SUMMARY
The increased incidence of drug-resistant tuberculosis has created an urgent necessity for the development of new and effective anti-tuberculosis drugs and for alternative therapeutic regimens. Clofazimine (CFZ) is a fat-soluble riminophenazine dye used in the treatment of leprosy worldwide. CFZ has also been used as a Group 5 drug in the treatment of tuberculosis (TB). A large cohort study from Bangladesh published in 2010 described a treatment regimen for multidrug-resistant tuberculosis (MDR-TB) including CFZ as being highly effective against MDR-TB. We searched multiple databases for studies published through February 2012 that reported use of CFZ in MDR- and extensively drug-resistant TB (XDR-TB) treatment regimens. We identified nine observational studies (6 MDR-TB and 3 XDR-TB) including patients with drug-resistant TB treated with CFZ. Overall, 65% (95% confidence interval [95%CI] 54–76) of the patients experienced favorable outcomes, defined as either cure or treatment completion. Using random effects meta-analysis, 65% (95%CI 52–79) of those with MDR-TB and 66% (95%CI 42–89) of those with XDR-TB experienced favorable treatment outcomes. High-quality prospective cohort studies and clinical trials examining the effect of CFZ as part of drug-resistant TB treatment regimens are needed.
Keywords: clofazimine, MDR-TB, XDR-TB, treatment outcome, systematic review
CLOFAZIMINE (CFZ), initially known as B663, was first synthesized in 1954 by Barry et al. as an anti-tuberculosis drug.1,2 In initial studies, the drug was thought to be ineffective in the treatment of tuberculosis (TB),3 and in 1955 Chang identified its effectiveness against Mycobacterium leprae.4 In recent years, as the global prevalence of drug-resistant TB has increased, researchers have sought to re-evaluate the chemotherapeutic role of CFZ as an anti-tuberculosis drug. The hypothesis is that if CFZ is of value in treating TB, its beneficial effects would be most easily observed in drug-resistant TB, as the companion drugs are likely to be less effective. The efficacy of CFZ in the treatment of drug-resistant TB is currently unclear. We performed this systematic review of the literature to review in vitro and animal data for CFZ, to identify all human studies utilizing CFZ for the treatment of drug-resistant TB, and to estimate treatment outcomes for multidrug-resistant (MDR-TB) and extensively drug-resistant TB (XDR-TB) of regimens that include CFZ.
METHODS
We searched PubMed, Embase and the Cochrane Library to identify studies in all languages that reported the use of CFZ for the treatment of MDR- or XDR-TB up to February 2012. The search terms used included ‘clofazimine,’ ‘tuberculosis’ and ‘treatment.’ Two reviewers (MG and MRO) independently screened the accumulated citations for relevance, reviewed full text articles and extracted data using a standardized form. In addition to database searches, we reviewed the meeting abstracts of the American Thoracic Society and the American College of Chest Physicians from 2010 to 2012, major pulmonary and textbooks on TB, reference lists and completed trials not yet published. References and related articles from studies that fit the study population were reviewed. Articles were searched in the above resources with the following search concepts with their synonyms; major search concepts included clofazimine, tuberculosis, outcomes, drug-resistant, multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB). These concepts were exploded to include all sub-headings of Medical Subject Headings (MeSH) as well as text searches for articles not yet indexed. No other search filters were used. We attempted to reduce publication bias by searching for abstracts of unpublished and non-English language studies. In addition to the literature on CFZ and TB, in vitro and animal studies were also comprehensively reviewed. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for the reporting of this systematic review.5
The following definitions were used to standardize outcome measurement: MDR-TB was defined as resistance to at least isoniazid (INH) and rifampin (RMP); XDR-TB was defined as resistance to INH and RMP, as well as to two of the most effective second-line drugs—a fluoroquinolone and at least one of the second-line injectable agents (amikacin, capreomycin or kanamycin); cure was defined as completion of treatment with five or more negative cultures over at least 12 months after the last positive culture; and completion was defined as completion of treatment with documented bacteriological conversion persisting through the end of treatment, but fewer than five negative cultures or <12 months of observation after the last positive culture.
