Abstract
Insulin-like growth factor (IGF) signaling has been implicated in the resistance to hormonal therapy in breast cancer. Using a model of postmenopausal, estrogen-dependent breast cancer, we investigated the antitumor effects of the dual IGF-1R/InsR tyrosine kinase inhibitor BMS-754807 alone and in combination with letrozole or tamoxifen. BMS-754807 exhibited antiproliferative effects in vitro that synergized strongly in combination with letrozole or 4-hydroxytamoxifen and fulvestrant. Similarly, combined treatment of BMS-754807 with either tamoxifen or letrozole in vivo elicited tumor regressions not achieved by single-agent therapy. Notably, hormonal therapy enhanced the inhibition of IGF-1R/InsR without major side effects in animals. Microarray expression analysis revealed downregulation of cell-cycle control and survival pathways and upregulation of erbB in response to BMS-754807 plus hormonal therapy, particularly tamoxifen. Overall, these results offer a preclinical proof-of-concept for BMS-754807 as an antitumor agent in combination with hormonal therapies in hormone-sensitive breast cancer. Cooperative cell-cycle arrest, decreased proliferation, and enhanced promotion of apoptosis may contribute to antitumor effects to be gauged in future clinical investigations justified by our findings.
Introduction
Hormonal therapies are front-line systemic therapies for patients with estrogen-responsive breast cancer (ERBC). The selective estrogen receptor modulator (SERM) tamoxifen, for instance, has shown improved survival in breast cancer patients for more than 25 years (1). However, resistance to therapies targeting the estrogen receptor signaling pathway represents a major clinical hurdle (2).
Mounting data suggests that the insulin-like growth factor (IGF) system is a major determinant in the development of resistance to therapies targeting estrogen signaling (3). As an estrogen-dependent gene, IGF-1 receptor (IGF-1R) expression is modulated by estrogen signaling (4). In addition, IGF-1, by a number of mechanisms, regulates estrogen receptor–dependent transcription (5). The combination of IGF-1 and estradiol synergistically stimulate growth of ERBC, and cross-talk pathways between these systems have implicated the IGF-1 system as a mechanism of resistance to endocrine therapy in breast cancer (6–9). Furthermore, the proliferative effects of IGF-1 can be attenuated by tamoxifen and cells that have been selected to become resistant to tamoxifen have increased responsiveness to the proliferative effects of IGF-1 (10). Recently, data has suggested that direct interactions between estrogen and IGF-1R may be important for mitogenic estrogen receptor signaling (11).
Thus, targeting both the IGF signaling pathway and the estrogen receptor pathway is an attractive strategy for enhancing the clinical activity of endocrine therapy, as well as preventing or delaying the development of resistance. Currently, it is unclear whether estrogen deprivation or estrogen receptor inhibition would have a greater antitumor effect in combination with IGF-1 blockade. This distinction becomes important as the 2 classes of approved endocrine therapies (aromatase inhibitors and SERMs, respectively) function by these differing mechanisms. Preclinical data with a monoclonal antibody (mAb) directed at the IGF-1R has shown enhancement of tamoxifen activity in vivo (12). However, in postmenopausal breast cancer patients, aromatase inhibitors are often used as first-line hormonal therapy due to superior activity over tamoxifen (13, 14). Thus, to optimize the selection of the most appropriate agent to investigate in combination with IGF-1 blockage, preclinical assessment of activity in an in vivo model is necessary.
In regards to blocking IGF signaling, the majority of current strategies aimed at blocking the IGF system focus on the IGF-1 receptor (IGF-1R). The IGF-1R is a transmembrane tyrosine kinase that is the major signaling receptor for the IGF-1 pathway (15). The functional receptor consists of 2 subunits (α and β) in a heterodimeric structure. Upon activation by the mitogenic ligands IGF-1 and IGF-2, the IGF-1R becomes autophosphorylated, stimulating the activation of downstream intracellular pathways (namely, the PI3K/AKT and Ras/MEK/ERK pathways) that lead to tumor proliferation, survival, and metastasis (16). In addition, the IGF-1R half-receptor can dimerize with the insulin receptor (InsR) tyrosine kinase, which shares a high degree of homology to the IGF-1R. Dimerization of these “hybrid-receptors” have different biological activity and ligand specificity (17). In particular, the fetal or A isoform of the InsR seems to have a more mitogenic role in cancer cell proliferation than its purely metabolic isoform B (18). The varying biological activities of the InsR isoforms are likely related to their differing affinities for IGF-1 system ligands. For instance, whereas the metabolic InsR isoform B only binds insulin at physiologic concentrations, the InsR isoform A is able to bind and be activated by IGF-2 (17). Thus, InsR isoform A through dimerization with IGF-1R or homodimerization may provide mitogenic stimuli to cancer cells through activation by IGF-2. Accumulated data has implicated the InsR isoform A, or the InsR total content, as being important in breast cancer progression and survival (19, 20). More recent data suggest it may also be a mechanism of resistance to therapies that specifically target the IGF-1 receptor, such as mAb therapies (21, 22). Patients with node-negative breast cancers, whose tumors express high InsR content, have worse disease-free survival than patients with even moderate InsR content (19). Early studies have also shown that approximately 80% of breast cancers have an InsR content higher than the median content found in the normal breast, and approximately 20% of cancers show InsR over 10-fold higher than the median value of the normal breast tissue (20). Early studies targeting the IGF-1 receptor in patients with refractory tumors have shown that mAb therapies may induce upregulation of insulin secretion, suggesting a compensatory mechanism which could possibly activate InsR signaling as a mechanism of resistance (23). Thus, if InsR isoform A expression is an important mechanism of proliferation of breast cancer cells and an important mechanism of resistance to IGF-1–targeted antibody therapy, dual kinase inhibitors of InsR and the IGF-1R may have a therapeutic advantage.
On the basis of these data, we conducted a preclinical study investigating the efficacy of a small molecule inhibitor of the IGF-1R and InsR, BMS-754807, in the estrogen-dependent, aromatase-expressing breast cancer model, MCF-7/AC-1 both with and without hormonal therapy (24, 25). Our hypothesis was that complete IGF blockade would increase the antitumor activity of hormonal therapy in ERBC. We have also used this model system to evaluate the tolerability of these treatments in vivo and carry out correlative studies to identify potential biomarkers of antitumor activity in response to IGF blockade.
Materials and Methods
Reagents
Phenol red–free modified IMEM, Dulbecco’s modified Eagle’s medium (DMEM), penicillin/streptomycin solution, 0.05% trypsin–EDTA solution, Dulbecco’s PBS, and geneticin (G418) were obtained from Life Technologies. FBS and charcoal/dextran–treated FBS were obtained from Hyclone/Thermo Scientific. Matrigel was purchased from BD Biosciences. Androstenedione, 4-hydroxytamoxifen (for in vitro use), tamoxifen (for in vivo use), and hydroxypropyl cellulose was purchased from Sigma Co. Enhanced chemiluminescence (ECL) kits and Hybond-ECL nitrocellulose membranes were purchased from Amersham Biosciences/GE Healthcare. IGF-1 LR3 was purchased from GroPep. Antibodies against p-AKT, AKT, p-IGF-IRβ/InRβ, IGF-IRβ, p-MAPK, mitogen—activated protein kinase (MAPK) were purchased from Cell Signaling Technology. An antibody against β-actin was purchased from Sigma-Aldrich. Antibodies against insulin Rβ, ERα were purchased from Santa Cruz Biotechnology. An antibody against Ki-67 was purchased from Abcam. Horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit antibodies were purchased form Invitrogen. MCF-7 human breast cancer cells stably transfected with the human aromatase gene were provided by Dr. Angela Brodie and Shiuan Chen (Beckman Research Institute of City of Hope, Duarte, California) as previously reported (26). Cells authentication was done using 16 loci short-tandem repeat profiling at the completion of included studies. Letrozole (Femara, CGS 20267) was kindly provided by Dr. D. Evans (Novartis Pharma).
