Abstract
Purpose
Bladder cancer is frequently diagnosed during a workup for hematuria. However, most patients with microscopic hematuria and many with gross hematuria are not appropriately referred to urologists. We hypothesized that in patients presenting with asymptomatic hematuria, the risk of having bladder cancer can be predicted with high accuracy. Towards this end, we analyzed risk factors in patients with asymptomatic hematuria and developed a nomogram for the prediction of bladder cancer presence.
Methods
Data from 1,182 consecutive subjects without a history of bladder cancer undergoing initial evaluation for asymptomatic hematuria were collected at three centers. Clinical risk factors including age, gender, smoking status, and degree of hematuria were recorded. All subjects underwent standard workup including voided cytology, upper tract imaging, and cystourethroscopy. Factors associated with the presence of bladder cancer were evaluated by univariable and multivariable logistic regression analyses. The multivariable analysis was used to construct a nomogram. Internal validation was performed using 200 bootstrap samples.
Results
Of the 1,182 subjects who presented with asymptomatic hematuria, 245 (20.7%) had bladder cancer. Increasing age (OR=1.03, p<0.0001), smoking history (OR=3.72, p<0.0001), gross hematuria (OR=1.71, p=0.002), and positive cytology (OR=14.71, p<0.0001) were independent predictors of bladder cancer presence. The multivariable model achieved 83.1% accuracy for predicting the presence of bladder cancer.
Conclusions
Bladder cancer presence can be predicted with high accuracy in patients who present with asymptomatic hematuria. We developed a nomogram to help optimize referral patterns (i.e., timing and prioritization) of patients with asymptomatic hematuria.
Keywords: urinary bladder neoplasms, hematuria, nomograms, early detection of cancer, carcinoma
Introduction
It is estimated that there will be 70,530 new cases and 14,680 deaths from bladder cancer in 2010 in the USA [1]. Risk factors for the development of bladder cancer include advanced age, male gender, tobacco use, and occupational exposures [2,3,4]. In most patients, bladder cancer is diagnosed during a workup for either microscopic or gross hematuria. All patients with gross hematuria should undergo a complete urologic evaluation, since approximately 10% will have bladder cancer [5]. The positive predictive value for microscopic hematuria is lower, between 2% and 5%. Approximately 9–18% of the general population will have some degree of hematuria during their lifetime [6]. As a result, guidelines for evaluation of asymptomatic microscopic hematuria are not universally agreed upon. The American Urological Association best-practice policy recommends cystourethroscopy for all patients with microscopic hematuria aged >35 years and for those aged <35 years with risk factors [7]. Others have recommended more restrictive criteria for identifying a subset of patients presenting with microscopic hematuria who should undergo urologic evaluation [8].
Alarmingly, many patients with hematuria are not appropriately referred to urologists for evaluation. A survey of 788 primary care physicians revealed that only 36% reported referring patients with microscopic hematuria to a urologist [9]. Even in patients with gross hematuria, referral rates were only 69–77%. An analysis of 926 consecutive patients with newly diagnosed hematuria from a health plan database revealed that only 47% of men and 28% of women were referred for urologic evaluation [10].
It has been demonstrated that an individual patient’s risk for bladder cancer depends on several factors. However, few studies have evaluated the combined effects of these factors [11,12]. Currently there are no models to quantify an individual’s risk of having bladder cancer after presenting with asymptomatic hematuria. To address this, we conducted a multi-institutional study of patients with asymptomatic hematuria to assess risk factors for bladder cancer and developed an internally validated predictive tool for clinical use.
Materials and Methods
Patients
This institutional review board-approved study was performed at three sites: EuromedClinic/Urologie24 (Fürth/Nürnberg, Germany), University of Tübingen (Tübingen, Germany), and General Hospital of Bolzano (Bolzano, Italy). Between 2000 (Fürth, n=434), 2002 (Bolzano, n=439), 2006 (Tübingen, n=309) and 2010, 1,182 consecutive patients with newly diagnosed asymptomatic hematuria without a history of bladder cancer were included.