Where available, we defined favorable outcomes in accordance with the World Health Organization (WHO) recommendations6 as patients who meet criteria either for cure or for treatment completion. However, not all studies used these outcome definitions and studies were not excluded on that basis.
All relevant articles were retrieved and independently assessed by two reviewers (MG and MRO). All articles that met these criteria were then exposed to a second stage of quality assessment. The US Preventive Services Task Force guidelines7 for grading the validity of individual studies for use in systematic reviews were applied to all potential articles; the Cochrane Collaboration tool for assessing risk of bias was also used.8 This is a scale that evaluated the generalizability, sample size, dropout rate, reproducibility and statistical methodology of each study. No studies were excluded due to quality concerns. Data extracted for each study included age, sex, treatment regimen, treatment duration, dates treated, percentage of retreatment cases, proportion of patients human immunodeficiency virus (HIV) tested, proportion of HIV-positive patients, adverse drug reactions, deaths, default, failure, treatment completion and cure.
Duplicate publications from the same cohort were excluded, and animal studies/case reports/case series with fewer than three patients were also excluded, from outcome analysis. If multiple published reports from the same cohort were available, we included only the report with the most detailed information on treatment protocol and outcomes. The results from animal studies, case reports and small case series were reviewed and are summarized in this article.
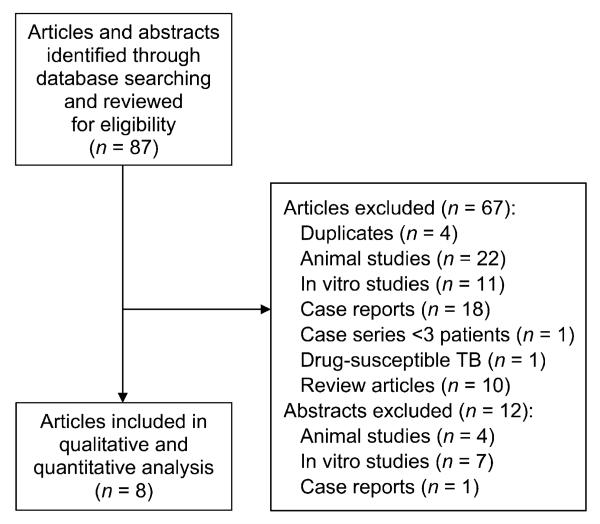
We summarized the proportion of patients who experienced favorable outcomes stratified by MDR-TB vs. XDR-TB status using a random effects model, which provides more conservative estimates than fixed effects modeling when heterogeneity is a concern.9 We visually assessed heterogeneity with use of forest plots, characterized the variation in study results attributable to heterogeneity (c-value) and statistically tested for heterogeneity (χ2 test).10 To evaluate for potential publication bias, we examined funnel plots and performed Egger’s regression test.11 Finally, to determine the influence of small studies on our effect estimates, we performed a sensitivity analysis removing studies with fewer than 15 individuals (Figure 1).
Figure 1.
Flow diagram of included studies.
RESULTS
Mechanism of action
It was initially thought that CFZ works by binding to the guanine bases of bacterial DNA, thereby inhibiting bacterial proliferation,12,13 but this theory has been replaced by the belief that it works through effects on intracellular redox cycling14 and membrane destabilization.15 By increasing reactive oxidant species, CFZ may act to promote killing of antibiotic tolerant M. tuberculosis persister organisms.16 In addition to antimicrobial activity, the drug has other pharmacological activities such as anti-inflammatory, pro-oxidative and immune-pharmacological properties.17 Synergistic effects of interferon-gamma and CFZ, as shown by Parak et al., may contribute to the anti-tuberculosis effect of the drug.18 CFZ reverses the inhibitory effect of M. tuberculosis-derived factors on phagocyte intracellular killing mechanisms which may also contribute to enhanced M. tuberculosis killing.19 Newer data suggest a potential synergistic effect of CFZ with pyrazinamide (PZA)20 and with clarithromycin (CLM)21 in M. tuberculosis killing, although the mechanism is unclear.
