Abstract
Pro-opiomelanocortin (POMC) and agouti-related protein (AGRP) neurons in the hypothalamus regulate various aspects of energy homeostasis and metabolism. POMC and AGRP neurons, respectively, agonize and antagonize melanocortin receptors on their common downstream neurons. However, it is unknown whether they also reciprocally stimulate and inhibit the same neurons by amino acid transmitters. Whereas AGRP neurons are mostly GABAergic, surprisingly, only a small population of POMC neurons has been found to be glutamatergic, and a significantly larger subpopulation to be GABAergic. To further examine amino acid phenotypes of POMC neurons, we studied mRNA expression for the glutamatergic marker, type 2 vesicular glutamate transporter (VGLUT2), and the GABA synthetic enzyme, glutamic acid decarboxylase 67 (GAD67), in POMC neurons of both rats and mice by using in situ hybridization techniques. In rats, approximately 58% of POMC neurons were labeled for VGLUT2 and 37% for GAD67 mRNA. In mice, approximately 43% of POMC neurons contained VGLUT2, and 54% contained GAD67 mRNA. In both species, a prominent mediolateral distribution pattern was observed at rostral and mid levels of the POMC cell group with VGLUT2–POMC neurons dominating in lateral portions and GAD67–POMC neurons in medial portions. These data demonstrate that both glutamatergic and GABAergic cells are present in comparably significant numbers among POMC neurons. Their glutamatergic or GABAergic phenotype may represent a major functional division within the POMC cell group.
INDEXING TERMS: VGLUT2, GAD, arcuate nucleus, POMC, glutamate, GABA
Pro-opiomelanocortin (POMC) and agouti-related protein (AGRP)-expressing neurons in the hypothalamic arcuate nucleus are two functionally antagonistic neuronal populations that have a critical role in the regulation of energy balance (Cone, 2005). POMC and AGRP neurons have highly overlapping projections (Bagnol et al., 1999; Haskell-Luevano et al., 1999) and exert opposing actions on the same downstream neurons via their respective peptide transmitters: α-melanocyte–stimulating hormone (α-MSH), the main cleavage product of POMC in the arcuate nucleus, activates melanocortin 3 and 4 receptors, whereas AGRP inhibits signaling through these receptors by acting either as an antagonist of α-MSH or as an inverse agonist (Cone, 2005; Low, 2011).
Although proper signaling through melanocortin receptors is indispensable for normal energy balance (Huszar et al., 1997; Chen et al., 2000), POMC and AGRP neurons also regulate the activity of downstream neurons by coreleasing amino acid neurotransmitters, such as the excitatory glutamate or the inhibitory γ-aminobutyric acid (GABA) (Tong et al., 2008; Wu et al., 2009; Hentges et al., 2009; Dicken et al., 2012). It is still unclear, however, whether POMC and AGRP neurons regulate their common downstream neurons antagonistically by fast synaptic transmission. While AGRP neurons are considered a largely homogeneous population of GABAergic cells (Vong et al., 2011), studies have demonstrated heterogeneity among POMC neurons, consisting of both GABAergic and glutamatergic subpopulations (Hentges et al., 2004; Hentges et al., 2009; Vong et al., 2011; Jarvie and Hentges, 2012; Dicken et al., 2012). In fact, recent studies on mice estimated that approximately 40% of POMC neurons express the GABA-synthesizing enzymes glutamic acid decarboxylase (GAD) 65 and 67 (Hentges et al., 2004; Hentges et al., 2009; Jarvie and Hentges, 2012), and only a small portion (7–10%) of POMC neurons were found to express the glutamatergic marker type 2 vesicular glutamate transporter (VGLUT2) (Vong et al., 2011; Jarvie and Hentges, 2012).
As GABAergic and glutamatergic subpopulations of POMC neurons may have substantially different regulatory functions, it is important to gain a better understanding of the amino acid neurotransmitter phenotype of these cells. It is still unknown what fast neurotransmitter ~50% of the mouse POMC neurons use (Jarvie and Hentges, 2012), and apart from reports of VGLUT2 protein in occasional POMC cells (Collin et al., 2003; Kiss et al., 2005), no data are available about the expression of GABAergic and glutamatergic markers in rat POMC neurons. In an attempt to obtain more complete neuroanatomical information about the glutamatergic and GABAergic subpopulations of POMC neurons in both species, we examined the expression of VGLUT2 and GAD67 mRNAs in POMC neurons, by using in situ hybridization in dual-labeling experiments.
MATERIALS AND METHODS
Animals
The experiments were carried out on adult male Sprague–Dawley rats (Taconic Farms, Germantown, NY) weighing 220–280 g and adult male C57Bl/6 mice (Taconic), weighing 19–21 g. Animals were housed under standard conditions (lights on between 0600 and 1800 hours, temperature 22 ± 1°C, rodent chow, and water ad libitum). All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Tufts Medical Center.
Generation of hybridization probes
The following template cDNA sequences were used for generating riboprobes for in situ hybridization: mouse POMC, corresponding to bases 532–1007 of GenBank Acc. No. NM_008895.3 (Jarvie and Hentges, 2012), kindly provided by Dr. Malcolm J. Low (University of Michigan, Ann Arbor); rat VGLUT2, 522–1400 of NM_053427.1 (Hrabovszky et al., 2004, 2005); mouse VGLUT2, 1,762–2,390 of NM_080853.3 (Hrabovszky et al., 2012); rat GAD67, 1,265–1,836 of NM_017007.1 (Hrabovszky et al., 2005), kindly provided by Dr. Sandra L. Petersen (University of Massachusetts, Amherst); and mouse GAD67, 317–892 of NM_008077.4. The mouse GAD67 cDNA construct was generated by a method described earlier, by using the PGEM T cloning kit (Promega, Madison, WI) (Hrabovszky et al., 2012). The POMC probe used in the dual-label in situ hybridization study was labeled with digoxigenin-11-UTP (Roche Applied Sciences, Basel, Switzerland) by in vitro transcription, using T7 RNA polymerase (Promega). Antisense riboprobes for rat and mouse VGLUT2 and GAD67 were synthesized by using SP6 or T7 RNA polymerase (Promega) in the presence of [35S]-UTP (PerkinElmer, Waltham, MA), and were purified with Mini Quick Spin RNA columns (Roche).
Combined in situ hybridization and immunofluorescence study
Tissue preparation for in situ hybridization and immunofluorescence
Rats (n = 4) and mice (n = 4) were deeply anesthetized with an overdose of pentobarbital (50 mg/kg for rats, 80 mg/kg for mice; Ovation Pharmaceuticals, Deerfield, IL) before perfusion. Rats were perfused transcardially with 20 ml 0.1 M phosphate-buffered saline (PBS; pH 7.4) followed by 120 ml 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4); mice were perfused with 5 ml PBS followed by 35 ml paraformaldehyde. The brains were removed and postfixed by immersion in the same fixative for 4 hours at room temperature. Tissue blocks containing the hypothalamus were cryoprotected in 20% sucrose in diethylpyrocarbonate (DEPC)-treated PBS at 4°C overnight, and then snap-frozen on dry ice. Serial 20-μm coronal sections through the rostrocaudal extent of the arcuate nucleus were cut on a Leica CM3050 S cryostat (Leica Microsystems, Nussloch, Germany), collected in cryoprotective solution (30% ethylene–glycol; 25% glycerol; 0.05 M phosphate buffer), and stored at −20°C until use.
