Abstract
Here we explored the impact of hydrogen sulfide (H2S) on biophysical properties of the primary human airway smooth muscle (ASM)–the end effector of acute airway narrowing in asthma. Using Magnetic Twisting Cytometry (MTC), we measured dynamic changes in the stiffness of isolated ASM, at the single-cell level, in response to varying doses of GYY4137 (1–10 mM). GYY4137 slowly released appreciable levels of H2S in the range of 10–275 µM, and H2S released was long lived. In isolated human ASM cells, GYY4137 acutely decreased stiffness (i.e. an indicator of the single-cell relaxation) in a dose-dependent fashion, and stiffness decreases were sustained in culture for 24h. Human ASM cells showed protein expressions of cystathionine-γ-lyase (CSE; a H2S synthesizing enzyme) and ATP-sensitive potassium (KATP) channels. The KATP channel opener pinacidil effectively relaxed isolated ASM cells. In addition, pinacidil-induced ASM relaxation was completely inhibited by the treatment of cells with the KATP channel blocker glibenclamide. Glibenclamide also markedly attenuated GYY4137-mediated relaxation of isolated human ASM cells. Taken together, our findings demonstrate that H2S causes the relaxation of human ASM and implicate as well the role for sarcolemmal KATP channels. Finally, given that ASM cells express intrinsic enzymatic machinery of generating H2S, we suggest thereby this class of gasotransmitter can be further exploited for potential therapy against obstructive lung disease.
Keywords: asthma, airway smooth muscle, single cell contraction, H2S, ATP-sensitive potassium channels
INTRODUCTION
H2S, the most recently-discovered gasotransmitter after NO and CO, has been reported to exert many physiological effects [1; 2]. H2S acts as a neuromodulator and/or neuroprotectant in the central nervous system and is involved with long-term potentiation in the hippocampus [3]. H2S has been shown to regulate insulin secretion [4; 5], promote angiogenesis [6] and protect cardiac muscle from oxidative stress [7; 8]. Among the many physiologic functions perhaps the most often reported is its mode of action on the vasculature [9; 10; 11; 12; 13; 14]. Specifically, H2S causes the relaxation of vascular smooth muscle via the ATP-sensitive potassium (KATP) channel [15].
In the lung, cystathionine-γ-lyase (CSE) is one of the major enzymes producing H2S [16] and the deficiency of CSE in mice polarizes T cells that renders mice more susceptible to allergen-induced airway hyperresponsiveness (AHR) [17]. AHR is the excessive narrowing of airways and is a cardinal feature of asthma contributing to disease morbidity [18]. Toward this end, administration of H2S donors has been shown to reduce the immune inflammatory response and AHR in animal models of asthma [17; 19]. In patients with asthma, Tian and colleagues [20] have recently reported a positive correlation between decline in lung function and decreases in CSE expression and endogenous plasma H2S concentration. Few studies have focused on the mechanistic actions of H2S in the lung-resident cells. Even though the role of KATP channels in regulating airway functions has been reported [21; 22; 23], the effects of H2S on airway smooth muscle (ASM), the end-effector of acute airway narrowing, are largely unexplored.
In this study, we explored the direct effects of GYY4137, an agent capable of generating H2S, on the biophysical properties of ASM using Magnetic Twisting Cytometry. Our findings showed that, at the single-cell level, GYY4137 causes ASM relaxation and that GYY4137-induced relaxation is mediated by H2S that acts to hyperpolarize ASM via, in part, opening the sarcolemmal KATP channel. Given the need for efficacious bronchodilators for treating obstructive lung diseases, H2S and its derived compounds may offer a promising new avenue for asthma therapy.
MATERIALS AND METHODS
Materials
DMEM-Ham's F-12 (1:1) was purchased from GIBCO (Grand Island, NY), and the synthetic arginine-glycine-aspartic acid (RGD) containing peptide was purchased from American Peptide Company (Sunnyvale, CA). Reagents were obtained from Sigma-Aldrich (St. Louis, MO) with the exception of GYY4137 and Glyburide (glibenclamide) which were purchased from Santa Cruz Biotechnology (Dallas, TX). All reagents (Na2S+9H2O, GYY4137, glibenclamide, pinacidil, cromakalim, diazoxide, and proparglyglycine) were reconstituted in either sterile distilled water or DMSO, frozen in aliquots, and diluted appropriately in serum-free media on the day of use.
