Abstract
The measurement of skeletal muscle protein fractional synthetic rate using an infusion of (1-13C)leucine and measuring the isotopic abundance of the tracer in skeletal muscle protein by preparative gas chromatography (GC)/ninhydrin isotope ratio mass spectrometry (IRMS) is laborious and subject to errors owing to contamination by 12C. The purpose of this study was to compare muscle (13C)leucine enrichment measured with the conventional preparative GC/ninhydrin IRMS approach to a new, continuous-flow technique using capillary GC/combustion IRMS. Quadriceps muscles were removed from four Sprague–Dawley rats after each was infused at a different rate with (1-13C)leucine for 6–8 h. Muscle leucine enrichment (at.% excess) measured by both methods differed by less than 4%, except at low (13C)leucine enrichments (<0.03 at.% excess). In addition, capillary GC/combustion IRMS was used to assess muscle (13C)leucine enrichment and fractional muscle protein synthesis rate in ten normal young men and women infused with (1,2-13C2)leucine for 12–14 h. This approach reduced the variability of the isotope abundance measure and gave estimates of muscle protein synthesis rate (0.050 ± 0.011% h−1 (mean ± SEM); range = 0.023–0.147% h−1) that agree with published values determined using the standard analytical approach. The measurement of (13C)leucine enrichment from skeletal muscle protein by capillary GC/combustion IRMS provides a simple, acceptable and practical alternative to preparative GC/ninhydrin IRMS.
INTRODUCTION
Over the last decade, measurement of the rate of skeletal muscle protein synthesis in vivo1–11 has been achieved by infusing subjects with a stable isotopically labeled amino acid (e.g. (1-13C)leucine, valine, phenylalanine) and measuring the rate of incorporation of the labeled amino acid into muscle protein. This requires biopsy sampling of the muscle tissue during the infusion of labeled amino acid, separating the amino acids from an acid hydrolysate of the tissue using preparative gas chromatography (GC) or liquid chromatography (LC), isolating the labeled amino acid using a post-column splitter and a liquid nitrogen trap or a fraction collector, releasing the labeled carboxyl group as CO2 by reaction with ninhydrin, and determining the 13CO2/12CO2 ratio using isotope ratio mass spectrometry (IRMS).12,13 Unfortunately, this approach is time and labor intensive, requires relatively large muscle samples (~25–100 mg) for adequate recovery of leucine and CO2, and has the potential for contamination with natural carbon, predominantly 12C, because of the numerous preparative steps.
In an effort to automate and simplify the established technique, a continuous-flow instrument has been developed (Isochrom-1, VG Instruments, Danvers, Massachusetts) that uses an in-line tandem system employing capillary GC to separate the amino acid of interest, direct combustion of the column effluent containing the amino acid, and subsequent direct determination of the 13CO2/12CO2 ratio in a dual-inlet isotope ratio mass spectrometer.14 The purpose of this investigation was to measure muscle (13C)leucine enrichment using this new approach and to compare the results with the established technique.
EXPERIMENTAL
Standard mixture
L-(1-13C)Leucine (99% at.%, Tracer Technologies, Somerville, Massachusetts) was mixed with natural leucine (Sigma Chemical Co., St Louis, Missouri) to make serial standard dilutions of (1-13C)leucine enrichment in the range expected in muscle hydrolysates obtained from subjects infused with this tracer (~ 1.08–1.19 at.% 1-13C in the carboxyl position, which is equivalent to ~0.001–0.011 at.% excess 13C in all carbons of the combusted chemical derivative of the leucine molecule). Analytical procedures for the standards were identical to those used for muscle tissue analysis described below.