Radioactive in situ hybridization and immunofluorescence
One day before hybridization, sections were transferred into PBS, mounted onto Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA), and desiccated overnight at 45°C. Sections were treated with 1 μg/ml proteinase K (Sigma-Aldrich, St. Louis, MO) in 0.1 M Tris buffer, pH 8.0, containing 0.05 M EDTA, for 30 minutes at 37°C. Then sections were washed in DEPC-treated PBS for 2 minutes, acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 minutes, rinsed in PBS, dehydrated in graded ethanol solutions (80, 95, 100%; 1 minute each), and placed into chloroform for 10 minutes. After immersion in 100% and 95% ethanol for 1 minute, sections were air-dried. A series of every sixth coronal section from each rat and mouse was hybridized with the rat or mouse VGLUT2 probe, respectively, and another series of sections was hybridized with the rat or mouse GAD67 probe. Probes were diluted in hybridization buffer (50% formamide, twofold concentration of standard sodium citrate buffer (SSC), 0.25 M Tris buffer, pH 8.0, Denhardt’s solution, 10% dextran sulfate, 0.5% sodium dodecyl sulfate, 265 μg/ml denatured salmon sperm DNA, 250 mM dithiothreitol) to a final concentration of 80,000 cpm/μl, and 20 μl was applied to the sections on each slide and covered with a plastic coverslip. Hybridization was performed overnight at 56°C in humidity chambers.
Following hybridization, the sections were washed in 1X SSC for 15 minutes, and then treated with ribonuclease A (25 μg/ml; Sigma-Aldrich) for 1 hour at 37°C, followed by additional stringency washes in 1X SSC (15 minutes), 0.5X SSC (15 minutes), and 0.1X SSC (2X 30 minutes) at 65°C. To prepare the sections for immunofluorescence, sections were treated with the mixture of 0.5% Triton X-100 and 0.5% H2O2 for 15 minutes, rinsed in PBS (3X 10 minutes), immersed in maleate buffer (0.1 M maleic acid, 0.15 M NaCl, pH 7.5) for 10 minutes, and then in 1% blocking reagent for nucleic acid hybridization (Roche Applied Sciences), diluted in maleate buffer. The sections were then incubated overnight at 4°C in a rabbit antiserum against the POMC precursor (Phoenix Pharmaceuticals, Burlingame, CA), diluted at 1:2,000 in blocking reagent, using CoverWell incubation chambers (Grace Bio-Labs, Bend, OR). After washes in PBS, sections were incubated for 4 hours at room temperature in Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) at 1:200 dilution. After rinses in PBS, sections were dehydrated in ascending series of ethanol (80, 95, 100%, 1 minute each), air-dried, and dipped into Kodak NTB autoradiography emulsion (Carestream Health, Rochester, NY). Emulsion coated-slides were placed in light-tight boxes containing desiccant, and stored at 5°C for 8 days (mouse GAD67 and VGLUT2) or 14 days (rat GAD67 and VGLUT2), after which the autoradiograms were developed by using Kodak D19 developer (Eastman Kodak, Rochester, NY). Slides were placed in ascending series of ethanol followed by xylenes (Sigma-Aldrich), and coverslipped with DPX mountant (Sigma-Aldrich).
Dual-label in situ hybridization study
Tissue preparation for dual-label in situ hybridization
Four mice were deeply anesthetized with ketamine–xylazine (ketamine: 75 mg/kg body weight; xylazine: 10 mg/kg body weight) and decapitated. The brains were removed and snap-frozen on powdered dry ice. Then 16-μm-thick coronal sections were cut from the hypothalamus through the rostrocaudal extent of the arcuate nucleus by using a Leica CM3050 S cryostat, thaw-mounted on Superfrost Plus glass slides (Fisher Scientific), and air-dried. The sections were stored at −80°C until processing for in situ hybridization.
Dual-label in situ hybridization
The sections were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 20 minutes, rinsed in 0.1 M PBS (pH 7.4) for 5 minutes, acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 minutes, and further processed for hybridization as described above. VGLUT2/POMC and GAD67/POMC dual-label hybridizations were carried out on adjacent series of sections from each brain, each series containing every seventh section. The POMC probe was diluted 1:50, and VGLUT2 and GAD67 probes were diluted to 60,000 cpm/μl in hybridization buffer. Hybridization was performed overnight at 56°C in humidity chambers.
Posthybridization treatments and processing of the sections for immunolabeling were identical to those described above. The sections were then incubated overnight in Fab fragments of peroxidase-conjugated sheep anti-digoxigenin antibody (Roche; diluted 1:100 in 1% blocking reagent) using CoverWell incubation chambers (Grace Bio-Labs). Sections were rinsed in PBS (3X 10 minutes), and the POMC hybridization signal was amplified by using the TSA Biotin Tyramide system (PerkinElmer) for 30 minutes. Sections were rinsed in PBS and incubated in Alexa Fluor 488–conjugated streptavidin (Life Technologies, Grand Island, NY) for 2 hours, diluted at 1:500 in 1% blocking reagent. Sections were rinsed in PBS, dehydrated, air-dried, and coated with Kodak NTB autoradiography emulsion. Exposure time was 7 days for GAD67, and 9 days for VGLUT2. The autoradiograms were developed, and the slides were coverslipped as described above.
Images and data analysis
For the in situ hybridization–immunofluorescence study, every 18th 20-μm-thick coronal section from the rats (six per rat), and every 12th section from the mice (four per mouse) were imaged for cell counting. For the dual-label in situ hybridization study, every 14th 16-μm-thick mouse section was imaged (five per mouse). Fluorescent images of the POMC labeling and darkfield images of the emulsion autoradiographs were captured through a 10× objective, by using a Zeiss Axioplan 2 microscope (Carl Zeiss, Gottingen, Germany) equipped with an RT SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Hybridization signals of VGLUT2 and GAD67 were analyzed in all POMC perikaryon profiles in a section bilaterally, by using ImageJ software (public domain at http://rsb.info.nih.gov/ij). To measure area fractions covered by silver grains, a threshold value was set for the darkfield image to separate bright pixels with silver-grain labeling from dark pixels. Then the corresponding fluorescent POMC image was overlaid, and all clearly labeled POMC neurons were assigned a number by using the multiple selection tool. POMC neurons were then individually outlined by using the fluorescent image, and the fraction of the cell area covered by silver grains (%Area value = pixels above threshold value/total pixels) was measured for every POMC cell by using the darkfield image. %Area values (silver grain coverage) were also measured over background areas that lacked VGLUT2 or GAD67 hybridization. Background areas for VGLUT2 included the subparaventricular zone, reticular thalamic nucleus, and ventral tuberomammillary nucleus; those for GAD67 included the paraventricular, ventromedial, and ventral premammillary nuclei. To be considered specifically labeled with the radioactive VGLUT2 or GAD67 probes, individual POMC neurons had to exhibit at least fivefold higher %Area values than background regions. These POMC cells were also evaluated subjectively to confirm positive labeling. Alternatively, dual labeling could also be rejected when the hybridization signal over the cell, based on its appearance and distribution, was more likely due to a partially overlapping cell or the spread of hybridization signal from neighboring cells.
Adobe Photoshop CS4 (Adobe Systems, San Jose, CA) was used to create and label composite images for publication and to modify brightness and contrast. The green Alexa 488 signal of hybridized POMC neurons in the dual-hybridization experiments was pseudo-colored to red for better visibility of double-labeled cells. Arcuate nucleus and retrochiasmatic area subregions were identified according to the nomenclatures of Paxinos and Watson (2007) and Franklin and Paxinos (2008). Line drawings representing the distribution of VGLUT2-and GAD67-positive POMC neurons were created with Corel Draw 12 (Corel, Ottawa, Canada). Cell profile sizes of POMC neurons were measured by using the outline of the fluorescent POMC labeling obtained from the analysis described above. The data from all analyzed POMC neurons were used for cell size measurements. Data are presented as mean ± SEM. One-way ANOVA and Bonferroni’s multiple comparison post hoc test were used to compare the profile sizes of VGLUT2-expressing and GAD67-expressing POMC neurons. Abercrombie’s formula was used to correct the cell counts for size difference among POMC neurons (Abercrombie, 1946; Guillery, 2002).
