Abstract
It is unclear whether cell culture methodology affects the corticosteroid sensitivity of chronic obstructive pulmonary disease (COPD) alveolar macrophages. We compared the effect of a short and a long isolation procedure on corticosteroid inhibition of lipopolysaccharide (LPS) stimulated cytokine release from COPD alveolar macrophages. We also investigated signalling pathways associated with macrophage activation during cell isolation. Macrophages cultured using a short isolation protocol released higher unstimulated levels of tumour necrosis factor (TNF)-α and chemokine C–X–C motif ligand (CXCL) 8; these macrophages were less sensitive to corticosteroid inhibition of LPS stimulated TNF-α and CXCL8 release when compared to a long isolation procedure. This was associated with increased p38 mitogen activated kinase (MAPK) activation. The p38 MAPK inhibitor, BIRB-796, significantly reduced unstimulated cytokine release. A key finding of this study was that both cell culture methods showed no difference in the corticosteroid sensitivity between COPD and control macrophages. We conclude that the culture of alveolar macrophages using a short isolation procedure alters cytokine production through p38 MAPK activation; this is associated with a change in corticosteroid sensitivity.
Keywords: Alveolar macrophage, Cytokines, Inflammation, Chronic obstructive pulmonary disease, Corticosteroids, p38 MAPK
1. Introduction
Alveolar macrophages play a key role in host defence to inhaled microbes and particulate matter (Gordon and Read, 2002). Macrophages are professional phagocytes which engulf invading antigens while minimising tissue damage (Murray and Wynn, 2011). These cells also regulate the immune response through the secretion of cytokines, chemokines, and growth factors.
COPD is characterised by an abnormal inflammatory response to inhaled noxious particles, commonly cigarette smoke (www.goldcopd.org). This inflammatory response involves increased numbers of macrophages in the airways (Hogg et al., 2004); these macrophages play a central role in co-ordinating pulmonary inflammation, for example by the secretion of CXCL8 which is known to be increased in the lungs of COPD patients (Keatings et al., 1996) and is a potent neutrophil chemoattractant (Kaur and Singh, 2013).
Inhaled corticosteroids (ICS) are the first choice anti-inflammatory treatment for COPD; these drugs improve lung function and reduce exacerbation rates (Calverley et al., 2007). However, many COPD patients treated with ICS have persistent airway inflammation and recurrent exacerbations (Bourbeau et al., 2007; Soriano et al., 2007). The effects of corticosteroids have been investigated using COPD alveolar macrophages cultured in vitro; it has been reported that corticosteroids have a reduced effect on cytokine production from COPD compared to control macrophages, leading to the suggestion that COPD macrophages have acquired corticosteroid resistance (Culpitt et al., 2003; Cosio et al., 2004). In contrast, we have repetitively found that the effects of corticosteroids on cytokine production from COPD and control macrophages are similar (Armstrong et al., 2009, 2011; Southworth et al., 2012; Higham et al., 2013; Lea et al., 2013; Plumb et al., 2013). We also observed that corticosteroids have a relatively limited effect on the macrophage secretion of certain inflammatory mediators that are centrally involved in the pathophysiology of COPD, such as CXCL8.
Alveolar macrophages are commonly isolated for culture by plate adherence. The duration of plate adherence may cause cell activation; plate adherence for 1 h results in higher levels of spontaneous TNF-α, CXCL8, and interleukin (IL)-6 production compared to plate adherence for 24 h (Tomlinson et al., 2012). These results demonstrate that alveolar macrophages show an increased level of activation soon after isolation; this was also associated with a failure to demonstrate an incremental response to subsequent LPS stimulation. Plate adherence itself may be the cause of this acute cellular activation, but the saline flushing required to retrieve macrophages from the lungs may also cause cell activation by osmotic or mechanical stress (Denkert et al., 1998; Aikawa et al., 2002; Shiratsuchi and Basson, 2005). A longer duration of culture for cell isolation appears to allow initial cell activation, due to either plate adherence or saline flushing, to settle over time.
It appears that the activation state and LPS responsiveness of macrophages differ according to the time of stimulation after isolation (Tomlinson et al., 2012). This phenomenon has not been studied in COPD alveolar macrophages. It is important to understand whether methodological differences in protocols for COPD macrophage culture used in different studies could lead to different states of cell activation that change corticosteroid sensitivity.
The aim of this study was to investigate the effect of cell culture methodology on the corticosteroid sensitivity of COPD alveolar macrophages. The effect of corticosteroids on cytokine production from unstimulated and LPS stimulated macrophages isolated using different protocols was investigated. We also investigated changes in cell signalling pathways during different macrophage isolation protocols.
2. Methods
2.1. Study subjects
43 patients undergoing surgical resection for lung cancer were recruited (Table 1). COPD was diagnosed based on ≥ 10 pack years smoking history, typical symptoms and airflow obstruction. Controls were smokers (S) with normal lung function. All subjects gave written informed consent. This research was approved by the local research ethics committee.
Table 1.