Cell culture
MCF-7 human breast cancer cells stably transfected with the human aromatase gene (MCF-7/AC-1 cells) as previously described (25) were routinely maintained in DMEM with 10% FBS, 1% penicillin/streptomycin solution, and 750 μg/mL G418. The culture medium was changed twice weekly.
MTS proliferation assay
The effect of the various drugs and hormones on MCF-7/AC-1 cellular growth was examined using the MTS proliferation assay (CellTiter 96 Aqueous; Promega), as previously described (27). Briefly, cells growing in regular media were transferred to IMEM containing 5% charcoal-stripped serum and 1% penicillin/streptomycin (charcoal-stripped serum in medium, CSSM) for 72 hours. This medium was used for all cell growth assays. The cells were detached from their flask using trypsin and seeded in 96-well plates at 1.0 × 103/mL (100 μL) on day 0. Twenty-four hours later, several concentrations of the hormones or drugs were added at doses indicated in the text. The cells were incubated for 6 days in the absence and presence of the tested drugs and hormones. At the end of the treatment, the MTS dye reduction was assessed as per the product information label. Proliferation was calculated as a percentage of the nondrug–treated controls. Experiments were done in at least triplicate. To evaluate the effect of combination treatment with BMS-754807 and tamoxifen or letrozole or fulvestrant, the method of Chou and Talalay was used to determine synergy as described previously (28). Median effect analysis was done using CalcuSyn software (Biosoft). Mean values of the combination index (CI) at the affected fractions of 50% (Fa50) and 75% (Fa75) are shown. A CI value significantly less than 1 indicates synergism, a CI not significantly different from 1 indicates addition, and a CI significantly higher than 1 indicates antagonism.
Postmenopausal intratumoral aromatase mice xenograft model
Female ovariectomized BALB/c athymic nude mice 4 to 6 weeks of age were obtained from Harlan Laboratories. The animals were housed in a pathogen-free environment under controlled conditions of light and humidity and received food and water. All animal studies were carried out according to the guidelines approved by the Animal Care Committee of the Mayo Clinic, Rochester, MN. Animals were allowed to acclimatize for 48 hours after shipment before tumor inoculation was done. For inoculation, subconfluent MCF-7/AC-1 cells were suspended in Matrigel (10 mg/mL) at 2.5 × 107 cells/mL. Each mouse was injected subcutaneously with 100 μL of cell suspension on each flank. Tumors were measured weekly with calipers, and volumes were calculated with the formula 4/3π x r12 x r2 (r1 < r2), in which r1 is the smaller radius. Treatments began when the tumors reached a measurable size (250–300 mm3). Mice randomized to treatment groups using JMP (SAS).
Mice received subcutaneous injection with vehicle, nonsteroidal aromatase inhibitor letrozole (10 μg/d) and antiestrogen tamoxifen (500 μg/d), which were all prepared as suspensions in 0.3% hydroxypropyl cellulose; IGF-1R/InsR inhibitor BMS-754807 (50 mg/kg/d) was prepared in PEG400:water (80:20) and was administered by daily gavage; the combinations of letrozole, and/or tamoxifen with BMS-754807 were given at the full doses. All treatments are given once daily for 28 days continuously. All animal groups were supplemented with androstenedione daily for the duration of the experiment.
After 28 days of treatment, 1 of the 2 flank tumors was surgically excised from each tumor-bearing animal. Animals were then maintained and observed until the study endpoint (300% tumor growth from treatment baseline), at which point the remaining tumor was resected at the time of animal sacrifice. After each resection, tumors were carefully excised from mouse tissue with portions frozen in OCT medium (Tissue-Tek) or placed in formalin for further analyses. Differences between the groups were analyzed by ANOVA with multiple comparison posttests using Prism (Graph Pad).
Mouse weight and blood glucose measurements
Blood glucose levels in all mice were measured from lateral tail vein pricks in the morning using an Ascensia Elite XL glucose meter (Bayer) and Glucometer Elite test strips (Fisher Scientific). One glucose measurement required approximately 3 μL of blood. Depending on the volume of blood that flowed out by a tail vein prick, 1 or 2 glucose measurements were taken. In the case of 2 measurements, the average was used in the calculations. Mouse weight was measured every other day.
Immunohistochemical staining
At autopsy, the tumors were resected and processed for routine gross and microscopic examination. The tumor tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Serial sections (5-μm thick) were cut on a microtome and mounted on glass slides. For histopathologic examination, every fourth section was dewaxed in Histoclear (National Diagnostic) and hydrated in graded alcohol solutions and distilled water for hematoxylin and eosin (H&E) staining and examined under a light microscope.
For immunohistochemical staining, prior to use, the slides were washed 3 times (5 minutes each) in xylene to remove paraffin, 3 times (5 minutes each) with 100%, 90%, and 70% ethanol (in that order), and hydrated in distilled water before tissue treatment. Antigens were retrieved by treatment for 45 minutes in 0.001 mol/L sodium citrate buffer (pH 6) in a water bath (95°C–99°C). The endogenous peroxides activity was inhibited with 3% hydrogen peroxide for 5 minutes. Nonspecific binding was blocked by incubation for 30 minutes with 2% bovine serum albumin in PBS; specimens were washed 5 times in PBS and incubated overnight at 4°C with different antibodies, including anti-human Ki-67 (1:500). After 5 further washes in PBS, specimens were incubated with second antibodies (Invitrogen). Staining reaction was carried out using HRP-mediated AEC (aminoethyl carbazole) or DAB immunostaining (Zymed Laboratories). Red or brown deposits indicted the sites of positive immunostaining and were counterstained with Mayer’s hematoxylin solution by standard procedures.
Western blot analysis
Inhibition of IGF-1R and insulin receptor phosphorylation and ERK/Akt pathways by BMS-754807 alone or combination with 4-OH tamoxifen (10 μmol/L), letrozole (10 μmol/L), or fulvestrant (100 nmol/L) were determined by Western blotting. Briefly, cells were cultured in IMEM steroid–reduced medium without phenol red for 24 hours, then subconfluent MCF-7/AC-1 cells were treated with either dimethyl sulfoxide (DMSO), BMS-754807 (10 μmol/L), or BMS-754807 (10 μmol/L) plus hormones for 24 hours in serum-free conditions. For the final 15 minutes of drug treatment, 10 nmol/L LongR3 IGF-I was added to the medium. Lysates were then prepared and analyzed by Western blotting.