ASM cell culture and characterization
Human bronchi were obtained from lungs unsuitable for transplantation in accordance with procedures approved by Committees on Studies Involving Human Beings from the University of Pennsylvania. Human ASM cells were prepared from these bronchi as described previously [24]. Unless otherwise specified, serum-deprived post-confluent cells were plated at 30,000 cells/cm2 on plastic wells (96-well Removawell, Immunlon II: Dynetech) previously coated with type I collagen (Vitrogen 100; Cohesion, Palo Alto, CA) at 500 ng/cm2. Cells were maintained in serum-free media at 37°C in humidified air containing 5% CO2 for 24h prior to experiments. These conditions have been optimized for seeding cultured cells on collagen matrix and for assessing their mechanical properties [25; 26; 27].
Magnetic twisting cytometry (MTC)
Dynamic changes in cell stiffness were measured as an indicator of the single-cell contraction and relaxation of isolated human ASM cells using MTC as described by us in detail elsewhere [25; 26; 27]. In brief, RGD-coated ferrimagnetic microbeads (4.5 µm in diameter) bound to the cytoskeleton through cell surface integrin receptors were magnetized horizontally and then twisted in a vertically aligned homogeneous magnetic field that was varying sinusoidally in time. This sinusoidal twisting magnetic field caused both a rotation and a pivoting displacement of the bead: as the bead moves, the cell develops internal stresses which in turn resist bead motions [28]. Lateral bead displacements in response to the resulting oscillatory torque were detected with a spatial resolution of ~5 nm, and the ratio of specific torque to bead displacements was computed and expressed here as the cell stiffness in units of Pascal per nm (Pa/nm).
Immunoblotting
The expression levels of different proteins were determined by Western blot as described previously [29]. Cells were grown to near confluence in 6 well plates and growth-arrested as described above. Cells were lysed in 1× RIPA buffer (Upstate) containing protease inhibitors (Roche) by mechanical scraping, and total protein concentration was determined (BioRad Protein Assay Reagent). Equal amounts of lysates from each sample were resolved by SDS PAGE, transferred to nitrocellulose membranes, and subsequently probed with the indicated primary antibody followed by HRP-conjugated anti-mouse (1:5000) or anti-goat (1:4000) antibody. Mouse anti-CTH (1:500; Santa Cruz Biotechnology) was used to detect cystathionine-γ-lyase (CSE) and goat anti-KIR6.1 (1:1000; Santa Cruz Biotechnology) was used to detect Kir6.1 subunit of the KATP channels in human ASM cells. Blots were developed using enhanced chemiluminescence and quantified using ImageJ (NIH).
H2S measurements
To trap H2S, zinc acetate (1% w/v) was added to media containing different concentration of GYY4137. After 5 min, the reaction was terminated with N,N-dimethyl-p-phenylenediamine sulfate (20 mM in 7.2 M HCl) and FeCl3 (30 mM in 1.2 M HCl). H2S in the sampled media interacts with N,N-dimethyl-p-phenylenediamine sulfate to form methylene blue. The absorbance of the resulting solution was determined at 670 nm after the mixture was kept in the dark for 20 min. H2S concentration in the culture media was calculated against the calibration curve of standard Na2S solutions.
Statistical analysis
Unless otherwise stated, we used Student’s t-test and the Analysis of Variance (ANOVA) with adjusting for multiple comparisons by applying the Bonferroni’s methods. To satisfy the normal distribution assumptions associated with ANOVA, cell stiffness data were converted to log scale prior to analyses. All analyses were performed in SAS V.9.2 (SAS Institute Inc., Cary, NC), and the 2-sided P-values less than 0.05 were considered significant.
