Abstract
Epidemiological studies have shown that lower urinary tract symptoms, including overactive bladder, commonly occur in both men and women, with an age-related increase in both sexes. Vascular endothelial dysfunction and urological symptoms are common in the metabolic syndrome; they also occur during the human ageing process and are independent risk factors for the development of atherosclerosis and hypertension. Pelvic arterial insufficiency may lead to impaired lower urinary tract perfusion and play an important role in the development of bladder dysfunction such as detrusor overactivity and overactive bladder. It seems reasonable, but has not been definitely established clinically, that chronic ischemia-related bladder dysfunction will progress to bladder underactivity. Studies in experimental models in rabbits and rats have shown that pelvic arterial insufficiency may result in significant bladder ischemia with reduced bladder wall oxygen tension, oxidative stress, increased muscarinic receptor activity, ultrastructural damage, and neurodegeneration. Several types of drug may be able to prevent some of these changes. Even if the α1-adrenoceptor blocker, silodosin, the phosphodiesterase type 5 inhibitor, tadalafil, the β3-α1-adrenoceptor agonist, mirabegron, and the free radical scavenger, melatonin, were unable to prevent the development of neointimal hyperplasia and consequent luminal occlusion in animal models, they all exerted a protecting effect on urodynamic parameters, and on the functional and morphological changes of the bladder demonstrable in vitro. The different mechanisms of action of the drugs suggest that many factors are involved in the pathogenesis of chronic ischemia-induced bladder dysfunction and can be targets for intervention. Since several of the agents tested are used clinically and effectively for relieving lower urinary tract symptoms, the results from animal models of chronic bladder ischemia seem to have translational value. Animal models may be of relevance for designing clinical studies to demonstrate if a certain drug may prevent progression of ischemia-related functional and morphological bladder changes.
Keywords: α1-adrenoceptor antagonists, animal models, β3-α1-adrenoceptor agonists, free radical scavengers, phosphodiesterase type 5 inhibitors
Introduction
Epidemiological studies have shown that lower urinary tract symptoms (LUTS), including overactive bladder (OAB), occur commonly in both men and women, with an age-related increase in both sexes [Irwin et al. 2011]. An estimated 45.2% of the 2008 worldwide population (4.3 billion) was affected by at least one LUTS and, by 2018, an estimated 2.3 billion individuals will be affected (18.4% increase). It is well-known that the causes of LUTS, including OAB, are multifactorial and involve many pathophysiologic mechanisms in both men and women [Roosen et al. 2009; Banakhar et al. 2012; Meng et al. 2012]. However, particularly in the elderly, ageing-associated changes in pelvic vasculature, such as atherosclerosis, may be an important contributing factor in both sexes [Ponholzer et al. 2006]. Vascular endothelial dysfunction occurs with ageing and is an independent risk factor for the development of atherosclerosis and hypertension [Herrera et al. 2010]. Moreover, the abdominal aorta and its branches, especially the bifurcation of the iliac arteries, are particularly vulnerable to atherosclerotic lesions [Tarcan et al. 1998]. The vascular supply to the human genitourinary tract, including the bladder, prostate, urethra and penis, is primarily derived from the iliac arteries, and atherosclerotic obstructive changes distal to the aortic bifurcation will have consequences for the distal vasculature and for lower urinary tract blood flow [Yamaguchi et al. 2014]. Pinggera and colleagues [Pinggera et al. 2008b] found that elderly patients with LUTS had a significant decrease in bladder blood flow in comparison with asymptomatic young individuals. These studies suggest that arterial occlusive disease and concomitant chronic bladder ischemia may produce bladder dysfunction, including detrusor overactivity (DO). However, despite intensive study in various animal models, the mechanisms behind changes in bladder function caused by chronic ischemia are incompletely known, and there is no established treatment. It has been suggested by animal studies, but has not been established clinically, that chronic ischemia-related bladder dysfunction will progress to bladder underactivity [Nomiya et al. 2013a; Sagawa et al. 2013]. Nevertheless, it would be desirable to treat not only LUTS, but also the progression of the morphological bladder changes induced by chronic ischemia.
It may be discussed whether the decreases in bladder blood flow during the micturition cycle demonstrated by, for example, Brading and colleagues [Brading et al. 1999] could contribute to bladder injury in certain situations. For example, in bladder outflow obstruction, there may be repeated episodes of prolonged detrusor ischemia which may cause ischemia-reperfusion injury [Greenland and Brading, 2000, 2001]. In an already ischemic bladder, it may be speculated that the decrease in bladder blood flow during a voiding cycle, particularly with high degrees of bladder filling, may create an ischemia-reperfusion episode. With time, such repeated episodes could add further damage to the bladder.
Treatment with α1-adrenoceptor (AR) blockers and phosphodiesterase type 5 (PDE5) inhibitors, such as tadalafil, sildenafil and vardenafil, have been shown to be effective for treating LUTS associated with benign prostatic hyperplasia (BPH) [Andersson et al. 2013a; Soler et al. 2013] and recently mirabegron, the β3-AR agonist, was approved as an effective treatment of OAB [Andersson et al. 2013b]. Theoretically, free radical scavengers could also offer interesting treatment options for LUTS/OAB [Meng et al. 2012; Soler et al. 2013]. The different mechanisms of action of these drugs support a multifactorial pathogenesis of LUTS/OAB. However, if chronic bladder ischemia (see below) is a common factor contributing to these disorders, the results from the animal models of chronic bladder ischemia may have translational value, and may be of relevance for designing clinical studies to demonstrate if a drug may prevent progression of ischemia-related functional and morphological bladder changes.