Specificity of hybridization probes and the POMC antiserum
Specificity of the rat VGLUT2, rat GAD67, and mouse VGLUT2 probes were reported previously by hybridizing with the sense transcripts that resulted in no labeling of brain tissue (Hrabovszky et al., 2004, 2005, 2012), and by showing identical distribution patterns to another probe targeting a non-overlapping segment of the same mRNA (Hrabovszky et al., 2004, 2005). Hybridization with the sense transcript of the mouse GAD67 probe sequence, carried out in parallel with the antisense probe, yielded no labeling. The hybridization pattern for each probe in this study was identical to published expression patterns of the corresponding mRNAs. The distribution of cell bodies labeled by the POMC antiserum or the mouse POMC hybridization probe was identical to the well-known distribution pattern of POMC neurons (Elias et al., 1999; Hentges et al., 2009; Jarvie and Hentges, 2012). The specificity of the rabbit POMC antiserum (Phoenix Pharmaceuticals), raised against the porcine POMC precursor (amino acids 27–52), was further confirmed by dual immunofluorescent labeling of rat and mouse hypothalamic sections using a sheep α-MSH antiserum (gift from Dr. Jeffrey B. Tatro, Tufts Medical Center, Boston, MA) (Elias et al., 1998). As expected, the α-MSH antiserum labeled fewer cell bodies than the POMC antiserum, but all α-MSH-immunoreactive cells were colabeled with the POMC antiserum.
RESULTS
VGLUT2 mRNA expression in rat POMC neurons
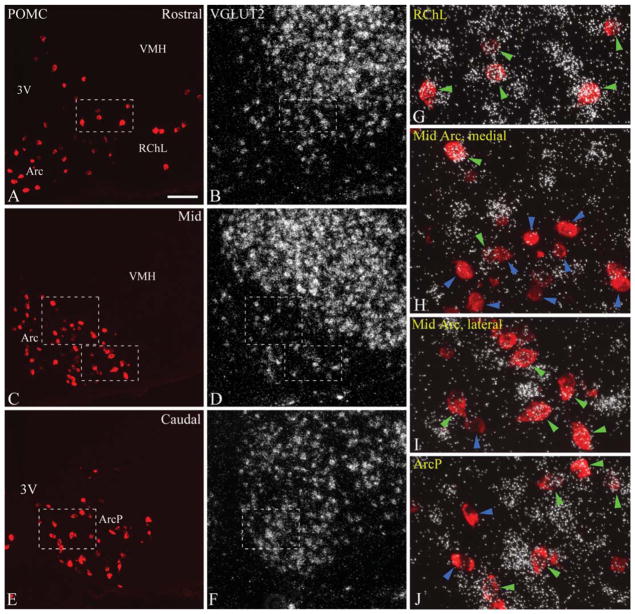
VGLUT2 expression was studied by using isotopic in situ hybridization in immunofluorescently labeled POMC neurons. VGLUT2 mRNA hybridization signal was readily detectable in a large population of POMC neurons. Examined in six rostrocaudal levels (levels 1–6), the distribution of VGLUT2-expressing POMC neurons was characteristically uneven within the POMC cell group. At the rostral pole of the arcuate nucleus and adjacent retrochiasmatic area (level 1), the vast majority of POMC cells expressed VGLUT2 mRNA (Figs. 1A,B,G 3A). At rostral and mid levels of the arcuate nucleus (levels 2–4), VGLUT2 mRNA was expressed in the vast majority of the more laterally located POMC neurons (Figs. 1C,D,H, 3B–D), distributed in the lateral arcuate nucleus and adjacent lateral retrochiasmatic area, and occasionally, within the ventromedial nucleus. The proportion of VGLUT2-expressing POMC neurons was lower in the medial and dorsal arcuate nucleus, where they were intermingled with several non-VGLUT2 POMC neurons (Figs. 1I, 3B–D). The lowest ratio of VGLUT2-expressing neurons was found in the posterior arcuate nucleus (levels 5 and 6) where VGLUT2-positive and VGLUT2-negative POMC neurons were closely situated and more intermingled (Figs. 1E,F,J,K, 3E,F). The percentage of VGLUT2-positive POMC neurons in different rostrocaudal levels is demonstrated in Table 1. Altogether, of 496.5 ± 18.9 POMC perikarya per rat, an average of 58.4 ± 2.2% was found to display VGLUT2 hybridization signal.
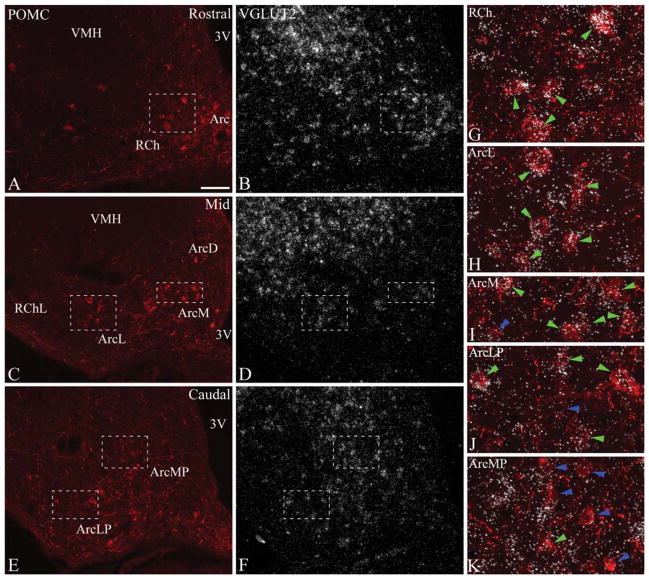
Figure 1.
A–F: Distribution of POMC and VGLUT2 mRNA at rostral (A,B), mid (C,D), and caudal (E,F) levels (corresponding to levels 1, 3, and 5, respectively) of the rat arcuate nucleus. POMC was detected by immunofluorescence, and VGLUT2 mRNA by radioactive in situ hybridization. VGLUT2 mRNA is expressed in all regions of the arcuate nucleus and the retrochiasmatic area, at varying densities and intensities. G–K: Higher magnification merged images of framed areas in A–F demonstrate examples of VGLUT2-positive (green arrowheads) and VGLUT2-negative POMC neurons (blue arrowheads). Note the high proportion of VGLUT2-expressing POMC neurons in the retrochiasmatic area (G), and in the lateral arcuate nucleus (H). 3V, third ventricle; Arc, arcuate nucleus; ArcD, dorsal arcuate nucleus; ArcL, lateral arcuate nucleus; ArcM, medial arcuate nucleus; ArcLP, posterolateral arcuate nucleus; ArcMP, posteromedial arcuate nucleus; RCh, retrochiasmatic area; RChL, lateral retrochiasmatic area; VMH, ventromedial hypothalamic nucleus. Scale bar in A = 100 μm for A–F; 25 μm for G–K.
