Abstract
To clarify the role of HDACs in erythropoiesis, expression, activity and function of class I (HDAC1, HDAC2, HDAC3) and class IIa (HDAC4, HDAC5) HDACs during in vitro maturation of human erythroblasts were compared. During erythroid maturation, expression of HDAC1, HDAC2 and HDAC3 remained constant and activity and GATA1 association (its partner of the NuRD complex), of HDAC1 increased. By contrast, HDAC4 content drastically decreased and HDAC5 remained constant in content but decreased in activity. In erythroid cells, pull down experiments identified the presence of a novel complex formed by HDAC5, GATA1, EKLF and pERK which was instead undetectable in cells of the megakaryocytic lineage. With erythroid maturation, association among HDAC5, GATA1 and EKLF persisted but levels of pERK sharply decreased. Treatment of erythroleukemic cells with inhibitors of ERK phosphorylation reduced by >90% the total and nuclear content of HDAC5, GATA1 and EKLF, suggesting that ERK phosphorylation is required for the formation of this complex. Based on the function of class IIa HDACs as chaperones of other proteins to the nucleus and the erythroid-specificity of HDAC5 localization, this novel HDAC complex was named nuclear remodeling shuttle erythroid (NuRSERY). Exposure of erythroid cells to the class II-selective HDAC inhibitor (HDACi) APHA9 increased γ/(γ+β) globin expression ratios (Mai et al., 2007), suggesting that NuRSERY may regulate globin gene expression. In agreement with this hypothesis, exposure of erythroid cells to APHA9 greatly reduced the association among HDAC5, GATA1 and EKLF. Since exposure to APHA9 did not affect survival rates or p21 activation, NuRSERY may represent a novel, possibly less toxic, target for epigenetic therapies of hemoglobinopaties and other disorders.
Keywords: Histone deacetylases, GATA1, EKLF, Erythropoiesis, Hemoglobin, Histone deacetylase inhibitors
1. INTRODUCTION
Histone deacetylases (HDACs) play a major role in the control of cell fate decisions by catalyzing deacetylation of lysine residues on histones and other proteins (Ahringer et al., 2000; Bolden et al., 2006; Delcuve et al., 2012). The human HDAC family includes 18 isoforms classified on the basis of their sequence similarity to enzymes from Saccharomyces cerevisiae into class I (HDAC1, HDAC2, HDAC3 and HDAC8), class IIa (HDAC4, HDAC5, HDAC7 and HDAC9), class IIb (HDAC6 and HDAC10) (Bolden et al., 2006), class III (sirtuins) (Haigis et al., 2006) and class IV (HDAC11) (Gao et al., 2002). Class I HDACs exert their functions as multiprotein complexes, which include transcription factors, that dock the complex to specific DNA sites and regulatory proteins (PKC and ERK) (Ahringer et al., 2000; Bolden et al., 2006; Delcuve et al., 2012).
Recent studies have implicated complexes including class I HDACs in the control of erythropoiesis. The first complex to be identified was the nucleosome remodeling complex (NuRD), an ATP-dependent chromatin remodeler (Tong et al., 1998) formed by HDAC1 and the erythroid-specific transcription factor GATA1 through the common obligatory partner FOG1 (Miccio et al., 2009). Acetylation of HDAC1 inhibits the enzymatic activity of the protein and determines whether the NuRD complex will repress (HDAC1) or activate (acetylated HDAC1) the expression of genes controlled by GATA1 (Yang et al., 2012). NuRD inhibits amplification of hematopoietic progenitor cells by suppressing expression of the transcription factor GATA2 (Fujiwarw et al., 2010) and promotes erythroid commitment and maturation by activating the expression of erythroid-specific genes (Wada et al., 2009; Gregory et al., 2010). An important interaction between EKLF and the Mi2β subunit of NuRD may be involved in regulating the restriction point between erythroid and megakaryocytic differentiation in progenitor cells bipotent for the two lineages (Siatecka et al., 2011). Class I HDACs have also been implicated in the regulation of globin gene expression. Bradner et al provided data suggesting that HDAC1 and HDAC2 are responsible for decreasing the γ/β globin gene expression ratio (Bradner et al., 2010). Additional studies have clarified that HDAC1 associated with NuRD is responsible for β globin gene activation but is dispensable for activation of γ globin (Miccio et al., 2010) while HDAC3 associated with nuclear receptor co-repressor (NCoR) is responsible for suppressing expression of γ globin (Mankidy et al., 2006).
Class II HDACs are high molecular weight proteins that shuttle other proteins between the nucleus and the cytoplasm (Sengupta et al., 2004; Fischle et al., 2002; Lahm et al., 2007). The role played by class II HDACs in erythroid maturation is overall poorly understood. Preliminary data provided by Watamoto et al. indicate that in murine erythroleukemic cells (MEL) HDAC5 and GATA1 form a complex that is dissociated upon induction to differentiation by N,N-hexamethylenebisacetamide (Watamoto et al., 2003). Using a loss-of-function approach in mice, Delehanty et al. have shown that HDAC5 is required for activation of the stress pathway in response to erythropoietin (EPO) challenges (Delehanty et al., 2012) and Mai et al. observed that treatment with the class II-selective HDAC inhibitor (HDACi) APHA9 increases the γ/(γ+β) globin gene expression ratio in human erythroid cells (Mai et al., 2007). The multiprotein complexes including class II HDACs that control erythroid maturation have still to be characterized.
To clarify the role played by class IIa HDACs in the control of human erythropoiesis, expression and activity of class I and class IIa HDACs during the maturation of erythroblasts generated ex vivo from cord blood or adult blood mononuclear cells was first compared. Next, changes in the levels of association between GATA1 and its NuRD partner HDAC1 during erythroid maturation were determined. Finally, the possibility that in erythroid cells GATA1 and EKLF might be associated with class IIa HDACs was investigated. During erythroid maturation the content of HDAC1, HDAC2 and HDAC3 remained constant. However, the activity and association with GATA1 of HDAC1 increased. By contrast, during erythroid maturation, the content of HDAC4 was greatly reduced. In addition, although the content of HDAC5 remained constant, its activity decreased. In proerythroblasts, a complex formed by HDAC5, GATA1, EKLF and pERK was identified. This complex was not detectable in cells of the megakaryocytic lineage. Although association among HDAC5, GATA1 and EKLF persisted with maturation, the levels of pERK were greatly reduced. Exposure of erythroid cell lines to inhibitors of ERK phosphorylation greatly reduced the total and nuclear content of HDAC5, GATA1 and EKLF. Formation of the complex was also greatly reduced by exposure to class II-selective HDAC inhibitors (HDACi) that also increased γ-globin expression. These results identify a novel HDAC complex, defined as nuclear remodeling shuttle erythroid (NuRSERY) that may represent a new target for epigenetic-based therapy of hemoglobinopaties.
2. MATERIALS AND METHODS
2.1. Human Subjects
Blood buffy coats from 15–23 different normal adult donors (AB) and from 7 low volume cord blood units (CB) were obtained from the Italian Red Cross Blood Bank, Rome, Italy, according to guidelines established by the institutional ethical committee for human subject studies.
2.2. Cell Processing
Mononuclear blood cells were separated by centrifugation at 400g × 30′ over Ficoll-Hypaque (Amersham-Pharmacia Biotec, Uppsala, Sweden).
2.3. Expansion of Human Erythroblasts in HEMA Culture
Human erythroblasts were obtained by culturing for 12–15 days blood mononuclear cells (106 cells/mL) in Iscove’s modified Dulbecco’s medium (IMDM, Mascia Brunelli, Milan, Italy) containing 20% of fetal bovine serum (FBS, Hyclone, Logan, UT, USA), Stem Cell Factor (SCF, 10 ng/mL) (Amgen, Thousand Oaks, CA), EPO (1 u/mL) (Epoetina alfa, Dompè Biotec, Milan, Italy), Interleukin-3 (IL-3, 1 ng/mL) (Bouty, Milan, Italy), dexamethasone (Dex, 10−6 M) and estradiol (10−6 M) (both from Sigma), as described (Migliaccio et al., 2002). Day 12–15 erythroblasts were collected and induced to mature by culture for 4 additional days with EPO (3 u/mL) and human recombinant insulin (10 ng/mL) (Calbiochem, Darmstadt, Germany). In selected experiments, the maturation cultures contained increasing concentrations of APHA9, UBHA24 and SAHA (Mai et al., 2007).
2.4. Expansion of Human Megakaryocytes (MK)
CD34pos cells purified from the blood of three adult donors (3 × 105/mL) were cultured for 12 days in serum-free X-Vivo medium (Lonza Walkersville, Inc., Walkersville, MD) supplemented with human IL-3 (3 ng/mL) and TPO (3% of a supernatant of BHK cells molecularly engineered to express the human TPO gene provided by K. Kaushansky). The cultures were replenished with fresh cytokines and medium every 3 days. The cultures generate within 12 days cells expressing high levels of the MK markers CD41a (which recognizes the GPIIb/IIIa complex) and CD42 (which recognizes GPIb) (Varricchio et al., 2012).