Subject demographics. Data shown are mean (sd). S: smokers, FEV1: forced expiratory volume in 1 s, FVC: forced vital capacity, and ICS: inhaled corticosteroid.
| S |
COPD |
|||||
|---|---|---|---|---|---|---|
| ELISA study 1 | ELISA study 2 | Western blot study | ELISA study 1 | ELISA study 2 | Western blot study | |
| n | 9 | 8 | 4 | 10 | 8 | 4 |
| GOLD stage I | n/a | n/a | n/a | 3 | 2 | 3 |
| GOLD stage II | n/a | n/a | n/a | 6 | 5 | 1 |
| GOLD stage III | n/a | n/a | n/a | 1 | 1 | 0 |
| Age (yrs) | 69.6 (5.5) | 67.9 (10.7) | 69.8 (8.5) | 68.4 (6.2) | 68.5 (8.1) | 67.3 (6.1) |
| Sex (M/F) | 4/5 | 3/5 | 4/0 | 6/4 | 5/3 | 2/2 |
| FEV1 (L) | 2.3 (0.5) | 1.9 (0.2) | 2.8 (0.4) | 1.8 (0.5) | 1.8 (0.6) | 2.0 (0.9) |
| FEV1 % predicted | 100.9 (18.1) | 86.5 (9.1) | 110.5 (37.7) | 71.5 (15.1) | 65.4 (16.1) | 85.5 (13.8) |
| FVC (L) | 3.1 (0.6) | 2.6 (0.3) | 3.9 (0.7) | 3.0 (0.6) | 3.3 (0.6) | 4.0 (1.9) |
| FEV1/FVC ratio (%) | 75.1 (5.7) | 82.2 (12.4) | 72.4 (8.6) | 61.1 (7.9) | 55.2 (16.3) | 49.4 (5.9) |
| Pack year history | 36.3 (26.3) | 46.4 (17.3) | 20.7 (5.0) | 64.7 (37.2) | 48.3 (30) | 45.3 (29.4) |
| Current/ex-smoker | 3/6 | 5/3 | 2/2 | 6/4 | 6/2 | 2/2 |
| ICS users | 0 | 0 | 0 | 4 | 4 | 0 |
2.2. Alveolar macrophage isolation
Alveolar macrophages were isolated from the lungs as previously described (Armstrong et al., 2009). Briefly, resected tissue was perfused with 0.1 M NaCl and the retrieved fluid was centrifuged (10 min, 400 g, room temperature). The cell pellet was re-suspended in RPMI-1640 media (Sigma-Aldrich, Poole, UK), layered over Ficoll-Paque (GE Healthcare, Buckinghamshire, UK), and centrifuged. Alveolar macrophage number and total cell number were assessed by trypan blue exclusion. Alveolar macrophages were re-suspended at a density of 1 × 106 per ml in RPMI-1640 media supplemented with 10% v/v foetal calf serum (Invitrogen, Paisley, UK), 2 mM l-glutamine (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich). The average number of non-adherent cells was 1.5 × 106 per ml.
2.3. Alveolar macrophage culture
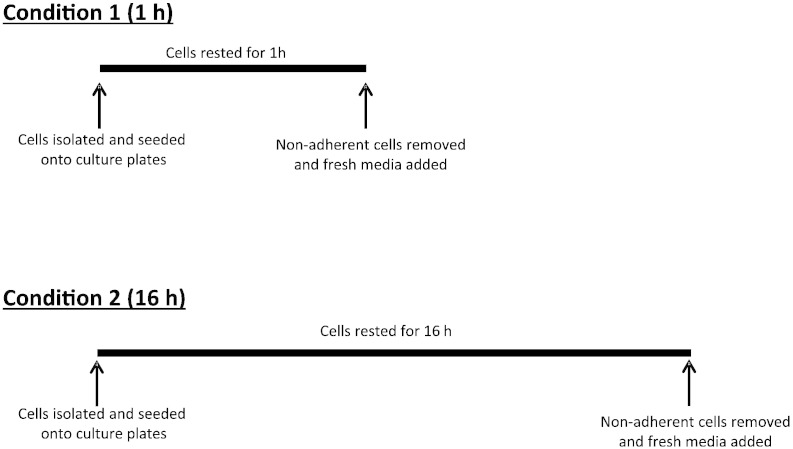
Freshly isolated macrophages were seeded onto flat bottomed 96-well plates at a concentration of 1 × 105 macrophages per well. There were two different culture conditions (Fig. 1); condition 1: macrophages were rested for 1 h in a 5% CO2 humidified atmosphere at 37 °C before non-adherent cells were removed and fresh medium was added (short incubation protocol). Condition 2: macrophages were rested for 16 h in a 5% CO2 humidified atmosphere at 37 °C and the following day non-adherent cells were removed and fresh medium was added (long incubation protocol).
Fig. 1.
A schematic representation of short (condition 1) and long (condition 2) incubation protocols used to isolate alveolar macrophages.
After the addition of fresh media, macrophages cultured under both conditions were cultured for a further 24 h with and without LPS (1 μg/ml Escherichia coli, O26:B6, Sigma-Aldrich). Cell culture supernatants were removed and analysed for TNF-α and CXCL8 by ELISA according to the manufacturer's instructions (R&D Systems, Abbingdon, UK).
2.3.1. The effect of dexamethasone on LPS stimulated cytokine release
After the addition of fresh media, macrophages cultured under both conditions (short and long incubation protocols) were treated with dexamethasone (0.1–1000 nM) or vehicle control (DMSO 0.005%, now referred to as “vehicle”) (both Sigma-Aldrich) for 1 h followed by LPS (1 μg/ml) stimulation for 24 h. Cell culture supernatants were removed and analysed for TNF-α and CXCL8 by ELISA.
2.3.2. The effects of dexamethasone and BIRB-796 on unstimulated cytokine release
After the addition of fresh media, macrophages cultured under both conditions were treated with either dexamethasone (1 μM), the p38 MAPK inhibitor BIRB-796 (1 μM, Sigma-Aldrich), or vehicle for 24 h (this timepoint was chosen to match the cell culture with LPS). Cell culture supernatants were removed and analysed for TNF-α, and CXCL8 by ELISA.