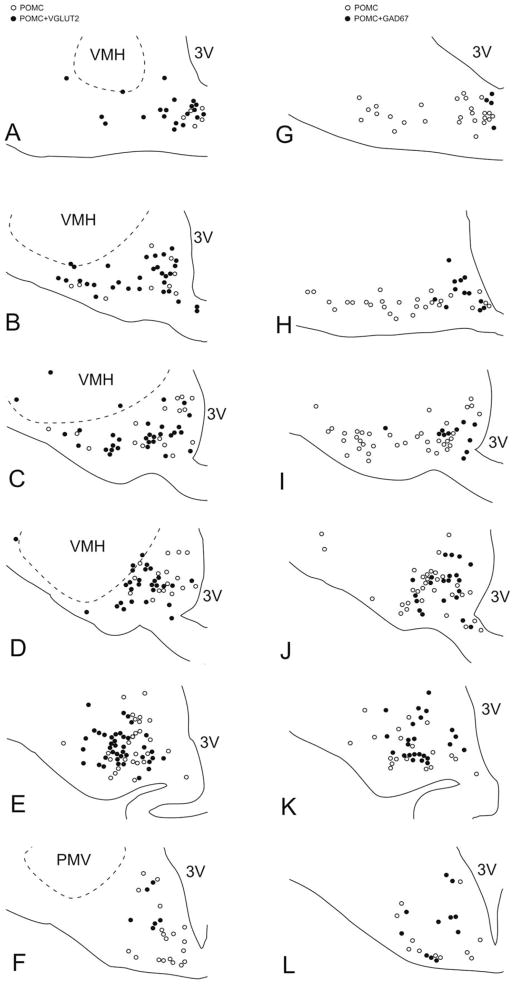
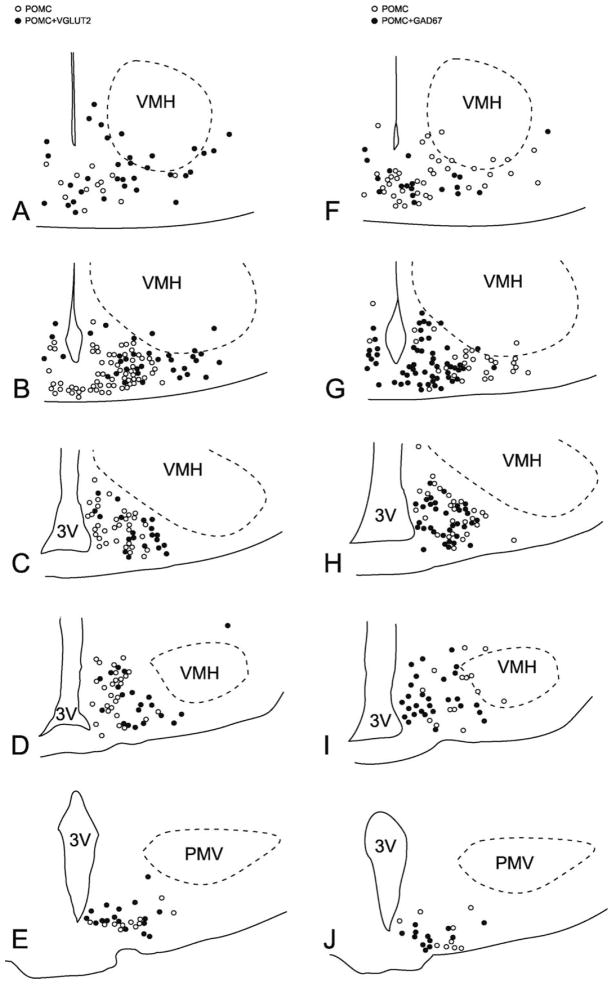
Figure 3.
Schematic maps illustrate the distribution of VGLUT2-expressing (A–F), and GAD67-expressing POMC neurons (G–L) from one rat, at the six different rostrocaudal levels examined. Open circles represent single-labeled POMC neurons, and filled circles represent VGLUT2-POMC neurons (A–F) or GAD67–POMC neurons (G–L). Note the highly complementary expression patterns of VGLUT2 and GAD67 in the POMC cell group. 3V, third ventricle; PMV, ventral premammillary nucleus; VMH, ventromedial hypothalamic nucleus. PMV and VMH borders were drawn based on their VGLUT2 mRNA expression.
TABLE 1.
Percentage of VGLUT2 and GAD67 mRNA Expression in POMC Neurons at Different Rostrocaudal Levels of the Rat Arcuate Nucleus–Retrochiasmatic Area1
| Level | % POMC neurons labeled for VGLUT2 | % POMC neurons labeled for GAD67 |
|---|---|---|
| 1 | 86.4 ± 1.9 | 8.5 ± 2.3 |
| 2 | 66.3 ± 3.5 | 30.0 ± 4.4 |
| 3 | 57.3 ± 2.9 | 29.8 ± 1.9 |
| 4 | 60.2 ± 2.9 | 43.8 ± 3.8 |
| 5 | 51.9 ± 4.4 | 46.0 ± 2.0 |
| 6 | 37.4 ± 7.3 | 57.3 ± 4.1 |
| Total | 58.4 ± 2.2 | 37.2 ± 2.2 |
Level 1, most rostral; level 6, most caudal, as shown in Figure 3. Abbreviations: GAD67, glutamic acid decarboxylase 67; POMC, pro-opiomelanocortin; VGLUT2, vesicular glutamate transporter 2.
GAD67 mRNA expression in rat POMC neurons
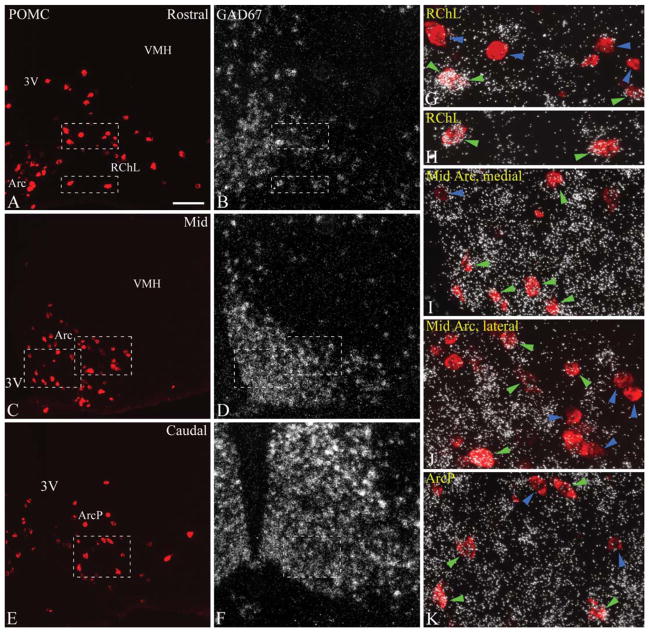
As seen with the same in situ hybridization–immunofluorescence technique, GAD67 mRNA was expressed in a significant subset of rat POMC neurons, but the extent of colocalization was lower than in the case of VGLUT2. The distribution of POMC neurons labeled for GAD67 mRNA was largely complementary to that of VGLUT2–POMC neurons (Fig. 3G–L). Most rostrally, GAD67 was expressed in only a few POMC cells (Figs. 2A,B,G, 3G). At rostral to mid levels (levels 2–4), GAD67-expressing POMC neurons were located primarily in the medial and dorsal arcuate nucleus, intermingled with POMC cells lacking the GAD67 signal (Figs. 2C,D,I, 3H–J). Dual-labeled neurons increased in number in caudal regions. POMC neurons in the lateral arcuate nucleus and lateral retrochiasmatic area rarely expressed GAD67 mRNA (Figs. 2C,D,H, 3H–J). The highest proportion of GAD67-expressing POMC neurons was found in the posterior arcuate nucleus, where POMC neurons with GAD67 mRNA were mixed with POMC neurons without hybridization signal (Figs. 2E,F,J, 3K,L). Table 1 shows the increase in the ratio of GAD67 mRNA-labeled POMC neurons from rostral to caudal levels. Altogether, of 492.0 ± 19.4 POMC perikarya per rat, an average of 37.2 ± 2.2% contained GAD67 hybridization signal.