Western blotting was done as previously described (27). Briefly, proteins were extracted from the tumor tissues by homogenization in buffer containing 50 mmol/L Tris (pH 7.4), 1 mmol/L EDTA, 150 mmol/L NaCl, and protease/phosphatase inhibitors (1 μg/mL phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, and 1 μg/mL leupeptin). The homogenates were centrifuged at 2,000 × g for 15 minutes at 4°C. After centrifugation at 10,000 × g for 5 minutes, the supernatants were separated and their protein concentrations were measured. The protein lysates were separated by 10% SDS-PAGE, transferred onto Immuno-Blot polyvinylidene difluoride membrane (catalog no. 162-0177; Bio-Rad), and Western blot analysis was done as described previously (27). The membranes were blocked with 5% milk in TBS [10 mmol/L Tris-HCl (pH 8.0) and 150 mmol/L NaCl] plus 0.05% Tween-20 overnight at 4°C and then incubated in 5% milk containing primary antibodies (1:1,000/1,500 for both antibodies or 1:2,500 dilution for Actin) overnight at 4°C. After incubation, membranes were washed 3 times (15 minutes each) with 5% milk, incubated with goat anti-rabbit IgG conjugated with HRP (1:5,000) in 5% milk for 1 hour at room temperature and washed 3 times (15 minutes each) in TBS. The bands were detected using an ECL Kit (Amersham). Densitometry was done using Genetools software (Syngene).
RNA isolation and gene expression profiling
Total RNA was isolated using TRIzol reagents (Catalog no. 15596-026; Invitrogen). One microgram of total RNA isolated from tumors surgically excised from each tumor-bearing animal after 28 days of treatment were used to generate gene expression data using Affymetrix HT-HG-U133A GeneChip (Affymetrix) according to the manufacturer’s instructions. The microarray data were analyzed by ANOVA using Partek software to identify genes differentially expressed between different treatment groups. Gene expression data was analyzed by Ingenuity Pathway Analysis to identify pathways that were up- or downregulated in response to in vivo treatments (Ingenuity Systems).
Statistical analysis
Error bars represent SEM. Differences in the mean of 2 samples were analyzed using Student unpaired t test. For comparisons between multiple samples, ANOVA with Tukey’s multiple comparisons posttest was used. For P values, differences less than 0.001 was considered extremely significant (***); 0.001 to 0.01 very significant (**); 0.01 to 0.05 significant (*). P values more than 0.05 were considered not significant (ns).
Results
MCF-7/AC-1 cells are estrogen-driven breast cancers cells in vitro and in vivo
The MCF-7/AC-1 cells were employed in a series of experiments due to the their responsiveness to estrogen and sensitivity to clinically estrogen-targeting agents, including the SERM tamoxifen, the aromatase inhibitor, letrozole, and the pure antiestrogen, fulvestrant. To confirm that the MCF-7/AC-1 cells were estrogen dependent in vitro, MCF-7/AC-1 cells were cultured in IMEM containing 5% charcoal-stripped serum, with or without the aromatase substrate androstenedione (1 nmol/L). After 6 days incubation, androstenedione stimulated a 4-fold growth in MCF-7/AC-1 cells (Fig. 1A). Furthermore, this androstenedione-induced, estrogen-dependent cell growth can be blocked by 4-hydroxytamoxifen in a dose-dependent manner (Fig. 1B). In vivo, AC-1 cells reliably formed xenografts that were palpable in 3 to 4 weeks upon androstenedione supplementation (Fig. 1C). Without androstenedione, xenografts did not reliably form. The uteri of AC-1 tumor-bearing mouse were substantially larger in the group receiving androstenedione compared with controls (Fig. 2A), supporting the evidence that androstenedione was being converted to estrogens in vivo and contributing to the proliferative effects on mouse endometrium (Fig. 2B). Thus, in our model, the tumors being generated are estrogen dependent and reliably heterotransplanted only in animals receiving androstenedione, supporting functional aromatase expression and estradiol production.
Figure 1.
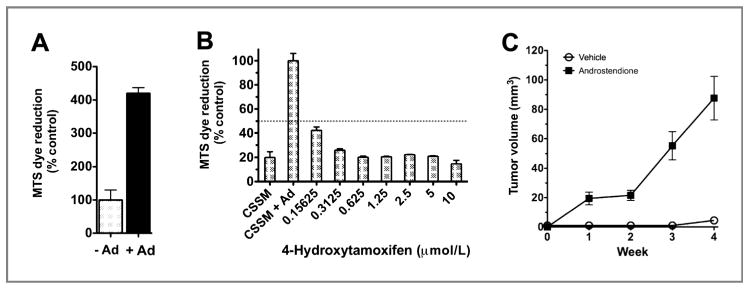
MCF-7/AC-1 cells are estrogen-driven in vitro and estrogen-dependent in vivo. Cells were cultured in IMEM steroid-reduced medium without phenol red for 2 days before plating. Cell proliferation was measured using an MTS assay, as described in Methods. A, effect of presence of androstenedione (Ad) on MCF-7/AC1 cell growth. Cell growth is expressed as the percentage of the cells compared with the control wells (untreated cells). Columns, mean; bars, SE. B, antiproliferative effect of increasing concentrations of 4-hydroxytamoxifen in the presence of 1 nmol/L androstenedione on MCF-7/AC1 cells. Cell proliferation is expressed as the percentage of the cells compared with the control wells (1 nmol/L androstenedione-treated cells). CSSM, untreated cells cultured in steroid-reduced medium. Columns, mean; bars, SE. C, MCF-7/AC-1 xenografts, each mouse received subcutaneous inoculations in 2 sites per flank with 100 μL of MCF-7/AC-1 cell suspension containing 2.5 × 106 cells. The mice were injected daily with supplemental androstenedione (100 μg/d) or vehicle from day 0. Tumors were measured with calipers weekly throughout experiment. Points, mean; bars, SE.
Figure 2.
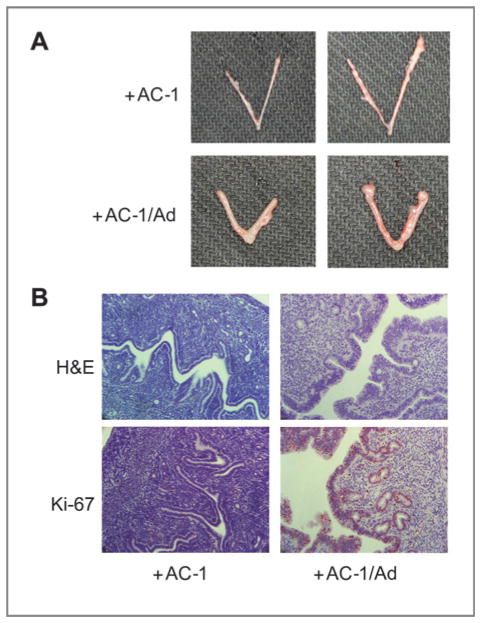
Uterotropic effects of mice bearing MCF-7/AC-1 tumors, with and without androstenedione. Each mouse received subcutaneous inoculations in 2 sites per flank with 100 μL of MCF-7/AC-1 cell suspension containing 2.5 × 106 cells. The mice were injected daily with supplemental androstenedione (100 μg/d) or vehicle from day 0. At day 28, the mice were sacrificed and uteri were removed from the mice, weighed, formalin-fixed, and embedded in paraffin. A, morphologic appearances of 2 representative uteri from ovariectomized MCF-7/AC-1 xenografts bearing mice were shown with or without androstenedione for 28 days. B, analysis of cell proliferation. Uterine cross-sections were examined by Ki-67 immunostaining. Reddish nuclear deposits indicate the sites of positive immunostaining (20×).