Animal studies
Animal experiments were carried out in adherence to the Guide for the Care and Use of Laboratory Animals as approved by the American Physiological Society. Two weeks prior to the infusion of leucine tracer, male Sprague–Dawley rats (285–320 g) had a Tygon catheter placed in a jugular vein for (1-13C)leucine infusion and a polyethylene catheter placed in a carotid artery for blood sampling using the techniques described previously.15 The catheters were kept patent by flushing with 500 μl heparinized saline (20 U ml−1). Four overnight fasted male rats were each infused with (1-13C)leucine at a different rate (prime = 0.6, 0.8, 1.6, 3.3 mg 100 g−1; constant infusion = 0.10, 0.20, 0.40 mg 100 g−1 h1 for 6 h; 0.64 mg 100 g−1 h−1 for 8 h). These infusion rates were chosen because they would result in muscle (13C)leucine enrichments spanning the range that would be expected in human muscles during an infusion of (1-13C)leucine. Immediately prior to the infusion and at half-hour intervals during the last 3 h of the infusion, blood samples (300 μl) were drawn for the measurement of plasma (1-13C)ketoisocaproic acid (KIC) enrichment. At the end of the infusion, the rats were anesthetized and the quadriceps muscles were removed and frozen in liquid nitrogen as previously described.16 Quadriceps muscles were also obtained from a control rat, not infused with tracer, to determine the natural abundance of 13C/12C in muscle leucine carbon. The quadriceps muscles were powdered with a mortar and pestle chilled with liquid nitrogen and lyophilized overnight.
Human studies
Seven young, healthy women (23 ± 1 years, 170 ± 6 cm, 63.3 ± 6.0 kg) and three men (23 ± 2 years, 177 ± 9 cm, 66.8 ± 2.8 kg) were recruited for this study, which was approved by the Human Studies Review Board at Washington University School of Medicine. Informed consent was obtained after the purpose and procedures were described. Each subject was admitted to the Clinical Research Center, where they received a 12–14 h intravenous infusion (prime = 7.5 μmol kg−1, constant infusion = 7.5 μmol kg h−1) of (1,2-13C2)leucine (97 at.% 13C, Tracer Technologies). Blood samples were taken prior to and at half-hour intervals during the last 4 h of the infusion and prepared for plasma (1,2-13C2)KIC analysis. The percutaneous needle biopsy technique17 was used to remove a muscle tissue sample from the vastus lateralis 1.5–2 h after the infusion started, and at the end of the infusion. The tissue sample was rinsed and blotted in normal saline, frozen in liquid nitrogen, and powdered as described above.
Analytical methods
Plasma (13C)KIC was isolated, derivatized and analyzed using positive chemical ionization (CI) GC/MS18 and the enrichment was used to represent precursor pool enrichment19 for the calculation of muscle protein synthesis rate (Ks; % h−1) using the equation;13 Ks = ((muscle (1-13C)leucine at.% excess × 100)/(plasma (1-13C)KIC at.% excess × elapsed time (h) between muscle samples)).
For preparative GC/ninhydrin IRMS, a portion of the rat muscle powder was prepared and analyzed for (1-13C)leucine enrichment using a modification of the previously described procedure.12 Instead of preparing the N-trifluoroacetyl isobutyl ester of the amino acids from the muscle hydrolysate, the tert-butyldimethylsilyl (t-BDMS) derivative was formed, as this derivative results in greater recovery of leucine from the post-column splitter. For capillary GC/combustion IRMS, the powdered rat and human muscle samples (15–30 mg frozen wet muscle) were homogenized in 1 ml 10% trichloroacetic acid, and centrifuged (5000 r.p.m.) so the supernatant could be removed and the pellet washed (4 ×) with 1 ml normal saline. The saline was removed and the pellet was hydrolyzed under nitrogen for 24–36 h with 2 ml of 6 N HCl. The hydrolysate was filtered (0.22 μm), then applied to an ion-exchange resin (Dowex AG-50W X8, 100–200 mesh, H+ form), and washed (4–6 ×) with 0.01 N HCl. The amino acids were eluted with 6 M NH4OH and the N-acetyl n-propyl (NAP) ester, prepared as described previously.20 NAP derivatives of the enriched leucine standards were prepared simultaneously.
For capillary GC/combustion IRMS (Isochrom I, VG Instruments) 2 μl of the derivatized amino acids were injected into a Hewlett-Packard 5980A gas chromatograph fitted with a 5% Phe Me capillary column (HP Ultra-2, 25 m, 0.32 mm i.d., 0.52 μm film thickness) and operated in the splitless mode. The carrier gas was helium maintained at a column head pressure of 10 p.s.i. The injector and FID temperatures were 250°C, and the oven temperature was programmed from 120 to 240°C at 10°C min−1, then 240–300°C at 30°C min−1, where it was held for 3–5 min. As leucine eluted from the capillary column (7.8 min), it was directed away from the FID using a pneumatically controlled splitter union and carried (helium) through an oxidation furnace tube (800°C) packed with CuO granules, where complete combustion to CO2 and water occurred. The water was subsequently removed in a trap (−100°C) and the CO2 carried by a capillary line to a dual-inlet isotope ratio mass spectrometer (SIRA II Series, VG Isogas). Pulses (6 × 30 s) of a calibrated reference CO2 gas (−8.467 δ13C, −9.481 δ18O) were introduced into the mass spectrometer by a capillary line before and after the combusted amino acid CO2 peak (Fig. 1). The fittings on the system were routinely checked for leaks with argon gas and the mass 40 ion current was always <10−12 A. The entire process (separation, reference/sample gas switches, data acquisition) was fully automated and controlled by the manufacturer’s computer software.