Microscopic hematuria was defined as three or more erythrocytes per high-power field (HPF) under white light microscopy from two of three properly collected urine specimens [6]. Mid-stream urine specimens were collected, immediately processed, and examined cytologically. Cytologic examination was performed by trained personnel at all three sites, with an experience of more than 3000 examinations per year. Negative and atypical cytology results were considered negative, while suspicious and positive results were considered positive. All patients underwent clinical examination including upper tract imaging and cystourethroscopy with biopsy of any suspicious lesions; they were considered positive for malignancy if one or more tumors were detected during initial cystourethroscopy or within the subsequent 3 months. Bladder cancer was diagnosed based on histological examination. All tumors were primary urothelial carcinoma. Surgical specimens were processed in accordance with standard pathological procedures. Histology and urinary cytology slides were reviewed at each institution with no knowledge of the clinical data. Genitourinary pathologists assigned pathologic stage according to the 2002 American Joint Cancer Committee TNM staging system and tumor grade according to the 2004 World Health Organization grading system.
Statistics
Descriptive statistics were calculated for the study cohort. Univariable and multivariable logistic regression analyses were performed to evaluate the association between bladder cancer and age, gender, smoking status (past/current vs. never), degree of hematuria (gross vs. microscopic), and cytology result (positive vs. negative). All five variables were included in the final multivariable model. Odds ratios (OR) and 95% confidence intervals (95% CI) were estimated from the models. The area under the curve (AUC) method was used to quantify the predictive accuracy of each variable on univariable analysis and the combined multivariable model. All AUC estimates were internally validated using 200 bootstrap samples [13,14]. The DeLong test was used to evaluate the increment in AUC achieved by adding cytology result to a multivariable model inclusive of age, gender, smoking status, and degree of hematuria. Regression coefficients from the multivariable model were used to generate a predictive nomogram [15]. Finally, a calibration plot was fitted to evaluate the extent of over- or under-estimation of the observed bladder cancer rate. All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SAS Version 9.2 (SAS Institute Inc., Cary, NC). The nomogram and calibration plot were constructed in R Version 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
The entire cohort included 919 men and 263 women (male-to-female ratio, 3.5). The median age was 65 years (range 18 – 93). Sixty-eight percent of subjects presented with microscopic hematuria, and 32% with gross hematuria. Of the 1182 subjects, 162 (13.7%) had a positive cytology result. Overall, 245 of 1182 (20.7%) subjects had bladder cancer; 138 (58.7%) had low-grade tumors and 97 (41.3%) had high-grade tumors. Stage distribution of the tumors was as follows: 12 (4.9%) PUNLMP, 148 (60.4%) pTa, 13 (5.3%) pTis, 44 (18.0%) pT1, 20 (8.2%) pT2, and 6 (2.4%) pT3.
Univariable logistic regression analyses identified older age (OR = 1.04, p < 0.0001), male gender (OR = 1.49, p = 0.03), past/current smoking history (OR = 3.38, p < 0.0001), gross hematuria (OR = 2.47, p < 0.0001), and positive urine cytology (OR = 16.12, p < 0.0001) as predictors of bladder cancer (Table 1). Urine cytology had the highest AUC (70.6%), followed by age (64.7%) and smoking status (64.6%).
Table 1.
Univariable and multivariable logistic regression analyses assessing the association between predictor variables and the presence of bladder cancer in 1182 patients.
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors of Bladder Cancer | OR | 95% CI | P-Value | AUC (%) | OR | 95% CI | P-Value | AUC (%) |
| Age (continuous) | 1.04 | (1.03, 1.06) | <0.0001 | 64.7 | 1.03 | (1.02, 1.05) | <0.0001 | 83.1% |
| Gender (male vs. female) | 1.49 | (1.04, 2.15) | 0.03 | 52.3 | 1.10 | (0.72, 1.68) | 0.66 | |
| Smoker (past/current vs. never) | 3.38 | (2.49, 4.58) | <0.0001 | 64.6 | 3.72 | (2.58, 5.37) | <0.0001 | |
| Hematuria (gross vs. microscopic) | 2.47 | (1.85, 3.30) | <0.0001 | 60.3 | 1.71 | (1.21, 2.41) | 0.002 | |
| Cytology (positive vs. negative) | 16.12 | (10.98, 23.66) | <0.0001 | 70.6 | 14.71 | (9.70, 22.28) | <0.0001 | |
AUC = area under the curve, CI = confidence interval, OR = odds ratio
AUC estimates are based on internal validation using 200 bootstrap samples.
