Abstract
Mouse embryonic stem cell (mESC)-derived neurons are a renewable cell source for investigation of neuronal circuits. Engineering circuit-tracing components into stem cells facilitates studies on mechanisms of synaptic coupling and circuitogenesis. This unit details methods for the generation of mESC-derived neurons harboring trans-synaptic viral tracing elements, which are used for investigation of synaptic connections within circuits in vitro, ex vivo, and in vivo. The first protocol describes procedures for feeder-free passaging of mESCs, modified to carry reporter and rabies virus tracing elements. The second protocol describes in vitro generation of neurons from these ESCs. The last protocols describe the use of ESC-derived neurons as “source cells” for rabies virus circuit-tracing to identify inputs onto synaptically connected neurons. Given the broad applicability, these protocols can be applied to investigate the ability of in vitro-derived neurons to establish/maintain synaptic connections in disease models, and/or with human-induced pluripotent stem cells.
Keywords: monosynaptic, embryonic stem cells, retrograde, rabies, circuit, neuron, synapse, TVA, rabies-G
INTRODUCTION
This unit describes the routine generation and maintenance of mouse embryonic stem cell (mESC)-derived neurons that harbor genetic elements necessary to perform transsynaptic tracing studies using genetically engineered rabies virus. Equipping stem cells with viral tracing elements combined with a fluorescent reporter, and further differentiating these cells into selective neuronal subtypes, allows precise infection and circuit tracing using stem cell-derived neurons as “source cells” for retrograde synaptic tracing studies in vitro, ex vivo, or in vivo in healthy neuronal tissue and disease models. These “source cells” establish synapses in vitro and in vivo and can easily be visualized after circuit integration by expression of a fluorescent marker. Subsequent infection of source cells by a modified rabies virus enables rapid and reliable identification of presynaptic inputs onto these cells. The methods discussed here are detailed descriptions of the experiments reported by Garcia et al. (2012). They can be used to generate neuronal lineages with circuit integration capabilities to study the mechanisms of synapse formation and circuit integration, as well as uncover finer details of brain circuit configuration. Finally, the described protocols can further be applied to human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs).
This unit begins with the feeder-free expansion and passaging of mESCs containing viral tracing elements on gelatin-coated culture plates, followed by in vitro differentiation into functional neurons. Transsynaptic viral tracing protocols are discussed using co-cultures of ESC-derived neurons with primary neurons, as well as brain slice explants. Although not described here, differentiated neurons can be further injected into different parts of healthy and disease model rodent brains to reveal intact neural circuitry in vivo.
NOTE: The following procedures are to be performed in a Class II biological safety cabinet.
NOTE: Aseptic technique, standard tissue culture facilities, and equipment for viral transduction and in vivo viral injection are required to carry out the steps described in these protocols.
BASIC PROTOCOL 1: EXPANSION AND PASSAGING OF mESCs ON FEEDER-FREE, GELATIN-COATED CULTURE PLATES
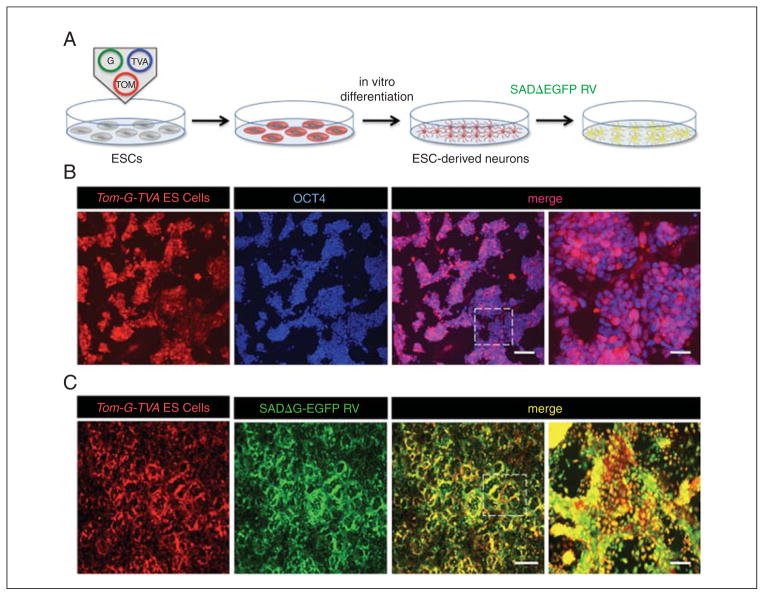
The protocol for the expansion and passaging of mESCs on feeder-free, gelatin-coated culture plates is described below. Traditionally, ESCs have been grown in culture on inactivated fibroblast feeder layers, but using feeders pose some notable drawbacks. For example, feeders make it challenging to passage pure ESC populations over time. Additionally, feeder layers provide heterogeneous growth factors, making it challenging to perform controlled experiments using feeder-dependent ESCs. Growing and passaging mESCs on gelatin-coated tissue culture dishes with media supplemented with leukemia inhibitory factor maintains cells in an undifferentiated state in the absence of feeder layers, which obviates some of these issues. However, in order to successfully accomplish healthy growth in the absence of feeder layers, the mESCs used for expansion and passaging should be of high quality and at least 80% of all mESCs should express the pluripotent stem cell marker Oct4 (Fig. 2D.15.1).
Figure 2D.15.1.
Experimental scheme and expression of genetically modified ESCs harboring transsynaptic viral tracing elements. (A) Experimental outline for in vitro transsynaptic tracing. (B) OCT4 expression pattern of ESCs harboring transsynaptic tracing components and fluorescent marker expression grown feeder-free on gelatinized tissue culture dishes (scale bars 100 μm and 30 μm, respectively). (C) Expression pattern of ESCs expressing viral tracing components 4 days post-infection with SADΔG-EGFP RV (scale bars 150 μm and 30 μm, respectively).