2.5. Cell Lines
Two erythroid (K562 and HEL) and two megakaryocytic (CMK and CMY) progenitor cell lines were investigated. The K562 cell line was derived from a patient with chronic myeloid leukemia (Lozzio et al., 1975) and harbors the Philadelphia (Ph) chromosome. The HEL cell line was derived from a patient with Hodgkin’s disease who later developed erythroleukemia (Martin et al., 1982) and carry the JAK2V617F mutation (Levine et al., 2005). K562 and HEL cells were provided by Dr. Papayannopoulou (Washington University, Seattle, WA, USA) on October 29th 1999 and March 3rd, 2009, respectively. The CMK and CMY cell lines were established from Down’s syndrome patients with acute megakaryoblastic leukemia (Sato et al., 1989; Miura et al., 1998) and carry the G4537A substitution at the intron-exon boundary of exon 2 of GATA1 that leads to synthesize a short form of the protein lacking the first 83 aminoacids of the amino-terminus domain (GATA1s) (Wechsler et al., 2002). CMK and CMY cells were provided by Drs. B Thompson (Northwestern University, Chicago, IL, USA) and M Weiss (Children Hospital, Philadelphia, PN, USA) in June 2013. All the cell lines were mass expanded upon arrival, cryopreserved in aliquots and stored in liquid nitrogen. All the experiments described in this paper were performed on cells expanded from thawed aliquots of the initial cryopreserved mass culture. Cell lines were cultured in IMDM supplemented with 10–20% FBS, antibiotics (1% penicillin-streptomycin, Lonza) and 1% of L-glutamine (Sigma) at 37°C in humidified atmosphere with 5% CO2. Erythroleukemic cells were treated with either with 100 μM of U0126 (ID50 = 70 nM for ERK1 and = 60 nM for ERK2, cat #9903 Cell signaling) or PD98059 (ID50 = 2 μM for ERK1 only, cat #9900 Cell Signaling) or Wortmannin (ID50 = 3 nM for PI-3K, cat #9951 Cell Signaling) for 24 h, unless otherwise stated.
2.6. Phenotypic Analysis
Cell maturation was analyzed by flow cytometry profiling based on CD36 [the thrombospondin receptor (Chen et al., 2007)] and CD235a (glycophorin A) expression and by morphological observations of cytocentrifuged smears (Shandon, Astmoor, UK) stained with May-Grünwald-Giemsa (Sigma).
2.7. RNA Isolation and Quantitative RT-PCR
Total RNA was isolated from 106 cells using TRIzol (Invitrogen, Carlsbad, CA) and reverse transcribed with the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA), as described by the manufacturer. Quantitative real-time PCR was carried out in a Prism 7700 Sequence Detection System (Applied Biosystems), using the TaqMan Master Mix containing AmpliTaq Gold DNA polymerase. Conditions for HDAC1, GATA1, GATA2 and γ/(γ+β) mRNA quantifications were already described (Varricchio et al., 2011). Each determination was performed in triplicate. mRNA levels were calculated with the 2-ΔCt method and expressed in arbitrary units considering the 2-ΔCt detected in controls as 1.
2.8. Western Blot (WB) and Immunoprecipitation (IP) studies
Whole cell extracts were prepared as described (Stellacci et al., 2009) and proteins (30 μg) separated on SDS-PAGE, transferred to nitrocellulose membranes which were then incubated with individual antibodies. The antibodies used in this WB study recognize pERK (#9101L Cell Signaling, Boston, MA, USA), ERK (#9102 Cell Signaling), anti-caspase-3 (#9662 Cell Signaling), GATA1 (C20 #1233 Santa Cruz Biotechnology, Santa Cruz, CA), EKLF (H-210 # 14034 Santa Cruz Biotechnology), ERα (HC-20 #543 Santa Cruz Biotechnology), GRα (H-300 #8992 Santa Cruz Biotechnology), HDAC1, HDAC2, and HDAC3 (#2062, #2540, #2632 Cell signaling), HDAC4 (#H9411 Sigma, Saint Louis, Missouri, USA), HDAC5 (#1439 Abcam, Cambridge, MA, USA), anti-p21Cip (C-19 #33780 Santa Cruz Biotechnology), anti-p27Kip1 (D696C12 #3686S Cell Signaling), GAPDH (#1616 Santa Cruz Biotechnology), Tubulin (#2144 Cell Signaling), Lamin B1 (#16048 Abcam) and HSP90 (#149 A/B Stress Mark Biosciences, Canada). The membranes were then incubated with appropriate horseradish peroxidase-coupled secondary antibodies (Calbiochem, San Diego, CA). Immune complexes were detected with an enhanced chemiluminescence kit (Amersham, Buckingamshire, UK). For IP studies, cell extracts (300–500 μg) were incubated with either anti-HDAC1, anti-HDAC5, anti-GATA1 or anti-EKLF antibodies overnight at 4°C with mixing and then with Ultralink Immobilized Protein A/G sepharose (Pierce Biotech, Rockford, IL) for 2 hr at room temperature. Immune complexes were dissociated by boiling the beads for 5 min in loading buffer, separated on SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by WB with various antibodies, as indicated (Varricchio et al., 2011). To increase specificity, EKLF was IP with a commercial antibody (H-210 #14034, Santa Cruz Biotechnology) and detected by WB with the 7B2 monoclonal antibody (Siatecka et al., 2010). In selected experiments, cells were subjected to sub-cellular cell fractionation with the NE-PER nuclear and cytosolic extraction kit (Thermo Scientific, Rockford, IL).
2.9. In vitro HDAC1 and HDAC5 activity determinations
HDAC1 and HDAC5 complexes were purified by IP with anti-HDAC1 or anti-HDAC5 antibodies from erythroblasts (7.5 × 105) lysed in IP buffer (50 mM Tris-HCl pH 7.0, 180 mM NaCl, 0.15% NP-40, 10% glycerol, 1.5 mM MgCl2, 1 mM NaMO4, 0.5 mM NaF) with a protease inhibitor cocktail (P2714-1BTL, Sigma). IP with an irrelevant IgG (Santa Cruz Biotechnology) was used as negative control. The levels of HDAC activity in the IP were determined using radiolabelled 3H-12 histone H4 peptides linked to streptavidin agarose beads as substrate, according to the supplier instructions (Upstate Biotechnology) (Mai et al., 2006). Lysates from HeLa cells were analysed in parallel as control.
2.10. Statistical Analysis
Statistical analysis was performed by Anova test with the computer software Origin 5.0 for Windows (Microcal Software, Inc., Northampton, MA, USA).
3. RESULTS
3.1 Changes in HDAC content and activity during the maturation of human erythroblasts
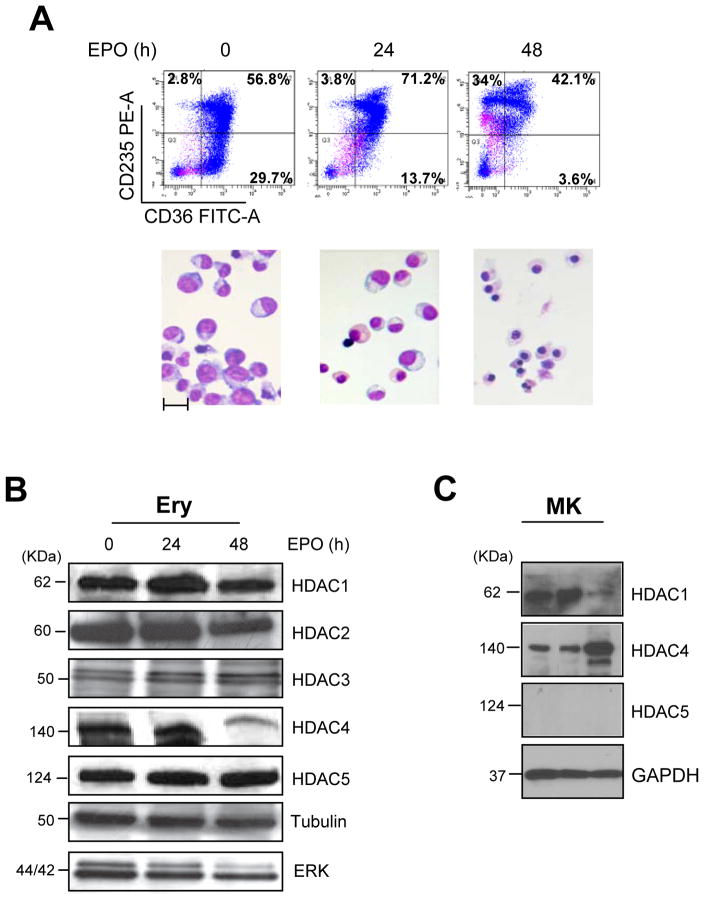
This study was performed on erythroid cells generated in HEMA culture by mononuclear cells from cord blood or adult blood and induced to mature in the presence of EPO for 0–96 h. The phenotype of the cell populations analyzed is described in Figure 1A. Three populations were analyzed: a) cells at day 12–15 of HEMA culture (0 h of EPO exposure) that contain > 87% CD36 positive cells, ~50% of which express CD235a and have proerythroblast morphology; b) cells exposed to EPO for 24 h, the majority of which (>70%) are positive for both CD36 and CD235a and express orthochromatic erythroblast morphology; c) cells exposed to EPO for 48 h that are all positive for CD235a but 34% of them no longer express CD36 and have the morphology of polychromatophilic erythroblasts.