Pharmacokinetics
CFZ has a half-life of approximately 70 days in humans,22 and average steady state concentrations are achieved at about 1 month. Autopsies performed on patients treated with CFZ have found crystallized CFZ in the intestinal mucosa, liver, spleen and lymph nodes.22 It has slow and variable (45–62%) absorption, and a substantial portion of the unchanged drug is excreted in the feces.22 The adult dose in published clinical literature varies from 50 to 300 mg daily,22 although the optimal dose for anti-tuberculosis treatment is unknown. Average peak serum concentrations for a single dose of 100 mg and 300 mg are respectively 0.7 and 1.0 μg/ml (Lamprene, Food and Drug Administration label, Basel, Switzerland). There is high inter- and intra-subject variability in the bioavailability of CFZ, but highest bioavailability occurs when taken with fatty meals.23 No dose change is recommended in renal disease, but dose adjustment may be necessary in patients with severe hepatic impairment. No specific laboratory monitoring is recommended in patients taking CFZ. Newer analogues14 with improved pharmacokinetics and alternative formulations24 (liposomal, nano-suspension, inhalational) of CFZ are being studied.
Animal studies
Animal data for the efficacy of CFZ have been inconsistent. CFZ has shown good anti-tuberculosis activity in murine models of TB disease, less in guinea pig models and no activity in the rhesus monkey model, despite a CFZ dosage of 100 mg/kg and high serum levels.25 Recent studies in mice show substantial killing with CFZ, and recent murine studies have shown that 3- and 4-drug combinations containing CFZ, particularly the combination of CFZ, PZA and TMC207, showed the greatest reduction in M. tuberculosis colony-forming unit counts of all regimens tested.20,26 Guinea pigs infected by intracardiac injection of M. tuberculosis did not show increased survival when treated with CFZ.27 Between-species differences in M. tuberculosis killing may be explained in part by differences in peak serum levels achieved; however, in the rhesus monkey model no significant killing was observed.28
Minimal inhibitory concentration studies
Minimal inhibitory concentrations (MICs) for CFZ are low in clinical M. tuberculosis strains; as clinical resistance is rare, the MIC breakpoint was derived from epidemiologic data rather than an MIC cut-off being associated with clinical failure. Clinical M. tuberculosis isolates have been found to have an MIC of between 0.12 and 0.25 μg/l for CFZ;29 1 μg/ml was identified as the breakpoint for CFZ resistance using the MGIT™ 960 method (BD, Sparks, MD, USA) for MDR-TB and XDR-TB isolates.30 Clinical resistance to CFZ is rare. Rastogi et al. noted that a clinical M. tuberculosis isolate was susceptible to CFZ even after the serial development of resistance to INH, fluoroquinolones, RMP, PZA and ethambutol during anti-tuberculosis treatment.31 Satana et al. showed that all 35 MDR-TB isolates tested were susceptible to CFZ,32 and only 2.9% resistance to CFZ was detected among 69 MDR-TB isolates in Russia.33 MIC cut-off points for susceptibility were determined using the epidemiological cut-off value based on the distribution of MICs in two different sets of clinical isolates rather than by using pharmacokinetic/pharmacodynamic data, as clinical outcome data are lacking. CFZ resistance was rare in both series (1/45 and 0/28 isolates),30,34 and therefore the validity of the proposed cut-offs is uncertain.