Materials
mESCs harboring viral tracing elements and fluorescent marker (Rabies-G, TVA receptor, tdTomato reporter; from here on referred to as Tom-G-TVA ESCs); such lines can be generated via homologous recombination, transfection, lentiviral transduction, or electroporation
Embryonic stem cell (ESC) medium (see recipe), prewarmed to 37°C
Tissue culture grade phosphate-buffered saline (PBS; Lonza), prewarmed to 37°C
0.05% trypsin/EDTA (Hyclone), prewarmed to 37°C
Fetal bovine serum (FBS; Hyclone)
Gelatin-coated 10-cm tissue culture dishes (see Support Protocol 1)
Stereomicroscope with fluorescent capability
37 °C water bath to warm media
37 °C humidified incubator with 5 % CO2
50 ml conical tubes
Benchtop centrifuge
1-ml pipet tips
-
Examine mESCs under a fluorescent stereomicroscope and determine cell confluence, undifferentiated morphology, colony formation, and reporter expression. Cells should be 90% confluent before splitting, and strongly express both the reporter plasmid and viral tracing components (Fig. 2D.15.1).
If thawing a new vial of mESCs, ensure that the cells are confluent before passaging. This allows for selection of quickly dividing, undifferentiated mESCs compared to differentiated lineages. For the next steps in this unit, it is essential that healthy, undifferentiated mESCs are used to ensure proper differentiation into neuronal lineages. Verification of viral tracing components via infection of pseudotyped rabies virus (RV) is strongly suggested to ensure expression of tracing vectors (Fig. 2D.15.1). -
Remove ESC medium and gently wash the mESCs with 10 ml prewarmed tissue culture grade PBS. Remove the PBS. Repeat this step once.
Washing mESC cultures with PBS removes any cell debris that would otherwise be carried over to a new culture, as well as remove any residual serum from the plate that could interfere with proper cell dissociation using low concentrations of trypsin. -
Add 1 ml prewarmed 0.05 % trypsin/EDTA solution to the plate. Incubate for 10 min at 37°C for 10 min.
A low concentration of trypsin/EDTA ensures the gentle dissociation of adherent ESCs. When dissociating ESCs, harsh treatments are discouraged to ensure the health of the cells, which is imperative for effective differentiation into neuronal lineages. Swirl the plate to ensure complete dissociation of the cells. Add 5 ml FBS to inactivate the trypsin.
-
Add the cell suspension into a 50-ml conical tube and centrifuge for 5 min at 180 × g, 24°C.
It is possible to plate the cells directly after trypsin inactivation without pelleting the cells first. However, the following additional steps are strongly encouraged to ensure passaging of healthy cells without contaminating cellular debris. Aspirate the supernatant. Take care not to aspirate the cell pellet.
-
Resuspend the pellet in 5 ml prewarmed ESC medium using a 1-ml pipet tip by pipetting up and down 30 times.
Avoid using a smaller-sized tip in order to prevent lysis of the cells. Split the cells 1:3 onto gelatin-coated culture dishes (see Support Protocol 1) with 10 ml ESC medium.
-
Maintain the cells at 37°C and continue splitting every 3 days.
Healthy ESCs are rapidly dividing, making it necessary to passage them frequently.
SUPPORT PROTOCOL 1: PREPARATION OF GELATIN-COATED TISSUE CULTURE DISHES
This protocol describes the preparation of gelatin-coated tissue culture dishes for effective feeder-free culture of undifferentiated mESCs. Coating plastic tissue culture dishes with gelatin facilitates the adhesion and growth of ESCs in the absence of feeder layers.
Materials
Tissue culture grade 0.1% gelatinized water (Millipore)
Tissue culture grade phosphate-buffered saline (PBS; Lonza)
10-cm plastic tissue culture dishes
-
Add 5 ml gelatinized water per tissue culture dish.
Ensure that the gelatin water covers the bottom of the tissue culture dish evenly. Incubate the dish for 30 min at room temperature.
Gently aspirate the gelatinized water.
-
Carefully wash the plate once with 5 ml PBS. Aspirate the PBS.
Take caution not to disrupt the newly formed layer of gelatin on the bottom of the dish. Use the gelatin-covered plates immediately and do not let them dry out.
BASIC PROTOCOL 2: IN VITRO NEURONAL INDUCTION OF mESCs HARBORING TRANSSYNAPTIC TRACING ELEMENTS
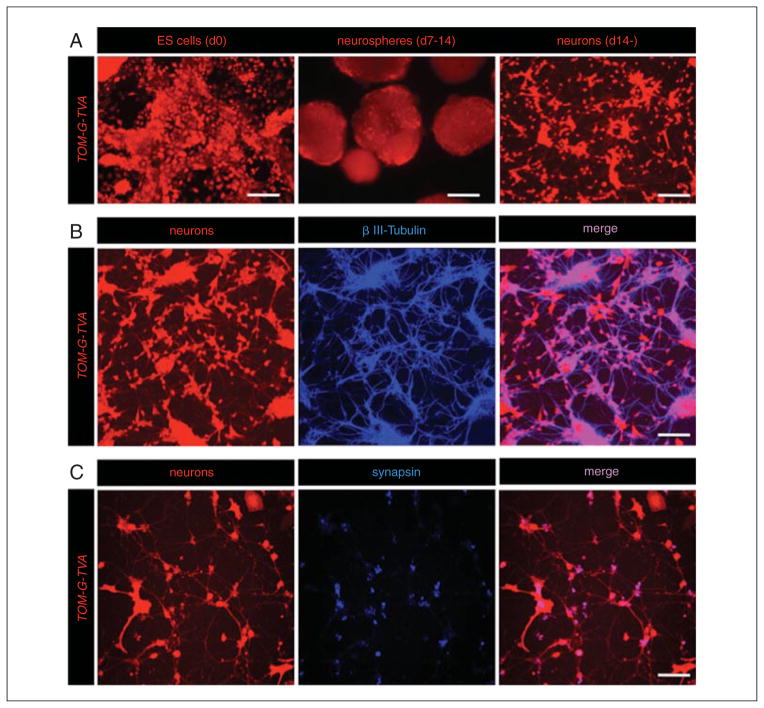
This protocol describes the high efficiency differentiation of mESCs harboring transsynaptic tracing elements into neuronal lineages (Fig. 2D.15.2), referred to as Tom-G-TVA neurons. One major advantage of using mESC cultures for neuronal differentiation is that high numbers of functional neurons can be efficiently generated using in vitro differentiation protocols. These in vitro–generated neurons can be used for the identification of synaptic connections in vitro and in vivo. This protocol can also be applied equally well to disease models, or applications that utilize cultured human induced pluripotent stem cells.