RESULTS
Na2S causes acute relaxation of isolated human airway smooth muscle cells
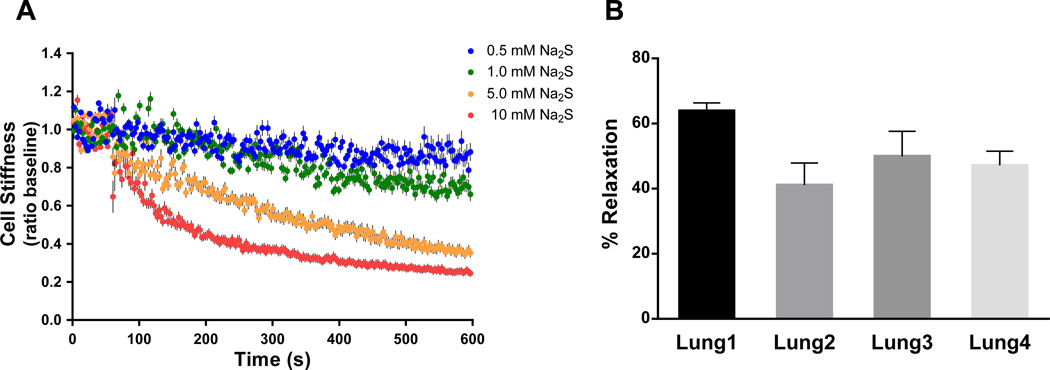
We tested first the effects of a well-known H2S donor, Na2S, on the stiffness of isolated human ASM cells. Addition of Na2S caused a rapid and dose-dependent decrease in cell stiffness (Figure 1A). The onset of stiffness decreases occurred as early as 2s following the addition of the highest dose of Na2S (10 mM). Decreases were significant from the baseline after 2s for 10 mM; 8s for 5 mM; 120s for 1 mM; and 178s for 0.5 mM, and continued for the duration of Na2S stimulation (Figure 1A). Using a mixed effect model to control for random effect due to the repeated measurements, we found significant group (i.e. dose) differences at 600s, except between 5 mM and 10 mM (P=0.139339). For individual cells obtained from three additional lung donors, Na2S (5 mM) markedly relaxed ASM (P<0.002, Signed Rank Test), resulting in ~40–60% relaxation (Figure 1B).
Figure 1. Effects of a fast-releasing H2S donor (Na2S) on single-cell mechanics of human ASM.
(A) Baseline stiffness was measured for the first 0–60s and changes in stiffness in response to addition of Na2S were measured continuously up to the indicated time (60–600s). For each cell, changes in stiffness in response to Na2S were normalized to its respective baseline stiffness. Cells were prepared from one donor lung (Lung 1). Data are presented as Mean±SE (0.5 mM Na2S, n=44; 1.0 mM Na2S, n=70; 5.0 mM Na2S, n=71; 10 mM Na2S, n=66 individual cell measurements). (B) Maximal stiffness reduction (i.e. % relaxation) induced by 5 mM Na2S. Cells were prepared from four different lung donors. Data are presented as Mean±SE (Lung 1, n=43; Lung 2, n=46; Lung 3, n=34; Lung 4, n=28).
GYY4137 causes sustained relaxation of isolated human airway smooth muscle cells
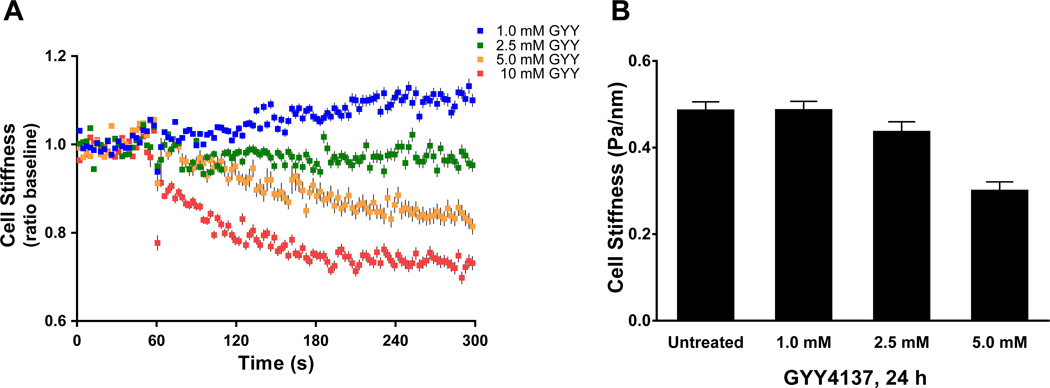
We next tested the effects of a water-soluble agent capable of releasing H2S, GYY4137 [30]. GYY4137 increased H2S concentration in a dose- and time-dependent manner (Supplementary Figure 1); GYY4137 (1–10 mM) acutely released ~10–275 µM of H2S. H2S released was sustained in culture over 24h (data not shown). In isolated human ASM, GYY4137 decreased cell stiffness in a dose-dependent manner (Figure 2). For acute exposure (Figure 2A), stiffness decreases were significant from the baseline for all doses of GYY4137 tested, except 1.0 mM GYY4137, with the maximal effect at 10 mM–an equivalent to ~275 µM of H2S. Human ASM cells exposed to GYY4137 for 24h also showed dose dependent decreases in cell stiffness (Figure 2B). When compared to time-matched untreated cells, however, we only found a significant (P<0.0001) reduction in cell stiffness at 5 mM GYY4137 which resulted in ~50% relaxation (Figure 2B).
