Abstract
Background
New technologies could facilitate changes in lifestyle and improve public health. However, no large randomized, controlled studies providing scientific evidence of the benefits of their use have been made. The aims of this study are to develop and validate a smartphone application, and to evaluate the effect of adding this tool to a standardized intervention designed to improve adherence to the Mediterranean diet and to physical activity. An evaluation is also made of the effect of modifying habits upon vascular structure and function, and therefore on arterial aging.
Methods/Design
A randomized, double-blind, multicenter, parallel group clinical trial will be carried out. A total of 1215 subjects under 70 years of age from the EVIDENT trial will be included. Counseling common to both groups (control and intervention) will be provided on adaptation to the Mediterranean diet and on physical activity. The intervention group moreover will receive training on the use of a smartphone application designed to promote a healthy diet and increased physical activity, and will use the application for three months. The main study endpoints will be the changes in physical activity, assessed by accelerometer and the 7-day Physical Activity Recall (PAR) interview, and adaptation to the Mediterranean diet, as evaluated by an adherence questionnaire and a food frequency questionnaire (FFQ). Evaluation also will be made of vascular structure and function based on central arterial pressure, the radial augmentation index, pulse velocity, the cardio-ankle vascular index, and carotid intima-media thickness.
Discussion
Confirmation that the new technologies are useful for promoting healthier lifestyles and that their effects are beneficial in terms of arterial aging will have important clinical implications, and may contribute to generalize their application in favor of improved population health.
Trial registration
Clinical Trials.gov Identifier: NCT02016014
Keywords: Physical activity, Food, Information and communication technologies, Arterial aging
Background
Benefits of physical activity
There is abundant scientific evidence of the biological and psychological benefits of physical activity [1]. On the other hand, low cardiopulmonary fitness is associated with an increased risk of cardiovascular morbidity-mortality, while improved physical fitness is associated with a decrease in mortality risk [2]. The results of a metaanalysis on the influence of physical activity upon blood pressure indicate a mean reduction of 3.8 mmHg in systolic blood pressure (SBP) and 2.6 mmHg in diastolic blood pressure (DBP) [3]. Aerobic exercise could attenuate arterial hardening as observed in the Baltimore Longitudinal Study, in which male athletes had a lesser pulse wave velocity (PWV), augmentation index (AIx) and SBP than sedentary individuals [4]. Improvement in carotid artery elasticity has also been recorded in previously sedentary subjects who started a physical exercise program [5]. Aerobic exercise has been related to a decrease in atherosclerosis progression in humans [6]. The results of the first phase of the EVIDENT study [7] have demonstrated an association between physical exercise and improved vascular structure and function [8]. An association has also been reported between the time people spend seated and mortality. However, the results are even more relevant on combining the time spent seated (6 hours a day) with low physical activity (< 24.5 METS/hours/week), yielding a relative risk (RR) of 1.94 (95% confidence interval (95% CI): 1.70-2.20) for women and 1.48 (95% CI: 1.33-1.65) for males, as opposed to those who spend less time seated and have greater activity [9]. The EVIDENT study has also found increased arterial stiffness to be related to the number of hours spent watching television [10]. On the other hand, physical activity shows an inverse correlation to the concentrations of fibrinogen [11].
Interventions for increasing physical activity
Although there is abundant evidence of the benefits of physical activity, the percentage of individuals who may be considered active is low in our setting [12]. In the United Kingdom, the prevalence of sedentarism reaches 60% in males and 74% in females [13], while in Spain the PEPAF (Experimental Program for Physical Activity Promotion) recorded a 75% sedentarism rate [14]. Different methods have been used in an attempt to promote physical activity, though with conflicting results. A Cochrane review concluded that there are no data to support the hypothesis that multiple-component community-based procedures are effective in increasing the physical activity of the population [15]. A recent metaanalysis of studies in the primary care setting has concluded that the promotion of physical activity in sedentary subjects increases the number of individuals who are classified as active subjects after 12 months (odds ratio (OR): 1.42; 95% CI: 1.17-1.73), though the effect has not been demonstrated over the long term [16,17]. The PEPAF study intervention resulted in an increase in physical activity versus the controls of 18 min./week (95% CI: 6–31 min./week), with a rise in METS/hours/week of 1.3 [95% CI: 0.4-2.2]. The proportion of individuals reaching the recommended minimum physical activity was 3.9% greater in the intervention group (1.2% to 6.9%) than in the control series. Few randomized, controlled clinical trials have evaluated the impact of interventions in terms of a decrease in sitting time, though an intervention designed to promote walking while working was able to reduce the daily sitting time by 21 minutes [18]. Evidence is even more limited in populations with chronic diseases, though an intervention in type 2 diabetics has recorded a reduction in daily sitting time of 23 minutes [19].
Benefits of the Mediterranean diet
The Mediterranean diet is presently regarded as the model of most healthy diet. It reflects the traditional eating habits of countries such as Greece, southern Italy and Spain. The main characteristics of this diet are: a) important consumption of cereals, legumes, nuts, fruit and vegetables; b) use of olive oil for cooking purposes and for the dressing of salads and vegetables (representing the main source of fat in the form of monounsaturated fatty acids); c) moderate to high consumption of fish; d) moderate to low consumption of chicken and dairy products; e) low consumption of red meats and meat products; and f) moderate alcohol consumption, mainly in the form of red wine with meals [20]. There is convincing evidence that the Mediterranean diet may have a positive effect upon endothelial function [21] and can lower peripheral blood pressure [22]. A correlation has also been established with vascular structure, as assessed by carotid intima-media thickness [23], as well as with decreased mortality due to any cause [24] and, more specifically, due to coronary disease [25]. The results of the EVIDENT I study found obese males to show the poorest adherence [26].
Interventions for improving eating habits (Mediterranean diet)
Few intervention studies with the Mediterranean diet have been carried out. A randomized clinical trial (the Lyon Diet Heart Study) [27], involving a modified model of the Mediterranean diet versus a control diet, concluded that the Mediterranean diet is associated with a marked reduction in cardiovascular mortality and in the incidence of cardiovascular complications among patients who have suffered myocardial infarction. However, prior to publication of the PREDIMED (Prevention with Mediterranean Diet) study [22], no randomized clinical trial had investigated whether the effects of the Mediterranean diet are superior to those of a low-fat diet in relation to the primary prevention of cardiovascular diseases. The PREDIMED study [22] was designed to determine whether the Mediterranean diet supplemented with virgin olive oil or nuts prevents the development of major cardiovascular complications in high vascular risk individuals, compared with a low-fat diet. In its initial phase, this study recorded an average increase of 1.8 points on the 14-point scale assessing adherence to the Mediterranean diet, versus an increase of 0.3 points in the control group [28]. During the first year, this implied a decrease in the prevalence of metabolic syndrome and its components and in the incidence of diabetes mellitus [28,29]. Furthermore, after four years of follow-up, the subjects in the intervention groups (Mediterranean diet) showed a 30% decrease in the incidence of major cardiovascular complications versus the control series [30].