Figure 2D.15.2.
In vitro generation of modified ESC-derived neurons. (A) Morphology of Tom-G-TVA ESCs during the neuronal differentiation protocol. ESCs are grown on gelatinized tissue culture dishes (day 0). ESCs form neurospheres in suspension culture (day 7 to 14) prior to dissociation and maintenance on PDL-coated glass coverslips (scale bars 30 μm, 150 μm, and 100 μm, respectively). (B) βIII-tubulin expression pattern of ESC-derived neurons harboring viral tracing components (scale bar 60 μm). (C) Synapsin expression pattern of ESC-derived neurons (scale bar 50 μm).
Materials
mESCs harboring viral tracing elements and fluorescent marker (rabies-G, TVA receptor, tdTomato reporter, referred to as Tom-G-TVA ESCs); such lines can be generated via homologous recombination, transfection, lentiviral transduction, or electroporation
ESC medium (see recipe), prewarmed to 37°C
Gelatinized tissue culture plates (see Support Protocol 1)
Trypan blue
Neurosphere (NS) medium (see recipe), prewarmed to 37°C
Retinoic acid (Sigma)
Dimethyl sulfoxide (DMSO; ATCC)
Tissue culture grade PBS (Lonza), prewarmed to 37°C
0.05% trypsin/EDTA (Hyclone), prewarmed to 37°C
Neurobasal (NB) medium (see recipe), prewarmed to 37°C
Poly-D-lysine (PDL)-coated glass coverslips (see Support Protocol 2)
70-μm cell strainer, optional
Hemacytometer
37 °C humidified incubator with 5 % CO2
10-cm bacteriological petri dishes (Greiner, cat. no. 633102)
50-ml conical tubes
5-, 10-, and 15-ml pipets
37°C water bath to warm media and other solutions
1-ml pipet tips
Benchtop centrifuge
Additional reagents and equipment for counting cells using a hemacytometer (Phelan, 2006)
Plate ESCs for differentiation
-
1
Grow Tom-G-TVA ESCs on gelatinized tissue culture plates to ~80% confluency in ESC medium.
At this step, it is essential to ensure the health of the ESCs and strong expression of transsynaptic tracing elements as previously suggested in Basic Protocol 1. -
2
Trypsinize and dissociate ESCs as described in Basic Protocol 1 (see steps 2 to 7). If desired, pass through a cell strainer to ensure a single-cell suspension.
It is important to dissociate the ESCs as close to a single-cell suspension as possible. The following differentiation protocol depends on single ESC colonies that will form neurospheres (NS) in suspension culture. -
3
Count ESCs using trypan blue and a hemacytometer (Phelan, 2006).
-
4
Plate 4 × 106 cells onto 10-cm bacteriological petri dishes in 15 ml prewarmed NS medium.
The use of this type of bacteriological petri dish ensures that the cells will form aggregates in suspension culture instead of adhering to the bottom of the plate. Only the cells that stay in suspension and form neurospheres will be used for the remainder of the protocol. Therefore, starting with a cell rich suspension culture will generate a high yield of neuronally differentiated cells.
Differentiate ESCs into neurons
-
5
Change NS medium after 2 days of aggregation by carefully transferring the entire NS suspension into a 50-ml conical tube. Transfer using a 25-ml pipet so as not to disintegrate the neurospheres.
-
6
Let neurospheres settle undisturbed for 3 to 5 min.
Do not centrifuge the neurospheres, as they are fragile. However, they are heavy enough that they will sink rapidly, allowing for media changes without the prior need to pellet cells first. -
7
Remove the supernatant and resuspend neurospheres gently in 15 ml prewarmed NS medium by pipetting up and down twice.
Do not use tips with narrow openings. Ideally, use a pipet-aid and a 5-ml pipet. -
8
Place the cell suspension back onto new petri dishes and place the dish back into the incubator.
-
9
Change the NS medium as described above (see step 5) after 4 days. Add retinoic acid (RA) 1:1000 (15 μl per 15 ml medium used) from 5 mM stock solution (in DMSO) (final concentration in neurosphere medium is 5 μM) to NS medium and place the dish back into the incubator.
RA is light sensitive and has a quick expiration date. Only prepare small aliquots and store in dark container at −20°C. Desiccate unused RA powder at −20°C. -
10
Change the NS medium again after 6 days of aggregation as in step 9 and resuspend in prewarmed neurosphere medium containing RA. Place the dish back in the incubator for 2 days.
Dissociate the neurospheres
-
11
Wash the neurospheres twice gently with 10 ml PBS.
PBS ensures that the neurospheres are serum-free for the following dissociation step, and that the trypsin works optimally to dissociate the neurospheres. -
12
Trypsinize the neurospheres by incubating them in 1 ml prewarmed 0.05% trypsin/EDTA solution for 5 min at 37°C in the water bath. During the incubation, gently shake the conical tube a few times.
Dissociation of the neurospheres can be readily observed at this step. It is important not to dissociate the cells too rapidly or too harshly, as this destroys the neuronally differentiated cells and impairs their function in future steps. -
13
Resuspend the dissociated neurospheres in 10 ml prewarmed NS medium to inactivate the trypsin.
-
14
Dissociate neurospheres by pipetting up and down gently using a 1-ml pipet tip until no neurospheres can be observed and all cells are in a homogeneous suspension.
This should take ~30 strokes. -
15
Centrifuge the cell suspension for 5 min at 180 × g, room temperature, using a benchtop centrifuge.
-
16
Remove as much supernatant as possible without aspirating the dissociated cells.
-
17
Resuspend the cell pellet in 2 ml NB medium with B27.
Plate neuronally differentiated ESCs
-
18
Count the cells and plate them at 2 × 105 cells per cm2 on poly-D-lysine (PDL)-coated glass coverslips (see Support Protocol 2). Aspirate the medium 2 hr after plating and replace with 2 ml fresh NB medium. Repeat this step once after 24 hr. Place the plate back in the incubator after each medium change.