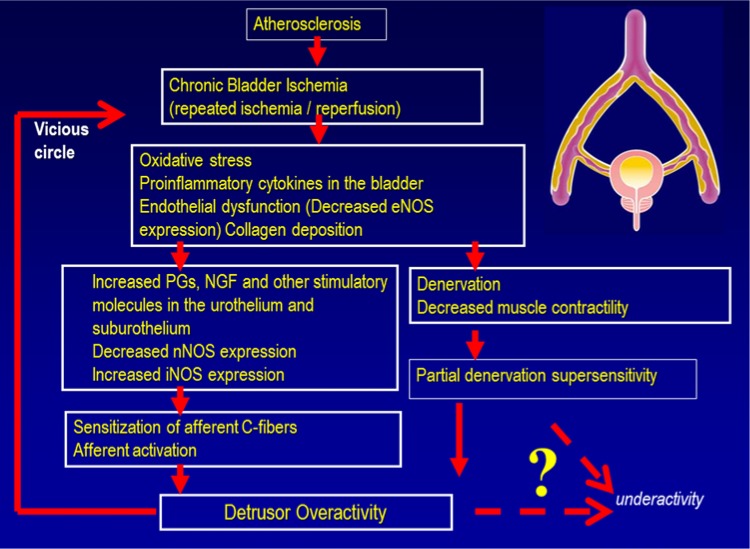
As discussed below, and illustrated in Figure 1, chronic ischemia (plus repeated ischemia/reperfusion episodes) could lead to oxidative stress and inflammatory changes in the bladder with release of agents such as prostaglandins and nerve growth factor which are importance for the generation of DO. Eventually, progressive vascular damage and ischemia may lead to denervation and decreases in detrusor contractility resulting in detrusor underactivity.
Figure 1.
Hypothesis; atherosclerosis-induced chronic bladder ischemia can lead to detrusor overactivity and possibly (eventually) to detrusor underactivity.
Animal models of chronic bladder ischemia
There are several published animal models of chronic bladder ischemia [Yamaguchi et al. 2014]. However, the effects of therapeutic interventions have been studied in only a few of these. Even if animal models cannot be expected to reflect a diagnosis based on symptoms, such as OAB, they may increase our understanding of the pathophysiology of the different disorders leading to, for example, OAB, and they can also be helpful in the development of effective treatments.
Rabbit models
Azadzoi and colleagues demonstrated that bilateral ligation of the vesical arteries led to extremely severe structural and functional changes, causing permanent damage to the bladder [Azadzoi et al. 1999a, 1999b]. They produced iliac artery occlusive disease using arterial balloon endothelial injury techniques and a 0.5% cholesterol diet in male rabbits. This showed that moderate ischemia caused frequent voiding, a significant increase in the frequency of spontaneous bladder contractions and increased contractile in vitro responses to carbachol and electrical field stimulation with moderate fibrosis in the bladder wall. Severe ischemia caused severe fibrosis, very weak bladder contraction, and decreased contractile responses to various stimuli. Azadzoi and colleagues [Azadzoi et al. 1999a, 1999b] also examined markers of oxidative injury and neural density in the ischemic bladder, and demonstrated that bladder hyperactivity under ischemic conditions involved noxious oxidative products and denervation. Thus there was an upregulation of stimulatory molecules, oxidative stress-sensitive genes, ultrastructural damage and neurodegeneration [Yamaguchi et al. 2014].
Yoshida and colleagues [Yoshida et al. 2010] examined the functional and histological bladder changes in the myocardial infarction-prone Watanabe heritable hyperlipidemia rabbit. They demonstrated that Watanabe heritable hyperlipidemia rabbits showed atherosclerotic changes in the iliac arteries, an increase in connective tissues in the bladder wall, frequent voiding and nonvoiding contractions, decreased detrusor contraction, denervation, and decreased in vitro contractile responses to carbachol and electrical field stimulation. The effects of therapeutic interventions with respect to bladder dysfunction do not seem to have been investigated in this model.
Rat models
Nomiya and colleagues [Nomiya et al. 2012a, 2012b] developed a rat model using techniques similar to those Azadzoi and colleagues [Azadzoi et al. 1999a, 1999b] had applied to rabbits. They found that endothelial injury of the iliac arteries combined with a 2% cholesterol diet for 8 weeks induced arterial occlusive disease and consequent bladder ischemia. Decreased contractile responses to potassium chloride (KCl), carbachol and nerve stimulation were observed. In addition, the animals exhibited increased collagen deposition in the muscle layer, elevated oxidative stress markers, upregulation of proinflammatory cytokines and decreased constitutive nitric oxide synthase (NOS) expression. These changes were associated with bladder hyperactivity defined as a significant increase in voiding frequency without effect on maximum pressure or residual volume.
Son and colleagues [Son et al. 2007] evaluated voiding function in a rat model where rats were fed a 1% cholesterol diet for 8 weeks, together with 2 weeks of NG-nitro-L-arginine methyl ester given to induce intimal changes. They found atherosclerotic changes in the iliac arteries and urodynamic bladder hyperactivity. Rahman and colleagues [Rahman et al. 2007] also examined bladder function in a rat model where rats were fed a 2% cholesterol and 10% lard diet alone for 6 months. These rats showed bladder hyperactivity with nonvoiding contractions, hypertrophy of the detrusor muscle and upregulation of P2X3, P2X1 and vanilloid receptor1 (TRPV1) expression. In these models, the effects of therapeutic interventions with respect to bladder dysfunction do not seem to have been investigated.