2.3.3. Phosporylation of p38 MAPK and NF-κB p65
Freshly isolated macrophages were seeded onto flat bottomed 6-well plates at a concentration of 1 × 106 macrophages per well for experiments investigating protein content by Western blot. Macrophages were cultured for 15 min, 30 min, 60 min (which represent the short incubation protocol and can be subdivided into 1a, 1b, and 1c respectively) and 16 h (condition 2), prior to non-adherent cell removal and the remaining cells were lysed. Samples were then prepared for Western blot.
2.4. Western blot
Cells were lysed in RIPA buffer [10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.25%] containing phosphatase (Sigma-Aldrich) and protease inhibitors (Calbiochem, Nottingham, UK). Cell lysates diluted in sample buffer [62.5 mM Tris, 10% glycerol, 1% SDS, 1% β-mercaptoethanol, and 0.01% bromphenol blue, pH 6.8] were electrophoresed on SDS polyacrylamide gels (10%) and transferred to Hy-bond ECL membranes (Whatman International Ltd, Kent, UK). Membranes were incubated with blocking buffer [5% dried milk in Tris buffered saline containing 0.1% Tween 20 (TBS/Tween 20)] for 1 h at room temperature and then incubated with primary antibodies (diluted in blocking buffer) at 4 °C overnight (rabbit anti-phospho-p38 MAPK [Thr180/Tyr182], rabbit anti-phospho-NF-κB p65 [Ser536]; Cell Signalling, Hertfordshire, UK, and rabbit anti-β-actin, Abcam, Cambridge, UK). After washing in TBS/Tween 20, the membranes were incubated for 60 min with a peroxidase-conjugated secondary antibody (diluted in wash buffer) (horseradish peroxidase-conjugated goat anti-rabbit, Cell Signalling), washed again, and the antibody-labelled proteins were visualized by enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK). Densitometric analysis was performed by normalising band density to that for β-actin using Quantity One v4.6.1 software (Bio-Rad, Hertfordshire, UK).
2.5. Data analysis
All statistical analysis was performed using GraphPad InStat software (GraphPad Software Inc., La Jolla, California, USA). Unstimulated CXCL8 release, and LPS stimulated TNF-α and CXCL8 release were normally distributed and therefore compared using a repeated measures ANOVA followed by a paired t-test. Dexamethasone dose response curves were compared using a repeated measures ANOVA followed by a Bonferonni post-test. Unstimulated TNF-α release was not-normally distributed and therefore compared using a Friedman test followed by a Wilcoxon matched pairs test. p < 0.05 was considered significant.
3. Results
3.1. Supernatant cytokine secretion
Alveolar macrophages isolated from 10 COPD patients and 9 S were used for the following experiments.
3.1.1. Unstimulated cytokine release
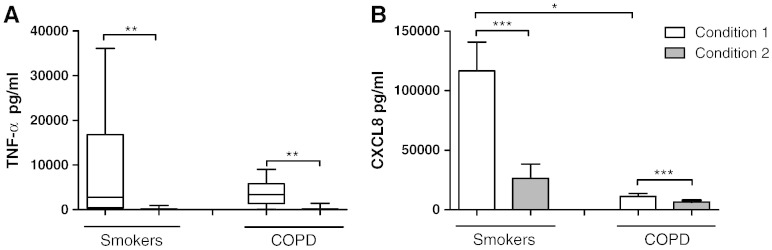
Alveolar macrophages isolated under condition 1 (1 h culture for adherence and isolation) and condition 2 (16 h culture for adherence and isolation) were then cultured for 24 h; supernatant TNF-α and CXCL8 levels under condition 1 were significantly higher (p < 0.001 for all comparisons) compared to condition 2, in both COPD patients and S (Fig. 2). COPD alveolar macrophages cultured under condition 1 had significantly lower levels of CXCL8 compared to S (p = 0.02), but there were no differences for CXCL8 between groups under condition 2. There were no differences between groups for TNF-α.
Fig. 2.
The effect of alternative culture conditions on unstimulated cytokine release. Macrophages from smoking controls (n = 9) and COPD patients (n = 10) were cultured under condition 1 (white bar) or condition 2 (grey bar) and left for a further 24 h. Culture supernatants were analysed for TNF-α (A) and CXCL8 (B). Data shown are median ± range (A) or mean ± SEM (B) where *, **, and *** = significantly higher levels of mediator release (p < 0.05, p < 0.01, and p < 0.001 respectively).
3.1.2. LPS stimulated cytokine release
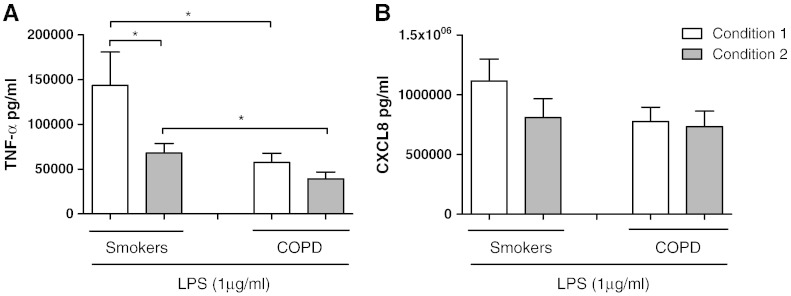
Alveolar macrophages isolated under conditions 1 and 2 were stimulated with LPS for 24 h (Fig. 3). LPS stimulation increased TNF-α and CXCL8 production under both conditions above matched unstimulated controls. COPD alveolar macrophages produced similar TNF-α levels when comparing conditions 1 and 2 (Fig. 3), while TNF-α production from S alveolar macrophages was significantly higher from condition 1 when compared to condition 2 (p = 0.03). TNF-α production from COPD alveolar macrophages cultured under conditions 1 and 2 was significantly lower than S alveolar macrophages (p = 0.048 and p = 0.04 respectively).
