Abstract
Inflammation is a normal immune response; but if the body's regulation of inflammation is dysfunctional, then it will have an adverse effect on the body. Although use of modern drugs for inflammation has a relieving effect, it is still unsatisfactory. Moreover, the emergence of drug-resistant strains and even new kinds of microorganisms is causing significant morbidity and mortality. Recently, more attention has been focused on herbal medicine to treat various diseases because of the ability of the herbs to affect multiple target signaling pathways and their multiple mechanisms of action. Thus, a large number of studies have reported on the anti-inflammatory and antimicrobial effects of the traditional Chinese herbs. Literature survey was performed by conducting systematic electronic search in PubMed, Science Direct, Google Scholar, and in books. This review has listed 11 heat-clearing Chinese herbs (HCCHs) including Scutellaria baicalensis (黃芩 Huáng Qín), Coptis chinensis (黃連 Huáng Lián), Flos Lonicerae (金銀花 Jīn Yín Hūa), Forsythia suspensa (連翹 Lián Qiào), Isatidis Folium (大青葉 Dà Qīn Yè), Radix Isatidis (板藍根 Bǎn Lán Gēn), Viola yedoensis (紫花地丁 Zǐ Huā Dì Dīn), Pulsatilla Radix (白頭翁 Bái Tóu Wēn), Andrographis paniculata (穿心蓮 Chuān Xīn Lián), Houttuynia cordata (魚腥草 Yú Xīng Cǎo), and Patrinia Herba (敗醬草 Bài Jiàn Cǎo), which have anti-inflammatory and antimicrobial effects, and has described their effects through different mechanisms of action and multiple targets. Their ability to affect multiple target signaling pathways and their potential mechanisms of action contributing to their anti-inflammatory and antimicrobial activity may be related to their action of removing heat and counteracting toxicity. Further studies are needed on the collection of HCCHs to know the detailed mechanism of action of herbs in this group for the assessment of effective drug.
Keywords: Anti-inflammatory activity, Antimicrobial activity, Heat-clearing Chinese herbs, Traditional Chinese Medicine
INTRODUCTION
Inflammation is a part of the immune response that can prevent infection through production of pro-inflammatory cytokines and generation of inflammatory mediators in response to microbial products.[1] Although inflammation is crucial to maintaining the health and integrity of an organism, when the inflammatory process is poorly controlled, it can cause massive tissue destruction and a series of chain reactions.[2,3,4] The current treatment of inflammatory disorders involves extensive use of nonsteroidal anti-inflammatory drugs and corticosteroids. Although use of modern drugs for inflammation has a relieving effect, it is still unsatisfactory.[5] Moreover, bacterial resistance to antibiotics and the emergence of new kinds of microorganisms are becoming an increasing problem all over the world, causing significant morbidity and mortality.[6,7] In order to combat this problem, novel antibiotic and anti-inflammatory compounds need to be found which are both effective and safe.
Traditional Chinese Medicine (TCM) has been used in China over thousands of years for the prevention and treatment of various diseases.[6,8,9] TCM uses yin–yang theory to explain the organizational structure, physiological functions, and pathological changes in the human body and to guide diagnosis and treatment of disease.[5,10] Although yin and yang are contradictory in nature, they depend on each other for existence. Keeping balance between yin and yang is very important to maintain the healthy state of human body. TCM theory states that the occurrence of the disease depends on the interaction between zheng qi (nonpathogenic qi) and xie qi (pathogenic qi). The idea of disease is the struggle between pathogenic qi and nonpathogenic qi; in this struggle process, there will be changes between yin and yang. TCM holds that variation between the evil aspect and healthy trend determines the occurrence of disease. Therefore, in TCM, inflammatory and antimicrobial therapy lies in strengthening the healthy trend and dispelling the evil aspect in order to keep a balanced state between yin and yang.[5,11]
Herbal medicine is one of the main components of TCM which has long been used for its multiple types of disease treatment. In recent times, it is making a rapid progress in scientific investigation and attracting great attention due to the good therapeutic effects and minimal side effects of the herbs.[6,8,12] Chinese herbs used in the treatment of diseases are grouped into many categories. One of these is heat-clearing Chinese herbs (HCCHs). Herbs in this group are mostly cold in nature and can clear away heat, purge fire, dry dampness, cool blood, and relieve toxic material. Their main action is clearing away interior heat, and thus they are considered to be antipyretic.[11,13] Because of all these properties, HCCHs may be effective in the treatment of inflammatory disease and microbial infection. This review tries to summarize the effect of HCCHs which have shown anti-inflammatory and antimicrobial activities and their mechanisms of action.