Mouse model
Shenfeld and colleagues [Shenfeld et al. 2005] examined contractility of bladder muscle strips from apolipoprotein E gene knockout mice known to spontaneously develop atherosclerosis. Despite apolipoprotein E gene knockout mice exhibiting massive atherosclerosis of the abdominal aortas and iliac arteries, there was no statistically significant differences compared with strips from control C57BI/6 mice. No information on cystometric parameters was reported, and the effects of therapeutic interventions with respect to bladder dysfunction were not investigated.
α1-Adrenoceptor blockade
In patients with LUTS associated with BPH, α1-AR antagonists are still the mainstay of medical treatment [Michel, 2010; Lepor et al. 2012; Andersson et al. 2013a]. Such antagonists were developed based on the hypothesis that these drugs improve voiding dysfunction due to relieving bladder outflow obstruction, since they were shown to relax prostatic smooth muscle and decrease urethral pressure in animal models. However, newer data indicate that their therapeutic effects, at least partly, occur independent of prostatic relaxation, perhaps involving direct effects on blood vessels, urothelium, afferent nerves, and/or smooth muscle of the urinary bladder [Michel, 2010]. On the other hand, it has been shown that in rabbits with chronic pelvic ischemia, there was an increased prostatic smooth muscle contraction in response to the α1-AR agonists, phenylephrine, and electrical field stimulation [Kozlowski et al. 2001; Azadzoi et al. 2003]. Similar results were obtained in the ischemic rat prostate [Zarifpour et al. unpublished information].
As a class, α1-AR -blockers cause little reduction of bladder outlet obstruction, and there seems to be little association between LUTS improvement by α1-AR antagonist treatment and changes in bladder outlet resistance [Barendrecht et al. 2008]. In addition, these drugs improve LUTS without bladder outflow obstruction. Pinggera and colleagues [Pinggera et al. 2008a] reported that α1-AR antagonists improved bladder blood flow and symptoms in patients with LUTS, and suggested that these drugs might ameliorate LUTS by increasing bladder blood flow.
Goi and colleagues [Goi et al. 2013] investigated the effects of the selective α1-AR antagonist, silodosin, on bladder blood flow and bladder function using the rat model of chronic bladder ischemia described by Nomiya and colleagues [Nomiya et al. 2012a, 2012b]. Using an osmotic pump, chronic bladder ischemia rats received either silodosin subcutaneously at a rate of 0.1 or 0.3 mg/, or vehicle for 8 weeks. For each α1-AR subtype, mRNA expression in bladder microvessels was examined by in situ hybridization. Bladder blood flow was measured using a laser speckle blood flow imager, and malondialdehyde in bladder tissue and 8-hydroxy-2-deoxyguanosine in urine were measured as markers of oxidative stress. The expression of all α1-AR subtype mRNA was observed in rat bladder microvessels. In rats with chronic bladder ischemia, silodosin abrogated the decreased bladder blood flow in the empty bladder and during bladder distension. This observation is in agreement with the effects of tamsulosin on bladder microcirculation in a rat ischemia-reperfusion model [Mizuno et al. 2010]. The levels of oxidative stress markers in the rats studied by Goi and colleagues [Goi et al. 2013] were significantly decreased by silodosin administration, and the drug ameliorated bladder overactivity. The basis for the improvement in bladder function was attributed to an improvement in bladder blood flow.
As mentioned previously, α1-AR antagonists have convincingly been shown to give symptomatic relief in male patients with LUTS. If this is related to an increase in blood flow such an effect would also be expected in women with LUTS and voiding symptoms. This was found to be the case in several clinical studies (one out of seven was randomized and placebo controlled) [Meyer and Brown, 2012]. In contrast, a randomized, double-blind, placebo-controlled study in women with OAB revealed no significant effect of tamsulosin [Robinson et al. 2007]. The divergent results may be explained by differences in the patient populations investigated. It may be that, as with men, α1-AR antagonists are more effective on voiding symptoms. Considering the multifactorial causes of OAB, it may also reflect that ischemia is only one of the factors contributing to this syndrome.
PDE-5 inhibition
The positive effects of PDE5-inhibitors (PDE5-I), alone or in combination with α1-AR antagonists, on male LUTS have been well documented [Martinez-Salamanca et al. 2011; Gacci et al. 2012]. Different PDE5-Is (e.g. sildenafil, vardenafil, tadalafil) alone or in combination with α1-AR antagonists (e.g. alfuzosin or tamsulosin) were compared with placebo or α1-AR antagonists alone [Martinez-Salamanca et al. 2011]. Gacci and colleagues [Gacci et al. 2012] performing a meta-analysis, demonstrated that the use of PDE5-Is alone was associated with a significant improvement in International Prostate Symptom Score (IPSS) at the end of the studies compared with placebo. The association of an α1-AR antagonist and a PDE5-I significantly improved IPSS and Qmax at the end of the studies compared with α1-AR antagonists alone. Studies on the effects of PDE5-Is on female LUTS/OAB do not seem to have been published.
