Summary
Chronic obstructive pulmonary disease (COPD) is a heterogeneous condition of the lungs and body. It is increasingly clear that spirometric measures of lung function alone are inadequate for a complete understanding of the impact of disease and are insufficient for the categorization of disease severity. Techniques in chest imaging and quantitative image analysis have advanced to the point where they can provide novel in-vivo insight into disease and potentially examine divergent responses to therapy. The following will review the strengths and limitations of some of the leading imaging techniques, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and optical coherence tomography (OCT). Following a brief explanation of the technique, each section will detail some of the potentially useful information obtained with these examinations. Future clinical care and investigation will likely include some combination of these imaging modalities and more standard assessments of disease severity.
Keywords: COPD, imaging, CT scan, MRI, PET, OCT
Introduction
Chronic obstructive pulmonary disease (COPD) is a condition defined as incompletely reversible expiratory airflow obstruction due to the exposure of noxious inhaled particulates.(1) While the severity of disease is assessed by the degree of lung function impairment, it is increasingly clear that COPD is a syndrome with numerous pulmonary and extra pulmonary manifestations such as emphysematous destruction of the lung parenchyma, lung cancer, remodeling of the airways and vasculature as well as cardiac impairment (2), cachexia, and bone demineralization. (3) There is great interest in the clinical and research communities to refine our understanding of the potential association of these processes and there is a belief that imaging may provide some of that insight.
The following chapter will review the insights gained by imaging in smoking related COPD. The unique contributions of various imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), optical coherence tomography (OCT), and positron emission tomography (PET) to a better understanding parenchymal, airway, and vascular disease will be explored. Finally, the current and future contributions of imaging to clinical care will be discussed.
Computed Tomography (CT)
Parenchymal Disease
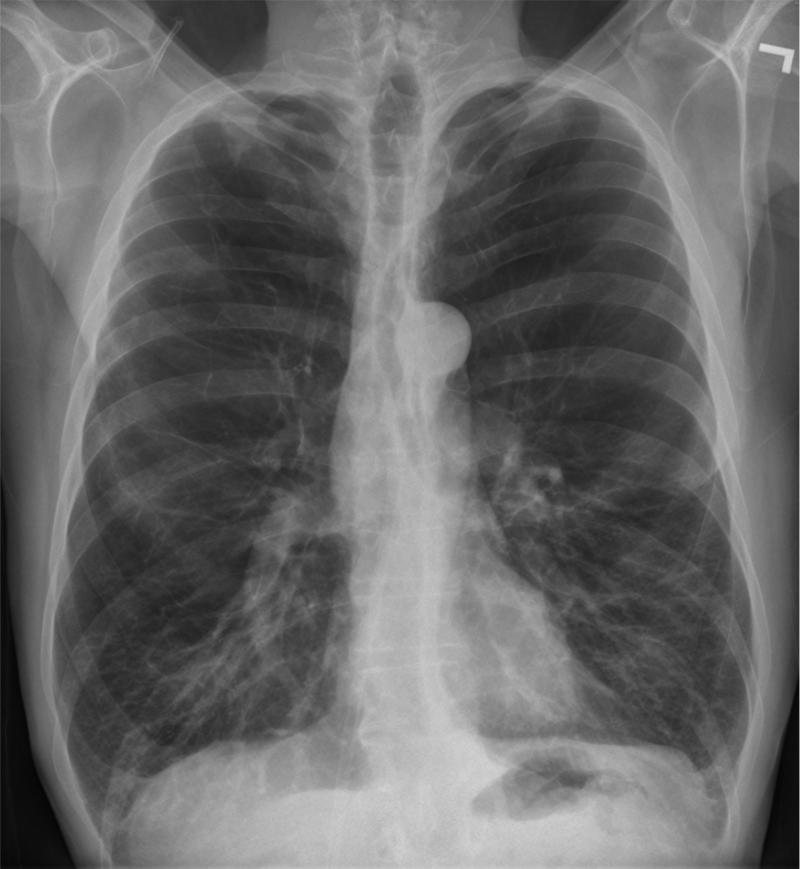
Smoking related destruction of the lung parenchyma is typically thought to manifest as emphysema.(4) Defined by its appearance in the secondary pulmonary lobule (the most fundamental structural component of the lung containing airways, lymphatics, vasculature, and parenchyma encapsulated in connective tissue), emphysema is visually classified as being centrilobular, panlobular, and paraseptal disease.(5) Initial roentgenologic studies of the lungs of smokers identified several cardiac signs for the presence of emphysema such as increased lucency of the lung fields, narrowing of the cardiac silhouette, and pruning of the peripheral vasculature (Figure 1A and B). Such findings are sensitive but lack the specificity required for large scale clinical and research applications.(6, 7)
Figure 1A and B.
