Abstract
In this study, we have examined the role of glutamine derivatives in reducing 5-fluorouracil (5-FU)-induced epithelial damage in an undifferentiated crypt intestinal cell line, IEC-6. In this model, we have investigated proliferation indirectly by detecting the enzyme-derived formazan dye from the tetrazolium salt WST-1 in viable cells at 24 and 48 h after 5-FU treatment. Migration was measured at 12 and 24 h after razor scraping of the cell monolayer. Cell death was measured by quantifying the percentage of apoptotic and necrotic figures by flow cytometry at 12 and 24 h following 5-FU challenge. Neither glutamine nor alanyl-glutamine prevented 5-FU-induced apoptosis and necrosis in IEC-6 cells at 12 and 24 h after 5-FU challenge. However, glutamine and alanyl-glutamine enhanced migration and proliferation when compared with 5-FU-treated controls (P <0.05). These new findings support our earlier study on the benefit of oral glutamine in enhancing epithelial recovery after 5-FU challenge.
Keywords: Alanyl-glutamine, Glutamine, 5-fluorouracil, Mucositis, Chemotherapy, Small intestine
Introduction
The small intestinal epithelium is composed of a diverse and dynamic population of cells, which have differential kinetics during intestinal adaptation against mucosal injury [1, 2]. Within the crypts, cells proliferate and migrate towards either the villi or to the bases of the crypts where they differentiate into a variety of cells such as enterocytes, goblet cells, and Paneth cells. Apoptosis is responsible for maintaining the balance between proliferation and elimination of the constantly renewing intestinal epithelial cells, which regenerate every 3 days [3, 4]. A variety of agents, including chemotherapeutic drugs, radiation, and intestinal pathogens are known to disturb the steady intestinal epithelium turnover [5–7]. 5-Fluorouracil (5-FU) is one of the most commonly used adjuvant chemotherapy drugs for colorectal cancer [8], but enteric inflammation accompanied by diarrhea is a commonly reported side-effect, sometimes leading to discontinuation of therapy [9]. Intestinal mucositis with diarrhea is often the dose-limiting adverse effect of 5-FU therapy and is likely accompanied by reduced enterocyte proliferation, migration, and a concomitantly increased apoptotic rate, resulting in intestinal malabsorption and disrupted intestinal barrier function, as reported by Daniele and others [10, 11].
Glutamine is the most abundant amino acid in blood with a concentration of 0.6 mM/l, which is higher than any other amino acid [12, 13]. Glutamine is known to be one of the main sources of energy for the intestinal epithelium by generating adenosine 5′-triphosphate (ATP). It is also the precursor of many other amino acids such as proline, arginine, citruline, and alanine, which play an important coadjuvant role during gut healing [13, 14]. The intestinal uptake of glutamine is enhanced when the body is subjected to stressful conditions, such as chemotherapy challenge [15, 16]. Glutamine supplementation in rats pretreated with toxic doses of methotrexate enables reduction in the severity of the intestinal mucositis [17] and improves intestinal permeability in patients treated with 5-FU and folinic acid [18]. Although glutamine has been shown to reduce diarrhea duration in children [19–21] and is generally recognized to be a conditionally essential amino acid in the critically ill patient [22], there remains a debate regarding the efficacy of glutamine due to its limited solubility, stimulating the pursuit of more stable glutamine compounds for clinical use [23, 24].
In a previous study, we showed that villus height was blunted due to cell proliferation arrest and that cell apoptosis increased within ileal crypts 24 h following 5-FU treatment in mice [25]. In this model, oral alanyl-glutamine, a more stable glutamine derivative, hastened ileal mucosal recovery following 5-FU challenge, by means of crypt hyperplasia. Although numerous studies have addressed the role of adjuvant glutamine therapy in intestinal mucositis [26], there is a scarcity of in vitro studies addressing the healing role of glutamine derivatives on 5-fluorouracil-induced cell damage.
In order to further investigate intestinal cell dynamics following 5-FU challenge, we have studied cell migration, proliferation, and apoptosis in a crypt intestinal cell line, IEC-6, pretreated with 5-FU, and further evaluated the role of glutamine and alanyl-glutamine supplementation in protecting IEC-6 cells from the 5-FU challenge in vitro.
Methods
Materials
Glutamine (Gln) and alanyl-glutamine (Ala-Gln) were obtained from Sigma (St. Louis, MO). The Annexin V ApopAlert kit was obtained from Clontech (Palo Alto, CA). Tetrazolium salt WST-1 reagent was obtained from Roche (Mannheim, Germany). 5-Fluorouracil was obtained from American Pharmaceutical Partners Inc. (Los Angeles, CA), at a concentration of 50 mg/ml in aqueous solution.
Cell Culture
Rat intestinal jejunal crypt cells (IEC-6, passages 20–35) were purchased from American Type Culture Collection (Rockville, MD) and were cultured at 37°C in a 5% CO2 incubator. The maintenance cell media was Dulbecco’s modified eagle media (DMEM; Gibco BRL, Grand Island, NY) supplemented with 5% fetal calf serum (FCS), 5 mg bovine insulin, 50 μg/ml penicillin/streptomycin (DMEM; Gibco BRL, Grand Island, NY), and a final concentration of 1 mM sodium pyruvate. The medium was changed thrice a week, according to standard culture protocols. Dulbecco’s modified eagle medium without glutamine (DMEM; Gibco BRL) was used whenever the supplementation effect of Gln or Ala-Gln was evaluated. The cultured cells were trypsinized with 0.25% ethylene-diamine-tetra-acetic acid (EDTA) trypsin when confluence was achieved.