Evaluation of arterial aging
Aging is associated with increased arterial stiffness, which can be evaluated with different clinical and biological tools. The accepted gold standard for assessing arterial stiffness is the carotid-femoral pulse wave velocity (PWV) [31], which has been correlated to increased morbidity-mortality in both patients with cardiovascular disease and in healthy individuals [32]. Central arterial pressure has also been shown to be more strongly correlated to cardiovascular morbidity and mortality than peripheral blood pressure [33]. The central and peripheral augmentation indexes are markers of arterial stiffness which together with aortic systolic pressure and aortic pulse pressure complement the information afforded by pulse wave velocity. The cardio-ankle vascular index (CAVI) also evaluates arterial stiffness, and is able to estimate the risk of atherosclerosis and vascular age. This parameter moreover is independent of arterial pressure [34,35].
Information technology as a support for improving health
Considering the trend in recent years in the use of mobile telephones, and the recent introduction of smartphones, it can be estimated that within 10 years, a full 80-90% of the population in developed countries will have one of these devices [36], and probably with many more applications than are available today. Information technology is finding many applications in the field of health and Medicine. Recently, short message service (SMS) communication has demonstrated its usefulness in helping people to stop smoking [37]. Smartphone applications designed to assist healthcare personnel both in training and in decision making processes in daily clinical practice and in emergency care are increasing, and there is already some evidence of their usefulness [38]. Such applications have also been used in patient care for monitoring biological parameters [39], detecting falls in the elderly [40], preventing cognitive impairment [41] and monitoring diabetes [42], as well as in cardiac rehabilitation [43] and also in the promotion of physical activity [44] and the management of [45]. Communication based on the use of mobile phones, and especially smartphones, has a strong potential to transform healthcare and clinical interventions in the community [36]. However, its effectiveness needs to be evaluated in multicenter clinical trials, as recommended by the United States Food and Drug Administration (FDA), in a way similarly to what is done with drug substances. A metaanalysis of the use of information technology in dietetic evaluation has concluded that it could improve dietary assessment in some population groups, though improved validity and reliability is required in evaluating micronutrients [46]. Many applications have been developed in the field of health, and specifically in relation to diet and exercise, destined for use by the general population. Although the acceptance and ease of use of such applications has been evaluated among young adults for the registry of eating habits and physical activity [47], they have not been tested in other population types, and little is known of their effectiveness in relation to health outcomes. Few studies have validated these tools, though some investigations are presently in course [48]. Even less work has been done to explore the impact of such applications in relation to increased physical activity and improved eating habits, and hence in terms of improvement of the health of the people who use them [49].
The present study aims to provide evidence of the effect which the new information and communication technologies, and specifically smartphone applications, could have as tools supporting changes in favor of more healthy lifestyles. An evaluation is also made of the effect of such changes upon arterial aging.
Objectives
The first objective of this study is to develop and validate a smartphone application supporting standardized counseling to increase physical activity and adapt eating habits to the recommendations of the Mediterranean diet. The second and central objective of the study is to quantify the effect attributable to the developed smartphone application in terms of the modification of habits, adherence to the Mediterranean diet, and increased physical activity. Lastly, an evaluation is made of the effect of the modification of habits upon vascular structure and function, and therefore on arterial aging.
Methods/Design
Design and setting
This is a randomized, multicenter, double-blind clinical trial involving two parallel groups, designed to evaluate the possible effects of adding an information and communication tool (intervention) in support of behavioral and educational counseling (control) to promote increased physical activity and adaptation of eating habits to the Mediterranean diet.
The study will include 6 groups of the Research Network on Preventive Activities and Health Promotion (REDIAPP) in Bilbao, Cuenca, Zaragoza, Valladolid, Barcelona and Salamanca (Spain), as a continuation of the EVIDENT project developed in 2010–2012.
Subjects
Study population
The study population will be selected from the EVIDENT project [7], comprising 1,553 subjects randomly selected in the primary care setting. The study will exclude patients over 70 years of age (due to difficulties in using information and communication tools among such individuals), those unable to do exercise or follow the Mediterranean diet, as well as those subjects who meet any of the exclusion criteria of the EVIDENT project (known coronary or cerebrovascular atherosclerotic disease; heart failure; moderate or severe chronic obstructive pulmonary disease; musculoskeletal disease that limited walking; advanced respiratory, renal, or hepatic disease; severe mental disease; treated oncological disease diagnosed in the 5 years) [7]. The rest of the subjects will be randomised on a centralized basis from Salamanca using the Epidat 4.0 software package to intervention (IG) and control groups (CG) with a ratio of 1/1. Of the 1553 subjects, it is estimated that 10-15% will be excluded; the resulting sample therefore will comprise 1,350 individuals. We estimate that another 10% will reject participation, i.e., we expect to include at least 1,215 subjects in the final sample.
Sample size
Estimation of sample size has been made for the main study endpoints. Regarding physical exercise, and assuming α = 0.05 and β = 0.10, with a standard deviation (SD) of 154, we would need 1,110 subjects (555 per group) to detect an increase of 30 counts/minute in IG versus CG. In turn, regarding the Mediterranean diet (MD), and assuming α = 0.05 and β = 0.10, with a standard deviation (SD) of 2, we would need 676 subjects (338 per group) to detect an increase of 0.5 points in the MD questionnaire in IG versus CG. The inclusion of 1,215 subjects is considered sufficient to detect clinically relevant differences in the main study endpoints, assuming the cluster effect of the design.
Variables and measurement instruments
The general and potentially effect-modifying variables, such as age, gender, occupation, smoking, alcohol consumption, personal history and drug use will be documented.
Mobile phone tool for evaluating healthy lifestyles
The developed tool is the result of an agreement between the company CGB and the GIAPCyL research group of the REDIAPP (RD12/0005/0004), through the Infosalud Fundation (Figures 1 and 2). The tool is a smartphone application with a user-friendly environment that is easy for adults to handle. It can be used to quickly evaluate the adaptation of living habits to healthy lifestyle recommendations referred to both eating and physical activity. An evaluation will be made of the quantity and quality of food intake according to standardized references, with the purpose of assessing adaptation of the eating habits of the individual to the Mediterranean diet. Based on adequate proportions of primary food elements, a personalized recommendation will be produced, depending on the entered intake characteristics. The application will generate detailed information on the eating deviations (diet composition and calories), with a view to facilitating changes in habits. Physical activity also will be evaluated, using an accelerometer included in the smartphone application, and which will count the steps taken over a period of 24 hours. The activity reported by the subject during periods in which the device is not used (e.g., swimming and other sports) also will be considered, assessing compliance with the exercise objectives and providing recommendations to increase activity and reach the target of 10,000 steps a day defining an active individual. A balance of activity and diet compliance will be made at the end of the day, and specific recommendations will be produced for the next day/days. The information will be stored in the device and will be downloaded on occasion of the control visits for subsequent analysis.
Figure 1.
Smartphone application (APP) EVIDENT II, main screen and physical activity.
Figure 2.
Smartphone application (APP) EVIDENT II, nutrition screens.