In the multivariable analysis, age (OR = 1.03, p < 0.0001), past/current smoking history (OR = 3.72, p < 0.0001), gross hematuria (OR = 1.71, p = 0.002), and positive urine cytology (OR = 14.71, p < 0.0001) were independent predictors of the presence of bladder cancer (Table 1). The bootstrap-corrected AUC (based on 200 bootstrap samples) of the multivariable model was 83.1%. The addition of cytology to a multivariable model inclusive of age, gender, smoking status, and degree of hematuria increased the AUC of the multivariable model from 73.8% to 83.1%, a gain of 9.3% (p < 0.0001).
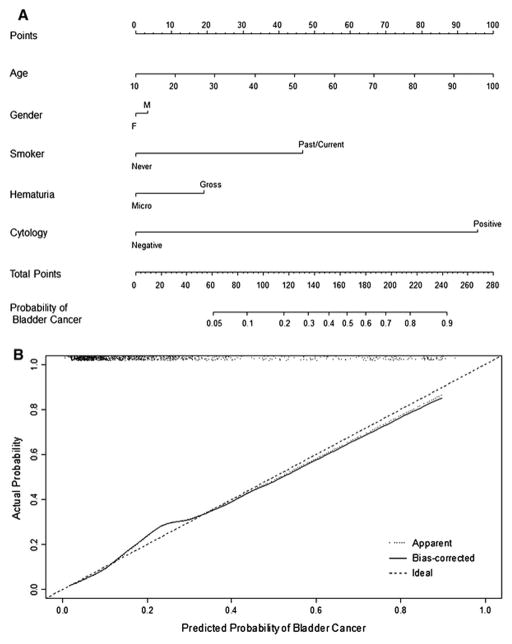
Figure 1A shows the multivariable nomogram, where age, gender, smoking status, degree of hematuria, and urine cytology define the risk of having bladder cancer. There is a linear increase in the risk of bladder cancer at cystourethroscopy with advancing age. With respect to bladder cancer risk, male gender contributes 3.5 risk points (not significant), gross hematuria contributes 19 risk points, past/current smoking history contributes 46 risk points, and positive urine cytology contributes 95 risk points.
Figure 1.
Figure 1A. Nomogram for the prediction of bladder cancer presence in patients with asymptomatic hematuria.
In this nomogram, age, gender, smoking history, degree of hematuria, and urine cytology define the risk of bladder cancer at cystourethroscopy. Nomogram instructions: To obtain the nomogram-predicted probability of bladder cancer at cystourethroscopy, locate patient values on each axis. Draw a vertical line to the ‘Points’ axis to determine how many points are attributed for each variable value. Sum the points for all variables. Locate the sum on the ‘Total Points’ line to assess the individual probability of bladder cancer at cystourethroscopy on the ‘Probability of Bladder Cancer’ line.
Figure 1B. Calibration plot.
In this calibration plot, the x-axis denotes the predicted probability and the y-axis denotes the observed fraction of bladder cancer. The 45° dashed line represents ideal predictions, while the solid line represents the internal validation (bias-corrected). The dotted line represents the uncorrected internal validation (apparent). The AUC was 83.1% and there was slight under- and over-estimation of the proportion of bladder cancer. The scatter plot at the top of the figure shows the distribution of the individual subject nomogram-predicted probabilities.