Wounding Assay of IEC-6 Cell Monolayers (Cell Migration)
IEC-6 cells were seeded in six-well plates at a concentration of 2 × 104 cells/well and cultivated in DMEM media with 5% FCS (Gibco BRL, Grand Island, NY). IEC-6 cells were confluent after 4 days following seeding. Wells were then scraped along their diameter and extending 30 mm in length to the right center corner using a sterile razor blade. Prior to scraping, 50% of the medium volume was removed from each study well. The medium was changed and the cells were then incubated for 1 h either with 5-fluorouracil, at concentrations of 0.3, 1, 3, 10, and 30 mM (selected based on previous study with IEC-6 cells challenged by 5-fluorouracil [27]) or standard Gln-containing media. Afterwards, wells were washed using DMEM. Wells were digitally photographed after 6, 12, and 24 h, and the number of cells in the longest migration line was counted and averaged according to the well surface area (adapted from McCormack and Brito et al. [28]). The digital pictures were captured and opened using Image Pro Plus v. 5.0 software (Media Cybernetics, Silver Spring, MD). A grid (each square = 0.1 mm2) was built, overlaying the digital images of the scraped area. The column of the farthest cell migration was chosen and IEC-6 adherent cells were tracked and identified by traced red dots for counting. The traced red dots within the column were changed to black on a white background and counted digitally by Image Pro Plus software. The resealing speed was also evaluated by averaging the length of the three longest lines of cells in each picture after 24 h.
Cell Proliferation
Cell proliferation was measured indirectly using the tetra-zolium salt WST-1 (4-[3-(4-iodophenyl)-2H-5-tetrazolio]-1-3-benzene disulfonate), according to the manufacturer recommendations. A 96-well plate was seeded with IEC-6 cells in a total concentration of 4 × 103 cells/well in 100 μl of normal DMEM. Cells were allowed to attach for 48 h, and then the wells were washed with 100 μl of DMEM without glutamine. The wells were incubated for 1 h with 1 mM 5-FU (because of the greatest efficacy seen in the migration study, as described below at the Results section) diluted in DMEM Gln-free medium or medium alone. Afterwards, the medium was removed and the wells were washed with Gln-free media and then supplemented or not with Gln or Ala-Gln (10 mM in DMEM Gln-free media).
After 24 and 48 h, wells were incubated for 4 h with 10 μl of the tetrazolium salt and the absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) microplate reader at 450 nm (reference range 420–480 nm). Tetrazolium salts are cleaved to formazan by mitochondrial enzymes in viable cells. Enhancement of the number of viable cells will result in an increase of the amount of the formazan dye, which is detectable by the ELISA reader. Therefore, this model indirectly measures the cell proliferation rate over time.
Flow Cytometry for Apoptosis and Necrosis
Apoptosis and necrosis were measured by flow cytometry analyses using the ApoAlert Annexin V kit. Annexin V is a molecule that binds to phosphatidylserine (PS) and, when conjugated to a fluorochrome, detects apoptotic cells expressing PS on the reversed membrane surface. For this protocol, propidium iodide (PI) was also used to detect necrotic and late apoptotic cells, which express PI inside the membrane. The cells were seeded on 12-well plates at a concentration of 5 × 105 cells/well. These cells were allowed to attach to the plate surface for 24 h. Afterwards, cells were washed with DMEM Gln-free media and either incubated or not with 5-fluorouracil for 1 h. Wells were then washed again and supplemented with Gln or Ala-Gln (both 10 mM) and then incubated for 6, 12, or 24 h. Cells were trypsinized, centrifuged, and washed with serum-containing media, before incubation with Annexin V. Cells were counted and diluted to 105–106 cells and rinsed with 1× binding buffer, and resuspended in 200 μl of binding buffer. Five microliters of Annexin V and 10 μl of PI were added and incubated for 5–15 min in the dark. The samples were then processed at the University of Virginia’s Flow Cytometry Core, using a Calibur (Becton Dickinson) dual-laser fluorescence-activated cell sorter (FACS).
Results
Effects of 5-Fluorouracil and Ala-Gln and Gln on IEC-6 Cell Migration
Pretreatment with 5-FU (for 1 h, diluted in standard media) inhibited cell migration in a dose-dependent fashion at 24 h (Fig. 1). The strongest migration inhibition was seen with 30 mM at 24 h (inhibition of 65.6% versus control). The dose of 1 mM of 5-FU blunted migration significantly at 24 h (inhibition of 47.1% versus control); hence, this dose was used to evaluate the effects of Ala-Gln and Gln supplementation on 5-FU-induced epithelial cell damage. Migration was inhibited by 1 mM of 5-FU by 55.5% compared to the control when Gln-free media was used after 24 h. The resealing speed, decreased significantly by 14.3% after 5-FU treatment: 413 ± 10.9 μm/24 h, N = 15, versus 354 ± 18.2 μm/24 h, N = 18 (P = 0.007, by unpaired Student t-test), an average speed reduction of 2.45 μm/h, as compared to IEC cells seeded under Gln-free media.
Fig. 1.