Adverse effects
In a retrospective review of 60 patients with MDR-TB treated with second-line agents, which included CFZ, there was a low rate of major adverse events and no discontinuation of treatment;35 40–50% of patients developed gastrointestinal intolerance.36 CFZ produces orange to brownish skin pigmentation in 75–100% of patients within a few weeks, as well as similar discoloration of most bodily fluids and secretions.36 These discolorations are reversible but may take months to years to disappear. Cases of icthyosis and skin dryness have also been reported in response to CFZ (8–28%).36
Cohort studies
The Table includes cohort studies utilizing CFZ. In 1993, Goble et al. treated 17 patients with MDR-TB with CFZ in Denver, but outcomes in these patients could not be discerned from the paper nor from personal correspondence with study authors.45 Geerligs et al. reported the 15-year experience of their TB treatment center in the Netherlands. In 39 MDR-TB patients, the majority treated with CFZ-containing TB regimens (mean treatment duration 608 days), the successful treatment percentage was 75%.37 In 2003, Mitnick et al. used CFZ in patients with 26 MDR-TB in Peru and reported a cure rate of 83%.38 In 2004, Senaratne et al. treated 14 MDR-TB patients in Sri Lanka with a combination of second-line anti-tuberculosis drugs that included CFZ; four patients were cured.39 In Spain, in 2005, Fortun et al. included CFZ as part of a regimen that included linezolid for 5 MDR-TB patients, with 100% culture conversion at 6 weeks.40 Mitnick et al. (Peru, 2008, n = 46) showed an 83% cure rate; the majority of these patients were treated with CFZ.41 Condos et al. (New York City, 2010, n = 7) evaluated linezolid as a part of a treatment regimen that included other Group 5 drugs (amoxicillin-clavulanate, CLM, CFZ, imipenem and linezolid), and six were cured.42 Van Deun et al. (2010, n = 427) used CFZ as a part of a 9-month multidrug regimen in the treatment of MDR-TB in Bangladesh, with a favorable outcome of 75.6%.43 Xu et al. (China, 2011, n = 39) included CFZ to treat MDR-TB and XDR-TB utilizing CFZ, and demonstrated a favorable outcome of 43.5%.44
Table.
Summary of cohort studies utilizing CFZ for drug-resistant TB
| Author, year, reference | Study location |
Study years | Sample size/ patients treated with CFZ |
HIV-positive patients/ total no. tested |
Age, years mean (±SD) |
Female % |
Median no. of drugs (range) |
Dose mg |
Patients with favorable outcome/ no. treated (%) |
|---|---|---|---|---|---|---|---|---|---|
| MDR-TB | |||||||||
| Geerligs et al., 200037 | Netherlands | 1985-1998 | 44/39 | 0/39 | 33 | 29.5 | 6 (4-9) | Not known | 33/44 |
| Mitnick et al., 200338 | Peru | 1996-1999 | 75/26 | 1/65 | 26.8 (±15) | 51 | 6 (5-9) | Not known | 21/26(83) |
| Senaratne, 200339 | Sri Lanka | 1997-2002 | 14/14 | Not available | 50 (±26) | 21.4 | 5-7 | Not known | 4/14(28.5) |
| Fortun et al., 200540 | Spain | 1999-2004 | 5/5 | 1/5 | ~26 | 20 | ~4 | 100 | 4/5 (80) |
| Van Deun et al., 201043 | Bangladesh | 1997-2007 | 427/427 in intensive and 244/427 in consolidative |
Not tested | 33.8 (±1.1) | 25 | ~5 | 50-100 | 323/427 (75.6) |
| Xu et al., 201144 | China | 2008-2011 | 20/20 | Not tested | 38 (±18) | 34.4 | 6 (4-7) | 100 | 8/20(40) |
| MDR-TB favorable outcome | 65% (95%CI 52-79) | ||||||||
| XDR-TB | |||||||||
| Condos et al., 200442 | USA | 2000-2005 | 7/3 | 1/7 | ~31 | 85.7 | ~7 | Not known | 3/3 (100) |
| Mitnick et al., 200841 | Peru | 1999-2002 | 48/46 | 0 | 32.0 (±9.9) | 35.4 | 5.3(4-7) | 200-300 | 28/46(60.8) |
| Xu et al., 201144 | China | 2008-2011 | 19/19 | Not tested | 38 (±16) | 34.4 | 6 (4-7) | 100 | 9/19(47.3) |
| XDR-TB favorable outcome | 66% (95%CI 42-89) | ||||||||
| Overall favorable outcome | 65% (95%CI 54-76) |
Proportion with favorable outcome in overall group, as outcome in CFZ strata was not available.
CFZ = clofazimine; TB = tuberculosis; HIV = human immunodeficiency virus; SD = standard deviation; MDR-TB = multidrug-resistant TB; CI = confidence interval; XDR-TB = extensively drug-resistant TB.