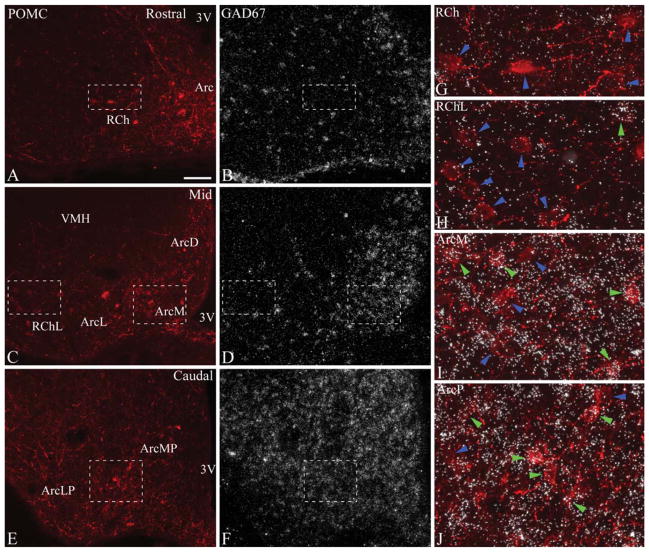
Figure 2.
A–F: Distribution of POMC and GAD67 mRNA at rostral (A,B), mid (C,D), and caudal (E,F) levels (corresponding to levels 1, 3, and 5, respectively) of the rat arcuate nucleus. POMC was detected by immunofluorescence, and GAD67 mRNA by radioactive in situ hybridization. GAD67 is extensively expressed in the arcuate nucleus, and less densely in the retrochiasmatic area. G–J: Higher magnification merged images of framed areas in A–F demonstrate examples of GAD67-positive (green arrowheads) and GAD67-negative POMC neurons (blue arrowheads). Most POMC neurons do not express GAD67 mRNA rostrally (G) and in the lateral retrochiasmatic area–lateral arcuate nucleus (H). GAD67 mRNA is expressed in several POMC neurons in the medial arcuate nucleus (I) and the posterior arcuate nucleus (J). 3V, third ventricle; Arc, arcuate nucleus; ArcD, dorsal arcuate nucleus; ArcL, lateral arcuate nucleus; ArcM, medial arcuate nucleus; ArcLP, posterolateral arcuate nucleus; ArcMP, posteromedial arcuate nucleus; ArcP, posterior arcuate nucleus; RCh, retrochiasmatic area; RChL, lateral retrochiasmatic area; VMH, ventromedial hypothalamic nucleus. Scale bar in A = 100 μm for A–F; 25 μm for G–J.
Size difference between VGLUT2- and GAD67-expressing POMC neurons in the rat
There was a notable variability in the size of POMC neurons, reported earlier by Meister et al. (2006), which seemed to correlate with their VGLUT2 or GAD67 expression and the different distribution patterns of these subpopulations. POMC neurons located most rostrally, and laterally at rostral to mid levels, were generally large. POMC neurons in the medial arcuate nucleus were generally smaller, and smallest in the dorsal arcuate nucleus. Large and small-sized POMC neurons were both present in the posterior arcuate nucleus. When the sizes of POMC cell body profiles from both hybridizations were compared, VGLUT2-expressing POMC neurons had an average profile size of 175.0 ± 1.9 μm2, which was very similar to the size of non-GAD67 POMC neurons (177.0 ± 1.8 μm2; P > 0.05), but significantly larger than non-VGLUT2 (146.8 ± 1.6 μm2; P < 0.001) and GAD67-expressing POMC neurons (150.9 ± 1.8 μm2; P < 0.001). The size of GAD67-expressing POMC neurons was similar to the size of non-VGLUT2 POMC neurons (150.9 ± 1.8 μm2 vs. 146.8 ± 1.6 μm2; P > 0.05).
Because larger cells are overrepresented against smaller cells in cell profile counts (Guillery, 2002), Abercrombie’s (1946) formula was applied to correct the percentages of our cell counts for the size difference of POMC neurons. Counting with the average profile diameter of the different POMC cells (VGLUT2-expressing: 17.3 μm; non-VGLUT2: 15.5 μm; GAD67-expressing: 15.7 μm; non-GAD67: 17.2 μm), the correction resulted in a less than 1% change in both the percentage of VGLUT2-expressing POMC neurons (57.5 ± 2.2% from 58.4 ± 2.2%), and the percentage of GAD67-expressing POMC neurons (38.1 ± 2.2% from 37.2 ± 2.2%).
VGLUT2 mRNA expression in mouse POMC neurons: results from two methods of differing sensitivity
With the combined in situ hybridization and immunofluorescence technique used in the rat, VGLUT2 mRNA was detected in a small subset, 13.8 ± 1.7%, of mouse POMC neurons. Most of these POMC neurons displayed only low-to-moderate levels of VGLUT2 hybridization signal. The highest numbers of VGLUT2-expressing POMC neurons were found most rostrally in the lateral retrochiasmatic area, but low numbers of double-labeled cells were observed at all levels of the arcuate nucleus.
Because of the low number of VGLUT2-expressing neurons in the arcuate nucleus observed by using this technique, we repeated the mouse experiments with a more sensitive approach for radioactive in situ hybridization, using nonperfused, fresh-frozen brain sections, as previously described by our group (Hrabovszky et al., 2012). Because the POMC antiserum barely labels POMC neurons in nonperfused tissue, a dual hybridization technique was carried out whereby POMC neurons were visualized by fluorescent in situ hybridization. The approach resulted in a significant improvement in the signal-to-noise ratio of the radioactive VGLUT2 hybridization, and, as anticipated, labeled more VGLUT2 mRNA-expressing neurons in the arcuate nucleus (Fig. 4A,B). Furthermore, many more VGLUT2-expressing POMC neurons were identified and with a higher intensity of VGLUT2 hybridization signal over individual POMC neurons than with the combined in situ hybridization–immunofluorescence approach (Table 2, Fig. 4C,D). In addition to the improved sensitivity for detecting VGLUT2 mRNA, the dual-hybridization method also labeled significantly more POMC cells, which was largely attributed to the fact that hybridization conditions reduce immunoreactivity, resulting in fewer clearly labeled POMC neurons by immunofluorescence. Figure 4 and Table 2 illustrate the superior sensitivity of the dual-hybridization method compared with the combined in situ hybridization and immunofluorescence method.
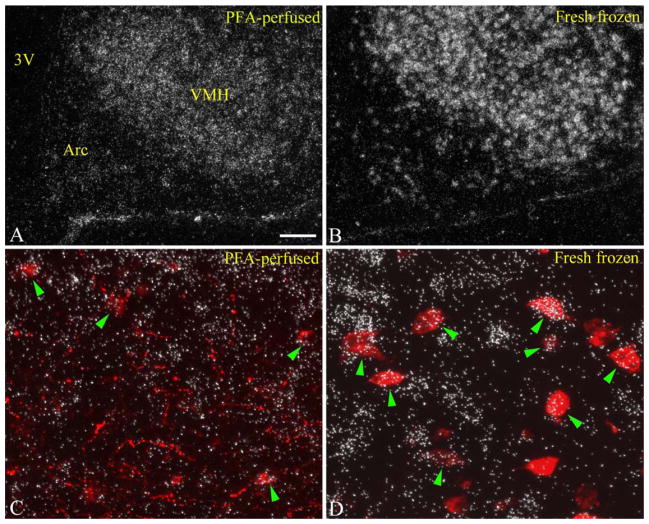
Figure 4.
A,B: In situ hybridization for VGLUT2 mRNA on paraformaldehyde-perfused (A), and fresh-frozen (B) brain sections from mice. Note the improved signal-to-noise ratio in the fresh-frozen section. VGLUT2 hybridization signal clearly and intensely labels numerous cells in the arcuate nucleus in the fresh-frozen section. C,D: Higher magnification dual-label images taken from the same region of the lateral retrochiasmatic area compare VGLUT2 signal intensities (silver grains) over individual POMC neurons (red fluorescence). Note the more intense VGLUT2 hybridization signal over POMC neurons in the fresh-frozen section (D) compared with the paraformaldehyde-perfused section (C). Due to the tissue-compressing effect of perfusion, the resolution of radioactive hybridization signal is also improved on the fresh-frozen tissue. 3V, third ventricle; Arc, arcuate nucleus; VMH, ventromedial hypothalamic nucleus. Scale bar in A = 100 μm for A,B; 25 μm for C,D. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE 2.
