Abstract
Stem cell-mediated tissue repair is a promising approach for many diseases. Mammalian intestine is an actively regenerating tissue such that epithelial cells are constantly shedding and underlying precursor cells are constantly replenishing the loss of cells. An imbalance of these processes will lead to intestinal diseases including inflammation and cancer. Mammalian intestinal stem cells (ISCs) are located in bases of crypts but at least two groups of cells have been cited as stem cells. Moreover, precursor cells in the transit amplifying zone can also proliferate. The involvement of multiple cell types makes it more difficult to examine tissue damage response in mammalian intestine. In adult Drosophila midgut, the ISCs are the only cells that can go through mitosis. By feeding pathogenic bacteria and stress inducing chemicals to adult flies, we demonstrate that Drosophila ISCs in the midgut can respond by increasing their division. The resulting enteroblasts, precursor cells for enterocytes and enteroendocrine cells, also differentiate faster to become cells resembling enterocyte lineage. These results are consistent with the idea that Drosophila midgut stem cells can respond to tissue damage induced by pathogens and initiate tissue repair. This system should allow molecular and genetic analyses of stem cell-mediated tissue repair.
The gastrointestinal (GI) tract is not only for nutrient absorption but also a major site of interaction between the host and environmental pathogens (Backhed et al., 2005; Macdonald and Monteleone, 2005; Radtke and Clevers, 2005). In addition to the numerous microbes and chemicals ingested during daily food intake, the GI tract also houses billions of commensal bacteria, which play important symbiotic roles with the host. The complex interaction between intestinal cells and microbes, both commensal and ingested, is essential for the well being of the host.
The epithelial lining of GI tract is essentially one to two-cell thick and the epithelium is constantly shedding cells due to aging or damage. Maintenance of the epithelial integrity requires replenishment of dead cells by proper division and differentiation of precursor cells (Crosnier et al., 2006; Scoville et al., 2008; Casali and Batlle, 2009). This tissue homeostasis is a highly regulated process, and Wnt, BMP and Notch signaling pathways have been implicated in mammalian intestinal cell maintenance and proliferation (Crosnier et al., 2006; Fodde and Brabletz, 2007; Nakamura et al., 2007). One possible mechanism for tissue homeostasis is perhaps based on adult stem cells. Intestinal stem cells (ISCs) divide asymmetrically in some way and give rise to progenitor cells, which in turn differentiate into various cell types in the intestine. Even thought ISCs in mouse intestine have been located to the base of each crypt, different markers have identified two groups of cells, namely +4 label retention cells and Lgr5-positive columnar base cells, as stem cells (Montgomery and Breault, 2008; Scoville et al., 2008; Casali and Batlle, 2009). In addition to the putative stem cells, precursors in the transit amplifying zone are also capable of dividing for the replenishment of ever shedding epithelial cells (Crosnier et al., 2006). Given the paucity of specific markers and the potential involvement of multiple cell types, how ISCs in mammals respond to environmental pathogens and mediate tissue repair needs further investigation (Barker et al., 2007; He et al., 2007; Sangiorgi and Capecchi, 2008; Scoville et al., 2008; Zhu et al., 2009).
Drosophila has been a very useful model organism for studying various aspects of stem cell biology including stem cell niche and asymmetric division (Kirilly and Xie, 2007; Egger et al., 2008). ISCs have recently been identified in Drosophila midgut and hindgut, equivalents of mammalian intestine and colon, respectively (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Takashima et al., 2008). The adult Drosophila midgut has approximately 1,000 ISCs that are distributed evenly along the gut and located basally to mature enterocytes. In Drosophila midgut, ISC is the only cell type that undergoes mitosis, while the differentiating enteroblasts undergo endoreplication. Coupled with the identification of an ISC-specific marker Delta, Drosophila midgut stem cells provide a relatively simple model to study biological responses of ISCs.
The Delta-Notch pathway plays a critical role in ISC fate determination (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007). Drosophila midgut ISC division is morphologically symmetrical, giving rise to two daughter cells that are initially similar. However, soon after division one cell retains high level of Delta and remains as an ISC, while the other cell quickly loses Delta and becomes an enteroblast (Ohlstein and Spradling, 2007). Active Delta in the newly formed ISC stimulates Notch signaling in the neighboring enteroblast to establish the proper asymmetric cell fate. Thus, the punctate staining of active Delta in cytoplasm serves as the only known ISC-specific marker (Bray, 2006; Ohlstein and Spradling, 2007). Enteroblasts function as precursor cells that can no longer divide but can differentiate into either enterocytes, the absorptive cells, or enteroendocrine cells, the hormone producing cells. Ninety percent of enteroblasts differentiate into enterocytes and ten percent into enteroendocrine cells (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007). The decision to differentiate into the two different cell types may depend on the strength of Notch signaling in the enteroblast, which in turn is determined by the level of Delta in the original ISC.