Scutellaria baicalensis (黃芩 Huáng Qín)
Scutellaria baicalensis is a species of flowering plant belonging to Lamiaceae family It is a heat-clearing, phlegm-removing herb, traditionally used to cool heat, drain fire, clear damp-heat, stop bleeding, calm the fetus, and descend yang.[11,13,14] The dry root part of Sc. baicalensis has many pharmacological effects including antipyretic, hepatoprotective, antihypertensive, diuretic, and antibiotic activities. It is mildly sedating and also used to treat dysentery and chronic hepatitis.[6,7,14,15]Sc. baicalensis has distinct effects in the treatment of inflammatory diseases; it alleviates inflammation by decreasing the expression of interleukin (IL)-1b, IL-6, and IL-12, and the production of tumor necrosis factor (TNF)-α and soluble intercellular adhesion molecule-1 (ICAM-1).[5,16] In Xie xin herbal decoction, huang qin, in combination with huang lian, inhibits nitric oxide (NO) production in vitro and in vivo in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Oroxylin A, which is a flavonoid found in dried root of Sc. baicalensis, has also shown good anti-inflammatory effect.[17,18] Moreover, Sc. baicalensis has antibacterial effect against Helicobacter pylori as well as inhibits the growth of Escherichia coli B, coagulase-negative staphylococci, and Saccharomyces cerevisiae.[7,15]
Coptis chinensis (黃連 Huáng Lián)
Coptis chinensis belongs to Ranunculaceae family. Traditionally, it has been used to drain fire, detoxify and disinfect, stop bleeding, cure eczema, burns, and ulcer, and to descend yang.[7,11,13,14,19] The main pharmacodynamic properties have long been recognized in the treatment of intestinal infections including acute gastroenteritis, cholera, and bacillary dysentery. It also used for treating various diseases including skin diseases, conjunctivitis, otitis, and hypertension.[14,19,20]C. chinensis has been demonstrated to have anti-inflammatory effects through different mechanisms. It inhibits TNF-induced Nuclear factor-kappaB (NF-kB) signaling in human keratinocytes by blocking the NF-kB–dependent pathway. It also decreases Th17 cytokine secretion and differentiation by activation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and down-regulation of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and retinoic acid-related orphan receptor ϒt (RORγt) expression. It also reduces Th1 cytokine secretion and differentiation by inhibition of protein 38 (p38) mitogen activated protein kinase (MAPK) and Jun N-terminal kinase (JNK) activation along with down-regulation of STAT1 and STAT4 activities.[21,22] In combination with other herbs, C. chinensis exhibited a good anti-inflammatory effect; the ethanol extract from Zuojin Pill inhibited inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), IL-6, IL-1β, and TNF-α expression by preventing the nuclear translocation of the NF-κB p50 and p65 subunits in RAW 264.7 cells.[23] Another Chinese medicinal formula, IBS-20, containing C. chinensis decreased LPS-stimulated pro-inflammatory cytokine secretion from JAWS II dendritic cells and also blocked the interferon gamma (IFNγ)-induced drop in transepithelial electric resistance which is an index of permeability, in fully differentiated Caco-2 monolayer.[24]C. chinensis has also significant antimicrobial activity against a variety of microorganisms including bacteria, viruses, fungi, protozoans, helminths, and Chlamydia, including Staphylococcus aureus, Pseudomonas aeruginosa, E. coli, Propionibacterium acnes, Streptococcus pneumoniae, Vibrio cholerae, Bacillus anthracis, Bacillus dysenteriae, and Sa. cerevisiae.[7,21,25] Berberine, the major active component of C. chinensis, was found to be bactericidal on V. cholera and capable of inhibiting bacterial adherence to mucosal or epithelial surfaces.[26]
