Abstract
Objective. To investigate the effect of Yangjing Capsule (YC) extract on proliferation of GC-1 spermatogonia (spg) cells and the mechanism. Methods. GC-1 spg cells were treated with 0.01, 0.1, and 1 mg/mL YC extract. MTT assay was performed to detect the cell viability. Flow cytometry was used to measure the cell cycle and apoptosis of GC-1 spg cells. Real-time PCR and western blot were applied to determine the mRNA and protein expression of Oct-4 and Plzf. Gfrα1 knockdown and LY294002 (PI3K inhibitor) were applied to explore the underlying mechanism. Results. After 48 h treatment of YC, the viability of GC-1 spg cells increased significantly and the ratio of apoptotic cells reduced significantly. The increased mRNA and protein expression of Oct-4 and Plzf suggested YC promoted self-renewal of GC-1 spg cells. Both Gfrα1 siRNAs and LY294002 treatments held back YC extract's stimulation effects on mRNA and protein expression of Oct-4 and Plzf and consequently inhibited the proliferation of GC-1 spg cells induced by YC extract. Conclusion. YC extract could stimulate the proliferation of GC-1 spg cells. Partly via Gfrα1, YC extract is able to trigger the activation of PI3K pathway and finally lead to self-renewal of GC-1 spg cells.
1. Introduction
About 15% of couples have problems in conceiving [1] and infertility beset around 80 million people worldwide by causing considerable psychological and financial burden. It is estimated that male reproductive dysfunction contributes to about half of infertile couples [2, 3]. Male infertility could be caused by various reasons, for instance, failure in spermatogenesis, epididymal maturation or storage of sperm, abnormal sperm delivery or accessory gland function, genetic or environmental factors, and sexual disorders [4, 5]. Among these causes, defects in spermatogenesis are the most common and account for 20%–25% of cases [6].
Spermatogenesis occurs in the seminiferous tubules of the testis with the seminiferous epithelium containing the developing germ cells and somatic Sertoli cells. Spermatogenesis is a continuous process by which the haploid germ cells are produced from differentiation of spermatogonial stem cells (SSCs) through mitosis and meiosis [7]. SSCs are responsible for sustaining fertility by tightly balancing between self-renewal and differentiation. With the establishment of balance, sperms are produced continually throughout the lifespan of an adult male [8–10]. Increased differentiation of SSCs at cost of self-renewal which destroys the balance would lead to male sterility [6]. Oct-4 and Plzf are considered to be responsible for preserving the pluripotency and self-renewal of stem cells [11, 12]. They are generally accepted as stem cell markers as they are downregulated during differentiation.
Self-renewal and differentiation of SSCs are strictly regulated by a combination of extrinsic gene signals from the niche as well as intrinsic signal pathways. Glial cell line-derived neurotrophic factor (Gdnf), secreted by Sertoli cells, is specifically responsible for the maintenance and self-renewal of SSCs [13, 14]. By binding to glycosylphosphatidylinositol- (GPI-) anchored cell surface molecule (Gfrα1), Gdnf is able to trigger PI3K/AKT pathway and eventually lead to self-renewal of SSCs [15, 16]. It has already been demonstrated that abnormal expression of extrinsic or intrinsic signaling molecules can give rise to impaired spermatogenesis [17, 18]. Oligozoospermia, asthenozoospermia, teratozoospermia, and azoospermia are the most common clinical manifestation of male infertility resulting from impaired spermatogenesis. In these cases, signal pathways regulating SSCs, such as PI3K/AKT pathway [19], are likely to be disrupted, thereby self-renewal of SSCs decreased and male infertility occurred.
The Yangjing Capsule (YC), which is composed of Herba Epimedii Brevicornus, Placenta Hominis, Rhizoma Polygonati Sibirici, Radix Rehmanniae Preparata, Angelica sinensis, and other components, has been suggested for the treatment of diseases of the reproductive system [20, 21]. Our previous clinical study found that YC was very effective for oligospermatism and can markedly increase sperm density for patients with a sperm density of over 5 × 106/mL [22]. While medical practice has proved the efficacy of Chinese herbal formulation, the underlying molecular mechanisms of YC improving male fertility remain elusive. Our present study is aimed to determine whether YC could promote self-renewal of SSCs and explore the targeted signal pathways involved.
2. Materials and Methods
2.1. Chemicals
Dulbecco's modified eagle's medium (DMEM), fetal bovine serum (FBS), and lyophilized trypsin-EDTA were obtained from GIBCO BRL (Grand Island, NY, USA). 3-[4,5-Dimethylthiazolyl-2]-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), sodium dodecyl sulfate (SDS), and Tris/HCl were purchased from Sigma (St. Louis, MO, USA). The whole protein extraction kits were purchased from Keygen (Keygen Biotech. Co. Ltd., Nanjing, China). Trizol reagent, PrimeScript RT Master Mix, and SYBR Green PCR Master Mix reagent kits were obtained from TaKaRa (TaKaRa Biotechnology, Dalian, China). The primers were synthesized by Invitrogen Life Tech (Carlsbad, CA, USA). Rabbit monoclonal anti-AKT (phospho S473), rabbit polyclonal anti-Plzf ab38739, and anti-Oct-4 ab18976 were from Abcam (Cambridge, MA, USA). Mouse monoclonal anti-β-actin was from Chemicon (Temecula, CA). Enhanced chemiluminescence was obtained from Amersham Biosciences (Uppsala, Sweden).