To obtain insights into the mechanisms of host self defense and tissue repair in the GI tract, we investigated how ISCs in adult Drosophila midgut respond to pathogens. Previous studies have shown that many microbes when introduced into body cavity by septic injury can cause lethality (Ip, 2005; Ferrandon et al., 2007; Lemaitre and Hoffmann, 2007; Dionne and Schneider, 2008; Vallet-Gely et al., 2008). Feeding of bacteria or viruses, however, rarely kill adult flies, demonstrating that the Drosophila GI tract has a strong barrier function or innate immune response. Nonetheless, two bacteria strains, Pseudomonas entomophila and Serratia marcescens, when fed to adult flies can cause lethality, albeit to varying degrees (Liehl et al., 2006; Nehme et al., 2007). The mechanism of pathogenesis is not entirely clear but depends at least partly on secreted proteases or epithelial invasion (Nehme et al., 2007; Buchon et al., 2009). Moreover, epithelial damage of the gut was observed after feeding of these bacteria. Meanwhile, an innate immune response to ingested microbes is the production of reactive oxygen species (ROS), which function as bactericidal molecules. ROS can also damage host cells, thus the balance between production and removal of ROS is essential for the health of the host (Ha et al., 2005a,b; Lee, 2008). ROS producing agents such as paraquat and hydrogen peroxide (H2O2) are therefore stress-inducing agents (Biteau et al., 2008; Choi et al., 2008). In this report, we show that oral feeding of pathogenic bacteria and stress-inducing agents can increase ISC division as well as enteroblast differentiation. These results demonstrate that adult Drosophila midgut can be used as a model to study stem cell-mediated tissue repair during pathogenic infection of the GI tract.
Materials and Methods
Drosophila stocks, bacteria strains, and feeding experiments
Information on Drosophila genes and stocks is available from Flybase (Bloomington, IN) (http://flybase.bio.indiana.edu). y1w*, CantonS and w1118 were used as wild type stocks for gut phenotypic comparison. UAS-mCD8GFP flies were obtained from the Bloomington stock center; esg-Gal4 and Su(H)Gbe-lacZ were as described (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007). Flies were maintained on cornmeal-yeast-molasses-agar media. Stocks were maintained at room temperature. For viability tests and feeding experiments, the flies were kept at 29°C. We usually used 50–100 flies per vial for viability tests and 10–50 flies per vial for gut phenotype induction. Feeding experiments involved using 3- to 5-day-old flies in an empty vial containing a piece of 2.5 cm × 3.75 cm chromatography paper (Fisher, Pittsburgh, PA). Five hundred microliters of 5% sucrose solution alone or with pathogens was used to wet the paper as feeding medium. Sucrose solution alone serves as the control for all experiments. Paraquat (Sigma, St. Louis, MO) and hydrogen peroxide (H2O2) (Fisher) were added in different amounts as indicated in the figures to the 5% sucrose solution. The bacterial growth medium 2×YT broth (MP Biochemicals, Solon, OH) was also used as a control for bacteria feeding experiments. A rifampicin resistant Pseudomonas entomophila strain was a generous gift from Bruno Lemaitre; Serratia marcescens Db11 was a generous gift from Christine Kocks. The bacteria were cultured overnight in 2×YT, concentrated and resuspended in 2×YT if necessary. The numbers of bacteria as indicated in the figures were mixed with the 5% sucrose solution for feeding. The feeding solution was changed every day.
For lineage analysis, GFP-marked intestinal stem cell clones from MARCM were generated as previously described (Lee and Luo, 2001). Fly stocks were crossed to generate offspring with the genotype: hsFLP; FRTG13 UAS-CD8GFP/FRTG13 tubulin-Gal80; tubulin Gal4/+. These stocks generated small number of GFP positive mitotic clones in midgut without a heat shock induction of the FLP recombinase. Only flies with all the correct chromosomes exhibited this low level of mitotic recombination and the GFP marked ISC divided and the cell nest gradually grew to include bigger cells as observed in older flies. These are consistent with having successful mitotic recombination, which by chance eliminates the repressor Gal80 in a mitotic stem cell and allows Gal4 driven GFP expression within that lineage only. For tissue damage experiments 3-day-old flies were set up for feeding in 29°C for 3 days before gut dissection.