Synergistic effect of BMS-754807 in combination with tamoxifen or letrozole on growth of MCF-7/AC-1 cells in vitro
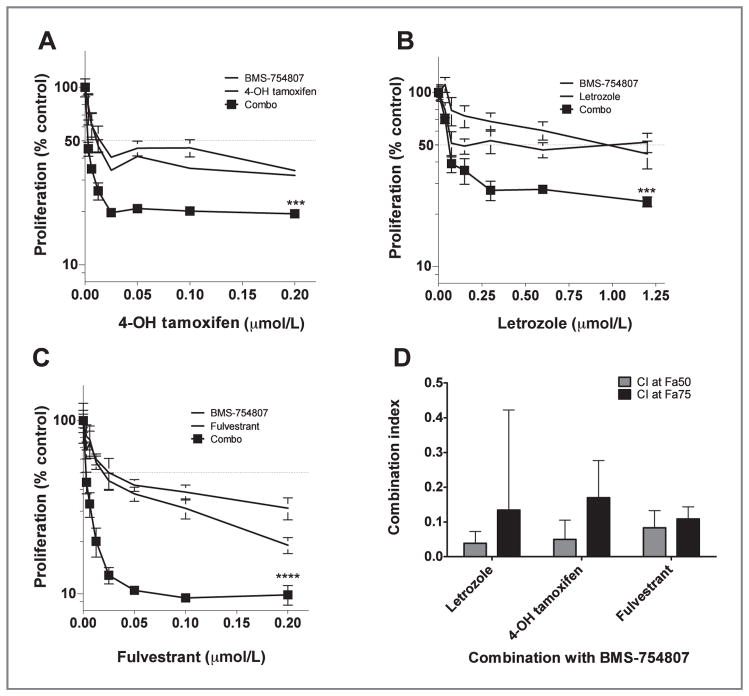
On the basis of the findings above, MCF-7/AC-1 cell proliferation was assessed by treatment with various concentrations of BMS-754807, 4-hydroxytamoxifen, letrozole, and fulvestrant either as single agent or 4-hydroxytamoxifen, letrozole, or fulvestrant in combination with BMS-754807 at a fixed ratio. At doses of the single agents that had modest antiproliferative effects, the combination treatment seemed to have a significant antiproliferative effect (P < 0.001; Fig. 3A and C). In comparison with the single-agent antiproliferative effects, the combination of BMS-754807 with letrozole, 4-hydroxytamoxifen, or fulvestrant was strongly synergistic at the 50% and 75% fraction affected (P < 0.001; Fig. 3D).
Figure 3.
Antiproliferative effects of BMS-754807 and/or hormonal therapy in vitro. MCF-7/AC-1 cells were cultured, and after 6 days, treatment cell proliferation measured as above. Proliferation was assessed in the presence of BMS-754807, hormonal therapies, and a fixed ratio of increasing amount of combinations, as indicated. Cell proliferation is expressed as the percentage of the cells compared with the control wells (1 nmol/L androstenedione-treated cells). A, 4-OH tamoxifen (***, P < 0.001); B, letrozole (***, P < 0.001); C, fulvestrant (****, P < 0.0001). Points, mean; bars, SE. D, CI values at the 50% (Fa50) and 75% (Fa75) fraction affected were plotted for the treatment combinations. All CI values plotted were significantly synergistic (P < 0.001). Columns, mean; bars, SE.
Enhanced effects of combined IGF blockade and hormonal therapy on AKT and ERK pathway signaling
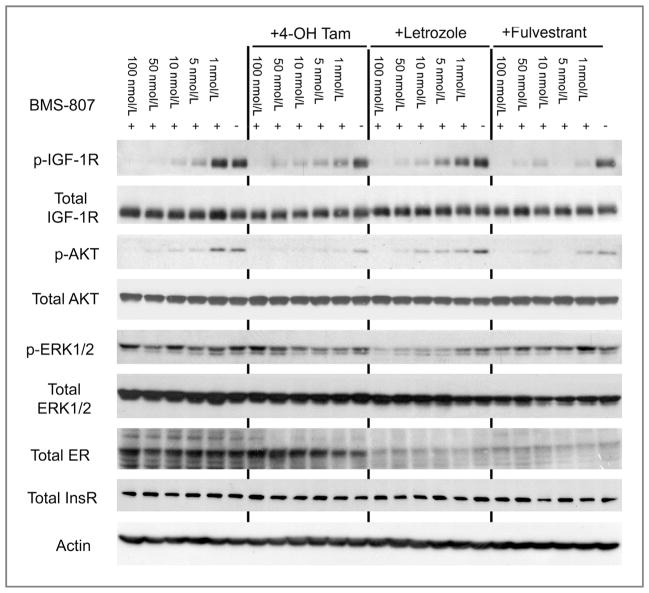
To investigate possible mechanisms for the synergy between BMS-754807 and hormonal therapies, we investigated the effects of BMS-754807 with and without hormonal therapies on the major proliferation and survival pathways of IGF signaling–AKT and ERK1/2 (Fig. 4; Supplementary Fig. S2). BMS-754807 alone very effectively inhibited pIGF-1R and pAKT and had modest effects on pERK1/2 in MCF-7/AC-1 cells in vitro. All hormonal therapies investigated enhanced the ability of BMS-754807 to decreased phosphorylation of IGF-1R and AKT in the low nanomolar range. 4-Hydroxytamoxifen seemed to be the most effective at enhancing the inhibition of AKT phosphorylation in combination with BMS-754807. Fulvestrant was the most effective hormonal therapy at enhancing the inhibition of IGF-1R phosphorylation by BMS-754807. Only letrozole seems to substantially reduce the phosphorylation of ERK1/2 in combination with BMS-754807. Total levels of IGF-1R, AKT, ERK1/2, and InsR were unchanged by BMS-754807 alone or in combination with 4-hydroxytamoxifen, letrozole, or fulvestrant. However, the estrogen receptor levels were decreased in the presence of fulvestrant and letrozole.
Figure 4.
Inhibition of IGF-1R/InsR, ERK/Akt phospho signaling pathways by BMS-754807 alone or combination with 4-OH Tam (10 μmol/L), letrozole (10 μmol/L), or fulvestrant (100 nmol/L). Briefly, cells were cultured in IMEM steroid–reduced medium without phenol red with 1 nmol/L androstenedione for 24 hours, then subconfluent MCF-7/AC-1 cells were treated with either DMSO, BMS-754807 at the indicated concentrations, or BMS-754807 plus hormones for 24 hours in serum-free conditions. For the final 15 minutes of drug treatment, 10 nmol/L LongR3 IGF-I was added to the medium. Lysates were then prepared and analyzed by Western blotting, as described in the Methods.