Physical activity
Physical activity will be measured by an accelerometer and by the 7-day physical activity recall (PAR). ActiGraph GT3X accelerometers (ActiGraph, Shalimar, FL, USA) will be used to evaluate principal endpoint of psychical activity, which have been previously validated [50-52]. ActiGraph is a monitor that uses a piezoelectric acceleration sensor to filter and convert the signals produced from the sensor in samples collected at a preset frequency in hertz. The samples are summed over a user-specified time sampling interval, called an “epoch”. Output from the ActiGraph is in the form of activity “counts,” where one count is equivalent to 16 milli-g per second, and where g is equal to 9.825 m · s - 2, the acceleration of gravity. Activity “counts” are recorded to the internal memory of accelerometers by converting acceleration units over a given epoch [53]. Subjects will wear the accelerometer fastened with an elastic strap to the right side of the waist for seven consecutive days with habitual physical activity, except for bathing and performing activities in the water. All subjects will be verbally instructed on how to use the accelerometer. The accelerometer will be set to record physical activity data every minute. The MAHUFFE software, available from: http://www.mrc-epid.cam.ac.uk/research/resources/materials-transfer-disclaimer/physical-activity-downloads/ (accessed: 30/12/2013), will be used to analyze the data. Sequences of 10 or more consecutive zero counts will be considered non-wearing time and excluded from the analyses. Inclusion criteria will be a minimum of 4 days of recording, including at least 1 weekend day and at least 600 registered minutes per day. The main outcome variable from the activity monitor will be the average intensity of physical activity (counts/minute), calculated with equal weighting given to each day (regardless of registered time per day). The intensity of physical activity will be determined according to the cut-off points proposed by Freedson [54], sedentary (<100 counts/minute), light (100–1952 counts/minute), moderate (1952–5724 counts/minute) vigorous (>5724 counts/minute) and very vigorous (>9498 counts/minute). Moderate-vigorous activity will be considered as activity accumulated from all bouts lasting at least 1 min.
The 7-day PAR is a general measure of physical activity, which has been recognized as valid and reliable tool in recent years and is widely used in epidemiological, clinical and behavior change studies. It consists of a semi-structured interview (10-15 minutes) in which participants provide an estimate of the number of hours dedicated to physical or occupational activities requiring at least a moderate effort in the past seven days. The categories collected are moderate, vigorous, and very vigorous physical activity. The amount of time dedicated to each activity is multiplied by the mean metabolic equivalents (METs) of each category: light activity 1.5, moderate 4, vigorous 6, and very vigorous 10. The sum of the products of the hours dedicated to each activity and its estimated mean energy expenditure (MET) provides an estimation of the kilocalories per kilogram used per day (kcal*kg-1 * d-1). The dose of physical exercise will be estimated in METs/hour/week and active persons were considered as those doing at least 30 minutes of moderate activity, five days a week, or at least 20 minutes of vigorous activity, 3 days a week or 450 MET · min · wk-1 [12]. Persons not reaching this level of physical activity were considered sedentary.
Questionnaire hours seated (Marshall): Evaluates the hours that the individual is sitting, in their work, in the displacements and at home, during the week and the weekend [55].
Nutrition
Adherence to the Mediterranean diet, principal endpoint of alimentation, will be measured using the validated 14-point Mediterranean Diet Adherence Screener (MEDAS) [56], developed by the PREDIMED study group. The MEDAS is a valid instrument for rapid estimation of adherence to the Mediterranean diet and may be useful in clinical practice. The 14-item screener includes 12 questions on food consumption frequency and two questions on food intake habits considered characteristic of the Spanish Mediterranean diet. Each question will be scored as 0 or 1. One point will be give for using olive oil as the principal source of fat for cooking, preferring white meat over red meat, or for consuming: 1) 4 or more tablespoons (1 tablespoon = 13.5 g) of olive oil/d; 2) 2 or more servings of vegetables/d; 3) 3 or more pieces of fruit/d; 4) < 1 serving of red meat or sausages/d; 5) < 1 serving of animal fat/d; 6) < 1 cup (1 cup = 100 ml) of sugar-sweetened beverages/d; 7) 7 or more servings of red wine/wk; 8) 3 or more servings of pulses/wk; 9) 3 or more servings of fish/wk; 10) fewer than 2 commercial pastries/wk; 11) 3 or more servings of nuts/wk; or 12) 2 or more servings/wk of a dish with a traditional sauce of tomatoes, garlic and onion. The end score will range from 0 to 14. Adequate adherence to the Mediterranean diet will be assumed when the total score is above or equal to 9 points [56].
The dietary habits of participants and information about dairy products were assessed using a semi-quantitative 137-item food-frequency questionnaire previously validated in Spain [57]. The questionnaire was based on the typical portion sizes that were multiplied by the consumption frequency for each food. This estimated frequency corresponds to the previous year at the time of the interview and it is divided into 9 intake frequencies ranging from never to more than 6 servings/day. This will be used to estimate daily energy intake, essential nutrients, vitamins, fiber, antioxidants and other nutrients.
Laboratory determinations
Venous blood sampling will be performed between 08:00 and 09:00 hours after the individuals fasted and abstained from smoking and the consumption of alcohol and caffeinated beverages for the previous 12 hours. Fasting plasma glucose, creatinine, uric acid, serum total cholesterol, HDL-cholesterol and triglyceride concentrations will be measured using standard enzymatic automated methods. LDL cholesterol will be estimated by the Friedewald equation when the direct parameter will be not available. Glycated haemoglobin will be measured with an immune-turbidimetric assay. High sensitive C-reactive protein levels and fibrinogen concentrations will be determined by immunoturbidimetric assay. Blood samples will be collected in the respective health centers, and all will be analyzed at the hospital of the city participating in external quality assurance programs of the Spanish Society of Clinical Chemistry and Molecular Pathology.
Anthropometric measurements
Body weight will be determined on two occasions using a homologated electronic scale (Seca 770; Medical scale and measurement systems, Birmingham, United Kingdom) following due calibration (precision ± 0.1 kg), with the patient wearing light clothing and shoeless. These readings will be rounded to 100 g. Height in turn will be measured with a portable system (Seca 222; Medical scale and measurement systems, Birmingham, United Kingdom), recording the average of two readings, and with the patient shoeless in the standing position. The values will be rounded to the closest centimeter. Body mass index (BMI) will be calculated as weight (kg) divided by height squared (m2). A value of > 30 kg/m2 will be taken to define obesity. Waist circumference will be measured using a flexible graduated measuring tape with the patient in the standing position without clothing. The upper border of the iliac crests will be located, and the tape will be wrapped around above this point, parallel to the floor, ensuring that it will be adjusted without compressing the skin. Adiposity indices, waist-height and waist-hip, will also be calculated.
Office or clinical blood pressure
Office blood pressure measurement will involve three measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP), using the average of the last two, with a validated OMRON model M10-IT sphygmomanometer (Omron Health Care, Kyoto, Japan), by following the recommendations of the European Society of Hypertension [58]. Pulse pressure will be estimated with the mean values of the second and third measurements.