Immunofluorescent staining and microscopy
Female flies were used for gut dissection, because of the bigger size but male flies were also used occasionally to check the phenotypes. The entire gastrointestinal tract was pulled from the posterior end directly into fixation medium containing 1×PBS and 4% Formaldehyde (Mallinckrodt Chemicals, Phillipsburg, NJ). Guts were fixed in this medium for 3 h; except for Delta staining the fixation was for 0.5 h. Subsequent rinses, washes and incubations with primary and secondary antibodies were done in a solution containing 1X PBS, 0.5% BSA, 0.1% Triton X-100 with 1:50 dilution of Horse serum for blocking. The following anti-sera were used: anti-Delta (monoclonal 1:100 dilution), anti-Prospero (monoclonal 1:50 dilution), all from Developmental Studies Hybridoma Bank; anti-phospho-histone H3 (rabbit 1:2,000 dilution) (Upstate Biotechnology, Millipore, Billerica, MA); anti-β-galactosidase (monoclonal 1:500 dilution) (Promega, Madison, WI); anti-β-galactosidase (rabbit 1:50,000) (Cappel, MP Biomedicals, Santa Ana, CA). Secondary antibodies were used in 1:2,000 dilution as follows: goat anti-mouse IgG conjugated to either Alexa 488 or Alexa 568, and goat anti-rabbit IgG conjugated to either Alexa 488 or Alexa 546 (Molecular probes, Eugene, OR). DAPI (Vectorshield, Vector Lab, Burlingame, CA) was used at 1:1 dilution in PBS. Most images were taken by a Nikon Spinning Disk confocal microscope (UMass Medical School Imaging Core Facility).
Results
Feeding of chemical and microbial pathogens causes dose dependent lethality
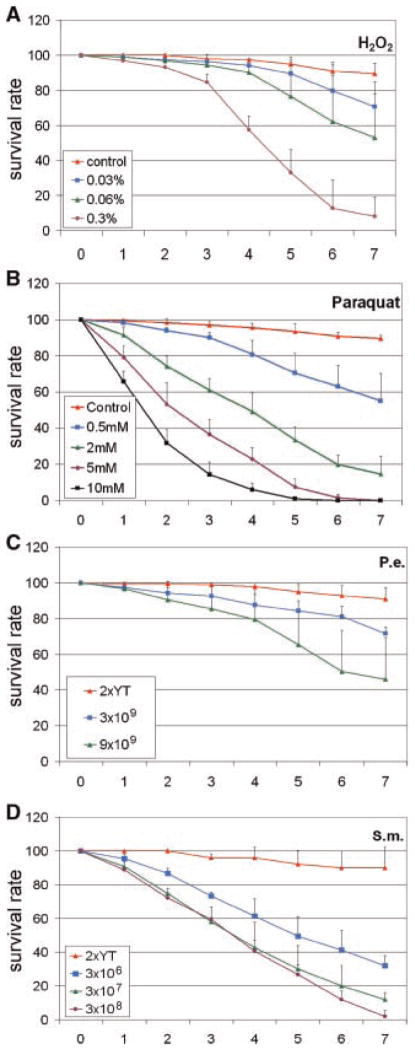
The uses of P. entomophila and S. marcescens as pathogenic bacteria, as well as paraquat and H2O2 as stress-inducing agents, have been previously described (Liehl et al., 2006; Nehme et al., 2007; Biteau et al., 2008; Choi et al., 2008). However, due to the variability of host response, we performed our lethality study using these reagents in order to obtain suitable feeding conditions for subsequent cellular assays. The minimum feeding solution contains 5% sucrose alone, which can sustain the viability of flies for more than 7 days albeit under nutritional starvation. The addition of bacteria growth medium 2×YT (2× yeast extract and tryptone) in the 5% sucrose provides sufficient nutrients and the flies stay well in this medium for more than 7 days. These two feeding solutions were used as controls. Inclusion of four experimental reagents in our feeding media caused dose dependent lethality when compared to controls (Fig. 1A–D). The use of 0.3% H2O2 in the feeding sucrose solution caused ~50% of fly killing in 4 days. We decided to use this feeding concentration for subsequent experiments because significant pathogenesis could be induced but a substantial number of flies were still alive after 4 days for tissue dissection. Paraquat feeding should induce similar oxidative stress in gut tissue. Indeed, we found that inclusion of 2 mM of paraquat in the sucrose solution caused a killing curve analogous to 0.3% of H2O2, thus we chose to use 2 mM paraquat for our subsequent feeding experiments. For bacteria feeding experiments, we included similar volume of 2×YT in the sucrose solution as control. The addition of 3 × 106 bacteria CFU of S. marcescens caused a strong killing effect, such that 60% of flies were killed within 4 days. Serial dilution of this bacteria caused gradually lower killing effects. P. entomophila appeared to be less pathogenic, and the use of 9 × 109 bacteria could only killed approximately 30% of flies in 4 days. This result is consistent with a previous report showing that adult flies have more resistance to P. entomophila than larvae (Liehl et al., 2006). Overall, these results establish that appropriate amount of pathogens can be used for feeding experiments and subsequent intestinal cell analysis.