The precise mechanism(s) of action of PDE5-Is on LUTS remains to be elucidated. PDE5-Is may act on several pathways including upregulating nitric oxide/cyclic guanosine monophosphate activity, downregulating Rho-kinase activity, modulating autonomic nervous system overactivity and bladder and prostate afferent nerves, increasing pelvic blood perfusion, and reducing inflammation. PDE5-Is have been shown to reduce nonvoiding contractions and bladder afferent nerve firing in decerebrate spinal cord-injured rats, and to reduce mechanosensitive afferent activities of both Aδ- and C-fibers in an irritated or overextended bladder model [Andersson et al. 2011; Giuliano et al. 2013].
To investigate the effect of tadalafil on chronic ischemia-related bladder dysfunction, Nomiya and colleagues [Nomiya et al. 2013b] used adult male Sprague-Dawley rats divided into control, arterial endothelial injury (AI), and AI with tadalafil treatment (AI-tadalafil) groups. The AI and AI-tadalafil groups underwent endothelial injury of the iliac arteries and received a 2% cholesterol diet following AI. AI-tadalafil rats received tadalafil (2 mg/kg/day) orally for 8 weeks after AI. The control group received a regular diet. After 8 weeks, urodynamic investigation was performed, and after sacrificing the animals, bladder tissue was harvested for pharmacological studies, and the iliac arteries and bladders were examined histologically. In the AI-tadalafil group neointimal formation and luminal occlusion were not prevented. However, there were significant improvements in all the functional and morphological parameters compared with the AI group. In the AI group, the micturition interval was significantly shorter (5.4 ± 0.5 versus 11.1 ± 1.1 min), and bladder capacity and voided volume were lower than in controls. In vitro, contractile responses of bladder strips to KCl, electrical field stimulation and carbachol were significantly lower after AI than in controls. The AI group showed a significantly increased percentage of collagen in the bladder wall (37.4 ± 1.8%) compared with the controls (21.5 ± 1.8%). Even though the direct mechanisms by which tadalafil protected bladder function and morphology were not established, these findings suggest (provided they can be translated into a clinical setting) that PDE5-Is may not only be able to reduce LUTS, but also have an effect on the progression of the underlying disorder. A factor that may limit the translational value of the results is that the tadalafil dose used is very high compared with what is used in humans.
Free radical scavenging
At high concentrations, free radicals and radical-derived, nonradical reactive species are hazardous for living organisms and damage all major cellular constituents. At moderate concentrations, however, nitric oxide (NO), superoxide anion, and related reactive oxygen species (ROS) play an important role as regulatory mediators in signaling processes [Dröge, 2002]. Oxidative stress is characterized by either short-term or chronic increase in the level of ROS, which, due to their high reactivity, induce oxidation of such vital cellular components as proteins, lipids, and nucleic acids; this process being the main cause of organ dysfunction [Dröge, 2002; Kirpatovsky et al. 2013]. Oxidative stress associated with chronic bladder ischemia may lead to bladder dysfunction and possibly even to underactive bladder. In most cases mitochondria, being responsible for ROS generation and transformation, play the key role in ischemic pathogenesis. Mice with mutation in the Immp2l gene have been shown to have high superoxide ion levels due to increased mitochondrial superoxide production. They also had bladder dysfunction, mainly characterized by emptying abnormalities in young males and increased detrusor activity in old females [Soler et al. 2010].
The need to maintain intracellular ROS at a level allowing normal intracellular signaling is an obvious strategy of ischemic organ protection [Dröge, 2002]. Studies in an experimental model in rabbits suggested that lack of perfusion and subsequent accumulation of oxidative stress and nitrosative elements in atherosclerosis-induced chronic bladder ischemia may contribute to deterioration of microvasculature, nerve fibers, epithelium and smooth muscle cells by lipid peroxidation, protein oxidation and DNA damage [Azadzoi et al. 2010, 2011]. Thus, control of oxidative stress as a prophylactic treatment may have beneficial effects on chronic ischemia-related bladder dysfunction.
Many studies have documented that melatonin, the major secretory product of the pineal gland, has potent endogenous free radical scavenging and antioxidative properties and protects against oxidative insults [Reiter et al. 2000]. Melatonin treatment was shown to reduce oxidative stress and to improve ageing-associated deficits in bladder function [Gómez-Pinilla et al. 2007a, 2007b, 2008]. It was also demonstrated that melatonin could reverse the reduced contractile responses of rat bladder strips to carbachol and prevent oxidative tissue damage following ischemia/reperfusion after clamping of the aorta [Sener et al. 2003].
Nomiya and colleagues [Nomiya et al. 2013b] investigated the potential therapeutic benefit of melatonin in chronic ischemia-related bladder dysfunction, using the previously described rat model. Adult male Sprague-Dawley rats were divided into control, arterial injury (AI) and AI with melatonin treatment low- (AI-ML)/high-dose (AI-MH) groups. AI, AI-ML and AI-MH groups underwent endothelial injury of the iliac arteries and received a 2% cholesterol diet following AI. AI-ML and AI-MH rats were treated with melatonin 2.5 or 20 mg/kg/day orally for 8 weeks after AI. The control group received a regular diet. After 8 weeks, urodynamic investigation was performed and bladder tissues and iliac arteries were processed for pharmacological studies, and examined by immunohistochemical and histological methods. Iliac arteries from AI rats displayed neointimal formation and luminal occlusion, and this was not prevented in the AI-ML and AI-MH groups. In the AI group, micturition interval was significantly shorter, and bladder capacity and voided volume lower than in the controls. Contractile responses of bladder strips to KCl, electrical field stimulation and carbachol were significantly lower after AI than in the controls. The AI bladders showed significantly increased collagen ratio, oxidative stress and iNOS expression, and decreased constitutive NOS expression compared with the controls. In the AI-ML group, the beneficial effects failed to reach statistical significance. However, in the AI-MH group, melatonin significantly improved oxidative stress and NOS expression, and there were significant improvements in all the functional and morphological parameters compared with the AI group. The mechanism by which chronic treatment with melatonin protected bladder function and morphology was suggested to be through its free radical scavenging and antioxidative properties. The melatonin doses used in this study were very high compared with what is usually prescribed for human use. This may limit the translational value of the results, which nevertheless illustrates that the treatment principle may be effective.