Figure 1.

Typical mass spectrometer tracing far CO2 gas (mass 44) obtained from the combustion of NAP–leucine and NAP–isoleucine isolated from human vastus lateralis muscle protein hydrolysate. Six reference gas (CO2) square-wave pulses are also shown. Assuming muscle tissue is 25% protein and 8% of this protein is leucine, the capillary GC/combustion IRMS instrument is capable of performing a 13CO2/12CO2 determination on 12–24 μg of NAP–leucine, which is equivalent to 1–2 μmol to CO2.
Statistics
The equation for the line, correlation coefficient, and 99% confidence limits for the enriched leucine standard curve were derived by linear regression analysis using a commercially available software package (Sigmaplot). At each level of tracer infusion, the rat muscle (13C)leucine enrichment determined by GC/combustion IRMS (4–6 injections per sample) was compared to the corresponding value determined by GC/ninhydrin IRMS (4 analyses per sample) using a two-tailed (t-test.
RESULTS
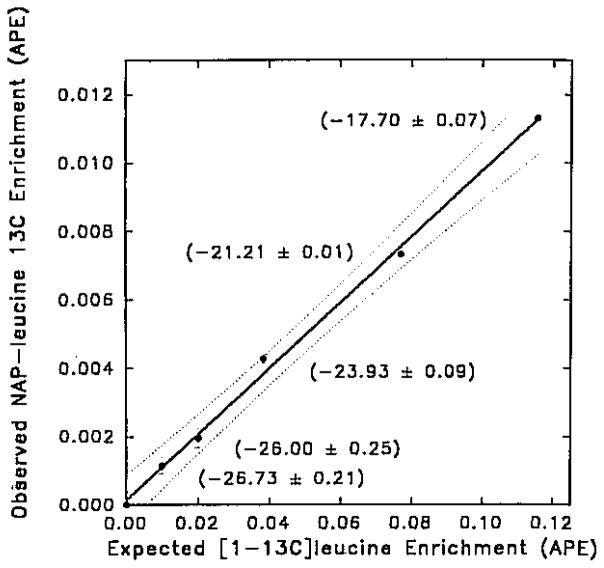
Figure 2 shows the highly linear (r = 0.998) relationship between the expected 13C enrichment in the carboxyl carbon atom of the enriched leucine standards and the observed 13C enrichment of the entire NAP–leucine molecule (11 carbon atoms) measured by capillary GC/combustion IRMS. The enrichment of the NAP–leucine derivative at natural 13C abundance was 1.08075 ± 0.00011 at.% (mean ± SD; n = 4), giving a intra-assay coefficient of variation of 0.01%, and a detection limit for (13C)leucine of 0.002 at.% excess (~1.7 delta). Repeated analysis (Table 1) of each standard measured over several weeks demonstrated an inter-assay reproducibility of 15–21% at low (<0.03 at.% excess) (1-13C)leucine enrichments, but 3% or less at (1-13C)leucine enrichments of 0.0466 at.% excess or greater.
Figure 2.
The relationship between the expected 13C enrichment (at.% excess) in the carboxyl leucine carbon and the observed 13C enrichment in N-acetyl n-propyl (NAP)–leucine (11 carbon atoms) measured by capillary GC/combustion IRMS. The equation for the line is Y = 0.0962X + 0.0001, r=0.998 (n = 21), and the 99th percentile confidence intervals are given by the dotted lines. The delta (δ) values versus PDB for NAP-leucine are shown in parentheses (mean ± SD) compared to a measured value of −27.71 ± 0.12 for natural NAP–leucine.
Table 1.