Fig. 1.
Dose dependent lethality caused by feeding pathogens. The four reagents used in the feeding experiments caused a dose dependent lethality when fed to adult flies over 7 days. The reagents used are (A) H2O2, (B) paraquat, (C) P. entomophila (P.e.), (D) S. marcescens (S.m.). The pathogens were included in various amounts as indicated in a 5% sucrose solution. The range of H2O2 used in the feeding solution was from 0.03 to 0.3% final concentration. The final concentration of paraquat used in the feeding solution was from 0.5 to 10 mM. The bacteria were cultured in 2×YT medium and the number of bacteria in CFU added to the feeding solution is as indicated. The control feeding in (C,D) was sucrose solution with same volume of 2×YT added as the bacteria fed samples. The final volume of the mixture was 0.5 ml, which was dropped on to a filter paper in a plastic vial for fly feeding. The percentage of flies left alive each day is expressed as survival rate, for 7 days. Flies were transferred to new vials with freshly prepared feeding solution every day. The error bars represent standard deviation.
Pathogen feeding increases the number of precursor cells in midgut
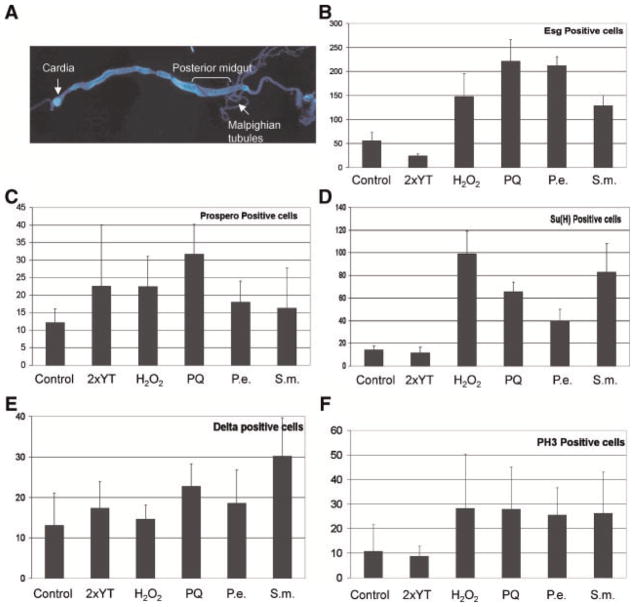
Based on the conditions established in our viability assays, we examined cellular phenotypes of dissected gut from live flies at earlier time of the killing curve, between 2 and 4 days, when most of the flies were still alive. We reason that at earlier time points the intestinal epithelium should be mostly intact and can mount appropriate responses towards pathogenic stimulation, while at later time the intestinal damage may be overwhelming and more complex responses may take place. The escargot promoter-directed Gal4 expression (esg-Gal4) coupled with UAS dependent mCD8GFP reporter (UAS-CD8GFP) can mark the cell membranes of intestinal precursor cells, including ISCs and enteroblasts (schematic illustration at the bottom of Fig. 2) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007). In control fly guts, GFP expression can be easily detected in some small cells either as individual cells or as pairs (Fig. 2A–D). We usually used 2- to 3-day-old flies for experiments but in older flies this esg-Gal4/UAS-GFP expression was detected in more precursor cells, suggesting more active division and enteroblast formation in the ISC nest (data not shown) (Biteau et al., 2008; Choi et al., 2008). Meanwhile, bigger nuclei show no such GFP signal and they are mature enterocytes that are polyploid. Some other small nuclei also show no GFP expression but usually stain positive for another marker Prospero and are thus enteroendocrine cells (Fig. 2M–P). After feeding with bacterial or chemical pathogens for 3 days, dissected guts show clearly increased GFP signals when compared to control samples (Fig. 2E–L). In addition to the apparent increase in the number of GFP positive cells, many GFP positive cells also had bigger sizes. The images shown in Figure 2 were all from the posterior midgut region. However, different regions of the midgut showed variable phenotypes. For example, H2O2 had a stronger phenotype in the anterior midgut, while paraquat has a stronger phenotype in the posterior midgut. Nonetheless, feeding of pathogens almost always increased GFP positive cells at least in some parts of the midgut. The increase of GFP-positive cells is a specific response, because the staining of enteroendocrine cells by Prospero did not show a similar increase (Fig. 2M–P). Therefore, all pathogen fed samples had detectable phenotypic changes, demonstrating that the pathogens somehow caused cell proliferation in the midgut.