Several other agents possessing such properties have been shown to have protective effects against ischemia-reperfusion bladder injuries, for example, co-enzyme Q [Juan et al. 2008; Kim et al. 2013], N-acetylcysteine [Shin et al. 2014], eviprostat [Matsui et al. 2012; Kawai et al. 2013] and edaravone [Matsumoto et al. 2010]. Some of these agents may also be useful for protection against the effects of chronic bladder ischemia.
β3-Adrenoceptor agonists
The clinical efficacy of the β3-AR agonist, mirabegron, is well established [Andersson et al. 2013a and 2013b; Sanford, 2013; Chapple et al. 2014]. Stimulation of β3-ARs relaxes detrusor smooth muscle, and consequently decreases afferent signaling from the bladder, improves bladder compliance on filling, and increases bladder capacity. The generally accepted molecular mechanism by which β-ARs mediate detrusor relaxation in most species, is activation of adenylyl cyclase and subsequent formation of cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA).
This enzyme, in turn, phosphorylates specific proteins resulting in detrusor relaxation. cAMP is one of the most potent signaling molecules to stabilize the vascular endothelial barrier [Schlegel and Waschke, 2013]. In hypoxic endothelial cells in culture, cAMP was shown to decrease through a reduction of adenylyl cyclase activity, and warm ischemia is known to decrease the cAMP concentration in the liver, heart, and kidney [Pinskey et al. 1993; Minor et al. 1999; Riera et al. 2001]. Therefore, it might be reasonable to assume that this also occurs in bladder tissue in chronic ischemia. Since urine storage and distension induce vessel compression and in turn worsen ischemia/hypoxia by consuming more energy during the voiding phase [Greenland et al. 2000; 2001], a cAMP decreasing effect can be thought of as a defensive mechanism under ischemic conditions. If mirabegron mediates bladder relaxation by increasing cAMP in the chronic ischemic bladder, this can be expected to affect the restrained cell metabolism under ischemic conditions, and raises the question of whether mirabegron treatment may have a negative impact on the chronic ischemic bladder. However, there is evidence suggesting that, in the bladder, K+ channels mediating hyperpolarization, particularly BKCa channels, may be more important in β3-AR mediated relaxation than cAMP [Frazier et al. 2005; Petkov, 2011].
Sawada and colleagues [Sawada et al. 2013] investigated the effect of mirabegron in a one of the previously described rat models of chronic ischemia-related bladder dysfunction [Nomiya et al. 2012a, 2012b]. They used male Sprague-Dawley rats which were divided into different groups: control (n = 10), arterial endothelial injury (AI; n = 16), and AI with mirabegron treatment (AI-mirabegron; n = 10). The AI and AI-mirabegron groups underwent endothelial injury of the iliac arteries and received a 2% cholesterol diet following AI. AI-mirabegron rats received mirabegron (10 mg/kg/day) orally for 8 weeks. The control group received a regular diet. After 8 weeks, urodynamic investigation was performed in awake animals. Pharmacological in vitro studies and histological examination of the iliac arteries and bladders were performed. The neointimal formation and luminal occlusion of the iliac arteries demonstrated by the ischemia in both AI and AI-mirabegron rats was not prevented by mirabegron treatment. Micturition interval (MI), bladder capacity (Bcap) and voided volume (VV) in the AI group were significantly less than in the control group (p < 0.01). In the AI-mirabegron group MI, Bcap and VV were significantly larger than in the AI group (p < 0.05), however, significantly less than in the control group (p < 0.05). Contractile responses of bladder strips to KCl, electrical field stimulation and carbachol were significantly lower after AI than in controls; responses in preparations from AI-mirabegron treated animals were similar to those of controls. The AI group showed a significantly higher percentage of bladder wall collagen (28.6 ± 1.57%) compared with the controls (8.65 ± 0.67%) and AI-mirabegron treated animals (17.2 ± 2.32%).
Even if the mirabegron dose used in this study may potentially limit the translational value of the results it seems as if in the chronically ischemic rat bladder, treatment with mirabegron protects bladder function and morphology, and if the results are valid for humans, they support β3-AR agonism as a potential treatment of chronic ischemia-related bladder dysfunction.
