Abstract
Objective. In this study, we investigated whether RAAS gene single nucleotide polymorphisms (SNPs) and their interactions were associated with end-stage renal stage (ESRD). Methodology and Results. This was a case-control study for 647 ESRD cases and 644 controls. AGT (M235T (rs699) and T174M (rs4762)), AGTR1 (A1166C (rs5186) and C573T (rs5182)), ACE (I/D (rs1799752) and G2350A (rs4343)), and CYP11B2 C-344T (rs1799998) were genotyped and compared between cases and controls to identify SNPs associated with ESRD susceptibility. Multifactor dimensionality reduction (MDR) was used to identify gene-gene interactions. Several RAAS genes were associated with ESRD: AGT M235T, ACE I/D, ACE G2350A, and CYP11B2 C-344T. By MDR analysis, a three-locus model (ACE ID/ACE G2350A/CYP11B2 C-344T) of gene-gene interaction was the best for predicting ESRD risk, and its maximum testing accuracy was 56.08% and maximum cross-validation consistency was 9/10. ESRD risk was higher with the simultaneous occurrence of ACE I/D DD-ACE G2350A AA. AGT, ACE, and CYP11B2 gene polymorphisms are associated with ESRD. Conclusions. The gene-gene interaction effects of ACE I/D, ACE G2350A, and CYP11B2 C-344T polymorphisms are more important than individual factors for ESRD development among Han Chinese.
1. Introduction
Taiwan has the third highest incidence and the highest prevalence of end-stage renal disease (ESRD) in the world. This not only burdens healthcare resources but also has a major impact on patients and their families [1]. ESRD is a complex phenotype, which results from the presence of underlying kidney disease, and superimposing inherited and environmental factors [2]. Among the predisposing genetic factors, renin-angiotensin-aldosterone system (RAAS) disruption is clearly involved in ESRD development [3].
The RAAS is a key blood pressure, renal hemodynamics, and volume homeostasis regulator [4]. Thus, genes that encode RAAS components are candidates for evaluating predisposition to renal disease development and progression [5]. Among RAAS candidate genes, angiotensinogen (AGT), angiotensin II type I receptor (AGTR1), angiotensin-converting enzyme (ACE), and aldosterone synthase (CYP11B2) genes appear to be particularly relevant to renal disease [6].
Several studies recently identified RAAS gene mutations and polymorphisms that affected host susceptibility to several diseases, including hypertension [7], type 2 diabetes [8], myocardial infarction [9], chronic kidney disease [6, 10], and ESRD [11]. Some studies also indicated that RAAS gene single nucleotide polymorphisms (SNPs) could alter homeostasis to an abnormal state [12, 13]. RAAS gene polymorphism involvement in kidney disease pathogenesis has been extensively studied in various populations [10, 11, 14, 15]. However, the influence of interactions among RAAS genes on ESRD susceptibility remains unknown. Some RAAS genes may have strong effects as “susceptibility loci” for renal disease development and progression, whereas some may have modest effects as “modifier genes” for endophenotypic expression. Compared with a single candidate gene approach, analysis of multiple candidate gene expression variations and their interactions may be a more powerful approach for studying complex diseases.
In this study, we genotyped seven different loci in four RAAS genes in ESRD patients and healthy controls and examined gene-gene interactions using multifactor dimensionality reduction (MDR) and a logistic regression model (LRM).
2. Methodology
2.1. Subject Recruitment
This case-control study included 647 ESRD patients (346 women; 301 men; mean age, 64.4 ± 14.7 years) receiving hemodialysis at Cardinal Tien Hospital and five other hemodialysis centers in Taipei, Taiwan. These patients were stable (without clinical complications) and had undergone hemodialysis for >6 months. Autoimmune disease, malignancy, and any acute or chronic infection were exclusion criteria. ESRD causes included diabetes mellitus (n = 256; 39.6%), chronic glomerulonephritis (n = 174; 26.9%), hypertensive nephropathy (n = 84; 13.0%), systemic nephropathy (n = 66; 10.2%), and unknown (n = 67; 10.3%).
We also enrolled 644 healthy controls (378 women; 266 men; mean age, 65.7 ± 13.6 years) with an estimated glomerular filtration rate (eGFR) of ≥60 mL/min/1.73 m2 and no proteinuria recruited from the Center of Physical Examination at Cardinal Tien Hospital. They had no evidence of kidney damage, including microalbuminuria or proteinuria, hematuria, and abnormal abdominal ultrasound. Clinical information and biochemistry test results were retrieved from hospital records.
2.2. Ethics Statement
This study was approved by the ethics committee of Cardinal Tien Hospital (CTH-100-3-5-025). After thoroughly explaining this study, written informed consent was obtained from all participants.
2.3. SNP Selection and Genotyping
AGT (M235T (rs699) and T174 M (rs4762)), AGTR1 (A1166C (rs5186) and C573T (rs5182)], ACE [I/D (rs1799752) and G2350A (rs4343)), and CYP11B2 C-344T (rs1799998) polymorphisms previously shown to be significantly associated with kidney diseases in genetic polymorphism studies of Chinese Han populations [10, 16] were selected. Genomic DNA was extracted from peripheral blood samples using standard procedures with proteinase K (Invitrogen, Carlsbad, CA, USA) digestion and phenol-chloroform extraction [17]. The above mentioned polymorphisms were screened by polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP). Primer design was based on published sequences [10, 16] or designed using primer Z software (http://genepipe.ngc.sinica.edu.tw/primerz/beginDesigndo).