Fig. 2.
Pathogen feeding induces a cell proliferation phenotype in adult midgut. The fly strain with GFP expression in midgut (esg-Gal4/UAS-mCD8GFP) was used for feeding experiment and gut dissection. The feeding was carried out for 3 days and guts from female flies were dissected. Representative phenotypes are shown here, all from the posterior midgut region as indicated in Figure 3A. The green signal in parts A–L is GFP, and the blue signal in all parts is DAPI for DNA staining. The red staining in parts M–P is anti-Prospero for enteroendocrine cells. The control feeding was 5% sucrose alone (A,B), or 5% sucrose plus equal amount of 2×YT as in bacteria feeding experiments (C,D). The feeding mixtures used were 0.3% H2O2 (E,F), 2 mM paraquat (G,H), 9 × 109 CFU of P. entomophila (I,J) and 3 × 106 CFU of S. marcescens (K,L). In control and 2×YT samples, the GFP marked precursor cells, including ISCs and enteroblasts, have small sizes and are fewer in number, dispersed among the enterocytes with bigger nuclei. Feeding of the four different reagents all showed increased GFP signal, with some larger-sized cells. Staining of Prospero indicates that the enteroendocrine cells do not show a similar increase with H2O2 (parts M–P) or the other three reagents (data not shown). The magnification of all the parts is the same, and the scale bar is 20 μm in part B.
Enteroblast accumulation is the major phenotypic change after pathogen feeding
To further assess the cell proliferation phenotype, we counted the number of GFP positive cells. The counting was performed on microscopic images taken from the posterior midgut region, as indicated by the bracket in Figure 3A. Both GFP-positive and-negative cells were counted. The number of GFP-positive cells per 100 negative cells was plotted as shown in Figure 3B. The result shows that feeding of H2O2 and paraquat increased the relative number of esg-Gal4/UAS-GFP positive cells approximately three- to fourfold over the sucrose control. Feeding of pathogenic bacteria increased the GFP positive cells by five- to eightfold when compared to the 2×YT control. On the other hand, the number of Prospero-positive enteroendocrine cells had no increase, except for paraquat feeding which showed a 2.5-fold increase (Fig. 3C). This quantification again demonstrates that pathogen feeding caused a cell proliferation phenotype in the midgut.
Fig. 3.
Quantification of cell types in midgut after pathogen feeding. Flies were fed with the four different reagents as indicated. Whole guts were dissected and stained. Immunoflourescent images were taken and positively and negatively stained cells were counted based on the images. Part A shows a DAPI stained midgut, and the posterior midgut region is indicated by the bracket. All images shown in Figure 2 and for cell counting in this figure were taken around this region of the gut, except for phospho-H3 staining, which was counted through the whole midgut. Part B, the GFP positive cells from the esg-Gal4/UAS-CD8GFP fly guts were counted in multiple images for each experiment and normalized by 100 unstained cells within each image revealed by DAPI staining and plotted as shown. Part C: Prospero positive cells were counted and plotted as number per 100 non-stained cells. Part D: β-galactosidase staining in Su(H)-lacZ guts and β-galactosidase-positive cells was counted and plotted as number per 100 non-stained cells. Part E: Delta-positive cells were counted and plotted as number per 100 non-stained cells. Part F: Phospho-H3-positive cells were counted in the whole gut and expressed as the average number of mitotic cells per gut. In all experiments we used 2 mM Paraquat, 0.3% H2O2, 9 × 109 CFU of P. entomophila and 3 × 106 CFU of S. marcescens. The final volume of the feeding mixture was 500 μl on a filter paper inside the vial. The controls were 5% sucrose alone and 2×YT in 5% sucrose. The error bars represent standard deviation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The expression of esg-Gal4/UAS-CD8GFP marks both ISCs and enteroblasts. To determine which cell type is responsible for the GFP-positive cell increase, we stained for enteroblast-specific marker Su(H)-lacZ and ISC-specific marker Delta. The positively stained cells were then counted and normalized with non-stained cells. The result showed that paraquat and H2O2 caused three- to fivefold increase in the number of cells stained positive for Su(H)-lacZ. The two bacteria strains causes two- to fourfold increase of Su(H)-lacZ-positive cells. Cell counts for Delta-positive staining revealed that there was less than twofold increase in the number of ISCs in guts of flies fed with the four reagents. Because the number of enteroblasts increased more than the number of ISCs, it suggests that feeding of pathogens increases ISC division to produce more daughter cells. Therefore, we stained the guts with phospho-histone3 (phospho-H3) antibody to assess cell division. Within the midgut, the only cell type that goes through mitosis is ISC. Enteroblasts cease mitosis although they still undergo endoreplication. Thus, phospho-H3 staining should mark those ISCs that have condensed chromosomes and are in the process of mitosis. Cell counts showed that paraquat and H2O2 treatment increased the number of mitotic cells by approximately threefold. The two bacterial strains used also increased the number by approximately 2.5-fold. Overall, the number of Delta-positive cells did not increase as much while the increase of phospho-H3-positive cells correlates better with the increases in enteroblast accumulation. These data suggest that pathogenic stimulation increases ISC division resulting in the formation of more enteroblasts.