Flos Lonicerae (金銀花 Jīn Yín Hūa)
Flos lonicerae is a honeysuckle flower belonging to Caprifoliaceae family. It is a widely used herb in China for the treatment of infection by exopathogenic wind-heat or epidemic febrile diseases.[11,14,27,28] The dried flower and buds of Flos Lonicerae have shown various pharmacological effects including anti-nociceptive, anti-diabetic, anti-tumor, antioxidant, anti-angiogenic, antipyretic, antiviral, and hepatoprotective activities.[6,29,30,31]Flos Lonicerae demonstrated anti-inflammatory properties through suppression of mediator release from the mast cells activated by secretagogues.[32] In addition, the n-butanol fraction containing Flos Lonicerae can alleviate inflammation better than celecoxib in carrageen- and croton oil-induced paw edema and ear edema.[29]Flos Lonicerae contains various active compounds that have marked anti-inflammatory effect, including luteolin (suppresses inflammatory mediator release by blocking NF-kB and MAPKs pathway activation in HMC-11 cells), chlorogenic acid (inhibits rat reflux esophagitis induced by pylorus and forestomach ligation), and loncerin (reduces edema by suppressing T cell proliferation, NO production from the macrophages, and shifting cellular immunity from Th1- toward Th2-type responses).[33,34,35]Flos Lonicerae has significant antimicrobial activity against diverse species of bacteria and fungi. It has inhibitory effect against H. pylori and Porphyromonas gingivalis,[15] and it treats candidal septic arthritis.[35] It also has antimicrobial effect against oral pathogens including Streptococcus mutans, Actinomyces viscosus, and Bacteroides melaninogenicus.[6]
Forsythia suspensa (連翹 Lián Qiào)
Forsythia suspensa is a flowering plant belonging to the family Oleaceae. Traditionally, it used to treat carbuncle, disperse lumps, and stagnation, and to expel wind and heat.[11,13,14] The fruit of F. suspensa has potent pharmacological actions such as antiviral, choleretic, antipyretic, hepatoprotective, antiemetic, and diuretic effects.[14,27]F. suspensa alleviates inflammation by reducing the anaphylactic antibodies, mast cell degranulation, and histamine release. It also significantly suppresses β-conglycinin–induced T lymphocyte proliferation and IL-4 synthesis.[36,37]F. suspensa fruit inhibits NO production and iNOS gene expression by its active components rengyolone, dibenzylbutyrolactone lignans, as well as its butanol fraction of the aqueous extract. It also inhibits TNF-α and COX-2 production.[38,39,40] Another bioactive agent, arctigenin, inhibits increase in capillary permeability and leukocyte recruitment into inflamed tissues, by reduction of the vascular leakage and cellular events through inhibition of production of inflammatory mediators such as NO and pro-inflammatory cytokines such as IL-1b, IL-6, TNF-α, and prostaglandin E2 (PGE2).[38,39,41] Moreover, F. suspensa inhibits NF-kB nucleus translocation through reduction in I-kappa-B (IkB) phosphorylation and suppression of NF-kB–regulated proteins, and also reduces the activation of MAPKs.[39,40,41] Various studies have reported the antimicrobial effect of F. suspensa. It has potent antibacterial activity against E. coli, Sta. aureus, Bacillus subtilis, Str. mutans, and Po. gingivalis and antifungal activity against Aspergillus flavus, Rhizopus stolonifer, Penicillium citrinum, Aspergillus niger, and Saccharomyces carlsbergensis.[6,42]F. suspensa suppresses influenza A virus–induced RANTES secretion by human bronchial epithelial cells to stop accumulation of inflammatory cells in the infective sites, which has been reported to play a crucial role in the progression of chronic inflammation and multiple sclerosis after viral infection.[27]