Sensitivity Difference Between the Combined In Situ Hybridization–Immunofluorescence and the Dual-Label In Situ Hybridization Method1
| In situ hybridization–immunofluorescence | Dual in situ hybridization | |
|---|---|---|
| Total POMC neurons/section | 55.4 ± 2.7 | 98.3 ± 2.4 |
| VGLUT2-expressing POMC neurons/section | 8.1 ± 1.0 | 42.0 ± 1.3 |
| % of VGLUT2-expressing POMC neurons | 13.8% ± 1.7 | 43.1% ± 1.6 |
| GAD67-expressing POMC neurons/section | 28.3 ± 2.8 | 53.0 ± 2.1 |
| % of GAD67-expressing POMC neurons | 54.0% ± 1.8 | 53.6% ± 0.7 |
Numbers of detected POMC, VGLUT2–POMC, and GAD67–POMC neurons, and observed percentages for VGLUT2-expressing and GAD67-expressing POMC neurons. Abbreviations: GAD67, glutamic acid decarboxylase 67; POMC, pro-opiomelanocortin; VGLUT2, vesicular glutamate transporter 2.
With dual-label in situ hybridization, VGLUT2 mRNA was found in a large number of POMC neurons. The vast majority of the most rostral POMC cells in the retrochiasmatic area rostral to the arcuate nucleus were VGLUT2-positive (data not shown). At the rostral pole of the arcuate nucleus (level 1), VGLUT2 mRNA-expressing POMC neurons were numerous in the lateral retrochiasmatic area, surrounding the ventral borders of the ventromedial nucleus, and were also found in the arcuate nucleus along with non-VGLUT2–POMC neurons (Figs. 5A,B,G, 7A). At mid levels of the arcuate nucleus (levels 2–4), VGLUT2 mRNA was observed in the majority of the laterally located POMC cells, and in POMC neurons at the dorsomedial border of the arcuate nucleus (Figs. 5C,D,I, 7B–D). A few VGLUT2-positive POMC neurons were also present in the medial arcuate nucleus, but most of the medially located POMC neurons were VGLUT2-negative (Figs. 5H, 7B–D). Caudally (level 5), approximately half of the POMC neurons were VGLUT2-positive, and intermingled with VGLUT2-negative POMC neurons (Figs. 5E,F,J, 7E). The proportion of VGLUT2-positive POMC neurons in different rostrocaudal levels is demonstrated in Table 3. In total, of the 488.8 ± 22.1 POMC neurons per mouse, 43.1 ± 1.6% contained VGLUT2 hybridization signal.
Figure 5.
A–F: Dual-label in situ hybridization demonstrates the distribution of POMC (red fluorescence) and VGLUT2 mRNA (silver grains) at rostral (A,B), mid (C,D), and caudal (E,F) levels (corresponding to levels 1, 3, and 5, respectively) of the mouse arcuate nucleus. VGLUT2 mRNA is expressed in numerous cells in the arcuate nucleus and the retrochiasmatic area. G–J: Higher magnification merged images of framed areas in A–F demonstrate examples of VGLUT2-positive (green arrowheads) and VGLUT2-negative POMC neurons (blue arrowheads). Note the high number of VGLUT2-expressing POMC neurons in the lateral retrochiasmatic area (G) and the lateral (I) and posterior arcuate nucleus (J), and the paucity of VGLUT2-positive POMC cells in the medial arcuate nucleus (H). 3V, third ventricle; Arc, arcuate nucleus; ArcP, posterior arcuate nucleus; RChL, lateral retrochiasmatic area; VMH, ventromedial hypothalamic nucleus. Scale bar in A = 100 μm for A–F; 25 μm for G–J.
Figure 7.
Schematic maps illustrate the distribution of VGLUT2-positive (A–E), and GAD67-positive POMC neurons (F–J) from one mouse at the five different rostrocaudal levels examined. Open circles represent single-labeled POMC neurons, and filled circles represent VGLUT2-POMC neurons (A–E) or GAD67–POMC neurons (F–J). Note the different distribution patterns of VGLUT2-expressing and GAD67-expressing POMC neurons. 3V, third ventricle; PMV, ventral premammillary nucleus; VMH, ventromedial hypothalamic nucleus.
TABLE 3.
Percentage of VGLUT2 and GAD67 mRNA Expression in POMC Neurons at Different Rostrocaudal Levels of the Mouse Arcuate Nucleus–Retrochiasmatic Area1
| Level | % POMC neurons labeled for VGLUT2 | % POMC neurons labeled for GAD67 |
|---|---|---|
| 1 | 54.7 ± 7.2 | 31.8 ± 2.1 |
| 2 | 36.1 ± 1.6 | 56.2 ± 1.1 |
| 3 | 36.0 ± 0.5 | 60.1 ± 2.3 |
| 4 | 45.7 ± 0.6 | 58.4 ± 2.5 |
| 5 | 53.2 ± 1.6 | 56.9 ± 2.0 |
| Total | 43.1 ± 1.6 | 53.6 ± 0.7 |
Level 1, most rostral; level 5, most caudal, as shown in Figure 7. Abbreviations: GAD67, glutamic acid decarboxylase 67; POMC, pro-opiomelanocortin; VGLUT2, vesicular glutamate transporter 2.
GAD67 mRNA expression in mouse POMC neurons
The percentages and distribution patterns of GAD67-positive POMC neurons found by combined in situ hybridization–immunofluorescence, and dual-label in situ hybridization were similar (Table 2), likely due to the higher, more easily detectable, levels of cellular GAD67 mRNA in POMC neurons. Results from the dual in situ hybridization study are presented in detail. GAD67-expressing neurons were rarely found among the most rostral POMC neurons in the retrochiasmatic area rostral to the arcuate nucleus (data not shown). At the most rostral part of the arcuate nucleus (level 1), GAD67-positive POMC neurons were found both in the arcuate nucleus and the adjacent lateral retrochiasmatic area (Figs. 6A,B,G,H, 7F). However, the majority of POMC neurons in the lateral retrochiasmatic area lacked GAD67 mRNA (Figs. 6G, 7F). At mid levels (levels 2–4), most POMC neurons in the medial part of the arcuate nucleus expressed GAD67 mRNA (Figs. 6C,D,I, 7G–I). Fewer GAD67-POMC neurons were found laterally, where several POMC neurons lacked the GAD67 hybridization signal (Figs. 6J, 7G–I). Most caudally (level 5), more than half of the POMC neurons expressed GAD67 mRNA, and they were intermingled with GAD67-negative POMC neurons (Figs. 6E,F,K, 7J). The percentage of GAD67-positive POMC neurons at each rostrocaudal level is presented in Table 3. Of 493.8 ± 13.0 POMC neurons per mouse, 53.6 ± 0.7% contained GAD67 hybridization signal.
Figure 6.
A–F: Dual-label in situ hybridization demonstrates the distribution of POMC (red fluorescence) and GAD67 mRNA (silver grains) at rostral (A,B), mid (C,D), and caudal (E,F) levels (corresponding to levels 1, 3, and 5, respectively) of the mouse arcuate nucleus. G–K: Higher magnification merged images of framed areas in A–F demonstrate examples of GAD67-positive (green arrowheads) and GAD67-negative POMC neurons (blue arrowheads). GAD67-positive POMC neurons are found in all subregions, most densely in the medial (I) and posterior arcuate nucleus (K). Note that several POMC neurons lack GAD67 mRNA in the lateral retrochiasmatic area (G) and lateral arcuate nucleus (J). 3V, third ventricle; Arc, arcuate nucleus; ArcP, posterior arcuate nucleus; RChL, lateral retrochiasmatic area; VMH, ventrome-dial hypothalamic nucleus. Scale bar in A = 100 μm for A–F; 25 μm for G–K.