Pathogenic stimulation does not affect cell fate determination
To ascertain that cell fates in midgut are not affected after pathogen feeding, we performed co-immunoflourescent staining for Delta and Su(H)-lacZ. In midguts of young flies, ISCs and enteroblasts after division are in close contact with each other for a short time. High level of β-catenin is present in the junctions of the two cells and E-cadherin is required to maintain this contact (Maeda et al., 2008). This close contact allows Delta-Notch signaling to occur properly between ISC and enteroblast for correct cell fate establishment. In control guts, Delta is detected only in ISC as punctate cytoplasmic staining (Fig. 4A). The neighboring enteroblast has Notch target gene Su(H)-lacZ expression, detected as b-galactosidase staining present in both cytoplasm and nucleus (Fig. 4B). After H2O2 feeding, more cells had nuclear and cytoplasmic β-galactosidase staining (Fig. 4D–F). These staining also became more apparent in cytoplasm likely due to the bigger cell size after pathogenic stimulation. Meanwhile, the cells that had Delta showed no β-galactosidase staining and had clear space surrounding the nuclei (indicated by arrows). This demonstrates that the Delta positive cells have no Su(H)-lacZ expression, and vice versa. The same non-overlap was observed in paraquat, P. entomophila and S. marcescens treated guts. These results suggest that the cell fate decision between ISC and enteroblast remains normal after feeding the various pathogens.
Fig. 4.
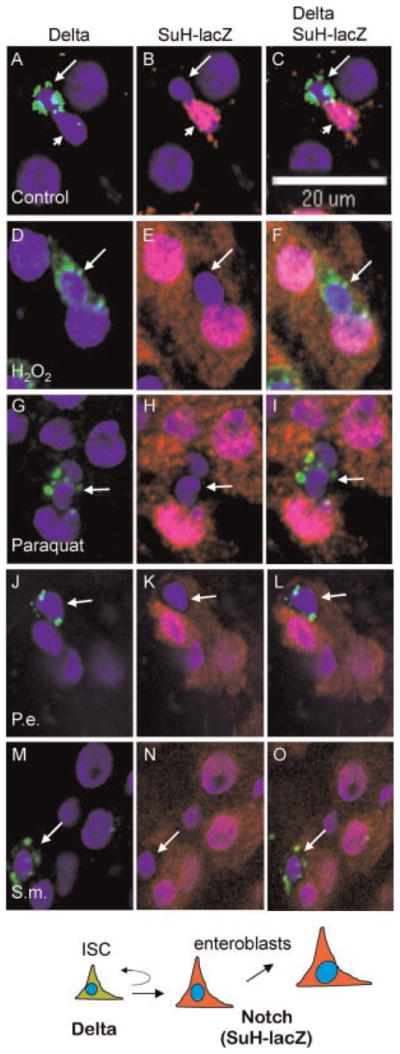
Pathogen feeding does not alter the cell fate decision. Dissected guts from Su(H)-lacZ flies fed with the various agents as indicated were used for immunofluorescent staining. Delta staining (green) and β-galactosidase staining (red) were performed together on the guts. Representative confocal images are shown here. In control samples, the Delta-positive cells (A, arrow) and the Su(H)-lacZ positive cells (B, arrowhead) are found next to each other and almost never overlap. (C) The Delta protein appears as punctate cytoplasmic staining. The β-galactosidase staining is both cytoplasmic and nuclear, thus overlaps extensively with DAPI staining (blue). In pathogen fed flies, the β-galactosidase staining increased substantially, consistent with the accumulation of more enteroblasts surrounding Delta-positive ISCs. There was also more obvious β-galactosidase staining (red) in cytoplasm, suggesting the cell size of enteroblasts has also increased. However, all Delta-positive cells clearly had no cytoplasmic β-galactosidase staining (indicated by arrows in parts D–O), and appeared to have empty space surrounding the nuclei. Over 100 Delta positive cells were counted in each experiment and no overlap of the staining was observed.