PCR amplification was performed as follows. Cycling conditions were an initial denaturation at 95°C for 5 min, followed by 35 denaturation cycles at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 10 min. PCR products were digested with the respective restriction endonucleases (New England Biolabs, MA, USA), and the resulting fragments were separated in 3.0% agarose gel containing 0.5 g/mL of ethidium bromide by electrophoresis at 100 V and visualized under UV light. Genotyping was performed after blinding for case or control status. Two independent investigators interpreted the images for each gel, and all ambiguous samples were analyzed again. To validate genotyping results, at least 10% of samples were randomly selected for repeated genotyping.
2.4. Statistical Analysis
The demographics were evaluated by Student's t-test for continuous variables and expressed as mean ± standard deviation (SD). Hardy-Weinberg equilibrium was assessed by a goodness-of-fit χ 2 test and performed to examine possible genotyping errors for each SNP among controls. Genotype and allele frequencies were compared between ESRD patients and healthy controls using a χ 2 test or Fisher's exact test as appropriate. LRM was used to estimate crude and adjusted (age, gender, body mass index, and smoking status) odds ratios (ORs) and 95% confidence intervals (CIs) as a measure of association with ESRD risk. Statistical analysis was performed using SPSS for Windows version 20.0 (SPSS, Chicago, IL, USA). Two-tailed P values of <0.05 were considered significant.
2.5. Gene-Gene Interaction Analysis
Gene-gene interactions among the loci were evaluated using multiple dimensionality reduction (MDR) and MDR-permutation testing software (version 1.0 beta). MDR reduces the dimensionality of multilocus information that has reasonable power to identify interactions among two or more loci in relatively small samples and improves the identification of polymorphism combinations associated with disease risk. Average prediction errors were calculated using permutation tests considered significant at P < 0.05. Stepwise logistic regression based on backward selection was used to confirm the results of interaction analyses.
3. Results
3.1. Study Population Demographic and Clinical Characteristics
Table 1 shows the demographic and clinical characteristics of this study population. No significant differences in gender, age, drinking status, and diastolic blood pressure were observed between the two groups, whereas significant differences were observed in other variables (P < 0.05).
Table 1.
Characteristics of study subjects.
| Case (n = 647) |
Control (n = 644) |
P value | |
|---|---|---|---|
| Male (%) | 301 (46.5%) | 266 (41.3%) | 0.059 |
| Age (years) | 64.4 ± 14.7 | 65.7 ± 13.6 | 0.100 |
| Body mass index (kg/m2) | 22.5 ± 3.9 | 24.7 ± 3.9 | <0.001 |
| Current or former smoker | 120 (18.5%) | 72 (11.2%) | <0.001 |
| Current or former drinker | 68 (10.5%) | 60 (9.3%) | 0.065 |
| Systolic blood pressure (mmHg) | 141.2 ± 34.8 | 126.4 ± 16 | <0.001 |
| Diastolic blood pressure (mmHg) | 75.3 ± 11.2 | 75.9 ± 11.1 | 0.322 |
| Fasting plasma glucose (mg/dL) | 153.6 ± 72.1 | 98.4 ± 23.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 5.7 ± 2.6 | 87.8 ± 14.8 | <0.001 |
| BUN (mg/dL) | 66.8 ± 19.3 | 15.6 ± 5.6 | <0.001 |
| Uric acid (mg/dL) | 7.2 ± 1.5 | 5.7 ± 1.3 | <0.001 |
| Serum creatinine (mg/dL) | 9.6 ± 2.6 | 0.8 ± 0.2 | <0.001 |
| Serum total cholesterol (mg/dL) | 165.3 ± 35.9 | 184.7 ± 34.4 | <0.001 |
| Serum triglyceride (mg/dL) | 157.4 ± 110.7 | 121.5 ± 98.4 | <0.001 |
3.2. Distributions of RAAS Gene Polymorphisms and Their Association with ESRD
AGT (M235T and T174M), AGTR1 (A1166C and C573T), ACE (I/D and G2350A), and CYP11B2 (C-344T) genotype distributions were all compatible with Hardy-Weinberg equilibrium for the controls (P > 0.05). Table 2 shows the genotype and allele frequencies of seven SNPs in the two groups. The genotype or allele frequencies for the AGT T174M and AGTR1 (A1166C and C573T) polymorphisms were not significantly different between groups. In addition, those SNPs in dominant and recessive modes were not significantly different (data not shown).
Table 2.
Genotype distribution of the RAAS polymorphisms among ESRD patients and control.
