Abstract
Aseptic loosening and other wear-related complications are one of the most frequent late reasons for revision of total knee arthroplasty (TKA). Periprosthetic osteolysis (PPOL) predates aseptic loosening in many cases indicating the clinical significance of this pathogenic mechanism. A variety of implant-, surgery-, and host-related factors have been delineated to explain the development of PPOL. These factors influence the development of PPOL due to changes in mechanical stresses within the vicinity of the prosthetic device, excessive wear of the polyethylene liner, and joint fluid pressure and flow acting on the peri-implant bone. The process of aseptic loosening is initially governed by factors such as implant/limb alignment, device fixation quality, and muscle coordination/strength. Later large numbers of wear particles detached from TKAs trigger and perpetuate particle disease, as highlighted by progressive growth of inflammatory/granulomatous tissue around the joint cavity. An increased accumulation of osteoclasts at the bone-implant interface, an impairment of osteoblast function, mechanical stresses, and an increased production of joint fluid contribute to bone resorption and subsequent loosening of the implant. In addition, hypersensitivity and adverse reactions to metal debris may contribute to aseptic TKA failure but should be determined more precisely. Patient activity level appears to be the most important factor when the long-term development of PPOL is considered. Surgical technique, implant design, and material factors are the most important preventative factors because they influence both the generation of wear debris and excessive mechanical stresses. New generations of bearing surfaces and designs for TKA should carefully address these important issues in extensive preclinical studies. Currently, there is little evidence that PPOL can be prevented with pharmacological interventions.
Keywords: total knee arthroplasty, osteolysis, aseptic loosening, wear particles, polyethylene, particle disease, joint fluid, knee biomechanics, implant alignment, patient-related factors, hypersensitivity, biomaterial solution, pharmacological interventions
Introduction
Total knee arthroplasty (TKA) relieves knee pain and improves function, and has a significant impact on the health-related quality of life. Currently, it is estimated that more than 1.5 million TKAs are performed worldwide each year (Health at a glance 2011; OECD indicators). However, some TKAs fail during the period of service, and require revision surgery. Revision surgery brings less satisfactory outcomes and increased risk for complications [1]. In addition, it is more expensive than primary operations. Assuming an increasing number of primary TKAs in the next decades, it is clear that the number of revision surgeries will also be increasing [2, 3]. This could have a significant economic impact on the health care system. Therefore, understanding current failure mechanisms of primary TKA, and especially the potential for prevention is critical to reduce an expected TKA revision burden.
Although infection, instability, and patellofemoral problems dominate as reasons for reoperation in the first five years after an index surgery, one of the most frequent late reasons for failure is aseptic loosening accompanied by osteolysis (Fig. 1), [4]. Chronologically, periprosthetic osteolysis predates aseptic loosening in the majority of cases creating conditions facilitating implant loosening via weakening of bone-implant interface. There is a relative paucity of reported studies related to the pathogenesis of osteolysis around TKA in comparison to total hip arthroplasty [5, 6]. Similar to total hip arthroplasty, the pathogenesis of aseptic loosening and osteolysis in TKA is multifactorial with contributions of surgeon-, patient-, and implant-related factors. However, there are also important differences between total hip arthroplasty and TKA that preclude a direct translation of results from hip to knee arthroplasty. These differences are related to joint anatomy and kinematics, biomechanical function, implant design, biomaterials and resulting tribology.
Figure 1.
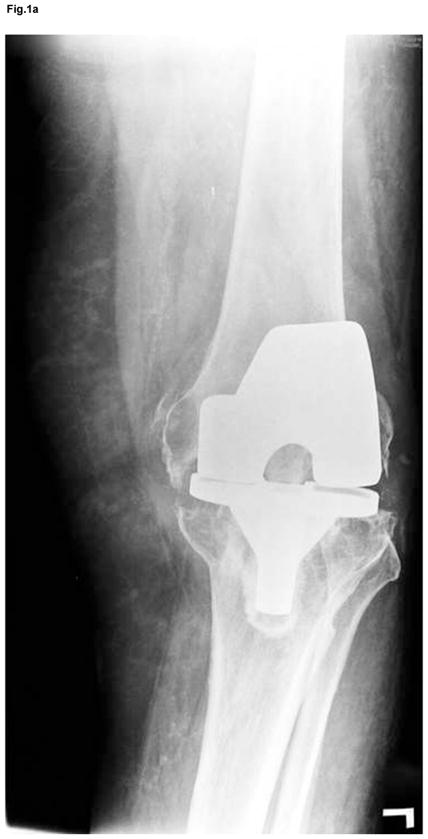
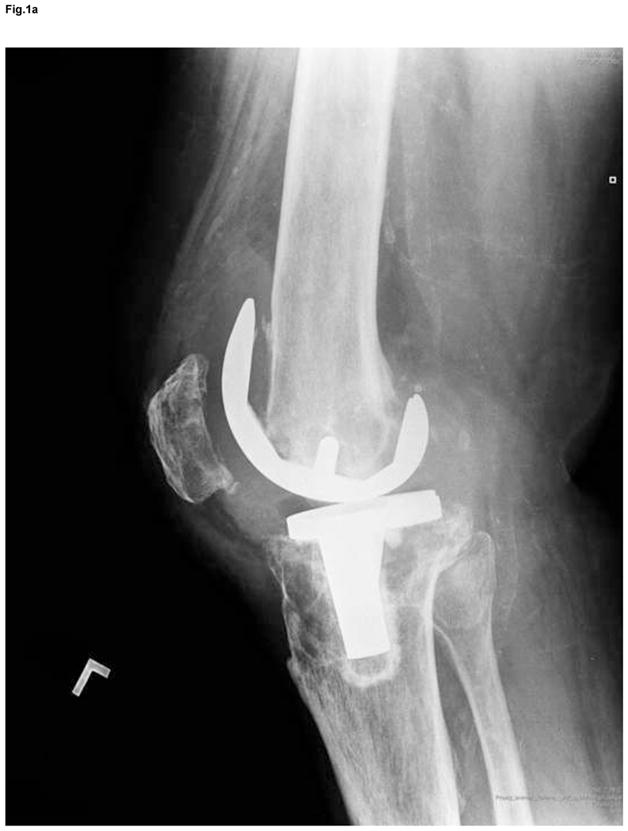
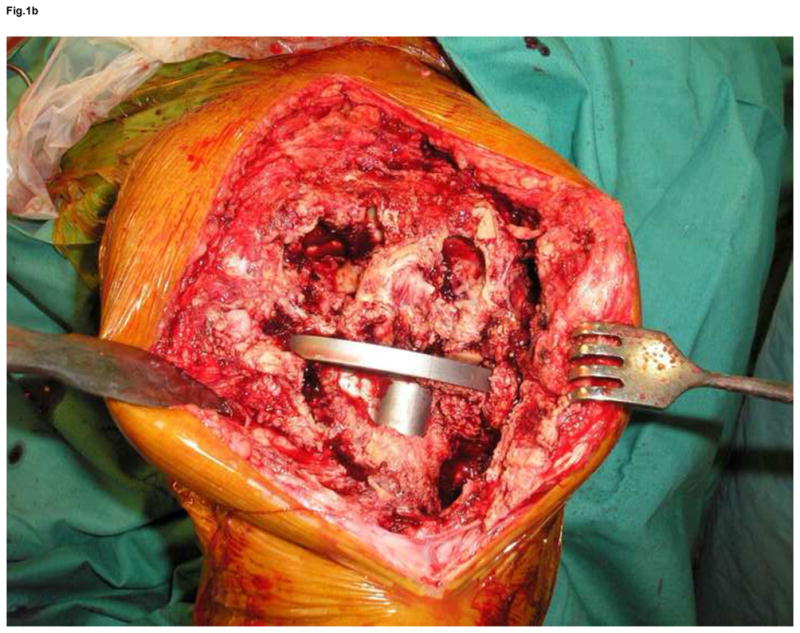
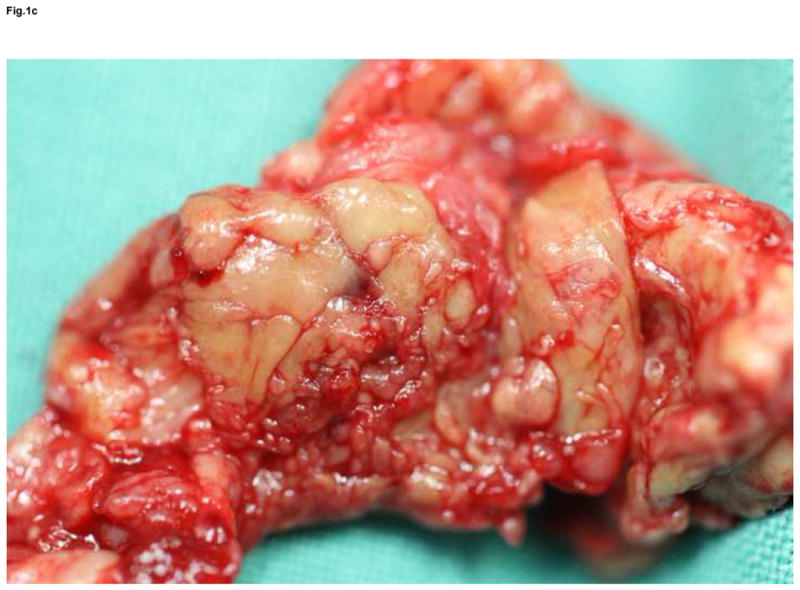
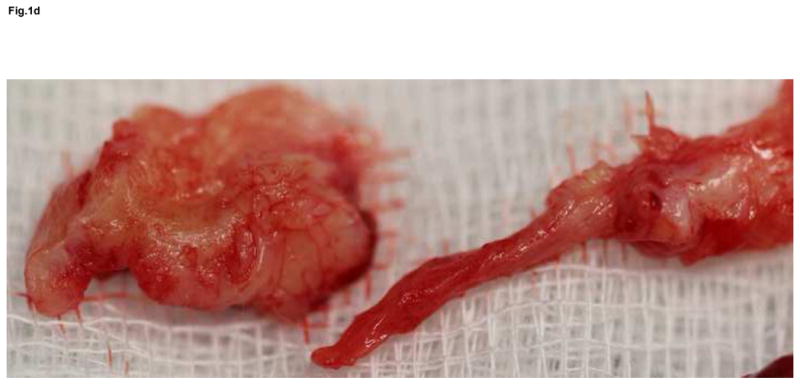
Anteroposterior and lateral x-ray of extensive osteolysis around the femoral and tibial component of TKA 11 years postoperatively (a); intraoperative view on the size of bone defects after removal of the failed femoral component and debridement of the osteolytic cavity (b); from the point a surgeon, the interface tissue membrane surrounding a failed TKA (c) appears to be similar to the same tissues from aseptically failed total hip arthroplasty (d).
The aim of this review is to summarize the present knowledge on the etiology and pathogenesis of periprosthetic osteolysis of TKA.
Early bone changes after TKA surgery
The critical structure supporting TKA components is the periprosthetic bone bed. The fate of the TKA depends on the long-term ability of bone to withstand mechanical stresses and strains without deterioration of its micro-architecture and density, which would jeopardize the integration and support of the implant. This depends at least partially on the magnitude of mechanical stresses, the bone tissue vitality, and the remodeling around the bone-implant interface.
The bone mineral density around a TKA decreases within several months after implantation. The loss in bone density reaches up to almost 23% within one year postoperatively. The reported values depend on the preoperative and post-surgical mechanical alignment, location of measurement, and on the method of assessment [7, 8]. Postoperative loss of bone density is the result of the surgical procedure, peri-operative inflammation and bone remodeling associated with postoperative alterations in mechanical load. On the other hand, there is no evidence that an extensive periosteal soft tissue release around the medial part of the proximal tibia is associated with an increased risk for bone damage. Regardless of the particular cause of an early bone mineral density loss, periprosthetic bone density generally normalizes in the majority of patients at the end of 3 years [9].