Increased enteroblast differentiation after pathogen feeding
We observed that many of the cells marked by esg-Gal4/UAS-GFP and Su(H)-lacZ were larger in size in pathogen fed samples than in the control samples. This suggests that in addition to the increase of stem cell division, the resulting enteroblasts may have faster differentiation into mature enterocytes, which are substantially bigger in size. To trace the fates of ISC and all subsequent cells, we performed lineage tracing by mosaic analysis with repressible cell marker (MARCM). This technique randomly allows Gal4 driven GFP marking of individual ISC lineage due to FLP-FRT-mediated mitotic recombination that removes the repressor Gal80 (Lee and Luo, 2001; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Guts of control MARCM flies fed with sucrose showed GFP expression in clusters with small number of cells (Fig. 5A–C). Under the same feeding condition the H2O2 treated flies had more GFP positive cells and were present in bigger clusters (Fig. 5D–F). Usually we found one cell exhibited punctate Delta staining in each cluster (arrow in all parts). Some clusters also had 2 or more Delta positive cells (data not shown), but it could be due to fusion of neighboring clones or some abnormal cell division. Most importantly, the GFP positive cells were mostly of bigger overall size and bigger nuclear size, comparing to the control GFP cells. These phenotypic changes were similar observed in guts of flies fed with paraquat, P. entomophila and S. marcescens (Fig. 5G–O). We quantified the number of GFP positive cells with bigger cell size. The result shown in Figure 5Q clear demonstrates that the number of differentiating or differentiated cells has increased by more than fourfold. Each isolated cluster should represent a single lineage originating from one ISC. Therefore, the result supports the idea that pathogenic feeding increases the number of cells produced by an ISC, which corroborates the results of increased ISC division. Moreover, the increase in size of most daughter cells suggests that pathogenic feeding also increases differentiation, possibly for tissue repair.
Fig. 5.
MARCM clonal analysis shows an increase in enteroblast differentiation. FLP-FRT-mediated mitotic recombination coupled with the Gal4/Gal80 chromosomes allows one of the two cells of a recent division to expressed GFP. If the newly formed ISC is genetically marked with this GFP expression, all subsequently derived cells will all be GFP positive, thus marking the whole lineage. If the newly formed enteroblast is genetically marked, it will not divide a gain and the GFP-marked cell will differentiate as an isolated cell. We counted only GFP-positive clusters, thus only events that mark ISCs initially. The guts were also stained for Delta (red). In control guts, the MARCM GFP-positive clusters had one Delta positive cell (A, arrow) and very few GFP positive cells that were also small and should represent enteroblasts. (B,C) Feeding with any of the four reagents increased the number of GFP-positive cells in each cluster (parts D–O), consistent with increased cell division. In isolated clusters, usually one Delta-positive cell was present, (arrow in all panels) suggesting one parental ISC gave rise to the other GFP-positive cells in the cluster. Moreover, the sizes of many of these GFP-positive daughter cells were bigger. Because the control and pathogen feeding were preformed for the same time interval (3 days) and at the same temperature (29°C), the results suggest that pathogen fed samples have increased enteroblast differentiation into bigger cells. Part P shows a schematic representation of differentiation from an ISC to a mature enterocyte. Part Q shows the counting of large (similar size as enterocytes) GFP-positive cells per gut in the indicated feeding experiments. Error bars represent standard deviation.
Discussion
We have shown that two stress-inducing chemicals and two pathogenic bacteria can induce ISC proliferation and enteroblast differentiation within a few days of feeding. Previous results also demonstrate two other tissue damaging agents in stimulating intestinal stem cells (Amcheslavsky et al., 2009; Buchon et al., 2009), albeit with different mechanism and responses. These gut phenotypes can be observed in times when less than 50% of fly death occurs. The overall gut morphology of the dissected flies that were still alive appeared rather normal, suggesting that tissue damage is still limited at this time. These results support the idea that pathogenic feeding causes tissue damage within the midgut and the ISCs respond by increasing their division and the resulting enteroblasts increase their differentiation. While it is also possible that these responses represent non-specific reaction to pathogens, we speculate that the stem cells are actively responding to tissue damage induced by the pathogens and are initiating repair. A recent report shows that feeding of a non-pathogenic bacterium, Erwinia carotovora, can induce the expression of the ligand Unpairded3 for the JAK-STAT pathway, which mediates cell proliferation in the midgut (Buchon et al., 2009). Moreover, insulin receptor signaling pathway is required for ISC proliferation (Amcheslavsky et al., 2009). Further analysis will show whether similar stimulation and repair mechanism occur after pathogenic bacteria-induced tissue damage.