| Genotypes | Case | Control | Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| AGT M235T | ||||||
| CC | 466 | 438 | 1 | 1 | ||
| CT | 169 | 182 | 0.87 (0.68–1.12) | 0.280 | 0.78 (0.59–1.03) | 0.075 |
| TT | 12 | 24 | 0.47 (0.23–0.95) | 0.036 | 0.24 (0.09–0.65) | 0.005 |
| T allele | 193 | 230 | 0.81 (0.65–0.99) | 0.044 | 0.70 (0.55–0.89) | 0.003 |
| AGT T174M | ||||||
| CC | 508 | 519 | 1 | 1 | ||
| CT | 134 | 120 | 1.14 (0.87–1.5) | 0.348 | 1.11 (0.82–1.51) | 0.484 |
| TT | 5 | 5 | 1.02 (0.29–3.55) | 0.973 | 1.19 (0.33–4.25) | 0.786 |
| T allele | 144 | 130 | 1.12 (0.87–1.43) | 0.393 | 1.11 (0.84–1.46) | 0.467 |
| AGTR1 A1166C | ||||||
| AA | 591 | 589 | 1 | 1 | ||
| AC | 56 | 55 | 1.01 (0.69–1.5) | 0.941 | 1.07 (0.7–1.63) | 0.761 |
| C allele | 56 | 55 | 1.01 (0.69–1.48) | 0.943 | 1.06 (0.7–1.61) | 0.766 |
| AGTR1 C573T | ||||||
| CC | 336 | 350 | 1 | 1 | ||
| CT | 267 | 240 | 1.16 (0.92–1.46) | 0.209 | 1.23 (0.96–1.59) | 0.105 |
| TT | 44 | 54 | 0.85 (0.55–1.3) | 0.450 | 0.84 (0.52–1.35) | 0.478 |
| T allele | 355 | 348 | 1.02 (0.86–1.21) | 0.812 | 1.05 (0.87–1.27) | 0.633 |
| ACE I/D | ||||||
| II | 231 | 281 | 1 | 1 | ||
| ID | 330 | 297 | 1.35 (1.07–1.71) | 0.012 | 1.42 (1.09–1.84) | 0.009 |
| DD | 86 | 66 | 1.59 (1.1–2.28) | 0.013 | 1.61 (1.08–2.4) | 0.019 |
| D allele | 502 | 429 | 1.27 (1.08–1.49) | 0.004 | 1.29 (1.08–1.54) | 0.005 |
| ACE G2350A | ||||||
| GG | 233 | 274 | 1 | 1 | ||
| GA | 299 | 276 | 1.27 (1.00–1.62) | 0.047 | 1.35 (1.03–1.76) | 0.029 |
| AA | 115 | 94 | 1.44 (1.04–1.99) | 0.028 | 1.62 (1.14–2.31) | 0.008 |
| A allele | 529 | 464 | 1.23 (1.05–1.44) | 0.011 | 1.31 (1.1–1.56) | 0.003 |
| CYP11B2 C-344T | ||||||
| TT | 384 | 340 | 1 | 1 | ||
| TC | 205 | 256 | 0.71 (0.55–0.92) | 0.010 | 0.70 (0.54–0.91) | 0.007 |
| CC | 58 | 48 | 1.07 (0.69–1.67) | 0.761 | 1.07 (0.69–1.68) | 0.756 |
| C allele | 321 | 352 | 0.88 (0.74–1.05) | 0.144 | 0.87 (0.72–1.06) | 0.178 |
Data were expressed as n (%) and have been adjusted by gender, age, BMI, and smoking status.
There was a significant association between the AGT M235T polymorphism and ESRD risk, with a mutation carrier having a lower risk (adjusted OR, 0.24; 95% CI, 0.09–0.65; P = 0.005). The genotype and allele distributions of ACE I/D and G2350A were significantly different between groups.
For ACE I/D, when genotype II was used as a reference, ID and DD genotypes were apparently associated with a higher ESRD risk (adjusted OR, 1.42; 95% CI, 1.09–1.84; P = 0.009; adjusted OR, 1.61; 95% CI, 1.08–2.40; P = 0.019, resp.). For ACE G2350A, when the GG genotype was used as a reference, GA and AA genotypes appeared to be associated with a higher ESRD risk (adjusted OR, 1.35; 95% CI, 1.03–1.67; P = 0.029; adjusted OR, 1.62; 95% CI, 1.14–2.31; P = 0.008, resp.).
Significant associations were found in the CYP11B2 C-344T polymorphism between groups. For CYP11B2 C-344T, the TC genotype compared with the TT genotype was a protective factor for ESRD (adjusted OR, 0.70; 95% CI, 0.54–0.91; P = 0.007).
3.3. RAAS Gene Polymorphisms Associated with Risk of Different ESRD Causes
We analyzed possible associations between RAAS gene polymorphisms and underlying ESRD etiology. After stratifying the ESRD patients according to different underlying causes for renal disease, an association was indicated for the AGT M235T genotype and glomerulonephritis (adjusted OR, 0.51; 95% CI, 0.31–0.85) but not for diabetic nephropathy, hypertensive nephropathy, systemic nephropathy, or nephropathy for unknown reasons.
There were significant differences in the genotypes of ACE I/D (ID/II, adjusted OR, 1.89; 95% CI, 1.31–2.71) and G2350A (GA/GG, adjusted OR, 1.65; 95% CI, 1.14–2.40; AA/GG, and adjusted OR, 2.04; 95% CI, 1.28–3.28) between patients with diabetic nephropathy and controls. The genotypes of ACE I/D (DD/II, adjusted OR, 2.21; 95% CI, 1.01–4.83) and G2350A (GA/GG, adjusted OR, 1.93; 95% CI, 1.02–3.63; AA/GG, and adjusted OR, 2.41; 95% CI, 1.11–5.22) were also significantly different for patients with hypertensive nephropathy compared with controls. The CYP11B2 genotype was significantly different between patients with systemic nephropathy and controls (adjusted OR, 0.50; 95% CI, 0.25–0.99). No associations were found between patients with different underlying causes of their renal disease and AGT T174M (Table 3).
Table 3.