Figure 1. HDACs expression during ex-vivo maturation of human erythroblasts.
A) Characterization of the maturation state of the erythroid cells used for the study. Flow cytometry profiling for CD36/CD235a expression (top panels) and morphology (by May-Grunwald/Giemsa staining, bottom panels) of human erythroblasts induced to mature with EPO for 0, 24 and 48 h, as indicated. Original magnifications 40X. The scale bar included in the micrograph corresponds to 35 μM.
B) WB analyses of HDAC1, HDAC2, HDAC3, HDAC4 and HDAC5 contents of erythroblasts treated with EPO for 0, 24 and 48 h (the same cells presented in A). Both Tubulin and total ERK were analyzed as loading controls. Expected molecular weights are indicated on the left. Similar results were observed in three additional experiments.
C) WB analyses of HDAC1, HDAC4 and HDAC5 content of megakaryocytes (MK) expanded ex-vivo from CD34pos cells purified from three separate AB donors. The biological features of the cells are described in Varricchio et al., 2012. GAPDH was analyzed as loading control.
D) WB analyses with the specific antibodies indicated on the right of nuclear and cytoplasmic fractions purified from proerythroblasts expanded from CB (Ery) and from erythroid (K562 and HEL) and megakaryocytic (CMK) progenitor cell lines. In the case of the cell lines, total cell extracts (T) were also analyzed for comparison. The fact that HDAC1, HDAC2, HDAC3 and GATA1 were readily detectable in nuclear extracts but not in total cell extracts of the three cell lines likely reflects the low abundance of these proteins in these cells. Laminin B and HSP90 were analyzed as loading and contamination control. Expected molecular weights are indicated on the left.
Human proerythroblasts contained robust levels of all HDACs analyzed (Figure 1B). With maturation, the content of HDAC1, HDAC2, HDAC3 and HDAC5 remained constant but that of HDAC4 decreased by 3-fold. Megakaryocytes (MK) expanded ex-vivo from three different donors also contained robust levels of HDAC1 but, by contrast with erythroid cells, contained robust levels of HDAC4 but undetectable levels of HDAC5 (Figure 1C).
Robust levels of H3-acetyl release activity were detected in IP with either anti-HDAC1 or anti-HDAC5 antibodies from human proerythroblasts (Table I). With erythroid maturation, the H3-acetyl release activity of IP with anti-HDAC1 antibodies increased >30% while that of IP with HDAC5 decreased by >80%. Since HDAC5 alone exerts a minor if any histone deacetylation activity (Fischle et al., 2002; Lahm et al., 2007), decrements in enzymatic activity of the HDAC5-IP likely reflect decreased association with its HDAC3 partner. In agreement with this hypothesis, robust levels of HDAC3 were IP with HDAC5 antibodies from the erythroid HEL cell line (data not shown).
Table I.
3H-Acetyl release activity of IP with HDAC1 and HDAC5 antibodies of lysates from human proerythroblasts.
| HDAC1 (epu) | HDAC5 (epu) | |
|---|---|---|
| Day 10 ProE | 11,474±275 | 7,729±520 |
| + EPO 24 h | 13,082±470* | 4,685±313* |
| + EPO 48 h | 14,448±807* | 1,162±53* |
| HeLa cells (positive control) | 7,888±25 | n.d. |
| Negative control (IgG) | 464±1 | 464±1 |
Results are normalized per cell number (extracts from 106 cells are analysed in each point) and are presented as mean (±SD) of three separate determinations. Similar results were obtained in an additional experiment.
p<0.01. n.d. = not done
In conclusion, during the maturation of human proerythroblasts, the content and activity of the class I HDACs investigated remained constant and even increased while the content (HDAC4) or activity (HDAC5) of class IIa containing complexes decreased.
3.2. Nucleus/Cytoplasm localization of HDACs, EKLF and GATA1 in primary erythroblasts and in erythroleukemic (K562 and HEL) and megakaryocytic (CMK and CMY) progenitor cell lines
To further characterize differences in HDAC content between erythroid cells and MK, the total, nuclear and cytoplasmic content of class I and class IIa HDACs of two erythroid (K562 and HEL) and two MK (CMK and CMY) progenitor cell lines were compared. Nuclear and cytoplasmic fractions from human proerythroblasts expanded from cord blood were also analyzed for comparison. Since EKLF is expressed in megakaryocyte/erythroid progenitors (Frontelo et al., 2007) and is localized both in the nucleus and in the cytoplasm of erythroid cells (Quadrini et al., 2008), these experiments included determinations of the cytoplasm/nuclear distribution of EKLF and GATA1, as control (Figure 1D and Supplemental Figure 1).
In proerythroblasts, as well as in erythroid cell lines, HDAC2 and GATA1 were localized mainly in the nucleus; HDAC1, HDAC3, HDAC5 and EKLF were localized mainly in the nucleus but detected also in the cytoplasm whereas HDAC4 was localized mainly in the cytoplasm (Figure 1D). By contrast, in progenitor MK cells, HDAC1, HDAC2 and GATA1 were detected only in the nucleus, HDAC4 and EKLF were localized mainly in the nucleus but detected also in the cytoplasm and HDAC5 was localized mainly in the cytoplasm (Figure 1D and Supplemental Figure 1).
These results indicate that HDAC5 and HDAC4 are the class IIa HDAC mainly localized in the nucleus of erythroid and megakaryocytic cells, respectively while GATA1 and EKLF are mainly localized in the nucleus of both cell types.
3.3. Increased association between HDAC1 and GATA1 during erythroid maturation
As an estimate of changes in levels of NuRD complex occurring with erythroid maturation, a series of IP with HDAC1 and GATA1 antibodies followed by WB were performed with erythroid cells from cord blood and adult blood (Figure 2A). WB analyses included EKLF, a factor known to bind GATA1 independently from the presence of DNA (Merika et al., 1995; Gregory et al., 1996) that may be indirectly associated with NuRD (Siatecka et al., 2011), and determination of γ/(γ+β) mRNA expression ratios as control.
Figure 2. In human erythroblasts, GATA1 is associated both with HDAC1 and EKLF.
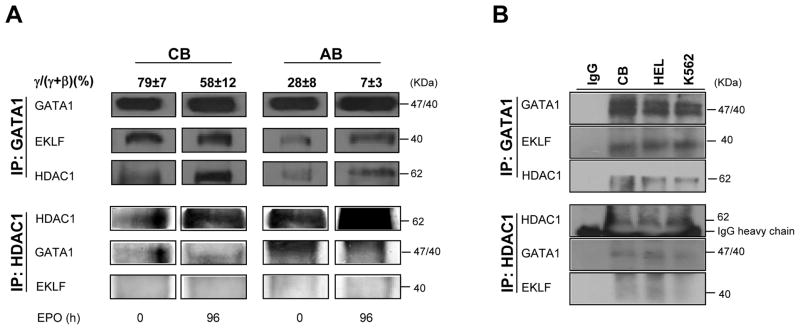
A) WB analyses with antibodies against GATA1, EKLF and HDAC1 of IP obtained with antibodies against GATA1 (top panels) and HDAC1 (bottom panels) of total cell lysates from erythroblasts from cord blood (CB) or adult blood (AB) induced to mature with EPO for 0 and 96 h, as indicated. The γ/(γ+β) expression ratios (in percent, mean±SD) expressed by the cells are indicated on the top. Similar results were obtained in six additional experiments, three with cells expanded from separate CB donors and three with cells expanded from separate AB donors.
B) WB analyses of GATA1 (top panel) and HDAC1 (bottom panel) IPs of total cell lysates from eythroblasts expanded from CB and of HEL and K562 cell lines. IP with IgG were used as negative controls.
Robust levels of GATA1 were IP with antibodies for HDAC1 and conversely HDAC1 was IP with GATA1 from lysates of proerythroblasts obtained ex-vivo from both cord blood and adult blood. In both cases, the levels of HDAC1 IP with the GATA1 antibodies increased with maturation (Figure 2A).