Central blood pressure and radial augmentation index
Central blood pressure (CBP) and radial augmentation index (rAIx) will be measured with Pulse Wave Application Software (B-pro (A-Pulse)) (Health STATS International) using tonometry to capture the radial pulse and by an equation to estimate central blood pressure (CASP). This device has been validated in asian hypertensives and healthy caucasians [59,60]. rAIx is a measurement taken directly from the late systolic shoulder of the peripheral arterial waveform, and is defined as the ratio of the difference between the 2nd peak and diastolic pressure to the difference between the 1st peak and diastolic pressure [61], it is age-dependent and could be a useful index of vascular aging [62]. rAIx will be calculated as follows: (Second peak systolic blood pressure [SBP2] - diastolic blood pressure [DBP])/(first peak SBP - DBP) × 100 (%) and it will be adjusted for heart rate at 75 bpm and it will be reported as rAIx75. Specific aspects regarding the validity and reliability of the measurement of central blood pressure and augmentation index have been reported elsewhere [60]. In brief, the intra-observer reliability was evaluated in 20 subjects by using the intraclass correlation coefficient (ICC), that showed values of r = 0.971 (95% CI: 0.923 to 0.989) for CASP and 0.952 (95% CI: 0.871 to 0.982) for rAIx. According to the Bland-Altman analysis, the mean difference for intraobserver agreement (95% limits of agreement) were -0.056 (-9.41 to 9.30) for CASP and 2.50 (-14.43 and 19.46) for rAIx. In 104 subjects we examined the agreement of measurements with SphygmoCor and found an ICC for CASP r = 0.972 (95%CI 0.959 to 0.981) and for rAIx r = 0.599 (95% CI: 0.409 to 0.728). In Bland–Altman analysis the mean difference for intraobserver agreement (95% limits of agreement) were 1.47 (0.47 to 2.47) in CASP and 5.85 (1.75 to 9.96) in rAIx.
Assessment of vascular structure by carotid intima media thickness (IMT)
Carotid ultrasound to assess C-IMT will be performed by two investigators trained for this purpose before starting the study. A Sonosite Micromax ultrasound device paired with a 5–10 MHz multi-frequency high-resolution linear transducer with Sonocal software will be used for performing automatic measurements of carotid IMT in order to optimize reproducibility. Measurements will be made of the common carotid after the examination of a 10 mm longitudinal section at a distance of 1 cm from the bifurcation, performing measurements in the proximal and in the distal wall in the lateral, anterior and posterior projections, following an axis perpendicular to the artery to discriminate two lines, one for the intima-blood interface and the other for the media-adventitious interface. A total of 6 measurements will be obtained of the right carotid and other 6 of the left carotid, using average values (average carotid IMT) and maximum values (maximum carotid IMT) automatically calculated by the software [63]. The measurements will be obtained with the subject lying down, with the head extended and slightly turned opposite to the examined carotid artery. The reliability was evaluated before the study began using the intraclass correlation coefficient, which showed values of 0.974 (95% CI: 0.935 to 0.990) for intra-observer agreement on repeated measurements in 20 subjects, and 0.897 (95% CI:0.740 to 0.959) for inter-observer agreement. According to the Bland-Altman analysis, the mean difference for intraobserver agreement (95% limits of agreement) was 0.022 (95% CI: -0.053 to 0.098) and intra-observer agreement was 0.012 (95% CI: -0.034 to 0.059). The average IMT will be considered abnormal if it measured > 0.90 mm, or if there will be atherosclerotic plaques with a diameter of 1.5 mm or a focal increase of 0.5 mm or 50% of the adjacent IMT [64].
Cardio-ankle vascular index and ankle-brachial index
Cardio-Ankle Vascular Index (CAVI) and Ankle-brachial index (ABI) will be measured using Vasera device VS-1500® (Fukuda Denshi). For the study, the lowest ankle-brachial index obtained will be considered. The pulse wave velocity (PWV) will be calculated, as well as Cardio-Ankle Vascular Index (CAVI), which gives a more accurate calculation of the atherosclerosis degree. CAVI integrates cardiovascular elasticity derived from the aorta to the ankle pulse velocity through an oscillometric method and it is used as a good measure of vascular stiffness and it doesn’t depend on blood pressure [35]. CAVI values will be automatically calculated by substituting the stiffness parameter β in the following equation to detect the vascular elasticity and the cardio-ankle PWV: Stiffness parameter β = 2ρ x 1/(Ps –Pd) x ln (Ps/Pd) x PWV2, where ρ is the blood density, Ps and Pd are SBP and DBP in mmHg, and the PWV is measured between the aortic valve and ankle. The average coefficient of the variation of the CAVI is less than 5%, which is small enough for clinical use and confirm that CAVI has favorable reproducibility [34]. Cardio-Ankle Vascular Index and Ankle-brachial index will be measured at rest.
Analysis of motivation to change
The model of Prochaska and Diclemente will be used [65]. The motivation stage will be classified on occasion of each interview, based on the following criteria: a) Pre-contemplation: The subjects are unaware that certain behaviors place their health at risk or that they have a health problem; alternatively, they are aware of the existence of a health problem but are reluctant to accept changes in behavior. b) Contemplation: The subjects are aware that certain behaviors place their health at risk or that they have a health problem, and agree to introduce changes within 6 months. c) Determination: The subjects seriously intend to change their behaviour in the near future (within 30 days). d) Action: The subjects are actively working upon changes in behavior that affect health or the identified health problem. e) Maintenance: The subjects routinely adopt the acquired behaviors. Maintenance is considered to have been reached when the new behavior persists for over 6 months. f) Relapse: The subjects start the cycle over again, i.e., they no longer adhere to the desired behavior.
Adherence to the tool
Adherence will be assessed by means of the Morisky-Green test [66], and by assessing the number and days of recordings in the device.
Intervention
Common to both groups
Counseling on physical activity Both groups (control and intervention) will receive counseling on physical activity with a view to favoring compliance with the current recommendations on physical activity in the general population. The intervention has demonstrated its effectiveness in the PEPAF study [14] and in the Prescribe Vida Saludable (PSV) program (Osakidetza). Counseling will consist of an individual visit lasting 15 minutes in which an explanation will be given of the health benefits of physical activity, with the recommendation to perform at least 30 minutes of moderate activity 5 days a week, or 20 minutes of vigorous activity three days a week. Counseling will be standardized in both groups, and the subjects will receive an informational brochure (Additional file 1) on the session. The first part (5 min.) will address the recommendations on physical exercise in relation to cardiovascular health. The second part (8 min.) will develop knowledge of the intensity of some specific physical activities such as walking, riding a bicycle, or other activities. The last part (2 min.) will be dedicated to answering questions and doubts. Specific advice will be given to reduce the number of hours spent sitting.
Nutritional counseling Both groups (control and intervention) will receive nutrition counseling aimed at favoring adherence to the MD. This intervention has been shown to be effective in the PREDIMED study [22]. Counseling will consist of an individual visit lasting 15 minutes in which the concepts of the MD will be explained, with insistence upon the importance of complying with each of the recommended points. Counseling will be standardized in both groups, and the subjects will receive an informational brochure (Additional file 1) on the session. The first part (3 min.) will develop the concept of the MD. The second part of the session (10 min.) will focus on each particular recommendation, with brief and clear messages. The last part of the interview (2 min.) will be dedicated to answering questions and doubts.