Effect of combination of BMS-754807 and tamoxifen or letrozole on growth of MCF-7/AC-1 cells xenografts in vivo
To determine whether the in vitro synergy translated into enhanced tumor regression and improvement in time to tumor progression, the effects of BMS-754807 in combination with hormonal therapy was investigated compared with single agents in vivo. As both tamoxifen and letrozole represent front-line breast cancer therapies with differing mechanisms (SERM vs. aromatase inhibitor, respectively), these agents were selected for in vivo investigations. MCF-7/AC-1 cells were injected subcutaneously into both flanks of athymic nude mice supplemented with androstenedione daily (100 μg/mouse/d s.c.). Once the tumors reached a measurable size of 250 to 300 mm3 (after 4–5 weeks), the mice were randomly assigned to 6 treatment groups so that mean tumor volumes were not significantly different at the start of treatment: control (vehicle alone), letrozole (10 μg/d s.c.), tamoxifen (500 μg/d s.c.), and BMS-754807 (50 mg/kg/d), BMS-754807 plus letrozole or plus tamoxifen. All treatments were given once daily for 28 days continuously. Animals of all groups were supplemented with androstenedione daily during the whole experiments.
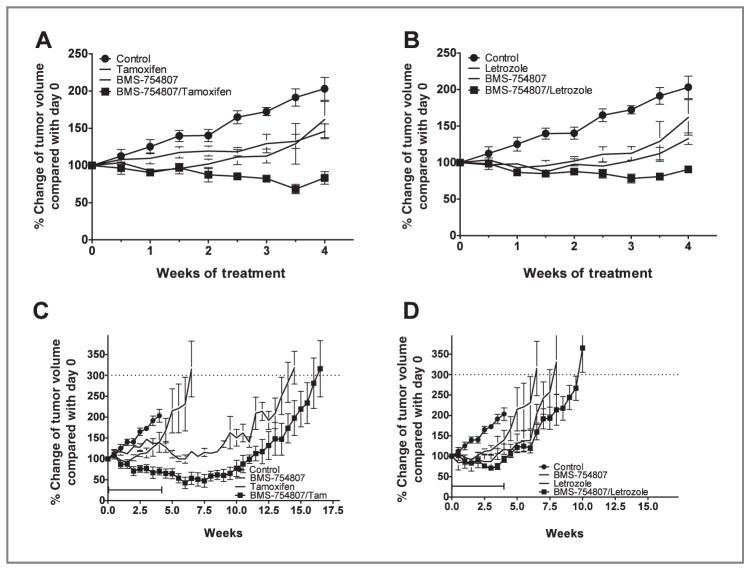
At the end of 28 days treatment, the tumors in the control group (203%) doubled in size after 4 weeks, whereas the tumors in the letrozole (P < 0.0005 vs. control), tamoxifen (P < 0.05 vs. control), or BMS-754807 (P < 0.005) groups showed significant growth inhibition compared with controls. There was no significant difference in the growth of the tumors in the single-agent treated groups. The tumors in mice receiving BMS-754807 with tamoxifen had significantly greater antitu-mor activity compared with either agent alone (Fig. 5A, P < 0.005 for BMS-754807/tamoxifen vs. BMS-754807; P < 0.0005 for BMS-754807/tamoxifen vs. tamoxifen). Similarly, tumors in mice receiving BMS-754807 with letrozole had greater antitu-mor activity than either agent alone (Fig. 5B, P < 0.005 for BMS-754807/letrozole vs. BMS-754807; P < 0.05 for BMS-754807/letrozole vs. letrozole). After 28 days treatment, there was no significant difference between BMS/tamoxifen and BMS/letrozole (P = 0.0514). After 28 days of treatment, the control cohort animals were sacrificed; in the experimental groups, 1 of the 2 flank tumors, which were the biggest, was surgically excised from each tumor-bearing animal. The mice were then followed without any treatment except daily androstenedione until the correlative endpoint (300% tumor growth from treatment baseline) was met. The tumor volumes were followed by once a week measurements. Times to the endpoint after 28 days treatment with BMS-754807/tamoxifen was significantly improved over tamoxifen alone (Fig. 5C; P = 0.0159) and BMS-754807 alone (P = 0.0031). There was no significant improvement in time to endpoint for the treatment of BMS-754807/letrozole over letrozole alone or BMS-754807 alone (Fig. 5D; P > 0.05).
Figure 5.
In vivo antitumor activity of single-agent BMS-754807, letrozole, tamoxifen, or in combinations. Ovariectomized female nu/nu mice between the ages of 7 to 8 weeks old were inoculated with MCF-7/AC-1 tumor cells in each flank with supplemental androstenedione (100 μg/d). Once the bilateral flank tumors grew such that the average of their left and right tumor volumes are between 250 to 300 mm3, mice were randomized to each treatment group and given various treatments for a total of 28 days; treatment groups consisted of 8 to 10 mice per group, and the experiments were repeated 3 times. The tumor volumes were measured twice weekly. At day 28, the average of the left and right tumor volumes of each mouse and percent change from its pretreatment average volume was determined, then 1 of the 2 flank tumors was surgically excised from each tumor-bearing animal. Animals were then observed until study endpoint. Tumor volume was measured at the intervals indicated and expressed as percentage change in tumor volume relative to start of treatment (baseline, day 0). A and B, at the end of study treatment, both the combination of letrozole + BMS-754807 and tamoxifen + BMS-754807 had significantly improved antitumor activity compared with the respective single agents. (bilateral tumor measurements). Points, mean; bars, SE. C and D, time to endpoint following treatment with BMS-754807 and/or hormonal therapy (single remnant tumor measurements). Points, mean; bars, SE.
Effects of BMS-754807 on mice blood glucose and weight change
As BMS-754807 is a dual inhibitor with activity against the IGF-1R and InsR kinase, we explored the tolerability of daily BMS-754807 as a single agent and in combination with hormonal therapy by assessing blood glucose and animal weight during the treatment. Comparisons betweens all treatment groups showed no significant difference between any of the treatment groups during the course of treatment (Fig. 6A; P = 0.5622). In contrast, treatment with BMS-754807/tamoxifen resulted in significant weight loss compared with all other treatment groups (Fig. 6B; P < 0.0001). However, the weight at treatment end was not different between the treatment groups (P = 0.2657).
Figure 6.