Inter-assay precision for five (13C)leucine–NAP standards measured by capillary GC/combustion IRMS over several weeks
| Standard no. | (1-13C)Leucine enrichment (at. % excess) | Coefficient of variation (no. of injections) |
|---|---|---|
| 1 | 0.0125 ± 0.0026 | 21 (8) |
| 2 | 0.0215 ± 0.0032 | 15 (5) |
| 3 | 0.0466 ± 0.0015 | 3 (4) |
| 4 | 0.0794 ± 0.0024 | 3 (5) |
| 5 | 0.1252 ± 0.0011 | 1 (5) |
Mean ± SD.
Table 2 compares the (1-13C)leucine enrichment values measured in rat muscle protein using capillary GC/combustion IRMS and the conventional preparative GC/ninhydrin IRMS at each level of tracer infusion. The (1-13C)leucine enrichments determined by both methods differed by less than 4%, except for the sample with the lowest enrichment (11%), and were not significantly different (p > 0.05). The fractional rate of muscle protein synthesis for these rat quadriceps muscles determined by capillary GC/combustion IRMS was 4.8% per day, which agrees with published values for quadriceps muscles from rats of similar weight determined using radio- and stable-labeled tracers (4.3–5.3% per day).21 As with the (1-13C)leucine standard solutions analyzed by capillary GC/combustion IRMS (Table 1), the rat muscle sample with the lowest enrichment (0.0288 at.% excess) had the highest variability (CV = 20%).
Table 2.
Comparison of (1-13C)leucine enrichment in rat quadriceps muscle protein measured by capillary GC/combustion IRMS and standard preparative GC/ninhydrin IRMS
| (1-13)Leucine infusion rate (mg 100 g−1 h−1 | (1-13C)Leucine enrichmenta (GC/combustion IRMS) | (1-13C)Leucine enrichmenta (GC/ninhydrin IRMS) |
|---|---|---|
| 0.10 for 6 h | 0.0288 ± 0.0059 (20) | 0.0256 ± 0.0003 (1.2) |
| 0.20 for 6 h | 0.0558 ± 0.0041 (7.3) | 0.0560 ± 0.0007 (1.2) |
| 0.40 for 6 h | 0.1068 ± 0.0029 (2.7) | 0.1042 ± 0.0104 (10) |
| 0.64 for 8 h | 0.1144 ± 0.0066 (5.8) | 0.1094 ± 0.0012 (1.2) |
Mean at.% excess ± SD for 4–6 injections on GC/combustion IRMS and 4 GC/ninhydrin IRMS analyses. Numbers in parentheses are the coefficients of variation.
Table 3 shows the method analytical precision and the calculated fractional muscle protein synthesis rates measured in men and women infused with (1,2-13C2)leucine. The duplicate measures differed by less than 5% in 5 of 10 subjects, 3 of 10 differed by 13–16%, and only 2 of 10 differed by 18–24%. These observations suggest that there was no relationship between replicate precision and isotope enrichment in the range of 0.0231–0.0803 at.% excess, and that measurement variabilty was reduced somewhat by the infusion of [1, 2-13C2]leucine.
Table 3.
Muscle (13C)leucine enrichment and fractional skeletal muscle protein synthesis rates measured in duplicate capillary GC/combustion IRMS analyses of the same muscle sample taken from human vastus lateralis during an infusion of (1,2-13C2)leucine
| Subject | Muscle (13C) leucine enrichment (at.% excess) | Muscle protein synthesis rate (% h−1) | |
|---|---|---|---|
| Sample 1 | Sample 2 | ||
| 1 | 0.0264 | 0.0264 | 0.035 |
| 2 | 0.0253 | 0.0286 | 0.033 |
| 3 | 0.0697 | 0.0719 | 0.070 |
| 4 | 0.0296 | 0.0310 | 0.040 |
| 5 | 0.0231 | 0.0231 | 0.025 |
| 6 | 0.0330 | 0.0379 | 0.046 |
| 7 | 0.0267 | 0.0311 | 0.037 |
| 8 | 0.0183 | 0.0216 | 0.023 |
| 9 | 0.0250 | 0.0311 | 0.044 |
| 10 | 0.0803 | 0.0781 | 0.147 |
The skeletal muscle fractional protein synthesis rates calculated from these values (Table 3) were in agreement with published values determined using the conventional analytical approach.1–13 For example, the published values for quadriceps muscle protein synthesis rate in healthy normal subjects are 0.035–0.063% h−1 (SD = 0.009) by Nair et al.,13 0.033–0.064% h−1 (SD = 0.012) by Gibson et al.,1 and 0.036–0.063% h−1 (SD = 0.011) by Nair et al.8 These ranges and standard deviations compare closely with the values reported in the present study (0.023–0.070% h−1, SD = 0.014) if subject no. 10 is excluded. Subject no. 10 completed a session of weight-lifting exercise 2 h prior to the initiation of the tracer infusion experiment. We believe that the prior bout of exercise was the cause of this subject’s elevated rate of muscle protein synthesis because (i) Carraro et al.11 have observed an increased rate of muscle protein synthesis during recovery from mild aerobic exercise, and (ii) we have observed an increase in muscle protein synthesis rate in an additional 7 individuals studied in the same manner as those reported here, after a session of resistance exercise (manuscript in Preparation).