PA and Lateral chest x-ray of a subject with severe COPD and emphysema. Note the lucency of the lung fields, flattening of the diaphragms, narrowed cardiac silhouette, and paucity of peripheral vascular markings.
With the introduction of CT into the medical sciences in the late 1970s, it became possible to visualize lung structure in vivo. One of the first applications of these imaging modalities was to develop subjective and objective methods for the assessment of emphysema.(8-11) Termed Density Mask Analysis, Muller and colleagues defined a Hounsfield Unit (HU) threshold in the CT image that dichotomized the lung into emphysematous (density less than that HU threshold) and non-emphysematous (density greater than that HU threshold) tissue.(12) This type of densitometric analysis was found to be predictive of clinically significant metrics of disease such as lung function and correlated with histopathologic assessments of emphysema on explanted lung tissue.(12, 13) With the caveat that the HU threshold used to delineate emphysematous from non-emphysematous tissue is subject to the image acquisition and reconstruction parameters, densitometric analysis of the lung parenchyma has become a cornerstone of radiologic characterization of lung disease in smokers.(14-18) (Figure 2). While densitometric analysis of the lung may provide global, regional, and lobar specific measures of emphysema on CT scan, when applied across a large region of lung a major limitation is its relative inability to differentiate emphysema subtype (centrilobular, panlobular, etc). This is most apparent when performing a head to head comparison of this technique with visual inspection of the lung parenchyma. While the visual analysis of the lung parenchyma suffers from intra and inter observer variability (19), in several investigations it has been demonstrated to correlate with pathology, lung function and predict outcome in clinical investigation.(8-11, 20, 21) In an effort to address this, the community of radiological and computer scientists has focused on developing techniques that may objectively identify emphysema type and distribution. These are based largely on patterns of local features in the secondary pulmonary lobule and for the purposes of this chapter will be collectively called textural analysis.
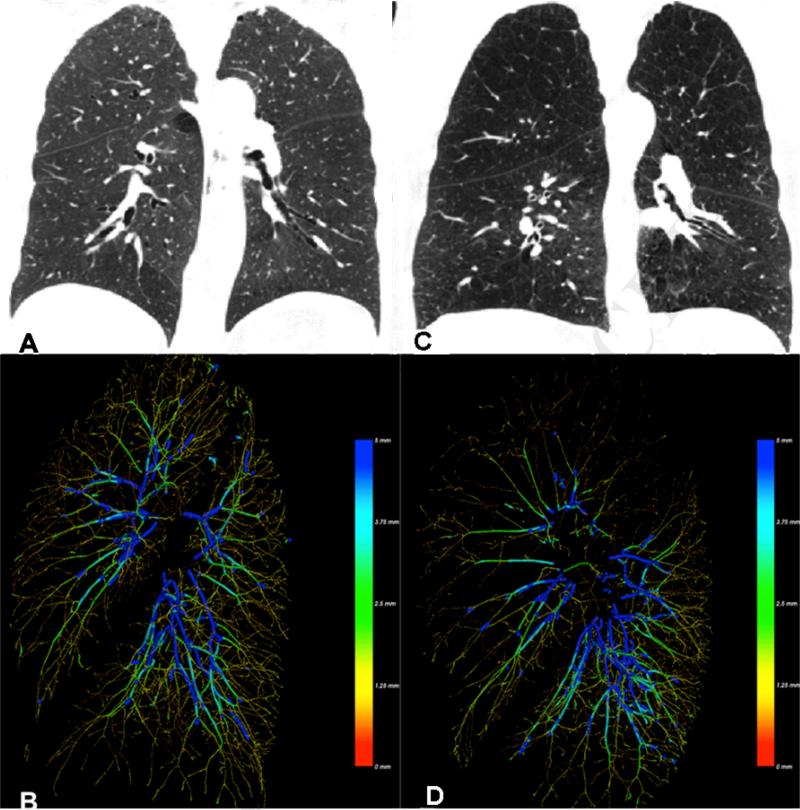
Figure 2.