AGT, AGTR1, ACE, and CYP11B2 gene polymorphisms with risk of different cause of ESRD.
| Diabetic nephropathy (n = 256) adjusted# OR (95% CI) |
Hypertensive nephropathy (n = 84) adjusted# OR (95% CI) |
Glomerulonephritis (n = 174) adjusted# OR (95% CI) |
Systemic nephropathy (n = 66) adjusted# OR (95% CI) |
Other&
(n = 67) adjusted# OR (95% CI) |
|
|---|---|---|---|---|---|
| AGT | |||||
| M235T | |||||
| CT/CC | 0.75 (0.52–1.09) | 1.07 (0.58–1.98) | 0.51 (0.31–0.85)* | 0.97 (0.5–1.89) | 1.08 (0.59–1.99) |
| TT/CC | 0.33 (0.09–1.14) | — | 0.18 (0.02–1.41) | — | 0.46 (0.06–3.55) |
| T174M | |||||
| CT/CC | 1.26 (0.84–1.88) | 1.10 (0.54–2.24) | 1.07 (0.66–1.75) | 0.79 (0.35–1.77) | 1.17 (0.58–2.36) |
| TT/CC | 0.94 (0.17–5.07) | — | 0.94 (0.10–8.55) | 2.58 (0.29–23.17) | 2.13 (0.23–19.54) |
| AGTR1 | |||||
| A1166C | |||||
| AC/AA | 0.79 (0.42–1.47) | 0.96 (0.37–2.51) | 1.49 (0.81–2.74) | 0.72 (0.22–2.43) | 1.59 (0.68–3.74) |
| CC/AA | — | — | — | — | — |
| C573T | |||||
| CT/CC | 1.30 (0.92–1.83) | 0.96 (0.54–1.70) | 1.32 (0.87–1.98) | 1.31 (0.69–2.49) | 1.00 (0.54–1.84) |
| TT/CC | 0.68 (0.33–1.39) | 0.76 (0.26–2.25) | 0.59 (0.24–1.46) | 1.62 (0.61–4.35) | 1.4 (0.55–3.58) |
| ACE | |||||
| ID | |||||
| ID/II | 1.89 (1.31–2.71)* | 1.16 (0.64–2.11) | 1.35 (0.89–2.05) | 0.83 (0.42–1.61) | 1.22 (0.66–2.26) |
| DD/II | 1.71 (0.97–3.00) | 2.21 (1.01–4.83)* | 1.03 (0.5–2.13) | 2.07 (0.93–4.64) | 1.99 (0.85–4.65) |
| G2350A | |||||
| GA/GG | 1.65 (1.14–2.40)* | 1.93 (1.02–3.63)* | 1.02 (0.66–1.56) | 1.92 (0.97–3.77) | 0.91 (0.49–1.68) |
| AA/GG | 2.04 (1.28–3.28)* | 2.41 (1.11–5.22)* | 1.13 (0.63–2.04) | 2.17 (0.9–5.25) | 1.12 (0.5–2.53) |
| CYP11B2 | |||||
| C-344T | |||||
| TC/TT | 0.75 (0.52–1.07) | 0.58 (0.32–1.04) | 0.73 (0.48–1.12) | 0.50 (0.25–0.99)* | 0.82 (0.44–1.55) |
| CC/TT | 1.11 (0.61–2.00) | 0.53 (0.16–1.78) | 0.75 (0.34–1.68) | 1.55 (0.62–3.85) | 2.26 (0.99–5.15) |
*P < 0.05, #adjusted for gender, age, BMI, and smoking status; &Others: for example, kidney stone, polycystic kidney disease, and so forth.
3.4. Evaluations of Gene-Gene Interactions: MDR and LRM
Table 4 summarizes the results of an exhaustive MDR analysis for evaluating all possible combinations of the studied polymorphisms. The best overall MDR model included the ACE I/D, ACE G2350A, and CYP11B2 C-344T polymorphisms. This model had a maximum testing accuracy of 56.08% and a maximum cross-validation consistency of 9 out of 10. This model was significant at the 0.01 level, which indicated that a model this good or better would be observed only once in 1,000 permutations; therefore, it was unlikely under the null hypothesis of no association. The distributions for cases and controls for each of the three-locus genotype combinations in the best MDR model are shown in Figure 1. Figure 2 shows the interaction maps of all genes based on entropy measures among individual variables. A strong interaction effect was found among ACE I/D-ACE G2350A and ACE G2350A-CYP11B2 C-344T, which had information gain values of 1.02% and 0.65%, respectively. Significance in two-way interactions (ACE I/D × ACE G2350A, ACE I/D × CYP11B2 C-344T, and ACE G2350A × CYP11B2 C-344T) was found using a LRM (data not shown). After adjusting for age, gender, body mass index, and smoking status, the interaction of ACE I/D and ACE G2350A SNPs with ESRD was maintained. When the wild-type ACE II-ACE GG genotype was used as a reference, the variant ACE I/D DD-ACE G2350A AA genotype had the greatest ESRD risk (adjusted OR, 3.13; 95% CI, 1.60–6.13; P = 0.001; Table 5).
Table 4.