(a) Diagram showing a representative digital image of the scraping area with superimposed grid (each square = 0.1 mm2), overlapping the column of farthest cell migration. IEC-6 adherent cells were tracked by traced red dots for counting. The traced red dots within the column were changed to black on a white background and counted digitally by Image Pro Plus software. A yellow line was drawn to depict the migration distance from the scraping point. (b) Dose-response and time-effect graphs of 5-fluorouracil (5-FU) at 6 and 24 h, showing inhibition of cell migration in vitro. IEC-6 cell monolayers were scraped to induce migration, and incubated for 1 h with 5-FU immediately after standard medium replacement, diluted in standard media at doses of 0.3, 1, 3, 10, or 30 mM. The bars represent means ± standard error (SE) for the number of migrating cells per square millimeter of scraped area. *Statistically significant difference (P < 0.05) compared to migrated cells in standard medium without 5-FU treatment (SM), determined by analysis of variance (ANOVA) and Bonferroni’s test
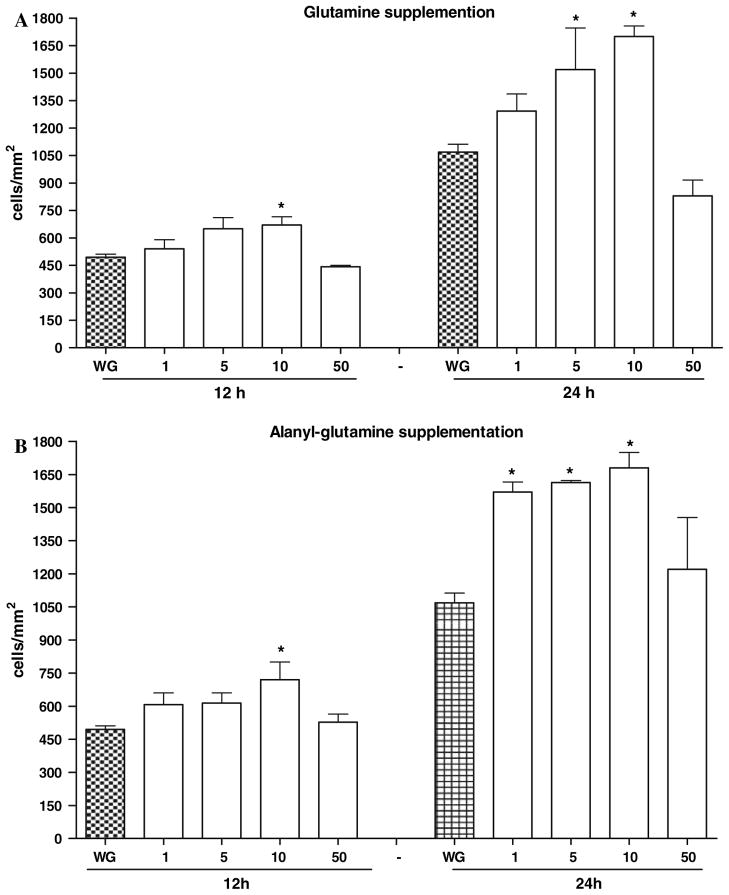
Both Ala-Gln and Gln supplementation (10 mM) significantly improved migration after 5-FU treatment (P < 0.001), by 83.1% and 49.7%, respectively, at 24 h (Figs. 2 and 3) and resealing speed by 25.4% and 15%, respectively, for Ala-Gln and Gln at 24 h (P <0.05, by unpaired Student’s t-test). Ala-Gln and Gln increased three- and twofold the average IEC-6 migration speed (μ/h), respectively, away from the scraping line, as compared to 5-FU. From the four supplementation doses tested for each nutrient, the peak dose of 10 mM was chosen, at both 12 and 24 h, for the remaining studies.
Fig. 2.
(a) Dose-response and time-effect graphs of glutamine (A) and alanyl-glutamine (B) at 12 and 24 h, in enhancing cell migration in vitro. IEC-cell monolayers were scraped to induce migration, and incubated with glutamine and alanyl-glutamine (1, 5, 10, and 50 mM) diluted in glutamine-free media, immediately after standard medium replacement. The bars represent means ± SE for the number of migrating cells per square millimeter of scraped area. *Statistically significant difference (P <0.05) compared to migrated cells in medium without glutamine (WG), determined by ANOVA and Bonferroni’s test
Fig. 3.