Isatidis folium (大青葉 Dà Qīng Yè)
Isatidis folium is a flowering plant belonging to the family Brassicaceae. The leaves of Isatidis Folium are traditionally used for the treatment of sore throat, redness of skin, and as an antipyretic.[13,14,27,43,44]Isatidis Folium has also been used to treat encephalitis, acute dysentery, hepatitis, measles, pneumonia, influenza, epidemic cerebrospinal meningitis, encephalitis B, viral pneumonia, mumps, and diabetics.[27,45,46] Tryptanthrin, an alkaloid isolated from Isatidis leaves, has shown anti-inflammatory effect due to its strong inhibitory effect on the COX-2 enzyme.[47] Several derivatives of hydroxycinnamic acid, including ferulic acid and sinapic acid, are also thought to be important to inhibit inflammation.[25]Isatidis Folium possesses valuable viricidal effect in the control of pseudorabies infection in swine.[48,49]
Radix Isatidis (板藍根 Bǎn Lán Gēn)
Isatidis radix belongs to the family Brassicaceae. Traditionally, it used to cool blood.[13,14,19] The dry root of Isatidis Radix has many pharmacological activities such as antibiotic, anti-diabetic, and immune-stimulating effects and is used to treat encephalitis B and viral infections.[48,50] Methanolic extracts of Isatidis Radix can significantly inhibit the release of inflammatory mediators from the macrophages, such as NO, PGE2, and pro-inflammatory cytokines.[51] Isatidis Radix has also been demonstrated to suppress the growth of E. coli and H. pylori and increases blood neutrophil phagocytosis of 32P-labeled Sta. aureus.[15,52,53] Syringic acid isolated from Isatidis Radix inhibited LPS-induced endotoxin shock.[51] Besides, Isatidis Radix is found to be clinically effective against the infections caused by various subtypes and strains of influenza viruses including Severe Acute Respiratory Syndrome (SARS).[44,50]
Viola yedoensis (紫花地丁 Zǐ Huā Dì Dīng)
Viola yedoensis is a flowering plant belonging to the violet family of Violaceae. Traditionally, it used to cool heat, and disinfect and detoxify.[11,13,14]V. yedoensis has several pharmacological effects including antibiotic, anti-inflammatory, and antipyretic activities. It can also be used for the treatment of skin diseases, i.e. eczema, impetigo, acne, pruritus, and cradle cap, and for upper respiratory tract infections with fever.[12,14] It has been reported to have antimicrobial activity against B. subtilis, Str. mutans, and Po. gingivalis.[54] It inhibits the replication of herpes simplex virus-1 and enterovirus 71 in the human neuroblastoma SK-N-SH cell line. Cyclotides from Viola are shown to be effective in inhibiting human immunodeficiency virus (HIV) replication.[50,55]
Pulsatilla radix (白頭翁 Bái Tóu Wēng)
Pulsatilla radix is a medicinal root plant of the Ranunculaceae. It used to cool heat, disinfect and detoxify, and clear damp-heat in TCM.[13,14] The root of Pulsatilla Radix has anti-inflammatory, antiparasitic, and antimicrobial action. It can treat dyspepsia, premenstrual tension, and psychosomatic disturbances.[14] A quinine-type compound, pulsaquinone, isolated from the aqueous ethanol extract of the roots of Pulsatilla Radix exhibited antimicrobial activities against an anaerobic non–spore-forming gram-positive bacillus, Pr. acnes, which is related to the pathogenesis of the inflamed lesions in a common skin disease, acne vulgaris.[56] Moreover, 4-hydroxy-3-methoxycinnamic acid of Pulsatilla Radix is found to have a selective growth inhibitor of the human intestinal bacteria, Clostridium perfringens and E. coli.[57]