Summary
Chronic bladder ischemia secondary to atherosclerosis and vascular dysfunction may be an important pathogenetic factor in age-related bladder dysfunction. Oxidative stress, proinflammatory cytokines in the bladder, increased collagen deposition, endothelial dysfunction (decreased eNOS expression), decreased nNOS and increased iNOS, increases of stimulatory molecules in the urothelium and lamina propria and sensitization of afferent nerves may on the one hand lead to DO/OAB and, via denervation (sensory and motor) and decreased muscle contractility, to underactive bladder. Different therapeutic principles, which clinically have shown positive effects in the treatment of LUTS/OAB, seem to have protective effects in a rat model of chronic bladder ischemia. Although the doses of the drugs used are high compared to the doses used in humans and that this may increase the risk of producing effects not seen in humans, this model may have translational value, and may be of relevance for designing clinical studies to demonstrate if a drug may prevent progression of ischemia-related functional and morphological bladder changes.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
Contributor Information
Karl-Erik Andersson, AIAS, Aarhus Institute of Advanced Studies, Aarhus University, Høegh-Guldbergs Gade 6B, building 1632, 8000 Aarhus C, Denmark; Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston Salem, NC, USA.
Masanori Nomiya, Division of Bioengineering and LUTD Research, Nihon University College of Engineering, Koriyama, Japan.
Norifumi Sawada, Department of Urology, Interdisciplinary Graduate School of Medicine, University of Yamanashi, Chuo City, Yamanashi, Japan.
Osamu Yamaguchi, Division of Bioengineering and LUTD Research, Nihon University College of Engineering, Koriyama, Japan.
References
- Andersson K., Chapple C., Cardozo L., Cruz F., Gratzke C., Lee K., et al. (2013a) Pharmacological treatment of urinary incontinence. In: 5th International Consultation on Incontinence, February 2012. Paris: ICUD-EAU [Google Scholar]
- Andersson K., de Groat W., McVary K., Lue T., Maggi M., Roehrborn C., et al. (2011) Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn 30: 292–301 [DOI] [PubMed] [Google Scholar]
- Andersson K., Martin N., Nitti V. (2013b) Selective β3-adrenoceptor agonists for the treatment of overactive bladder. J Urol 190: 1173–1180 [DOI] [PubMed] [Google Scholar]
- Azadzoi K., Babayan R., Kozlowski R., Siroky M. (2003) Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol 170: 659–663 [DOI] [PubMed] [Google Scholar]
- Azadzoi K., Chen B., Radisavljevic Z., Siroky M. (2011) Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol 186: 2115–2122 [DOI] [PubMed] [Google Scholar]
- Azadzoi K., Radisavljevic Z., Golabek T., Yalla S., Siroky M. (2010) Oxidative modification of mitochondrial integrity and nerve fiber density in the ischemic overactive bladder. J Urol 183: 362–369 [DOI] [PubMed] [Google Scholar]
- Azadzoi K., Tarcan T., Kozlowski R., Krane R., Siroky M. (1999a) Overactivity and structural changes in the chronically ischemic bladder. J Urol 162: 1768–1778 [PubMed] [Google Scholar]
- Azadzoi K., Tarcan T., Siroky M., Krane R. (1999b) Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol 161:1626–1635 [PubMed] [Google Scholar]
- Banakhar M., Al-Shaiji T., Hassouna M. (2012) Pathophysiology of overactive bladder. Int Urogynecol J 23: 975–982 [DOI] [PubMed] [Google Scholar]
- Barendrecht M., Abrams P., Schumacher H., de la Rosette J., Michel M. (2008) Do alpha1-adrenoceptor antagonists improve lower urinary tract symptoms by reducing bladder outlet resistance? Neurourol Urodyn 27: 226–230 [DOI] [PubMed] [Google Scholar]
- Brading A., Greenland J., Mills I., McMurray G., Symes S. (1999) Blood supply to the bladder during filling. Scand J Urol Nephrol Suppl 201: 25–31 [PubMed] [Google Scholar]
- Chapple C., Cardozo L., Nitti V., Siddiqui E., Michel M. (2014) Mirabegron in overactive bladder: A review of efficacy, safety, and tolerability. Neurourol Urodyn 33: 17–30 [DOI] [PubMed] [Google Scholar]
- Dröge W. (2002) Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95 [DOI] [PubMed] [Google Scholar]
- Frazier E., Mathy M., Peters S., Michel M. (2005) Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J Pharmacol Exp Ther 313: 260–267 [DOI] [PubMed] [Google Scholar]
- Gacci M., Corona G., Salvi M., Vignozzi L., McVary K., Kaplan S., et al. (2012) A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 61: 994–1003 [DOI] [PubMed] [Google Scholar]
- Giuliano F., Ückert S., Maggi M., Birder L., Kissel J., Viktrup L. (2013) The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol 63: 506–516 [DOI] [PubMed] [Google Scholar]