Figure 2. Effects of a slow-releasing H2S donor (GYY4137) on single-cell mechanics of human ASM.
(A) Baseline stiffness was measured for the first 0–60s and changes in stiffness in response to addition of GYY4137 were measured continuously up to the indicated time (60–300s). For each cell, changes in stiffness in response to GYY4137 were normalized to its respective baseline stiffness. Data are presented as Mean±SE (1.0 mM GYY, n=841; 2.5 mM GYY, n=794; 5.0 mM GYY, n=1017; 10 mM GYY, n=832 individual cell measurements obtained from 4 different lung donors). (B) Stiffness changes in response to varying doses of GYY4137 measured at 24h. Data are presented as Geometric Mean±SE (time-matched, untreated, n=530; 1.0 mM GYY, n=522; 2.5 mM GYY, n=433; 5.0 mM GYY, n=462).
Human airway smooth muscle cells express functional KATP channels
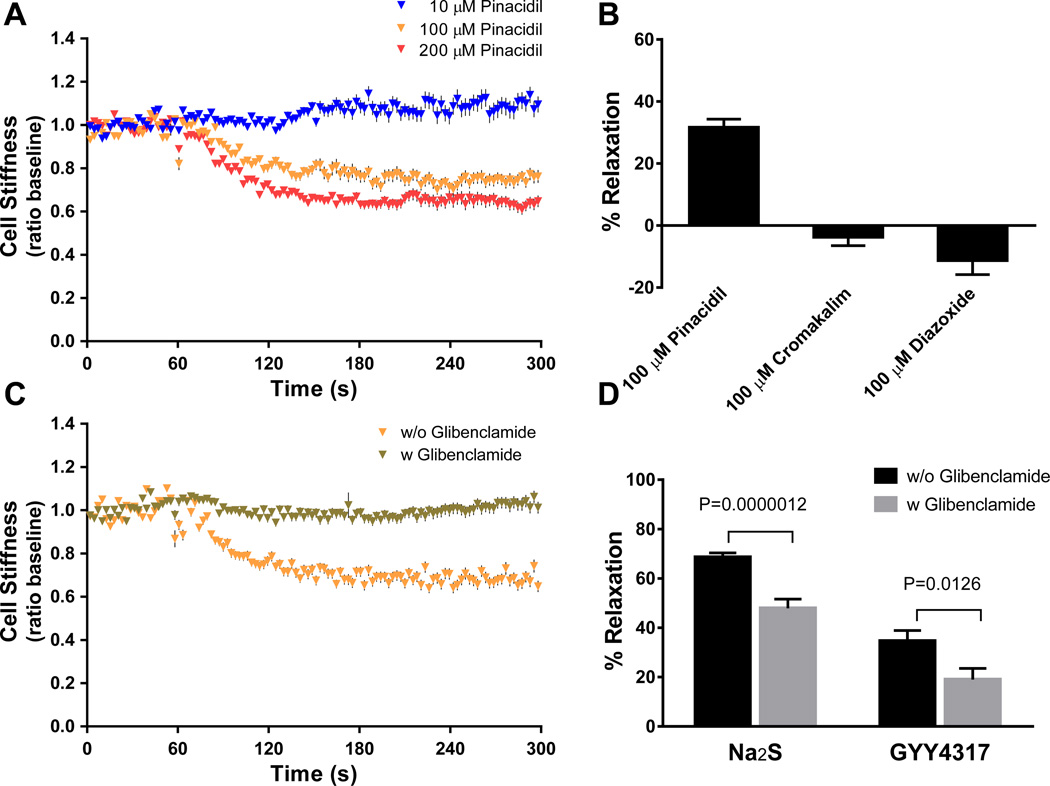
Since in vascular smooth muscle H2S activates KATP channels [15], we next explored the expression, signaling and function of KATP channels in ASM. Primary human ASM cells expressed KATP channels as assessed by Western blot (Supplementary Figure 2). Whereas the KATP channel antagonist glibenclamide had no effect on cell stiffness (data not shown), the KATP channel opener pinacidil caused dynamic decreases in cell stiffness in a dose-dependent manner (Figure 3A). At 100 µM, pinacidil effectively decreased cell stiffness (P<0.0001) but, interestingly, other KATP channel openers, cromakalim and diazoxide, failed to decrease the stiffness of isolated human ASM (Figure 3B). Whereas pinacidil and cromakalim are relatively nonselective and target both sarcolemmal and mitochondrial KATP channels, diazoxide is relatively selective for the mitochondrial KATP channel [31; 32; 33]. It is interesting to note that diazoxide caused appreciable increases in cell stiffness (P=0.014, Signed Rank Test). In addition, pinacidil-induced stiffness decreases were abolished by the treatment of cells with glibenclamide (Figure 3C). Strikingly, compared with respective untreated cells, human ASM cells treated with glibenclamide also exhibited attenuated relaxation responses to both Na2S and GYY4137 (Figure 3D). These findings demonstrate that human ASM cells express functional KATP channels and that H2S acts to relax human ASM, presumably via the sarcolemmal KATP channel.