Mechanical theories underlying periprosthetic osteolysis around TKA
Development of periprosthetic osteolysis can be linked to the long-term influence of mechanical forces on both, the joint replacement device and the bone bed. Under ideal conditions, the interface between the implant and bone bed should withstand repeated mechanical stresses associated with activities of daily living. Along this line, the major factors that would limit the mechanical stability of TKA and its longevity are the accuracy of the surgical reconstruction, the fixation of the implant to the bone bed, and factors influencing bone vitality and remodeling.
There is considerable evidence that relates abnormal mechanical stresses/strains to prosthesis/knee malalignment [10–12]. Abnormal forces associated with a limb/prosthetic malalignment can degrade the bone cement layer anchoring implants to the bone [13]. The integrity of the bone–cement interface is especially critical to implant survival [14–16]. In this line, deterioration of bone structure around the areas of cement fixation observed in some TKA postmortem specimens might be understood as a reaction to long-term overload of bone [17]. On the other hand, little is known about intraoperative- and postoperative factors supporting bone vitality and remodeling after the TKA surgery.
What influences the magnitude of axial forces at the knee joint
The magnitude of force generated at the knee joint during each step is directly proportional to body weight. Using an instrumented knee implant in five subjects, average peak resultant forces were determined in vivo [18]. They were highest during stair descend (~350% BW), followed by stair ascending (~300% BW), and level walking (~250% BW). Chair rising and sitting down generated similar peak forces as level walking (~250% BW and ~225% BW, respectively). Every extra kg of body weight is at least linearly related to contact force, although, applying a regression model, it has been recently suggested that every kg will generate up to 40 N more compression at the tibio-femoral articulation [19]. This could explain, at least in part, the higher incidence of radiolucent lines around TKA and the increased rate of aseptic loosening observed in severely obese patients compared to those who are moderately overweight [20]. On the other hand, obese patients walk on average less in comparison to those who are non-obese [21]. Thus, cumulative overload may be decreased in obese patients.
An optimal surgical alignment of the limb axis/TKA allows for a better distribution of mechanical forces to the surrounding bone bed and, vice versa, limb/prosthesis malalignment can result in localized mechanical overload with increased polyethylene wear, an induction of bone cement cracks, and prosthetic bone bed damage (Fig. 2). Even small deviations in the alignment of the tibial component, as little as 3°, an overall neutral alignment of the limb may lead to an earlier failure and the acceleration of polyethylene wear [13]. Similarly, a tibial component malalignment increases the risk of medial bone collapse and implant failure [22, 23]. Poor axis and component alignment, combined with poor fixation, may lead to micromotion and early symptomatic loosening.
Figure 2.
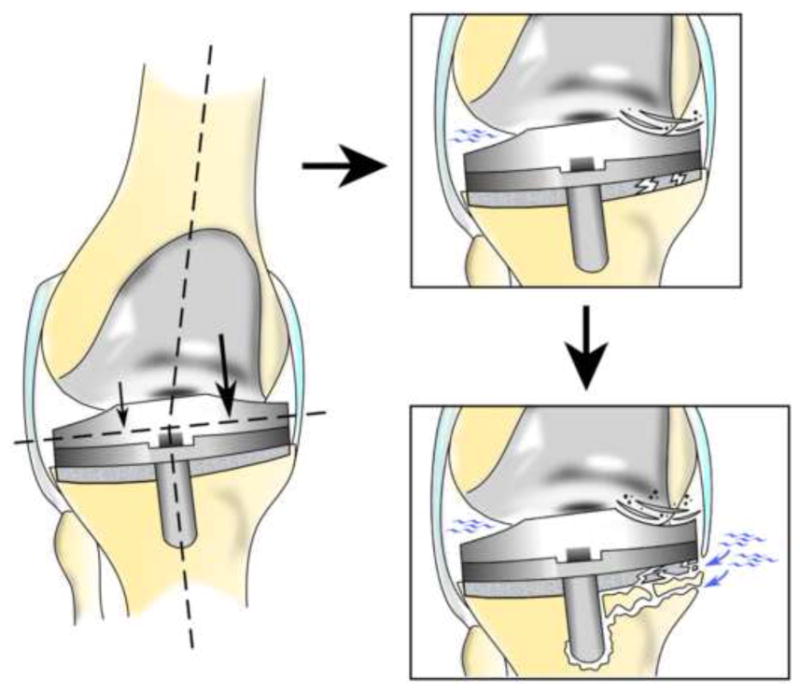
Any deviation from the optimal alignment of the limb axis can lead to a localized increase in surface contact pressure. This may lead to increased polyethylene wear, mechanical overload of the underlying bone with direct bone damage, as well as bone cement crack initiation and growth opening the prosthetic bone bed to the ingress of joint fluid.
Finally, the integrity and function of the muscles of the lower limb influence the magnitude of contact forces in the prosthetic knee during walking [24]. Several studies have shown the importance of the quadriceps and gastrocnemius muscles in the outcome of TKA [25, 26]. Both, vasti and gastrocnemii muscle groups are able to create higher forces in a knee joint when they are co-activated simultaneously. These muscles induce peak forces in the tibio-femoral joint during the gait cycle, which are about 2.5 to 2.8 times the body weight, or approximately up to 2100N for a 75kg patient [27]. Even muscles, which do not cross the joint, can indirectly influence the contact forces to the extent that they affect the gait pattern and ground reaction forces [28]. Accordingly, the gluteus maximus, soleus and other muscles can greatly influence knee joint loading [29]. Muscles around the knee do not only form a dynamic unit enabling movement, but are also responsible for energy absorption to prevent overload of the knee [30]. Muscles in physically well-conditioned patients can therefore decrease the total load applied to a TKA. The main stabilizer of the knee joint is the quadriceps muscle group and weakness or atrophy greatly reduces their protective potential. Impaired muscle function with related gait disturbances is potentially dangerous for implant survival and sometimes responsible for residual disability in patients after TKA [24, 31].
Accumulation of mechanically induced changes
There is no doubt that cumulative mechanically induced changes in the bone-cement-implant interface and the polyethylene liner contribute to the failure of TKA (thus the phrase “wear is a result of load and use”). The physical activity of the patient strongly affects the implant survival in terms of aseptic loosening and periprosthetic osteolysis, as in hip arthroplasty. Schmalzried et al. showed large inter-individual differences in daily activity, with the most active patient taking 3.2 million steps per year whereas the least active patient took only 72,000 steps per year [32]. There is a significant difference between men and women in walking activity; men appear to be more active than women. Additionally, patients who were less than sixty years old were more active than those who were sixty years old or above. Overloading seems to correlate at least partially with higher activity levels and, along this line, younger age and male gender may be at increased risk for aseptic loosening and osteolysis in TKA. It would be useful to determine an “index of usage”, which would integrate the type and level of a various activities for a particular patient (age, gender, body weight, job activities, hobbies etc.).
A typical example of the role of cumulative changes can be seen at the bone-cement-implant interface (Fig. 2). At first, during each step, high stresses are generated at this interface without any signs of damage. However, after some time, dependent on size/duration of stresses and interface properties, these stresses accumulate leading to crack initiation and propagation, and ultimately to fatigue of the bone-cement-implant interface [33]. There are several reports that show how high stresses/strains in the bone cement can lead to material fatigue, with cement creeping and cracking, reduction of its stiffness, as well as debonding from the implant, increasing micro-motion at the interface [34–37]. Little is known on the mechanisms of sequential trabecular resorption adjacent to cemented TKA compromising potentially the fixation of cemented component to the bone bed [17]. However, it is important to distinguish failure due to cumulative stresses at an originally firm bone-implant interface from the early debonding, which can occur at the tibial component [15, 16].
The role of hydrodynamic pressure in creation of bone defects
With every step, polyethylene particles are released from the top and bottom surfaces of the polyethylene liner. These particles and the joint movement stimulate the formation of a pseudosynovial lining membrane secreting fluid [38] which is accumulated in the TKA joint cavity creating conditions for the generation of hydrodynamic forces influencing the periprosthetic joint space during each movement in the knee [39]. Intermittent waves of fluid pressures act on the accessible bone bed, soft tissues and implant-bone interface (Fig. 3).
Figure 3.
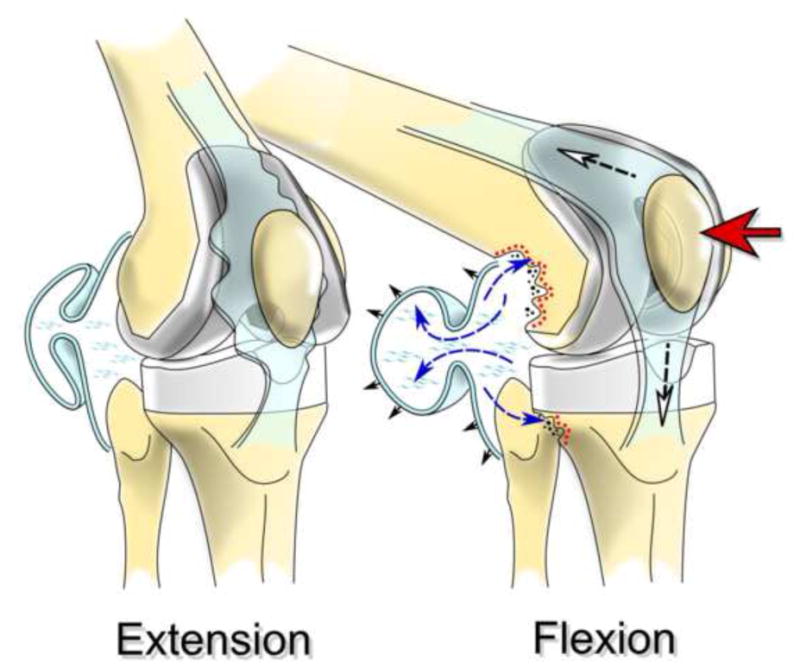
Increased hydrostatic pressure accompanying movement of the knee joint can distribute implant-derived wear products throughout the effective joint space and induce chronic inflammation, osteoclast formation and osteolysis.
Hydrodynamic forces can directly damage bone surfaces around an implant especially in those parts of the knee with a spongy bone surface (“places of the smallest resistance”). In one rabbit model it was demonstrated that an intraarticular pressure of 150 mm Hg can directly induce osteocyte death and prevent new bone formation [40]. Other studies have further investigated the effects of capsular pressure and high velocity fluid flow on bone resorption and implant debonding induced by hydrodynamic phenomena [41–44]. The size of the pressure (and velocity of the flow) in a particular TKA depends not only on the fluid volume, but also on the size of the joint space, joint position, mechanical compliance of the joint capsule and the drainage of the joint fluid into the periarticular lymphatic vessels and interstitial tissue space [45, 46]. Fluid pressure inside a hip joint cavity can reach up to 776 mm Hg during different mechanical maneuvers [47], but similar data are not available from TKA.
Biological theories of periprosthetic osteolysis
Alternative theories suggest that periprosthetic osteolysis is the result of predominantly biological mechanisms occurring around TKA. In this context, “biological” refers to ischemia, inflammation, fibroblast-, osteoblast-, osteocyte-, osteoclast-driven, and probably also allergic factors (Fig. 4). Wear debris generated from TKA surfaces plays an important role to trigger osteolysis [48]. Other factors that may be important, but cannot be readily analyzed include “non-particle” interactions between the implant and surrounding tissues that are generally included in the term of “biocompatibility”. These may be related to biochemical reactions on the implant surface with host proteins/cells/tissues leading to an either adaptive or maladaptive phenotype of periprosthetic tissues including a fibrous capsule formation around an implant [49, 50]. Additionally, the quality of the initial implant fixation, surgical trauma, specific implant-bone loading pattern, implant stiffness, characteristics of the bone bed, synovial and interstitial fluid pressure waves, and the exposure to microbe-derived pathogen-associated molecular patterns from indolent biofilm or endogenous damage-associated molecular patterns stimulation may play a role [15, 16, 51]. Finally, genetic susceptibility to osteolysis and aseptic loosening at the site of total hip arthroplasty has been reported, while data related to aseptically failed TKA are not available [52, 53].