The calibration plot in Figure 1B shows the performance characteristics of the multivariable model for predicting bladder cancer. The minimal underestimation increases for individuals with a nomogram-predicted probability of >15% and reaches a maximum for those with a predicted probability of 24%. There is slight overestimation for individuals with a nomogram-predicted probability ≥40%. The scatter plot on the top of Figure 1B demonstrates that the majority of subjects had relatively low predicted probabilities of bladder cancer. Specifically, 75% of subjects had a predicted probability of bladder cancer below 25%.
Discussion
The early detection and timely treatment of bladder cancer is critical [16,17]. In the Western world, approximately 25% of patients with bladder cancer present with muscle-invasive disease at diagnosis. If bladder cancers can be detected while confined to the mucosa or lamina propria, they can often be successfully treated via relatively nonmorbid means with good survival estimates and quality of life. Screening for bladder cancer is not currently recommended, mainly due to the low prevalence of the disease [18]; therefore, most patients are diagnosed after presenting with hematuria. However, hematuria is fairly common, given that an estimated 9–18% of the population will present with hematuria during their lifetime [6]. The high prevalence of hematuria and lack of awareness have resulted in low or delayed referral of patients with asymptomatic hematuria to urologists [9,10]. Another possibility for these suboptimal referral patterns is a perceived small chance of malignancy in patients with microscopic hematuria. Increased education of primary care physicians with quantification of bladder cancer risk may result in improved referral rates.
Delays in the diagnosis and treatment of bladder cancer have been shown to adversely affect survival [19]. One study demonstrated that delay from onset of symptoms to referral is associated with advanced tumor stage and decreased survival [20]. Recently, it was demonstrated that a delay in the diagnosis of bladder cancer increased the risk of death from disease independent of tumor stage and grade [21]. Identification of those patients with asymptomatic hematuria at highest risk of bladder cancer may increase awareness of bladder cancer risk, enabling more timely referral and diagnosis.
We developed a highly accurate, well-calibrated nomogram to predict the individual risk of bladder cancer for a patient presenting with asymptomatic hematuria. This is to be distinguished from rate ratios or sensitivity thresholds that apply to groups of patients [22]. The accuracy of this tool compares favorably to that of others commonly used in medicine for clinical decision making regarding risk of cancer and need for diagnostic procedures [23]. Utilization of this nomogram will assist primary care physicians and other healthcare practitioners in determining a patient’s risk of bladder cancer. Indeed, the risk of bladder cancer is nontrivial even in patients with seemingly few risk factors. A hypothetical 60-year old male patient with a history of smoking who presents with microscopic hematuria and negative urine cytology has approximately a 17.5% chance of having bladder cancer.
We confirmed that malignant cytology, advanced age, and smoking history are strong predictors of bladder cancer in patients with asymptomatic hematuria [24,25]. The reported sensitivity of voided urine cytology for detecting bladder cancer ranges from 40% to 76% and is dependent on a number of factors, such as tumor severity (stage and grade), disease prevalence, and the expertise of the cytopathologist [26]. Furthermore, cytology has poor sensitivity for detecting low grade tumors, which represent the most common type of bladder cancer. Nevertheless, positive cytology was a strong predictor of bladder cancer, as evidenced by an OR of 14.7 in the multivariable model and a contribution of 95 risk points in the nomogram.
In our study, patients who presented with gross hematuria had a 31.5% chance of having bladder cancer; this is consistent with a meta-analysis that demonstrated a positive predictive value of 22% for gross hematuria [27]. While it is generally accepted that patients with gross hematuria should undergo evaluation, it is more difficult to individualize care for patients with microscopic hematuria. Urinalysis is commonly obtained by primary care physicians and microscopic hematuria is sometimes present in apparently normal individuals [6]. The positive predictive value of microscopic hematuria for detecting patients with bladder cancer ranges from 2–8% [6,28]. Nevertheless, given the higher prevalence of microscopic hematuria relative to gross hematuria, workup for microscopic hematuria results in a significant number of bladder cancer diagnoses. For example, in our cohort, the total number of bladder cancer cases originating from patients with microscopic hematuria and gross hematuria were similar.