Food and water borne diseases, as well as intestinal inflammation and cancer, continue to be a major health concern worldwide (Backhed et al., 2005; Macdonald and Monteleone, 2005; Radtke and Clevers, 2005). An organism’s barrier epithelia are designed to manage continuous contact with microbes and other harmful reagents. Our intention is to use Drosophila as a model to study intestinal responses to stress caused by oral ingestion of pathogenic bacteria and compare the phenotype with known stress-inducing agents such as paraquat and hydrogen peroxide. Previous reports show that bacteria and stress-inducing agents cause pathological changes in adult Drosophila midgut (Liehl et al., 2006; Nehme et al., 2007; Biteau et al., 2008; Choi et al., 2008). These studies employed different conditions, such as a non-pathogenic bacterial strain E. carotovora or a shorter time course for paraquat feeding. Our experimental condition and subsequently induced phenotypes reported here should complement those reports. A detectable phenotype is the increase in cell division, which causes accumulation of enteroblasts in the midgut. The increase in number of ISC based on Delta staining is not as high and cannot account for the increased number of enteroblats/daughter cells, suggesting that individual ISC division rate has increased. We have also provided evidence that the differentiation of enteroblasts to bigger cells occurs with higher frequency within the same experimental time. On the other hand, the number and morphology of enteroendocrine cells did not show significant difference. Based on these observations we conclude that the oxidative stress caused by bacteria and chemicals has accelerated cell division as well as differentiation to form more enterocytes, consistent with epithelial repair after pathogenic damage.
Previous reports documented that epithelial damage is associated with feeding of the two pathogenic bacteria, P. entomophila and S. marecescens (Liehl et al., 2006; Nehme et al., 2007). These bacteria can elicit complex reactions in the midgut, and thus we are unsure of the mechanism by which ISC proliferation is brought about by these pathogens. A logical interpretation of the phenotypes, however, is a damage response where the gut tries to replenish lost enterocytes or those whose functioning is damaged by oxidative stress. It has been known that the fly gut employs an antioxidant system as an immune response against ingested microbes (Ha et al., 2005a,b; Lee, 2008). Therefore, bacterial feeding should mimic some aspects of the oxidative stress phenotypes. We observe that different oxidizing agents, paraquat and hydrogen peroxide, also show prominent and similar phenotypes in the fly gut. Paraquat has been used as an herbicide. It is a highly toxic compound that is absorbed rapidly across the mammalian small intestine brush border and is known to trigger Parkinson’s disease like symptoms in rats (Ossowska et al., 2006). We hope our work will lead to a better understanding of the mechanisms that lead to the observed oxidative stress phenotype and further develop Drosophila as a model system to study intestinal pathogenesis.
Acknowledgments
We thank Tzumin Lee for MARCM flies. The work in Y.T. Ip laboratory was supported by grants from NIH (DK75545 and GM53269) and Worcester Foundation for Biomedical Research. Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
Literature Cited
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. Notch signalling: A simple pathway becomes complex. Nature Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Schneider DS. Models of infectious diseases in the fruit fly Drosophila melanogaster. Dis Model Mech. 2008;1:43–49. doi: 10.1242/dmm.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Chell JM, Brand AH. Insights into neural stem cell biology from flies. Philos Trans R Soc Lond B Biol Sci. 2008;363:39–56. doi: 10.1098/rstb.2006.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005a;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, Lee WJ. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005b;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT. Drosophila innate immunity goes viral. Nat Immunol. 2005;6:863–864. doi: 10.1038/ni0905-863. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Xie T. The Drosophila ovary: An active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- Lee WJ. Bacterial-modulated signaling pathways in gut homeostasis. Sci Signal. 2008;1:pe24. doi: 10.1126/stke.121pe24. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2006;2:e56. doi: 10.1371/journal.ppat.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells. 2008;13:1219–1227. doi: 10.1111/j.1365-2443.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Smialowska M, Kuter K, Wieronska J, Zieba B, Wardas J, Nowak P, Dabrowska J, Bortel A, Biedka I, Schulze G, Rommelspacher H. Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: Implications for Parkinson’s disease. Neuroscience. 2006;141:2155–2165. doi: 10.1016/j.neuroscience.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: Intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Vallet-Gely I, Lemaitre B, Boccard F. Bacterial strategies to overcome insect defences. Nat Rev Microbiol. 2008;6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]