- Goi Y., Tomiyama Y., Nomiya M., Sagawa K., Aikawa K., Yamaguchi O. (2013) Effects of silodosin, a selective α1A-adrenoceptor antagonist, on bladder blood flow and bladder function in a rat model of atherosclerosis induced chronic bladder ischemia without bladder outlet obstruction. J Urol 190: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla P., Gomez M., Hedlund P., Swärd K., Hellstrand P., Camello P., et al. (2007a) Effect of melatonin on age associated changes in Guinea pig bladder function. J Urol 177: 1558–1561 [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla P., Gomez M., Sward K., Hedlund P., Hellstrand P., Camello P., et al. (2008) Melatonin restores impaired contractility in aged guinea pig urinary bladder. J Pineal Res 44: 416–425 [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla P., Pozo M., Camello P. (2007b) Aging impairs neurogenic contraction in guinea pig urinary bladder: role of oxidative stress and melatonin. Am J Physiol Regul Integr Comp Physiol 293: R793–R803 [DOI] [PubMed] [Google Scholar]
- Greenland J., Brading A. (2001) The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol 165: 245–248 [DOI] [PubMed] [Google Scholar]
- Greenland J., Hvistendahl J., Andersen H., Jörgensen T., McMurray G., Cortina-Borja M., et al. (2000) The effect of bladder outlet obstruction on tissue oxygen tension and blood flow in the pig bladder. BJU Int 85: 1109–1114 [DOI] [PubMed] [Google Scholar]
- Herrera M., Mingorance C., Rodríguez-Rodríguez R., Alvarez de Sotomayor M. (2010) Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152 [DOI] [PubMed] [Google Scholar]
- Irwin D., Kopp Z., Agatep B., Milsom I., Abrams P. (2011) Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 108: 1132–1138 [DOI] [PubMed] [Google Scholar]
- Juan Y., Hydery T., Mannikarottu A., Kogan B., Schuler C., Leggett R., et al. (2008) Coenzyme Q10 protect against ischemia/reperfusion induced biochemical and functional changes in rabbit urinary bladder. Mol Cell Biochem 311: 73–80 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Oka M., Kyotani J., Oyama T., Matsumoto S., Kakizaki H. (2013) Effect of the phytotherapeutic agent eviprostat on the bladder in a rat model of bladder overdistension/emptying. Neurourol Urodyn 32: 1031–1037 [DOI] [PubMed] [Google Scholar]
- Kim J., Jang H., Bae J., Lee J. (2013) Effects of coenzyme Q10 on bladder dysfunction induced by chronic bladder ischemia in a rat model. J Urol 189: 2371–2376 [DOI] [PubMed] [Google Scholar]
- Kirpatovsky V., Plotnikov E., Mudraya I., Golovanov S., Drozhzheva V., Khromov R., et al. (2013) Role of oxidative stress and mitochondria in onset of urinary bladder dysfunction under acute urine retention. Biochemistry (Mosc) 78: 542–548 [DOI] [PubMed] [Google Scholar]
- Kozlowski R., Kershen R., Siroky M., Krane R., Azadzoi K. (2001) Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol 165: 1019–1026 [PubMed] [Google Scholar]
- Lepor H., Kazzazi A., Djavan B. (2012) α-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol 22: 7–15 [DOI] [PubMed] [Google Scholar]
- Martinez-Salamanca J., Carballido J., Eardley I., Giuliano F., Gratzke C., Rosen R., et al. (2011) Phosphodiesterase type 5 inhibitors in the management of non-neurogenic male lower urinary tract symptoms: critical analysis of current evidence. Eur Urol 60: 527–535 [DOI] [PubMed] [Google Scholar]
- Matsui T., Oka M., Fukui T., Tanaka M., Oyama T., Sagawa K., et al. (2012) Suppression of bladder overactivity and oxidative stress by the phytotherapeutic agent, Eviprostat, in a rat model of atherosclerosis-induced chronic bladder ischemia. Int J Urol 19: 669–675 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Hanai T., Shimizu N., Sugimoto K., Uemura H. (2010) Effect of edaravone on ischemia/reperfusion injury in rat urinary bladder - changes in smooth muscle cell phenotype and contractile function. Aktuelle Urol 41(Suppl. 1): S46–S49 [DOI] [PubMed] [Google Scholar]
- Meng E., Lin W., Lee W., Chuang Y. (2012) Pathophysiology of overactive bladder. LUTS 4(Suppl. 1): 38–55 [DOI] [PubMed] [Google Scholar]
- Meyer L., Brown J. (2012) Tamsulosin for voiding dysfunction in women. Int Urol Nephrol 44: 1649–1656 [DOI] [PubMed] [Google Scholar]
- Michel M. (2010) The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: alpha-blockers in the treatment of male voiding dysfunction - how do they work and why do they differ in tolerability? J Pharmacol Sci 112: 151–157 [DOI] [PubMed] [Google Scholar]
- Minor T., Akbar S., Tolba R. (1999) Preservation of livers from non-heart-beating donors: modulation of cAMP signal and organ viability by glucagon. Transplant Proc 1999. 31: 1068 [DOI] [PubMed] [Google Scholar]
- Mizuno H., Yamamoto T., Okutsu H., Ohtake A., Sasamata M., Matsukawa Y., et al. (2010) Effect of tamsulosin on bladder microcirculation in a rat ischemia-reperfusion model, evaluated by pencil lens charge-coupled device microscopy system. Urology 76: 1266, e1–5 [DOI] [PubMed] [Google Scholar]
- Nomiya M., Burmeister D., Sawada N., Campeau L., Zarifpour M., Keys T., et al. (2013a) Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J Urol 754–761 [DOI] [PubMed] [Google Scholar]
- Nomiya M., Burmeister D., Sawada N., Campeau L., Zarifpour M., Yamaguchi O., et al. (2013b) Effect of melatonin on chronic bladder-ischaemia-associated changes in rat bladder function. BJU Int 112: E221–E230 [DOI] [PubMed] [Google Scholar]
