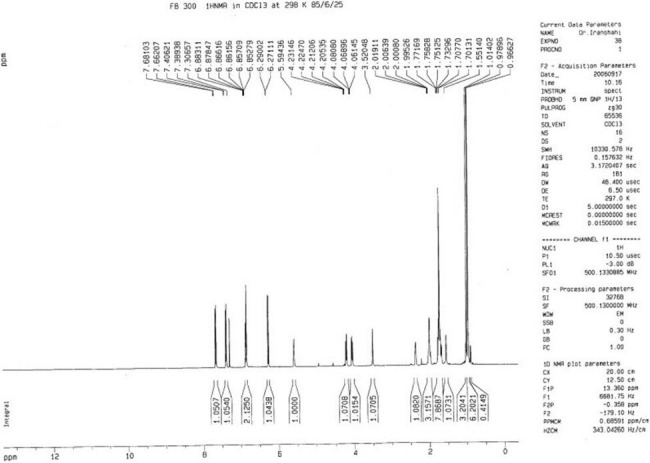
The 1H-NMR spectrum of mogoltacin (2)
Figure 5.
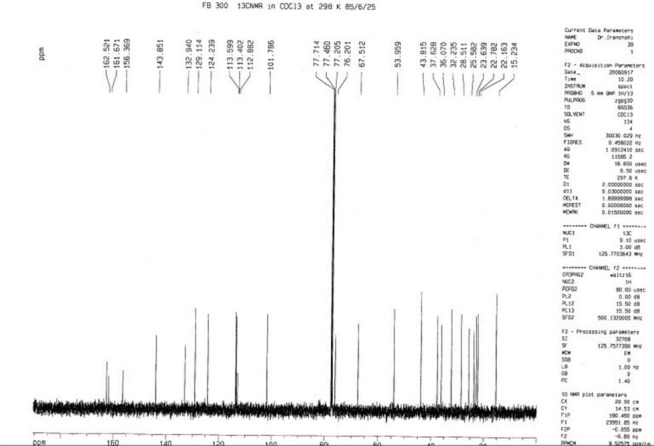
The 13C-NMR spectrum of mogoltacin (2)
The NMR spectral data of 3 were very similar to those of 2, except for the stereochemistry of C-3′. Thus, the differences between the 1H NMR of 2 and 3 appeared in the signals due to protons on C-2′, C-3′ and the methyl groups of 13′ and 14′. In 3, the signals for the H-2′ and H-3′ appeared at δH 1.67 and δH 3.31, respectively, and those for the methyl groups of 13′ and 14′ appeared at δH 1.05 and 0.93, respectively. In 2, the corresponding signals appeared at δH 1.69 (H-2′), 3.52 (H-3′), 1.02 (H-13′), and 0.97 (H-14′), respectively. ROESY experiments confirmed the stereochemistry of 3′-OH as β and α conformers for compounds 2 and 3, respectively. Therefore, compound 3 was determined as feselol [Tables 1 and 2 and Figures 6 and 7].
Figure 6.
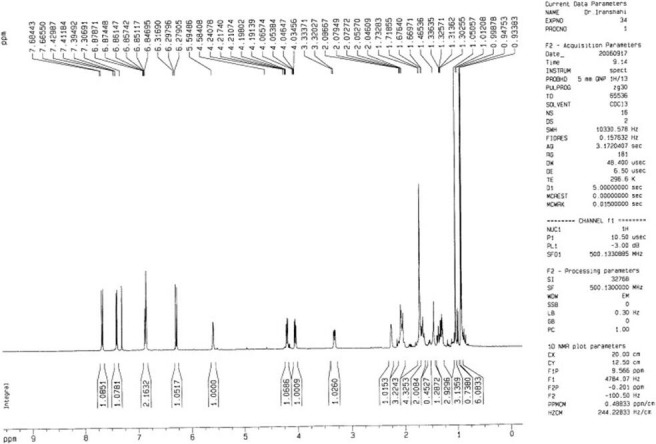
The 1H-NMR spectrum of feselol (3)
Figure 7.