This step is only necessary if cells are not used for rodent transplantation or seeding onto slice cultures. Basic Protocol 3 explains the process of co-seeding ESC-derived neurons with primary cortical cultures for synapse formation and viral circuit tracing studies in vitro. Basic Protocol 4 describes the process of seeding ESC-derived neurons onto brain slices for ex vivo circuit tracing studies. -
19
Culture the TOM-G-RITVA neurons (2 × 105 cells per cm2) in neurobasal medium and change the medium every 4 days to 2 ml fresh NB medium. Place the plate back in the incubator after each medium change.
From this step on, the cells should be of neuronal identity and are referred to as TOM-G-RITVA neurons.ESC-derived neurons express markers of mature neurons as early as 7 days post-plating onto PDL-coated glass coverslips. Marker expression enriches with additional time in culture (Fig. 2D.15.2). If no neuronal marker expression is evident 14 days post-plating, the differentiation is considered unsuccessful and one should refer to the troubleshooting guide (Table 2D.15.1) for helpful hints. Once plated, ESC-derived neurons have been successfully cultured in vitro for up to 40 days.
Table 2D.15.1.
Troubleshooting Guide for Common Problems During ESC Differentiation and Viral Tracing
| Problem | Possible cause | Solution |
|---|---|---|
| Poor ESC cell growth on gelatin-coated plates | First feeder-free passage on gelatin-coated plates Bottom of culture dish is not covered with gelatin Cells are differentiating |
Passage at least three times and evaluate cell growth Ensure that gelatin-water covers bottom of dish during dish preparation Add sufficient LIF to media |
| Neurospheres attach to plate | Bacteriological petri dishes promote attachment Density of cells in suspension is too high |
Obtain petri dishes from suggested manufacturer Start suspension culture with suggested number of dissociated ESCs |
| ESCs do not differentiate into neuronal lineage with high yield | Decreased pluripotent marker expression in starting ESC culture Retinoic acid is of insufficient quality Retinoic acid was omitted |
Obtain a new batch of ESCs with high pluripotent marker expression Obtain a new batch of retinoic acid Restart suspension culture |
| ESC-derived neurons do not express GFP on RV infection | ESCs do not express sufficient amounts of fluorescent reporter, rabies-G, and TVA receptor | Verify expression via fluorescent reporter expression, ESC viral infection, and immunoblotting |
| Transsynaptic tracing is not observed | ESC-derived neurons do not make sufficient connections with host neurons | Allow a minimum of 5 days for ESC-derived neurons to form connections with primary neurons |
| Primary neuronal culture dies upon RV infection | RV is allowed to spread longer than 14 days and kills cells | Perform observation/experimentation as early as 5–7 days post RV infection but no longer than 10 days |
SUPPORT PROTOCOL 2: PREPARATION OF POLY-D-LYSINE (PDL)-COATED GLASS COVERSLIPS
The use of PDL-coated glass coverslips for the growth and maintenance of ESC-derived and primary neuronal cultures facilitates axon and dendrite outgrowth, and aids in cellular adhesion to the culture dish. Both ESC-derived and primary neurons form extensive dendritic arborizations as early as 5 days in culture on PDL-coated dishes (Fig. 2D.15.2).
Materials
Autoclaved deionized water
Poly-D-lysine (PDL) aliquots (see recipe)
Tissue culture grade phosphate-buffered saline (PBS; Lonza)
18-mm circular glass coverslips kept in 100% ethanol
Forceps, sterile
12-well tissue culture plates
Tissue culture hood with UV capability
37 °C humidified incubator with 5% CO2
-
Carefully remove a glass coverslip using sterile forceps from the ethanol storage solution and place into a well of the 12-well tissue culture plate. Repeat this step until the desired number of wells are filled with glass coverslips.
Commercially available glass coverslips are not provided sterile, which is necessary for contamination-free tissue culture procedures. Keeping coverslips in 100% ethanol prevents growth of microorganisms. Rinse the coverslips inside the wells by washing three times, each time with 2 ml deionized water. After each wash, aspirate the water.
Let the coverslips air-dry in the tissue culture hood for 30 min by removing the lid of the dish.
During the last 15 min, close the hood and turn on the UV lamp.
Take one aliquot of PDL and warm it to 37°C for 5 min.
-
Add 300 μl PDL directly onto the glass coverslip.
Ensure that the PDL covers the glass coverslip entirely. -
Incubate at least 1 hr to overnight at 37°C in the incubator.
This time frame allows for a sufficient coat of PDL to form on the glass coverslip. Aspirate the PDL and rinse once carefully with 1 ml tissue culture grade PBS.
-
Aspirate PBS and use immediately to plate neurons.
It is highly important to not let the PDL dry out in order to ensure proper adhesion and neurite formation of the newly plated cells.
BASIC PROTOCOL 3: SEEDING mESC-DERIVED NEURONS WITH PRIMARY NEURONS AND IN VITRO TRANSSYNAPTIC VIRAL TRACING
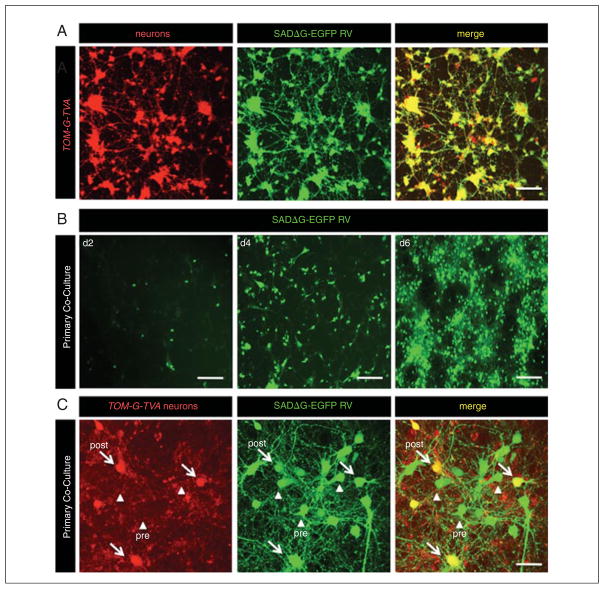
ESC-derived neurons harboring transsynaptic viral tracing elements constitute a ready source of cells for circuit tracing studies both in vitro and in vivo. Co-seeding primary cortical or hippocampal neurons with modified ESC-derived neurons allows for precise analysis of synapse and circuit formation in healthy neurons, as well as neurons obtained from disease models. Viral transduction takes place using genetically engineered rabies virus (Wickersham et al., 2007a) (Fig. 2D.15.3). In this protocol, the use of wild-type cortical neurons for in vitro transsynaptic tracing using ESC-derived neurons is outlined. This protocol can be further applied to iPSC-derived neurons from human disease models.