The percentage of adverse events attributable to CFZ in these eight cohort studies was 11.4%, and resulted in discontinuation of the drug in <1% of patients. The CFZ dose used in these eight studies varied from 50 to 100 mg daily, but the majority of patients (84.1%) received 100 mg.
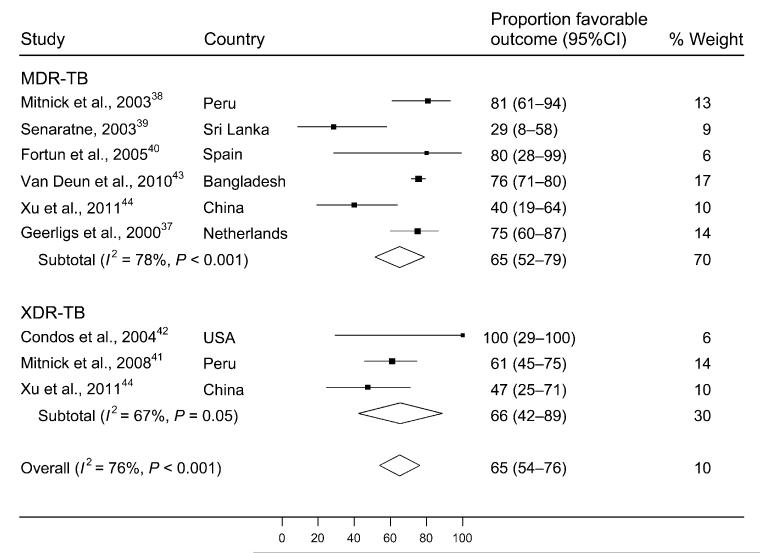
A random effects meta-analysis demonstrated that 65% (95% confidence interval [95%CI] 54–76) of all patients treated with CFZ-containing regimens experienced favorable outcomes, defined as either cure or treatment completion (Figure 2). In stratified analysis, 65% (95%CI 52–79) of those with MDR-TB and 66% (95%CI 42–89) of those with XDR-TB experienced favorable treatment outcomes. The heterogeneity of this effect estimate (I2 value) was 76% of the variance (P < 0.001), indicating significant heterogeneity. Egger’s regression test for publication bias was not statistically significant (P = 0.18). Reassessment of favorable outcomes following removal of the smaller studies (n < 15) from the analysis resulted in no change in the overall summary statistic, and an approximate 10% change in the stratified analysis (from 65% [95%CI 52–79] to 68% [95%CI 50–86] for patients with MDR-TB, and from 66% [95%CI 42–89] to 57% [95%CI 44–69] for patients with XDR-TB). This indicates that small study effects (i.e., systematically higher effect estimates for smaller relative to larger studies) may have influenced summary estimates for XDR- but not MDR-TB.
Figure 2.
Proportion of patients with favorable outcomes among studies including clofazamine in MDR- or XDR-TB treatment regimens. CI = confidence interval; MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant TB.
DISCUSSION
CFZ is a major component of drug regimens used in the treatment of leprosy.46 Our systematic review is to our knowledge the first review of the literature with respect to CFZ in the treatment of drug-resistant TB. Although in vitro and animal studies are not uniformly positive, the majority of those studies and most MIC studies suggest that CFZ has a measurable anti-tuberculosis effect. CFZ may be useful in the treatment of MDR-TB as an additional agent to prevent amplification of resistance on treatment and improve outcome. Preliminary data suggest that CFZ may improve killing of persister M. tuberculosis and have synergy with existing antimycobacterial agents.
A 2004 review of the treatment of drug-resistant TB and the 2008 guidelines from the WHO for the treatment of drug-resistant TB lists CFZ as a Group 5 drug, defined as a potentially useful agent with conflicting animal or clinical evidence, or an agent with unclear efficacy due to possible cross-resistance.6,47 A 2009 systematic review and meta-analysis of treatment outcomes among patients with MDR-TB,48 and a 2010 systematic review and meta-analysis of treatment outcomes in patients with XDR-TB did not examine the effects of individual agents such as CFZ.49 In the study from Bangladesh,43 CFZ was a prominent part of a multidrug regimen and may have played a role in the successful shortening of the duration of treatment of MDR-TB. Theoretically, CFZ may have contributed to the elimination of persistent organisms and thus have facilitated success of the 9-month regimen due to its extended half-life and lipophilicity. The role of CFZ in the treatment of XDR-TB has not been well defined.