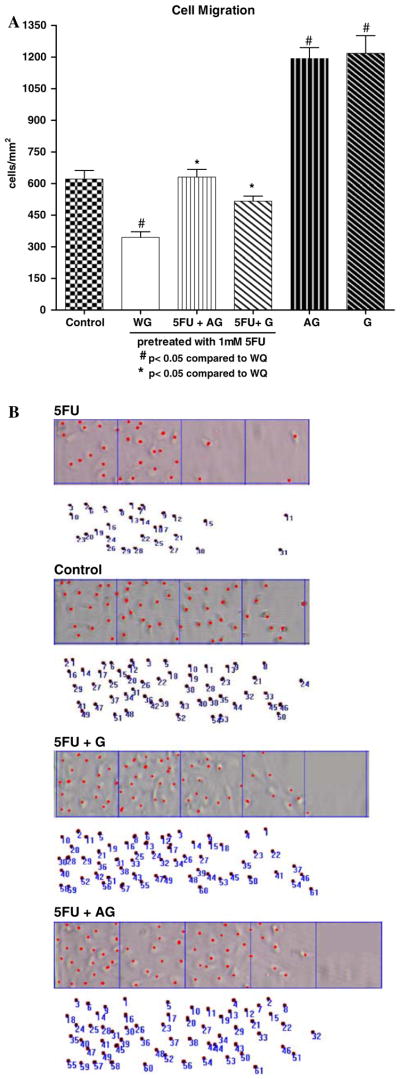
(a) Protective effect of glutamine (G) and alanyl-glutamine (AG) (10 mM) in cell migration following 24 h of 5-FU challenge, in enhancing cell migration in vitro. IEC-6 cell monolayers were scraped to induce migration, and incubated with glutamine and alanyl-glutamine diluted in glutamine-free media, immediately after standard medium replacement. The bars represent means ± SE for the number of migrating cells per square millimeter of scraped area. *P <0.05 compared to migrated cells in glutamine-free media without treatment (WG), #P <0.05, compared to migrated cells in standard media pretreated with 1 mM of 5-FU, by ANOVA and Bonferroni’s test. (B) Representative migration columns of adherent IEC-6 cells from the experimental groups 24 h following the 5-FU challenge. The control group is shown at the top, representing migrating cells not challenged with 5-FU embedded in standard media
Effect of 5-Fluorouracil and Ala-Gln and Gln on Cell Proliferation
5-FU significantly reduced proliferation at 24 and 48 h in previously exposed cells compared to the control group without glutamine (18.9% at 24 h and 38.6% at 48 h; P <0.05). Ala-Gln and Gln, at a dose of 10 mM, enhanced cell proliferation compared to the control cells seeded in glutamine-free medium at both 24 and 48 h (55.9% and 91%, respectively, for Ala-Gln, and 60.8 and 91.3%, respectively, for Gln); see Fig. 4. Ala-Gln (10 mM) increased cell proliferation following 5-FU pretreatment at a rate of 23.2% and 38.6% after 24 and 48 h, respectively. Supplementation with 10 mM of Gln increased cell proliferation at a rate of 25.1% and 29.2%, after 24 and 48 h, respectively, as detected by the WST-1 technique. At 50 mM concentration, both Ala-Gln and Gln did not increase cell proliferation at 12- and 24-h incubations.
Fig. 4.
Effect of glutamine (G) and alanyl-glutamine (AG) on cell proliferation assay by detected absorbance using an ELISA microplate reader at 450 nm in 96-well seeded IEC-6 cells, following 1 h of 5-fluorouracil (5-FU) exposure (1 mM). 5-FU was diluted in DMEM Gln-free media. After 24 and 48 h, wells were incubated for 4 h with 10 μl of the tetrazolium salt and the absorbance was measured. *P <0.05 compared to untreated control in glutamine-free media (WG) at 24 and 48 h, #P <0.05, compared to standard media pretreated with 1 mM of 5-FU at 24 and 48 h, by Student’s t test and ANOVA and Bonferroni’s test, respectively
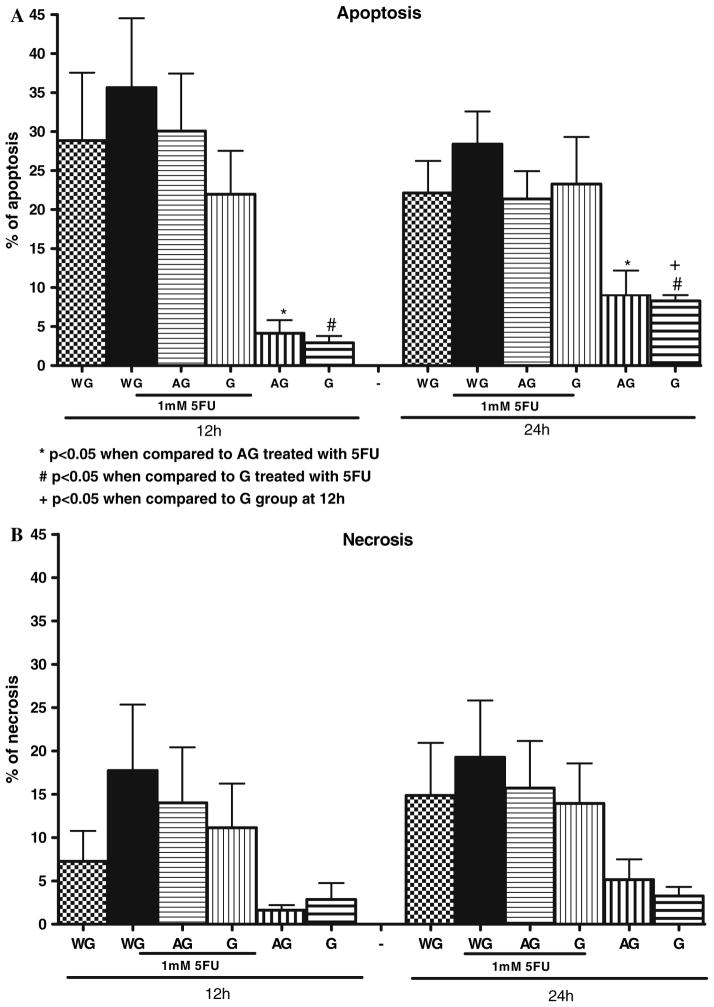
Lack of Effect of Ala-Gln and Gln on 5-FU-Induced Apoptosis and Necrosis
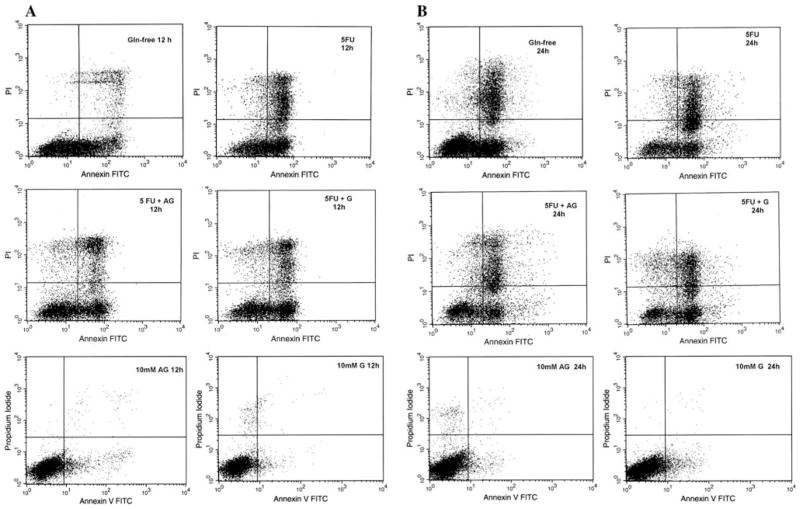
Triplicate flow cytometry analyses demonstrated a significant increase in apoptosis in IEC-6 cells after 1 h of 1 mM 5-FU exposure. The apoptotic rate peaked at 12 h (35.6%), P = 0.01 versus either the untreated control containing Gln or Ala-Gln-enriched media. No further increase was observed at 24 h, following 5-FU treatment. Additionally, we could not find a significant difference in apoptotic rates before 12 h following 5-FU exposure (data not shown) as compared to the respective controls. The lack of glutamine in the medium for 12 and 24 h increased significantly the apoptosis rates to 28.8% and 22.1%, respectively, when compared with Gln-enriched medium (10 mM of Gln), which showed an apoptosis rate of 2.9% and 8% at 12 and 24 h, respectively. There was no significant difference between the group without glutamine, and therefore under stressful conditions, and the group treated with 1 mM of 5-FU after 1 h (Figs. 5 and 6). Apoptotic cells were positive for annexin V labeled with fluorescein isothiocyanate (V-FITC) only (lower right quadrant) and cells that were both necrotic and apoptotic were both positive for annexin V-FITC and propidium iodide (upper right quadrant). The viable cells did not stain