Best gene-gene interaction models identified by the MDR method.
| Locus no. | Best model | Testing Bal. Acc. (%) | CVC† | P-value* |
|---|---|---|---|---|
| 1 | ACE I/D | 0.5179 | 6/10 | 0.6520 |
| 2 | ACE I/D, ACE G2350A | 0.5537 | 10/10 | 0.0280 |
| 3 | ACE I/D, ACE G2350A, CYP11B2 C-344T | 0.5608 | 9/10 | 0.0060 |
| 4 | AGTR1 C573T, ACE I/D, ACE G2350A, CYP11B2 C-344T | 0.5499 | 7/10 | 0.0560 |
| 5 | AGT M235T, AGTR1 C573T, ACE I/D, ACE G2350A, CYP11B2 C-344T | 0.5568 | 7/10 | 0.0150 |
| 6 | AGT M235T, AGT T174M, AGTR1 C573T, ACE I/D, ACE G2350A, CYP11B2 C-344T | 0.5290 | 6/10 | 0.4100 |
| 7 | AGT M235T, AGT T174M, AGTR1 A1166C, AGTR1 C573T, ACE I/D, ACE G2350A, CYP11B2 C-344T | 0.5227 | 10/10 | 0.5500 |
*Interactions were validated based on 1000 permutations; †CVC: cross-validation consistency.
Figure 1.
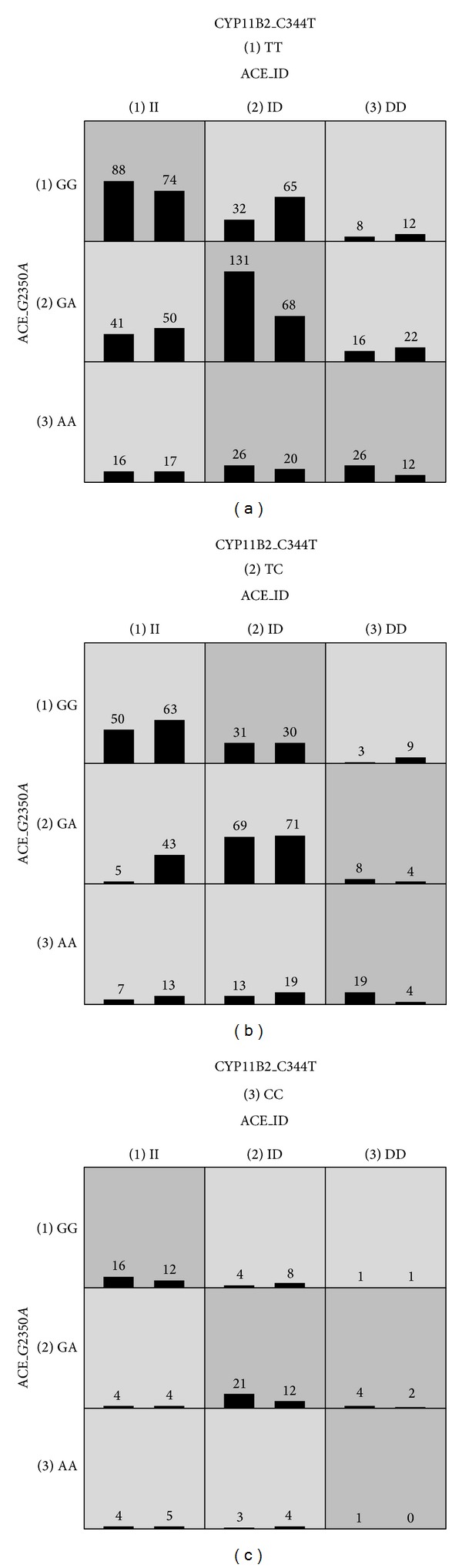
The distribution of cases (left bars) and controls (right bars) for each genotype combination from the three SNPs (ACE I/D, ACE G2350A, and CYP11B2 C-344T) identified in the overall best model by MDR analysis.
Figure 2.
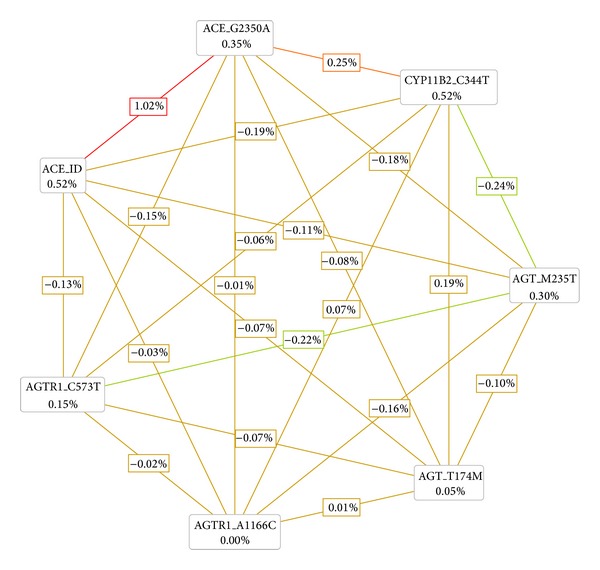
Interaction map for ESRD risk. The values inside boxes indicate information gain (IG) of individual attribute or main effects, whereas values between nodes exemplify IG of pairwise combinations of attributes or interaction effects. A positive entropy (plotted in red or orange) indicates interaction while a negative (plotted in green) indicates redundancy.
Table 5.