Andrographis paniculata (穿心蓮 Chuān Xīn Lián)
Andrographis paniculata is also known as nemone chinensi and belongs to Acanthaceae family.[11] The active compounds isolated from An. paniculata, including diterpene, lactone, and andrographolide, have shown anti-inflammatory, anti-allergic, immune-stimulatory, and antiviral activities.[58,59]An. paniculata alleviates inflammation by inhibiting iNOS, TNF-α, IL-1b, IL-6, and IL-12 expression and NO production by down-regulation of p38MAPKs signaling pathways.[5,60] It also suppresses influenza A virus-induced RANTES secretion by human bronchial epithelial cells.[27]
Houttuynia cordata (魚腥草 Yú Xīng Cǎo)
Houttuynia cordata is one of the two species in the genus Houttuynia and belongs to the family Saururaceae.[14] It has many pharmacological effects including immune-stimulating, anti-inflammatory, antibiotic, antiviral, diuretic, analgesic, and hemostatic effects. It also used to treat pneumonia, bronchitis, colitis, urogenital tract infections, and chronic obstructive respiratory diseases, and topically to treat herpes simplex.[61]
Patrinia Herba (敗醬草 Bài Jiàn Cǎo)
Patrinia herba is a medicinal herb belongs to family of Valerianaceae.[12,14] It has antibiotic, hepatoprotective, sedating, and hypnotic effects, and it can be used to treat mumps.[14]Patrinia Herba can inhibit adjuvant-induced inflammation and hyperalgesia. In rats, it attenuates Freund's adjuvant (CFA)–induced hyperalgesia and facilitates the recovery from hyperalgesia, and also reduces edema.[62]
CONCLUSION
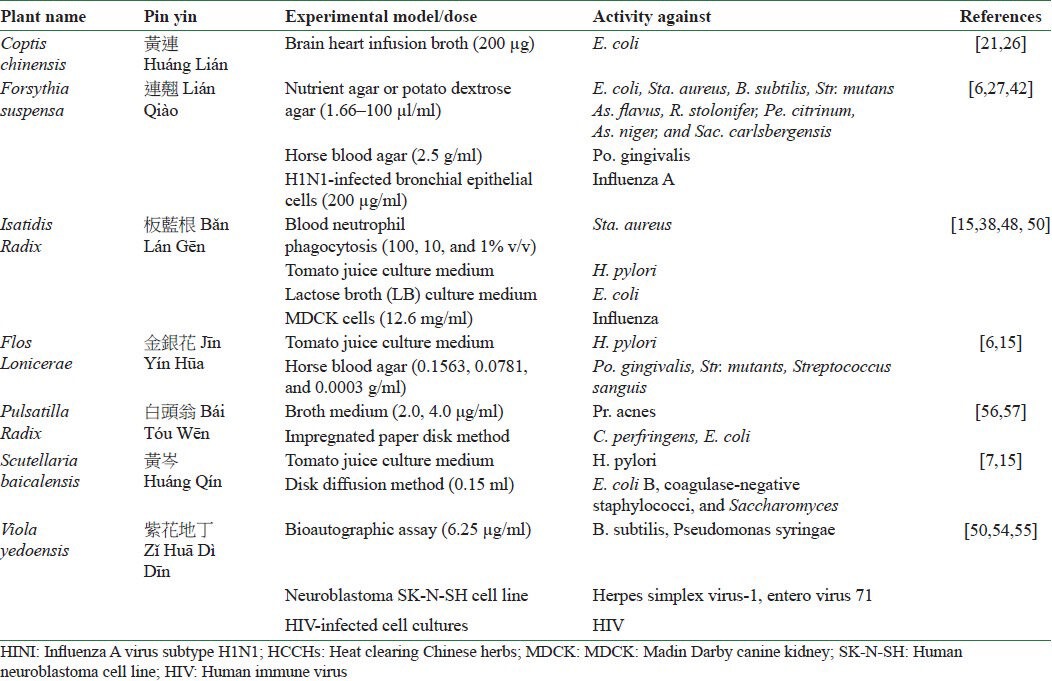
Investigation of the functions of different Chinese herbs by modern research has allowed us to determine the importance of using Chinese herbs for treatment of many diseases. Various studies have revealed that HCCHs are used for treating inflammatory and microbial diseases due to their multiple active ingredients. Since inflammation is the result of interaction of various inflammatory mediators, HCCHs can exert anti-inflammatory effect through different mechanisms of action including inhibition of inflammatory cytokines and mediators, blocking of inflammatory signaling, and interfering with chemokines [Table 1]. Moreover, HCCHs have also shown antimicrobial effect through inhibition of microbial adherence to mucosal or epithelial surfaces, inhibition of endotoxin shock, and selective inhibition of microbial growth [Table 2]. Collectively, all the above mechanisms are likely to be important for the anti-inflammatory and antimicrobial activity of HCCHs. This review reveals the anti-inflammatory and antimicrobial effects of HCCHs, in general, from different aspects and through different mechanisms. This may be linked to their action of removing heat and fire and counteracting toxicity. Therefore, further studies are needed on the collection of HCCHs to find the detailed mechanism of action of herbs in this group and to determine whether their nature of clearing away heat is related to their anti-inflammatory and antimicrobial effects according to Chinese medical theory, rather than focusing on simple gradient or single herbs for the assessment of effective therapeutic drugs from HCCHs.
Table 1.
Anti-inflammatory effects of HCCHs
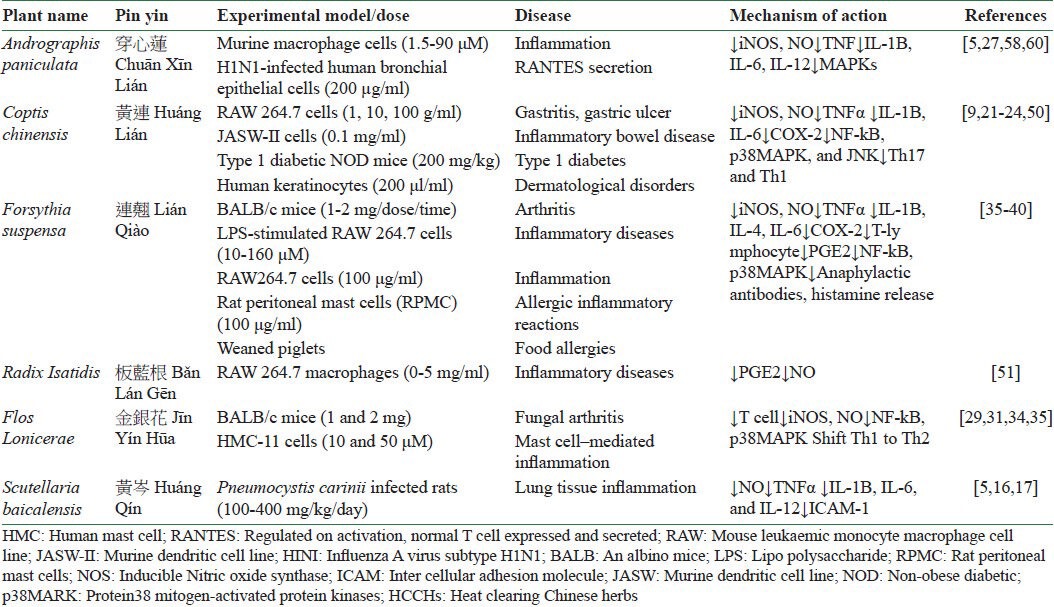
Table 2.