for annexin V-FITC or with propidium iodide. Gln (10 mM) and Ala-Gln (10 mM) failed to reduce significantly the apoptotic cell death due to 5-FU pretreatment (Gln: 21.9% and Ala-Gln: 30.08% at 12 h; Gln: 23,2% and Ala-Gln: 21.3% at 24 h), although a trend was observed toward apoptotic and necrotic decline, especially at 12 h (Figs. 5 and 6). The apoptosis rate of 8% observed at 24 h in the Gln-enriched media group (10 mM of Gln) showed a significant increase when compared with the apoptosis rate of 2.9% at 12 h. There was no significant difference in apoptosis in the rate of the Ala-Gln-enriched group (10 mM of Ala-Gln) at 12 and 24 h.
Fig. 5.
Apoptosis and necrosis induced by 1 h 5-fluorouracil (5-FU) pretreatment (at 1 mM, diluted in glutamine-free media) were not inhibited by alanyl-glutamine (AG) and glutamine (G) at 12 (a) and 24 h (b) in IEC-6 cells. Cells were stained with FITC-conjugated annexin V and propidium iodide and analyzed by flow cytometry. Results are shown as density plots with propidium iodide versus annexin V-FITC. Viable cells have low annexin-V-FITC and low propidium iodide staining (lower left quadrant), apoptotic cells have high annexin V-FITC and low propidium iodide staining (lower right quadrant), and necrotic cells have high propidium iodide and annexin V-FITC staining (upper right quadrant)
Fig. 6.
Effect of glutamine (G) and alanyl-glutamine (AG) on apoptosis (a) and necrosis (b) by flow cytometry in IEC-6 cells, following 1 h of 5-fluorouracil (5-FU) exposure (1 mM). 5-FU was diluted in DMEM Gln-free media. *P <0.05 compared to media enriched with 10 mM of AG, pretreated with 1 mM of 5-FU at 12 and 24 h, #P <0.05, compared to media enriched with 10 mM of G, pretreated with 1 mM of 5-FU at 12 and 24 h, + P <0.05 compared to media enriched with 10 mM of G at 12 h, by Student’s t test
Discussion
In this study we have shown that IEC-6 cells, which represent a rat crypt undifferentiated cell line, when previously exposed to 5-FU treatment, exhibited mitotic arrest, increased apoptotic rates, and migration inhibition in a dose-dependent manner, corroborating clinical findings on intestinal barrier impairment seen with 5-FU chemotherapy [29, 30]. Cell proliferation, migration, and apoptosis at the crypt level are critically important in modulating dynamics of enterocyte turnover within the mucosa, especially following severe catabolic states, in order to repave the epithelial lining layer lost due to cell shedding at the villus level [31].
In our model, glutamine and alanyl-glutamine supplementation improved cell proliferation and migration in IEC-6 cells previously treated with 5-FU. Glutamine cooperates with distinct growth factors within the injured mucosa to enhance crypt turnover activity and to be involved in mitotic signaling pathways, ultimately leading to intestinal barrier healing by shifting the balance toward more proliferative instead of cell death programming responses [32, 33]. In addition, critical growth factors, such as the insulin-like growth factor-1 (IGF-1), are involved in intestinal mucosal healing following injury, and have now been associated with increased amino acid transporters in the enterocyte membrane [34, 35]. Our findings further support the strategy of exogenous glutamine administration in an attempt to supply adequate fuel to the rapidly proliferating immune and intestinal cells, following endogenous glutamine tissue deprivation in the severely ill, as observed in successful clinical trials with glutamine-enriched oral hydration therapy and parenteral supplementation [16, 21, 36, 37]. Furthermore, the observed beneficial effects in 5-FU-injured intestinal cells in this study suggests a possible protective or reparative role of glutamine or alanyl-glutamine supplementation during chemotherapy in clinical situations.