Figure 3. Mechanistic actions of H2S on single-cell mechanics of human ASM: Role for KATP channels.
(A) Dynamic changes in cell stiffness in response to 10 µM (n=133), 100 µM (n=133) and 200 µM (n=141) pinacidil. (B) Maximal stiffness reduction (i.e. % relaxation) induced by 100 µM pinacidil (n=150), cromakalim (n=123), and diazoxide (n=120). (C) Dynamic changes in cell stiffness in response to 100 µM pinacidil with prior treatments of cells with (n=236) or without (n=150) glibenclamide (10min, 100 µM). (D) Maximal ASM relaxation at 300s induced by H2S donors with prior treatments of cells with or without glibenclamide (n=119–167 individual cell measurements). Data are presented as Mean±SE.
Human airway smooth muscle cells express functional cystathionine-γ-lyase (CSE)
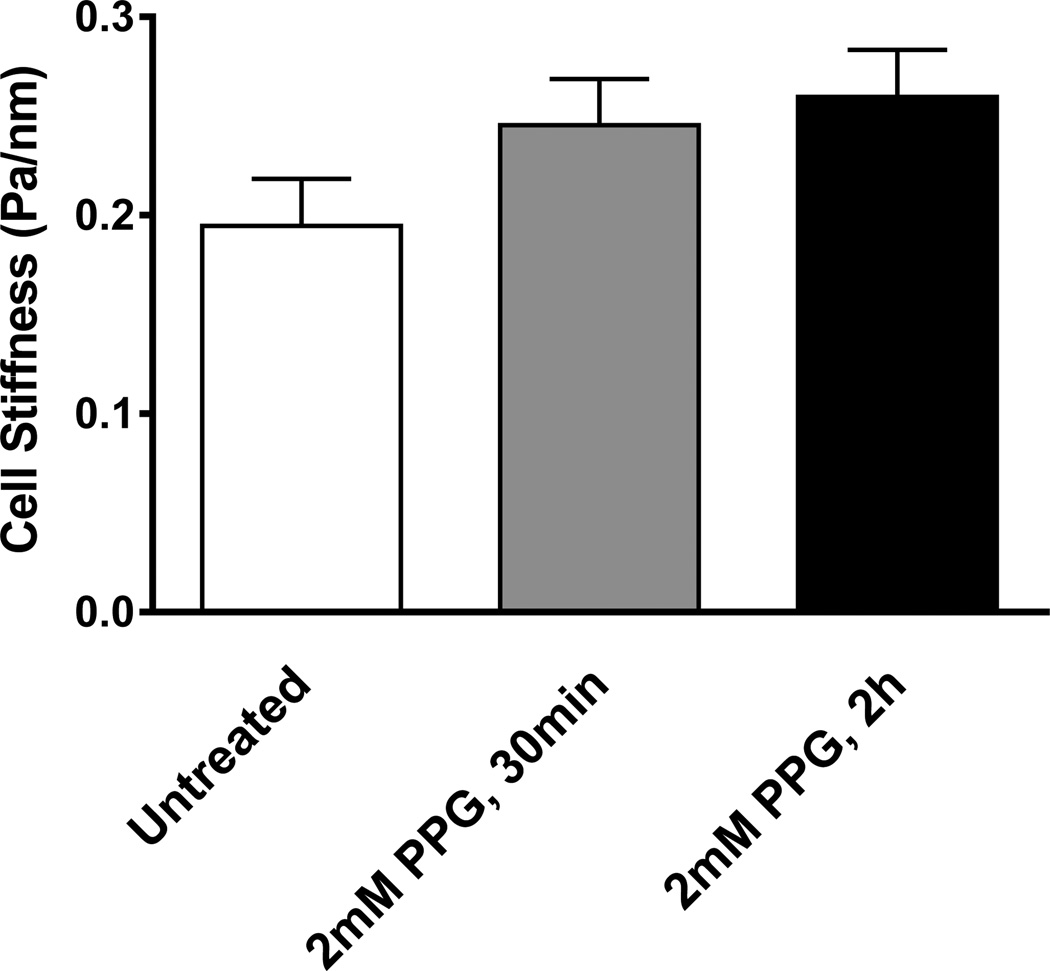
Finally, we asked whether human ASM cells express intrinsic enzymatic machinery of generating H2S. As depicted in Supplementary Figure 2, human ASM cells showed protein expression of CSE. It is interesting to note that under serum-deprived condition, which has been shown to enhance contractile function of ASM in culture [25; 27], we found noticeable decreases in protein expression of CSE. Consistent with this notion, inhibiting CSE with DL-propargylglycine (PPG) caused appreciable increases in ASM stiffness (Figure 4). Together, these findings demonstrate that human ASM cells are capable of generating H2S and that endogenous H2S generated via CSE may regulate physiologic homeostasis of ASM tone.
Figure 4. Effects of pharmacological inhibition of CSE on ASM stiffness.
Cells were untreated (n=272) or treated with 2 mM proparglyglycine (PPG) for 30min (n=287) and 2h (n=348), and the stiffness measured by MTC. Data are presented as Geometric Mean±SE. Stiffness of treated groups were significantly greater than untreated group (P<0.001). There were no statistical differences between 30min and 2h treatment groups.
DISCUSSION