Antimicrobial effects of HCCHs
ACKNOWLEDGMENT
This study was supported by Tianjin University of Traditional Chinese Medicine (TUTCM).
REFERENCES
- 1.Paul WE. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2003. Fundamental Immunology. [Google Scholar]
- 2.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, et al. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146:9–39. doi: 10.1016/j.jep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Wong RW, Hägg U, Samaranayake L, Yuen MK, Seneviratne CJ, Kao R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study. Int J Oral Maxillofac Surg. 2010;39:599–605. doi: 10.1016/j.ijom.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach FS. Anti-microbial properties of Scutellaria baicalensis and Coptis chinensis, two traditional Chinese medicines. Biosci Horiz. 2011;4:9. [Google Scholar]
- 8.Li T, Peng T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antiviral Res. 2013;97:1–9. doi: 10.1016/j.antiviral.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan MH, Chiou YS, Tsai ML, Ho CT. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1:17. doi: 10.1016/s2225-4110(16)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu LM, Chen X, Xu JT. Determination methods for inspection of the complexion in traditional Chinese medicine: A review. Zhong Xi Yi Jie He Xue Bao. 2009;7:701–5. doi: 10.3736/jcim20090801. [DOI] [PubMed] [Google Scholar]
- 11.Tang Decai XJ. Shanghai: Shanghai University of Traditional Chinese Medicine; 2006. Science of Chinese Materia Medica. [Google Scholar]
- 12.Kong DX, Li XJ, Tang GY, Zhang HY. How many traditional Chinese medicine components have been recognized by modern Western medicine? A chemoinformatic analysis and implications for finding multicomponent drugs. Chem Med Chem. 2008;3:233–6. doi: 10.1002/cmdc.200700291. [DOI] [PubMed] [Google Scholar]
- 13.Franzblau SG, Cross C. Comparative in vitro antimicrobial activity of Chinese medicinal herbs. J Ethnopharmacol. 1986;15:279–88. doi: 10.1016/0378-8741(86)90166-2. [DOI] [PubMed] [Google Scholar]
- 14.Beijing: People's Medical Publishing House; 2005. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 15.Du P, Zhu S, Lu P. Antibacterial activity of 20 kinds of Chinese medicinal materials for Helicobacter pylori in vitro. Zhong Yao Cai. 2001;24:188–9. [PubMed] [Google Scholar]
- 16.Zhou L, Zhou BY. Influence of baicalin on TNF-alpha and soluble intercellular adhesion molecule-1 in rats infected with Pneumocystis carinii. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:144–7. [PubMed] [Google Scholar]
- 17.Xu G. Treatment of reflux laryngopharyngitis with modified banxia xiexin tang (Pinellia decoction for draining the heart): A report of 40 cases. J Tradit Chin Med. 2006;26:127–31. [PubMed] [Google Scholar]
- 18.Li L, Bao H, Wu J, Duan X, Liu B, Sun J, et al. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int Immunopharmacol. 2012;13:15–22. doi: 10.1016/j.intimp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J Ethnopharmacol. 2009;126:5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17:1562–4. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- 21.Enk R, Ehehalt R, Graham JE, Bierhaus A, Remppis A, Greten HJ. Differential effect of Rhizoma coptidis and its main alkaloid compound berberine on TNF-alpha induced NFkappaB translocation in human keratinocytes. J Ethnopharmacol. 2007;109:170–5. doi: 10.1016/j.jep.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–9. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang QS, Cui YL, Dong TJ, Zhang XF, Lin KM. Ethanol extract from a Chinese herbal formula, “Zuojin Pill”, inhibit the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 mouse macrophages. J Ethnopharmacol. 2012;141:377–85. doi: 10.1016/j.jep.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Grinchuk V, Ip SP, Che CT, Fong HH, Lao L, et al. Anti-Inflammatory Activities of a Chinese Herbal Formula IBS-20 In Vitro and In Vivo. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/491496. 491496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng W, Luo D. Chinese herbs and anti-infection immunity. Int J Biosci. 2012;2:11. [Google Scholar]