Figure 2D.15.3.
In vitro transsynaptic viral tracing of TOM-G-TVA ESC-derived neurons as “source cells.” (A) EGFP expression pattern of a pure culture of ESC-derived neurons 5 days post-infection with SADΔG-EGFP RV (scale bars 100 μm). (B) EGFP expression pattern of primary neuronal co-culture 2, 4, and 6 days post RV infection. Initially, only “source cells” are infected, rapidly followed by synaptically connected primary neurons (scale bars 120 μm). (C) Confocal image of in vitro transsynaptic tracing showing “source cells” (yellow) and presynaptically connected primary neurons (green) 5 days post RV infection (scale bar 20 μm).
Materials
Dissection medium (DM; see recipe), ice-cold
Digestion solution (DS; see recipe), prewarmed to 37°C
2.5% trypsin (Hyclone), prewarmed to 37°C
Timed-pregnant C57BL/6 females (pups E18 on dissection day)
Isoflurane, optional
DNase aliquots (see recipe), prewarmed to 37°C
Trypsin inhibitor (Sigma)
Trypan blue
Neurobasal (NB) medium (see recipe), prewarmed to 37°C
PDL-coated glass coverslips (see Support Protocol 2)
FUdR (see recipe)
SADΔG-EGFP rabies virus (produced as described in Wickersham et al., 2007a,b)
TOM-G-RITVA ESC-derived neurons (see Basic Protocol 2)
37°C water bath to warm media and other solutions
Sterile sharp and blunt forceps and scissors for dissection
10-cm tissue culture dishes
0.20-μm syringe filter
Centrifuge
37 °C humidified incubator with 5% CO2
1-ml pipet tips
Hemacytometer
12-well plates
50-ml conical tubes
Additional reagents and equipment for euthanizing the mice (Donovan and Brown, 2006) and counting cells using a hemacytometer (Phelan, 2006)
Dissect brain tissue
-
1
Prewarm 8 ml DM, DS, and 2.5% trypsin in a water bath at 37°C for 10 to 15 min.
-
2
Anesthetize mice with isoflurane or sacrifice via cervical dislocation (see Donovan and Brown, 2006).
Ensure that the time from sacrificing the pregnant dam until the following dissection step are minimized, as this greatly impacts the yield of healthy primary neuronal cultures. -
3
One by one, using sterile forceps and scissors, quickly remove pups from the embryonic sac and decapitate so that the heads fall into ice-cold DM in a 10-cm tissue culture dish.
-
4
Hold the pup head down through the eyes using sterile forceps and carefully tease apart the skin and open the skull to expose the brain. Scoop the brain out using blunt forceps and place into a new 10-cm dish with ice-cold DM.
-
5
Hold the brain by the stem using forceps and separate the hemispheres.
-
6
Remove the vessel membrane (pia mater) from the cortex. Turn the hemisphere over to expose the hippocampus. If the hippocampus is desired, collect it from six pups. If the cortex is desired for primary cultures, collect cortices from the same number of animals.
Depending on the desired type of primary cultures and experimental design, hippocampi and cortices can be used equally well. Using six animals ensures that sufficient live and healthy neurons will be present for plating post cellular dissociation.
Dissociate brain tissue
-
7
Combine DS with trypsin, add two aliquots of DNase, mix, and filter through a 0.20-μm syringe filter.
The addition of DNase ensures that the dissociation of primary neurons will not cause the solution to become too viscous to work with. -
8
Centrifuge the pieces of dissected brain for 2 min at 180 × g, room temperature.
-
9
Gently remove the supernatant.
Do not aspirate the supernatant completely to avoid inadvertently aspirating the pellet. -
10
Add 5 to 6 ml prewarmed trypsin solution to the brain pieces. Swirl the tubes to break up the pellet.
-
11
Incubate for 8 min at 37°C.
-
12
During the incubation, dissolve 50 mg trypsin inhibitor in 8 ml prewarmed DM. Mix and syringe filter.
-
13
Centrifuge the digested pieces of hippocampus or cortex for 2 min at 180 × g, room temperature. Remove the supernatant.
-
14
Add 5 ml trypsin inhibitor solution. Swirl the tube to gently break up the pellet.
-
15
Incubate for 2 min at 37°C.
-
16
Centrifuge the solution for 2 min at 180 × g, room temperature. Remove the supernatant.
-
17
Wash the pellet with 10 ml ice-cold DM. Resuspend by swirling. Do not pipet up and down.
-
18
Centrifuge as above and remove the supernatant.
-
19
Add one aliquot of DNase to 5 ml ice-cold DM. Mix and syringe filter.
-
20
Resuspend the pellets in 3.5 ml DM/DNase solution. Triturate gently by pipetting up and down 30 times with a 1-ml pipet tip. Avoid forming bubbles.
-
21
Centrifuge the cell suspension for 10 min at 180 × g, room temperature.
-
22
Resuspend pellets in 10 ml ice-cold DM. Count the cells using trypan blue and a hemacytometer. Keep the cell suspension on ice during this time.
-
23
Calculate the number of cells/ml.
Co-seed primary neurons with ESC-derived neurons
-
24
Add 1.5 ml cold NB medium into each well of a 12-well plate with PDL-covered glass coverslips.
-
25
Prepare a cell suspension so that 0.5 ml contains 2 × 105 primary neurons together with 0.5% ESC-derived neurons (generated in Basic Protocol 2) per well.
-
26
Add 0.5 ml of the cell suspension into each well and place the plate into the incubator.
This will provide a total volume of 2 ml per well. Use 2 ml in every subsequent medium change. -
27
Change the medium to new NB medium after 24 hr and place the dish back into the incubator.
Change the medium shortly after seeding primary neurons to remove dead cells and cellular debris that could contaminate the culture. -
28
Replace the medium every 4 days and watch for glial growth.
-
29
After day 7, add FUdR to prewarmed NB medium to stop cell division and inhibit glial cell growth.