Joint effect of ACE I/D and ACE G2350A from ESRD.
| Genotypes | Case | Control | Crude OR (95% CI) | P value | Adjusted# OR (95% CI) | P value# |
|---|---|---|---|---|---|---|
| I/D | ||||||
| II | ||||||
| G2350A | ||||||
| GG | 154 | 149 | 1 | 1 | ||
| GA | 67 | 103 | 0.63 (0.43–0.92) | 0.017 | 0.68 (0.46–1.05) | 0.084 |
| AA | 12 | 22 | 0.53 (0.25–1.11) | 0.090 | 0.40 (0.16–1.00) | 0.050 |
| ID | ||||||
| G2350A | ||||||
| GG | 50 | 97 | 0.5 (0.33–0.75) | 0.001 | 0.52 (0.33–0.84) | 0.007 |
| GA | 221 | 151 | 1.42 (1.04–1.92) | 0.026 | 1.56 (1.09–2.22) | 0.015 |
| AA | 28 | 28 | 0.97 (0.55–1.71) | 0.910 | 1.13 (0.58–2.21) | 0.715 |
| DD | ||||||
| G2350A | ||||||
| GG | 27 | 35 | 0.75 (0.43–1.29) | 0.297 | 0.82 (0.43–1.54) | 0.536 |
| GA | 42 | 43 | 0.95 (0.58–1.53) | 0.818 | 1.11 (0.64–1.91) | 0.717 |
| AA | 46 | 16 | 2.78 (1.51–5.13) | 0.001 | 3.13 (1.60–6.13) | 0.001 |
#Adjusted for gender, age, BMI, and smoking status.
4. Discussion and Conclusions
We presented statistical evidence for significant interactions among the ACE I/D, ACE G2350A, and CYP11B2 C-344T genes and the ESRD risk. These results were corroborated by permutation testing. Four genetic polymorphisms in AGT, ACE, and CYP11B2 were found to be significantly associated with the ESRD risk; however, these single SNP analyses did not remain significant after correction for multiple testing (data not shown). Our data also indicated that underlying ESRD etiology differed based on RAAS genes.
Several studies investigated relationships between RAAS gene polymorphisms and ESRD. Many of these focused on the associations of single polymorphisms among RAAS genes with ESRD. The AGT M235T gene polymorphism, which is correlated with circulating and cellular AGT concentrations, has been implicated in ESRD etiology and investigated in epidemiologic studies [18, 19]. The AGTR1 gene was independently associated with renal disease progression and cardiovascular disease [20, 21]. Several studies reported that ACE serum and plasma levels were influenced by ACE I/D [22] and G2350A [23] polymorphisms. An increased ACE level is associated with renal disease pathogenesis. The CYP11B2 C-344T polymorphism was associated with serum aldosterone levels, urinary aldosterone excretion, blood pressure, and left ventricular size and mass [24]. These genetic polymorphisms of key components of the RAAS provide a basis for studying the relationship between genetic variants and the development of renal damage in individual subjects. However, these findings remain controversial, and contributions of interactions on ESRD may provide an explanation. The magnitude of an effect is likely missed if genes are individually examined without considering possible interactions. Evaluating interactions increases the power to detect these effects and also aids in understanding genetic influences on the biological and biochemical pathways that underlie the disease. One study reported on the interactions at the cellular level [25]. This suggests that a deeper insight derived at the statistical level is relevant at the biological level.
An increasing number of studies have focused on interactions, which can be partly attributed due to new statistical theories [26–28]. MDR is a powerful method for analyzing interactions and has been successfully applied in many genetic studies of complex diseases [29, 30]. MDR pools genotypes into “high-risk” and “low-risk” groups to reduce multidimensional data into one-dimension. Our results suggested a three-way interaction between ACE I/D, ACE G2350A, and CYP11B2 C-344T. However, this interaction for susceptibility was not confirmed by LRM, which only found a two-way interaction. A possible reason for this is that MDR did not detect the interaction defined by “deviation from the multiplicative” as in the LRM. Significant results from MDR only implied that the combination of markers contributed to an increased or decreased risk of disease and the effect between these markers could be either multiplicative or a deviation from multiplicative. Additional studies will be needed to establish the underlying mechanisms that explain the possible interactions among ACE I/D, G2350A, and CYP11B2 C-344T polymorphisms for ESRD predisposition.
Recently, many research articles have focused on RAAS gene polymorphisms associated with risk of different ESRD causes. Reis et al. showed that the AGT M235T TT genotype may be linked to a DN risk in the Turkish population [31]. ACE I/D gene polymorphism was associated with DN and reported for Northeast Asians [32]. Song et al. found that CYP11B2 C-344T polymorphism was associated with renal dysfunction progression only in female patients with IgA nephropathy [33]. The C-344T polymorphism was a risk factor for accelerated progression in a Polish population with primary chronic glomerulonephritis [34]. In the present study, we also analyzed possible associations between RAAS gene polymorphisms and underlying ESRD etiology. ACE (I/D and G2350A) was associated with diabetic nephropathy (DN) and hypertensive nephropathy. AGT M235T and CYP11B2 C-344T were associated with glomerulonephritis and systemic nephropathy, respectively. However, the cause of ESRD and interindividual differences in susceptibility remains elusive. More studies are needed to clarify the cause and inter-individual differences in ESRD susceptibility.
Our results suggest that AGT, ACE, and CYP11B2 gene polymorphisms are associated with ESRD and that an interaction effect of ACE I/D, ACE G2350A, and CYP11B2 C-344T polymorphisms may play a more important role than individual factors for ESRD development. A higher ESRD risk was found for the simultaneous occurrence of ACE DD-ACE AA. This investigation was done with Han Chinese patients. The applicability of our results to other ethnic groups is uncertain and warrants further study.
Acknowledgment
This study was supported by grants from the Cardinal Tien Hospital and the Bureau of Health Promotion, Taiwan (CTH-102-1-2C07, CTH-101-1-2C03, DOH102-HP-1103, DOH101-HP-1103).