Figure 4.
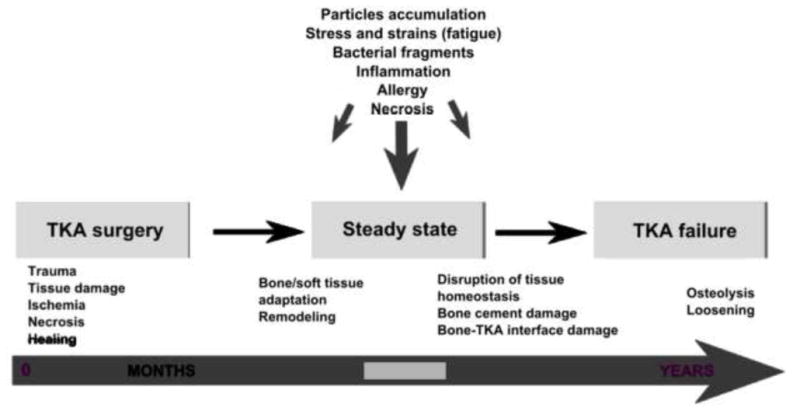
Several lines of evidence suggest that periprosthetic osteolysis as a time-related process is closely associated with mechanical and biological etiologic factors. However, in the majority of total knee arthroplasties this process and revision are postponed to the second or third decade after the surgery or may not emerge at all, with the patient escaping this fate.
Source and type of prosthetic particles
Despite clear clinical similarities between periprosthetic osteolysis around the total hip arthroplasty and TKA, there are also important dissimilarities, at least in terms of type and size of particles. The generation of wear particles starts immediately after surgery, even in cases with well-fixed and oriented components, as in total hip arthroplasty [54]. Frictional forces resulting from a rolling/sliding motion of the knee joint are the main source of wear debris, and all four major wear mechanisms, i.e. adhesive wear, abrasive wear (including third-body wear), surface fatigue and tribo/electrochemical wear may contribute [55]. In TKA, the range of particle size and mechanisms of wear are different from those seen in total hip arthroplasty. Shanbhag et al. found that polyethylene particles detached from TKA surfaces are larger and more irregular in shape than those produced in total hip arthroplasty [56]. Others correlated in size and number of polyethylene particles with the type of surface damage [57]. Smaller, more biologically active particles are generally created during repeated rolling, sliding and rotational motion on bearing surfaces by adhesion and abrasion, while larger particles are produced as a result of surface fatigue, namely delamination (flaky debris) and pitting (round debris). A high proportion of the small particles is generated by back-side wear [58–60]. Recent studies reported a decrease in survivorship of rotating platform knees, at least in part, because of increased backside wear [61]. Differences in conformity between total hip and knee arthroplasty may also explain the disparity observed in the size of the wear particles [59, 62].
In comparison to the hip replacement, the TKA offers generally a higher surface area for wear processes. For instance, a 28-mm polyethylene cup in total hip arthroplasty has a surface area of 1232 mm2 while TKA liner for a 64 mm tibial component can have a surface area of around 2500 mm2. Under the assumption that each milligram of polyethylene contains up to 1.3×1010 of submicron particles [63], the total number of particles detached from TKAs can be enormous. However, the particular particle load depends on the particle size and the total wear of a polyethylene liner and thus, may differ between implant designs [64]. In fact, the distribution of wear products around TKA is partially a function of a larger joint space available in comparison to hip arthroplasty. As a result, knee replacements could demonstrate a lower number of particles per unit of surface compared to hip replacements. Additionally, particles can accumulate in dissecting popliteal cyst-like structures [65].
Additionally, some differences can be related to the manufacturing of polyethylene bearings in TKA. Although a number of studies demonstrated that compression-molded inserts have a better wear-resistance than machined components when articulating against the femoral condyles (front side wear), other studies suggested that these polyethylenes can have problems with backside wear [66–68]. The main mechanism responsible for the failure of polyethylene inserts in TKAs is a combination of mechanical stresses and persistent oxidative degradation, with the latter facilitating the fatigue of the former. Oxidation is tightly associated with particular processing and sterilization methods of the polyethylene [59].
Another difference lies in the coefficient of friction (μ). In the case of TKA, abnormally high tractive-rolling forces may act at the tibial plateau in comparison to the natural joint (μ = 0.03–0.10 versus μ = 0.002–0.03). Tractive-rolling forces are unique to the knee joint and do not occur in the hip which undergoes pure sliding only. Generally, large tractive forces can increase polyethylene wear. Higher tractive forces may be induced by abnormal gait patterns and muscle function, which alter the normal tangential shear stresses at the interface. Repeated rolling combined with increased tractive forces during joint movement may therefore facilitate fatigue damage [24, 69].
Chronic inflammation and the development of osteolysis
The size of osteolytic lesions and the subsequent risk for aseptic loosening depend in part on the host’s response to polyethylene, polymethylmethacrylate, and metallic wear particles. Accordingly, their features (origin, particle size, concentration etc.) are key parameters of particle disease. In this line, prosthetic particles stimulate cells within periprosthetic tissues after opsonization by host proteins (which may include damage-associated molecular patterns or alarmins) and/or bacterial by-products (pathogen/microbe-associated molecular patterns). Particle-host protein/bacterial fragment complexes are phagocytosed by macrophages (and other cells of the innate immune system), or stimulate these cells by non-phagocytic processes [70]. For instance, it has been suggested that cobalt ions can directly stimulate cells via dimerization and activation of Toll-like receptor 4, similar to lipopolysaccharide (endotoxin) [71, 72]. In response to phagocytosis/non-phagocytic stimulation, the macrophages (other cells) express chemokines, cytokines, proteolytic enzymes, reactive oxygen and nitrogen species, prostaglandin E2 and other substances of inflammation, leading eventually to numerical/functional predominance of osteoclasts in induced periprosthetic bone multicellular units at bone-implant interface (Fig. 5). Simultaneously, inflammatory signals direct the growth of pseudosynovial/interface and granulomatous tissues, and also the secretion of large amounts of joint fluid, all contributing to the expansion of resorption cavities around the TKA [73, 74].
Figure 5.
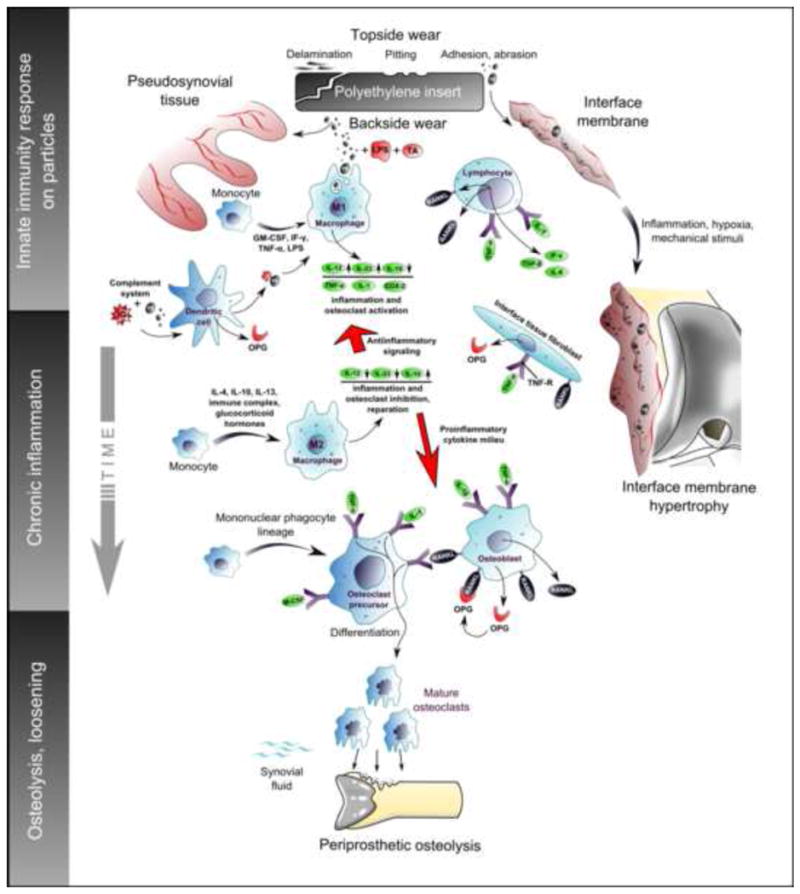
Polyethylene particles detached from TKA surfaces activate several cell lines of innate and adaptive immunity. Polarization of macrophages into classical M1 phenotype is responsible for triggering and perpetuation of chronic inflammation, which facilitates growth of the interface membrane. Alternatively polarized M2 macrophages produce anti-inflammatory cytokines and homeostatic repair molecules. Thus, the fate of implant and periprosthetic tissues may depend on the balance between M1 and M2 polarized macrophages. Similar rules could regulate also the behavior of fibrocytes, fibroblasts and other pertinent cell populations.
Abbrev.: COX2 - Cyclooxygenase-2; GM-CSF-granulocyte macrophage colony stimulating factor; IFN-γ – interferon gamma; Ig – immunoglobulin; IL- interleukin; LPS-lipopolysaccharide; M-CSF – macrophage colony stimulating factor; OPG – osteoprotegerin; RANKL – receptor activator of nuclear factor kappa-B ligand; TA - teichoic acid; TGF-β - transforming growth factor beta; TNF-α – tumor necrosis factor alpha
Prosthetic particles are not the only source of inflammatory signals around TKA. Low pH and hypoxia can also contribute to this fate [75, 76]. Santavirta et al. introduced a concept of the ischemic-reperfusion injury of the interface membrane that might contribute to premature aseptic loosening [77]. Important sources of hypoxia are increased oxygen consumption by local inflammatory cells and impaired blood supply as a result of the surgical procedure and the presence of a large avascular implant in bone. A hypoxic environment induces the expression of hypoxia-inducible factors (HIF-1α, HIF-2α) and heat shock proteins that adapt the gene expression and cell metabolism to lower oxygen availability within the hypoxic tissue. Several studies show that ischemia stimulates macrophages and fibroblasts to express inflammatory cytokines [78–80]. Recently, it was shown that there was an increased expression of VEGF in fibroblasts from a failed total hip arthroplasty compared to controls with osteoarthritis [81]. Other studies show that the mechanism of cell death (proportion of necrosis to apoptosis) in the periprosthetic tissues could also contribute to the inflammatory environment around an implant and risk for TKA failure [82–86].
Delayed type T-cell mediated hypersensitivity has been investigated in the failure of total joint arthroplasty [87, 88]. All metal prostheses, in particular MoM gliding surface and junctions, can release metal ions following tribocorrosion in body fluids and these might fuel allergic reactions [89]. Hypersensitivity can be defined as an abnormal immunological reaction upon exposure to a specific substance [90, 91]. In contrast to toxic reactions, there is no simple dose-response relationship, with higher doses being more potent than lower doses, because even small amounts of allergen can cause strong reactions, i.e. hypersensitivity. Metal ions released can bind to self-peptides located in the antigen-binding groove of the major histocompatibility complex class II molecule, modifying the self so that it is recognized as non-self by T cells. Immune reactions described adjacent to metal implants could be Th1 dominant. Hence, increased levels of IFN-γ, TNF- α, IL-18 and other cytokines should be observed in metal hypersensitive joint arthroplasty patients [92]. However, the Th2 and Th17 hypotheses have not been adequately evaluated yet. Second, it is difficult to prove that an adverse host response is immunologically mediated. A typical T-lymphocyte rich immunohistopathology, associated with positive epicutaneous patch test to a specific implant-derived allergens and healing of the response upon exchange of the implant to an immunologically inert implant could be considered as minimal requirements for the demonstration of hypersensitivity. This should preferably be combined with the demonstration of oligoclonal T cell activation in peri-implant tissues. Third, it is still not known if and how much classical hypersensitivity to metallic particles contributes to osteolysis and aseptic loosening in TKA. A strong argument against the hypersensitivity theory is the clinical finding of a relatively low revision rate related to “allergic” loosening. Given that 10% of the population is hypersensitive to the materials found in jewelry and joint replacements, and metallic debris and ions can induce allergic reaction in periprosthetic tissues [93], surgeons should be revising hundreds of thousands of patients with total hip and knee replacements worldwide due to “allergic osteolysis and aseptic loosening”. Hypersensitivity could be one of the mechanisms leading to failure, but the role for hypersensitivity as one of the mechanisms responsible for aseptic loosening remains open until new methods enable reliable classification of aseptically failed TKA to allergic and non-allergic cases.