-
30
Once extensive dendritic arborizations are visible, which will take ~10 to 14 days post-seeding, transduce the culture with low-titer SADΔG-EGFP rabies virus (6 × 103 viral particles) by directly adding the virus to the tissue culture plate containing the same medium from the previous medium change. Change the medium after 24 hr and place the plate back into the incubator.
-
31
Observe transsynaptic tracing using GFP expression in red and green “source cells,” as well as presynaptic primary neurons (green only, Fig. 2D.15.3).
BASIC PROTOCOL 4: SEEDING mESC-DERIVED NEURONS ONTO BRAIN SLICES FOR TRANSSYNAPTIC VIRAL TRACING
In vitro neuronal tracing studies can help elucidate valuable information about synaptogenesis and circuit formation using primary neuronal cultures in both healthy and disease model neurons. However, in order to determine precise patterns of synaptic connectivity in vivo, slice explants and neuronal transplantation directly onto brain tissue provide a more tractable approach. In this protocol, we describe the generation of brain slice explants and the subsequent transplantation of modified ESC-derived neurons for transsynaptic viral tracing studies. This protocol can be used for the generation of slice explants from healthy and diseased rodent brain tissue, combined with transsynaptic circuit analysis.
Materials
C57BL/6 wild-type mice or any disease model of interest
Isoflurane
10-ml syringe (28-G needle) filled with ice-cold GBSS
Modified Gey’s balanced salt solution (GBSS; see recipe), ice-cold
Slice culture medium (see recipe), prewarmed to 37°C
Neurobasal (NB) medium (see recipe), prewarmed to 37°C
TOM-G-RITVA ESC-derived neurons (see Basic Protocol 2)
SADΔG-EGFP rabies virus (produced as described in Wickersham et al., 2007a,b)
Dissecting tray
Sterile dissection scissors, bone scissors, and blunt and sharp forceps
Vibratome (Leica)
30-mm Millicell-CM culture membranes (Millipore)
6-well tissue culture plates
37 °C, 5% CO2 incubator
Dissect brain tissue
-
1
Deeply anesthetize the mouse with isoflurane.
-
2
Secure the mouse to the bottom of a tray, ventral side facing up.
-
3
Locate the sternum and make a central incision to expose the heart using sterile dissection scissors. Snip the right atrium using sterile dissection scissors to allow for drainage of blood.
-
4
Lightly pierce the left ventricle with the 10-ml syringe and slowly perfuse the mouse with the ice-cold GBSS.
During a good perfusion, the liver will visibly lighten and dark blood will leave the right atrium. The purpose of this step is to cool the brain and keep the tissue healthy during the dissection procedure. -
5
Make a cross-length incision using dissecting scissors at the base of the neck and remove the skin from the skull.
-
6
Using bone scissors, make a deep incision into the bone between the two orbits, followed by a length-wise incision from the ear canals to the eye socket, bilaterally. Locate the lambda suture on the skull and superficially cut alongside it. Locate the central suture and cut alongside it.
The skull plates should now be easily removable using forceps. -
7
Invert the animal. The brain will loosen from the olfactory bulbs. Using sharp forceps, carefully dissect the optic and cranial nerves, followed by the brain stem/spinal cord.
-
8
Quickly place the brain in ice-cold GBSS.
The entire dissection procedure should be performed quickly in order to ensure the health of the neuronal tissue.
Culture the brain slices
-
9
Cut transverse slices (300 μm) with a Vibratome. Collect the brain slices in GBSS.
The region of the brain to be removed for subsequent slice culture highly depends on the experiment to be performed. In Garcia et al. (2012), primary slice cultures were made from the midbrain, including regions of the striatum and hypothalamus, although olfactory bulb and cerebellar cultures are possible using this technique. -
10
Place the desired slices onto Millicell-CM culture membranes (Millipore) and transfer to 6-well plates filled with 1.2 ml slice culture medium.
Avoid adding too much culture medium, as this will cause the slices to float. Slices will need to adhere to the culture medium for optimum health. -
11
Incubate the cultures in 5% CO2, 95% atmospheric air at 37°C.
-
12
After 3 days, change the medium to prewarmed NB/B27.
Seed brain slice explants with ESC-derived neurons
-
13
Seed slices with 1 × 104 TOM-G-RITVA ESC-derived neurons by gently removing the desired number of cells from suspension and placing directly onto brain slices.
-
14
Transduce the slices with low-titer SADΔG-EGFP rabies virus (6 × 103 viral particles) 5 days post-seeding as described in Basic Protocol 3, step 30.
This time frame ensures that dendritic arborizations are formed by the seeded neurospheres, and that synaptic connections are established.Transsynaptic tracing can be observed as early as 3 days post-infection. -
15
Change the culture medium to fresh NB/B27 medium every 3 days for the duration of the experiment.
Slice cultures can be kept alive for 2 to 4 weeks.
REAGENTS AND SOLUTIONS
For culture recipes and steps, use sterile tissue culture-grade water and perform all steps in a tissue culture hood.