2.2. Preparation of YC Extract
The YC is composed of 11 traditional Chinese drugs: 13.3% Yinyanghuo (Herba Epimedii Brevicornus), 13.3% Muli (Concha Ostreae (calcined)), 13.3% Wangbuliuxing (Semen Vaccariae Segetalis), 10% Huangqi (Radix Astragali Mongolici), 10% Danggui (Radix Angelicae Sinensis), 6.7% Huangjing (Rhizoma Polygonati Sibirici), 6.7% Shayuanzi (Semen Astragali Complanati), 6.7% Ziheche (Placenta Hominis), 6.7% Shudihuang (Radix Rehmanniae Preparata), 6.7% Lizhihe (Semen Litchi), and 6.7% Shuizhi (Hirudo). The YC extract was prepared based on the methods described by Kao et al. [23]. The content of the YC (equivalent to 10 g of crude drug) was extracted with distilled water and subsequently subjected to ultrasonic extraction for 45 min. The supernatant was collected and centrifuged at 13,000 g and 4°C for 30 min to collect the supernatant, which was concentrated to 100 mL with a rotary evaporator at 60°C. The final concentration of the YC extract corresponded to 100 mg/mL of the crude herbal dose. The pH of the extract was adjusted to 7.0, and the extract was sterilized by filtration and stored at −80°C until use.
2.3. siRNA Transfection and PI3K Inhibition of GC-1 Spg Cells
GC-1 spg cells were cultured in DMEM, supplemented with 10% FBS, 50 IU/mL penicillin, and 50 μg/mL streptomycin, and then incubated in a 5% CO2 incubator (Thermo Fisher Scientific, Rochester, USA) at 37°C. For determining effects of YC extract on the self-renewal, GC-1 spg cells were treated with 0, 0.01, 0.1, and 1 mg/mL YC extract and 20 ng/mL Gdnf (used as positive control), respectively. After 48 h, cells were collected for assay of proliferation, cell cycle, and apoptosis. RNAs and protein were prepared to detect the expression of Plzf and Oct-4 by quantitative PCR and western blot. To explore the underlying mechanism, Gfrα1 knockdown and PI3K inhibition were performed. For Gfrα1 knockdown, 19-nucleotide siRNA sequences (sense: 5′-GCC CUC ACA GGC UUC UGU U-3′ and antisense: 3′-CGG GAG UGU CCG AAG ACA A-5′) targeting mouse Gfrα1 sequence (GCC CTC ACA GGC TTC TGT T) were designed using BLOCK-iT RNAi Designer (Invitrogen) and synthesized by Invitrogen [24]. The Stealth RNAi negative control obtained from Invitrogen was used as a control for monitoring nonsequence-specific effects. 50 pmol Stealth RNAi negative control and Gfrα1 siRNA were transfected into GC-1 spg cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. At 48 h after transfection, cells were treated with 1 mg/mL YC extract for 48 h and collected for proliferation assay, quantitative PCR, and western blot. For PI3K inhibition, GC-1 spg cells were exposed by 50 μM LY294002 (targeting the ATP-binding site of the PI3K) for abrogating PI3K activation. 2 h later, 1 mg/mL YC extract was added and cells were collected for proliferation assay, quantitative PCR, and western blot.
2.4. MTT Assay of Cell Proliferation
Cells were seeded in 96-well plate for 48 h treatment of 0, 0.01, 0.1, and 1 mg/mL YC extract, 20 ng/mL Gdnf, 50 μM LY294002, and 50 pmol Gfrα1 siRNA, respectively (n = 5). MTT (5 mg/mL) was added with 20 μL to each well and incubated for another 4 h before it was discarded. Then the purple-blue MTT formazan precipitate was dissolved in 100 μL dimethyl sulfoxide (DMSO). The absorbance (OD) was measured at 490 nm. The proliferation ratio was calculated by the following formula: proliferation ratio (%) = (average ODtreatment group/average ODcontrol group − 1) × 100%.
2.5. Cell Cycle and Apoptosis Assay
Cells were incubated in 6-well plates for 48 h treatment. Then cells were digested and washed. Cell cycle and apoptosis analysis were measured with flow cytometer (BD, Franklin, NJ, USA) according to the instructions of the Cycletest Plus DNA assay kit and Annexin V-FITC/PI apoptosis detection kit, respectively. The percentages of cells in G0/G1 phase, S phase, and G2/M phase were evaluated by the ModFit software (BD, Franklin, NJ, USA). The percentages of early stage and late stage apoptosis were evaluated with CellQuest software (BD, Franklin, NJ, USA). Total apoptosis rate was equal to early stage apoptosis plus late stage apoptosis.