Apart from classical delayed type hypersensitivity, metal ions can induce degradation of self-proteins so that instead of dominant and tolerogenic antigenic determinants cryptogenic and immune activating determinants are produced and by this way peripheral tolerance is lost [94]. However, currently there is no indication of such a phenomenon in aseptic implant loosening. Finally, adverse reactions to metal debris can arise, apart from direct toxic and necrotic effects at higher concentrations, via direct homodimerization and stimulation of TLR4 by cobalt ions (Co2+) exerting a lipopolysaccharide-like effect on TLR4-equipped cells [71, 72].
Is the particle disease in knee arthroplasty similar to hip arthroplasty?
The majority of research on periprosthetic osteolysis has been based on studies of total hip arthroplasties. However, from a pathobiological point of view it is likely that the host’s response to a TKA and wear debris is not completely different from the host’s response to a total hip arthroplasty. From a purely surgical point of view, the interface surrounding the aseptically loosened TKA appears to be similar to tissues around aseptically failed total hip arthroplasty (Fig. 1). In fact, these tissues are similar in terms of cellular profile, structure, chemokine/cytokine signaling, and enzyme profiles [95]. On the other hand, we revealed a lower number of inflammatory and osteoclastogenic cytokine transcripts in pseudosynovial tissues from aseptically failed TKAs compared to total hip arthroplasties (unpublished data).
Osteolysis as a result of increased osteoclast activity
Briefly, this concept is based on the evidence that wear debris stimulates an innate host immune response leading to chronic low-grade inflammation and increased osteoclast formation, accumulation and maturation [70]. Alternatively, an increased accumulation of hyperactive osteoclasts around bone-implant interface could be related to the stimulation of osteocytes by peri-implant joint fluid pressure [44, 96]. The osteoblast phenotype around implant may change so that it supports the osteoclast differentiation and inhibits bone formation [97]. This would further contribute to a local predominance of bone resorption over formation in periprosthetic bone [98].
TNF-α and IL-1β trigger and other molecules perpetuate and regulate inflammatory pathways involved in particle disease. Based on that, the fate of TKA induced inflammation depends at least partially on the result of macrophage polarization, and number and status of fibroblasts in the pseudosynovial/interface membrane (Fig. 6), [99–101]. The high sphingosine-1-phosphate concentration in blood plasma leads to migration (egress) of circulating monocytes to bone marrow [102] and probably to peri-implant tissues as well. In periprosthetic tissues macrophage-colony stimulating factor (M-CSF) works as a survival factor for monocytes. Chemokines like CCL2 (acting via CCR2 or CCL4) and CXCL12 (acting via CXCR4) can further attract monocyte/macrophages from blood to the periprosthetic synovial-like fibrous membrane and then to the bone-implant interface [103]. Finally, on the bone surface the RANKL-RANK-OPG interaction plays an essential role in osteoclastogenesis [104], with TNF-α supporting survival and differentiation of osteoclasts, whereas IL-1β also supports osteoclast activation. Osteoblasts, bone marrow stromal cells, fibroblasts, lymphocytes and mast cells in the periprosthetic membranes are the major producers of RANKL [105–108]. Elevated RANKL expression was identified in the interface membranes retrieved from patients revised due to osteolysis [109–111]. On the other hand, osteoprotegerin (OPG) blocks the maturation of osteoclasts and thereby decreases their resorption [104]. The local ratio between RANKL and osteoprotegerin could relate to the size of the osteolytic defect [112, 113]. On the other hand, the RANKL-RANK-OPG axis is not the only signaling pathway regulating osteoclast maturation and activity [114]. Recently, it was revealed that another co-stimulatory molecule called OSCAR could play an important role in osteoclast differentiation, maturation and activity [115]. Despite the vast body of evidence that supports the role of the osteoclast number and activity in the process of periprosthetic bone resorption around total hip arthroplasty, there is only weak evidence for the same mechanism in association with aseptically failed TKA.
Figure 6.
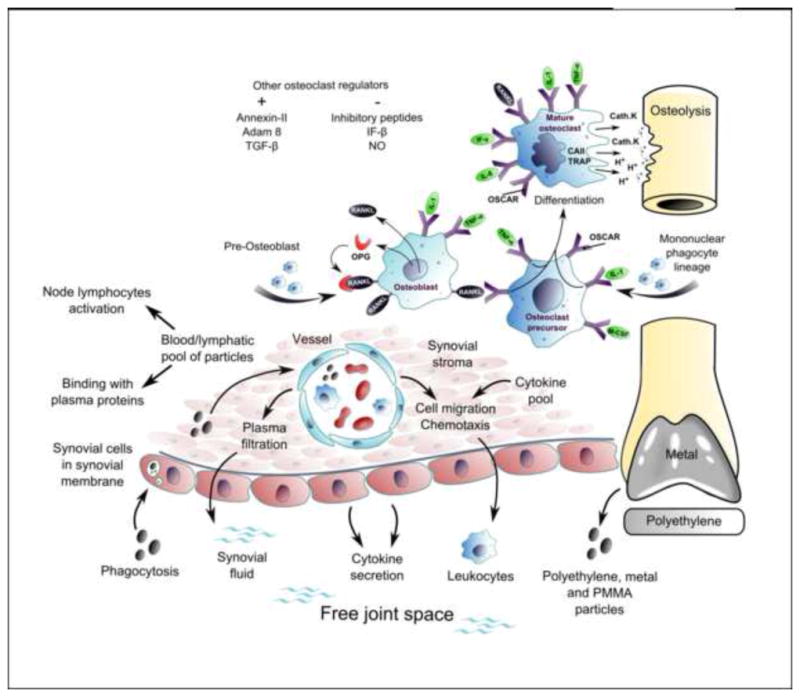
The key feature of periprosthetic osteolysis is an over-activation of bone resorbing osteoclasts, which occurs upon the assembly, activation and imbalanced function of the bone multicellular units around bone-implant interface, leading to net loss of bone. Precursors of osteoclasts (mononuclear monocyte/macrophage lineage) are recruited from blood/bone marrow pool with participation of various chemokines such as CCL2, CCL5, CXCL12, and CX3CL1. Osteoclast differentiation, maturation, activity and apoptosis are under strict control of a number of genes/signaling molecules.
Abbrev.: CAII – carbonic anhydrase II; Cath. K – cathepsin K; GM-CSF - granulocyte macrophage colony stimulating factor; IFN- β, γ – interferon beta, gamma; Ig – immunoglobulin; IL- interleukin; OPG – osteoprotegerin; OSCAR – osteoclast- associated receptor; PMMA – polymethyl methacrylate; RANKL – receptor activator of nuclear factor kappa-B ligand; TGF-β - transforming growth factor beta; TNF-α – tumor necrosis factor alpha; TRAP – tartrate-resistant acid phosphatase
Integral theory of periprosthetic osteolysis around TKA
Based on the above, it is clear that mechanical and biological mechanisms do not act separately in the processes of osteolysis and aseptic loosening. Accordingly, osteolysis and aseptic loosening of TKA can be considered to represent the result of a harmful combination of mechanical and biological events leading to failure of the bone-implant interface and the underlying bone bed. Both etiologic factors are operative from the early postsurgical time to the final event of aseptic loosening. For example, wear particles from the polyethylene liner trigger a complex host response with increased secretion of joint fluid (“particle-induced synovitis”) produced by fibroblast-like type B synoviocytes of the surface layer of a pseudosynovial tissue around the TKA joint cavity. High joint fluid pressure can induce tissue ischemia and even direct bone necrosis [46, 116, 117]. Additionally, increased joint fluid pressure could influence the osteoclast differentiation, their cytokine gene expression and may also lead to a lack of anabolic effect in bone remodeling [118].
The amount of joint fluid together with the pressure generated contributes in part to the expansion of “effective joint space”. This facilitates the delivery of wear particles, inflammatory mediators, signaling molecules and other substances to areas distant from the bone-implant interface further intermixing mechanical and biological processes around TKA.
Osteolysis as a result of interface membrane growth
Voids at the cement-bone interface are always immediately present after TKA surgery. Existing “empty spaces” at the bone-implant interface are eventually filled by fibrous tissue containing only a few cells and blood vessels. Repeated mechanical stress on the membrane during each movement step induces trauma and hypoxic conditions leading to proliferation of fibroblasts that synthesize an abundant extracellular matrix to adapt to the stresses and strains around the implant. It has been shown that the mechanical environment can induce the differentiation of mesenchymal stem cells via up- and/or down-regulation of mechano-sensitive genes and in this way biomechanical loads can influence tissue differentiation resulting in excessive formation of fibrous tissue [119–121]. Interestingly, macrophages significantly increase their expression of TNF-α, IL-1β and IL-6 when they are treated conjointly with polyethylene particles and pressure [122, 123]. Taking this into account, it must be concluded that mechanical conditions can modify the proliferation and expression capacity of distinct cell groups participating in periprosthetic osteolysis and aseptic loosening. As a result, the interface membrane grows around the implant and becomes hypertrophic and highly infiltrated by macrophages, fibroblasts, mast cells, and multi-nucleated giant cells by the time of revision surgery [124, 125].
Recently, an alternative mechanism of periprosthetic osteolysis has been proposed; this assumes that pressurized fluid flow along the bone implant interface stimulates sensors of osteocytes in the periprosthetic bone, subsequently inducing the expression of cytokines involved in osteoclast differentiation [96]. Taken together, the examples above and other data support the concept of aseptic loosening and osteolysis as a condition caused by mechanical factors and biological host responses acting in synergy.
Clinical impact of research on periprosthetic osteolysis
Many aspects of periprosthetic osteolysis and aseptic loosening remain to be elucidated because of their very complex and multifactorial pathogenesis. Due to the same reasons, it may be that some causative agents are patient specific and not applicable to the general population. On the other hand, some “pathogenic” conditions have been revealed and may be used for preventative or even curative intervention.
Patient-related factors
Patient-related risk factors like age, gender, primary diagnosis, and individual susceptibility to osteolysis and chronic inflammation cannot be influenced by any known intervention. Even factors like patient weight are difficult to influence. Some other factors that have shown to increase polyethylene wear and/or mechanical stresses at the implant/bone interface can be at least partially influenced. For instance, the type, extent and intensity of physical activity could be modified to avoid a premature TKA failure. However, there is a relative paucity of literature data directly relating polyethylene wear to patient activity in TKA, and many recommendations are based on simplified biomechanical models [126] which solely provide information on level walking. Although this is the most frequent dynamic activity of daily living, it does not exclude the possibility that other less frequent activities may be more damaging. More representative wear scars have been obtained during in vitro testing, when other activities of daily living were included and generated wear rates were higher [127]. Gait kinematics has shown to be variable among patients, even for the same knee implant design [128, 129]. It is likely that this has effects on polyethylene wear and implant longevity. Therefore, a preventative intervention could include the training of muscles around the knee and hip together with gait analysis. The correction of gait may have also potential to reduce the loads at the knee joint [130].
Implant-related factors
Improved implant design and material properties can significantly decrease wear rate in TKA [59]. Although less conforming designs with flatter tibial surfaces result in increased polyethylene fatigue-wear, a recent retrieval study concluded that more conformity actually may increase surface fatigue damage [62]. Constraint-induced stresses due to knee kinematics were held responsible for this finding and thought to offset the lower contact stresses from compressive forces.