Our systematic review of 599 patients with drug-resistant TB who were treated with CFZ showed a cumulative favorable outcome proportion of 65%. This point estimate was unchanged in an analysis stratified by MDR- vs. XDR-TB, although we could locate only three studies in the literature where CFZ was incorporated into treatment regimens for XDR-TB. The high concentration of CFZ achieved in macrophages,50,51 the ability of CFZ to reverse the inhibitory effect of M. tuberculosis-derived factors on phagocyte intracellular killing mechanisms19 and the possible synergistic effects of CFZ with gamma interferon,18 PZA20 and CLM,21 may improve treatment outcomes in drug-resistant TB. Compared to a meta-analysis of MDR-TB treatment outcomes, our estimate of successful outcome in MDR-TB treatment with CFZ is slightly higher (65%, 95%CI 52–79 vs. 54%, 95%CI 43–68), but there is broad overlap of the CIs.
The long half-life of CFZ is a pharmacokinetic characteristic that may improve its efficacy.22 The low cost of CFZ is an additional advantage.52 The limited supply of CFZ presents a potential problem, as the majority of the global supply is directed towards the treatment of leprosy.53 Adverse effects of CFZ are generally minor and rarely life-threatening. Although the optimal dose of CFZ is unknown, based on clinical experience and the published literature, we would recommend a dose of 100 mg/day except in patients with a weight < 33 kg, where 50 mg/day should be used. In a study of M. leprae, the authors did note a correlation between CFZ dosage and adverse events,44 and a decrease in dose was found to reduce the severity of the adverse drug event.
Limitations of our study include significant heterogeneity for our primary outcome measure. We used empirical random effects weighting, performed a sensitivity analysis excluding all studies contributing <15 eligible individuals, and separately synthesized data for MDR- and XDR-TB patients to minimize heterogeneity. Our results are also limited by the observational nature of the studies included. There may have been selection bias in that patients who received CFZ were systematically different from those who did not, in terms of disease severity, access to expanded drug-susceptibility testing, and/or accompanying agents in the treatment regimen. Treatment outcome definitions were not uniform throughout the different clinical studies in our analysis, and this limitation may have biased the results. Limitations inherent to all systematic reviews also apply to our study, including the inability to take into account individual intra-study biases.
Prospective cohort studies and a clinical trial utilizing CFZ in the treatment of drug-resistant TB have been initiated.39 Prospective cohort studies and randomized controlled trials are needed to determine the efficacy of CFZ in the treatment of MDR- and XDR-TB, optimize dosing and define the role of CFZ-containing drug regimens in the treatment of drug-resistant TB.
Acknowledgements
MG and MRO were supported by the Albert Einstein Global Health Center. MRO was supported by a career development award from the Einstein/Montefiore Institute for Clinical and Translational Research, and the Stony Wold-Herbert foundation. NP and MRO were supported by the Centre for AIDS Programme of Research. These funding sources played no role in the study design or data analysis.
Footnotes
Conflict of interest: none declared.