2.6. RNA Isolation and Real-Time PCR
Cells at a density of 4 × 105/well were plated in 6-well plates for 48 h treatment. The total RNA was extracted using Trizol reagent, measured by spectrometry at an OD260/280, and reverse transcribed into cDNA in a total volume of 20 μL with PrimeScript RT Master Mix. All of the RT-PCR reactions were performed with a CFX96 real-time PCR system (Bio-Rad Laboratories, Hercules, CA) using SYBR Green chemistry (Bio-Rad Laboratories). GAPDH was selected as the control. The primer sequences were as follows: GAPDH, sense: 5′-AGG TTG TCT CCT GCG ACT TCA-3′ and antisense: 5′-GGG TGG TCC AGG GTT TCT TAC T-3′; Plzf, sense: 5′-CAC ACA GGC AGA CCC ATA CT-3′ and antisense: 5′-TTT GTG GCT CTT GAG TGT GC-3′; OCT-4, sense: 5′-CTT GCT GCA GAA GTG GGT GGA GGA A-3′ and antisense: 5′-CTG CAG TGT GGG TTT CGG GCA-3′ [25, 26]. The reactions were performed at 94°C for 3 min followed by 40 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The final extension was carried out for 5 min at 72°C. A melting curve analysis was performed to confirm the products. The relative abundances of the target mRNAs were calculated using the 2−ΔΔCt method. The data were expressed as the percentage of control (100%).
2.7. Protein Extraction and Western Blot Analysis
Cells were seeded in 60 mm dishes at a density of 1 × 106/well for 48 h treatment. The cells were harvested, washed three times with precooled PBS, and treated with cell lysis buffer for western blot analysis. After centrifugation at 12.000 g at 4°C for 20 min, the supernatants were collected and stored at −80°C until analysis.
The concentrations of protein were measured by the Bio-Rad Bradford assay (Bio-Rad Laboratories, Hercules, CA). The proteins were normalized to 50 μg/lane, separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred to nitrocellulose membranes. After treatment with blocking solution (5% skim milk powder in Tris-buffered saline) at 37°C for 1 h, the membranes were incubated overnight with the primary antibodies rabbit monoclonal anti-pAKT (1 : 5000), rabbit polyclonal anti-Plzf (1 : 500), rabbit polyclonal anti-Oct-4 (1 : 400), or mouse monoclonal anti-β-actin (1 : 5000) at 4°C. After washing with TBS three times, the membranes were incubated with HRP-conjugated secondary antibodies (1: 5000) at 37°C for 1 h and examined using enhanced chemiluminescence. The relative protein levels in each sample were normalized to the levels of β-actin to standardize the variations in loading. Densitometric analyses of the scanned immunoblotting images were performed using a Quantity One image system. The data are expressed as a percentage of the control (100%).
2.8. Statistical Analysis
Data were analyzed using a SPSS 16.0 statistical package. All results were expressed as mean ± standard deviation (S.D.). One-way analysis of variance (ANOVA) was used to analyze the difference between groups, followed by Dunnett's t-test. P < 0.05 was considered as statistically significant.
3. Results
3.1. Effects of YC Extract on Proliferation of GC-1 Spg Cells
The effects of YC extract on cell proliferation are shown in Figure 1. Gdnf, responsible for proliferation of SSCs, was used as positive control. In the presence of 0.01, 0.1, and 1 mg/mL YC extract for 48 h, OD values increased in a dose dependent manner. The proliferation rates of GC-1 spg cells increased by 13.38%, 32.47%, and 46.04%, respectively. In the presence of 20 ng/mL Gdnf for 48 h, the proliferation rates of GC-1 spg cells significantly increased by 47.98%. There are no evident changes observed with the treatment of 50 μM LY294002 or 50 pmol Gfrα1 siRNA.
Figure 1.
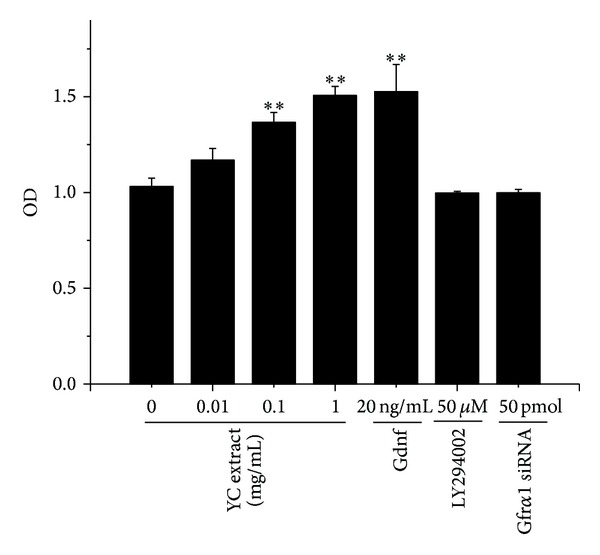
Effects of YC extract on proliferation of GC-1 spg cells. Cells were treated with 0, 0.01, 0.1, and 1 mg/mL YC extract, 20 ng/mL Gdnf, 50 μM LY294002, and 50 pmol Gfrα1 siRNA, respectively. **Significantly different from control at P < 0.01, n = 5.
3.2. Effects of YC Extract on Cell Cycle of GC-1 Spg Cells
Results of cell cycle assay of GC-1 spg cells are shown in Figure 2. The percentage of S phase in control group was 30.52% (Figure 2(a)), and after 48 h treatment of different doses of YC extract, the percentage of S phase evidently rose to 37.45%, 41.47%, and 46.30%, respectively (Figures 2(b), 2(c), and 2(d)). A decline of percentage of G0/G1 phase was also observed. These data suggested that YC extract could advance the cell cycle from the G1 phase to the S phase and promote proliferation of spg cells.
Figure 2.