Digestion solution (DS)
176 mg NaHCO3 (4.3 mM)
2.98 mg HEPES (25 mM)
4 g NaCl (137 mM)
186 mg KCl (5 mM)
1.25 g Na2HPO4 (7 mM)
Add deionized water to 500 ml
Filter through 0.45-μm bottle filter
Store up to 60 days at 4°C
Dissection medium (DM)
250 ml HBSS+
600 mg (10 mM) HEPES
3 g (33.3 mM) glucose
25 μl gentamicin (50 mg/ml; Lonza)
Filter through a 0.45-μm bottle filter
Store up to 60 days at 4°C
DNase aliquots
Dissolve DNaseI (Sigma, cat. no. D5025) in 50 mlHBSS+
Sterilize by filtration
Divide into 1-ml aliquots
Store up to 1 year at −20°C
Embryonic stem cell (ESC) medium
500 ml G-MEM (Lonza)
50 ml fetal bovine serum (FBS; Invitrogen)
5 ml GlutaMAX (Invitrogen)
5 ml penicillin/streptomycin (Corning, cat. no. 30-001-CL)
5 ml sodium pyruvate (Invitorgen)
0.5 ng/ml LIF (Millipore)
560 μl of 1 M 2-Mercaptoethanol
Filter through a 0.45-μm bottle filter
Store up to 30 days at 4°C
FUdR stock solution (1000×)
25 mg (10 mM) FUdR (Sigma, cat. no. F-503)
24 mg (10 mM) uridine (Sigma, cat. no. U-3003)
Add deionized water to 10 ml
Filter through a 0.45-μm bottle filter
Store up to 60 days at 4°C
Hanks plus (HBSS+)
500 ml HBSS (Lonza)
600 mg (10 mM) HEPES
3 g (33.3 mM) glucose
25 μl gentamicin (50 mg/ml; Lonza)
Filter through 0.45-μm bottle filter
Store for up to 60 days at 4°C
Modified Gey’s balanced salt solution (GBSS)
500 ml GBSS (Lonza)
2.5 g D-glucose
5 ml of 3 M KCl
10 μl gentamicin (50 mg/ml; Lonza)
Store up to 6 months at 4°C
Neurobasal (NB) medium
500 ml Neurobasal medium (Invitrogen)
25 ml fetal bovine serum (FBS; Invitrogen)
5 ml GlutaMAX (Invitrogen)
10 ml B27 supplement (Invitrogen)
10 μl gentamicin (50 mg/ml; Lonza)
Filter through a 0.45-μm bottle filter
Store up to 30 days at 4°C
Neurosphere (NS) medium
500 ml G-MEM (Lonza)
50 ml fetal bovine serum (FBS; Invitrogen)
5 ml GlutaMAX (Invitrogen)
5 ml penicillin/streptomycin (Corning, cat. no. 30-001-CL)
5 ml sodium pyruvate (Invitrogen)
560 μl of 1 M 2-mercaptoethanol
Filter through a 0.45-μm bottle filter
Store up to 30 days at 4°C
Poly-D-lysine (PDL) (500 ml of 1 mg/ml stock)
Dissolve poly-D-Lysine (Sigma, cat. no. P0899) in 500 ml tissue culture grade ddH2O, mix well in the original water bottle. Sterilize by filtration, divide into 300-μl aliquots. Store up to 1 year at −80°C.
Slice culture medium
250 ml MEM (Lonza)
125 ml horse serum (Hyclone)
125 ml HBSS
30 mM D-glucose
7.5 % NaHCO3
0.01% ascorbic acid
10 μl gentamicin (50 mg/ml; Lonza)
Store up to 30 days at 4°C
COMMENTARY
Background Information
The discovery of adult neurogenesis in the mammalian brain fifty years ago opened many new avenues of inquiry into the survival, differentiation, and integration of new neurons into existing brain circuitry. For the first time, a model of continued circuit integration after birth allows for the precise investigation of cues necessary for circuit formation and synaptogenesis in the adult brain. This phenomenon provides valuable information towards the development of novel cell-based therapeutic approaches for damaged or diseased nervous tissue. One major challenge towards cell replacement therapies in the adult brain is that, unlike in the embryonic milieu of large-scale organogenesis, adult neurons must recognize and respond to cues for integration into a specific locale within a pre-defined synaptic network. Complicating matters, the precise synaptic networks into which adult neurons integrate are themselves only coarsely defined. With advancements in microscopy, a growing panel of immunohistochemical markers, and the development of innovative molecular strategies to manipulate neurons, the ability to ascertain the ultimate fate of transplanted neurons in the adult brain is within reach. These endeavors can be accelerated by the availability of straightforward protocols describing technical approaches and potential applications of combined imaging, histological, and molecular strategies in studies investigating the underlying mechanisms of circuit integration and synapse formation.
In recent times, it has become possible to manipulate and differentiate ESCs into diverse cellular subtypes. ESC-derived neurons have become highly valuable in both in vitro and in vivo studies of neurodegenerative disease models. Moreover, ESC-derived neurons provide a renewable source of neurons for studies investigating novel cell-based therapeutic approaches. Further, it is now possible to reprogram adult tissue into pluripotent stem cells, termed induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006; Takahashi et al., 2007), and to differentiate these cells into neuronal subtypes, in efforts to develop cell-based therapies using patient-derived tissues. However, one major limitation in the advancement of cell-based therapies for degenerative neurological diseases stems from a lack of knowledge about the precise circuitries involved in pathogenesis. Therefore, in order to realize the goal of cell-based therapies for neurodegenerative diseases, it is important to target and manipulate new neurons in the adult brain, in hopes of understanding the mechanisms of their survival and integration.
Toward uncovering the precise circuitries in diverse regions of the brain, Wickersham et al. (2007a,b) developed a novel transsynaptic viral tracing strategy using genetically engineered rabies virus (RV). RV is a negative strand enveloped RNA virus that travels retrogradely to presynaptic neurons. Two major modifications took place to make RV useful for neuronal tracing experiments. First, the glycoprotein G, which mediates infectivity and transsynaptic spread, was replaced with the cDNA encoding EGFP, thereby marking infected cells GFP positive. Next, the RV envelope was modified for expression of the ENVA protein, which confers specificity of infection only to neurons ectopically expressing the avian TVA receptor, whose target cell surface receptor is not normally found on mammalian cells. Therefore, by expressing the TVA receptor, neurons can be engineered as specific targets of RV and, by harboring the cDNA for glycoprotein G, they also enable a single jump of RV particles to the presynaptic inputs of the targeted neuron. Thus, such neurons serve as “source cells” for transsynaptic viral tracing elements.
The protocols in this unit describe the modification and manipulation of mouse embryonic stem cells to obtain neural progenitor cells that integrate into existing brain circuitry, as reported by Garcia et al. (2012). Others have reported various specialized protocols for the generation of neural progenitors, including subtype-specific neural cells, and the transplantation of derived neural precursors into animal models of brain injury or disease (Bissonnette et al., 2011; Caiazzo et al., 2011; Cho et al., 2011). Additionally, reports have described genetic manipulations of neurons in in vitro models of neurodegenerative disease to study specific dysfunction in the relevant circuits (Tonnesen et al., 2011). The present protocols extend these studies to functional analysis of transplanted neural progenitors in vivo. Successful integration of these cells in vitro and in vivo can be assessed by a variety of means, including morphological analysis, neurochemical profiling, and electrophysiological measurements. We include details on how to apply this technology in conjunction with other methods, such as rabies virus circuit tracing (Wickersham et al., 2007a,b; Callaway, 2008), in order to deepen investigations of the synaptic networks into which neural stem cells terminally integrate.