Previously, it was found that the method of manufacturing and sterilization of polyethylene affects its clinical performance. In general, based on registry studies and clinical cohorts, it is possible to identify implants with long implant survival and those should be preferred in clinical practice over those without long-term clinical follow-up data. Great expectations have been placed on the potential use of highly cross-linked polyethylenes (HXPEs) in clinical practice. However, currently there is only limited evidence in favor of their use in TKA [131]. Cross-linking decreases the fatigue resistance of polyethylene [132]. Additionally, some in vitro studies demonstrate that HXPE wear debris could induce higher specific functional activity in comparison to non-HXPE debris [133]. Alternative bearings made of modern ceramic or even completely new biomaterials such as polyetheretherketone might play a role in the future [134]. Currently, there is very limited experience with ceramic-polyethylene bearings in TKA and even less with ceramic-on-ceramic TKA [135, 136]. Regarding the latter, a main concern is related to fragility of the ceramic-ceramic bearings, which could face high peak impact forces in the knee joint. Femoral components made of oxidized zirconium (OxZr, a metallic Zr-Nb2.5 alloy base, an oxygen enriched metal transition zone and an oxidized Zr ceramic surface) have been proposed as another alternative to reduce wear in TKA [137, 138].
If hypersensitivity contributes to loosening it should be possible to utilize implants with surface treatment aimed at avoiding the dissolution of metallic allergens from the body of an implant. There is some evidence that this could be one approach to the hypersensitivity problem [139, 140]. However, this solution requires valid screening methods identifying all suitable candidates for such implants preoperatively [141, 142].
Surgeon-related factors
A meticulous surgical technique is essential to minimize the risk for premature failure due to aseptic loosening, osteolysis, and other complications in TKA [15, 16]. Known factors such as the failure to restore the mechanical axis of the limb, poor component alignment, and/or instability of TKA, all potentially result in increased loading forces across the bone-implant interface and have shown to correlate with polyethylene damage [143]. Computer-assisted devices (navigation) were introduced into clinical practice to improve the position of TKA components and alignment of the limb as well as to avoid local implant/polyethylene overload. Assuming these tools are reliable and cost-effective, their wide usage could decrease the revision burden in the future. Studies demonstrating more reproducible implantation of TKA components in patients that were operated using navigation are currently available [144]. However, long-term data showing better clinical survival of navigated surgery over non-navigated are not available [145].
The bone–cement interface is especially critical in terms of the implant survival [146]. However, consensus on what is the single best technique for cementation in TKA still remains unclear. Assuming inter-surgeon differences in this parameter, the outcome of TKA might be biased. Further research in the field of the optimal cementing technique is highly warranted [147]. Surgeon education and training opportunities should focus on achieving optimal implant position and restoration of the correct mechanical axis of TKA as well as providing a durable implant-cement-bone interface.
Pharmacological interventions
Understanding the pathogenesis of periprosthetic osteolysis enables the development of effective preventative and therapeutic measures. Basically, pharmacological interventions could target chronic inflammation and osteoclast maturation/survival. The main problem is “how and when” to deliver these agents to the growing synovial-like interface membranes and the imbalanced bone multicellular units around the TKA. Systemic administration could represent an overtreatment in this context.
One large recent study indicates that early postoperative systemic administration of bisphosphonates could decrease the risk for aseptic loosening in TKA [148]. In addition, a recent meta-analysis of 14 randomized controlled trials, including two conducted on patients with TKA, reports a moderate therapeutic effect of early bisphosphonate administration on periprosthetic bone loss after total joint arthroplasty [149]. This could be due to a blocking of the early postoperative bone resorption related to surgical trauma, inflammation and disuse osteopenia. There is also at least one clinical study showing a positive effect of long-term administration of alendronate on femoral bone density in total hip arthroplasty [150]. In this line, some concern exists related to the long-term administration of bisphosphonates that could be associated with bone necrosis and atypical fractures in long bones [151]. However, the risk for atypical femoral fracture seems to be only about 5 cases per 10,000 patient-years [152].
Experimental studies show that a blockade of RANK signaling with soluble RANK:Fc (which binds to RANKL and prevents this ligand mediating RANK signaling) prevents wear debris osteolysis [153, 154]. Similar data are available for other osteolysis-targeted therapies, e.g. anti-cytokine (e.g. anti-TNF, anti-IL1/-IL-6), anti-enzyme (e.g. inhibition of MMP-9) or hormonal (e.g. parathyroid hormone, calcitonin), [155–163]. However, none of these strategies is yet approved for treatment of periprosthetic osteolysis and aseptic loosening in TKA.
Recently, it was suggested that statins could decrease the risk of aseptic loosening in total hip arthroplasty [164]. The mechanism of their action is currently not completely elucidated. Statins affect the mevalonate pathway producing farnesyl- and geranylgeranyl pyrophosphates, which post-translationally modulate bone metabolism regulating small G proteins. Additionally, this could be associated with attenuation of the particle-induced activation of monocytes and/or production of IL-6 [165, 166]. Finally, anabolic steroids have positive effects on the musculoskeletal system, therefore, some investigators have used them in order to increase the muscle size and strength and diminish early bone resorption [167]. However, again there is still insufficient clinical research evidence to recommend this practice.
Future research directions
In order to reduce the generation of wear particles, future research should be directed towards a better understanding of the biomechanics and kinematics of the TKA. This data should be based on important activities of daily life, including recreational activities, and could facilitate the planning of more relevant pre-clinical wear testing scenario. Along this line, it is necessary to develop new models and paradigms to improve our understanding of mechanical forces and kinematics of osteolysis and aseptic loosening of TKA. Based on this concept, clinicians would have the opportunity to include patient specific data from biomechanical and kinematic examinations into his/her evaluation and indication for TKA. After surgery, these instruments could also facilitate the monitoring of the performance and outcome of a TKA.
Further, research activities on tissue responses that occur with TKA implants and their wear byproducts will shed new information on avenues for the prevention and treatment of adverse outcomes. It seems that local tissues around TKA face somewhat different stresses, strains and particle loads than tissues around a total hip arthroplasty. It is also important to determine the type and extent of cross-talk between the pro-inflammatory and tissue-resident homeostatic cells that control the damaging processes associated with particle disease. Knowledge on the context-dependent regulation of monocyte/macrophages, fibroblasts, and other cell populations in the periprosthetic tissues may offer new therapeutic potential in the clinical management of particle-induced periprosthetic osteolysis. Determining the key tissue protective mechanisms that underlie the host accommodation to biomechanical stresses and prosthetic particles/ions is a first step. Thereafter, new biomaterial strategies, which combine biomimetic material with homeostatic modulators, may provide long-term maintenance of functional tissues around total joint arthroplasties.
Additionally, further progress in the understanding of osteolysis and aseptic loosening of TKAs could be facilitated with better analyses of potential risk factors for known pathogenic mechanisms. Patient-, implant-, and surgeon-related factors should be combined in a multivariate analysis. Data from knee arthroplasty registries should be part of the information used in this complex scientific framework. Analyzing data from large numbers of successful and unsuccessful TKA cases is a starting point to decrease the knowledge gap in this field. Ultimately, a thorough, interdisciplinary evaluation of implants currently in use, together with material scientists, biomechanical engineers, implant designers and surgeons may lead to vast future improvements in TKA survivorship.
Acknowledgments
The work on the review was supported by IGA Ministry of Health Czech Republic (IGA MZ CR NT/11049), Sigrid Jusélius Foundation, Finska Läkaresällskapet, the Danish Foundation for Strategic Research, Helsinki University Central Hospital and Orton Orthopaedic Hospital of the Invalid Foundation, and NIH grants 2R01AR055650-05, 1R01AR063717-01, and 1R01AR059843-02.
References
- 1.Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. The Journal of arthroplasty. 2011;26:615–20. doi: 10.1016/j.arth.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA: the journal of the American Medical Association. 2012;308:1227–36. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen AB, Mehnert F, Odgaard A, Schroder HM. Existing data sources for clinical epidemiology: The Danish Knee Arthroplasty Register. Clinical epidemiology. 2012;4:125–35. doi: 10.2147/CLEP.S30050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paxton EW, Furnes O, Namba RS, Inacio MC, Fenstad AM, Havelin LI. Comparison of the Norwegian knee arthroplasty register and a United States arthroplasty registry. The Journal of bone and joint surgery American volume. 2011;93 (Suppl 3):20–30. doi: 10.2106/JBJS.K.01045. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SK, Chu A, Ranawat AS, Slamin J, Ranawat CS. Osteolysis after total knee arthroplasty. The Journal of arthroplasty. 2007;22:787–99. doi: 10.1016/j.arth.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Naudie DD, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2007;15:53–64. doi: 10.5435/00124635-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Windisch C, Windisch B, Kolb W, Kolb K, Grutzner P, Roth A. Osteodensitometry measurements of periprosthetic bone using dual energy X-ray absorptiometry following total knee arthroplasty. Archives of orthopaedic and trauma surgery. 2012;132:1595–601. doi: 10.1007/s00402-012-1601-9. [DOI] [PubMed] [Google Scholar]
- 8.Soininvaara TA, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM, Kroger HP. Periprosthetic tibial bone mineral density changes after total knee arthroplasty: one-year follow-up study of 69 patients. Acta orthopaedica Scandinavica. 2004;75:600–5. doi: 10.1080/00016470410001493. [DOI] [PubMed] [Google Scholar]
- 9.Petersen MM, Nielsen PT, Lauritzen JB, Lund B. Changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty. A 3-year follow-up of 25 knees. Acta orthopaedica Scandinavica. 1995;66:513–6. doi: 10.3109/17453679509002305. [DOI] [PubMed] [Google Scholar]
- 10.Wong J, Steklov N, Patil S, Flores-Hernandez C, Kester M, Colwell CW, Jr, et al. Predicting the effect of tray malalignment on risk for bone damage and implant subsidence after total knee arthroplasty. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29:347–53. doi: 10.1002/jor.21221. [DOI] [PubMed] [Google Scholar]
- 11.Perillo-Marcone A, Taylor M. Effect of varus/valgus malalignment on bone strains in the proximal tibia after TKR: an explicit finite element study. Journal of biomechanical engineering. 2007;129:1–11. doi: 10.1115/1.2401177. [DOI] [PubMed] [Google Scholar]