In spite of the abundance of literature describing the impact of H2S on various vascular tissues (aorta, pulmonary artery, mesenteric artery, hepatic vessels), very limited reports seem to have covered its effect on ASM. Since the vascular smooth muscle reports hold out a possible therapeutic pathway for chronic drug-resistant hypertension, there seemed to be a need to explore the effects of H2S on ASM for possible therapeutic pathways for asthmatics, especially those for whom the classical β-adrenergic receptor agonists are less effective. To our knowledge, this report is the first such study on human ASM.
Using MTC, we measured functional changes in human ASM cells in response to Na2S and GYY4137. Na2S rapidly decreased cell stiffness in a dose-dependent fashion, with maximal relaxation attained within 600s with 5 mM Na2S. In addition, both acute and chronic exposures to GYY4137 caused marked decreases in cell stiffness and, for chronic exposures, decreases were equally efficacious as that of Na2S. Human ASM cells showed protein expression of CSE and KATP channels. Pinacidil, but not cromakalim and diazoxide, caused marked decreases in cell stiffness which were completely inhibited by glibenclamide. Glibenclamide attenuated in turn Na2S- and GYY4137-induced stiffness decreases in isolated human ASM cells.
In the present study, we used two agents capable of generating H2S (Na2S and GYY4137) and, for Na2S, used what might be considered excessively high concentrations–perhaps too high for physiological significance. However, Na2S releases approximately one third of H2S [34], and H2S readily undergoes oxidation at normal levels of oxygen. For this reason, we bubbled our various solutions of Na2S with 10% O2/balance N2 in an effort to minimize loss of the precursor and H2S. In experiments determining the amount of H2S released by GYY4137, nevertheless, we found that 1 mM GYY4137 acutely released ~10 µM H2S; 2.5 mM released ~100 µM H2S; 5.0 mM released ~225 µM H2S; and 10 mM released ~275 µM H2S (Supplementary Figure 1). H2S released by various doses of GYY4137 was sustained in culture over 24h (data not shown) and, importantly, the concentrations of H2S released were within the range reported by others [35; 36].