Fig. 3.
The effect of alternative culture conditions on LPS stimulated cytokine release. Macrophages from smoking controls (n = 9) and COPD patients (n = 10) were cultured under condition 1 (white bar) or condition 2 (grey bar) and stimulated with LPS (1 μg/ml) for 24 h. Culture supernatants were analysed for TNF-α (A) and CXCL8 (B). Data shown are mean ± SEM where * = significantly higher levels of mediator release (p < 0.05).
There were no differences observed in the levels of LPS stimulated CXCL8 when comparing conditions 1 and 2 (Fig. 3). There were also no differences when comparing COPD patients to S.
3.1.3. Dexamethasone inhibition of LPS stimulated cytokine release
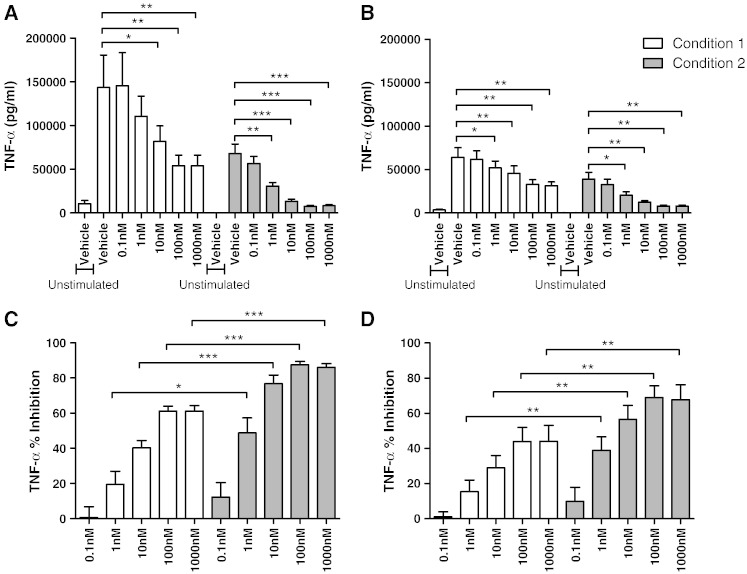
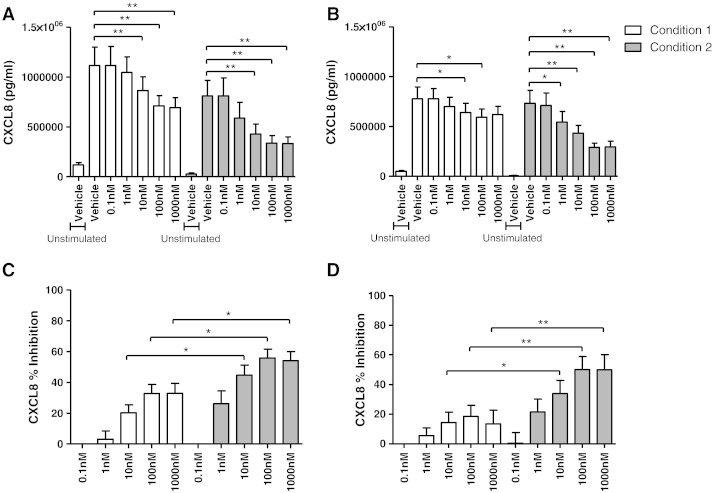
Alveolar macrophages isolated under conditions 1 and 2 were subsequently cultured with dexamethasone (0.1–1000 nM) for 1 h followed by LPS stimulation for 24 h. TNF-α and CXCL8 production from both COPD and S alveolar macrophages was significantly inhibited by dexamethasone. However, the level of TNF-α and CXCL8 inhibition in both COPD and S alveolar macrophages cultured under condition 2 was significantly higher than condition 1 (at concentrations 1–1000 nM for TNF-α and 10–1000 nM for CXCL8; Figs. 4 and 5 respectively). The effects of dexamethasone on TNF-α and CXCL8 production were similar in COPD patients compared to S; although there was a numerically greater effect of dexamethasone at some concentrations, these differences were not statistically significant e.g. at 1000 nM dexamethasone, the mean difference for TNFα inhibition was 17% and 21% (conditions 1 and 2 respectively; p > 0.05), while the mean difference for CXCL8 inhibition was 20% and 18% for CXCL8 (conditions 1 and 2 respectively; p > 0.05).
Fig. 4.
The effect of alternative culture conditions on dexamethasone inhibition of LPS stimulated TNF-α release. Macrophages from smoking controls (A and C; n = 9) and COPD patients (B and D; n = 10) were cultured under condition 1 (white bar) or condition 2 (grey bar), pre-treated with vehicle control (0.005% DMSO) or dexamethasone (0.1–1000 nM) for 1 h before being stimulated with LPS (1 μg/ml) for 24 h. Culture supernatants were analysed for TNF-α. Data shown are mean ± SEM of absolute TNF-α levels (A and B) or percentage inhibition of TNF-α (C and D). *, **, and *** = significant mediator reduction below vehicle control (p < 0.05, p < 0.01, and p < 0.001 respectively) (A and B) or significantly higher level of inhibition compared to respective dexamethasone concentration under condition 1 (C and D).