Panels A and C provide a coronal view of the lungs of a smoker with normal lung function (Panel A) and one with moderate emphysema (Panel C). Panels B and D provide volumetric reconstructions of the left lung pulmonary vasculature from a left mid axillary view. Vessels are color coded by diameter. Notice the loss of vasculature and thinning of the vessels in regions most affected by emphysema.
Texture analysis of the lung involves the selection of a discrete region of interest (ROI) within the lung field and then assessing several parameters in this constrained region such as density and the patterns of changes in density. Using such an approach, several groups of investigators have demonstrated that this technique could accurately identify disease type (using visual analysis as a gold standard) and provide more robust measures of lung disease for correlation in clinical investigation.(22-26) Indeed Xu et al demonstrated that such a technique could provide information that surpassed that provided by visual inspection.(27) While the sensitivity of textural analysis is potentially superior to visual inspection, it is computationally costly and until a more parsimonious approach to tissue classification is developed it is limited to smaller scale investigations.
Finally, it is increasingly clear that the manifestations of smoking related parenchymal disease are not limited to low attenuating tissue on CT scan. Recently Lederer et al demonstrated that a subset of smokers are more likely to have high attenuating inflammatory, fibrotic and atelectatic regions of the lung that are associated with a restrictive spirometric pattern of lung function.(28) In a subsequent study, these lesions were found to be associated with reductions in lung volume and were inversely associated with emphysema.(29) Those smokers with such interstitial lung abnormalities (ILA) also tended to have pseudo normalization of their spirometry, likely from the mitigating effects of these abnormalities on reduced lung elastic recoil caused by emphysema. Further work is needed to determine the complete nature of ILA in smokers but a subset of these subjects may progress to clinically overt interstitial lung disease.(30) Also, given the common noxious exposure (tobacco smoke), a deeper understanding of the mechanisms that lead the lung down a fibrotic rather than emphysematous pathway of remodeling may offer insight into overall disease susceptibility.
Airway Disease
In obstructive lung disease, the site of expiratory airflow limitation is believed to be the small airways, those less than 2mm in diameter.(31) While this is beyond the resolution of clinical CT scanning, prior investigation suggests that radiological assessments of the central airways reflects remodeling in the lung periphery.(31) There are several metrics of central airway morphology in smokers. These include the external or total bronchial area (TBA), the wall area (WA), the lumen area (Ai), the wall thickness (WT) and the wall area percent (WA%: 100 * (wall area)/(lumen + wall area)). In one of the first systematic analysis of airway morphology in smokers, Nakano et al assessed the apical segment of the right upper lobe (RB1) in 114 smokers. In their analysis, they found that those subjects with the greatest WA% (increased ratio of wall area to lumen area) had the lowest FEV1 expressed as a percent of predicted.(32) Based upon this investigation, the WA% has become the most commonly employed metric for clinical investigation largely because it has consistently provided the strongest correlation to spirometric measures of lung function. In a subsequent investigation, this same group demonstrated that central airway remodeling apparent on CT reflected distal histopathologic remodeling of the small airways, those with great central airway wall thickening had more small airway disease.(33) More recent work has suggested that the more peripheral the radiological assessments of airway structure in smokers (measures performed in airway generations closer to the small airways) the stronger the correlation with lung function.(34) While this finding has compelled investigators to examine more and more distal airways, such measures are limited by the resolution of the CT images. For this reason, the most accurate measures of airway morphology are obtained from the 3rd generation segmental and possibly 4th generation subsegmental airways.