Specific of the intervention group
The subjects in the IG will receive a smartphone for three months, corresponding to the intervention period. A first visit lasting 15 minutes will be used to provide training in the use of the device, which should be employed daily for the full three-month period of the intervention. The investigator will instruct the participants on the use of the tool that evaluates food intake, on how to enter the information and receive the recommendations, and on how to use of the accelerometer and read the generated information – with the recommendation to reach a total of 10,000 daily steps. The subjects will be required to daily enter food intake (breakfast, lunch, afternoon snack, and dinner), selecting the dishes and foods from the application menu. Regular physical activity will be recorded with the accelerometer of the device, together with due registry of those activities performed without the smartphone (swimming, football, etc.). Lastly, the final daily summary will be reviewed, with a balance of food intake and physical activity, and the device will offer a recommended plan for the next days, with a view to improving eating habits and increasing physical activity. A new visit will be held one week after supplying the device, in order to confirm that it is being used correctly, and to clarify any possible doubts. The smartphone will be returned after three months, coinciding with the common review visit.
Blinding strategy
The investigator performing randomization and intervention in the IG will be different from the investigator conducting evaluation and the rest of interventions, blinding will be maintained during the study, and the investigator performing data analysis also will be blinded. Due to the nature of the study, the subjects cannot be blinded to the intervention.
Project schedule
The project is planned as three yearly periods with subsequent follow-up to evaluate the long-term effects of the intervention. In a previous phase conducted in 2013, the software for the smartphones was developed by the company CGB, and a prior pilot evaluation was carried out in Salamanca among users of both sexes with different ages and cultural levels, to confirm the practical feasibility of the tool and introduce any necessary changes. In the present project, pilot evaluation will be made in 2014–2015, along with recruitment of all the subjects and conduction of the first visits, while in 2015–2016 we will complete the follow-up visits (12 months) – with conclusion of the operative phase of the project on 31 December 2016.
The flow chart (Figure 3) shows the phases of the clinical trial.
Figure 3.
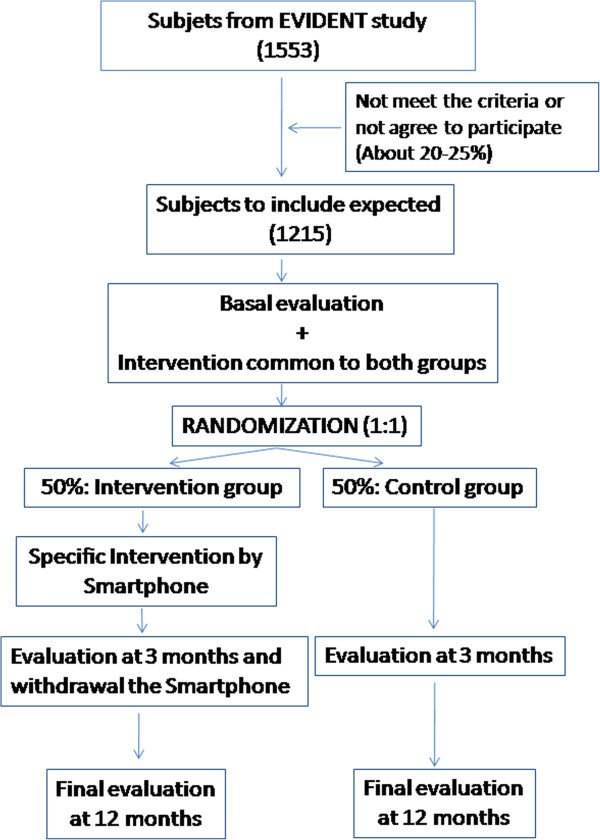
Flow - chart of EVIDENT II clinical trial.
Statistical analysis
The results will be expressed as the mean ± standard deviation for quantitative variables or using the frequency distribution for qualitative variables. Analysis of the results will be made on an intent-to-treat (ITT) basis. Statistical normality will be tested using the Kolmogorov-Smirnov test. Use will be made of the chi-squared test to analyze the association between independent qualitative variables, along with the McNemar test for paired samples. The Student t-test will be used for the comparison of means between two groups, and the paired t-test will be applied to assess changes within one same group. Alternatively, the corresponding nonparametric tests will be used, as required. The relationship between quantitative variables will be analyzed using Pearson’s correlation coefficient or Spearman coefficient in the case of asymmetrically distributed variables. Multivariate linear regression analysis and logistic regression analysis will be used to analyze the variables determining the changes in physical activity and eating habits, and in the arterial stiffness parameters (PWV, AIx and CAVI). In order to analyze the effect of the intervention, comparison will be made of the changes observed in the control group (CG) versus the intervention group (IG), with estimation of the Cohen d statistic, adjusting for the variables that may influence the results. Logistic regression will analyze the odds ratio (OR) for achieving the objectives of diet and exercise compliance. A multilevel analysis will be performed to determine the effect of the different recruitment centers. Gender will be contemplated in the analysis to assess differences between males and females in terms of adherence to the smartphone application, and in the middle- and long-term results. Likewise, an analysis will be made according to the degree of motivation at baseline assessment and adherence to use of the smartphone. The effect of the intervention could be modified by age, gender, cultural and socioeconomic level, body mass index (BMI) and certain disease conditions, as well as by the baseline lifestyles, which will be controlled in the analysis. The contrasting of hypotheses establishes α = 0.05. The IBM-SPSS version 20.0 statistical package will be used throughout.
Quality control
In order to ensure data quality, the nursing professionals in charge of data collection will receive specific training. Regular external monitoring will then be performed in the six health centers to verify adequate application of methods, both in performing the different examinations and collecting the information.
Methodological limitations
The study follows all the recommendations of the CONSORT, though in view of the nature of the intervention, the participating subjects will not be blinded to the intervention. Since we are dealing with lifestyle modifications, the analysis of the main results referred to the Mediterranean diet is based on self-declared data, even though use will be made of validated tools and the general food questionnaire, which may serve as quality control. In any case, objective data will be available in the case of physical exercise (accelerometer). Difficulties in using the application may increase the number of dropouts in the intervention group.
Ethical and legal issues
The study has been approved by the clinical research ethics committee (CEIC) of the healthcare area of Salamanca (“CEIC of Area de Salud de Salamanca”, 21 June 2013), as coordinating center, and by the ethics committees of the collaborating centers (“CEIC of Aragón (CEICA), CEIC of IDIAP Jordi Gol, CEIC of Euskadi (CEIC-E), CEIC of the Area integrada de salud de Talavera de la Reina and CEIC of the Área de Salud de Valladolid Oeste”). Subjects will be required to sign the informed consent prior to inclusion in the study, in accordance with the Declaration of Helsinki [67]. Subjects will be informed of the objectives of the project and of the risks and benefits of the examinations made. None of the examinations pose life-threatening risks for the type of subjects to be included in the study. The study includes the obtainment of biological samples; the study subjects therefore will be informed in detail. The confidentiality of the recruited subjects will be ensured at all times in accordance with the provisions of current legislation on personal data protection (15/1999 of 13 December, LOPD), and the conditions contemplated by Act 14/2007 on biomedical research.