The fact that neither cromakalim nor diazoxide produced ASM relaxation (Figure 3B) was somewhat surprising. Allen and colleagues [37] found cromakalim both hyperpolarized and relaxed guinea-pig trachealis muscle. Moreover, since agents that suppress potassium permeability (4-aminopyridien, procaine, and TEA) reduced both the hyperpolarization and the relaxation, the opening of plasmalemmal K+ channels was proposed as the mechanism; but no particular K+ channel was designated. Further, cromakalim plus a second KATP channel opener, Y-26763, have been shown to reduce the tension of a carbachol-induced contraction of the rat trachea [23]. In isolated human ASM cells, however, the KATP channel opener, pinacidil, but not cromakalim or diazoxide, effectively relaxed ASM (Figure 3A,B). Moreover, since the relaxing effect of pinacidil was blocked by glibenclamide (Figure 3C), and since the relaxing effects of both Na2S and GYY4137 were attenuated by glibenclamide (Figure 3D), we conclude that in this preparation of human ASM cells the responsible K+ channel is the KATP channel. This conclusion is supported by the positive Western blot signal for Kir6.1 (Supplementary Figure 2). In addition, the fact that diazoxide, which is selective for SUR1-based plasma KATP channels and the mitochondrial KATP channel, failed to relax isolated human ASM further implicates the involvement of a SUR2-based sarcolemmal KATP channel.
The action of H2S on KATP channels is well documented and reviewed recently [1; 2; 38]. In fact the precise loci of activity have been thoroughly explored [39]. The exhaustive exploration of Mustafa et al. [35] have identified that physiological sulfhydration of Kir6.1-cysteine-43 in the KATP channel in mice activates the channel causing hyperpolarization. But this study also pointed to a role in vasorelaxation for the intermediate and small conductance potassium channels. The vasorelaxation also identified H2S as a major endothelial derived hyperpolarizing factor (EDHF) [40]. Consistent with these studies, our findings demonstrate that smooth muscle in the airways reacts quite similarly to that in the vasculature to H2S. Moreover, our findings show that human ASM cells have an intrinsic enzymatic machinery of generating H2S. To what extent, if any, CSE expression differs from healthy and asthmatic ASM is currently under study.
Asthma is a debilitating inflammatory disorder characterized by excessive contraction of ASM and narrowing of the airways. Although agents directed at K+ channels have been studied and found to be ineffective (as reviewed in [41]), given the need for efficacious bronchodilators for treating obstructive lung diseases, H2S and its derived compounds can be further exploited for asthma therapy.
Supplementary Material
HIGHLIGHTS.
GYY4137 released H2S in the range of 10–275 µM, and H2S released was long lived.
GYY4137 acutely relaxed ASM, and the relaxation was sustained in culture for 24h.
Human ASM cells showed protein expressions of CSE and KATP channels.
Glibenclamide completely inhibited pinacidil-induced ASM relaxation.
Glibenclamide markedly attenuated GYY4137-mediated ASM relaxation.
ACKNOWLEDGEMENTS
This work was supported by NHLBI grants: HL107361 (to S.S.A.), HL114471 (to R.A.P and S.S.A.), and HL50712 (to R.F). S.S.A was also supported by American Asthma Foundation (Sandler: 108183) grant. Human tissue used for this research project was provided by the National Disease Research Interchange.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
R.F., R.W., and S.S.A. conceived the study; R.F., B.D.S., D.Y.L., and J.Y.K. performed single-cell mechanics; G.Y. and R.W. measured H2S concentration and manuscript preparation; Y.C.L. and M.R.H. performed Western blots; D.B.F. provided cromakalim and diazoxide, and his expert insights into the regulation of KATP channels; and R.A.P. provided isolated human ASM cells and manuscript preparation. R.F. and S.S.A. wrote the manuscript. S.S.A. directed all studies, data analysis and interpretation, and is the primary author of the manuscript.