DISCUSSION
In the present study, we provide a comparative description of VGLUT2 and GAD67 mRNA expression in hypothalamic POMC neurons of the rat and mouse. We observed that approximately 60% of rat POMC neurons express VGLUT2, and ~40% express GAD67 mRNA. In mice, ~45% of POMC neurons express VGLUT2, and approximately 55% express GAD67 mRNA. These findings suggest that significant glutamatergic and GABAergic subpopulations of POMC neurons both exist. It should be emphasized, however, that only male rats and mice were used for this study, and the organization of VGLUT2- and GAD67-expressing POMC neurons may be somewhat different in females.
VGLUT2- and GAD-expressing POMC neurons in the rat
The present study is the first detailed investigation of glutamatergic and GABAergic marker expression in POMC neurons in the rat hypothalamus. Previous studies reported the presence of VGLUT2 protein in some of the POMC cell bodies (Collin et al., 2003; Kiss et al., 2005), and a lack of immunoreactivity for GAD enzymes or GABA (Ovesjo et al., 2001). Because VGLUT2 and the GAD enzymes are localized preferentially in neuronal fibers and are less concentrated in the cell body, estimating the coexpression of these markers in neuronal subpopulations requires in situ hybridization for the respective mRNAs that are localized in the cell body. Because VGLUT2 is the predominant vesicular glutamate transporter expressed in the mediobasal hypothalamus (Ziegler et al., 2002), and GAD67 is expressed in the same pattern in the arcuate nucleus as the other GABA-synthetic enzyme isoform, GAD65 (Esclapez et al., 1993; Feldblum et al., 1993), VGLUT2 and GAD67 mRNAs provide good markers to identify the glutamatergic and GABAergic subsets of POMC neurons. The present findings revealed a marked heterogeneity among POMC neurons, with VGLUT2 mRNA being expressed in about 58% and GAD67 mRNA in 37% of POMC neurons. These two subpopulations had different distribution patterns with a low degree of overlap. VGLUT2-expressing POMC neurons dominated over GAD67-expressing POMC neurons in the retrochiasmatic area and the arcuate nucleus at the most rostral level. Similarly, the glutamatergic dominance was also observed at mid levels in the lateral retrochiasmatic area and the lateral arcuate nucleus, whereas GAD67 mRNA was expressed in only a few POMC neurons in these areas. Overlapping distributions of VGLUT2-expressing and GAD67-expressing POMC neurons were found in the medial and dorsal arcuate nucleus at mid levels, and in the posterior arcuate nucleus. However, an increasing abundance of GAD67-expressing and a decreasing ratio of VGLUT2-expressing POMC neurons was identified toward the caudal end. These complementary distribution patterns suggest that the majority POMC neurons express either only VGLUT2 or only GAD67 at detectable levels, but not both markers, indicating the existence of distinct glutamatergic and GABAergic POMC cell populations. Nevertheless, a minor subset of rat POMC neurons may express both VGLUT2 and GAD67, similar to what was found in mice (Jarvie and Hentges, 2012).
A notable difference between VGLUT2-expressing and GAD67-expressing POMC neurons was also in their size, with VGLUT2-expressing POMC neurons being significantly larger than GAD67-expressing POMC neurons. A similar size difference between GABAergic and glutamatergic POMC neurons was observed previously in mice (Hentges et al., 2009). This finding further corroborates that glutamatergic and GABAergic POMC neurons are two distinct cell types with different properties.
VGLUT2- and GAD-expressing POMC neurons in the mouse
In mice, VGLUT2 mRNA was detected in approximately 43% of POMC neurons, and GAD67 mRNA expression was found in 54% of POMC cells. Similar to what was observed in the rat, VGLUT2- and GAD67-expressing POMC neurons had partially different distribution patterns. A conspicuous mediolateral distribution was observed in the rostral two-thirds of the POMC cell group, where the majority of VGLUT2-POMC neurons were found in more lateral positions, whereas GAD67-POMC neurons tended to concentrate in the medial parts of the cell group. In the posterior arcuate nucleus, the distributions of VGLUT2- and GAD67-expressing POMC neurons were highly overlapping.
Putting these results into the context of published data, we observed a similar, but somewhat higher, ratio (54%) of GAD67-expressing mouse POMC neurons than reported in previous studies (Hentges et al., 2004; Hentges et al., 2009; Jarvie and Hentges, 2012). The proportion of GAD-expressing POMC neurons has been consistently estimated to be around 40% by three different approaches. Accordingly, by dual in situ hybridization, Hentges et al. (2004) found that 35% of POMC neurons expressed GAD67 mRNA, and in another study that 42% of DsRed-labeled POMC neurons expressed the GAD67–green fluorescent protein (GFP) transgene in a double transgenic mouse strain (Hentges et al., 2009). Most recently, Jarvie and Hentges (2012) have reported that 45% and 38% of POMC neurons express GAD65 and GAD67 mRNA, respectively, as detected by fluorescent in situ hybridization in enhanced (E)GFP-labeled POMC neurons. In agreement with Hentges et al. (2009), who reported the conspicuous absence of GAD67-expression from a rostral population of POMC neurons, we found the fewest number of GAD67-expressing POMC neurons rostrally in the retrochiasmatic area.
In contrast to earlier studies, the present study describes a much larger subpopulation, approximately 43%, of mouse POMC neurons that express VGLUT2 mRNA. Previously, VGLUT2 expression was detected in only 7% of POMC neurons by fluorescent in situ hybridization, (Jarvie and Hentges, 2012), and in 10% of POMC neurons (Vong et al., 2011) using a double transgenic mouse strain. By combined radioactive in situ hybridization and immunofluorescence on paraformaldehyde-perfused brain sections, we also found that only 14% of POMC neurons were labeled for VGLUT2. However, by using a more sensitive method of dual fluorescent and radioactive in situ hybridization carried out on fresh-frozen sections, a substantially higher number of VGLUT2-expressing POMC neurons was detected, comprising ~43% of arcuate POMC neurons. Having compared these two methods, we believe that the potential masking effect of paraformaldehyde perfusion that cannot be reversed by proteinase K treatment may hinder the detection of low levels of cellular VGLUT2 mRNA. Therefore, the use of fresh-frozen tissue is necessary for the sensitive detection of VGLUT2 mRNA in POMC neurons in the mouse brain. Additionally, our results indirectly suggest that the findings by Jarvie and Hentges (2012), showing that in situ hybridization more sensitively detects the expression of neurotransmitter phenotypic markers than transgenically expressed fluorescent reporter proteins, are also valid for VGLUT2.
Functional implications for glutamatergic and GABAergic POMC neurons
Hypothalamic POMC and AGRP neurons have long been known as an endogenous agonist–antagonist system, respectively stimulating and inhibiting melanocortin receptors on the same downstream neurons (Cone, 2005). In keeping with this concept, it was recently demonstrated that α-MSH potently stimulates the firing frequency of hypothalamic paraventricular nucleus neurons by a direct postsynaptic mechanism, whereas AGRP inhibits the firing frequency of these neurons (Ghamari-Langroudi et al., 2011). It would be logical to assume that POMC and AGRP neurons also regulate their common downstream neurons oppositely by glutamate and GABA. The large number of glutamatergic POMC cells found in the present study strongly supports the hypothesis that at least some downstream cell populations are excited by glutamate from POMC neurons whereas they are inhibited by GABA from AGRP neurons.