Discussion
The exponential development of smartphone applications in the field of healthcare has not been accompanied by sufficient scientific evidence of the usefulness of such applications in improving the health of the population. As has already been called upon by different organisms, and more specifically by the FDA, it seems necessary to conduct experimental studies similar to those used in pharmacological treatments, in order to obtain sufficient scientific evidence of the possible usefulness of these tools.
The EVIDENT I study obtained evidence of the association between healthy lifestyles - particularly physical activity and diet – and vascular structure and function. Specifically, less physical activity or prolonged sedentarism was associated to greater aging of the vascular system [10,68].
The EVIDENT II study developed a smartphone application that has already been tested in a prior pilot phase designed to assess its reliability in the recording and analysis of diet and physical activity, as well as in relation to the counseling needed to improve these parameters and come closer to the international recommendations referred to the compliance with the Mediterranean diet and physical activity equivalent to at least 10,000 steps a day.
With this project we hope to demonstrate that regular use of this application for at least three months is able to modify certain habits, resulting in eating habits more in line with the Mediterranean diet, which has already demonstrated its benefits in terms of cardiovascular morbidity-mortality [30]. Likewise, we hope to increase the physical activity of the participants and to raise the proportion of individuals considered to be active, as this should imply multiple improvements in health – as has already been demonstrated in different studies [1-3].
Although over the short term it will be difficult to demonstrate the health benefits of the changes in diet and physical activity, over the middle term we hope to demonstrate that the use of this tool can not only improve living habits but also afford benefits in terms of vascular function - improving the parameters that assess arterial stiffness, such as AIx, CAVI and PWV.
Therefore, the results of this study could lead to a strategy based on the use of new technologies to delay arterial aging, combining increased physical activity and eating habits adapted to the Mediterranean diet. This strategy based on the incorporation of new technologies that are widely disseminated among the population, would result in healthy lifestyles and could constitute a powerful tool against vascular aging.
Abbreviations
ABI: Ankle-brachial index; Aix: Augmentation index; BMI: Body mass index; CAVI: Cardio-ankle vascular index; CIT: Communication and information technology; DBP: Diastolic blood pressure; PEPAF: Experimental program for Physical Activity Promotion; ESH: European Society of Hypertension; IMT: Intima-media thickness; EVIDENT: Lifestyles and vascular aging; MET: Metabolic equivalent; PREDIMED: Prevention by Mediterranean diet; PWV: Pulse wave velocity; PAR: Physical activity recall; REDIAPP: Research Network on Preventive Activities and Health Promotion; SBP: Systolic blood pressure.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conception of the idea for the study: LG-O and JIR-R. Development of the protocol, organization and funding: LG-O, JIR-R, MÁG-M, JAER-S, JAM-F, DP-A, CM-C, AG-A, NGonzález-Viejo, EI-SN and YS. Writing of the manuscript: JIR-R, LG-O and MAG-M. All the authors have read the draft critically, to make contributions, and have approved the final text.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Informational brochure to support the common intervention.
Contributor Information
José I Recio-Rodríguez, Email: donrecio@gmail.com.
Carlos Martín-Cantera, Email: Carlos.Martin@uab.es.
Natividad González-Viejo, Email: natigonviejo@gmail.com.
Amparo Gómez-Arranz, Email: amparoaga@hotmail.com.
Maria S Arietaleanizbeascoa, Email: MARIASOLEDAD.ARIETALEANIZBEASCOASARABIA@osakidetza.net.
Yolanda Schmolling-Guinovart, Email: yschmolling@gmail.com.
Jose A Maderuelo-Fernandez, Email: jmaderuelo@saludcastillayleon.es.
Diana Pérez-Arechaederra, Email: arechaederra@yahoo.es.
Emiliano Rodriguez-Sanchez, Email: Emiliano@usal.es.
Manuel A Gómez-Marcos, Email: magomez@usal.es.
Luis García-Ortiz, Email: Lgarciao@usal.es.
Acknowledgments
We are grateful to all professionals participating in the EVIDENT II study.
Coordinating center: L Garcia-Ortiz, JI Recio-Rodriguez, MA Gómez-Marcos, and MC Patino-Alonso from the Primary Care Research Unit of La Alamedilla Health Center, Salamanca, Spain.
Participating centers:
La Alamedilla Health Center, (Health Service of Castilla y León): E. Rodríguez-Sánchez, JA. Maderuelo-Fernández. JA. Iglesias-Valiente, D. Pérez-Arechaederra, S. Mora-Simón, C. Agudo-Conde, MC. Castaño-Sánchez, C. Rodríguez-Martín, B. Sánchez-Salgado, A. de Cabo-Laso.
Passeig de Sant Joan Health Center, (Catalan Health Service): C. Martín Cantera, J. Canales Reina, E. Rodrigo de Pablo, M L. Lasaosa Medina, MJ. Calvo Aponte, A. Rodríguez Franco, C. Martin Borras, A. Puig Ribera, R. Colominas Garrido, E. Puigdomenech Puig. Poble Sec Health Center, (Catalán Health Service): J. Anton Alvarez. Ca N’Oriac Health Center, (Catalan Health Service): M. Romaguera Bosch. Sant Roc Health Center, (Catalán Health Service): N. Curos Bernet, P Martinez Visa.
Rio Tajo Health Center, (Castilla-La Mancha Health Service): Y.Schmolling-Guinovart, B.Rodríguez-Martin, JB. Calderón-Ubeda, JL. Menéndez-Obregón, A. Segura-Fragoso, C. Zabala-Baños, V. Martínez-Vizcaíno, A. Fernández del Rio, M. Martínez-Andrés
Casa de Barco Health Center, (Health Service of Castilla y León): C. Fernández Alonso, A. Gómez Arranz, E. Ibáñez Jalón, A. de la Cal de la Fuente, M. Menéndez Suarez, I. Enrique Cabero, I. Repiso Gento, MR. Álvarez Posadas.
Torre Ramona Health Center, (Health Service of Aragon): N. González-Viejo, JF. Magdalena-Belio, L. Otegui-Ilarduya, FJ. Rubio-Galán, A. Melguizo-Bejar, I. Sauras-Yera, MªJ. Gil-Train, M. Iribarne-Ferrer, I. Liñares-Varela. MÁ. Lafuente-Ripolles.
Primary Care Research Unit of Bizkaia, Basque Health Service-Osakidetza: G. Grandes, A. Sanchez, V. Arce, MS. Arietaleanizbeascoa, N. Mendizabal, E. Iturregui-San Nicolás, P.Escondrillas-Wencel, J.Rodriguez-Morua.
CGB Computer Company, Salamanca, Spain, contributed to the technical development of APP EVIDENT II. C González-Blanco, P Sánchez-Álvarez, JA Rodríguez-Valle.
Sources of funding
This project was financed by the Carlos III Health Institute of the Ministry of Health of Spain (FIS:PI13/00618, PI13/01526, PI13/00058, PI13/01635, PI13/02528, PI12/01474,RETICS RD12/0005) and the Autonomous Government of Castilla and León (GRS 770/B/13) and Infosalud Fundation.