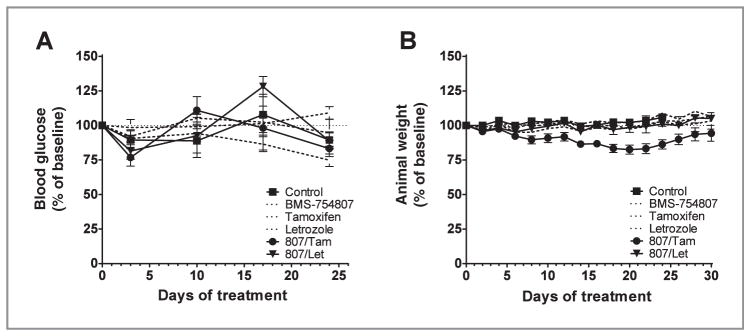
Glucose homeostasis and animal weight during in vivo therapy. During administration of 28 days of vehicle, BMS-754807, letrozole, tamoxifen, or their combinations, both (A) glucose and (B) weight of all animals on study were measured and recorded. Investigators were blinded to treatment groups by using implanted identification chips. Points, mean; bars, SE
Gene expression profiling reveal modulation of cell cycle and prosurvival pathway genes
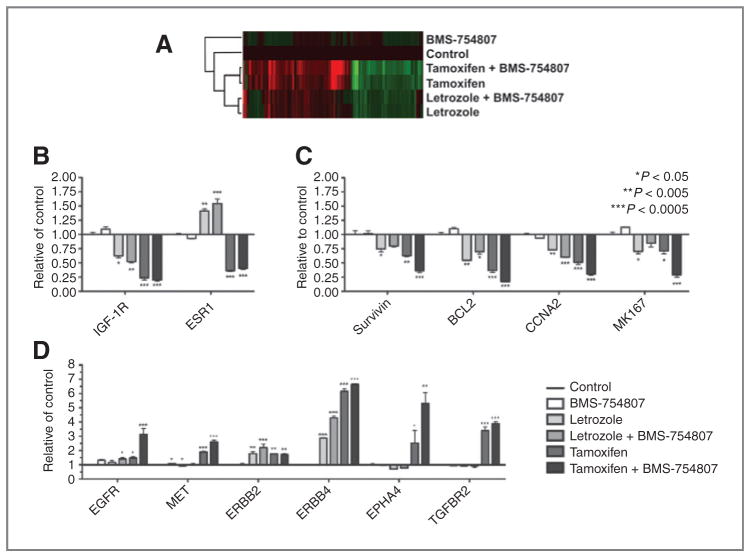
To define the mechanisms of enhanced activity in combination of targeting both the estrogen receptor (ER) and IGF-1R/InsR, we conducted a microarray study on tumors that were surgically excised from tumor-bearing animal after 28 days of treatment. Statistical analyses were done to identify 868 probe sets representing 698 unique genes that were significantly modulated by any treatment with false discovery rate = 5% and 2-fold changes when compared with the untreated control (Supplementary Table S1). Both hormonal agents had similar change in gene expression with some in different directions, and the combination of BMS-754807 with tamoxifen further enhanced the changes in gene expression patterns (Fig. 7A). Both letrozole and tamoxifen decreased IGF-1R expression and the latter had more significant effects, whereas ER expression was only downregulated by tamoxifen, not by letrozole (Fig. 7B). BMS-754807 did not alter expression of either IGF-1R or ER expression, nor did it substantially affect the changes in expression because of hormonal therapy. Significant changes were shown after study treatments in genes involved survival and proliferation (Fig. 7C and D). Among the cell cycle and survival genes, BIRC5 (gene for survivin), BCL2, CCNA2 (gene for Cyclin A2), and MKI67 (gene for Ki-67) were downregulated by study treatments, though these effects were most profound in the BMS-754807 + tamoxifen treatment group, particularly for MKI67. Receptor tyrosine kinases EGFR, ERBB2, ERBB4, MET, EPHA4, and TGFBR2 were upregulated in response to BMS-754807 + tamoxifen and, to a lesser extent, other study treatments. For example, both hormonal agents increased ERBB4 expression, which was enhanced by BMS-754807; EPHA4, MET, and TGFBR2 expression were only affected by tamoxifen, not by letrozole. BMS-754807 in combination with tamoxifen extended the upregulation of these genes; whereas, epidermal growth factor receptor (EGFR) expression only modestly changed in response to single drug treatment but increased substantially by tamoxifen/BMS-754807 in combination.
Figure 7.
Genes significantly modulated by either single hormonal agent or in combination with BMS-754807. RNA isolated from resected tumors at treatment end (day 28) was used for DNA microarray experiments, as described in Methods. A, heat map showing the expression pattern changes and clustering of 698 genes by protocol treatment. B, changes in IGF-1R and ER expression by protocol treatment. Columns, mean; bars; SD. C, changes in cell cycle, proliferation, and prosurvival genes that represent the most significant changes in response to protocol treatment. D, changes in receptor tyrosine kinase genes that represent the most significant changes in response to protocol treatment.
Ingenuity Pathway Analysis was done to compare the involvement of biological functions and canonical signaling pathways of genes modulated by each treatment condition (Supplementary Fig. S1). The top significant differences in biological functions (e.g., cell cycle, growth and proliferation, and cell death) and canonical signaling pathways (e.g., ATM signaling and p53 signaling) were shown in Supplementary Fig. S1A and B, respectively. Genes that were further modulated by tamoxifen and BMS-754807 were involved in cell-cycle regulation (e.g., CCNA2, CCNB1, CCNB2, CCNE2, CDC6, CDC7 CDK2, CHEK1, and CHEK2), antiapoptosis (e.g., BAG3, BCL2, and BIRC5), and cell proliferation (e.g., Ki67 and PCNA).
Discussion
We have shown that targeting IGF signaling has antitumor activity in combination with hormonal therapies. This is clinically significant, as resistance to hormonal therapy is a common clinical problem that limits survival in patients with breast cancer (2). As agents targeting IGF-1R/InsR, such as BMS-754807 are currently undergoing clinical investigation, these data would support the combination of IGF-targeted therapy with hormonal therapy. Interestingly, BMS-754807 seems to synergize with hormonal therapies agents that target ER signaling by different mechanisms with similar efficiency. This would suggest that IGF signaling has a role in estrogen signaling downstream of the ER. Indeed, despite having similar in vitro and in vivo efficacy during treatment, letrozole in combination with BMS-754807 decreased ER expression, whereas tamoxifen in combination with BMS-754807 led to increased ER expression by Western blotting (Fig 4). This contrasted the RNA expression of ER in both cases, suggesting compensatory mechanisms of regulation. In regards to the IGF targeting component, initial data suggests that the method of IGF targeting does indeed matter. Preliminary data with the same MCF-7/AC-1 model suggests that using a mAb against the IGF-1R does not enhance the efficacy of hormonal therapy in vivo (29, 30). Although unclear as to why this may be different, mAb therapy against the IGF-1R induced upregulation of InsR A expression, which is an IGF signaling receptor (31). This upregulation of InsR-A expression is not substantially modulated in response to BMS-754807 either as a single agent or in combination with hormonal therapy. This may also explain the recent negative clinical trial of the anti–IGF-1R mAb AMG-479 in combination with hormonal therapy in patients with breast cancer (32). These data in summation would suggest that targeting the InsR is a critical component of enhancing the antitumor effects of hormonal therapy targeting in breast cancer.
In regards to targeting the InsR, there are hypothetical concerns about the safety and tolerability of this approach (33). This largely contributed to the initial suggestion in the field of selectively targeting IGF signaling with an anti–IGF-1R mAb to avoid metabolic dysregulation and represented the most viable strategy at the time. However, ironically, among the most common side effects from anti–IGF-1R–targeted mAbs is hyperglycemia (22, 34). Importantly, early investigations with IGF-1R/InsR targeting small molecule inhibitors have shown reasonable tolerability with mild adverse event profiles that include manageable hyperglycemia (35–37). Clinical investigations will be needed to determine whether this is tolerable and is an effective method of IGF signaling blockade in combination with hormonal therapy.