- 26.Sun D, Abraham SN, Beachey EH. Influence of berberine sulfate on synthesis and expression of Pap fimbrial adhesin in uropathogenic Escherichia coli. Antimicrob Agents Chemother. 1988;32:1274–7. doi: 10.1128/aac.32.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko HC, Wei BL, Chiou WF. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J Ethnopharmacol. 2006;107:205–10. doi: 10.1016/j.jep.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Chai XY, Li SL, Li P. Quality evaluation of Flos lonicerae through a simultaneous determination of seven saponins by HPLC with ELSD. J Chromatogr A. 2005;1070:43–8. doi: 10.1016/j.chroma.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Kang M, Jung I, Hur J, Kim SH, Lee JH, Kang JY, et al. The analgesic and anti-inflammatory effect of WIN-34B, a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J Ethnopharmacol. 2010;131:485–96. doi: 10.1016/j.jep.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Qian ZM, Li HJ, Li P, Ren MT, Tang D. Simultaneous qualitation and quantification of thirteen bioactive compounds in Flos lonicerae by high-performance liquid chromatography with diode array detector and mass spectrometry. Chem Pharm Bull (Tokyo) 2007;55:1073–6. doi: 10.1248/cpb.55.1073. [DOI] [PubMed] [Google Scholar]
- 31.Qian ZM, Wen XD, Li HJ, Liu Y, Qin SJ, Li P. Analysis of interaction property of bioactive components in Flos Lonicerae Japonicae with protein by microdialysis coupled with HPLC-DAD-MS. Biol Pharm Bull. 2008;31:126–30. doi: 10.1248/bpb.31.126. [DOI] [PubMed] [Google Scholar]
- 32.Chan BC, Hon KL, Leung PC, Sam SW, Fung KP, Lee MY, et al. Traditional Chinese medicine for atopic eczema: Penta Herbs formula suppresses inflammatory mediators release from mast cells. J Ethnopharmacol. 2008;120:85–91. doi: 10.1016/j.jep.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Kang OH, Choi JG, Lee JH, Kwon DY. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules. 2010;15:385–98. doi: 10.3390/molecules15010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku SK, Seo BI, Park JH, Park GY, Seo YB, Kim JS, et al. Effect of Lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J Gastroenterol. 2009;15:4799–805. doi: 10.3748/wjg.15.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Han Y. Antiarthritic effect of lonicerin on Candida albicans arthritis in mice. Arch Pharm Res. 2011;34:853–9. doi: 10.1007/s12272-011-0520-6. [DOI] [PubMed] [Google Scholar]
- 36.Hao Y, Li D, Piao X, Piao X. Forsythia suspensa extract alleviates hypersensitivity induced by soybean beta-conglycinin in weaned piglets. J Ethnopharmacol. 2010;128:412–8. doi: 10.1016/j.jep.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 37.Kim MS, Na HJ, Han SW, Jin JS, Song UY, Lee EJ, et al. Forsythia fructus inhibits the mast-cell-mediated allergic inflammatory reactions. Inflammation. 2003;27:129–35. doi: 10.1023/a:1023865727780. [DOI] [PubMed] [Google Scholar]
- 38.Kang HS, Lee JY, Kim CJ. Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol. 2008;116:305–12. doi: 10.1016/j.jep.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, Cho BJ, Park TW, Park BE, Kim SJ, Sim SS, et al. Dibenzylbutyrolactone lignans from Forsythia koreana fruits attenuate lipopolysaccharide-induced inducible nitric oxide synthetase and cyclooxygenase-2 expressions through activation of nuclear factor-kappab and mitogen-activated protein kinase in RAW264.7 cells. Biol Pharm Bull. 2010;33:1847–53. doi: 10.1248/bpb.33.1847. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Kim DH, Baek SH, Lee HJ, Kim MR, Kwon HJ, et al. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-kappaB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem Pharmacol. 2006;71:1198–205. doi: 10.1016/j.bcp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Shin S, Kim H, Han S, Kim K, Kwon J, et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-kappaB pathways. J Inflamm (Lond) 2011;8:16. doi: 10.1186/1476-9255-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XQ, Zhang XF, Lee KS. Antimicrobial Activity of the Extracts of Forsythia suspensa and Dendranthema indicum. Agric Chem Biotechnol. 2005;48:3. [Google Scholar]
- 43.Ho YL, Kao KC, Tsai HY, Chueh FY, Chang YS. Evaluation of antinociceptive, anti-inflammatory and antipyretic effects of Strobilanthes cusia leaf extract in male mice and rats. Am J Chin Med. 2003;31:61–9. doi: 10.1142/S0192415X03000783. [DOI] [PubMed] [Google Scholar]