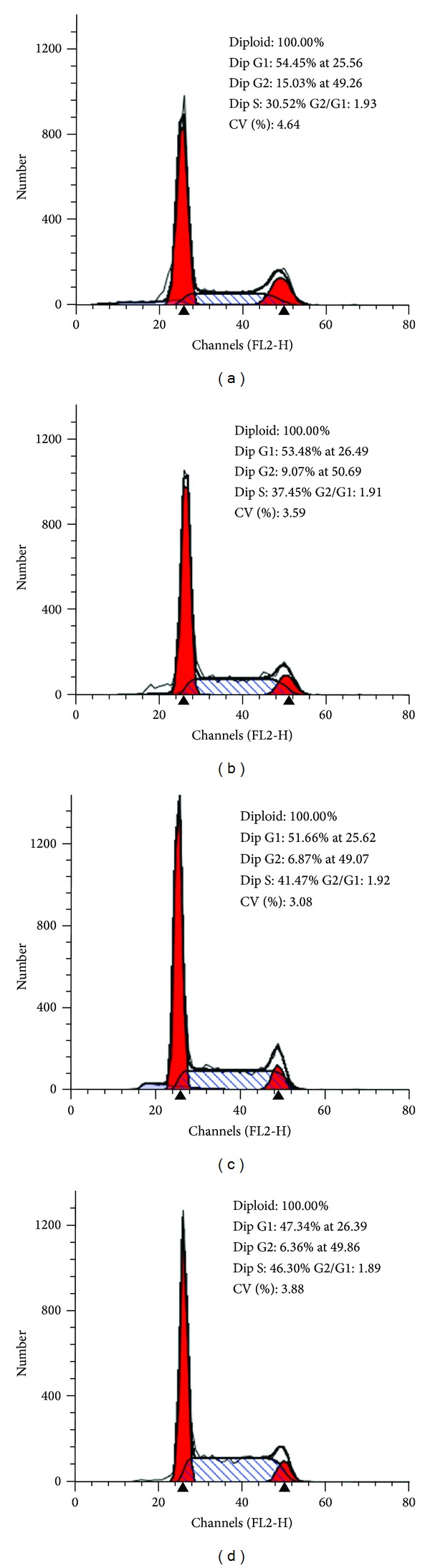
Effects of YC extract on cell cycle of GC-1 spg cells. Cells were treated with 0 (a), 0.01 (b), 0.1 (c), and 1 (d) mg/mL YC extract for 48 h, respectively. The analysis was performed in triplicate and representative data was shown.
3.3. Effects of YC Extract on Apoptosis of GC-1 Spg Cells
As shown in Figure 3, total apoptosis rate was equal to early stage apoptosis (LR) plus late stage apoptosis (UR). The apoptosis rates are 9.68 (a), 8.95 (b), 5.31 (c), and 2.38 (d), respectively. 0.01 mg/mL YC extract had no evident effect on apoptosis of GC-1 spg cells. However, 0.1 and 1 mg/mL YC extract notably inhibited cell apoptosis.
Figure 3.
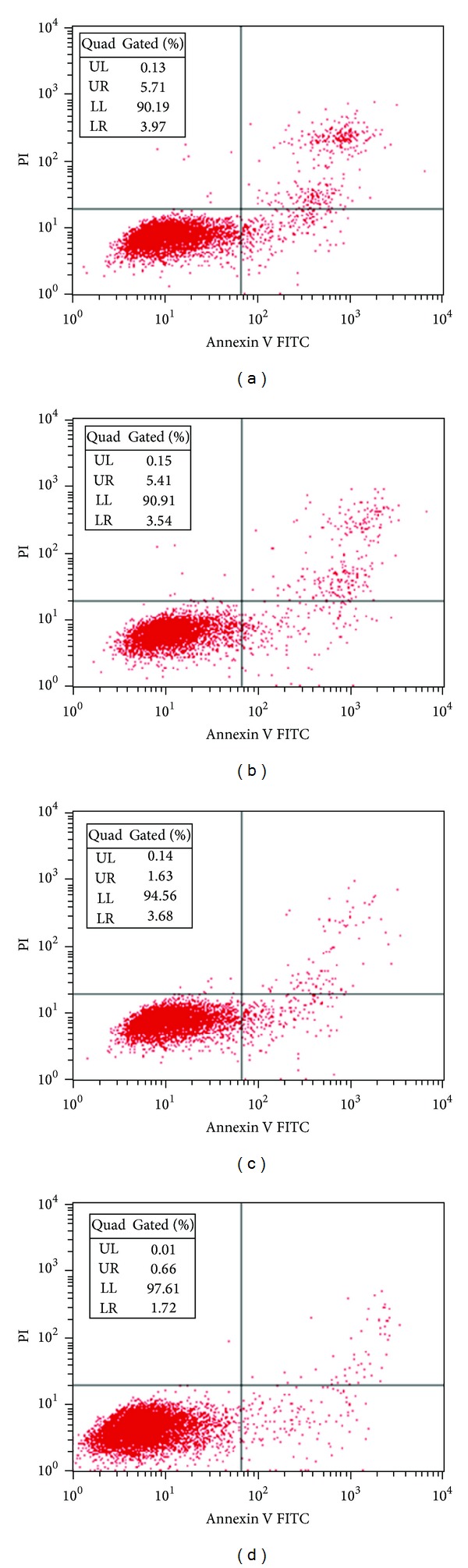
Effects of YC extract on apoptosis of GC-1 spg cells. Cells were treated with 0 (a), 0.01 (b), 0.1 (c), and 1 (d) mg/mL YC extract for 48 h, respectively. Annexin V+/PI− population (LR in diagram) indicated early apoptosis, and annexin V+/PI+ population (UR in diagram) indicated late apoptosis. The analysis was performed in triplicate and representative data was shown.