References
- 1.Barry VC, Belton JG, Conalty ML, et al. A new series of phenazines (rimino-compounds) with high antituberculosis activity. Nature. 1957;179:1013–1015. doi: 10.1038/1791013a0. [DOI] [PubMed] [Google Scholar]
- 2.Barry VC, Buggle K, Byrne J, Conalty ML, Winder F. Absorption, distribution and retention of the riminocompounds in the experimental animal. Ir J Med Sci. 1960;416:345–352. doi: 10.1007/BF02945619. [DOI] [PubMed] [Google Scholar]
- 3.Barry VC, Conalty ML. Anti-tuberculosis activity in the phenazine series. II. N3-substituted anilinoaposafranines (riminocompounds) and some derivatives. Am Rev Tuberc. 1958;78:62–73. doi: 10.1164/artpd.1958.78.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Chang YT. Chemotherapy of murine leprosy. IV. The effects of amithiozone (TB1/698), p-aminosalicylic acid (PAS), B 283 (a phenazine pigment), five antibiotics and three diphenylthiourea compounds on mouse leprosy. Int J Lepr. 1955;23:167–180. [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Emergency update 2008. WHO; Geneva, Switzerland: 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2008.402. [Google Scholar]
- 7.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21:1525–1537. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison NE, Marley GM. Clofazimine binding studies with deoxyribonucleic acid. Int J Lepr Other Mycobact Dis. 1976;44:475–481. [PubMed] [Google Scholar]
- 13.Arbiser JL, Moschella SL. Clofazimine: a review of its medical uses and mechanisms of action. J Am Acad Dermatol. 1995;32:241–247. doi: 10.1016/0190-9622(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 14.Yano T, Kassovska-Bratinova S, Teh JS, et al. Reduction of clofazimine by mycobacterial type 2 NADH: quinone oxido-reductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011;286:10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliva B, O’Neill AJ, Miller K, Stubbings W, Chopra I. Anti-staphylococcal activity and mode of action of clofazimine. J Antimicrob Chemother. 2004;53:435–440. doi: 10.1093/jac/dkh114. [DOI] [PubMed] [Google Scholar]
- 16.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci USA. 2012;109:12147–12152. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy VM, O’Sullivan JF, Gangadharam PR. Antimycobacterial activities of riminophenazines. J Antimicrob Chemother. 1999;43:615–623. doi: 10.1093/jac/43.5.615. [DOI] [PubMed] [Google Scholar]
- 18.Parak RB, Wadee AA. The synergistic effects of gamma interferon and clofazimine on phagocyte function: restoration of inhibition due to a 25 kilodalton fraction from Mycobacterium tuberculosis. Biotherapy. 1991;3:265–272. doi: 10.1007/BF02171691. [DOI] [PubMed] [Google Scholar]
- 19.Wadee AA, Anderson R, Rabson AR. Clofazimine reverses the inhibitory effect of Mycobacterium tuberculosis derived factors on phagocyte intracellular killing mechanisms. J Antimicrob Chemother. 1988;21:65–74. doi: 10.1093/jac/21.1.65. [DOI] [PubMed] [Google Scholar]
- 20.Tasneen R, Li SY, Peloquin CA, et al. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Wang B, Zhao WJ, et al. A study on the activity of clofazimine with antituberculous drugs against Mycobacterium tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33:675–678. Chinese. [PubMed] [Google Scholar]
- 22.Holdiness MR. Clinical pharmacokinetics of clofazimine. A review. Clin Pharmacokinet. 1989;16:74–85. doi: 10.2165/00003088-198916020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Nix DE, Adam RD, Auclair B, Krueger TS, Godo PG, Peloquin CA. Pharmacokinetics and relative bioavailability of clofazimine in relation to food, orange juice and antacid. Tuberculosis (Edinb) 2004;84:365–373. doi: 10.1016/j.tube.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Adams LB, Sinha I, Franzblau SG, Krahenbuhl JL, Mehta RT. Effective treatment of acute and chronic murine tuberculosis with liposome-encapsulated clofazimine. Antimicrob Agents Chemother. 1999;43:1638–1643. doi: 10.1128/aac.43.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dooley KE, Obuku EA, Durakovic N, Belitsky V, Mitnick C, Nuermberger EL. World Health Organization Group 5 drugs for the treatment of drug-resistant tuberculosis: unclear efficacy or untapped potential? J Infect Dis. 2012 Aug. doi: 10.1093/infdis/jis460. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams K, Minkowski A, Amoabeng O, et al. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2012;56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steenken W, Jr, Montalbine V, Smith MM. Antituberculous activity of rimino compound of the phenazine series. Am Rev Respir Dis. 1960;81:764–767. doi: 10.1164/arrd.1960.81.5.764. [DOI] [PubMed] [Google Scholar]