- 12.Ritter MA, Davis KE, Davis P, Farris A, Malinzak RA, Berend ME, et al. Preoperative malalignment increases risk of failure after total knee arthroplasty. The Journal of bone and joint surgery American volume. 2013;95:126–31. doi: 10.2106/JBJS.K.00607. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A, Lee GY, Steklov N, Colwell CW, Jr, Ezzet KA, D’Lima DD. Effect of tibial component varus on wear in total knee arthroplasty. The Knee. 2012;19:560–3. doi: 10.1016/j.knee.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Lachiewicz PF, Soileau ES. The rates of osteolysis and loosening associated with a modular posterior stabilized knee replacement. Results at five to fourteen years. The Journal of bone and joint surgery. 2004;86-A:525–30. doi: 10.2106/00004623-200403000-00010. American volume. [DOI] [PubMed] [Google Scholar]
- 15.Arsoy D, Pagnano MW, Lewallen DG, Hanssen AD, Sierra RJ. Aseptic tibial debonding as a cause of early failure in a modern total knee arthroplasty design. Clinical orthopaedics and related research. 2013;471:94–101. doi: 10.1007/s11999-012-2467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pijls BG, Valstar ER, Nouta KA, Plevier JW, Fiocco M, Middeldorp S, et al. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta orthopaedica. 2012;83:614–24. doi: 10.3109/17453674.2012.747052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mann KA, Miller MA, Pray CL, Verdonschot N, Janssen D. A new approach to quantify trabecular resorption adjacent to cemented knee arthroplasty. Journal of biomechanics. 2012;45:711–5. doi: 10.1016/j.jbiomech.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, et al. Loading of the knee joint during activities of daily living measured in vivo in five subjects. Journal of biomechanics. 2010;43:2164–73. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis and rheumatism. 2005;52:2026–32. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 20.Berend ME, Ritter MA, Hyldahl HC, Meding JB, Redelman R. Implant migration and failure in total knee arthroplasty is related to body mass index and tibial component size. The Journal of arthroplasty. 2008;23:104–9. doi: 10.1016/j.arth.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Stickles B, Phillips L, Brox WT, Owens B, Lanzer WL. Defining the relationship between obesity and total joint arthroplasty. Obesity research. 2001;9:219–23. doi: 10.1038/oby.2001.24. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda S, Miura H, Nagamine R, Urabe K, Harimaya K, Matsunobu T, et al. Changes in knee alignment after total knee arthroplasty. The Journal of arthroplasty. 1999;14:566–70. doi: 10.1016/s0883-5403(99)90078-5. [DOI] [PubMed] [Google Scholar]
- 23.Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, et al. Tibial component failure mechanisms in total knee arthroplasty. Clinical orthopaedics and related research. 2004:26–34. doi: 10.1097/01.blo.0000148578.22729.0e. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg HJ, Foucher KC, Andriacchi TP, Wimmer MA. Direct comparison of measured and calculated total knee replacement force envelopes during walking in the presence of normal and abnormal gait patterns. Journal of biomechanics. 2012;45:990–6. doi: 10.1016/j.jbiomech.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HJ, Fernandez JW, Akbarshahi M, Walter JP, Fregly BJ, Pandy MG. Evaluation of predicted knee-joint muscle forces during gait using an instrumented knee implant. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27:1326–31. doi: 10.1002/jor.20876. [DOI] [PubMed] [Google Scholar]
- 26.Lin YC, Walter JP, Banks SA, Pandy MG, Fregly BJ. Simultaneous prediction of muscle and contact forces in the knee during gait. Journal of biomechanics. 2010;43:945–52. doi: 10.1016/j.jbiomech.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki K, Neptune RR. Individual muscle contributions to the axial knee joint contact force during normal walking. Journal of biomechanics. 2010;43:2780–4. doi: 10.1016/j.jbiomech.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zajac FE, Gordon ME. Determining muscle’s force and action in multi-articular movement. Exercise and sport sciences reviews. 1989;17:187–230. [PubMed] [Google Scholar]
- 29.Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. Journal of biomechanics. 2006;39:2623–30. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nature reviews Rheumatology. 2011;7:57–63. doi: 10.1038/nrrheum.2010.195. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clinical biomechanics. 2003;18:871–6. doi: 10.1016/s0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 32.Schmalzried TP, Szuszczewicz ES, Northfield MR, Akizuki KH, Frankel RE, Belcher G, et al. Quantitative assessment of walking activity after total hip or knee replacement. The Journal of bone and joint surgery American volume. 1998;80:54–9. [PubMed] [Google Scholar]
- 33.Waanders D, Janssen D, Mann KA, Verdonschot N. The effect of cement creep and cement fatigue damage on the micromechanics of the cement-bone interface. Journal of biomechanics. 2010;43:3028–34. doi: 10.1016/j.jbiomech.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DG, Miller MA, Mann KA. Creep dominates tensile fatigue damage of the cement-bone interface. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:633–40. doi: 10.1016/j.orthres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Cheng K, Pruitt L, Zaloudek C, Ries MD. Osteolysis caused by tibial component debonding in total knee arthroplasty. Clinical orthopaedics and related research. 2006;443:333–6. doi: 10.1097/01.blo.0000196044.42413.c7. [DOI] [PubMed] [Google Scholar]
- 36.Waanders D, Janssen D, Miller MA, Mann KA, Verdonschot N. Fatigue creep damage at the cement-bone interface: an experimental and a micro-mechanical finite element study. Journal of biomechanics. 2009;42:2513–9. doi: 10.1016/j.jbiomech.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JY, Tozzi G, Chen J, Contal F, Lupton C, Tong J. Bone-cement interfacial behaviour under mixed mode loading conditions. Journal of the mechanical behavior of biomedical materials. 2010;3:392–8. doi: 10.1016/j.jmbbm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Saari H, Santavirta S, Nordstrom D, Paavolainen P, Konttinen YT. Hyaluronate in total hip replacement. The Journal of rheumatology. 1993;20:87–90. [PubMed] [Google Scholar]
- 39.Skripitz R, Aspenberg P. Pressure-induced periprosthetic osteolysis: a rat model. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2000;18:481–4. doi: 10.1002/jor.1100180322. [DOI] [PubMed] [Google Scholar]
- 40.Van der Vis HM, Aspenberg P, Marti RK, Tigchelaar W, Van Noorden CJ. Fluid pressure causes bone resorption in a rabbit model of prosthetic loosening. Clinical orthopaedics and related research. 1998:201–8. [PubMed] [Google Scholar]
- 41.Fahlgren A, Bostrom MP, Yang X, Johansson L, Edlund U, Agholme F, et al. Fluid pressure and flow as a cause of bone resorption. Acta orthopaedica. 2010;81:508–16. doi: 10.3109/17453674.2010.504610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahlgren A, Johansson L, Edlund U, Aspenberg P. Direct ex vivo measurement of the fluid permeability of loose scar tissue. Acta of bioengineering and biomechanics/Wroclaw University of Technology. 2012;14:47–51. [PubMed] [Google Scholar]
- 43.Johansson L, Edlund U, Fahlgren A, Aspenberg P. Fluid-induced osteolysis: modelling and experiments. Computer methods in biomechanics and biomedical engineering. 2011;14:305–18. doi: 10.1080/10255842.2010.484808. [DOI] [PubMed] [Google Scholar]
- 44.Mann KA, Miller MA. Fluid-structure interactions in micro-interlocked regions of the cement-bone interface. Computer methods in biomechanics and biomedical engineering. 2013 doi: 10.1080/10255842.2013.767336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nade S, Newbold PJ. Factors determining the level and changes in intra-articular pressure in the knee joint of the dog. The Journal of physiology. 1983;338:21–36. doi: 10.1113/jphysiol.1983.sp014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alidousti H, Taylor M, Bressloff NW. Do capsular pressure and implant motion interact to cause high pressure in the periprosthetic bone in total hip replacement? Journal of biomechanical engineering. 2011;133:121001. doi: 10.1115/1.4005455. [DOI] [PubMed] [Google Scholar]
- 47.Hendrix RW, Wixson RL, Rana NA, Rogers LF. Arthrography after total hip arthroplasty: a modified technique used in the diagnosis of pain. Radiology. 1983;148:647–52. doi: 10.1148/radiology.148.3.6878678. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clinical orthopaedics and related research. 2001:71–7. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Rujitanaroj PO, Jao B, Yang J, Wang F, Anderson JM, Wang J, et al. Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1A1) expression by nanofiber-mediated siRNA gene silencing. Acta biomaterialia. 2013;9:4513–24. doi: 10.1016/j.actbio.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt DR, Kao WJ. The interrelated role of fibronectin and interleukin-1 in biomaterial-modulated macrophage function. Biomaterials. 2007;28:371–82. doi: 10.1016/j.biomaterials.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 51.Lahdeoja T, Pajarinen J, Kouri VP, Sillat T, Salo J, Konttinen YT. Toll-like receptors and aseptic loosening of hip endoprosthesis-a potential to respond against danger signals? Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28:184–90. doi: 10.1002/jor.20979. [DOI] [PubMed] [Google Scholar]
- 52.Gallo J, Mrazek F, Petrek M. Variation in cytokine genes can contribute to severity of acetabular osteolysis and risk for revision in patients with ABG 1 total hip arthroplasty: a genetic association study. BMC medical genetics. 2009;10:109. doi: 10.1186/1471-2350-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mostardi RA, Kovacik MW, Ramsier RD, Bender ET, Finefrock JM, Bear TF, et al. A comparison of the effects of prosthetic and commercially pure metals on retrieved human fibroblasts: the role of surface elemental composition. Acta biomaterialia. 2010;6:702–7. doi: 10.1016/j.actbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Cooper HJ, Ranawat AS, Potter HG, Foo LF, Koob TW, Ranawat CS. Early reactive synovitis and osteolysis after total hip arthroplasty. Clinical orthopaedics and related research. 2010;468:3278–85. doi: 10.1007/s11999-010-1361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wimmer MA. Wear of the polyethylene component created by rolling motion of the artificial knee joint. Aachen: Shaker-Verlag; 1999. [Google Scholar]
- 56.Shanbhag AS, Bailey HO, Hwang DS, Cha CW, Eror NG, Rubash HE. Quantitative analysis of ultrahigh molecular weight polyethylene (UHMWPE) wear debris associated with total knee replacements. Journal of biomedical materials research. 2000;53:100–10. doi: 10.1002/(sici)1097-4636(2000)53:1<100::aid-jbm14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 57.Hirakawa K, Bauer TW, Yamaguchi M, Stulberg BN, Wilde AH. Relationship between wear debris particles and polyethylene surface damage in primary total knee arthroplasty. The Journal of arthroplasty. 1999;14:165–71. doi: 10.1016/s0883-5403(99)90120-1. [DOI] [PubMed] [Google Scholar]
- 58.Brandt JM, MacDonald SJ, Bourne RB, Medley JB. Retrieval analysis of modular total knee replacements: factors influencing backside surface damage. The Knee. 2012;19:306–15. doi: 10.1016/j.knee.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Fisher J, Jennings LM, Galvin AL, Jin ZM, Stone MH, Ingham E. 2009 Knee Society Presidential Guest Lecture: Polyethylene wear in total knees. Clinical orthopaedics and related research. 2010;468:12–8. doi: 10.1007/s11999-009-1033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crowninshield RD, Wimmer MA, Jacobs JJ, Rosenberg AG. Clinical performance of contemporary tibial polyethylene components. The Journal of arthroplasty. 2006;21:754–61. doi: 10.1016/j.arth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Namba RS, Inacio MC, Paxton EW, Ake CF, Wang C, Gross TP, et al. Risk of revision for fixed versus mobile-bearing primary total knee replacements. The Journal of bone and joint surgery American volume. 2012;94:1929–35. doi: 10.2106/JBJS.K.01363. [DOI] [PubMed] [Google Scholar]
- 62.Wimmer MA, Laurent MP, Haman JD, Jacobs JJ, Galante JO. Surface damage versus tibial polyethylene insert conformity: a retrieval study. Clinical orthopaedics and related research. 2012;470:1814–25. doi: 10.1007/s11999-012-2274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKellop HA, Campbell P, Park SH, Schmalzried TP, Grigoris P, Amstutz HC, et al. The origin of submicron polyethylene wear debris in total hip arthroplasty. Clinical orthopaedics and related research. 1995:3–20. [PubMed] [Google Scholar]