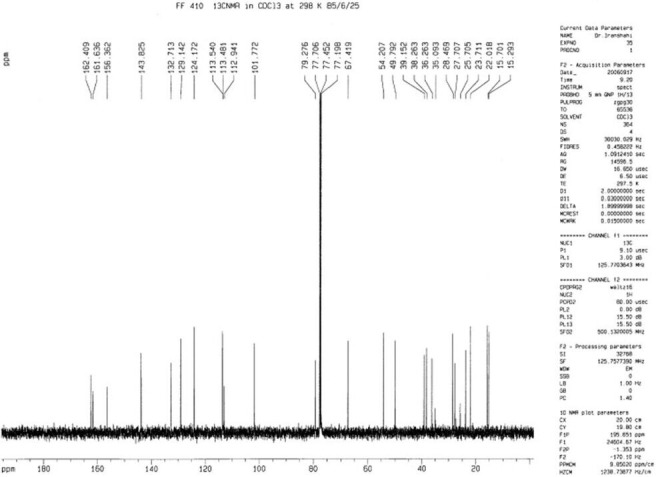
The 13C-NMR spectrum of feselol (3)
Based on a previous report on the P-gp inhibitory effect of diportlandin [4 in Figure 1], a sesquiterpene coumarin from Euphorbia portlandica,[3] and conferone (1),[4] we decided to test the activity of conferone, feselol, and mogoltacin on doxorubicin uptake in MCF-7/Dox cells. It should be pointed out that all of the tested compounds in this study and the previous one[3] contain a drimane-type sesquiterpene moiety linked to 7-hydroxycoumarin or 7-hydroxycoumarin derivatives.
Although breast cancer cells are usually sensitive to chemotherapy, P-gp overexpression and consequent resistance to chemotherapy is commonly observed in advanced breast tumors. Cells overexpressing P-gp are characterized by a lower capacity to accumulate and retain anticancer drugs than the sensitive ones. Three hours after exposure to Dox, the MCF-7/Dox cells showed an intracellular concentration of Dox 12.1-fold lower than the parent sensitive MCF-7 cells. This marked difference in the intracellular Dox content demonstrates the overexpression of resistant human breast cancer cell line in drug efflux pumps. In a previous study, it was shown that diportlandin (4), a sesquiterpene coumarin with structural similarity to those of our tested compounds, has significant effect in inhibiting the efflux pump activity mediated by P-gp, as compared to the positive control VPM.[3] In 2006, Barthomeuf et al. reported that conferone (1) enhances the uptake of vinblastine in a mammalian cell line expressing the MDR1 gene, evidencing for the first time that certain sesquiterpene ethers from Ferula may have some potency for the treatment of MDR malignancies.[4] Furthermore, it was observed that the ability to inhibit P-gp transport is a common feature of various sesquiterpene coumarins from Ferula and that conferone may modulate MDR1 gene expression.[16] In the present study, it was verified that 3 h of exposure to feselol, mogoltacin, and conferone at 10 and 25 μM, or to 10 μM VPM singly, was not toxic for MCF-7 and MCF-7/Dox. Exposure of MCF-7/Dox to feselol, mogoltacin, conferone, or VPM enhanced Dox uptake [Figure 8]. This finding probably demonstrates that the tested coumarins have the capacity to inhibit P-gp–mediated drug efflux in human breast cancer cells. The chemosensitizing effects of coumarin derivatives were dose dependent, but varied with the structure of the chemical. At 25 μM, all coumarins restored at least 50% of the reference uptake (uptake by sensitive cells); but at 10 μM, their potency varied in the order of conferone > mogoltacin > feselol. At this concentration, all compounds were less potent than the positive control. In sensitive MCF-7 cells, the uptake of Dox by cells was not affected by the addition of coumarins at 10 μM concentration. However, it was decreased by VPM at 10 μM and by the coumarins at 25 μM. This decrease may be due to the enhanced cytotoxicity of the combination in sensitive MCF-7 cells, which caused less cell density and, therefore, less intracellular Dox to be assayed [Figure 2].
Figure 8.
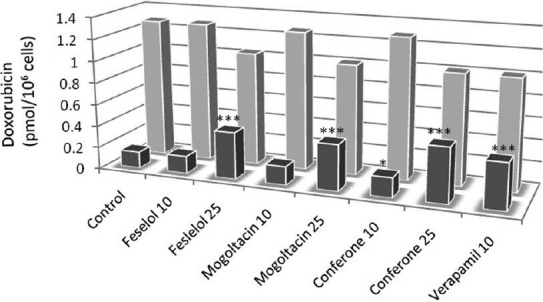
Enhanced accumulation of Dox in MCF-7/Dox cells by conferone, feselol, mogoltacin, and VPM. MCF-7/Dox (dark) and sensitive MCF-7 (light) cells were incubated with Dox (10 μM) in the presence of the indicated concentrations of feselol, mogoltacin, and conferone. Control cells were not incubated with any compound. VPM (10 μM) was used as positive control. Each experiment was performed in triplicate. P-values refer to the comparison between each tested compound and the negative control group. *P< 0.05; ***P< 0.001
The present study probably proves that conferone, mogoltacin, and feselol suppress P-gp–mediated drug efflux in highly resistant human breast cancer cells. The most interesting finding is the possibility to establish the first structure–activity relationship, since all of the mentioned compounds have an identical carbon skeleton in their structures. On the other hand, previous findings[4,9] demonstrate that drimane-type sesquiterpene coumarins have low toxicity and could serve as promising lead compounds for designing novel chemosensitizing agents. Although the toxicological profile of the tested phytochemicals is yet to be determined, it should be pointed out that Ferula species which contain these compounds have been traditionally used as medicinal plants, spices, and foods for a long time and, thus, could be considered as potentially safe.