- 28.Friedman LN. Tuberculosis: current concepts and treatment. CRC Press; Boca Raton, FL, USA: 1994. [Google Scholar]
- 29.Mauch H, Reichert B, Ruf B, Brehmer W. Resistance testing of M. avium-intracellulare and M. tuberculosis of AIDS patients with new drugs and drug combinations. Pneumologie. 1990;44(Suppl 1):504–506. German. [PubMed] [Google Scholar]
- 30.van Ingen J, Simons S, de Zwaan R, et al. Comparative study on genotypic and phenotypic second-line drug resistance testing of Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:2749–2753. doi: 10.1128/JCM.00652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rastogi N, Ross BC, Dwyer B, et al. Emergence during unsuccessful chemotherapy of multiple drug resistance in a strain of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 1992;11:901–907. doi: 10.1007/BF01962370. [DOI] [PubMed] [Google Scholar]
- 32.Satana D, Coban AY, Uzun M. Testing susceptibility of multi-drug-resistant Mycobacterium tuberculosis to second-line drugs by use of blood agar. J Clin Microbiol. 2010;48:4291–4293. doi: 10.1128/JCM.00688-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balabanova Y, Ruddy M, Hubb J, et al. Multidrug-resistant tuberculosis in Russia: clinical characteristics, analysis of second-line drug resistance and development of standardized therapy. Eur J Clin Microbiol Infect Dis. 2005;24:136–139. doi: 10.1007/s10096-004-1268-4. [DOI] [PubMed] [Google Scholar]
- 34.Schön T, Juréen P, Chryssanthou E, et al. Wild-type distributions of seven oral second-line drugs against Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2011;15:502–509. doi: 10.5588/ijtld.10.0238. [DOI] [PubMed] [Google Scholar]
- 35.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:648–655. [PubMed] [Google Scholar]
- 36.Moore VJ. A review of side-effects experienced by patients taking clofazimine. Lepr Rev. 1983;54:327–335. doi: 10.5935/0305-7518.19830039. [DOI] [PubMed] [Google Scholar]
- 37.Geerligs WA, van Altena R, de Lange WCM, van Soolingen D, van der Werf TS. Multidrug-resistant tuberculosis: longterm treatment outcome in the Netherlands. Int J Tuberc Lung Dis. 2000;4:758–764. [PubMed] [Google Scholar]
- 38.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 39.Senaratne WV. Outcome of treatment of multidrug-resistant tuberculosis. Ceylon Med J. 2004;49:86–87. doi: 10.4038/cmj.v49i3.3246. [DOI] [PubMed] [Google Scholar]
- 40.Fortun J, Martin-Davila P, Navas E, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56:180–185. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 41.Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–574. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest. 2008;134:187–192. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 43.Van Deun A, Maug AK, Salim MA, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 44.Xu HB, Jiang RH, Xiao HP. Clofazimine in the treatment of multidrug-resistant tuberculosis. Clin Microbiol Infect. 2011;18:1104–1110. doi: 10.1111/j.1469-0691.2011.03716.x. [DOI] [PubMed] [Google Scholar]
- 45.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR., Jr. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–532. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. WHO Expert Committee on Leprosy . Technical Report Series 874. WHO; Geneva, Switzerland: 1998. Seventh report. [PubMed] [Google Scholar]
- 47.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 48.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy VM, Nadadhur G, Daneluzzi D, O’Sullivan JF, Gangadharam PR. Antituberculosis activities of clofazimine and its new analogs B4154 and B4157. Antimicrob Agents Chemother. 1996;40:633–636. doi: 10.1128/aac.40.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jagannath C, Reddy MV, Kailasam S, O’Sullivan JF, Gangadharam PR. Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis: in vitro, intra-cellular, and in vivo studies. Am J Respir Crit Care Med. 1995;151:1083–1086. doi: 10.1164/ajrccm/151.4.1083. [DOI] [PubMed] [Google Scholar]
- 52.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 53.Peloquin CA. Shortages of antimycobacterial drugs. N Engl J Med. 1992;326:714. doi: 10.1056/NEJM199203053261018. [DOI] [PubMed] [Google Scholar]