As expected (Merika et al., 1995; Gregory et al., 1996), EKLF was readily detected in IP with GATA1 antibodies (Figure 2) and conversely GATA1 was readily detected in IP with EKLF antibodies (data not shown). The amounts of EKLF in IP with GATA1 antibodies of erythroblasts from cord blood [γ/(γ+β) expression ratios 79%] were 2-times greater than those IP from adult blood cells [γ/(γ+β) mRNA expression ratios 28%]. With maturation, the amounts of EKLF IP with GATA1 antibodies remained constant in cord blood and increased in adult blood (Figure 2 and data not shown). In spite of numerous attempts, EKLF was never detected in IP with HDAC1 antibodies (Figure 2) and HDAC1 was never detected in IP with EKLF antibodies (data not shown). Therefore, it is unlikely that the association between EKLF and GATA1 was the consequence of an indirect association of EKLF with the NuRD complex.
These results indicate that proerythroblasts from cord blood and adult blood contain equivalent amounts of NuRD and that the content of this complex increases with maturation. They also identify an association between EKLF and GATA1 that may involve a HDAC complex still to be identified.
3.4. Identification of a novel HDAC complex formed by HDAC5, GATA1, EKLF and pERK
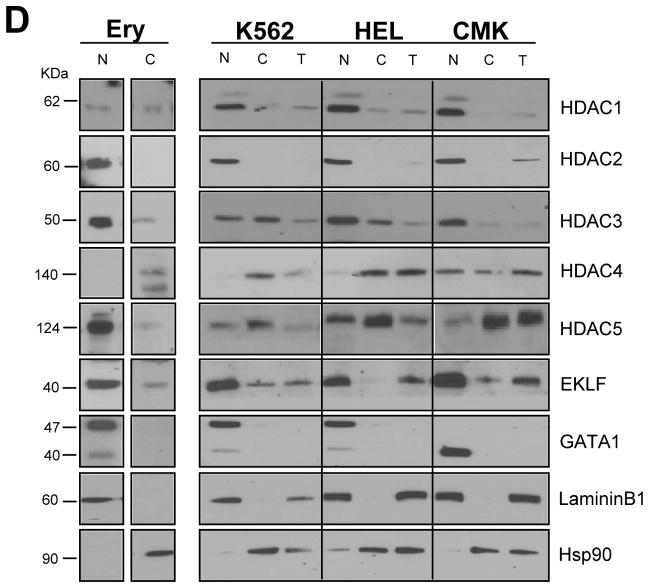
To clarify whether the complex formed by EKLF and GATA1 might include HDACs other than HDAC1, a series of IP with HDAC4 and HDAC5 followed by WB analyses for EKLF and GATA1 were performed (Figure 3 and data not shown). This analysis included pERK, a regulatory protein often associated with class II HDACs (Sengupta et al., 2004), the estrogen receptor (ERα) as positive control of IP with the GATA1 antibody (Blobel et al., 1995; Blobel et al., 1996), and the glucocorticoid receptor (GRα), as a negative control.
Figure 3. Identification of NuRSERY, a complex formed by HDAC5, EKLF, GATA1 and pERK present in erythroid cells.
A) WB analysis with antibodies against HDAC5, GATA1, EKLF and pERK of IP obtained with antibodies for HDAC5, EKLF and GATA1 (indicated on the top) of total cell lysates from human erythroblasts. IP were also analyzed with antibodies for ERα (positive control for GATA1 IP) and GRα (negative control). Additional controls were represented by IP with IgG and WB of lysates from HeLa cells. The asterisks indicate WB with antibodies used for the IP. Expected molecular weights are indicated on the left.
B) WB analysis for HDAC5, GATA1 and EKLF of IP with HDAC5 antibodies, and with IgG as negative control, of total cell lysates from human erythroblasts (1st IP) and of IP (2nd IP) with GATA1 antibodies, and with IgG as negative control, of supernatants from the 1st HDAC5 and IgG IPs, as indicated. The results presented in A and B are representative of those obtained in two separate experiments analyzed in duplicate.
C) WB analyses for EKLF of IPs with HDAC5 and GATA1 antibodies of cord blood-derived proerythroblasts (CB, left panel) and HEL (right panel) cells. Total lysates (T) from HEL and K562 cells were analyzed on the same gel for comparison. EKLF IP from cord blood-derived erythroblasts migrate as a 40KD band, that IP from HEL cells migrate as two bands of 47 and 40 KD while that from total cell lysates migrate as a 47KD band.
D) WB analysis with GATA1, EKLF, HDAC5 and ERK of GATA1 IPs obtained from human proerythroblasts expanded from CB and from HEL, K562 and CMK cells. IgGs were used as negative controls.
Robust levels of GATA1, EKLF and pERK were consistently detected in IP with HDAC5 antibodies. Conversely, HDAC5 and pERK were consistently present and HDAC4 never detected in IP with antibodies for both EKLF and GATA1 (Figure 3A and data not shown).
The robust levels of EKLF observed in IP obtained with HDAC5 and GATA1 antibodies suggests that EKLF is directly associated with both proteins. This hypothesis was confirmed by performing a second IP with GATA1 antibody on the supernatant of the first IP with HDAC5 antibodies (Figure 3B). This second IP contained barely detectable levels not only of HDAC5 but also of GATA1 and EKLF, a strong indication that the first IP with HDAC5 antibodies had pulled down the majority of these proteins, including EKLF.
Although EKLF IP with all the different antibodies migrated with apparently similar motility (Figure 2 and 3A), EKLF pulled down with GATA1 and HDAC5 antibodies may represent a subset of the protein that had undergone specific post-transcriptional modification(s). To test this hypothesis, the motility of EKLF pulled down with HDAC5 and GATA1 antibodies from cord blood-derived proerythroblasts and from HEL cells was compared with that of EKLF detected in total cell extracts from HEL and K562 cell lines (Figure 3C). Indeed, EKLF pulled down with the two antibodies from proerythroblasts migrated as a single band faster than the band detected in total cell lysates while that pulled down from HEL cells migrated as a double band, one corresponding to the band observed in total cell lysates and the other one corresponding to the band observed in pull down from proerythroblasts. These results suggest that EKLF associated with the HDAC5 complex may undergo post-transcriptional modifications and that the extent of these modifications may be regulated. Further experiments performed as part of a separate study will clarify whether these modifications are represented by de-acethylation and whether they are mediated by recruitment of HDAC3 to the complex.
To test the erythroid specificity of the association between GATA1, EKLF and HDAC5, IPs with the GATA1 antibody of lysates from cord blood-derived proerythroblasts, K562, HEL and CMK cells were probed by WB with EKLF and HDAC5. The GATA1 IP of all the erythroid cells analyzed contained great levels of both EKLF and HDAC5 while that from the MK progenitor CMK cell line did not (Figure 3D).
As expected (Blobel et al., 1995; Blobel et al., 1996), ERα was detected only in IP with GATA1 antibodies and GR was not detectable.
In conclusion, these experiments identify a novel erythroid specific HDAC complex formed by HDAC5, GATA1, EKLF and ERK. On the basis of the biological activity of class IIa HDACs, this complex was defined as nuclear remodeling shuttle erythroid (NuRSERY).
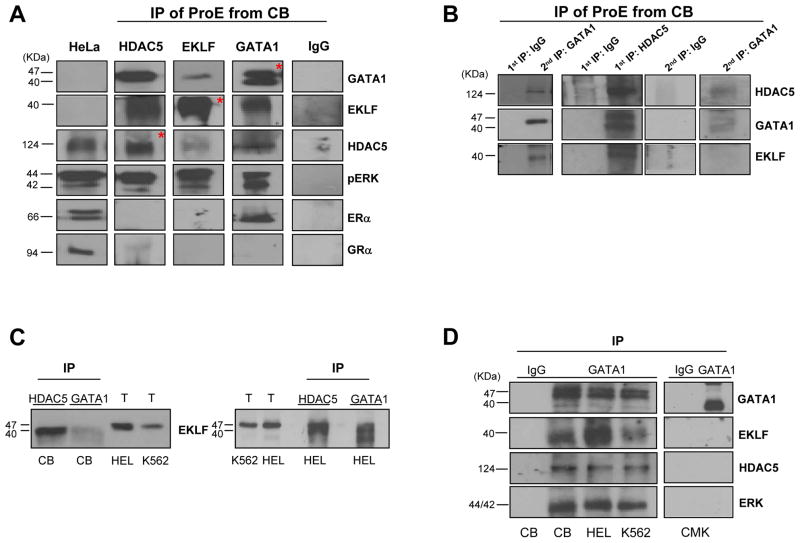
3.5 The levels of ERK phosphorylation regulates the content of the components of the NuRSERY complex
To assess the function played by ERK phosphorylation on the formation of the NuRSERY complex, the content of HDAC5, GATA1 and EKLF in HEL and K562 cells exposed for 24 h to the ERK phosphorylation inhibitor U0126 [100 μM] and PD98059 [100 μM], or to the PI-3K inhibitor Wortmannin [100 μM] as control, was investigated (Figure 4). The levels of pERK were greatly reduced by exposure to U0126 in both HEL and K562 cells, modestly reduced by exposure to PD98059 (an inhibitor known to be less potent than U0126) only in K562 cells and not affected by exposure to Wortmannin in both cell lines (Figure 4A and B). The great reductions in levels of pERK observed in HEL and K562 exposed to U0126 were associated with 5-fold reductions in the content of HDAC5, GATA1 and EKLF while the content of HDAC1 and HDAC4, analyzed as control did not change. By contrast, exposure to PD98059 or to Wortmannin did not alter the content of HDAC5, GATA1 and EKLF.