- 64.Utzschneider S, Paulus A, Datz JC, Schroeder C, Sievers B, Wegener B, et al. Influence of design and bearing material on polyethylene wear particle generation in total knee replacement. Acta biomaterialia. 2009;5:2495–502. doi: 10.1016/j.actbio.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. The Journal of arthroplasty. 1992;7:37–41. doi: 10.1016/0883-5403(92)90030-t. [DOI] [PubMed] [Google Scholar]
- 66.Won CH, Rohatgi S, Kraay MJ, Goldberg VM, Rimnac CM. Effect of resin type and manufacturing method on wear of polyethylene tibial components. Clinical orthopaedics and related research. 2000:161–71. doi: 10.1097/00003086-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 67.Naudie DD, Rorabeck CH. Sources of osteolysis around total knee arthroplasty: wear of the bearing surface. Instructional course lectures. 2004;53:251–9. [PubMed] [Google Scholar]
- 68.Currier BH, Currier JH, Collier JP, Mayor MB. Effect of fabrication method and resin type on performance of tibial bearings. Journal of biomedical materials research. 2000;53:143–51. doi: 10.1002/(sici)1097-4636(2000)53:2<143::aid-jbm3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 69.Wimmer MA, Andriacchi TP. Tractive forces during rolling motion of the knee: implications for wear in total knee replacement. Journal of biomechanics. 1997;30:131–7. doi: 10.1016/s0021-9290(96)00112-1. [DOI] [PubMed] [Google Scholar]
- 70.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate immunity. 2013;19:213–24. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konttinen YT, Pajarinen J. Surgery: Adverse reactions to metal-on-metal implants. Nature reviews Rheumatology. 2012;9:5–6. doi: 10.1038/nrrheum.2012.218. [DOI] [PubMed] [Google Scholar]
- 72.Tyson-Capper AJ, Lawrence H, Holland JP, Deehan DJ, Kirby JA. Metal-on-metal hips: cobalt can induce an endotoxin-like response. Annals of the rheumatic diseases. 2012 doi: 10.1136/annrheumdis-2012-202468. [DOI] [PubMed] [Google Scholar]
- 73.Neale SD, Athanasou NA. Cytokine receptor profile of arthroplasty macrophages, foreign body giant cells and mature osteoclasts. Acta orthopaedica Scandinavica. 1999;70:452–8. doi: 10.3109/17453679909000980. [DOI] [PubMed] [Google Scholar]
- 74.Krohmer G, Koleganova N, Hadjicostas PT, Fink B, Berger I. Degenerative changes of the interface membrane as a possible reason for prosthesis loosening. Histology and histopathology. 2008;23:925–33. doi: 10.14670/HH-23.925. [DOI] [PubMed] [Google Scholar]
- 75.Konttinen YT, Takagi M, Mandelin J, Lassus J, Salo J, Ainola M, et al. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2001;16:1780–6. doi: 10.1359/jbmr.2001.16.10.1780. [DOI] [PubMed] [Google Scholar]
- 76.Arnett TR. Acidosis, hypoxia and bone. Archives of biochemistry and biophysics. 2010;503:103–9. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 77.Santavirta S, Takagi M, Gomez-Barrena E, Nevalainen J, Lassus J, Salo J, et al. Studies of host response to orthopedic implants and biomaterials. Journal of long-term effects of medical implants. 1999;9:67–76. [PubMed] [Google Scholar]
- 78.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clinical immunology. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Naylor AJ, Filer A, Buckley CD. The role of stromal cells in the persistence of chronic inflammation. Clinical and experimental immunology. 2013;171:30–5. doi: 10.1111/j.1365-2249.2012.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, et al. Redox control of inflammation in macrophages. Antioxidants & redox signaling. 2013 doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waris V, Sillat T, Waris E, Virkki L, Mandelin J, Takagi M, et al. Role and regulation of VEGF and its receptors 1 and 2 in the aseptic loosening of total hip implants. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2012;30:1830–6. doi: 10.1002/jor.22138. [DOI] [PubMed] [Google Scholar]
- 82.Huk OL, Zukor DJ, Ralston W, Lisbona A, Petit A. Apoptosis in interface membranes of aseptically loose total hip arthroplasty. Journal of materials science Materials in medicine. 2001;12:653–8. doi: 10.1023/a:1011254029864. [DOI] [PubMed] [Google Scholar]
- 83.Meinecke I, Pap G, Mendoza H, Drange S, Ender S, Strietholt S, et al. Small ubiquitin-like modifier 1 [corrected] mediates the resistance of prosthesis-loosening fibroblast-like synoviocytes against Fas-induced apoptosis. Arthritis and rheumatism. 2009;60:2065–70. doi: 10.1002/art.24633. [DOI] [PubMed] [Google Scholar]
- 84.Yang F, Wu W, Cao L, Huang Y, Zhu Z, Tang T, et al. Pathways of macrophage apoptosis within the interface membrane in aseptic loosening of prostheses. Biomaterials. 2011;32:9159–67. doi: 10.1016/j.biomaterials.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 85.Sabbatini M, Piffanelli V, Boccafoschi F, Gatti S, Reno F, Bosetti M, et al. Different apoptosis modalities in periprosthetic membranes. Journal of biomedical materials research Part A. 2010;92:175–84. doi: 10.1002/jbm.a.32349. [DOI] [PubMed] [Google Scholar]
- 86.Reno F, Sabbatini M, Masse A, Bosetti M, Cannas M. Fibroblast apoptosis and caspase-8 activation in aseptic loosening. Biomaterials. 2003;24:3941–6. doi: 10.1016/s0142-9612(03)00276-x. [DOI] [PubMed] [Google Scholar]
- 87.Thomas P, Braathen LR, Dorig M, Aubock J, Nestle F, Werfel T, et al. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009;64:1157–65. doi: 10.1111/j.1398-9995.2009.01966.x. [DOI] [PubMed] [Google Scholar]
- 88.Evans EM, Freeman MA, Miller AJ, Vernon-Roberts B. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. The Journal of bone and joint surgery. 1974;56-B:626–42. doi: 10.1302/0301-620X.56B4.626. British volume. [DOI] [PubMed] [Google Scholar]
- 89.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bulletin of the NYU hospital for joint diseases. 2009;67:182–8. [PubMed] [Google Scholar]
- 90.Thyssen JP, Schalock P. Contribution of allergic and other metal ion mediated responses to loosening of a total hip arthroplasty. In: Fokter SK, editor. Recent advances in arthroplasty. Rijeka: Croatia; 2012. pp. 339–43. [Google Scholar]
- 91.Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis: contact, atopic, occupational, drug: official journal of the American Contact Dermatitis Society, North American Contact Dermatitis Group. 2011;22:65–79. [PubMed] [Google Scholar]
- 92.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burkandt A, Katzer A, Thaler K, Von Baehr V, Friedrich RE, Ruther W, et al. Proliferation of the synovial lining cell layer in suggested metal hypersensitivity. In vivo. 2011;25:679–86. [PubMed] [Google Scholar]
- 94.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annual review of immunology. 1993;11:729–66. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 95.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. The Journal of bone and joint surgery British volume. 1998;80:531–9. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 96.Nam D, Bostrom MP, Fahlgren A. Emerging Ideas: Instability-induced Periprosthetic Osteolysis Is Not Dependent on the Fibrous Tissue Interface. Clinical orthopaedics and related research. 2013 doi: 10.1007/s11999-013-2896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Atkins GJ, Welldon KJ, Holding CA, Haynes DR, Howie DW, Findlay DM. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials. 2009;30:3672–81. doi: 10.1016/j.biomaterials.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 98.Fritton K, Ren PG, Gibon E, Rao AJ, Ma T, Biswal S, et al. Exogenous MC3T3 Preosteoblasts Migrate Systemically and Mitigate the Adverse Effects of Wear Particles. Tissue engineering Part A. 2012;18:2559–67. doi: 10.1089/ten.tea.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buckley CD. Why does chronic inflammation persist: An unexpected role for fibroblasts. Immunology letters. 2011;138:12–4. doi: 10.1016/j.imlet.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends in immunology. 2012 doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, et al. Macrophages-Key cells in the response to wear debris from joint replacements. Journal of biomedical materials research Part A. 2013 doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. The Journal of experimental medicine. 2010;207:2793–8. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gibon E, Ma T, Ren PG, Fritton K, Biswal S, Yao Z, et al. Selective inhibition of the MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2012;30:547–53. doi: 10.1002/jor.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyce BF, Rosenberg E, de Papp AE, Duong le T. The osteoclast, bone remodelling and treatment of metabolic bone disease. European journal of clinical investigation. 2012;42:1332–41. doi: 10.1111/j.1365-2362.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 105.Haynes DR, Crotti TN, Loric M, Bain GI, Atkins GJ, Findlay DM. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology. 2001;40:623–30. doi: 10.1093/rheumatology/40.6.623. [DOI] [PubMed] [Google Scholar]
- 106.Ali AS, Lax AS, Liljestrom M, Paakkari I, Ashammakhi N, Kovanen PT, et al. Mast cells in atherosclerosis as a source of the cytokine RANKL. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2006;44:672–4. doi: 10.1515/CCLM.2006.117. [DOI] [PubMed] [Google Scholar]
- 107.Koreny T, Tunyogi-Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis and rheumatism. 2006;54:3221–32. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 108.Mandelin J, Li TF, Hukkanen M, Liljestrom M, Salo J, Santavirta S, et al. Interface tissue fibroblasts from loose total hip replacement prosthesis produce receptor activator of nuclear factor-kappaB ligand, osteoprotegerin, and cathepsin K. The Journal of rheumatology. 2005;32:713–20. [PubMed] [Google Scholar]
- 109.Ramage SC, Urban NH, Jiranek WA, Maiti A, Beckman MJ. Expression of RANKL in osteolytic membranes: association with fibroblastic cell markers. The Journal of bone and joint surgery American volume. 2007;89:841–8. doi: 10.2106/JBJS.F.00655. [DOI] [PubMed] [Google Scholar]
- 110.Sabokbar A, Itonaga I, Sun SG, Kudo O, Athanasou NA. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2005;23:511–9. doi: 10.1016/j.orthres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Crotti TN, Smith MD, Findlay DM, Zreiqat H, Ahern MJ, Weedon H, et al. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials. 2004;25:565–73. doi: 10.1016/s0142-9612(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 112.Granchi D, Pellacani A, Spina M, Cenni E, Savarino LM, Baldini N, et al. Serum levels of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand as markers of periprosthetic osteolysis. The Journal of bone and joint surgery American volume. 2006;88:1501–9. doi: 10.2106/JBJS.E.01038. [DOI] [PubMed] [Google Scholar]
- 113.Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N, et al. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. The American journal of pathology. 2003;163:2021–31. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, et al. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. The Journal of experimental medicine. 2013;210:i1. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. The Journal of clinical investigation. 2011;121:3505–16. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Robertsson O, Wingstrand H, Kesteris U, Jonsson K, Onnerfalt R. Intracapsular pressure and loosening of hip prostheses. Preoperative measurements in 18 hips. Acta orthopaedica Scandinavica. 1997;68:231–4. doi: 10.3109/17453679708996690. [DOI] [PubMed] [Google Scholar]
- 117.Jawed S, Gaffney K, Blake DR. Intra-articular pressure profile of the knee joint in a spectrum of inflammatory arthropathies. Annals of the rheumatic diseases. 1997;56:686–9. doi: 10.1136/ard.56.11.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nilsson A, Norgard M, Andersson G, Fahlgren A. Fluid pressure induces osteoclast differentiation comparably to titanium particles but through a molecular pathway only partly involving TNFalpha. Journal of cellular biochemistry. 2012;113:1224–34. doi: 10.1002/jcb.23456. [DOI] [PubMed] [Google Scholar]