Abbreviations
- ACE:
Angiotensin-converting enzyme
- AGT:
Angiotensinogen
- AGTR1:
Angiotensin II type I receptor
- CYP11B2:
Aldosterone synthase
- eGFR:
Estimated glomerular filtration rate
- ESRD:
End-stage renal stage
- LRM:
Logistic regression model
- MDR:
Multifactor dimensionality reduction
- PCR:
Polymerase chain reaction
- RAAS:
Renin-angiotensin-aldosterone system
- RFLP:
Restriction fragment length polymorphism
- SD:
Standard deviation
- SNPs:
Single nucleotide polymorphisms.
Conflict of Interests
The authors declare no conflict of interests.
Authors' Contribution
Sui-Lung Su, Hsin-Yi Yang, Chia-ChaoWu, and Kuo-Cheng Lu conceived and designed the experiments. Hsin-Yi Yang, Yuh-Feng Lin, Chi-An Hsu, and Kuo-Cheng Lu performed the experiments. Sui-Lung Su, Hsin-Yi Yang, Chia-ChaoWu, Chin Lin, Yuh-Feng Lin, Ching-Huang Lai, and Kuo-Cheng Lu analyzed the data. Sui-Lung Su, Hsin-Yi Yang, Yuh-Feng Lin, Sen-Yeong Kao, and Kuo-Cheng Lu contributed reagents/materials/analysis tools. Sui-Lung Su, Hsin-Yi Yang, Chia-ChaoWu, and Kuo-Cheng Lu wrote the paper.
References
- 1.US Renal Data System. 2012 Annual Data Report. 2012 http://www.usrds.org/
- 2.Ogata S, Yorioka N, Gilbertson DT, Chen S-C, Foley RN, Collins AJ. Genetic and environmental effects and characteristics of Japanese end-stage renal disease patients. Hemodialysis International. 2009;13(supplement 1):S8–S12. doi: 10.1111/j.1542-4758.2009.00412.x. [DOI] [PubMed] [Google Scholar]
- 3.Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. American Journal of Medicine. 2004;116(4):263–272. doi: 10.1016/j.amjmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. American Journal of Hypertension. 1999;12(12, part 3):205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 5.Rüster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. Journal of the American Society of Nephrology. 2006;17(11):2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 6.Kujawa-Szewieczek A, Kocierz M, Piecha G, Kolonko A, Chudek J, Wiȩcek A. Gene polymorphisms of renin-angiotensin-aldosterone system components and the progression of chronic kidney diseases. Postepy Higieny i Medycyny Doswiadczalnej. 2010;64:423–438. [PubMed] [Google Scholar]
- 7.Song SB, Jin HS, Hong KW, et al. Association between renin-angiotensin-aldosterone system-related genes and blood pressure in a Korean population. Blood Press. 2011;20(4):204–210. doi: 10.3109/08037051.2011.555074. [DOI] [PubMed] [Google Scholar]
- 8.Prasad P, Tiwari AK, Kumar KMP, et al. Chronic renal insufficiency among Asian Indians with type 2 diabetes: I. Role of RAAS gene polymorphisms. BMC Medical Genetics. 2006;7, article 42 doi: 10.1186/1471-2350-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaël Nossent A, Hansen JL, Doggen C, Quax PHA, Sheikh SP, Rosendaal FR. SNPs in microRNA binding sites in 3′-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. American Journal of Hypertension. 2011;24(9):999–1006. doi: 10.1038/ajh.2011.92. [DOI] [PubMed] [Google Scholar]
- 10.Su S-L, Lu K-C, Lin Y-F, et al. Gene polymorphisms of angiotensin-converting enzyme and angiotensin II Type I receptor among chronic kidney disease patients in a Chinese population. Journal of the Renin-Angiotensin-Aldosterone System. 2012;13(1):148–154. doi: 10.1177/1470320311430989. [DOI] [PubMed] [Google Scholar]
- 11.Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, Ksiazek A. Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrology Dialysis Transplantation. 2006;21(4):979–983. doi: 10.1093/ndt/gfk012. [DOI] [PubMed] [Google Scholar]
- 12.Sayed-Tabatabaei FA, Oostra BA, Isaacs A, Van Duijn CM, Witteman JCM. ACE polymorphisms. Circulation Research. 2006;98(9):1123–1133. doi: 10.1161/01.RES.0000223145.74217.e7. [DOI] [PubMed] [Google Scholar]
- 13.Patel SK, Wai B, Ord M, et al. Association of ACE2 genetic variants with blood pressure, left ventricular mass, and cardiac function in caucasians with type 2 diabetes. American Journal of Hypertension. 2012;25(2):216–222. doi: 10.1038/ajh.2011.188. [DOI] [PubMed] [Google Scholar]
- 14.Anbazhagan K, Sampathkumar K, Ramakrishnan M, Gomathi P, Gomathi S, Selvam GS. Analysis of polymorphism in Renin Angiotensin System and other related genes in South Indian chronic kidney disease patients. Clinica Chimica Acta. 2009;406(1-2):108–112. doi: 10.1016/j.cca.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Thameem F, Voruganti VS, He X, et al. Genetic variants in the renin-angiotensin system genes are associated with cardiovascular-renal-related risk factors in Mexican Americans. Human Genetics. 2008;124(5):557–559. doi: 10.1007/s00439-008-0581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang HY, Lu KC, Fang WH, et al. Impact of interaction of cigarette smoking with angiotensin-converting enzyme polymorphisms on end-stage renal disease risk in a Han Chinese population. Journal of Renin-Angiotensin-Aldosterone System. 2013 doi: 10.1177/1470320313481837. [DOI] [PubMed] [Google Scholar]