Figure 4. Exposure of erythroleukemic cell lines to inhibitors of ERK phosphorylation greatly reduces the content of components of NuRSERY.
A) WB analyses with antibodies specified on the right of total cell extracts from HEL cells untreated or exposed for 24 h either to U0126 [100 μM] or PD98059 [100 μM], as indicated. GAPDH was used as loading control. Exposure to U0126, but not to PD98059 [100 μM], reduced the levels of ERK phosphorylation and the content of HDAC5 and GATA1 but did not affect the content of HDAC4 and HDAC1.
B) WB analyses with antibodies specified on the right of total cell extracts from K562 cells untreated or exposed for 24 h to either U0126 [100 μM] or PD98059 [100 μM] (Exp I) and U0126 [100 μM] or Wortmannin [100 μM] (Exp II), as indicated. GAPDH was used as loading control. Exposure to U0126, but not to PD98059 or wortmannin, reduced the levels of ERK phosphorylation and HDAC5, GATA1 and EKLF content without affecting HDAC1 content.
C) WB analyses with antibodies specified on the right of nuclear extracts from K562 cells untreated or exposed to U0126 [100 μM] for 1, 2, 4, 6 and 24 h, as indicated. Laminin B was used as loading control. Exposure to U0126 reduced the nuclear levels of ERK phosphorylation within 1 h and the nuclear content of HDAC5, GATA1 and EKLF by 6 h. HDAC5 was still detected but GATA1 and EKLF became barely detectable after 24 h of exposure to the inhibitor.
To clarify whether inhibition of ERK phosphorylation reduces the nuclear content of the different proteins, WB analyses of nuclei from K562 cells exposed to U0126 for 1, 2, 4, 6 and 24 h were performed (Figure 4C). U0126 reduced the nuclear levels of pERK within 1 h. However, reductions in HDAC5, GATA1 and EKLF content were not observed before 6 h. By 24 h, HDAC5 was still detected but GATA1 and EKLF were no longer detectable in the nuclei of these cells.
There results suggests that the levels of ERK phosphorylation regulate formation of the NuRSERY complex and retention of GATA1 and EKLF in the nuclei.
3.6 Identification of the biological function of NuRSERY
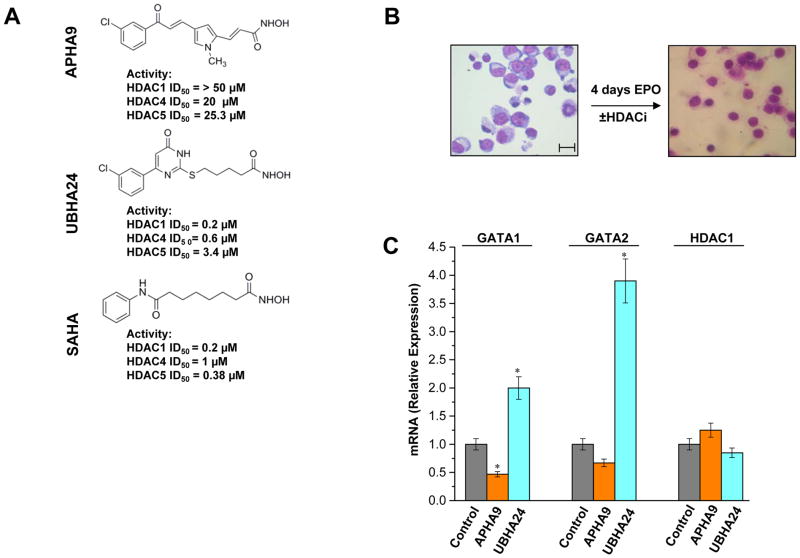
The availability of the crystal structure of the catalytic domain of the HDAC-like enzyme from the bacteria Aquifex Aeolicus (Finnin et al., 1999) allowed the definition of the pharmacophore model for HDACi and the synthesis of new generation HDACis with class specificity. By screening a library of more than 25 HDACis we identified APHA9, a class II-selective HDACi and UBHA24, a pan-HDACi, that were both capable to increase γ/(γ+β) globin expression ratio at levels similar to those induced by SAHA in erythroid cells obtained from healthy volunteers and thalassemic patients (Supplemental Figure 2 and Mai et al., 2007). APHA9 exerted its effects by increasing both β-globin (modestly) and γ-globin (robust) mRNA while UBHA24 exerted its effects by reducing the levels of β-globin mRNA. Since this first publication, the class II-selectivity of APHA9 and the pan-inhibitory activity of UBHA24 have been confirmed in additional publications (Mai et al., 2003; Mai et al., 2005; Mai et al., 2007; Simonetti et al., 2007; Duong et al., 2008; Ragno et al., 2008). The molecular structure and ID50 on human HDAC1, HDAC4 and HDAC5 of these compounds is compared to that of the pan-HDACi SAHA in Figure 5A. Increases of γ/(γ+β) globin expression ratios by suppressing β-globin expression induced by UBHA24 are consistent with the ability of this compound to inhibit HDAC1 (Miccio et al., 2010). By contrast, the complex changes in globin gene expression induced by the class II-selective HDACi APHA9 could not be explained with current knowledge on the biological activity of class IIa HDACs in erythropoiesis. The identification of NuRSERY suggested to us that APHA9 may increase γ/(γ+β) globin expression ratio because inhibition of HDAC5 activity altered the physiological balance between GATA1 and EKLF in erythroid cells. To test this hypothesis we first performed experiments to exclude that APHA9 does not inhibit HDAC1 indirectly, by suppressing its expression. Then we compared the levels of NuRSERY and of its individual components in erythroid cells induced to mature with or without APHA9 (or UBHA24 as control). Of note, progression of erythroid maturation was not altered by neither APHA9 nor UBHA24 (Figure 5B) while a 20% reduction in survival was observed only in cells exposed to UBHA24 (Supplemental Figure 2).
Figure 5. Pharmacological inhibition of class II and pan-HDACs does not affect erythroid maturation and HDAC1 mRNA expression.
A) Molecular structure and in vitro ID50 on HDAC1, HDAC4 and HDAC5 purified from human erythroid cells of the class II-selective HDACi APHA9 and pan-HDACi UBHA24. The molecular structure and ID50 of SAHA are reported for comparison. These results are similar to those obtained with the equivalent HDACs purified from other human cell types (Duong et al., 2008; Mai et al., 2003; Mai et al., 2005; Ragno eta al., 2008).
B) Morphology of human proerythroblasts exposed to EPO for 0 or 96 h (4 days) either alone or in the presence of APHA9 and UBHA24. The presence of the HDACi did not affect progression of human erythroblasts to the polychromatophilic state after 4 days of exposure to EPO. The scale bar included in the micrograph corresponds to 35 μM.
C) Real-time RT-PCR determination of the levels of GATA1, GATA2 and HDAC1 mRNA in erythroblasts exposed for 96 h to EPO either alone or in the presence of APHA9 (2 μM), and UBHA24 (0.2 μM). Results are presented as relative units with respect to cells exposed to EPO alone and are presented as mean (±SD) of values observed in three separate experiments. Values statistically different (p<0.05) from controls are indicated by *.
The expression of HDAC1 mRNA in cells exposed to APHA9 and UBHA24 is compared in Figure 5C. These experiments included determinations of the expression of GATA2, a gene known to be suppressed by HDAC1-NuRD (Fujiwara et al., 2010; Yang et al., 2012) and GATA1, as control. Neither APHA9 nor UBHA24 altered the expression of HDAC1. UBHA24 significantly increased by 4-fold GATA2 expression but expression of this gene was not affected by APHA9. By contrast, expression of GATA1 was modestly (by 2-fold) reduced by APHA9 and modestly (2-fold) increased by UBHA24. These results indicate that APHA9 does not inhibit, neither direct or indirectly, HDAC1 activity in vivo.