The existence of both glutamatergic and GABAergic POMC neurons, however, implies a functional heterogeneity between these two populations; in fact, the glutamate versus GABA phenotypes may represent a major functional division within the POMC cell group. Glutamatergic and GABAergic POMC neurons may be recipients of distinct sets of hormonal and neuronal inputs, and may send selective outputs to separate regions of the brain. Functionally different subsets of POMC neurons have been identified recently, including separate populations of leptin-responsive and insulin-responsive POMC neurons (Williams et al., 2010). POMC neurons regulated by serotonin also represent a population distinct from the leptin-regulated POMC neurons (Sohn et al., 2011). Interestingly, leptin-activated POMC neurons in mice are mainly located laterally in the retrochiasmatic area and arcuate nucleus (Sohn et al., 2011; Williams et al., 2010), a region where the majority of POMC neurons expresses VGLUT2 but not GAD67. Furthermore, leptin-activated POMC neurons in the rat are particularly concentrated most rostrally and in the lateral arcuate nucleus, but are less frequent in the medial arcuate nucleus (Elias et al., 1999). This distribution pattern is highly reminiscent of the distribution of VGLUT2-expressing POMC neurons in the rat. These data raise the possibility that leptin acts selectively on glutamatergic, but not on GABAergic, POMC cells in both species. Thus, identifying the hormonal and neural signals that may differently regulate glutamatergic and GABAergic POMC cells would be key to understanding the distinct roles of these two cell types.
A further understanding of the potentially different regulatory functions of glutamate- and GABA-releasing POMC cells would be enhanced by identifying the projection patterns of both cell types. Hypothalamic POMC neurons project to a wide variety of brain regions (Bagnol et al., 1999; Haskell-Luevano et al., 1999), and it has been suggested that subpopulations of POMC neurons innervate distinct target sites (King and Hentges, 2011). Melanocortin peptides have been associated with several functions other than energy homeostasis and metabolism, including central regulation of cardiovascular function, sexual function, inflammation, and the fever response (Tatro and Sinha, 2003; Cone, 2005; Bertolini et al., 2009). It would be intriguing to speculate that these functions may be regulated through distinct projection sites of POMC neurons, originating selectively from excitatory or inhibitory POMC cells.
POMC neurons have also been reported to regulate neuronal functions locally, within the arcuate nucleus. Using channelrhodopsin-labeled POMC neurons, both GABA and glutamate release could be induced from POMC terminals onto other neurons in the arcuate nucleus, including other POMC neurons (Dicken et al., 2012). However, GABA was more frequently released than glutamate (Dicken et al., 2012). This, together with earlier data demonstrating symmetric-type, inhibitory synaptic contacts between POMC neurons in the rat arcuate nucleus (Chen and Pelletier, 1983), suggests that GABAergic POMC neurons establish more intranuclear connections than glutamatergic POMC neurons, and possibly have an autoregulatory function to inhibit POMC neuronal activity. The local projections of GABAergic POMC neurons may also inhibit AGRP cells. However, a recent study by Atasoy et al. (2012) found no synaptic responses from AGRP or POMC neurons following photostimulation of channelrhodopsin-labeled POMC neurons or axons, arguing against the possibility that GABAergic POMC neurons exert significant local actions.
Although the differing distribution patterns of VGLUT2- and GAD67-expressing POMC neurons suggest that they largely form distinct subpopulations, in some areas their distribution overlaps, raising the possibility of dual phenotypic, VGLUT2- and GAD-expressing, POMC neurons. Indeed, Jarvie and Hentges (2012) observed a small number of POMC neurons that expressed both VGLUT2 and GAD65 mRNAs. It is possible, therefore, that there may be a third subtype of POMC neuron in which the glutamate- or GABA-releasing properties are not entirely predetermined but are rather a function of the type of regulatory signals acting on the cell. A similar plasticity phenomenon has been suggested in a subregion of the preoptic area where VGLUT2 and GAD are coexpressed in the same neurons, with the amount of VGLUT2 and vesicular GABA transporter proteins in their axons oppositely regulated by estrogen (Ottem et al., 2004). It is an intriguing possibility that some glutamatergic and GABAergic POMC neurons form part of a single, dynamic cell population rather than two permanent neuronal subsets with distinct physiological roles and amino acid neurotransmitter phenotypes.
CONCLUSIONS
In conclusion, these data demonstrate that both glutamatergic and GABAergic cells comprise significant, distinct subpopulations of hypothalamic POMC neurons, implying comparable physiological significance for each cell type. Future studies will be required to determine whether glutamatergic and GABAergic POMC neurons participate in different regulatory functions.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: DK-3721; Grant sponsor: The Dr. Gerald J. and Dorothy R. Friedman New York Foundation for Medical Research (to R.M.L.); Grant sponsor: The Hilda and Preston Davis Foundation for eating disorders Research (postdoctoral fellowship award to G.W.).
We thank Dr. Melissa Chee for reviewing the manuscript and for her valuable comments.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no known or potential conflict of interest to disclose.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: GW. Acquisition of data: GW. Analysis and interpretation of data: GW. Study supervision and review of data: RML. Drafting of the manuscript: GW. Critical revision of the manuscript for important intellectual content: GW, EH, RML. Statistical analysis: GW. Funding: RML, GW. Administrative, technical, and material support: EH.
LITERATURE CITED
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini A, Tacchi R, Vergoni AV. Brain effects of melanocortins. Pharmacol Res. 2009;59:13–47. doi: 10.1016/j.phrs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chen YY, Pelletier G. Demonstration of contacts between proopiomelanocortin neurons in the rat hypothalamus. Neurosci Lett. 1983;43:271–276. doi: 10.1016/0304-3940(83)90200-8. [DOI] [PubMed] [Google Scholar]
- Collin M, Backberg M, Ovesjo ML, Fisone G, Edwards RH, Fujiyama F, Meister B. Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci. 2003;18:1265–1278. doi: 10.1046/j.1460-9568.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Tobin AJ, Houser CR. Comparative localization of mRNAs encoding two forms of glutamic acid decarboxylase with nonradioactive in situ hybridization methods. J Comp Neurol. 1993;331:339–362. doi: 10.1002/cne.903310305. [DOI] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. San Diego: Academic Press; 2008. Compact. [Google Scholar]
- Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci U S A. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology. 1999;140:1408–1415. doi: 10.1210/endo.140.3.6544. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24:1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Turi GF, Kallo I, Liposits Z. Expression of vesicular glutamate transporter–2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology. 2004;145:4018–4021. doi: 10.1210/en.2004-0589. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Halasz J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Wittmann G, Kallo I, Fuzesi T, Fekete C, Liposits Z. Distribution of type 1 cannabinoid receptor-expressing neurons in the septal-hypothalamic region of the mouse: colocalization with GABAergic and glutamatergic markers. J Comp Neurol. 2012;520:1005–1020. doi: 10.1002/cne.22766. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J Comp Neurol. 2012;520:3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS One. 2011;6:e25864. doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J, Csaba Z, Csaki A, Halasz B. Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur J Neurosci. 2005;21:2111–2119. doi: 10.1111/j.1460-9568.2005.04012.x. [DOI] [PubMed] [Google Scholar]
- Low MJ. Agnostic about in vivo inverse agonism of agouti-related peptide. Endocrinology. 2011;152:1731–1733. doi: 10.1210/en.2011-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Gomuc B, Suarez E, Ishii Y, Durr K, Gillberg L. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur J Neurosci. 2006;24:2731–2740. doi: 10.1111/j.1460-9568.2006.05157.x. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen L. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovesjo ML, Gamstedt M, Collin M, Meister B. GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. J Neuroendocrinol. 2001;13:505–516. doi: 10.1046/j.1365-2826.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. San Diego: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatro JB, Sinha PS. The central melanocortin system and fever. Ann N Y Acad Sci. 2003;994:246–257. doi: 10.1111/j.1749-6632.2003.tb03187.x. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]