- 17.Poncz M, Solowiejczyk D, Harpel B. Construction of human gene libraries from small amounts of peripheral blood: analysis of β-like globin genes. Hemoglobin. 1982;6(1):27–36. doi: 10.3109/03630268208996930. [DOI] [PubMed] [Google Scholar]
- 18.Zhou TB, Yin SS, Qin YH. Association of angiotensinogen M235T gene polymorphism with end-stage renal disease risk: a meta-analysis. Molecular Biology Reports. 2013;40(2):765–772. doi: 10.1007/s11033-012-2114-x. [DOI] [PubMed] [Google Scholar]
- 19.Huang H-D, Lin F-J, Li X-J, Wang L-R, Jiang G-R. Genetic polymorphisms of the renin-angiotensin-aldosterone system in Chinese patients with end-stage renal disease secondary to IgA nephropathy. Chinese Medical Journal. 2010;123(22):3238–3242. [PubMed] [Google Scholar]
- 20.Vašků A, Souček M, Tschöplová S, Stejskalová A. An association of BMI with A (-6) G, M235T and T174M polymorphisms in angiotensinogen gene in essential hypertension. Journal of Human Hypertension. 2002;16(6):427–430. doi: 10.1038/sj.jhh.1001409. [DOI] [PubMed] [Google Scholar]
- 21.Campbell CY, Fang BF, Guo X, et al. Associations between Genetic Variants in the ACE, AGT, AGTR1 and AGTR2 Genes and Renal Function in the Multi-Ethnic Study of Atherosclerosis. American Journal of Nephrology. 2010;32(2):156–162. doi: 10.1159/000315866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. Journal of Clinical Investigation. 1990;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keavney B, McKenzie CA, Connell JMC, et al. Measured haplotype analysis of the angiotensin-I converting enzyme gene. Human Molecular Genetics. 1998;7(11):1745–1751. doi: 10.1093/hmg/7.11.1745. [DOI] [PubMed] [Google Scholar]
- 24.Barbato A, Russo P, Siani A, et al. Aldosterone synthase gene (CYP11B2) C-344T polymorphism, plasma aldosterone, renin activity and blood pressure in a multi-ethnic population. Journal of Hypertension. 2004;22(10):1895–1901. doi: 10.1097/00004872-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Babu M, Musso G, Díaz-Mejía JJ, Butland G, Greenblatt JF, Emili A. Systems-level approaches for identifying and analyzing genetic interaction networks in Escherichia coli and extensions to other prokaryotes. Molecular BioSystems. 2009;5(12):1439–1455. doi: 10.1039/B907407d. [DOI] [PubMed] [Google Scholar]
- 26.Kardia SLR, Bielak LF, Lange LA, et al. Epistatic effects between two genes in the renin-angiotensin system and systolic blood pressure and coronary artery calcification. Medical Science Monitor. 2006;12(4):CR150–CR158. [PubMed] [Google Scholar]
- 27.Qi Y, Niu W, Zhu T, Zhou W, Qiu C. Synergistic effect of the genetic polymorphisms of the renin-angiotensin- aldosterone system on high-altitude pulmonary edema: a study from Qinghai-Tibet altitude. European Journal of Epidemiology. 2008;23(2):143–152. doi: 10.1007/s10654-007-9208-0. [DOI] [PubMed] [Google Scholar]
- 28.Tsai C-T, Hwang J-J, Ritchie MD, et al. Renin-angiotensin system gene polymorphisms and coronary artery disease in a large angiographic cohort: Detection of high order gene-gene interaction. Atherosclerosis. 2007;195(1):172–180. doi: 10.1016/j.atherosclerosis.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genetic Epidemiology. 2003;24(2):150–157. doi: 10.1002/gepi.10218. [DOI] [PubMed] [Google Scholar]
- 30.Ritchie MD, Hahn LW, Roodi N, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. American Journal of Human Genetics. 2001;69(1):138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reis KA, Ebinç FA, Koç E, et al. Association of the angiotensinogen M235T and APO e gene polymorphisms in turkish type 2 diabetic patients with and without nephropathy. Renal Failure. 2011;33(5):469–474. doi: 10.3109/0886022X.2011.568133. [DOI] [PubMed] [Google Scholar]
- 32.Tarnow L, Gluud C, Parving H-H. Diabetic nephropathy and the insertion/deletion polymorphism of the angiotensin-converting enzyme gene. Nephrology Dialysis Transplantation. 1998;13(5):1125–1130. doi: 10.1093/ndt/13.5.1125. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Narita I, Goto S, et al. Gender specific association of aldosterone synthase gene polymorphism with renal survival in patients with IgA nephropathy. Journal of Medical Genetics. 2003;40(5):372–376. doi: 10.1136/jmg.40.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlik M, Mostowska A, Lianeri M, et al. Association of aldosterone synthase (CYP11B2) gene -344T/C polymorphism with the risk of primary chronic glomerulonephritis in the Polish population. Journal of the Renin-Angiotensin-Aldosterone System. 2013 doi: 10.1177/1470320313489588. [DOI] [PubMed] [Google Scholar]