Fig. 5.
The effect of alternative culture conditions on dexamethasone inhibition of LPS stimulated CXCL8 release. Macrophages from smoking controls (A and C; n = 9) and COPD patients (B and D; n = 10) were cultured under condition 1 (white bar) or condition 2 (grey bar), pre-treated with vehicle control (0.005% DMSO) or dexamethasone (0.1–1000 nM) for 1 h before being stimulated with LPS (1 μg/ml) for 24 h. Culture supernatants were analysed for CXCL8. Data shown are mean ± SEM of absolute CXCL8 levels (A and B) or percentage inhibition of CXCL8 (C and D). * and ** = significant mediator reduction below vehicle control (p < 0.05 and p < 0.01 respectively) (A and B) or significantly higher level of inhibition compared to respective dexamethasone concentration under condition 1 (C and D).
3.2. Activation of p38 MAPK and NF-κB p65
We hypothesised that the increased unstimulated release of TNF-α and CXCL8 from alveolar macrophages cultured under condition 1 may be due to increased activation of the p38 MAPK or NF-κB pathways. The reduced effects of dexamethasone may also be due to activation of these pathways. Alveolar macrophages from 4 COPD patients and 4 S were cultured for 15 min, 30 min, 60 min (conditions 1a, 1b, and 1c respectively), and 16 h (condition 2).
The 60 min time point reflects the point at which pathway activation can be observed following the short incubation protocol before the addition of stimulants or drugs. The 15 min and 30 min time points were included to ensure the window of activation was not missed. Likewise, the 16 h time point reflects the point at which pathway activation can be observed following the long incubation protocol before the addition of stimulants or drugs.
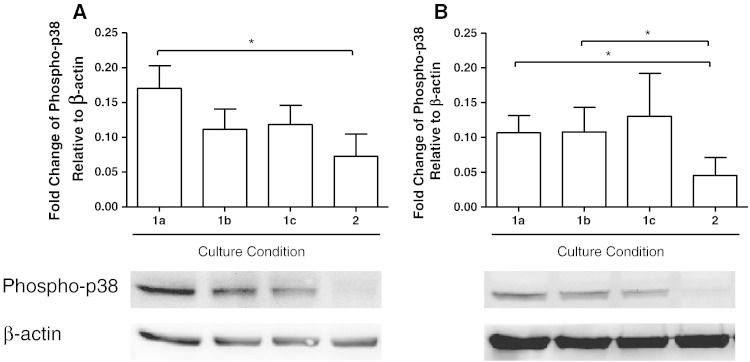
The levels of phosphorylated p38 MAPK were significantly higher in COPD macrophages cultured for 15 and 30 min compared to macrophages cultured for 16 h (p = 0.03 and p = 0.02 respectively; Fig. 6). There was a numerical trend for increased p38 MAPK activation in macrophages cultured for 60 min compared to 16 h, but this did not reach statistical significance (p = 0.1). Similarly, the levels of phosphorylated p38 MAPK were statistically higher in macrophages from S cultured for 15 min compared to 16 h (p = 0.03), with numerical trends evident in macrophages cultured for 30 and 60 min that did not reach statistical significance compared to 16 h (p = 0.3 and p = 0.2 respectively).
Fig. 6.
The effect of alternative culture conditions on the phosphorylation of p38 MAPK. Macrophages from smoking controls (A; n = 4) and COPD patients (B; n = 4) were cultured for 15 min (1a), 30 min (1b), 60 min (1c), or 16 h (2). Data shown are mean ± SEM of phosphorylated p38 MAPK protein band density normalised to β-actin. Representative Western blot protein bands are shown below respective conditions on the chart. * = significant increase in p38 MAPK levels (p < 0.05).
There was no difference due to the duration of cell culture in the levels of phosphorylated NF-κB p65 in COPD or S macrophages (Fig. 7).
Fig. 7.
The effect of alternative culture conditions on the phosphorylation of NF-κB p65. Macrophages from smoking controls (A; n = 4) and COPD patients (B; n = 4) were cultured for 15 min (1a), 30 min (1b), 60 min (1c), or 16 h (2). Data shown are mean ± SEM of phosphorylated NF-κB p65 protein band density normalised to β-actin. Representative Western blot protein bands are shown below respective conditions on the chart.
3.3. Dexamethasone and BIRB-796 inhibition of unstimulated cytokine release
Having observed that p38 MAPK activation occurs in unstimulated cells isolated using condition 1, we investigated whether p38 MAPK activation contributes to increased unstimulated TNF-α and CXCL8 release by using BIRB-796, a p38 MAPK inhibitor. Alveolar macrophages from 8 COPD patients and 8 S were isolated using conditions 1 and 2, and then cultured with dexamethasone (1 μM), BIRB-796 (1 μM), or vehicle control for 24 h.