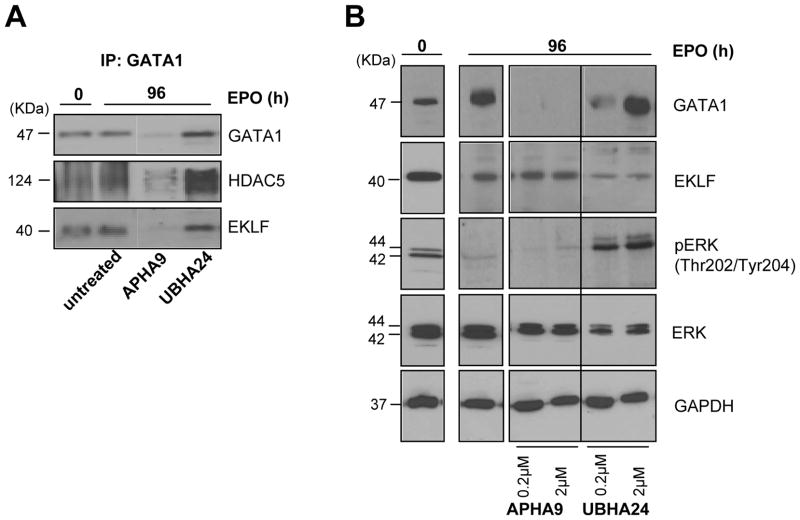
To clarify whether exposure to APHA9 had modified the association among partners of NuRSERY, IP with GATA1 antibodies of immature erythroid cells and of erythroid cells induced to mature in the absence or presence of APHA9 or UBHA24 were performed (Figure 6A). These experiments provided also information on the levels of NuRSERY in erythroid cells at different stages of maturation. Robust levels of HDAC5 and EKLF were IP with GATA1 antibodies from proerythroblasts and from erythroid cells induced to mature with EPO for 4 days. The presence of UBHA24 did not affect the levels of HDAC5 and EKLF pulled down with the GATA1 antibodies. In contrast, GATA1, HDAC5 and EKLF were all barely detectable in IP with GATA1 antibodies of cells induced to mature in the presence of APHA9. These results suggest that exposure to APHA9 greatly reduces the presence of NuRSERY in erythroid cells.
Figure 6. Decreased content of partners of the NuRSERY complex in erythroblasts exposed for 4 days to EPO in combination with the class II-selective HDACi APHA9.
A) IP with anti-GATA1 antibodies probed with antibodies against GATA1, HDAC5 and EKLF of whole cell extracts from human proerythroblasts (0 h) or from erythroblasts exposed for 96 h to EPO either alone or with APHA9 (2 μM), as indicated. Results obtained with cells exposed to UBHA24 (2 μM) are reported for comparison. The same cells as those analyzed in Figure 5.
B) WB analysis with antibodies specific for GATA1, EKLF, pERK and ERK of whole cell extracts from human proerythroblasts (0 h) or from erythroblasts obtained after 96 h of exposure to EPO either alone or in combination with increasing concentration of APHA9 or UBHA24, as indicated. GAPDH was analyzed as loading control. The expected molecular weights are indicated on the left. The same cells as those analyzed in A.
The great reduction in GATA1 content observed in erythroblasts exposed to APHA9 for 4 days that had matured normally (Figure 5B) is in apparent contradiction with the observation that hypomorphic GATA1 mutations in mice delay maturation (Ghinassi et al., 2007). This apparent contradiction may be due to the fact that in these experiments GATA1 content was measured only at the end of the maturation process and we have no information on what this content was during the first 6–2 h when the bulk of genes required for erythroid maturation are transcribed. The observation that treatment of K562 cells with U0126 reduced the nuclear content of HDAC5 and GATA1 after 6 h suggests that the GATA1 content may have been normal also during the first 6 h of exposure of proerythroblasts to APHA9.
The content of the partners of the NuRSERY complex in erythroid cells induced to mature with or without APHA9 or UBHA24 was further characterized by WB (Figure 6B). As expected, mature erythroid cells expressed 3-times more GATA1 and slightly reduced levels of EKLF than immature cells. Mature erythroid cells expressed barely detectable levels of pERK, the first element of cKIT signalling (Varricchio et al., 2012), and this reduction may reflect the absence of SCF from the differentiation media. The presence of APHA9 reduced GATA1 content to barely detectable levels but did not alter the content of EKLF or induced pERK. By contrast, exposure to UBHA24 did not affect GATA1 content while reducing by 5-fold the content of EKLF and inducing ERK phosphorylation.
These results indicate that APHA9 deeply affects the content and association of the different components of NuRSERY.
3.7 Characterization of signaling pathways differentially regulated in erythroid cells by APHA9 and UBHA24
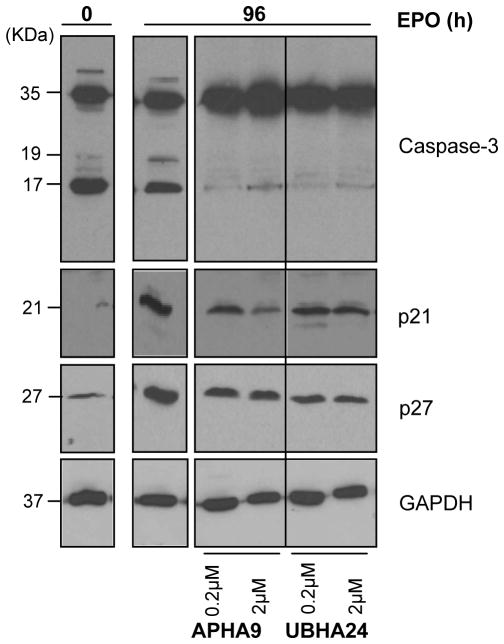
GATA1 exerts multiple controls on the biology of erythroid cells, including survival. In spite of barely detectable GATA1 content, erythroid cells exposed to APHA9 had normal survival rates while the survival of those exposed to UBHA24, which expressed normal levels of GATA1, was reduced by 20% [Supplemental Figure 2 and (Mai et al., 2007)]. Since the levels of GATA1 mRNA in cells exposed to APHA9 were modestly (2-fold) lower than normal (Figure 5C), experiments were performed to clarify the mechanism reducing GATA1 content in erythroid cells exposure to APHA9 and the GATA1-independent signaling pathways that may regulate survival of erythroid cells exposed to UBHA24. These experiments involved determinations of the levels of Caspase-3 activation, the enzyme responsible for GATA1 degradation (De Maria et al., 1999), and the p21 and p27 content of immature erythroid cells and of erythroid cells induced to mature in the absence or presence of APHA9 or UBHA24 (Figure 7).
Figure 7. Effect of APHA9 and UBHA24 on Caspase-3 activation and p21 and p27 content in erythroblasts exposed to EPO for 96 h.
WB analysis with antibodies for Caspase-3, p21 and p27 of whole cell extracts from human proerythroblasts (0 h) and from erythroblasts obtained after 96 h of exposure to EPO either alone or in combination with increasing concentration of APHA9 or UBHA24, as indicated. GAPDH was analyzed as loading control. The same cells as those analyzed in Figure 5. Expected molecular weights are indicated on the left.
Erythroid maturation was associated with decreased levels of Caspase 3 activation and increased p21 (by 10-fold) and p27 (by 5-fold) content. Reductions in Caspase-3 activation may be in part responsible for the increased GATA1 content observed when erythroid cells mature (Figure 6B) while increased levels of p21 and p27 may be responsible for blocking proliferation of these cells (Zupkovitz et al., 2010; Dai et al., 2013; Jiang et al., 2013). Exposure to APHA9 (lanes 3 and 4) decreased (by 4-fold) Caspase-3 activation, suggesting that the reductions of GATA1 content observed in the cells exposed to this HDACi (Figure 6B) were mediated by a Caspase-3 independent mechanism still to be identified. Exposure to APHA9 also reduced p21 (by 5–10-fold) and p27 (by 2-fold) content. Reduced p21 content, the p53 target protein (Papetti et al., 2010), may explain why, in spite of their barely detectable GATA1 content erythroid cells treated with APHA9 have normal survival rates. Cells exposed to UBHA24 (lanes 5–6) expressed reduced activation of Caspase-3 but contained more (2-fold) p21 and less (5- to 10-fold) p27 than cells exposed to APHA9. The difference in p21 and p27 expression observed between cells exposed to APHA9 and UBHA24 may be responsible for the reduced cell survival observed in cultures supplemented with this compound (Dai et al., 2013).
In conclusion, class-II selective and pan-HDACi had different potency in activating death pathways in erythroid cells.
4. DISCUSSION
The data presented here identify NuRSERY, a novel erythroid-specific HDAC complex formed by HDAC5, GATA1, EKLF and pERK, and provide insights on the role played by class IIa HDACs in erythropoiesis.