The mechanism of synergy between antiestrogen hormonal therapy and BMS-754807 seems to be multifactorial. We suggest several mechanisms on the basis of the expression analyses that show differences between genes that are modulated in response to BMS-754807 with tamoxifen and compare this to the effect of BMS-754807 with letrozole, which had no improvement in time to study endpoint (Fig. 5C and D). This is an intriguing comparison as the improvements in the primary endpoints were similar for BMS-754807 with either tamoxifen or letrozole (Fig. 5A and B). One can speculate that this results from the described “withdrawal” response to tamoxifen. Tumor response after withdrawal of tamoxifen was first described by Legault-Poisson and colleagues (38) and then by other investigators (39–43). Prospective data has also shown that up to 40% of patients progressing on tamoxifen had a response or prolonged stability after tamoxifen withdrawal (44). Withdrawal responses from aromatase inhibitors, however, are not as well described (45, 46). These clinical findings may explain our results following the completion of therapy.
Overall, the DNA microarray data from BMS-754807/tamoxifen-treated tumors suggested a stronger repression of proliferative, cell cycle, DNA damage, and survival markers than other treatments investigated, including BMS-754807/letrozole. BMS-754807 in combination with tamoxifen had a substantial decrement in Ki-67 gene expression at the end of treatment in comparison with all other treatments, including BMS-754807 in combination with letrozole (Fig. 7C). It is possible that the enhanced effects of the Ki-67 downregulation after 28 days of treatment with BMS-754807 and tamoxifen led to the extended benefits of the combination beyond the end of treatment that were not seen with BMS-754807 in combination with letrozole. Future studies will be needed to determine whether this effect contributed to the long-term benefits of the BMS-754807 plus tamoxifen combination. In addition, canonical pathway analyses for hereditary breast cancer, ATM, DNA damage, and cell-cycle check point signaling were strikingly upregulated in response to BMS-754807 + tamoxifen. Western blotting also suggested that the combination of BMS-754807 with 4-hydroxytamoxifen maximally blocked survival signaling through AKT (Fig. 4). Thus, future biomarker evaluations in studies investigating BMS-754807 in combination with hormonal therapy should include evaluation of cell cycle, DNA damage, survival, and proliferation markers, including Ki-67.
Expression of the erbB family of receptors, as well as other receptor tyrosine kinases identified in Fig. 7D, may also be important biomarkers of resistance and represent adaptive mechanisms of escape in response to IGF and estrogen-targeted therapy. Modulation of erbB receptors has been previously described as an adaptive pathway to IGF blockade and may be sufficient for tumor cells to overcome IGF-targeting strategies, such as BMS-754807 (27, 47). As erbB-targeted therapies are available, this may support the rationale for combinations of BMS-754807 with either EGFR- or HER2-targeted therapy in tumors expressing these proteins.
In summary, IGF-1R/InsR inhibition with BMS-754807 enhances the antitumor effects of hormonal therapy in vitro and in vivo. These preclinical data led us to propose a phase II noncomparative clinical trial investigating the combination of BMS-754807 and letrozole or single-agent BMS-754807 in patients with locally advanced/metastatic, estrogen receptor–positive, nonsteroidal aromatase inhibitor–resistant breast cancer (ClinicalTrials.gov number NCT01225172) which is ongoing. These preclinical data also suggest that BMS-754807 with other hormonal agents including tamoxifen or fulvestrant warrant further investigation.
Supplementary Material
Acknowledgments
Grant Support
This work was supported in part by Mayo Clinic Breast SPORE (CA116201-03, P. Haluska, X. Hou), NIH K12 (CA090628-05, P. Haluska), Mayo Clinic Cancer Center (CA15083, P. Haluska), and Bristol Myers Squibb (P. Haluska).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Confiicts of Interest
F. Huang, K.A. Reeves, A. Greer, F.G. Finckenstein, J.M. Carboni, and M.M. Gottardis are employees of Bristol-Myers Squibb.
References
- 1.Baum M, Brinkley DM, Dossett JA, McPherson K, Patterson JS, Rubens RD, et al. Improved survival among patients treated with adjuvant tamoxifen after mastectomy for early breast cancer. Lancet. 1983;2:450. doi: 10.1016/s0140-6736(83)90406-3. [DOI] [PubMed] [Google Scholar]
- 2.Gee JM, Howell A, Gullick WJ, Benz CC, Sutherland RL, Santen RJ, et al. Consensus statement. Workshop on therapeutic resistance in breast cancer: impact of growth factor signalling pathways and implications for future treatment. Endocr Relat Cancer. 2005;12 (Suppl 1):S1–7. doi: 10.1677/erc.1.01054. [DOI] [PubMed] [Google Scholar]
- 3.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–87. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 4.Maor S, Mayer D, Yarden RI, Lee AV, Sarfstein R, Werner H, et al. Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumor cells: involvement of transcription factor Sp1. J Endocrinol. 2006;191:605–12. doi: 10.1677/joe.1.07016. [DOI] [PubMed] [Google Scholar]
- 5.Cascio S, Bartella V, Garofalo C, Russo A, Giordano A, Surmacz E. Insulin-like growth factor 1 differentially regulates estrogen receptor-dependent transcription at estrogen response element and AP-1 sites in breast cancer cells. J Biol Chem. 2007;282:3498–506. doi: 10.1074/jbc.M606244200. [DOI] [PubMed] [Google Scholar]
- 6.Hamelers IH, van Schaik RF, van Teeffelen HA, Sussenbach JS, Steenbergh PH. Synergistic proliferative action of insulin-like growth factor I and 17 beta-estradiol in MCF-7S breast tumor cells. Exp Cell Res. 2002;273:107–17. doi: 10.1006/excr.2001.5430. [DOI] [PubMed] [Google Scholar]
- 7.Lee AV, Weng CN, Jackson JG, Yee D. Activation of estrogen receptor-mediated gene transcription by IGF-I in human breast cancer cells. J Endocrinol. 1997;152:39–47. doi: 10.1677/joe.0.1520039. [DOI] [PubMed] [Google Scholar]
- 8.Milano A, Dal Lago L, Sotiriou C, Piccart M, Cardoso F. What clinicians need to know about antioestrogen resistance in breast cancer therapy. Eur J Cancer. 2006;42:2692–705. doi: 10.1016/j.ejca.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Li X, Burghardt R, Smith R, 3rd, Safe SH. Role of estrogen receptor (ER) alpha in insulin-like growth factor (IGF)-I-induced responses in MCF-7 breast cancer cells. J Mol Endocrinol. 2005;35:433–47. doi: 10.1677/jme.1.01858. [DOI] [PubMed] [Google Scholar]