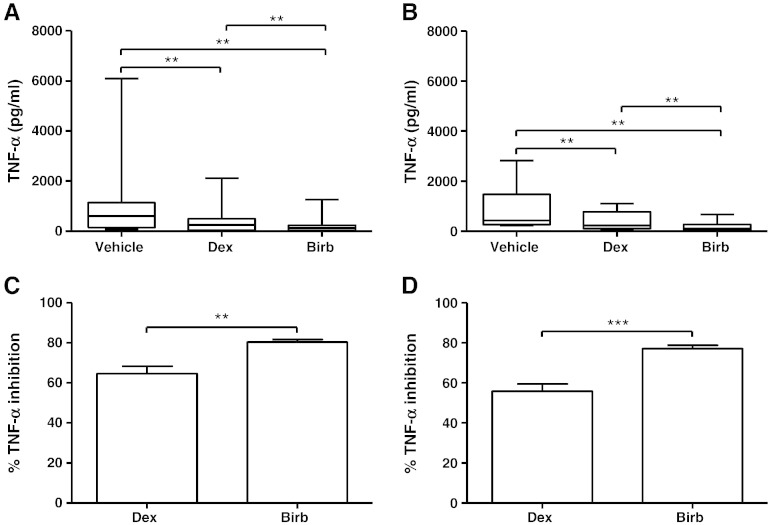
The levels of TNF-α released from macrophages cultured under condition 2 were below the lower limit of detection of the ELISA. The release of TNF-α from COPD and S macrophages cultured under condition 1 was inhibited by BIRB-796, with the effect of this p38 MAPK inhibitor being greater than dexamethasone in both COPD and S macrophages (p = 0.0004 and p = 0.002 respectively; Fig. 8).
Fig. 8.
The effect of dexamethasone and BIRB-796 on unstimulated TNF-α release. Macrophages from smoking controls (n = 8; A and C) and COPD patients (n = 8; B and D) were cultured under condition 1 and then treated with vehicle (0.005% DMSO), dexamethasone (1 μM), or BIRB-796 (1 μM) for 24 h. Culture supernatants were analysed for TNF-α. Data shown are median ± range of absolute TNF-α levels (A and B) or mean ± SEM of percentage inhibition of TNF-α (C and D). ** = significant reduction in TNF-α levels compared to vehicle control (A and B) (p < 0.01). ** and *** = significantly higher level of inhibition (C and D) (p < 0.01 and p < 0.001 respectively).
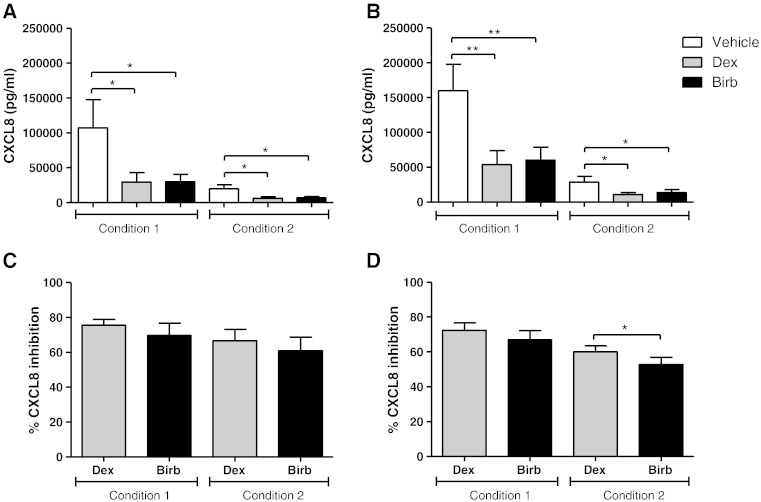
The levels of CXCL8 were quantifiable from macrophages cultured under both conditions, and were significantly inhibited by dexamethasone and BIRB-796 (Fig. 9). The effects of dexamethasone on CXCL8 inhibition were greater than BIRB-796 in COPD macrophages cultured under condition 2 (p = 0.03) but these drugs had similar effects under condition 1 (Fig. 9). There was no difference between BIRB-796 and dexamethasone on CXCL8 production from S macrophages cultured under conditions 1 and 2. There was no difference in the level of inhibition of TNF-α and CXCL8 when comparing COPD patients to S.
Fig. 9.
The effect of dexamethasone and BIRB-796 on unstimulated CXCL8 release. Macrophages from smoking controls (n = 8; A and C) and COPD patients (n = 8; B and D) were cultured under condition 1 or 2 and then pre-treated with vehicle (DMSO 0.005%), dexamethasone (1 μM), or BIRB-796 (1 μM) for 24 h. Culture supernatants were analysed for CXCL8. Data shown are mean ± SEM of absolute CXCL8 levels or percentage inhibition of CXCL8. * and ** = significant reduction in CXCL8 levels compared to vehicle control (A and B) (p < 0.01). * = significantly higher level of inhibition (C) (p < 0.05 and p < 0.01 respectively).
4. Discussion
The isolation of alveolar macrophages from COPD patients and S using a short incubation time (1 h) resulted in significantly different cytokine production and corticosteroid sensitivity compared to a long incubation time (16 h). Short isolation times caused higher unstimulated levels of TNF-α and CXCL8 and reduced sensitivity to corticosteroid after LPS stimulation. These findings were associated with increased p38 MAPK activation with a short isolation protocol. It appears that p38 MAPK activation occurs immediately upon isolation, and that short isolation protocols result in p38 MAPK dependent cytokine production that is associated with corticosteroid insensitivity.
The increased levels of phosphorylated p38 MAPK after a short isolation time may be due to mechanical or osmotic stress (Denkert et al., 1998; Aikawa et al., 2002; Shiratsuchi and Basson, 2005) that occurs during the isolation process. Our Western blot experiments showed that p38 MAPK activation was diminished after the long isolation protocol. However, it is possible that the elevated phosphorylated p38 MAPK levels soon after retrieval from the lungs during the short isolation protocol may reflect the in vivo state; immunohistochemistry studies have shown that alveolar macrophages in lung tissue sections from COPD patients have elevated levels of phosphorylated p38 MAPK compared to controls (Renda et al., 2008; Gaffey et al., 2013). Our view is that the short isolation procedure caused p38 MAPK activation due to mechanical and osmotic stress, but a degree of p38 MAPK activation was probably also present because of the in vivo pulmonary environment in COPD.