Our novel findings with alanyl-glutamine supplementation in vitro further support our previous study, in which we confirmed the benefit of the more stable alanyl-glutamine on intestinal recovery following a single dose of 5-FU (150 mg/kg) treatment in mice, mostly associated with increased mitotic index and crypt hyperplasia. However, in contrast to our in vitro data, glutamine failed to accelerate mucosal healing in our murine model, which may suggest the superiority of the dipeptide over the more poorly soluble compound, glutamine, in vivo [25]. Interestingly, both in vivo and in vitro studies did not find a role of glutamine or alanyl-glutamine in preventing 5-FU-induced apoptosis in crypt cells 24 h after the challenge, at the peak time of the apoptotic curve, which is believed to precede chemotherapy-induced crypt hypoplasia [38]. Other studies have also described a possible lack of effect of glutamine supplementation in preventing apoptosis in vitro during chemotherapy treatment with methotrexate [39], supporting our findings, and suggesting that glutamine may act through different pathways on preventing cell death. Glutamine modulation of cell apoptosis appears to recruit cell-type-specific signaling, involving the intrinsic and extrinsic pathways [40, 41] and its deprivation was shown to induce apoptosis in vitro [42–44]. The role of glutamine deprivation in the significant increase in apoptotic rates is corroborated by the fact that IEC-6 cells seeded for 24 h in a glutamine-enriched media, without media change, show a higher apoptotic cell death as compared to 12 h, suggesting that a high metabolic consumption of glutamine by the cells rapidly depletes this conditionally required nutrient from the media. Nonetheless, the rate of apoptosis was unchanged in IEC-6 seeded in alanyl-glutamine enriched media, under the same conditions, suggesting better bio-availability of this dipeptide as a source of glutamine [45]. Although a trend toward reduction of apoptosis was seen, we could not show a significant glutamine effect in our in vitro model. Other studies, including our own [46, 47], have found a protective role of glutamine in preventing cell apoptosis seen with toxins, atrophy or cytokines [47–49] in other more differentiated intestinal cell lines. However, we postulate that apoptosis at the level of undifferentiated stem cells is critical to the effects of 5-FU in preventing the emergence of mutant cell lines in the crypts, which would prevent further neoplasic growth. Nevertheless, glutamine may still protect the intestinal mucosa even during chemotherapy, since it is not believed to negatively affect tumor growth [50].
In this current study, we have addressed the role of glutamine derivatives in IEC-6 cells following 5-FU challenge, evaluating cell migration, proliferation, and apoptosis using a specific undifferentiated intestinal cell line. This study further clarifies crypt cell dynamics following 5-FU-induced epithelial cell damage seen in vivo. The optimal dose of 10 mM of glutamine and alanyl-glutamine show enhanced crypt cell proliferation following 5-FU challenge. The same concentration proved to be beneficial in our previous studies [47, 51] with Clostridium difficile toxin A-induced epithelial injury. This concentration used in the medium far exceeds the physiological serum concentration, confirming the high requirement for these peptides for cell growth and adaptation following severe epithelial injury.
Further studies are needed to define the molecular mechanisms involved in intestinal cell viability and injury repair, including studies of the WNT signaling pathway and R-spondin-1 [52], promitotic factors which may interact with glutamine to hasten intestinal recovery. Additionally, the interaction with other critical guttrophic nutrients such as zinc and arginine are critical targets for developing more efficient strategies to alleviate malabsorption and diarrhea due to undesirable intestinal mucositis, and to rebuild the small intestinal barrier, work which is currently underway in our laboratory.
Acknowledgments
This study was supported by the following grants from the National Institutes of Health: ABC Grant no. 5 D43 TW001136, and GIDRT Grant no. 5 D43 TW006578.