3.4. Effects of the YC Extract on the Expression of Oct-4 and Plzf mRNAs and Proteins
Oct-4 and Plzf were chosen as stem cell markers to confirm the undifferentiated status of GC-1 spg cells. Figures 4(a) and 4(b) showed that the expression of Oct-4 and Plzf mRNAs increased significantly by exposure of 0.1, 1 mg/mL YC extract and 20 ng/mL Gdnf. Accordingly, Figure 4(c) showed that the expression of Oct-4 and Plzf proteins increased evidently at the same condition. The result suggested that YC extract could promote self-renewal of GC-1 spg cells.
Figure 4.
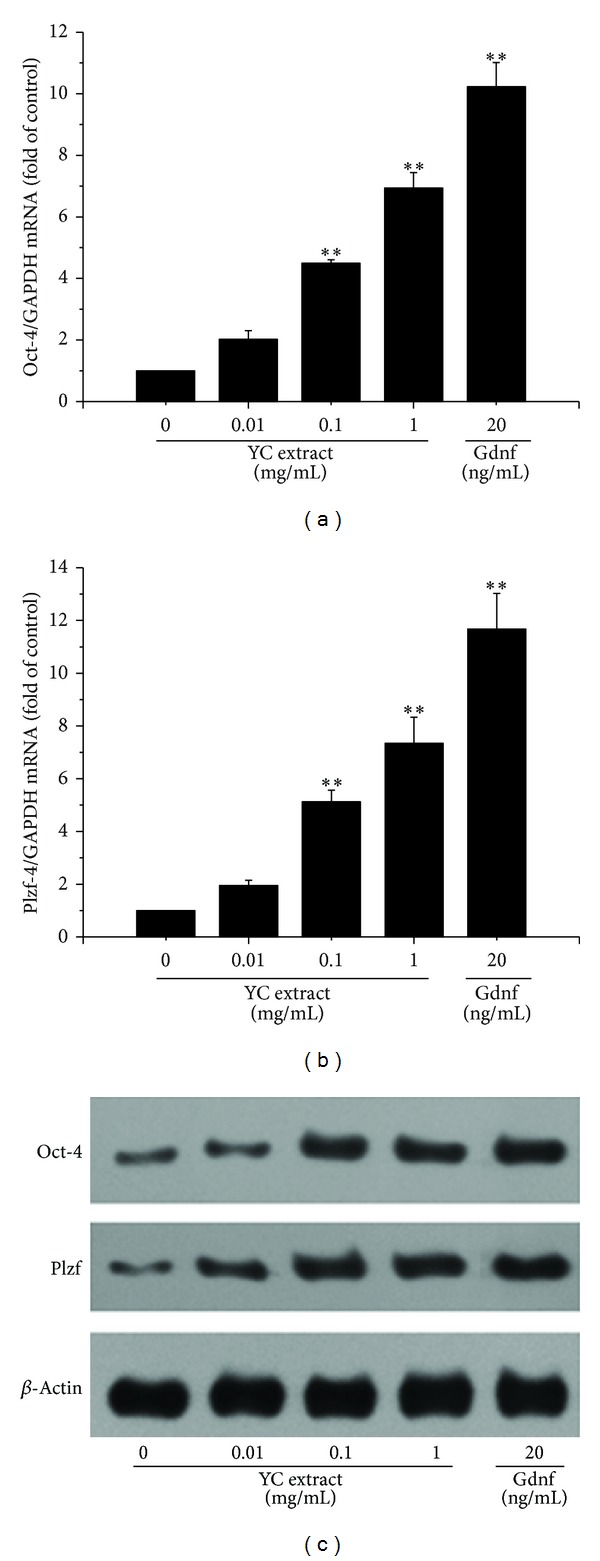
Effects of the YC extract on the expression of Oct-4 and Plzf mRNAs and proteins in GC-1 spg cells. GC-1 spg cells were treated with 0.01, 0.1, and 1 mg/mL YC extract or 20 ng/mL Gdnf for 48 h. Expression of mRNAs was detected by real-time PCR. Expression of proteins was detected by western blot. The data are expressed as the percentage of the control (100%). **Significantly different from control at P < 0.01. The analysis was performed in triplicate and representative bands were shown.
3.5. Gfrα1 Knockdown or PI3K Inhibition Blocks YC Extract Induced Proliferation of GC-1 Spg Cells
To investigate the mechanism of YC extract induced proliferation of GC-1 spg cells, Gfrα1 knockdown and PI3K inhibition were performed. As shown in Figure 5, YC extract significantly stimulated the cell proliferation. However, both Gfrα1 siRNA and LY294002, an inhibitor of PI3K, could evidently abrogate the stimulative effect of YC extract.
Figure 5.