Activation of the p38 MAPK pathway promotes inflammation through increasing inflammatory gene expression, stabilising mRNAs, and enhancing protein translation (Saklatvala, 2004). However, this pathway is corticosteroid insensitive in alveolar macrophages; it has been reported that corticosteroids do not inhibit phosphorylation of p38 MAPK or its downstream target, heat shock protein 27, in LPS stimulated alveolar macrophages of COPD patients and controls (Armstrong et al., 2011). In addition, administration of prednisolone to COPD patients did not suppress p38 MAPK activation in whole blood cultured ex-vivo (Singh et al., 2010). The increased activation of p38 MAPK in freshly isolated macrophages may therefore explain reduced corticosteroid inhibition of LPS stimulated TNF-α and CXCL8 observed using condition 1; the longer these macrophages were “rested” in cell culture, the phosphorylated levels of p38 MAPK reduced, and corticosteroid sensitivity increased.
Increased activation of the p38 MAPK pathway may also explain the increased unstimulated release of TNF-α and CXCL8 from macrophages isolated with a short incubation time. BIRB-796 reduced unstimulated TNF-α and CXCL8 release by approximately 80% and 70% respectively, confirming the p38 MAPK dependency of unstimulated cytokine production. Interestingly, under condition 1 the effect of BIRB-796 was greater than dexamethasone on TNF-α production, and equal to dexamethasone on CXCL8 production. This further demonstrates the important role of p38 MAPK activation during condition 1.
It has previously been demonstrated that COPD macrophages display progressive downregulation of classically activated (M1) genes including CXCL9, CXCL10, CXCL11 and IL-1β, and progressive upregulation of alternatively activated (M2) genes such as matrix metalloproteinase (MMP) 2 and MMP7 compared to S (Shaykhiev et al., 2009). Classically activated macrophages are known to be more pro-inflammatory, whereas alternatively polarised macrophages are known to have greater anti-inflammatory and tissue repair functions (Gordon, 2003; Gordon and Taylor, 2005; Martinez et al., 2009). Consequently, a reduced LPS stimulated pro-inflammatory response of COPD alveolar macrophages compared to controls has also been previously reported. Similarly, here we report that LPS stimulated TNF-α production from COPD alveolar macrophages was reduced compared to S, although there was no difference for CXCL8.
TNF-α release from LPS stimulated S macrophages cultured under condition 1 was higher than from macrophages cultured under condition 2. A probable explanation for this is the increased p38 MAPK activation during condition 1. TNF-α release from LPS stimulated COPD alveolar macrophages was similar when comparing culture conditions; this may be due to the blunted TNF-α response observed in COPD patients compared to S, with a lower window to detect differences between cell culture conditions.
There was no difference in the corticosteroid sensitivity of alveolar macrophages when comparing COPD patients and S, supporting our previous observations. Despite earlier studies proposing reduced corticosteroid sensitivity of COPD macrophages compared to controls (Culpitt et al., 2003; Cosio et al., 2004), we have consistently shown that the degree of corticosteroid inhibition of LPS stimulated cytokine production does not differ between macrophages from COPD patients and controls (Armstrong et al., 2009, 2011; Southworth et al., 2012; Higham et al., 2013; Lea et al., 2013; Plumb et al., 2013). In addition, Plumb et al. reported that glucocorticoid receptor (GR) function, examined by GR phosphorylation and transactivation in response to beclomethasone-17-monopropionate (17-BMP), is similar in macrophages from COPD patients and controls. We also confirm previous observations that LPS stimulated CXCL8 production is less sensitive to corticosteroid inhibition than other cytokines. Corticosteroid sensitivity is regulated by an innate transcriptional programme which is dependent on the stimulating signal and varies between inflammatory genes. Ogawa et al. reported that dexamethasone suppressed only approximately half of all LPS induced genes in healthy murine macrophages (Ogawa et al., 2005). This demonstrates the innate restrictions on corticosteroid inhibition, and probably explains the natural variations in the effects of corticosteroids between different inflammatory proteins secreted by COPD alveolar macrophages.
In conclusion, we have shown that the protocols used to isolate and culture alveolar macrophages from COPD patients and S can alter cytokine production through p38 MAPK activation, which is associated with a change in corticosteroid sensitivity after LPS stimulation. We believe these results reflect a change in macrophage activation due to the isolation procedure. However, the removal of non-adherent cells may also influence macrophage behaviour in cell culture and the study of both populations would be informative. Nevertheless, both cell culture methods showed no difference in corticosteroid sensitivity between COPD and control macrophages, in agreement with recent publications (Higham et al., 2013; Lea et al., 2013; Plumb et al., 2013). Our results highlight the importance of using consistent cell culture conditions in such experiments: different protocols can change corticosteroid sensitivity.
Acknowledgements
This work was kindly funded by GlaxoSmithKline and the BBSRC.