Contributor Information
Manuel B. Braga-Neto, Institute of Biomedicine and Clinical Research Unit-University Hospital, Federal University of Ceará, Fortaleza, Brazil
Cirle A. Warren, Center for Global Health, School of Medicine, Division of Infectious Diseases and International Health, MR4, Lane Road, Room 3148, Charlottesville, VA 22908, USA
Reinaldo B. Oriá, Email: rbo5U@virginia.edu, Institute of Biomedicine and Clinical Research Unit-University Hospital, Federal University of Ceará, Fortaleza, Brazil. Department of Morphology, Federal University of Ceará, Fortaleza, Brazil
Manuel S. Monteiro, Institute of Biomedicine and Clinical Research Unit-University Hospital, Federal University of Ceará, Fortaleza, Brazil
Andressa A. S. Maciel, Institute of Biomedicine and Clinical Research Unit-University Hospital, Federal University of Ceará, Fortaleza, Brazil
Gerly A. C. Brito, Department of Morphology, Federal University of Ceará, Fortaleza, Brazil. Department of Pharmacology, Federal University of Ceará, Fortaleza, Brazil
Aldo A. M. Lima, Institute of Biomedicine and Clinical Research Unit-University Hospital, Federal University of Ceará, Fortaleza, Brazil. Department of Pharmacology, Federal University of Ceará, Fortaleza, Brazil
Richard L. Guerrant, Email: rlg9a@virginia.edu, Institute of Biomedicine and Clinical Research Unit-University Hospital, Federal University of Ceará, Fortaleza, Brazil. Center for Global Health, School of Medicine, Division of Infectious Diseases and International Health, MR4, Lane Road, Room 3148, Charlottesville, VA 22908, USA
References
- 1.Potten CS. Kinetics and possible regulation of crypt cell populations under normal and stress conditions. Bull Cancer. 1975;62:419–430. [PubMed] [Google Scholar]
- 2.Potten CS. Cell cycles in cell hierarchies. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:257–278. doi: 10.1080/09553008514552541. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS, Booth C. The role of radiation-induced and spontaneous apoptosis in the homeostasis of the gastrointestinal epithelium: a brief review. Comp Biochem Physiol B Biochem Mol Biol. 1997;118:473–478. doi: 10.1016/s0305-0491(97)00219-8. [DOI] [PubMed] [Google Scholar]
- 4.Clatworthy JP, Subramanian V. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech Dev. 2001;101:3–9. doi: 10.1016/s0925-4773(00)00557-8. [DOI] [PubMed] [Google Scholar]
- 5.Roncucci L, Pedroni M, Vaccina F, Benatti P, Marzona L, De PA. Aberrant crypt foci in colorectal carcinogenesis. Cell and crypt dynamics. Cell Prolif. 2000;33:1–18. doi: 10.1046/j.1365-2184.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becciolini A, Balzi M, Fabbrica D, Potten CS. Cell kinetics in rat small intestine after exposure to 3 Gy of gamma-rays at different times of the day. Int J Radiat Biol. 1996;70:281–288. doi: 10.1080/095530096145012. [DOI] [PubMed] [Google Scholar]
- 7.Naughton PJ, Grant G, Ewen SW, Spencer RJ, Brown DS, Pusztai A, Bardocz S. Salmonella typhimurium and Salmonella enteritidis induce gut growth and increase the polyamine content of the rat small intestine in vivo. FEMS Immunol Med Microbiol. 1995;12:251–258. doi: 10.1111/j.1574-695X.1995.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelder W, Hospers GA, Plukker JT. Effects of 5-fluoro-uracil adjuvant treatment of colon cancer. Expert Rev Anticancer Ther. 2006;6:785–794. doi: 10.1586/14737140.6.5.785. [DOI] [PubMed] [Google Scholar]
- 9.Beretta GD, Milesi L, Pessi MA, Mosconi S, Labianca R. Adjuvant treatment of colorectal cancer. Surg Oncol. 2004;13:63–73. doi: 10.1016/j.suronc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Daniele B, Secondulfo M, De VR, Pignata S, De ML, Delrio P, Palaia R, Barletta E, Tambaro R, Carratu R. Effect of chemotherapy with 5-fluorouracil on intestinal permeability and absorption in patients with advanced colorectal cancer. J Clin Gastroenterol. 2001;32:228–230. doi: 10.1097/00004836-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Duncan M, Grant G. Oral and intestinal mucositis—causes and possible treatments. Aliment Pharmacol Ther. 2003;18:853–874. doi: 10.1046/j.1365-2036.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 12.Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002;18:761–766. doi: 10.1016/s0899-9007(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 13.Oehler R, Roth E. Regulative capacity of glutamine. Curr Opin Clin Nutr Metab Care. 2003;6:277–282. doi: 10.1097/01.mco.0000068962.34812.ac. [DOI] [PubMed] [Google Scholar]
- 14.Neu J, DeMarco V, Li N. Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69–75. doi: 10.1097/00075197-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 15.O’Connell MJ. Current status of adjuvant therapy for colorectal cancer. Oncology (Williston Park) 2004;18:751–755. [PubMed] [Google Scholar]
- 16.Elia M, Lunn PG. The use of glutamine in the treatment of gastrointestinal disorders in man. Nutrition. 1997;13:743–747. doi: 10.1016/s0899-9007(97)83037-7. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro-Filho BA, Lima IP, Araujo DH, Cavalcante MC, Carvalho GH, Brito GA, Lima V, Monteiro SM, Santos FN, Ribeiro RA, Lima AA. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci. 2004;49:65–72. doi: 10.1023/b:ddas.0000011604.45531.2c. [DOI] [PubMed] [Google Scholar]
- 18.Daniele B, Perrone F, Gallo C, Pignata S, De MS, De VR, Barletta E, Tambaro R, Abbiati R, D’Agostino L. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity a double blind, placebo controlled, randomised trial. Gut. 2001;48:28–33. doi: 10.1136/gut.48.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yalcin SS, Yurdakok K, Tezcan I, Oner L. Effect of glutamine supplementation on diarrhea, interleukin-8 and secretory immunoglobulin A in children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2004;38:494–501. doi: 10.1097/00005176-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lima AA, Guerrant RL. Glutamine for childhood malnutrition: is it needed? J Pediatr Gastroenterol Nutr. 2005;40:526–527. doi: 10.1097/00005176-200504000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Carneiro-Filho BA, Bushen OY, Brito GA, Lima AA, Guerrant RL. Glutamine analogues as adjunctive therapy for infectious diarrhea. Curr Infect Dis Rep. 2003;5:114–119. doi: 10.1007/s11908-003-0046-2. [DOI] [PubMed] [Google Scholar]