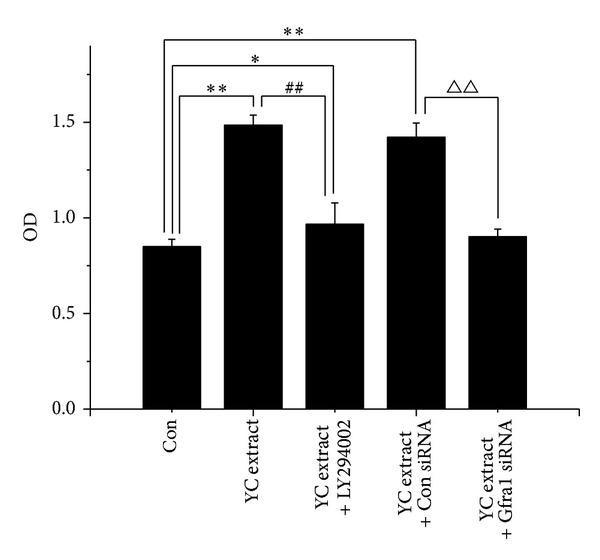
Gfrα1 knockdown or PI3K inhibition blocks YC extract induced proliferation of GC-1 spg cells. GC-1 cells were exposed to control blank, 1 mg/mL YC extract, 1 mg/mL YC extract with 50 μM LY294002, and 1 mg/mL YC extract with or without 50 pmol Gfrα1 siRNA. *P < 0.05; **P < 0.01 compared with control group; ## P < 0.01 compared with YC extract group; △△ P < 0.01 compared with YC extract and control siRNA group, n = 5.
3.6. Gfrα1 Knockdown or PI3K Inhibition Blocks YC Extract Induced Upregulation of Oct-4 and Plzf Expression
The results of Figure 6 showed that both Gfrα1 siRNA and LY294002 could abolish the upregulation of Oct-4 and Plzf expression at levels of mRNA and protein induced by YC extract. Meanwhile, upregulation of pAKT protein expression induced by YC extract, evidence for PI3K pathway activation, was also suppressed by Gfrα1 siRNA and LY294002. Combined with the results above, it could be inferred that Gfrα1 and PI3K played crucial roles in the promotion of self-renewal induced by YC extract.
Figure 6.
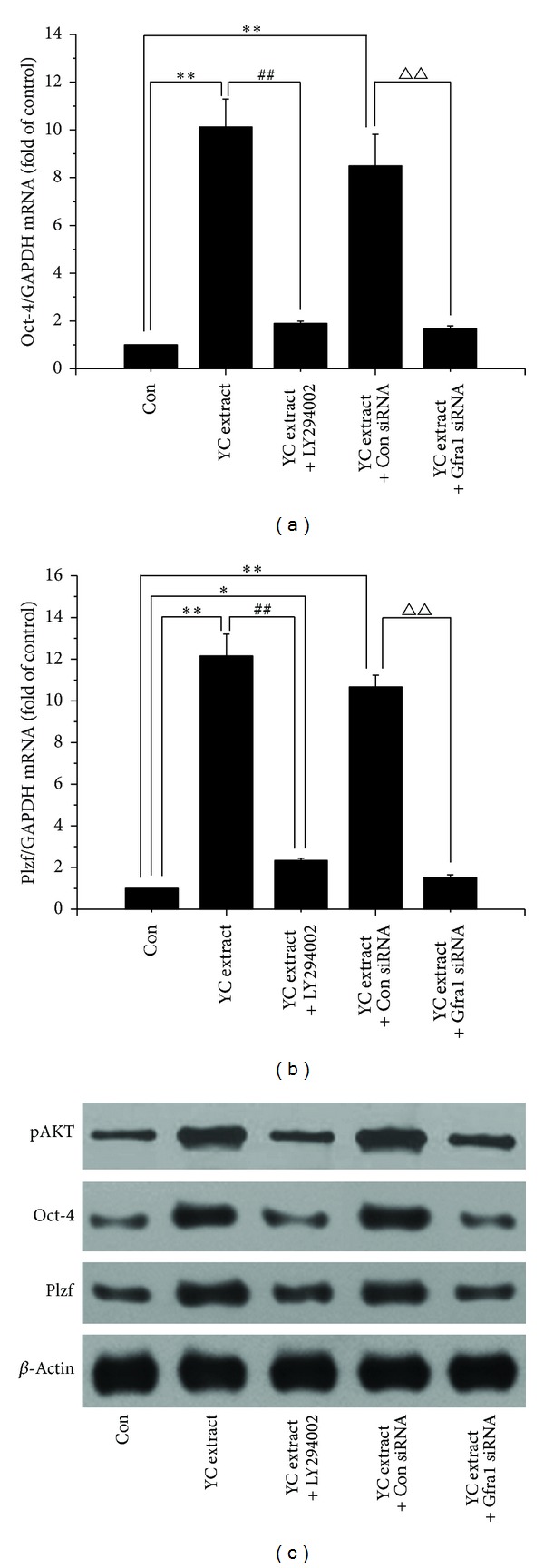
Gfrα1 knockdown or PI3 K inhibition blocks YC extract induced upregulation of Oct-4 and Plzf expression. GC-1 cells were exposed to control blank, 1 mg/mL YC extract, 1 mg/mL YC extract with 50 μM LY294002, and 1 mg/mL YC extract with or without 50 pmol Gfrα1 siRNA. *P < 0.05; **P < 0.01 compared with control group; ## P < 0.01 compared with YC extract group; △△ P < 0.01 compared with YC extract and control siRNA group. The analysis was performed in triplicate and representative bands were shown.