Investigators have begun to look beyond airway wall thickening to assess airway disease in smokers. Included in these efforts are quantitative assessments of mural density or attenuation.(35) The premise behind these investigations is that as an airway wall thickens or remodels both the shape and contents of the wall change. Normal bronchial cartilage may be gained or lost and normal connective tissue replaced by scar. Preliminary investigation suggests that airway wall attenuation may provide additional information regarding airway disease in smokers.(35) Further work is needed to comprehensively understand the scanner to scanner variability and the influence of body habitus on these measures.
Recently, Hogg et al introduced a new paradigm for airway disease in smokers. Not only does airway remodeling lead to luminal obstruction and expiratory airflow obstruction but there appears to be an outright loss of airways in advanced COPD.(36) Using micro CT to examine resected lung tissue, this group demonstrated that subjects with advanced emphysema may have lost up to 90% of their terminal bronchioles. In addition to expiratory airway collapse due to loss of elastic recoil and fixed luminal obstruction of the small airways, a third potential mechanism for increased resistance to flow is the loss of parallel pathways.
Based upon these findings Diaz et al examined the chest CT scans of 50 smokers enrolled in the Lung Tissue Research Consortium (LTRC) and found that those subjects with more advanced emphysema have pruning of the central airways on CT scan (loss of airways in generations 5-8).(37) Further, even after adjustment for densitometric measures of emphysema, the total airway count (TAC: sum of airway generations visible in the 3rd to 8th generation starting from the apical segment of the right upper lobe) was an independent predictor of the BODE score which is a validated multidimensional measure of mortality in COPD. Further histological validation is needed to determine the extent to which airway loss manifests in the more proximal airway tree however airway drop out may be a marker of the extreme of airway disease.
As CT acquisition times have decreased it is now feasible to perform more dynamic inspiratory/expiratory CT scanning of the chest. The addition of an expiratory image allows for the quantitative detection of the hallmark of a COPD, gas trapping. Visually, this may appear as mosaicism suggesting local gas trapping due to an admixture of emphysema and airway disease. Using a HU threshold of -856 (attenuation value for normal) the expiratory image can also be quantitatively assessed where all tissue below this value are designated as exhibiting gas trapping.(38) While a current limitation of such an approach is the inability to differentiate the effects of emphysema and airway remodeling, new techniques are being developed which may allow for the “subtraction” of emphysema giving the user a clearer picture of the impact of small airway disease.
Vascular Remodeling
Pulmonary vascular disease is an independent predictor of morbidity and mortality in COPD. It is estimated that 30 to 70% of subjects with COPD have clinically significant burdens of disease and recent work has demonstrated that pathologic pulmonary vascular remodeling is found even in smokers with normal lung function.(39-45) The mechanisms for this process likely include inflammation, hypoxic vasoconstriction, and outright loss of parallel pathways due to emphysematous destruction of the tissue. While the standard visual assessment of pulmonary vascular remodeling includes measurements of the diameter of the main pulmonary artery, more recent investigations have capitalized on the ability of CT imaging to provide detailed measures of structure. These studies have demonstrated that remodeling of the distal intra-parenchymal pulmonary vasculature yields compelling insights into the relation of vascular disease and emphysema, the effect of pulmonary vascular disease on pulmonary artery pressure, and a potential link between pulmonary vascular remodeling and atherosclerotic disease.(46-48)
More recently, Alford et al undertook an investigation of pulmonary vascular remodeling in the very earliest stages of smoking related lung disease.(49) In a cohort of 43 subjects (17 normals, 12 smokers with no emphysema and normal lung function, 12 smokers with very mild emphysema), using central venous boluses of iodinated contrast agent, this group was able to demonstrate that pulmonary vascular remodeling could be detected and quantitatively assessed at its very earliest stages.(49) While such an approach is not amenable to population based studies, the findings of this study are consistent with the hypothesis that emphysema may begin as a vascular disease leading to a regional loss of tissue.