With erythroid maturation, content (HDAC1, 2 and 3) and activity (HDAC1) of class I HDACs increased. Also association between HDAC1 and GATA1, a reflection of the content of the NuRD complex increased with maturation. By contrast, content (HDAC4) and activity (HDAC5) of class IIa HDACs decreased during the maturation of human proerythroblasts (Figure 1 and Table I). Reductions in HDAC5 de-acetylation activity are probably a reflection of decreased association with its HDAC3 partner. In addition, human proerythroblasts contained abundant levels of NuRSERY, a complex formed by HDAC5 with GATA1 and EKLF, the two major erythroid-specific transcription factors, and pERK, the first element of the SCF signaling pathway (Varricchio et al., 2012) (Figure 3). With erythroid maturation, HDAC5, GATA1 and EKLF remained associated but the levels of pERK sharply decreased (Figure 6). Data on human embryonic kidney cells and osteosarcoma cells indicate that pERK mediates the phosphorylation of the nuclear localization domain of HDAC5 that activates the deacetylation activity of this enzyme. Since HDAC5 does not have deacetylation activity per se, this observation may reflect the fact that pERK, by facilitating the nuclear localization of HDAC5, favors its association with HDAC3. This interpretation suggests that the reduced levels of pERK observed in mature erythroid cells, by causing migration of HDAC5 in the cytoplasm, results in its dissociation from HDAC3 and are responsible for the reduced levels of lysine releasing activity observed in IP with HDAC5 antibodies from these mature cells (Table I). Support for this interpretation is provided by the observation that exposure to an ERK1/2 inhibitor reduced the total and nuclear content of all the components of NuRSERY in erythroleukemic cell lines. Overall, these results suggest that complexes containing class IIa HDACs play a role more important than those containing class I HDACs in the control of early stages of erythroid maturation. We propose that NuRSERY promotes early stages of erythroid maturation by regulating the concentration and acetylation state of GATA1 and EKLF in the nucleus. In this process, HDAC5 plays direct and indirect roles. Its direct activity is to shuttle GATA1 and EKLF from the cytoplasm to the nucleus. Once NuRSERY is in the nucleus, HDAC5 determines deacetylation of GATA1 and EKLF indirectly by recruiting HDAC3 to the complex. This process is susceptible to two levels of controls. One level is regulated by the levels of ERK phosphorylation probaly induced by SCF and determines the shuttling activity of HDAC5 favoring the nuclear localization of NuRSERY (Figure 1D and 4). The other level, to be investigated in a dedicated study, controls the recruitment to the complex of HDAC3 and regulates the deacetylation levels of GATA1 and EKLF.
The association between GATA1 and EKLF, a reflection of the cell content of NuRSERY, was greater in proerythroblasts from cord blood than in those from adult blood (Figure 2). This observation suggests that NuRSERY may control globin genes expression. This hypothesis was confirmed by the observation that APHA9, a small molecule with class II-selective HDACi activity increased γ(γ+β) mRNA expression ratio (Supplemental Figure 2 and Mai el al., 2007), and reduced GATA1 content and its association with other partners (EKLF and HDAC5) of the NuRSERY complex (Figure 6). Since HDAC5 is responsible for deacetylation of GATA1 (Delehanty et al., 2012) necessary for nuclear retention (Greco et al., 2011), it is likely that APHA9, by inhibiting HDAC5 activity, increased GATA1 acetylation determining its translocation and degradation in the cytoplasm reducing the levels of the protein detectable by WB (Figure 6).
Reductions in GATA1 content in response to APHA9 explain some of the complex effects exerted by this compound on globin gene expression. APHA9 increased both γ-globin (robustly) and β-globin (modestly) mRNA levels. Since GATA1 is the factor responsible for docking HDAC1-NuRD to the γ-globin locus that suppresses its transcription (Botardi et al., 2009), reductions in GATA1 content were likely responsible for the increased γ-globin expression. However, GATA1 is also responsible for docking the acetylated form of HDAC1 (AcHDAC1)-NuRD to the β-globin locus that activates β-globin expression (Yang et al., 2012). Therefore, reductions in GATA1 content should also reduce β-globin expression. However, in addition to the AcHDAC1-NuRD complex, expression of the β-globin gene is activated by AcEKLF (Sengupta et al., 2008). The observation that EKLF is part of NuRSERY suggests that HDAC5, throw its association with HDAC3, may deacetylate not only AcGATA1 but also AcEKLF. We suggest that APHA9, by inhibiting HDAC5, increased the nuclear concentration of AcEKLF inducing modest increases in β-globin expression. Additional proteomic analyses aimed to identify all the substrates of HDAC5 in erythroid cells are necessary to test this hypothesis.
The important role played by HDACs as epigenomic regulators of gene transcription and cell signaling and their susceptibility to chemical inhibition have inspired numerous studies exploring the therapeutic potential of HDACi not only as inducers of hemoglobin F expression in hemoglobinopathies (Higgs et al., 2012) but also in other disorders including cancer (Glozak et al., 2007). In spite of the encouraging results obtained so far, all the clinical studies with HDACi are plagued by numerous side effects, the most prominent of which is anemia. The plasticity of the chemical structure of HDACi predicts that the identification of the mechanism(s) responsible for anemia in these therapies will allow identification of compounds beneficially lacking unwanted erythroid effects. The observation that in murine models HDAC2 is responsible for the nuclear condensation necessary for erythrocyte enucleation (Ji et al., 2010) provided a mechanism for the anemia induced by therapies with pan- or class I-selective HDACis. The signaling analyses presented in Figure 7 suggest that these HDACis may also determine anemia by activating the apoptosis pathway. In fact, the use of HDACi as apoptosis-induced agents in cancer therapy relays on the ability of these compounds to target p21 and p27 (Glozak et al., 2007). The pan-HDACi UBHA24 targeted p21 and p27 and reduced survival also of erythroid cells. By contrast the class II-selective HDACi APHA9 increased p21 content, inducing apoptosis, in breast cancer cells (Duong et al., 2008) but did not reduce p21 expression nor affect survival of erythroid cells (Figure 7 and Supplemental Figure 2). The lack of apoptosis-inducing effects in erythroid cells observed with this compound suggests that class II-selective HDACis may be more suitable for epigenomic therapy of cancer than class I-selective or pan-HDACis.
Supplementary Material
Western blot analyses with the antibodies specified on the right of nuclear, cytoplasmic and total fractions of the megakaryocytic CMY progenitor cell line.
Results are presented as mean (±SD) of those observed in at least three independent experiments. * = p<0.05 with respect to control by paired t test. These data represents a summary of those previously published in (Mai et al., 2007) and are presented here only for comparison.
Acknowledgments
This study was supported by grants from NHLBI (HL116329-01), NCI (CA108671-01), Blueprint (contract no. 282510), EPIGEN (MIUR-CNR), and AIRC (contract no. 11812 and IG-10413). We thank Dr. Drs. B. Thompson and M. Weiss for providing the CMY and CMK cell lines and Drs. M. Vitale and E. Masselli for providing extracts from ex-vivo expanded human MK.
Footnotes
Conflict of interest
The authors declared no competing financial or other interests.