4. Discussion
YC extract has been used for male infertility therapy in clinic for years, but the mechanisms still remain unclear. Our results suggested that YC extract could stimulate self-renewal of SSCs and protect SSCs from apoptosis, thus improving spermatogenesis.
Oct-4 is considered to be the master transcription factor responsible for preserving the pluripotency and self-renewal of stem cells [11, 25]. It is downregulated during differentiation with loss of pluripotency [27–29] and generally accepted as a stem cell marker. Plzf has also drawn wide attention due to its role of regulating differentiation. Plzf is claimed to be essential for maintaining pluripotent properties and self-renewal as its expression is confined to stem cells and early progenitor cells [12, 30, 31]. While Plzf is expressed at high level in undifferentiated pluripotent stem cells, its expression declined when differentiation started. Our results showed that 48 h treatment of YC extract not only promoted the proliferation of GC-1 spg cells but also promoted self-renewal of cells proved by enhanced expression of Oct-4 and Plzf mRNAs and proteins, which were quite similar to the effect of Gdnf treatment (Figures 1 and 4). As a matter of course, we deduced that YC extract probably functioned in the similar ways as Gdnf.
Gdnf, secreted by Sertoli cells, is so far the only known paracrine factor specifically responsible for the maintenance and self-renewal of SSCs in vivo [13, 14]. Lin et al. reported that Gdnf is a member of the transforming growth factor beta (TGF-β) superfamily [32]. Gdnf signals through a multicomponent receptor complex are composed of the Ret receptor tyrosine kinase and Gfrα1 [33, 34]. Gdnf can trigger various downstream signal pathways to promote cell survival and self-renewal via GPI-linked protein Gfrα1 [35, 36]. Based on the comparable effects of YC extract and Gdnf, we speculated that Gfrα1 also plays a key role in YC extract's biological effects. To confirm our hypothesis, we used siRNA to knockdown Gfrα1 expression in current research. Figure 5 showed that Gfrα1 knockdown almost entirely abolished the proliferous effect of GC-1 cells induced by YC extract. Corresponding with this, Gfrα1 knockdown also abrogated the elevated expression of Oct-4 and Plzf mRNAs and proteins induced by YC extract (Figure 6). On the basis of these results, we inferred that YC extract exerts biological effects partly via Gfrα1.
To further investigate the mechanism of YC extract, we treated GC-1 cells with 50 μM LY294002 to inhibit PI3K pathway before the treatment of YC extract. PI3K pathway has been demonstrated to play a central role in the Gdnf induced self-renewal of SSCs [37, 38]. The binding of Gdnf to Gfrα1 triggers phosphorylation of SRC-kinase family proteins followed by activation of PI3K/AKT pathway and eventually leads to self-renewal of SSCs via expression of N-myc gene [15, 16]. As has been stated, like Gdnf, YC extract also induces self-renewal via Gfrα1, and activation of PI3K pathway is most likely very crucial in YC extract's effects. As expected, pAKT protein expression increased evidently after treatment of YC extract standing for PI3K pathway activation (Figure 6). PI3K pathway inhibition evidently removed the proliferous effect of GC-1 spg cells induced by YC extract which was shown in Figure 5. Consistent with this, the upregulated Oct-4 and Plzf mRNAs and proteins also dropped down by LY294002 treatment (Figure 6).
In summary, YC extract could improve spermatogenesis by means of promoting self-renewal of SSCs and protecting SSCs from apoptosis, which were verified by cell proliferation and upregulation of OCT-4 and Plzf expression after the treatment of YC extract. To explore possible mechanism, we performed Gfrα1 knockdown and PI3K inhibition before application of YC extract. The results showed that both Gfrα1 knockdown and PI3K inhibition were able to evidently remove the cell proliferation and upregulation of OCT-4 and Plzf expression induced by YC extract. Therefore, we concluded that, partly via Gfrα1, YC extract could trigger the activation of PI3K pathway and finally lead to self-renewal of SSCs. However, as YC extract is composed of multiple components, further studies are needed to identify the primary effective component.
Acknowledgments
This work was supported by the Chinese National Natural Science Foundation (no. 81273760, no. 81302969) and a Postgraduate Innovation Program of the Jiangsu Province Education Department (no. CXLX13_583). The authors thank the State Key Laboratory of Reproductive Medicine, the Clinical Center of Reproductive Medicine, and the Nanjing Medical University for providing laboratory and technological assistance.
Conflict of Interests
There is no conflict of interests regarding the publication of this paper.