With the advances and large scale application of CT scanning in clinical investigation there have been several recent studies that have provided compelling insight into the clinical and functional impact of smoking related lung disease. For example, using CT scans, epidemiologic, and clinical data from the Multi Ethnic Study of Atherosclerosis (MESA), Barr et al clearly demonstrated that emphysema and its associated hyperinflation compromises cardiac function through reductions in left ventricular filling, possibly due to occult pulmonary vascular remodeling.(2) More recently Han and colleagues depicted the very complex relationship between radiological emphysema, airway disease, and acute exacerbations of COPD.(50) The results of this investigation may allow clinicians and clinical investigators to identify who is at greatest risk for an AECOPD to enrich both clinical studies and maximize preventive therapies in the outpatient setting. Lastly, to mention one of the most direct applications of CT imaging of the chest in therapeutic intervention, a trial of bronchoscopically placed one-way valves to achieve minimally invasive volume reduction demonstrated that those subjects with incomplete interlobar fissures had the lowest chance of procedural benefit likely due to collateral ventilation.(51)
Possibly the greatest critique of CT imaging to date has been the lack of a clear vision of how quantitative assessments of parenchymal, airway, and vascular disease may guide the clinical care of patients with COPD. While the quantitative CT scan is not yet integrated into clinical practice the results of several recent investigations have provided new understanding of disease and it is through this understanding that new therapies and therapeutic approaches to care will be discovered. These advances don't come without concern. The clinical and research community is increasingly aware of the risks associated with the radiation exposure necessary for CT acquisition. While the estimates of the associations between the dose of radiation and risk of cancer vary, this risk may be as high as 1/80 lifetime risk from a single CT.(52, 53)
Finally, mention must be made regarding the overlap or co-occurrence of lung cancer and COPD. While it is unclear if the origins of cancer are found in airway or parenchymal remodeling it is believed that smokers with COPD who have emphysema on their CT are at the highest risk for developing cancer.(54-56) There are now several studies utilizing screening CT scans of the chest to determine if early detection and presumably early intervention will reduce cancer related mortality.(57-60) One of the largest and most recent studies, the National Lung Screening Trial (NLST) found a 20% relative reduction in mortality in subjects undergoing annual screening CT scans.(61) While these results are quite compelling, there are limitations to screening CT scans. Given the cost of each CT, it is impractical and impossible to screen the general population of smokers. Clearly further refinements of who is at greatest risk for the development of cancer need to be undertaken to develop a more focused screening algorithm. Also, screening CT scans of the chest of smokers leads to the detection of a large number of false positive nodules that may require further evaluation.(62-64) In addition to the added cost of these procedures comes the morbidity associated with lung biopsy and fiberoptic bronchoscopic approaches to obtaining tissue for histopathology diagnosis.(64)
Magnetic Resonance Imaging (MRI)
The basis for MRI imaging is the perturbation of protons (hydrogen atoms) by a burst of radio waves. A strong magnetic field is applied to the tissue which aligns the protons within. The brief application of a radio wave then forces these protons out of alignment. The energy emitted by the proton during this and the process of returning to alignment is detected by the scanner and converted into the image displayed for clinical use. Unlike CT scanning, no ionizing radiation is used to generate the image. Given this obvious advantage, MRI has the potential to perform detailed real time evaluations of tissue motion which are then related to global and local tissue mechanics. A limiting factor for the application of MR to the lung is, however, the lung architecture itself. The lung is primarily a gas filled structure whose density (and therefore concentration of protons) is well below that of solid organs such as the brain or liver. Because of this limitation, a good deal of research in lung imaging has been focused on the development and application of inhaled and intravenous contrast agents to enhance data collection.