This nomogram enables practitioners to better quantify an individual’s risk of bladder cancer than interpreting risk factors in isolation. For example, the combination of the risk factors in the nomogram performs better than gross hematuria or malignant cytology alone. Traditionally, physician judgment has formed the basis for risk estimation, patient counseling, and decision making. However, clinicians’ estimates are often biased due to both subjective and objective confounders [29]. Predictive tools have been shown to perform better than clinical judgment when predicting probabilities of outcome [29,30]. That said, physician input is obviously essential in medical decision making, both for the measurement of predictive variables, and for the interpretation and application of prediction tools in clinical practice. The tool presented in this study can serve as a basis for individual patient counseling and clinical decision making based on data from a large cohort of patients with asymptomatic hematuria. We hope that use of this nomogram will result in increased and earlier referrals of patients with hematuria to urologists, as it may help illustrate the non-trivial risk of having bladder cancer, even for patients with minimal risk factors. This might improve patient care through earlier diagnosis resulting in more timely treatment.
It is possible that inclusion of other variables might have improved the performance of the nomogram. For example, smoking history was categorized as “current/history of” or “never.” Quantification of tobacco use could have improved the accuracy of our model. Furthermore, addition of additional risk factors and occupational or other chemical exposures would likely also improve the model’s predictive accuracy [4]. However, very few patients present with significant exposures.
There are a number of potential limitations of our study. While this is a multi-institutional study, the results may not be readily generalizable to other centers. Our cohort has a higher prevalence of bladder cancer (31.5% in subjects with gross hematuria, 15.7% in microscopic hematuria) than is typically cited in the literature. There is likely an increased probability of bladder cancer in our cohort based on local referral patterns. Our nomogram was internally validated using a bootstrapping method [14]; we are in the process of externally validating our model using an independent prospective cohort of patients referred to urologists for asymptomatic hematuria.
In conclusion, we developed a highly accurate, well-calibrated nomogram to predict the risk of bladder cancer in patients with asymptomatic hematuria. Using this nomogram, primary care physicians and other healthcare providers will be able to quantify the risk of bladder cancer for individual patients. Education of both patients and providers regarding bladder cancer risk will hopefully result in increased and more timely referrals, especially for those patients at highest risk.
Acknowledgments
We would like to thank Dr. Madhu Mazumdar for her supervision of the statistical analysis. This research was performed under the auspices of the International Bladder Cancer Network (IBCN). Dr. Paul J. Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996).
Footnotes
Conflict of Interest Statement
The authors certify that they have no actual or potential conflict of interest in relation to this article.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, Steineck G, Kogevinas M, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007;25:285–295. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 4.Silverman DT, Levin LI, Hoover RN, Hartge P. Occupational risks of bladder cancer in the United States: I. White men. J Natl Cancer Inst. 1989;81:1472–1480. doi: 10.1093/jnci/81.19.1472. [DOI] [PubMed] [Google Scholar]
- 5.Sutton JM. Evaluation of hematuria in adults. JAMA. 1990;263:2475–2480. [PubMed] [Google Scholar]
- 6.Grossfeld GD, Litwin MS, Wolf JS, Hricak H, Shuler CL, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology. 2001;57:599–603. doi: 10.1016/s0090-4295(01)00919-0. [DOI] [PubMed] [Google Scholar]
- 7.Davis R, Jones SJ, Barocas DA, Castle EP, Lang EK, et al. American Urological Association Guidelines May 2012. 2012. DIAGNOSIS, EVALUATION and FOLLOW-UP OF ASYMPTOMATIC MICROHEMATURIA (AMH) IN ADULTS: AUA GUIDELINE. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348:2330–2338. doi: 10.1056/NEJMcp012694. [DOI] [PubMed] [Google Scholar]