References
- 1.Anawalt BD. Approach to male infertility and induction of spermatogenesis. The Journal of Clinical Endocrinology & Metabolism. 2013;98:3532–3542. doi: 10.1210/jc.2012-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holden CA, McLachlan RI, Pitts M, et al. Men in Australia Telephone Survey (MATeS): a national survey of the reproductive health and concerns of middle-aged and older Australian men. The Lancet. 2005;366(9481):218–224. doi: 10.1016/S0140-6736(05)66911-5. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TJ, Pera RR, Turek PJ. The genetics of male infertility. Seminars in Reproductive Medicine. 2009;27(2):124–136. doi: 10.1055/s-0029-1202301. [DOI] [PubMed] [Google Scholar]
- 4.O’Flynn O’Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertility and Sterility. 2010;93(1):1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 5.Vicari E, Calogero AE, Condorelli RA, Vicari LO, La Vignera S. Male accessory gland infection frequency in infertile patients with chronic microbial prostatitis and irritable bowel syndrome. International Journal of Andrology. 2012;35(2):183–189. doi: 10.1111/j.1365-2605.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 6.Singh SR, Burnicka-Turek O, Chauhan C, Hou SX. Spermatogonial stem cells, infertility and testicular cancer. Journal of Cellular and Molecular Medicine. 2011;15(3):468–483. doi: 10.1111/j.1582-4934.2010.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12201–12206. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caires K, Broady J, McLean D. Maintaining the male germline: regulation of spermatogonial stem cells. Journal of Endocrinology. 2010;205(2):133–145. doi: 10.1677/JOE-09-0275. [DOI] [PubMed] [Google Scholar]
- 9.Caires KC, de Avila J, McLean DJ. Endocrine regulation of spermatogonial stem cells in the seminiferous epithelium of adult mice. BioResearch Open Access. 2012;1:222–230. doi: 10.1089/biores.2012.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biology of Reproduction. 2010;82(2):363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhartiya D. Are mesenchymal cells indeed pluripotent stem cells or just stromal cells? OCT-4 and VSELs biology has led to better understanding. Stem Cells International. 2013;2013:6 pages. doi: 10.1155/2013/547501.547501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa GM, Avelar GF, Rezende-Neto JV, et al. Spermatogonial stem cell markers and niche in equids. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0044091.e44091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann M-C, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; Influence of GDNF. Developmental Biology. 2005;279(1):114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. Journal of Clinical Investigation. 2011;121(9):3456–3466. doi: 10.1172/JCI57984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. Journal of Biological Chemistry. 2007;282(35):25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brehm R, Steger K. Regulation of sertoli cell and germ cell differentation. Advances in Anatomy Embryology and Cell Biology. 2005;181:1–93. [PubMed] [Google Scholar]
- 18.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136(7):1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann M-C. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Molecular and Cellular Endocrinology. 2008;288(1-2):95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin B, Huang Y, Xia X, Wang X, Yang X, Zhou Z. Effect of Yangjing capsule and Xinxibao on sperm DNA integrity of patients with male infertility. Chinese Journal of Andrology. 2006;20(12):45–49. [Google Scholar]
- 21.Sun D, Cui Y, Jin B, et al. Effects of the yangjing capsule extract on steroidogenesis and apoptosis in mouse leydig cells. Evidence-Based Complementary and Alternative Medicine. 2012;2012:10 pages. doi: 10.1155/2012/985457.985457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin B, Huang Y, Yang X, et al. Clinical observation on treatment of oligospermatism with Yangjing Capsule. Journal of Nanjing University of Traditional Chinese Medicine. 2006;22:286–289. [Google Scholar]
- 23.Kao S-T, Yeh C-C, Hsieh C-C, et al. The Chinese medicine Bu-Zhong-Yi-Qi-Tang inhibited proliferation of hepatoma cell lines by inducing apoptosis via G0/G1 arrest. Life Sciences. 2001;69(13):1485–1496. doi: 10.1016/s0024-3205(01)01226-7. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Jiang J, Hofmann M-C, Dym M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biology of Reproduction. 2007;77(4):723–733. doi: 10.1095/biolreprod.107.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhartiya D, Kasiviswanathan S, Unni SK, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. Journal of Histochemistry and Cytochemistry. 2010;58(12):1093–1106. doi: 10.1369/jhc.2010.956870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung M, Pei J, Pei Y, Jhanwar SC, Pass HI, Testa JR. The promyelocytic leukemia zinc-finger gene, PLZF, is frequently downregulated in malignant mesothelioma cells and contributes to cell survival. Oncogene. 2010;29(11):1633–1640. doi: 10.1038/onc.2009.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell PA, Perez-Iratxeta C, Andrade-Navarro MA, Rudnicki MA. Oct4 targets regulatory nodes to modulate stem cell function. PLoS ONE. 2007;2(6, article e553) doi: 10.1371/journal.pone.0000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22(2):225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- 29.Lengner CJ, Welstead GG, Rudolf Jaenisch RJ. The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle. 2008;7(6):725–728. doi: 10.4161/cc.7.6.5573. [DOI] [PubMed] [Google Scholar]
- 30.Costoya JA, Hobbs RM, Barna M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nature Genetics. 2004;36(6):653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 31.Song W, Zhu H, Li M, et al. Promyelocytic leukaemia zinc finger maintains self-renewal of male germline stem cells (mGSCs) and its expression pattern in dairy goat testis. Cell Proliferation. 2013;46:457–468. doi: 10.1111/cpr.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 33.Jing S, Wen D, Yu Y, et al. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell. 1996;85(7):1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 34.Treanor JJS, Goodman L, De Sauvage F, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382(6586):80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 35.Grasso M, Fuso A, Dovere L, et al. Distribution of GFRA1-expressing spermatogonia in adult mouse testis. Reproduction. 2012;143(3):325–332. doi: 10.1530/REP-11-0385. [DOI] [PubMed] [Google Scholar]
- 36.Trupp M, Scott R, Whittemore SR, Ibáñez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. Journal of Biological Chemistry. 1999;274(30):20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 37.Braydich-Stolle L, Kostereva N, Dym M, Hofmann M-C. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Developmental Biology. 2007;304(1):34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Kanatsu-Shinohara M, Inoue K, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134(10):1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]