Parenchyma
While the strength of CT is its ability to accurately reflect details in tissue architecture, it is limited in its ability to detect function. Generally tissue that appears normal on CT scan is assumed to make full contribution to overall lung function. Several recent MR studies using inhaled hyperpolarized noble gases such as 3Helium and 129Xenon have offered new insight into lung structure and function and have great promise for demonstrating the falsity of this assumption.
The promise of hyperpolarized gases for imaging has been known for over 25 years and it was not until the late 1990s that their application pulmonary research began to move towards its true potential.(65) To perform such experiments, a sample of Helium or Xenon is hyperpolarized using a laser and then upon inhalation will initially distribute throughout the gas containing regions of the lung. The initial diffusivity of the gas can then be assessed to provide quantitative information about lung structure such as mean linear intercept, surface-to-volume ratio, airway radii, and number of alveoli.(66) 129Xenon has the added advantage of being freely diffusible across the alveolar capillary membrane and several investigators have demonstrated that this diffusion and wash out in the capillary bed can be readily distinguishable. The integration of these steps, diffusion of gas into the alveolus, transfer across the alveolar-capillary membrane, and then removal by capillary blood flow allows quantitative insight into one of the most basic functions of the lung, matching of ventilation and perfusion.(67-72)
Another interesting property of hyperpolarized noble gases is their increased rate of decay in the presence of oxygen. What may appear to be a limitation to pulmonary research has been in turn demonstrated to offer additional information about function. Due to regional heterogeneity in ventilation perfusion matching in the lung, oxygen tension is not uniform throughout the gas containing regions of the parenchyma. Detection and quantification of this by measuring the differential rates of decay of 3He or 129Xe allows for an in-vivo assessment of the most fundamental aspect of lung function (Figure 3).(73-75)
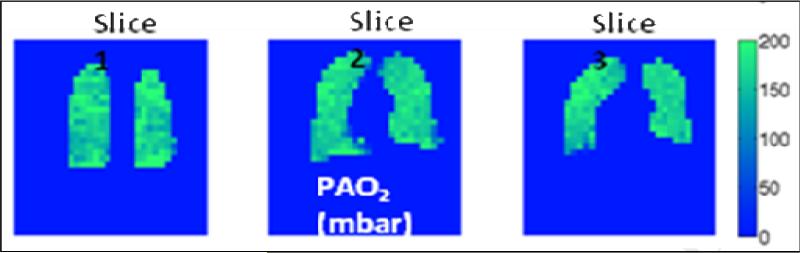
Figure 3.
3D coronal maps of a healthy human subject depicting regional differences in oxygen tension using 3He. At right is a color coded bar of oxygen tension in mbar (1mbar=0.75mm Hg). Slide courtesy of Dr. Samuel Patz.
Airways
Unlike CT, MR based investigations of the airways do not tend to focus on the morphology of the more central tracheobronchial tree. Rather, MR is more readily used to interrogate the flow of gas throughout the lung. The resulting flow of gas (diminished in more diseased areas of the lung) can be quantified and presented as a ventilation defect volume (VDV).(76-79) Such a measure may reflect regions afflicted by either emphysema or airway disease, or potentially an admixture of the two. Again, unlike CT scan, this assessment is not dependent on proximal airway structure reflecting distal remodeling, rather it is a direct measure of the properties of the distal lung parenchyma and small airways.