- 22.Kelly D, Wischmeyer PE. Role of L-glutamine in critical illness: new insights. Curr Opin Clin Nutr Metab Care. 2003;6:217–222. doi: 10.1097/00075197-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Furst P. New developments in glutamine delivery. J Nutr. 2001;131:2562S–2568S. doi: 10.1093/jn/131.9.2562S. [DOI] [PubMed] [Google Scholar]
- 24.Buchman AL. Glutamine for the gut: mystical properties or an ordinary amino acid? Curr Gastroenterol Rep. 1999;1:417–423. doi: 10.1007/s11894-999-0024-4. [DOI] [PubMed] [Google Scholar]
- 25.Carneiro-Filho BA, Oria RB, Wood RK, Brito GA, Fujii J, Obrig T, Lima AA, Guerrant RL. Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition. 2004;20:934–941. doi: 10.1016/j.nut.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Miller AL. Therapeutic considerations of L-glutamine: a review of the literature. Altern Med Rev. 1999;4:239–248. [PubMed] [Google Scholar]
- 27.Hirata K, Horie T. Stimulation of intestinal epithelial restitution by prostaglandin E(1) analogue. Cancer Chemother Pharmacol. 2003;51:216–220. doi: 10.1007/s00280-003-0576-1. [DOI] [PubMed] [Google Scholar]
- 28.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992;263:G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- 29.Daniele B, Secondulfo M, De VR, Pignata S, De ML, Delrio P, Palaia R, Barletta E, Tambaro R, Carratu R. Effect of chemotherapy with 5-fluorouracil on intestinal permeability and absorption in patients with advanced colorectal cancer. J Clin Gastroenterol. 2001;32:228–230. doi: 10.1097/00004836-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Fata F, Ron IG, Kemeny N, O’Reilly E, Klimstra D, Kelsen DP. 5-fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer. 1999;86:1129–1134. doi: 10.1002/(sici)1097-0142(19991001)86:7<1129::aid-cncr5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y, Wu ZH. The anabolic effects of recombinant human growth hormone and glutamine on parenterally fed, short bowel rats. World J Gastroenterol. 2002;8:752–757. doi: 10.3748/wjg.v8.i4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler TR, Mantell MP, Chow JC, Rombeau JL, Smith RJ. Gut adaptation and the insulin-like growth factor system: regulation by glutamine and IGF-I administration. Am J Physiol. 1996;271:G866–G875. doi: 10.1152/ajpgi.1996.271.5.G866. [DOI] [PubMed] [Google Scholar]
- 34.Alexander AN, Carey HV. Insulin-like growth factor-I stimulates Na+-dependent glutamine absorption in piglet enterocytes. Dig Dis Sci. 2002;47:1129–1134. doi: 10.1023/a:1015010728696. [DOI] [PubMed] [Google Scholar]
- 35.Ray EC, Avissar NE, Sax HC. Growth factor regulation of enterocyte nutrient transport during intestinal adaptation. Am J Surg. 2002;183:361–371. doi: 10.1016/s0002-9610(02)00805-x. [DOI] [PubMed] [Google Scholar]
- 36.Mithieux G. New data and concepts on glutamine and glucose metabolism in the gut. Curr Opin Clin Nutr Metab Care. 2001;4:267–271. doi: 10.1097/00075197-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 38.Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–637. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Jr, Ko TC. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. doi: 10.1016/s1091-255x(00)80022-0. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs BC, Bode BP. Stressing out over survival: glutamine as an apoptotic modulator. J Surg Res. 2005;131:26–40. doi: 10.1016/j.jss.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392–401. doi: 10.1002/jcp.20339. [DOI] [PubMed] [Google Scholar]
- 42.Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Jr, Ko TC. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. doi: 10.1016/s1091-255x(00)80022-0. [DOI] [PubMed] [Google Scholar]
- 43.Paquette JC, Guerin PJ, Gauthier ER. Rapid induction of the intrinsic apoptotic pathway by L-glutamine starvation. J Cell Physiol. 2005;202:912–921. doi: 10.1002/jcp.20194. [DOI] [PubMed] [Google Scholar]
- 44.Mates JM, Segura JA, Alonso FJ, Marquez J. Pathways from glutamine to apoptosis. Front Biosci. 2006;11:3164–3180. doi: 10.2741/2040. [DOI] [PubMed] [Google Scholar]
- 45.Kircher B, Eibl G, Enrich B, Latzer K, Herold M, Niederwieser D. The role of L-alanyl-L-glutamine in the immune response in vitro. Wien Klin Wochenschr. 2002;114:702–708. [PubMed] [Google Scholar]
- 46.Brito GA, Carneiro-Filho B, Oria RB, Destura RV, Lima AA, Guerrant RL. Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig Dis Sci. 2005;50:1271–1278. doi: 10.1007/s10620-005-2771-x. [DOI] [PubMed] [Google Scholar]
- 47.Carneiro BA, Fujii J, Brito GA, Alcantara C, Oria RB, Lima AA, Obrig T, Guerrant RL. Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect Immun. 2006;74:81–87. doi: 10.1128/IAI.74.1.81-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Jr, Ko TC. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. doi: 10.1016/s1091-255x(00)80022-0. [DOI] [PubMed] [Google Scholar]
- 49.Evans ME, Jones DP, Ziegler TR. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr. 2003;133:3065–3071. doi: 10.1093/jn/133.10.3065. [DOI] [PubMed] [Google Scholar]
- 50.Decker GM. Glutamine: indicated in cancer care? Clin J Oncol Nurs. 2002;6:112–115. doi: 10.1188/02.CJON.112-115. [DOI] [PubMed] [Google Scholar]
- 51.Brito GA, Carneiro-Filho B, Oria RB, Destura RV, Lima AA, Guerrant RL. Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig Dis Sci. 2005;50:1271–1278. doi: 10.1007/s10620-005-2771-x. [DOI] [PubMed] [Google Scholar]
- 52.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]