- 9.Nieder AM, Lotan Y, Nuss GR, Langston JP, Vyas S, et al. Are patients with hematuria appropriately referred to Urology? A multi-institutional questionnaire based survey. Urol Oncol. 2010;28:500–503. doi: 10.1016/j.urolonc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: does a gender disparity exist? Urology. 2008;72:498–502. doi: 10.1016/j.urology.2008.01.086. discussion 502–493. [DOI] [PubMed] [Google Scholar]
- 11.Summerton N, Mann S, Rigby AS, Ashley J, Palmer S, et al. Patients with new onset haematuria: assessing the discriminant value of clinical information in relation to urological malignancies. Br J Gen Pract. 2002;52:284–289. [PMC free article] [PubMed] [Google Scholar]
- 12.Lotan Y, Capitanio U, Shariat SF, Hutterer GC, Karakiewicz PI. Impact of clinical factors, including a point-of-care nuclear matrix protein-22 assay and cytology, on bladder cancer detection. BJU Int. 2009;103:1368–1374. doi: 10.1111/j.1464-410X.2009.08360.x. [DOI] [PubMed] [Google Scholar]
- 13.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. p. xvi.p. 436. [Google Scholar]
- 14.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Lotan Y, Shariat SF, Schmitz-Drager BJ, Sanchez-Carbayo M, Jankevicius F, et al. Considerations on implementing diagnostic markers into clinical decision making in bladder cancer. Urol Oncol. 2010;28:441–448. doi: 10.1016/j.urolonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Shariat SF, Lotan Y, Vickers A, Karakiewicz PI, Schmitz-Drager BJ, et al. Statistical consideration for clinical biomarker research in bladder cancer. Urol Oncol. 2010;28:389–400. doi: 10.1016/j.urolonc.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svatek RS, Lotan Y, Karakiewizc PI, Shariat SF. Screening for bladder cancer using urine-based tumor markers. Minerva Urol Nefrol. 2008;60:247–253. [PubMed] [Google Scholar]
- 19.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115:988–996. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace DM, Bryan RT, Dunn JA, Begum G, Bathers S. Delay and survival in bladder cancer. BJU Int. 2002;89:868–878. doi: 10.1046/j.1464-410x.2002.02776.x. [DOI] [PubMed] [Google Scholar]
- 21.Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Skolarus TA, et al. Delays in diagnosis and bladder cancer mortality. Cancer. 2010 doi: 10.1002/cncr.25310. [DOI] [PubMed] [Google Scholar]
- 22.Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14:4400–4407. doi: 10.1158/1078-0432.CCR-07-4713. [DOI] [PubMed] [Google Scholar]
- 23.Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer. 2008;113:3075–3099. doi: 10.1002/cncr.23908. [DOI] [PubMed] [Google Scholar]
- 24.Badalament RA, Hermansen DK, Kimmel M, Gay H, Herr HW, et al. The sensitivity of bladder wash flow cytometry, bladder wash cytology, and voided cytology in the detection of bladder carcinoma. Cancer. 1987;60:1423–1427. doi: 10.1002/1097-0142(19871001)60:7<1423::aid-cncr2820600702>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, et al. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105:300–308. doi: 10.1111/j.1464-410X.2009.09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karakiewicz PI, Benayoun S, Zippe C, Ludecke G, Boman H, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU Int. 2006;97:997–1001. doi: 10.1111/j.1464-410X.2006.06036.x. [DOI] [PubMed] [Google Scholar]
- 27.Buntinx F, Wauters H. The diagnostic value of macroscopic haematuria in diagnosing urological cancers: a meta-analysis. Fam Pract. 1997;14:63–68. doi: 10.1093/fampra/14.1.63. [DOI] [PubMed] [Google Scholar]
- 28.Messing EM, Madeb R, Young T, Gilchrist KW, Bram L, et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107:2173–2179. doi: 10.1002/cncr.22224. [DOI] [PubMed] [Google Scholar]
- 29.Ross PL, Gerigk C, Gonen M, Yossepowitch O, Cagiannos I, et al. Comparisons of nomograms and urologists’ predictions in prostate cancer. Semin Urol Oncol. 2002;20:82–88. doi: 10.1053/suro.2002.32490. [DOI] [PubMed] [Google Scholar]
- 30.Walz J, Gallina A, Perrotte P, Jeldres C, Trinh QD, et al. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100:1254–1258. doi: 10.1111/j.1464-410X.2007.07130.x. [DOI] [PubMed] [Google Scholar]