- Nomiya M., Sagawa K., Yazaki J., Takahashi N., Kushida N., Haga N., et al. (2012a) Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol Urodyn 31: 185–189 [DOI] [PubMed] [Google Scholar]
- Nomiya M., Yamaguchi O., Andersson K., Sagawa K., Aikawa K., Shishido K., et al. (2012b) The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn 31: 195–200 [DOI] [PubMed] [Google Scholar]
- Petkov G. (2011) Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinggera G., Mitterberger M., Pallwein L., Schuster A., Herwig R., Frauscher F., et al. (2008a) alpha-Blockers improve chronic ischaemia of the lower urinary tract in patients with lower urinary tract symptoms. BJU Int 101: 319–324 [DOI] [PubMed] [Google Scholar]
- Pinggera G., Mitterberger M., Steiner E., Pallwein L., Frauscher F., Aigner F., et al. (2008b) Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. BJU Int 102: 470–474 [DOI] [PubMed] [Google Scholar]
- Pinsky D., Oz M., Liao H., Morris S., Brett J., Sciacca R., et al. (1993) Restoration of the cAMP second messenger pathway enhances cardiac preservation for transplantation in a heterotopic rat model. J Clin Invest 92: 2994–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponholzer A., Temml C., Wehrberger C., Marszalek M., Madersbacher S. (2006) The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur Urol 50: 581–586 [DOI] [PubMed] [Google Scholar]
- Rahman N., Phonsombat S., Bochinski D., Carrion R., Nunes L., Lue T. (2007) An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int 100: 658–563 [DOI] [PubMed] [Google Scholar]
- Reiter R., Tan D., Osuna C., Gitto E. (2000) Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci 7: 444–458 [DOI] [PubMed] [Google Scholar]
- Riera M., Torras J., Cruzado J., Lloberas N., Liron J., Herrero I., et al. (2001) The enhancement of endogenous cAMP with pituitary adenylate cyclase-activating polypeptide protects rat kidney against ischemia through the modulation of inflammatory response. Transplantation 72: 1217–1223 [DOI] [PubMed] [Google Scholar]
- Robinson D., Cardozo L., Terpstra G., Bolodeoku J. and the Tamsulosin Study Group. (2007) A randomized double-blind placebo-controlled multicentre study to explore the efficacy and safety of tamsulosin and tolterodine in women with overactive bladder syndrome. BJU Int 100: 840–845 [DOI] [PubMed] [Google Scholar]
- Roosen A., Chapple C., Dmochowski R., Fowler C., Gratzke C., Roehrborn C., et al. (2009) A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol 56: 810–819 [DOI] [PubMed] [Google Scholar]
- Sagawa K., Aikawa K., Nomiya M., Ogawa S., Akaihata H., Takahashi N., et al. (2013) Impaired detrusor contractility in a rat model of chronic bladder ischemia. Urology 81: 1379, e9–e14 [DOI] [PubMed] [Google Scholar]
- Sanford M. (2013) Mirabegron: a review of its use in patients with overactive bladder syndrome. Drugs 73: 1213–1225 [DOI] [PubMed] [Google Scholar]
- Sawada N., Nomiya M., Hood B., Koslov D., Zarifpour M., Andersson K. (2013) Protective effect of a β3-adrenoceptor agonist on bladder function in a rat model of chronic bladder ischemia. Eur Urol 64: 664–671 [DOI] [PubMed] [Google Scholar]
- Schlegel N., Waschke J. (2013) cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res (Published online: 10 December 2013). [DOI] [PubMed] [Google Scholar]
- Sener G., Sehirli A., Paskaloglu K., Dulger G., Alican I. (2003) Melatonin treatment protects against ischemia/reperfusion-induced functional and biochemical changes in rat urinary bladder. J Pineal Res 34: 226–230 [DOI] [PubMed] [Google Scholar]
- Shenfeld O., Meir K., Yutkin V., Gofrit O., Landau E., Pode D. (2005) Do atherosclerosis and chronic bladder ischemia really play a role in detrusor dysfunction of old age? Urology 65: 181–184 [DOI] [PubMed] [Google Scholar]
- Shin J., Kim G., Song K., Na Y., Sul C., Lim J. (2014) Protective effect of N-acetylcysteine against ischemia/reperfusion injury in rat urinary bladders. Cell Biochem Funct 32: 24–30 [DOI] [PubMed] [Google Scholar]
- Soler R., Andersson K., Chancellor M., Chapple C., de Groat W., Drake M., et al. (2013) Future direction in pharmacotherapy for non-neurogenic male lower urinary tract symptoms. Eur Urol 64: 610–621 [DOI] [PubMed] [Google Scholar]
- Soler R., Füllhase C., Lu B., Bishop C., Andersson K. (2010) Bladder dysfunction in a new mutant mouse model with increased superoxide–lack of nitric oxide? J Urol 183: 780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H., Lee S., Park W., Park K., Park S., Kang M., et al. (2007) New unstable bladder model in hypercholesterolemia rats. Urology 69: 186–190 [DOI] [PubMed] [Google Scholar]
- Tarcan T., Azadzoi K., Siroky M., Goldstein I., Krane R. (1998) Age-related erectile and voiding dysfunction: the role of arterial insufficiency. Br J Urol 82(Suppl. 1): 26–33 [DOI] [PubMed] [Google Scholar]
- Yamaguchi O., Nomiya M., Andersson K. (2014) Functional consequences of chronic bladder ischemia. Neurourol Urodyn 33: 54–58 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Masunaga K., Nagata T., Satoji Y., Shiomi M. (2010) The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn 29: 1350–1354 [DOI] [PubMed] [Google Scholar]