- 119.Michalopoulos E, Knight RL, Korossis S, Kearney JN, Fisher J, Ingham E. Development of methods for studying the differentiation of human mesenchymal stem cells under cyclic compressive strain. Tissue engineering Part C, Methods. 2012;18:252–62. doi: 10.1089/ten.tec.2011.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang P, Wu Y, Jiang Z, Jiang L, Fang B. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. International journal of molecular medicine. 2012;29:1083–9. doi: 10.3892/ijmm.2012.934. [DOI] [PubMed] [Google Scholar]
- 121.Kaivosoja E, Barreto G, Levon K, Virtanen S, Ainola M, Konttinen YT. Chemical and physical properties of regenerative medicine materials controlling stem cell fate. Annals of medicine. 2012;44:635–50. doi: 10.3109/07853890.2011.573805. [DOI] [PubMed] [Google Scholar]
- 122.MacQuarrie RA, Fang Chen Y, Coles C, Anderson GI. Wear-particle-induced osteoclast osteolysis: the role of particulates and mechanical strain. Journal of biomedical materials research Part B, Applied biomaterials. 2004;69:104–12. doi: 10.1002/jbm.b.20031. [DOI] [PubMed] [Google Scholar]
- 123.Bosetti M, Masse A, Navone R, Cannas M. Biochemical and histological evaluation of human synovial-like membrane around failed total hip replacement prostheses during in vitro mechanical loading. Journal of materials science Materials in medicine. 2001;12:693–8. doi: 10.1023/a:1011216509099. [DOI] [PubMed] [Google Scholar]
- 124.De Man FH, Tigchelaar W, Marti RK, Van Noorden CJ, Van der Vis HM. Effects of mechanical compression of a fibrous tissue interface on bone with or without high-density polyethylene particles in a rabbit model of prosthetic loosening. The Journal of bone and joint surgery American volume. 2005;87:1522–33. doi: 10.2106/JBJS.D.01882. [DOI] [PubMed] [Google Scholar]
- 125.El-Warrak AO, Olmstead M, Schneider R, Meinel L, Bettschart-Wolfisberger R, Akens MK, et al. An experimental animal model of aseptic loosening of hip prostheses in sheep to study early biochemical changes at the interface membrane. BMC musculoskeletal disorders. 2004;5:7. doi: 10.1186/1471-2474-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kuster MS, Grob K, Gachter A. Knee endoprosthesis: sports orthopedics possibilities and limitations. Der Orthopade. 2000;29:739–45. doi: 10.1007/s001320050520. [DOI] [PubMed] [Google Scholar]
- 127.Benson LC, DesJardins JD, LaBerge M. Effects of in vitro wear of machined and molded UHMWPE tibial inserts on TKR kinematics. Journal of biomedical materials research. 2001;58:496–504. doi: 10.1002/jbm.1046. [DOI] [PubMed] [Google Scholar]
- 128.Ngai V, Wimmer MA. Kinematic evaluation of cruciate-retaining total knee replacement patients during level walking: a comparison with the displacement-controlled ISO standard. Journal of biomechanics. 2009;42:2363–8. doi: 10.1016/j.jbiomech.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cottrell JM, Babalola O, Furman BS, Wright TM. Stair ascent kinematics affect UHMWPE wear and damage in total knee replacements. Journal of biomedical materials research Part B, Applied biomaterials. 2006;78:15–9. doi: 10.1002/jbm.b.30451. [DOI] [PubMed] [Google Scholar]
- 130.Metcalfe A, Stewart C, Postans N, Barlow D, Dodds A, Holt C, et al. Abnormal loading of the major joints in knee osteoarthritis and the response to knee replacement. Gait & posture. 2013;37:32–6. doi: 10.1016/j.gaitpost.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 131.Lachiewicz PF, Geyer MR. The use of highly cross-linked polyethylene in total knee arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19:143–51. doi: 10.5435/00124635-201103000-00003. [DOI] [PubMed] [Google Scholar]
- 132.Gencur SJ, Rimnac CM, Kurtz SM. Fatigue crack propagation resistance of virgin and highly crosslinked, thermally treated ultra-high molecular weight polyethylene. Biomaterials. 2006;27:1550–7. doi: 10.1016/j.biomaterials.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 133.Fisher J, McEwen HM, Tipper JL, Galvin AL, Ingram J, Kamali A, et al. Wear, debris, and biologic activity of cross-linked polyethylene in the knee: benefits and potential concerns. Clinical orthopaedics and related research. 2004:114–9. doi: 10.1097/01.blo.0000148783.20469.4c. [DOI] [PubMed] [Google Scholar]
- 134.Sonntag R, Reinders J, Kretzer JP. What’s next? Alternative materials for articulation in total joint replacement. Acta biomaterialia. 2012;8:2434–41. doi: 10.1016/j.actbio.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 135.Bal BS, Garino J, Ries M, Oonishi H. Ceramic bearings in total knee arthroplasty. The journal of knee surgery. 2007;20:261–70. doi: 10.1055/s-0030-1248055. [DOI] [PubMed] [Google Scholar]
- 136.Iida T, Minoda Y, Kadoya Y, Matsui Y, Kobayashi A, Iwaki H, et al. Mid-term clinical results of alumina medial pivot total knee arthroplasty. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012;20:1514–9. doi: 10.1007/s00167-011-1734-3. [DOI] [PubMed] [Google Scholar]
- 137.Laskin RS. An oxidized Zr ceramic surfaced femoral component for total knee arthroplasty. Clinical orthopaedics and related research. 2003:191–6. doi: 10.1097/01.blo.0000093003.90435.1f. [DOI] [PubMed] [Google Scholar]
- 138.Innocenti M, Civinini R, Carulli C, Matassi F, Villano M. The 5-year results of an oxidized zirconium femoral component for TKA. Clinical orthopaedics and related research. 2010;468:1258–63. doi: 10.1007/s11999-009-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thomsen M, Rozak M, Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: disappearance of symptoms after revision with a special surface-coated TKA--a case report. Acta orthopaedica. 2011;82:386–8. doi: 10.3109/17453674.2011.579521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bergschmidt P, Bader R, Mittelmeier W. Metal hypersensitivity in total knee arthroplasty: revision surgery using a ceramic femoral component - a case report. The Knee. 2012;19:144–7. doi: 10.1016/j.knee.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 141.Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. The Journal of bone and joint surgery British volume. 2012;94:1126–34. doi: 10.1302/0301-620X.94B8.28135. [DOI] [PubMed] [Google Scholar]
- 142.Schalock PC, Menne T, Johansen JD, Taylor JS, Maibach HI, Liden C, et al. Hypersensitivity reactions to metallic implants - diagnostic algorithm and suggested patch test series for clinical use. Contact dermatitis. 2012;66:4–19. doi: 10.1111/j.1600-0536.2011.01971.x. [DOI] [PubMed] [Google Scholar]
- 143.Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clinical orthopaedics and related research. 1994:31–43. [PubMed] [Google Scholar]
- 144.Hoppe S, Mainzer JD, Frauchiger L, Ballmer PM, Hess R, Zumstein MA. More accurate component alignment in navigated total knee arthroplasty has no clinical benefit at 5-year follow-up. Acta orthopaedica. 2012;83:629–33. doi: 10.3109/17453674.2012.747923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim YH, Park JW, Kim JS. Computer-Navigated Versus Conventional Total Knee Arthroplasty: A Prospective Randomized Trial. The Journal of bone and joint surgery. 2012 doi: 10.2106/JBJS.L.00142. American volume. [DOI] [PubMed] [Google Scholar]
- 146.Ritter MA, Herbst SA, Keating EM, Faris PM. Radiolucency at the bone-cement interface in total knee replacement. The effects of bone-surface preparation and cement technique. The Journal of bone and joint surgery American volume. 1994;76:60–5. doi: 10.2106/00004623-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 147.Cawley DT, Kelly N, Simpkin A, Shannon FJ, McGarry JP. Full and surface tibial cementation in total knee arthroplasty: a biomechanical investigation of stress distribution and remodeling in the tibia. Clinical biomechanics. 2012;27:390–7. doi: 10.1016/j.clinbiomech.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 148.Prieto-Alhambra D, Javaid MK, Judge A, Murray D, Carr A, Cooper C, et al. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. Bmj. 2011;343:d7222. doi: 10.1136/bmj.d7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin T, Yan SG, Cai XZ, Ying ZM. Bisphosphonates for periprosthetic bone loss after joint arthroplasty: a meta-analysis of 14 randomized controlled trials. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23:1823–34. doi: 10.1007/s00198-011-1797-5. [DOI] [PubMed] [Google Scholar]
- 150.Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27:183–8. doi: 10.1002/jor.20748. [DOI] [PubMed] [Google Scholar]
- 151.Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA: the journal of the American Medical Association. 2010;304:1480–4. doi: 10.1001/jama.2010.1360. [DOI] [PubMed] [Google Scholar]
- 152.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. The New England journal of medicine. 2011;364:1728–37. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 153.Childs LM, Paschalis EP, Xing L, Dougall WC, Anderson D, Boskey AL, et al. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17:192–9. doi: 10.1359/jbmr.2002.17.2.192. [DOI] [PubMed] [Google Scholar]
- 154.Aspenberg P, Agholme F, Magnusson P, Fahlgren A. Targeting RANKL for reduction of bone loss around unstable implants: OPG-Fc compared to alendronate in a model for mechanically induced loosening. Bone. 2011;48:225–30. doi: 10.1016/j.bone.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 155.Yamanaka Y, Clohisy JC, Ito H, Matsuno T, Abu-Amer Y. Blockade of JNK and NFAT pathways attenuates orthopedic particle-stimulated osteoclastogenesis of human osteoclast precursors and murine calvarial osteolysis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31:67–72. doi: 10.1002/jor.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 157.Looney RJ, Schwarz EM, Boyd A, O’Keefe RJ. Periprosthetic osteolysis: an immunologist’s update. Current opinion in rheumatology. 2006;18:80–7. doi: 10.1097/01.bor.0000198004.88568.96. [DOI] [PubMed] [Google Scholar]
- 158.Chen D, Zhang X, Guo Y, Shi S, Mao X, Pan X, et al. MMP-9 inhibition suppresses wear debris-induced inflammatory osteolysis through downregulation of RANK/RANKL in a murine osteolysis model. International journal of molecular medicine. 2012;30:1417–23. doi: 10.3892/ijmm.2012.1145. [DOI] [PubMed] [Google Scholar]
- 159.Mao X, Pan X, Peng X, Cheng T, Zhang X. Inhibition of titanium particle-induced inflammation by the proteasome inhibitor bortezomib in murine macrophage-like RAW 264. 7 cells. Inflammation. 2012;35:1411–8. doi: 10.1007/s10753-012-9454-5. [DOI] [PubMed] [Google Scholar]
- 160.Liu F, Zhu Z, Mao Y, Liu M, Tang T, Qiu S. Inhibition of titanium particle-induced osteoclastogenesis through inactivation of NFATc1 by VIVIT peptide. Biomaterials. 2009;30:1756–62. doi: 10.1016/j.biomaterials.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 161.Fahlgren A, Yang X, Ciani C, Ryan JA, Kelly N, Ko FC, et al. The effects of PTH, loading and surgical insult on cancellous bone at the bone-implant interface in the rabbit. Bone. 2013;52:718–24. doi: 10.1016/j.bone.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shin DK, Kim MH, Lee SH, Kim TH, Kim SY. Inhibitory effects of luteolin on titanium particle-induced osteolysis in a mouse model. Acta biomaterialia. 2012;8:3524–31. doi: 10.1016/j.actbio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 163.Kauther MD, Bachmann HS, Neuerburg L, Broecker-Preuss M, Hilken G, Grabellus F, et al. Calcitonin substitution in calcitonin deficiency reduces particle-induced osteolysis. BMC musculoskeletal disorders. 2011;12:186. doi: 10.1186/1471-2474-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thillemann TM, Pedersen AB, Mehnert F, Johnsen SP, Soballe K. The risk of revision after primary total hip arthroplasty among statin users: a nationwide population-based nested case-control study. The Journal of bone and joint surgery American volume. 2010;92:1063–72. doi: 10.2106/JBJS.H.01805. [DOI] [PubMed] [Google Scholar]
- 165.Valles G, Perez C, Bore A, Martin-Saavedra F, Saldana L, Vilaboa N. Simvastatin prevents the induction of interleukin-6 gene expression by titanium particles in human osteoblastic cells. Acta biomaterialia. 2013;9:4916–25. doi: 10.1016/j.actbio.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 166.Laing AJ, Dillon JP, Mulhall KJ, Wang JH, McGuinness AJ, Redmond PH. Statins attenuate polymethylmethacrylate-mediated monocyte activation. Acta orthopaedica. 2008;79:134–40. doi: 10.1080/17453670710014888. [DOI] [PubMed] [Google Scholar]
- 167.Cardozo CP, Qin W, Peng Y, Liu X, Wu Y, Pan J, et al. Nandrolone slows hindlimb bone loss in a rat model of bone loss due to denervation. Annals of the New York Academy of Sciences. 2010;1192:303–6. doi: 10.1111/j.1749-6632.2009.05313.x. [DOI] [PubMed] [Google Scholar]