References
- Varo Cenarruzabeitia JJ, Martinez Hernandez JA, Martinez-Gonzalez MA. Benefits of physical activity and harms of inactivity. Med Clin (Barc) 2003;121(17):665–672. doi: 10.1016/S0025-7753(03)74054-8. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136(7):493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 Pt 1):1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270–1275. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Lakka TA, Laukkanen JA, Rauramaa R, Salonen R, Lakka HM, Kaplan GA, Salonen JT. Cardiorespiratory fitness and the progression of carotid atherosclerosis in middle-aged men. Ann Intern Med. 2001;134(1):12–20. doi: 10.7326/0003-4819-134-1-200101020-00008. [DOI] [PubMed] [Google Scholar]
- Garcia-Ortiz L, Recio-Rodriguez JI, Martin-Cantera C, Cabrejas-Sanchez A, Gomez-Arranz A, Gonzalez-Viejo N, Iturregui-San Nicolas E, Patino-Alonso MC, Gomez-Marcos MA. Physical exercise, fitness and dietary pattern and their relationship with circadian blood pressure pattern, augmentation index and endothelial dysfunction biological markers: EVIDENT study protocol. BMC Public Health. 2010;10:233. doi: 10.1186/1471-2458-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ortiz L, Recio-Rodriguez JI, Schmidt-Trucksass A, Puigdomenech-Puig E, Martinez-Vizcaino V, Fernandez-Alonso C, Rubio-Galan J, Agudo-Conde C, Patino-Alonso MC, Rodriguez-Sanchez E, Gomez-Marcos MA. Relationship between objectively measured physical activity and cardiovascular aging in the general population - The EVIDENT trial. Atherosclerosis. 2014;233(2):434–440. doi: 10.1016/j.atherosclerosis.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, Colditz GA, Thun MJ. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. 2010;172(4):419–429. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Romaguera-Bosch M, Grandes G, Menendez-Suarez M, Lema-Bartolome J, Gonzalez-Viejo N, Agudo-Conde C, Garcia-Ortiz L. Association of television viewing time with central hemodynamic parameters and the radial augmentation index in adults. Am J Hypertens. 2013;26(4):488–494. doi: 10.1093/ajh/hps071. [DOI] [PubMed] [Google Scholar]
- Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, Martinez-Vizcaino V, Martin-Borras C, de-la-Cal-Dela-Fuente A, Sauras-Llera I, Sanchez-Perez A, Agudo-Conde C, Garcia-Ortiz L. Relationship between physical activity and plasma fibrinogen concentrations in adults without chronic diseases. PLoS ONE. 2014;9(2):e87954. doi: 10.1371/journal.pone.0087954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005;1:CD003180. doi: 10.1002/14651858.CD003180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandes G, Sanchez A, Sanchez-Pinilla RO, Torcal J, Montoya I, Lizarraga K, Serra J. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169(7):694–701. doi: 10.1001/archinternmed.2009.23. [DOI] [PubMed] [Google Scholar]
- Baker PRA, Francis DP, Soares J, Weightman AL, Foster C. Community wide interventions for increasing physical activity. Cochrane Database Syst Rev. 2011;4:CD008366. doi: 10.1002/14651858.CD008366.pub2. [DOI] [PubMed] [Google Scholar]
- Orrow G, Kinmonth AL, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;344:e1389. doi: 10.1136/bmj.e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, White M, Mathers JC, Sniehotta FF. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11(1):75. doi: 10.1186/1741-7015-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson ND, Puig-Ribera A, McKenna J, Brown WJ, Burton NW, Cooke CB. Do walking strategies to increase physical activity reduce reported sitting in workplaces: a randomized control trial. Int J Behav Nutr Phys Act. 2009;6:43. doi: 10.1186/1479-5868-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greef KP, Deforche BI, Ruige JB, Bouckaert JJ, Tudor-Locke CE, Kaufman JM, De Bourdeaudhuij IM. The effects of a pedometer-based behavioral modification program with telephone support on physical activity and sedentary behavior in type 2 diabetes patients. Patient Educ Couns. 2011;84(2):275–279. doi: 10.1016/j.pec.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez MA, Sanchez-Villegas A. The emerging role of Mediterranean diets in cardiovascular epidemiology: monounsaturated fats, olive oil, red wine or the whole pattern? Eur J Epidemiol. 2004;19(1):9–13. doi: 10.1023/b:ejep.0000013351.60227.7b. [DOI] [PubMed] [Google Scholar]
- Fuentes F, Lopez-Miranda J, Perez-Martinez P, Jimenez Y, Marin C, Gomez P, Fernandez JM, Caballero J, Delgado-Lista J, Perez-Jimenez F. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. Br J Nutr. 2008;100(1):159–165. doi: 10.1017/S0007114508888708. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Murie-Fernandez M, Irimia P, Toledo E, Martinez-Vila E, Buil-Cosiales P, Serrano-Martinez M, Ruiz-Gutierrez V, Ros E, Estruch R, Martinez-Gonzalez MA. Carotid intima-media thickness changes with Mediterranean diet: a randomized trial (PREDIMED-Navarra) Atherosclerosis. 2011;219(1):158–162. doi: 10.1016/j.atherosclerosis.2011.06.050. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. doi: 10.1136/bmj.311.7018.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez MA, Fernandez-Jarne E, Serrano-Martinez M, Marti A, Martinez JA, Martin-Moreno JM. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: an operational healthy dietary score. Eur J Nutr. 2002;41(4):153–160. doi: 10.1007/s00394-002-0370-6. [DOI] [PubMed] [Google Scholar]
- Patino-Alonso MC, Recio-Rodriguez JI, Magdalena Belio JF, Colominas-Garrido R, Lema-Bartolome J, Gomez Arranz A, Agudo-Conde C, Gomez-Marcos MA, Garcia-Ortiz L. Factors Associated with Adherence to the Mediterranean Diet in the Adult Population. J Acad Nutr Diet. 2013. doi:10.1016/j.jand.2013.07.038. [DOI] [PubMed]
- de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. doi: 10.1161/01.CIR.99.6.779. [DOI] [PubMed] [Google Scholar]
- Salas-Salvado J, Fernandez-Ballart J, Ros E, Martinez-Gonzalez MA, Fito M, Estruch R, Corella D, Fiol M, Gomez-Gracia E, Aros F, Flores G, Lapetra J, Lamuela-Raventos R, Ruiz-Gutierrez V, Bullo M, Basora J, Covas MI. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168(22):2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- Salas-Salvado J, Bullo M, Babio N, Martinez-Gonzalez MA, Ibarrola-Jurado N, Basora J, Estruch R, Covas MI, Corella D, Aros F, Ruiz-Gutierrez V, Ros E. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34(1):14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Pharm D, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med. 2013;369(7):676–7. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13(2):101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18(11):924–938. doi: 10.5551/jat.7716. [DOI] [PubMed] [Google Scholar]