- 10.Santen RJ, Song RX, Masamura S, Yue W, Fan P, Sogon T, et al. Adaptation to estradiol deprivation causes up-regulation of growth factor pathways and hypersensitivity to estradiol in breast cancer cells. Adv Exp Med Biol. 2008;630:19–34. doi: 10.1007/978-0-387-78818-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endo-crinology. 2007;148:4091–101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–73. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 13.Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–58. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 15.Sepp-Lorenzino L. Structure and function of the insulin-like growth factor I receptor. Breast Cancer Res Treat. 1998;47:235–53. doi: 10.1023/a:1005955017615. [DOI] [PubMed] [Google Scholar]
- 16.Dupont J, LeRoith D. Insulin and insulin-like growth factor I receptors: similarities and differences in signal transduction. Horm Res. 2001;55 (Suppl 2):22–6. doi: 10.1159/000063469. [DOI] [PubMed] [Google Scholar]
- 17.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–95. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 18.Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, et al. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–9. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 19.Mathieu MC, Clark GM, Allred DC, Goldfine ID, Vigneri R. Insulin receptor expression and clinical outcome in node-negative breast cancer. Proc Assoc Am Physicians. 1997;109:565–71. [PubMed] [Google Scholar]
- 20.Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L, et al. Elevated insulin receptor content in human breast cancer. J Clin Invest. 1990;86:1503–10. doi: 10.1172/JCI114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avnet S, Sciacca L, Salerno M, Gancitano G, Cassarino MF, Longhi A, et al. Insulin receptor isoform A and insulin-like growth factor II as additional treatment targets in human osteosarcoma. Cancer Res. 2009;69:2443–52. doi: 10.1158/0008-5472.CAN-08-2645. [DOI] [PubMed] [Google Scholar]
- 22.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–40. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 23.Haluska P, Shaw H, Batzel GN, Molife LR, Adjei AA, Yap TA, et al. Phase I dose escalation study of the anti-IGF-1R monoclonal antibody CP-751,871 in patients with refractory solid tumors. ASCO Meeting Abstracts. 2007;25:3586. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 24.Jelovac D, Macedo L, Handratta V, Long BJ, Goloubeva OG, Ingle JN, et al. Effects of exemestane and tamoxifen in a postmenopausal breast cancer model. Clin Cancer Res. 2004;10:7375–81. doi: 10.1158/1078-0432.CCR-04-0565. [DOI] [PubMed] [Google Scholar]
- 25.Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006;66:7775–82. doi: 10.1158/0008-5472.CAN-05-3984. [DOI] [PubMed] [Google Scholar]
- 26.Zhou DJ, Pompon D, Chen SA. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–54. [PubMed] [Google Scholar]
- 27.Haluska P, Carboni JM, TenEyck C, Attar RM, Hou X, Yu C, et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7:2589–98. doi: 10.1158/1535-7163.MCT-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Haluska P, Hou X, Huang F, Harrington SC, Greer A, Macedo L, et al. Complete IGF signaling blockade by the dual-kinase inhibitor, BMS-754807, is sufficient to overcome tamoxifen and letrozole resistance in vitro and in vivo. Cancer Res. 2009;69:402. [Google Scholar]
- 30.Hou X, Harrington SC, Macedo LF, Weroha SJ, Brodie A, Haluska P. Hormonal therapies differentially enhance insulin receptor isoform A and erbB receptor up-regulation in response to IGF-1R inhibitor MK-0646 in vivo. Proc Amer Assoc Cancer Res; 2009 Apr 18–22; Denver, CO. 2009. p. 2812. [Google Scholar]
- 31.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–88. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman PA, Ferrero JM, Bourgeois H, Kennecke H, De Boer R, Jacot W, et al. A randomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC) Cancer Res. 2010;70:S1–4. [Google Scholar]
- 33.Zhang H, Yee D. The therapeutic potential of agents targeting the type I insulin-like growth factor receptor. Expert Opin Investig Drugs. 2004;13:1569–77. doi: 10.1517/13543784.13.12.1569. [DOI] [PubMed] [Google Scholar]
- 34.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials–early lessons. J Mammary Gland Biol Neoplasia. 2008;13:471–83. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carden CP, Kim ES, Jones RL, Alam SM, Johnson FM, Stephens AW, et al. Phase I study of intermittent dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR) in patients with advanced solid tumors. J Clin Oncol. 2010;28:abstractno. 2530. [Google Scholar]
- 36.Desai J, Solomon BJ, Davis ID, Lipton LR, Hicks R, Scott AM, et al. Phase I dose-escalation study of daily BMS-754807, an oral, dual IGF-1R/insulin receptor (IR) inhibitor in subjects with solid tumors. J Clin Oncol. 2010;28:abstract no. 3104. [Google Scholar]
- 37.Evans T, Lindsay CR, Chan E, Tait B, Michael SA, Day S, et al. Phase I dose-escalation study of continuous oral dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR), in patients with advanced solid tumors. J Clin Oncol. 2010;28:abstract no. 2531. [Google Scholar]
- 38.Legault-Poisson S, Jolivet J, Poisson R, Beretta-Piccoli M, Band PR. Tamoxifen-induced tumor stimulation and withdrawal response. Cancer Treat Rep. 1979;63:1839–41. [PubMed] [Google Scholar]
- 39.Belani CP, Pearl P, Whitley NO, Aisner J. Tamoxifen withdrawal response. Report of a case. Arch Intern Med. 1989;149:449–50. [PubMed] [Google Scholar]
- 40.Stein W, 3rd, Hortobagyi GN, Blumenschein GR. Response of meta-static breast cancer to tamoxifen withdrawal: report of a case. J Surg Oncol. 1983;22:45–6. doi: 10.1002/jso.2930220112. [DOI] [PubMed] [Google Scholar]
- 41.Kristensen CA, Kristjansen PE, Brunner N, Quistorff B, Spang-Thomsen M. Growth inhibition in response to estrogen withdrawal and tamoxifen therapy of human breast cancer xenografts evaluated by in vivo 31P magnetic resonance spectroscopy, creatine kinase activity, and apoptotic index. Cancer Res. 1995;55:4146–50. [PubMed] [Google Scholar]
- 42.Howell A, Dodwell DJ, Anderson H, Redford J. Response after withdrawal of tamoxifen and progestogens in advanced breast cancer. Ann Oncol. 1992;3:611–7. doi: 10.1093/oxfordjournals.annonc.a058286. [DOI] [PubMed] [Google Scholar]
- 43.Canney PA, Griffiths T, Latief TN, Priestman TJ. Clinical significance of tamoxifen withdrawal response. Lancet. 1987;1:36. doi: 10.1016/s0140-6736(87)90717-3. [DOI] [PubMed] [Google Scholar]
- 44.Taylor SGt, Gelman RS, Falkson G, Cummings FJ. Combination chemotherapy compared to tamoxifen as initial therapy for stage IV breast cancer in elderly women. Ann Intern Med. 1986;104:455–61. doi: 10.7326/0003-4819-104-4-455. [DOI] [PubMed] [Google Scholar]
- 45.Bhide SA, Rea DW. Metastatic breast cancer response after Exemes-tane withdrawal: a case report. Breast. 2004;13:66–8. doi: 10.1016/j.breast.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Cigler T, Goss PE. Aromatase inhibitor withdrawal response in metastatic breast cancer. J Clin Oncol. 2006;24:1955–6. doi: 10.1200/JCO.2005.03.4108. [DOI] [PubMed] [Google Scholar]
- 47.Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–70. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.