DISCUSSION
These observations indicate that capillary GC/combustion IRMS provides a rapid, acceptable alternative to preparative GC/ninhydrin IRMS for the measurement of (13C)leucine enrichment in skeletal muscle samples and for the determination of the fractional rate of muscle protein synthesis in rat and human skeletal muscle. However, at (1-13C)leucine enrichments below about 0.03 at.% excess the variability associated with capillary GC/combustion IRMS is greater than the variability of preparative GC/ninhydrin IRMS. In part, this reflects the fact that the capillary GC/combustion IRMS analysis is carried out on a different portion of the isotope standard curve. The ninhydrin reaction selectively removes the carboxyl carbon of leucine, and therefore, 13C/12C ratios are measured for a single carbon. The combustion process, on the other hand, burns the entire NAP–leucine molecule to CO2, effecting an 11-fold dilution of 13C at the first carbon position with natural abundance 13C/12C of the remaining carbons of the derivatized molecule.
The dilution of the label may not be a severe limitation for several reasons. Multiple injections of the same muscle sample can be made and this can improve the measurement precision of capillary GC/combustion IRMS to some extent. Replicate injections are not an analytical problem because a complete sample analysis (GC separation, combustion, IRMS and data processing) requires only 15 min. This is a trivial inconvenience in comparison to the time and effort required to perform the same tasks using the preparative GC/ninhydrin IRMS technique. Moreover, the variability introduced by the dilution effect can be reduced by infusing leucine containing more than one labeled carbon atom. For example, doubling the amount of tracer administered (e.g. (1,2-13C2)leucine) or increasing the duration of the leucine tracer infusion will increase muscle (13C)leucine enrichment proportionately. The measured values, therefore, will fall on the less variable portion of the standard curve. The dilution effect can also be minimized by selecting a chemical derivative that adds fewer carbons to the amino acid. The latter two approaches remain to be tested while, in the present study, the first approach succeeded in labeling muscle protein leucine to a level measurable with precision sufficient for biological studies in man. Further, the capillary GC/combustion IRMS approach can be applied easily to the measurement of 13C-labeled amino acid incorporation into proteins with faster turnover rates, such as albumin, fibronectin and apolipoprotein B. Since these proteins turn over at rates far faster than the very slowly turning over structural muscle proteins, the same isotope infusion rates used in the present study would result in protein isotopic enrichments in the high precision area of the standard curve.
The close agreement between the capillary GC/combustion IRMS and preparative GC/ninhydrin IRMS measures of muscle (13C)leucine enrichment (Table 2) suggest that contamination of the sample with 12C during the numerous preparative steps is not a serious problem. However, the additional sample transfers that occur during the preparative GC/ninhydrin IRMS process results in a combined loss of leucine and CO2 (~30%), which partially explains the slightly larger muscle sample requirements associated with this approach. Despite the appreciable loss of leucine and CO2 during the preparative GC/ninhydrin IRMS process, the present findings suggest that the measurement of 13C enrichment was not affected.
Finally, the precision of the preparative GC/ninhydrin IRMS method is optimal when large muscle samples (≥50 mg) are available and large amounts of CO2 are recovered during the ninhydrin reaction. More recently, enrichment measures have been accomplished on smaller muscle samples (20–50 mg) using the t-BDMS chemical derivative. By comparison, the capillary GC/combustion IRMS technique requires only 15–30 mg of wet muscle, and replicate analyses can be performed on one tissue sample. This is particularly important for human studies where biopsy specimens are commonly 50 mg or less in size.