These protocols introduce one possible application of a combined molecular approach to understanding the mechanisms underlying synaptic integration of mature neurons in the adult mammalian brain. Potential applications of this basic approach are broad and include other genetic modifications of neural stem cells, including light- or xenobiotic-responsive membrane ion channels that enable in vivo circuit manipulations in the brains of animal models. Such manipulations will provide new tools for the investigation into both structural and functional connectivity of neurons at unparalleled levels of control and resolution within living brain tissue. These studies could provide much-needed groundwork to translate basic understanding of normal synaptic development in the adult brain to clinically relevant applications, including progress towards cell-based replacement therapies for neurodegenerative disease.
Critical Parameters and Troubleshooting
ESC and iPSC culture heavily depends on the use of healthy and rapidly dividing cells, free from contamination. In order to ensure the successful differentiation of ESCs and iPSCs into neuronal lineages using suspension culture, it is imperative to examine the starting culture prior to differentiation. Through morphological examination and molecular analysis of stem cells marker expression (e.g., OCT4, SOX2, KLF, C-MYC), ESCs and iP-SCS should be verified to be healthy and in an undifferentiated state. Furthermore, to ensure that healthy and rapidly dividing cells are used for the outlined protocols, cells should be maintained at high percentage confluence.
ESCs and iPSCs can be genetically modified to express constructs via a variety of methodologies, which include homologous recombination of a targeting vector [the preferred method by Garcia et al. (2012) to ensure stable protein expression], electroporation, viral transduction, or stable transfection. Whichever method is used to express the viral targeting and reporter constructs, verification of protein expression is recommended. If ESCs and iPSCs express the targeting construct, fluorescent marker expression should be readily visible and cells should be susceptible to transduction by the modified RV variant SADΔG-EGFP (Fig. 2D.15.1).
Healthy ESCs strongly expressing transsynaptic tracing elements are grown in suspension culture in the absence of leukemia inhibitory factor prior to addition of retinoic acid, which initiates differentiation into neuronal lineages. During this step, it is critical to form single-cell suspensions that give rise to individual neurospheres. During the differentiation process, precise timing of media changes and addition of retinoic acid are essential to ensure the sufficient differentiation of ESCs and iPSCs into neuronal stem cells and/or functional neurons.
Using primary neuronal cultures and slice explants to study in vitro and ex vivo circuit integration provides convincing evidence that genetically modified ESC-derived neurons can be used for in vivo circuit tracing experiments. To ensure that the primary culture and slice explant experiments succeed, it is essential to maintain the cultures free of contaminants and perform frequent media changes in a sterile hood.
Upon circuit integration and synapse formation, genetically modified ESCs and iPSCs harboring rabies-G, TVA, and a fluorescent reporter can be used as “source cells” for transsynaptic tracing studies (Garcia et al., 2012). In order to ensure effective circuit tracing, ESC-derived neurons must be allowed sufficient time to form dendritic arborizations and synaptic connections. This is especially important when cells are transplanted in vivo. Upon viral infection, neurons rapidly generate high amounts of RV particles, which label infected cell green and jump to presynaptic neurons within days. The most efficient viral tracing is observed at 7 days post-infection. Infected neurons remain healthy for up to 10 days post-infection, making it possible to perform further circuit analysis.
Anticipated Results
This unit describes protocols for the efficient generation of genetically modified ESC-derived neurons and the use of these neurons in circuit tracing studies in vitro and in vivo. If followed accordingly, one can generate high numbers of ESC-derived neurons with 90% purity. As in most in vitro differentiation protocols, a small number of glial cells will be observed in the culture. However, these cells are minimal and will not contaminate the experimental results. Further, use of the chemotherapeutic FUdR in culture media can help suppress glial proliferation. Upon RV infection, presynaptic connections are rapidly identified via strong and reliable EGFP expression. Although we have tested the ESC-derived neurons in co-culture and slice explants from wild-type tissue, we believe the protocols described here are very well suited for use in neuronal disease models.
Time Considerations
Prior passaging of genetically modified ESCs harboring tracing elements should be performed to ensure the health and undifferentiated state of the ESC culture. If frozen, cells should be passaged at least twice before undergoing differentiation (3 days per passage). The differentiation protocol requires a total of 14 days in suspension culture before ESC-derived neurons can be plated or transplanted. Upon plating, it is highly suggested to allow sufficient time for dendritic arborizations and synapse formation prior to RV infection. Five days should be a minimum timeframe allowed to visualize synaptogenesis. After RV infection, transsyanptic tracing is observed as early as 3 to 5 days post-infection, with optimal transsynaptic viral spread observed at 7 days. Thus, from beginning of differentiation to the final viral circuit tracing result, ~4 weeks is necessary to execute this protocol.
Acknowledgments
This work was supported through the McNair Medical Institute, NINDS award 1F31NS081805 to I.G. and NINDS 1R01NS078294 to B.R.A.
Literature Cited
- Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–811. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EG, Zaremba JD, McKercher SR, Talantova M, Tu S, Masliah E, Chan SF, Nakanishi N, Terskikh A, Lipton SA. MEF2C enhances dopaminergic neuron differentiation of human embryonic stem cells in a parkinsonian rat model. PLoS One. 2011;6:e24027. doi: 10.1371/journal.pone.0024027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J, Brown P. Euthanasia. Curr Protoc Immunol. 2006;73:1.8.1–1.8.4. doi: 10.1002/0471142735.im0108s73. [DOI] [PubMed] [Google Scholar]
- Garcia I, Huang L, Ung K, Arenkiel BR. Tracing synaptic connectivity onto embryonic stem cell-derived neurons. Stem Cells. 2012;30:2140–2151. doi: 10.1002/stem.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC. Techniques for mammalian cell tissue culture. Curr Protoc Mol Biol. 2006;74:A.3F.1–A.3F.18. doi: 10.1002/0471142727.mba03fs74. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Parish CL, Sorensen AT, Andersson A, Lundberg C, Deisseroth K, Arenas E, Lindvall O, Kokaia M. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One. 2011;6:e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods. 2007a;4:47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007b;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]