- 44.Liu JF, Jiang ZY, Wang RR, Zheng YT, Chen JJ, Zhang XM, et al. Isatisine A, a novel alkaloid with an unprecedented skeleton from leaves of Isatis indigotica. Org Lett. 2007;9:4127–9. doi: 10.1021/ol701540y. [DOI] [PubMed] [Google Scholar]
- 45.Zou P, Hong Y, Koh HL. Chemical fingerprinting of Isatis indigotica root by RP-HPLC and hierarchical clustering analysis. J Pharm Biomed Anal. 2005;38:514–20. doi: 10.1016/j.jpba.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Qiao CZ, Liu S, Hang HM. Evaluation on antiendotoxic action and antiviral action in vitro of tetraploid Isatis indigotica. Zhongguo Zhong Yao Za Zhi. 2000;25:327–9. [PubMed] [Google Scholar]
- 47.Kong WJ, Zhao YL, Shan LM, Xiao XH, Guo WY. Investigation on the spectrum-effect relationships of EtOAc extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:109–14. doi: 10.1016/j.jchromb.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 48.Lin CW, Tsai FJ, Tsai CH, Lai CC, Wan L, Ho TY, et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsuan SL, Chang SC, Wang SY, Liao TL, Jong TT, Chien MS, et al. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol. 2009;123:61–7. doi: 10.1016/j.jep.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao HF, Lu MC, Chang HC, Wei CC, Kao CH, Chen ZH, et al. Effects of herbal medicinal formulas on suppressing viral replication and modulating immune responses. Am J Chin Med. 2010;38:173–90. doi: 10.1142/S0192415X10007749. [DOI] [PubMed] [Google Scholar]
- 51.Shin EK, Kim DH, Lim H, Shin HK, Kim JK. The anti-inflammatory effects of a methanolic extract from Radix Isatidis in murine macrophages and mice. Inflammation. 2010;33:110–8. doi: 10.1007/s10753-009-9164-9. [DOI] [PubMed] [Google Scholar]
- 52.Hu S, Cai W, Ye J, Qian Z, Sun Z. Influence of medicinal herbs on phagocytosis by bovine neutrophils. Zentralbl Veterinarmed A. 1992;39:593–9. doi: 10.1111/j.1439-0442.1992.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 53.Kong W, Zhao Y, Shan L, Xiao X, Guo W. Thermochemical studies on the quantity-antibacterial effect relationship of four organic acids from Radix Isatidis on Escherichia coli growth. Biol Pharm Bull. 2008;31:1301–5. doi: 10.1248/bpb.31.1301. [DOI] [PubMed] [Google Scholar]
- 54.Xie C, Kokubun T, Houghton PJ, Simmonds MS. Antibacterial activity of the Chinese traditional medicine, Zi Hua Di Ding. Phytother Res. 2004;18:497–500. doi: 10.1002/ptr.1497. [DOI] [PubMed] [Google Scholar]
- 55.Wang CK, Colgrave ML, Gustafson KR, Ireland DC, Goransson U, Craik DJ. Anti-HIV cyclotides from the Chinese medicinal herb Viola yedoensis. J Nat Prod. 2008;71:47–52. doi: 10.1021/np070393g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho SC, Sultan MZ, Moon SS. Anti-acne activities of pulsaquinone, hydropulsaquinone, and structurally related 1, 4-quinone derivatives. Arch Pharm Res. 2009;32:489–94. doi: 10.1007/s12272-009-1402-z. [DOI] [PubMed] [Google Scholar]
- 57.Lee HS, Beon MS, Kim MK. Selective growth inhibitor toward human intestinal bacteria derived from Pulsatilla cernua root. J Agric Food Chem. 2001;49:4656–61. doi: 10.1021/jf010609z. [DOI] [PubMed] [Google Scholar]
- 58.Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant agents from Andrographis paniculata. J Nat Prod. 1993;56:995–9. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- 59.Melchior J, Palm S, Wikman G. Controlled clinical study of standardized Andrographis paniculata extract in common cold-a pilot trial. Phytomedicine. 1997;3:315–8. doi: 10.1016/S0944-7113(97)80002-5. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Wang ZT, Ge BX. Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways. Int Immunopharmacol. 2008;8:951–8. doi: 10.1016/j.intimp.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Chen M, Gan L, Lin S, Wang X, Li L, Li Y, et al. Alkaloids from the root of Isatis indigotica. J Nat Prod. 2012;75:1167–76. doi: 10.1021/np3002833. [DOI] [PubMed] [Google Scholar]
- 62.Wei F, Zou S, Young A, Dubner R, Ren K. Effects of four herbal extracts on adjuvant-induced inflammation and hyperalgesia in rats. J Altern Complement Med. 1999;5:429–36. doi: 10.1089/acm.1999.5.429. [DOI] [PubMed] [Google Scholar]