ACKNOWLEDGMENTS
The authors wish to thank “La ligue contre le cancer,” in particular, the Cantal and Puy de Dome Committees for their support and partial financial contribution to this work. In addition, this research was partially supported by a grant (85029) from Mashhad University of Medical Sciences Research Council.
REFERENCES
- 1.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting P-glycoprotein. Cancer Control. 2003;10:159–65. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 2.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr Relat Cancer. 2003;10:43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 3.Madureira AM, Molnar A, Abreu PM, Molnar J, Ferreira MJ. A new sesquiterpene-coumarin ether and a new abietane diterpene and their effects as inhibitors of p-glycoprotein. Planta Med. 2004;70:828–33. doi: 10.1055/s-2004-827231. [DOI] [PubMed] [Google Scholar]
- 4.Barthomeuf C, Demeule M, Grassi J, Saidkhodjaev A, Beliveau R. Conferone from Ferula schtschurowskiana enhances vinblastine cytotoxicity in MDCK-MDR1 cells by competitively inhibiting P-glycoprotein transport. Planta Med. 2006;72:634–9. doi: 10.1055/s-2006-931574. [DOI] [PubMed] [Google Scholar]
- 5.Abd El-Razek MH, Ohta S, Hirata T. Terpenoid coumarins of the genus Ferula. Heterocycles. 2003;60:689–716. [Google Scholar]
- 6.Iranshahi M, Shahverdi AR, Mirjani R, Amin GR, Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Naturforsch. 2004;59:506–8. doi: 10.1515/znc-2004-7-809. [DOI] [PubMed] [Google Scholar]
- 7.Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito C, Furukawa H, et al. Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta Med. 2008;74:147–50. doi: 10.1055/s-2008-1034293. [DOI] [PubMed] [Google Scholar]
- 8.Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 2007;68:554–61. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Shahverdi AR, Saadat F, Khorramizadeh MR, Iranshahi M, Khoshayand MR. Two matrix metalloproteinase from Ferula persica var. persica. Phytomedicine. 2006;13:712–7. doi: 10.1016/j.phymed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Oughlissi-Dehak K, Lawton P, Michalet S, Bayet C, Darbour N, Hadji-Mahammed M, et al. Sesquiterpenes from aerial parts of Ferula vesceritensis. Phytochemistry. 2008;69:1933–8. doi: 10.1016/j.phytochem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Iranshahi M, Rezaee R, Sahebkar AH, Bassarello C, Piacente S, Pizza C. Sesquiterpene coumarins from the fruits of Ferula badrakema. Pharm Biol. 2009;47:344–7. [Google Scholar]
- 12.Barthomeuf C, Debiton E, Mshvildadze V, Kemertelidze E, Balansard G. In vitro activity of Hederacolchiside A1 compared with other saponins from Hedera colchica against the proliferation of human carcinoma and melanoma cells. Planta Med. 2002;68:672–5. doi: 10.1055/s-2002-33807. [DOI] [PubMed] [Google Scholar]
- 13.Huang M, Liu G. The study of innate drug resistance of human hepatocellular carcinoma Bel7402 cell line. Cancer Lett. 1999;135:97–105. doi: 10.1016/s0304-3835(98)00280-8. [DOI] [PubMed] [Google Scholar]
- 14.Murray RD, Mendez J, Brown SA. 1st ed. New York: John Wiley and Sons Inc; 1982. The natural coumarins; p. 555. [Google Scholar]
- 15.Lee E, Mabry TJ. Sesquiterpene coumarin ethers of Ferula tingitana. J Nat Prod. 1985;48:326–7. doi: 10.1021/np50038a024. [DOI] [PubMed] [Google Scholar]
- 16.Bayet-Robert M, Iranshahi M, Thivat E, Durando X, Chollet P, Barthomeuf C. Une coumarine sesquiterpenique, la conférone, inhibe à la fois l’activité de transport et l’expression transcriptionnelle de la P-glycoprotéine. 2nd scientific meeting of CLARA, Grenoble, France, 2007, 19-20. Bull Cancer. 2007;94:S70–1. [Google Scholar]