In conclusion, continuous-flow capillary GC/combustion IRMS can provide a rapid, acceptable, practical alternative to preparative GC/ninhydrin IRMS for the measurement of muscle (13C)leucine enrichment and muscle protein synthesis rate.
Acknowledgments
This work was supported by NIH grants RR00954, HD20805 and AG05562. K. E. Yarasheski was supported by a Career Development Award (AG00444). Richard Berger provided technical assistance with the mass spectrometry, and Timothi Beth is gratefully acknowledged for his expertise with the rat surgical techniques.
References
- 1.Gibson JNA, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Clin Sci. 1987;72:503. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- 2.Morrison WL, Gibson JNA, Scrimgeour C, Rennie MJ. Clin Sci. 1988;75:415. doi: 10.1042/cs0750415. [DOI] [PubMed] [Google Scholar]
- 3.Rennie MJ, Edwards RHT, Millward DJ, Wolman SL, Halliday D, Matthews DE. Nature. 1982;296:165. doi: 10.1038/296165a0. [DOI] [PubMed] [Google Scholar]
- 4.Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Clin Sci. 1989;76:447. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- 5.Gibson JNA, Smith K, Rennie MJ. Lancet. 1988;ii:767. doi: 10.1016/s0140-6736(88)92417-8. [DOI] [PubMed] [Google Scholar]
- 6.Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. J Appl Physiol. 1989;66:498. doi: 10.1152/jappl.1989.66.1.498. [DOI] [PubMed] [Google Scholar]
- 7.Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Am J Physiol. 1992;262:E261. doi: 10.1152/ajpendo.1992.262.3.E261. ([Endocrinol. Metab. 25) [DOI] [PubMed] [Google Scholar]
- 8.Nair SK, Welle SL, Halliday D, Campbell RG. J Clin invest. 1988;82:198. doi: 10.1172/JCI113570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Clin Sci. 1988;75:481. doi: 10.1042/cs0750481. [DOI] [PubMed] [Google Scholar]
- 10.Rennie MJ, Edwards RHT, Halliday D, Matthews DE, Wolman SL, Millward DJ. Clin Sci. 1982;63:519. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 11.Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Am J Physiol. 1990;259:E470. doi: 10.1152/ajpendo.1990.259.4.E470. (Endocrinol. Metab. 22) [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Scrimgeour CM, Bennet WM, Rennie MJ. Biomed Environ Mass Spectrom. 1988;17:267. doi: 10.1002/bms.1200150704. [DOI] [PubMed] [Google Scholar]
- 13.Nair KS, Halliday D, Griggs RC. Am J Physiol. 1988;254:E208. doi: 10.1152/ajpendo.1988.254.2.E208. (Endocrinol. Metab. 17) [DOI] [PubMed] [Google Scholar]
- 14.Silfer JA, Engel MH, Macko SA, Jumeau EJ. Anal, Chem. 1991;63:370. [Google Scholar]
- 15.Jacob R, Barrett E, Plewe G, Fagin KD, Sherwin RS. J Clin Invest. 1989;83:1717. doi: 10.1172/JCI114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarasheski KE, Lemon PWR, Gilloteaux J. J Appl Physiol. 1990;69:434. doi: 10.1152/jappl.1990.69.2.434. [DOI] [PubMed] [Google Scholar]
- 17.Evans WJ, Phinney SD, Young VR. Med Sci Sports Exercise. 1982;14:101. [PubMed] [Google Scholar]
- 18.Matthews DE, Schwarz HP, Yang RD, Motil KJ, Young VR, Bier DM. Metabolism. 1982;31:1150. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- 19.Watt PW, Lindsay Y, Scrimgeour CM, Chien PAF, Gibson JNA, Taylor DJ, Rennie MJ. Proc Nat Acad Sci USA. 1991;88:5892. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams RF. J Chromatogr. 1974;95:189. doi: 10.1016/s0021-9673(00)84078-9. [DOI] [PubMed] [Google Scholar]
- 21.Waterlow JC, Garlick PJ, Millward DJ. Protein Turnover in Mammalian Tissues and in the Whole Body. North-Holland; Amsterdam: 1978. [Google Scholar]