REFERENCES
- 1.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 2.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 3.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu LY, Yang W, Jia XM, Yang GD, Duridanova D, Cao K, Wang R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest. 2009;89:59–67. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Yang GD, Jia XM, Wu LY, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 9.Bianca RDD, Sorrentino R, Mirone V, Cirino G. Hydrogen sulfide and erectile function: a novel therapeutic target. Nat Rev Urol. 2011;8:286–289. doi: 10.1038/nrurol.2011.45. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YQ, Ndisang JF, Tang GH, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 11.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Bosc LVG, Walker BR, Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am J Physiol. 2013;304:H1446–H1454. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streeter E, Hart J, Badoer E. An investigation of the mechanisms of hydrogen sulfide-induced vasorelaxation in rat middle cerebral arteries. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:991–1002. doi: 10.1007/s00210-012-0779-2. [DOI] [PubMed] [Google Scholar]
- 13.White BJO, Smith PA, Dunn WR. Hydrogen sulphide-mediated vasodilatation involves the release of neurotransmitters from sensory nerves in pressurized mesenteric small arteries isolated from rats. Br J Pharmacol. 2013;168:785–793. doi: 10.1111/j.1476-5381.2012.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao WM, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 15.Zhao WM, Zhang J, Lu YJ, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous K-ATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YH, Wang R. The message in the air: Hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol. 2012;184:130–138. doi: 10.1016/j.resp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang GS, Wang PP, Yang GD, Cao QH, Wang R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol. 2013;182:1188–1195. doi: 10.1016/j.ajpath.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Woolcock AJ, Peat JK. Epidemiology of bronchial hyperresponsiveness. Clin Rev Allergy. 1989;7:245–256. doi: 10.1007/BF02914477. [DOI] [PubMed] [Google Scholar]
- 19.Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–123. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Tian M, Wang Y, Lu YQ, Yan M, Jiang YH, Zhao DY. Correlation between serum H2S and pulmonary function in children with bronchial asthma. Mol Med Rep. 2012;6:335–338. doi: 10.3892/mmr.2012.904. [DOI] [PubMed] [Google Scholar]
- 21.Challiss RA, Patel N, Arch JR. Comparative effects of BRL 38227, nitrendipine and isoprenaline on carbachol- and histamine-stimulated phosphoinositide metabolism in airway smooth muscle. Br J Pharmacol. 1992;105:997–1003. doi: 10.1111/j.1476-5381.1992.tb09091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamei K, Nabata H, Kuriyama H. Effects of KC 399, a novel ATP-sensitive K+ channel opener, on electrical and mechanical responses in dog tracheal smooth muscle. J Pharmacol Exp Ther. 1994;268:319–327. [PubMed] [Google Scholar]
- 23.Miyata S, Shibata O, Saito M, Tsuda A, Shibata S, Makita T, Sumikawa K. Effects of ATP-sensitive potassium channel openers on the contractile and phosphatidylinositol responses of the rat trachea. J Anesth. 2002;16:279–283. doi: 10.1007/s005400200043. [DOI] [PubMed] [Google Scholar]
- 24.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An SS, Kim J, Ahn K, Trepat X, Drake KJ, Kumar S, Ling G, Purington C, Rangasamy T, Kensler TW, Mitzner W, Fredberg JJ, Biswal S. Cell stiffness, contractile stress and the role of extracellular matrix. Biochem Biophys Res Commun. 2009;382:697–703. doi: 10.1016/j.bbrc.2009.03.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87:148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 29.Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, Damera G, Panettieri RA, DuBose TD, An SS, Penn RB. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol. 2012;166:981–990. doi: 10.1111/j.1476-5381.2011.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide - Releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 31.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 32.Liu YG, Sato T, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels - Novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 33.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O'Rourke B. Mitochondrial ROMK Channel Is a Molecular Component of MitoK(ATP) Circ Res. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan H, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 35.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 37.Allen SL, Boyle JP, Cortijo J, Foster RW, Morgan GP, Small RC. Electrical and mechanical effects of BRL34915 in guinea-pig isolated trachealis. Br J Pharmacol. 1986;89:395–405. doi: 10.1111/j.1476-5381.1986.tb10273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peers C, Bauer CC, Boyle JP, Scragg JL, Dallas ML. Modulation of ion channels by hydrogen sulfide. Antioxid Redox Signal. 2012;17:95–105. doi: 10.1089/ars.2011.4359. [DOI] [PubMed] [Google Scholar]
- 39.Jiang B, Tang G, Cao K, Wu L, Wang R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid Redox Signal. 2010;12:1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 40.Tang GH, Yang GD, Jiang B, Ju YJ, Wu LY, Wang R. H2S is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal. 2013;19:1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 41.Janssen LJ, Killian K. Airway smooth muscle as a target of asthma therapy: history and new directions. Respir Res. 2006;7:123. doi: 10.1186/1465-9921-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.