References
- Aikawa R., Nagai T., Kudoh S., Zou Y., Tanaka M., Tamura M., Akazawa H., Takano H., Nagai R., Komuro I. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension. 2002;39:233. doi: 10.1161/hy0202.102699. [DOI] [PubMed] [Google Scholar]
- Armstrong J., Sargent C., Singh D. Glucocorticoid sensitivity of lipopolysaccharide-stimulated chronic obstructive pulmonary disease alveolar macrophages. Clin. Exp. Immunol. 2009;158:74. doi: 10.1111/j.1365-2249.2009.03986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J., Harbron C., Lea S., Booth G., Cadden P., Wreggett K.A., Singh D. Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2011;338:732. doi: 10.1124/jpet.111.180737. [DOI] [PubMed] [Google Scholar]
- Bourbeau J., Christodoulopoulos P., Maltais F., Yamauchi Y., Olivenstein R., Hamid Q. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007;62:938. doi: 10.1136/thx.2006.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley P.M., Anderson J.A., Celli B., Ferguson G.T., Jenkins C., Jones P.W., Yates J.C., Vestbo J., for the TORCH Investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356:775. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Cosio B.G., Tsaprouni L., Ito K., Jazrawi E., Adcock I.M., Barnes P.J. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 2004;200:689. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt S.V., Rogers D.F., Shah P., De Matos C., Russell R.E., Donnelly L.E., Barnes P.J. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003;167:24. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- Denkert C., Warskulat U., Hensel F., Haussinger D. Osmolyte strategy in human monocytes and macrophages: involvement of p38MAPK in hyperosmotic induction of betaine and myoinositol transporters. Arch. Biochem. Biophys. 1998;354:172. doi: 10.1006/abbi.1998.0661. [DOI] [PubMed] [Google Scholar]
- Gaffey K., Reynolds S., Plumb J., Kaur M., Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur. Respir. J. 2013;42:28. doi: 10.1183/09031936.00170711. [DOI] [PubMed] [Google Scholar]
- GOLD . vol. 2013. 2013. (Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease). [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S.B., Read R.C. Macrophage defences against respiratory tract infections. Br. Med. Bull. 2002;61:45. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Higham A., Lea S., Plumb J., Maschera B., Simpson K., Ray D., Singh D. The role of the liver X receptor in chronic obstructive pulmonary disease. Respir. Res. 2013;14:106. doi: 10.1186/1465-9921-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg J.C., Chu F., Utokaparch S., Woods R., Elliott W.M., Buzatu L., Cherniack R.M., Rogers R.M., Sciurba F.C., Coxson H.O., Pare P.D. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:2645. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Kaur M., Singh D. Neutrophil chemotaxis caused by chronic obstructive pulmonary disease alveolar macrophages: the role of CXCL8 and the receptors CXCR1/CXCR2. J. Pharmacol. Exp. Ther. 2013;347:173. doi: 10.1124/jpet.112.201855. [DOI] [PubMed] [Google Scholar]
- Keatings V.M., Collins P.D., Scott D.M., Barnes P.J. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 1996;153:530. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Lea S., Plumb J., Metcalfe H., Spicer D., Woodman P., Fox J.C., Singh D. The effect of PPAR-gamma ligands on in vitro and in vivo models of COPD. Eur. Respir. J. 2013;43:409. doi: 10.1183/09031936.00187812. [DOI] [PubMed] [Google Scholar]
- Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009;27:451. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lozach J., Benner C., Pascual G., Tangirala R.K., Westin S., Hoffmann A., Subramaniam S., David M., Rosenfeld M.G., Glass C.K. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J., Robinson L., Lea S., Banyard A., Blaikley J., Ray D., Bizzi A., Volpi G., Facchinetti F., Singh D. Evaluation of glucocorticoid receptor function in COPD lung macrophages using beclomethasone-17-monopropionate. PLoS One. 2013;8:e64257. doi: 10.1371/journal.pone.0064257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renda T., Baraldo S., Pelaia G., Bazzan E., Turato G., Papi A., Maestrelli P., Maselli R., Vatrella A., Fabbri L.M., Zuin R., Marsico S.A., Saetta M. Increased activation of p38 MAPK in COPD. Eur. Respir. J. 2008;31:62. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004;4:372. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Shaykhiev R., Krause A., Salit J., Strulovici-Barel Y., Harvey B.G., O'Connor T.P., Crystal R.G. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 2009;183:2867. doi: 10.4049/jimmunol.0900473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Basson M.D. Activation of p38 MAPKalpha by extracellular pressure mediates the stimulation of macrophage phagocytosis by pressure. Am. J. Physiol. Cell Physiol. 2005;288:C1083. doi: 10.1152/ajpcell.00543.2004. [DOI] [PubMed] [Google Scholar]
- Singh D., Smyth L., Borrill Z., Sweeney L., Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J. Clin. Pharmacol. 2010;50:94. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- Soriano J.B., Sin D.D., Zhang X., Camp P.G., Anderson J.A., Anthonisen N.R., Buist A.S., Burge P.S., Calverley P.M., Connett J.E., Petersson S., Postma D.S., Szafranski W., Vestbo J. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest. 2007;131:682. doi: 10.1378/chest.06-1696. [DOI] [PubMed] [Google Scholar]
- Southworth T., Metryka A., Lea S., Farrow S., Plumb J., Singh D. IFN-gamma synergistically enhances LPS signalling in alveolar macrophages from COPD patients and controls by corticosteroid-resistant STAT1 activation. Br. J. Pharmacol. 2012;166:2070. doi: 10.1111/j.1476-5381.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson G.S., Booth H., Petit S.J., Potton E., Towers G.J., Miller R.F., Chain B.M., Noursadeghi M. Adherent human alveolar macrophages exhibit a transient pro-inflammatory profile that confounds responses to innate immune stimulation. PLoS One. 2012;7:e40348. doi: 10.1371/journal.pone.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]