- Boulos MN, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online. 2011;10:24. doi: 10.1186/1475-925X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward MG, Roberts I. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49–55. doi: 10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D, Clark N, Soar J, Padkin A, Stoneham A, Perkins GD, Nolan J. A randomised control trial to determine if use of the iResus(c) application on a smart phone improves the performance of an advanced life support provider in a simulated medical emergency. Anaesthesia. 2011;66(4):255–262. doi: 10.1111/j.1365-2044.2011.06649.x. [DOI] [PubMed] [Google Scholar]
- Neubert S, Arndt D, Thurow K, Stoll R. Mobile real-time data acquisition system for application in preventive medicine. Telemed J E Health. 2010;16(4):504–509. doi: 10.1089/tmj.2009.0123. [DOI] [PubMed] [Google Scholar]
- Yamada M, Aoyama T, Okamoto K, Nagai K, Tanaka B, Takemura T. Using a Smartphone while walking: a measure of dual-tasking ability as a falls risk assessment tool. Age Ageing. 2011;40(4):516–519. doi: 10.1093/ageing/afr039. [DOI] [PubMed] [Google Scholar]
- Dufau S, Dunabeitia JA, Moret-Tatay C, McGonigal A, Peeters D, Alario FX, Balota DA, Brysbaert M, Carreiras M, Ferrand L, Ktori M, Perea M, Rastle K, Sasburg O, Yap MJ, Ziegler JC, Grainger J. Smart phone, smart science: how the use of smartphones can revolutionize research in cognitive science. PLoS One. 2011;6(9):e24974. doi: 10.1371/journal.pone.0024974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciemins E, Coon P, Sorli C. An analysis of data management tools for diabetes self-management: can smart phone technology keep up? J Diabetes Sci Technol. 2010;4(4):958–960. doi: 10.1177/193229681000400427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worringham C, Rojek A, Stewart I. Development and feasibility of a smartphone, ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PLoS One. 2011;6(2):e14669. doi: 10.1371/journal.pone.0014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin C, Bock B. Desired features of smartphone applications promoting physical activity. Telemed J E Health. 2011;17(10):801–803. doi: 10.1089/tmj.2011.0055. [DOI] [PubMed] [Google Scholar]
- Gan KO, Allman-Farinelli M. A scientific audit of smartphone applications for the management of obesity. Aust N Z J Public Health. 2011;35(3):293–294. doi: 10.1111/j.1753-6405.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- Ngo J, Engelen A, Molag M, Roesle J, Garcia-Segovia P, Serra-Majem L. A review of the use of information and communication technologies for dietary assessment. Br J Nutr. 2009;101(Suppl 2):S102–112. doi: 10.1017/S0007114509990638. [DOI] [PubMed] [Google Scholar]
- Wohlers EM, Sirard JR, Barden CM, Moon JK. Smart phones are useful for food intake and physical activity surveys. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5183–5186. doi: 10.1109/IEMBS.2009.5333721. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y, Komatsu J, Suarez L, Vittinghoff E, Haskell W, Noorishad T, Pham K. The mPED randomized controlled clinical trial: applying mobile persuasive technologies to increase physical activity in sedentary women protocol. BMC Public Health. 2011;11:933. doi: 10.1186/1471-2458-11-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free C, Phillips G, Felix L, Galli L, Patel V, Edwards P. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Res Notes. 2010;3:250. doi: 10.1186/1756-0500-3-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson EL Jr, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med Sci Sports Exerc. 1995;27(6):934–940. [PubMed] [Google Scholar]
- Matthews CE, Keadle SK, Sampson J, Lyden K, Bowles HR, Moore SC, Libertine A, Freedson PS, Fowke JH. Validation of a Previous-Day Recall Measure of Active and Sedentary Behaviors. Med Sci Sports Exerc. 2013;45(8):1629–1638. doi: 10.1249/MSS.0b013e3182897690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring) 2007;15(10):2371–2379. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- Chen KY, Bassett DR Jr. The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc. 2005;37(11 Suppl):S490–500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094–1102. doi: 10.1249/MSS.0b013e3181c5ec18. [DOI] [PubMed] [Google Scholar]
- Schroder H, Fito M, Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Lamuela-Raventos R, Ros E, Salaverria I, Fiol M, Lapetra J, Vinyoles E, Gomez-Gracia E, Lahoz C, Serra-Majem L, Pinto X, Ruiz-Gutierrez V, Covas MI. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141(6):1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ballart JD, Pinol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez-Bauer M, Martinez-Gonzalez MA, Salas-Salvado J, Martin-Moreno JM. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103(12):1808–1816. doi: 10.1017/S0007114509993837. [DOI] [PubMed] [Google Scholar]
- O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23(4):697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an N-point moving average method. J Am Coll Cardiol. 2011;57(8):951–961. doi: 10.1016/j.jacc.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Garcia-Ortiz L, Recio-Rodriguez JI, Canales-Reina JJ, Cabrejas-Sanchez A, Gomez-Arranz A, Magdalena-Belio JF, Guenaga-Saenz N, Agudo-Conde C, Gomez-Marcos MA. Comparison of two measuring instruments, B-pro and SphygmoCor system as reference, to evaluate central systolic blood pressure and radial augmentation index. Hypertens Res. 2012;35(6):617–623. doi: 10.1038/hr.2012.3. [DOI] [PubMed] [Google Scholar]
- Munir S, Guilcher A, Kamalesh T, Clapp B, Redwood S, Marber M, Chowienczyk P. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008;51(1):112–118. doi: 10.1161/HYPERTENSIONAHA.107.096016. [DOI] [PubMed] [Google Scholar]
- Kohara K, Tabara Y, Oshiumi A, Miyawaki Y, Kobayashi T, Miki T. Radial augmentation index: a useful and easily obtainable parameter for vascular aging. Am J Hypertens. 2005;18(1 Pt 2):11S–14S. doi: 10.1016/j.amjhyper.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, Agudo-Conde C, Gomez-Sanchez L, Gomez-Sanchez M, Rodriguez-Sanchez E, Garcia-Ortiz L. Protocol for measuring carotid intima-media thickness that best correlates with cardiovascular risk and target organ damage. Am J Hypertens. 2012;25(9):955–961. doi: 10.1038/ajh.2012.72. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- Tuah NA, Amiel C, Qureshi S, Car J, Kaur B, Majeed A. Transtheoretical model for dietary and physical exercise modification in weight loss management for overweight and obese adults. Cochrane Database Syst Rev. 2011;10 doi: 10.1002/14651858.CD008066.pub2. CD008066. [DOI] [PubMed] [Google Scholar]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- WMA. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Garcia-Ortiz L, Recio-Rodriguez JI, Puig-Ribera A, Lema-Bartolome J, Ibanez-Jalon E, Gonzalez-Viejo N, Guenaga-Saenz N, Agudo-Conde C, Patino-Alonso MC, Gomez-Marcos MA. Blood Pressure Circadian Pattern and Physical Exercise Assessment by Accelerometer and 7-Day Physical Activity Recall Scale. Am J Hypertens. 2013. doi: 10.1093/ajh/hpt159. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Informational brochure to support the common intervention.