Competing interests
The authors have declared that no competing interests exists
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahringer J. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 2000;16:351–6. doi: 10.1016/s0168-9525(00)02066-7. [DOI] [PubMed] [Google Scholar]
- Blobel GA, Sieff CA, Orkin SH. Ligand-dependent repression of the erythroid transcription factor GATA-1 by the estrogen receptor. Mol Cell Biol. 1995;15:3147–53. doi: 10.1128/mcb.15.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel GA, Orkin SH. Estrogen-induced apoptosis by inhibition of the erythroid transcription factor GATA-1. Mol Cell Biol. 1996;16:1687–94. doi: 10.1128/mcb.16.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–84. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Bottardi S, Ross J, Bourgoin V, Fotouhi-Ardakani N, Affar el B, et al. Ikaros and GATA-1 combinatorial effect is required for silencing of human gamma-globin genes. Mol Cell Biol. 2009;29:1526–37. doi: 10.1128/MCB.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gao Z, Zhu J, Rodgers GP. Identification of CD13+CD36+ cells as a common progenitor for erythroid and myeloid lineages in human bone marrow. Exp Hematol. 2007;35:1047–55. doi: 10.1016/j.exphem.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Liu Y, Liu J, Wen X, Xu Z, et al. A novel CyclinE/CyclinA-CDK Inhibitor targets p27(Kip1) degradation, cell cycle progression and cell survival: Implications in cancer therapy. Cancer Lett. 2013;33:103–12. doi: 10.1016/j.canlet.2013.01.025. [DOI] [PubMed] [Google Scholar]
- De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–93. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. 2012;4:5. doi: 10.1186/1868-7083-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delehanty LL, Bullock GC, Goldfarb AN. Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1. Blood. 2012;120:4219–28. doi: 10.1182/blood-2011-10-387050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong V, Bret C, Altucci L, Mai A, Duraffourd C, et al. Specific activity of class II histone deacetylases in human breast cancer cells. Mol Cancer Res. 2008;6:1908–19. doi: 10.1158/1541-7786.MCR-08-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- Frontelo P, Manwani D, Galdass M, et al. Novel role for EKLF in megakaryocyte lineage commitment. Blood. 2007;110:3871–3880. doi: 10.1182/blood-2007-03-082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Lee HY, Sanalkumar R, Bresnick EH. Building multifunctionality into a complex containing master regulators of hematopoiesis. Proc Natl Acad Sci U S A. 2010;107:20429–34. doi: 10.1073/pnas.1007804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem. 2002;277:25748–55. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- Ghinassi B, Sanchez M, Martelli F, Amabile G, Vannucchi AM, Migliaccio G, Orkin SH, Migliaccio AR. The hypomorphic Gata1low mutation alters the proliferation/differentiation potential of the common megakaryocytic-erythroid progenitor. Blood. 2007;109:1460–71. doi: 10.1182/blood-2006-07-030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–32. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- Greco TM, Yu F, Guise AJ, Cristea IM. Nuclear import of histone deacetylase 5 by requisite nuclear localization signal phosphorylation. Mol Cell Proteomics. 2011;10:M110 004317. doi: 10.1074/mcp.M110.004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RC, Taxman DJ, Seshasayee D, Kensinger MH, Bieker JJ, Wojchowski DM. Functional interaction of GATA1 with erythroid Kruppel-like factor and Sp1 at defined erythroid promoters. Blood. 1996;87:1793–1801. [PubMed] [Google Scholar]
- Gregory GD, Miccio A, Bersenev A, Wang Y, Hong W, et al. FOG1 requires NuRD to promote hematopoiesis and maintain lineage fidelity within the megakaryocytic-erythroid compartment. Blood. 2010;115:2156–66. doi: 10.1182/blood-2009-10-251280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379:373–83. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- Ji P, Yeh V, Ramirez T, Murata-Hori M, Lodish HF. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95:2013–21. doi: 10.3324/haematol.2010.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Wang X, Liu X, Li F. Gene delivery of cyclin-dependent kinase inhibitors p21 (Waf1) and p27 (Kip1) suppresses proliferation of MCF-7 breast cancer cells in vitro. Breast Cancer. 2013 doi: 10.1007/s12282-012-0438-y. In Press. [DOI] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007;104:17335–40. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- Mai A, Massa S, Pezzi R, Rotili D, Loidl P, Brosch G. Discovery of (aryloxopropenyl)pyrrolyl hydroxyamides as selective inhibitors of class IIa histone deacetylase homologue HD1-A. J Med Chem. 2003;46:4826–4829. doi: 10.1021/jm034167p. [DOI] [PubMed] [Google Scholar]
- Mai A, Massa S, Pezzi R, et al. Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl) pyrrolyl hydroxyamides. J Med Chem. 2005;48:3344–3353. doi: 10.1021/jm049002a. [DOI] [PubMed] [Google Scholar]
- Mai A, Jelicic K, Rotili D, Di Noia A, Alfani E, et al. Identification of two new synthetic histone deacetylase inhibitors that modulate globin gene expression in erythroid cells from healthy donors and patients with thalassemia. Mol Pharmacol. 2007;72:1111–23. doi: 10.1124/mol.107.036772. [DOI] [PubMed] [Google Scholar]
- Mai A, Massa S, Rotili D, Simeoni S, Ragno R, et al. Synthesis and biological properties of novel, uracil-containing histone deacetylase inhibitors. J Med Chem. 2006;9:6046–56. doi: 10.1021/jm0605536. [DOI] [PubMed] [Google Scholar]
- Mankidy R, Faller DV, Mabaera R, Lowrey CH, Boosalis MS, et al. Short-chain fatty acids induce gamma-globin gene expression by displacement of a HDAC3-NCoR repressor complex. Blood. 2006;108:3179–86. doi: 10.1182/blood-2005-12-010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982;216:1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Merika M, Orkin SH. Functional synergy and physical interactions of the erythroid transcription factor GATA-1 with the Kruppel family proteins Sp1 and EKLF. Mol Cell Biol. 1995;15:2437–2447. doi: 10.1128/mcb.15.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miccio A, Blobel GA. Role of the GATA-1/FOG-1/NuRD pathway in the expression of human beta-like globin genes. Mol Cell Biol. 2010;30:3460–70. doi: 10.1128/MCB.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miccio A, Wang Y, Hong W, Gregory GD, Wang H, et al. NuRD mediates activating and repressive functions of GATA-1 and FOG-1 during blood development. Embo J. 2009;29:442–56. doi: 10.1038/emboj.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G, Di Pietro R, di Giacomo V, Di Baldassarre A, Migliaccio AR, et al. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol Dis. 2002;28:169–80. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- Miura N, Sato T, Fuse A, et al. Establishment of a new human megakaryoblastic cell line, CMY, with chromosome 17p abnormalities. Int J Mol Med. 1998;1:559–563. doi: 10.3892/ijmm.1.3.559. [DOI] [PubMed] [Google Scholar]
- Papetti M, Wontakal SN, Stopka T, Skoultchi AI. GATA-1 directly regulates p21 gene expression during erythroid differentiation. Cell Cycle. 9:1972–80. doi: 10.4161/cc.9.10.11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrini KJ, Gruzglin E, Bieker JJ. Non-random subcellular distribution of variant EKLF in erythroid cells. Exp Cell Res. 2008;314:1595–1604. doi: 10.1016/j.yexcr.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragno R, Simeoni S, Rotili D, et al. Class II-selective histone deacetylase inhibitors. Part 2: alignment-independent GRIND 3-D QSAR, homology and docking studies. Eur J Med Chem. 2008;43:621–632. doi: 10.1016/j.ejmech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Sato T, Fuse A, Eguchi M, et al. Establishment of a human leukaemic cell line (CMK) with megakaryocytic characteristics from a Down’s syndrome patient with acute megakaryoblastic leukaemia. Br J Haematol. 1989;72:184–190. doi: 10.1111/j.1365-2141.1989.tb07681.x. [DOI] [PubMed] [Google Scholar]
- Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- Sengupta T, Chen K, Milot E, Bieker JJ. Acetylation of EKLF is essential for epigenetic modification and transcriptional activation of the beta-globin locus. Mol Cell Biol. 2008;28:6160–70. doi: 10.1128/MCB.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;18:2044–54. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siatecka M, Lohmann F, Bao S, Bieker JJ. EKLF directly activates the p21WAF1/CIP1 gene by proximal promoter and novel intronic regulatory regions during erythroid differentiation. MolCell Biol. 30:2811–2822. doi: 10.1128/MCB.01016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti G, Passariello C, Rotili D, Mai A, Garaci E, Palamara AT. Histone deacetylase inhibitors may reduce pathogenicity and virulence in Candida albicans. FEMS Yeast Res. 2007;7(8):1371–1380. doi: 10.1111/j.1567-1364.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- Stellacci E, Di Noia A, Di Baldassarre A, Migliaccio G, Battistini A, Migliaccio AR. Interaction between the glucocorticoid and erythropoietin receptors in human erythroid cells. Exp Hematol. 2009;37:559–72. doi: 10.1016/j.exphem.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JK, Hassig CA, Schnitzler GR, Kingston RE, Schreiber SL. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–21. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- Varricchio L, Masselli E, Alfani E, Battistini A, Migliaccio G, et al. The dominant negative beta isoform of the glucocorticoid receptor is uniquely expressed in erythroid cells expanded from polycythemia vera patients. Blood. 2011;118:425–36. doi: 10.1182/blood-2010-07-296921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio L, Tirelli V, Masselli E, Ghinassi B, Saha N, et al. The expression of the glucocorticoid receptor in human erythroblasts is uniquely regulated by KIT ligand: implications for stress erythropoiesis. Stem Cells Dev. 2012;21:2852–65. doi: 10.1089/scd.2011.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Kikuchi J, Nishimura N, Shimizu R, Kitamura T, Furukawa Y. Expression levels of histone deacetylases determine the cell fate of hematopoietic progenitors. J Biol Chem. 2009;284:30673–83. doi: 10.1074/jbc.M109.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watamoto K, Towatari M, Ozawa Y, Miyata Y, Okamoto M, et al. Altered interaction of HDAC5 with GATA-1 during MEL cell differentiation. Oncogene. 2003;22:9176–84. doi: 10.1038/sj.onc.1206902. [DOI] [PubMed] [Google Scholar]
- Wechsler J, Greene M, McDevitt MA, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32:148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- Yang T, Jian W, Luo Y, Fu X, Noguchi C, et al. Acetylation of histone deacetylase 1 regulates NuRD corepressor complex activity. J Biol Chem. 2012;287:40279–91. doi: 10.1074/jbc.M112.349704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, et al. The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Mol Cell Biol. 2010;30:1171–81. doi: 10.1128/MCB.01500-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analyses with the antibodies specified on the right of nuclear, cytoplasmic and total fractions of the megakaryocytic CMY progenitor cell line.
Results are presented as mean (±SD) of those observed in at least three independent experiments. * = p<0.05 with respect to control by paired t test. These data represents a summary of those previously published in (Mai et al., 2007) and are presented here only for comparison.