Vasculature
A strength of MRI is its ability to assess organ motion through the continuous or gated acquisition of data. As an example, ECG gated MR has become a standard for the calculation of cardiac function and offers more reproducible investigations of both right and left ventricular function than cardiac echo.(80-83) Given increasing interest in the interdependence of heart and lung in diseases such as COPD, an in-vivo tool to assess ventricular impairment is of great interest to clinical investigators. MR also has been used to assess the distensibilty of the central vessels.(84, 85) Previous investigations in pulmonary hypertension suggest that such measures offer prognostic value for therapeutic intervention.(86) Finally, the true source of pulmonary vascular compromise in COPD is likely the distal small vessels. As mentioned previously, remodeling at these sites has been observed even in smokers with normal lung function.(43, 44) While direct morphologic assessment of the vascular at this level is beyond the resolution of clinical MR, techniques such as dynamic contrast enhanced MR perfusion may offer a solution.(87-89) The premise to this technique is that after an intravenous contrast agent is introduced into the pulmonary arterial circulation, its local concentration will diminish proportionally to the ability of the lung to carry it away in the circulating blood volume. The more prolonged the decline in contrast concentration the lower regional flow and therefore the greater relative regional pulmonary vascular resistance. Such a technique has been applied with in pulmonary embolism or suspected pulmonary embolism.(87, 90) Further work is needed to apply and validate this technique to pulmonary vascular disease associated with smoking.
There is great promise in the applications of MR to investigations of smoking related lung disease. This comes from both the novel applications of inhaled and intravenous contrast agents as well as the remarkable ability it provides for resolving lung structure. Recently Kirby et al demonstrated that in a longitudinal assessment of 20 subjects (15 smokers, 5 normals), 3He proved to be a more sensitive measure of disease progression in the smokers over a 2 year period than standard spirometric and plethysmographic measures of lung function.(91) Additional ongoing work at multiple institutions suggests that these techniques may improve our ability to monitor disease progression and response to therapy. With time, MRI may play a very significant role in both investigation and clinical care of patients with COPD.
Positron Emission Tomography (PET)
Positron Emission Tomography or PET is a nuclear medicine technology based upon the detection of regionalized concentrations of a positron emitting radionuclide. The localization of this tracer is dependent upon the type of biologically active molecule that serves as its carrier. A commonly used molecule for clinical medicine is glucose which is taken up by the most metabolically active tissues. While PET has been widely used by clinicians for the detection and monitoring of malignancy, its applications in lung disease such as COPD have provided new insights into disease pathology and potentially pathogenesis.
Recently Vidal Melo and colleagues demonstrated that there is significant heterogeneity of lung perfusion in mild and moderate COPD even after adjusting for regional changes in lung density and ventilation.(92) Similar to work published by Alford et al, these changes in regional perfusion appear to precede visible changes to the lung structure such as emphysema suggesting that at least part of the progression of parenchymal disease in COPD is due to vascular remodeling.(49) Further work is needed to establish the relationship of longitudinal changes in regional lung perfusion and disease progression but as the authors suggest, assessments of vascular morphology and perfusion may indeed be a valuable biomarker for COPD.
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) is an imaging method based upon the refraction of light as it passes through tissues. A fiberoptic probe with a light source is introduced into the airways via a bronchoscope and the light patterns reflected by the tissue of interest are then reconstructed into an image. Unlike CT, MRI, or PET, OCT has the ability to resolve structures on the order of micrometers and can essentially provide in-vivo images of tissue histology (Figure 4). The primary strength of OCT is in examining airway morphology which thus readily lends itself to airway disease in COPD. Recently Coxson et al demonstrated in smokers that while CT measures of airway wall thickness correlate with lung function, it significantly overestimates airway size.(93) OCT was a more sensitive measure of disease and simultaneously provided data on airway wall morphology and subepithelial remodeling and collagen deposition. While OCT is not amenable to large scale population based studies, it's ability to detect and monitor airway disease in smokers makes it a natural candidate for the investigation of pharmaceutical agents thought to reduce mural inflammation.(94)
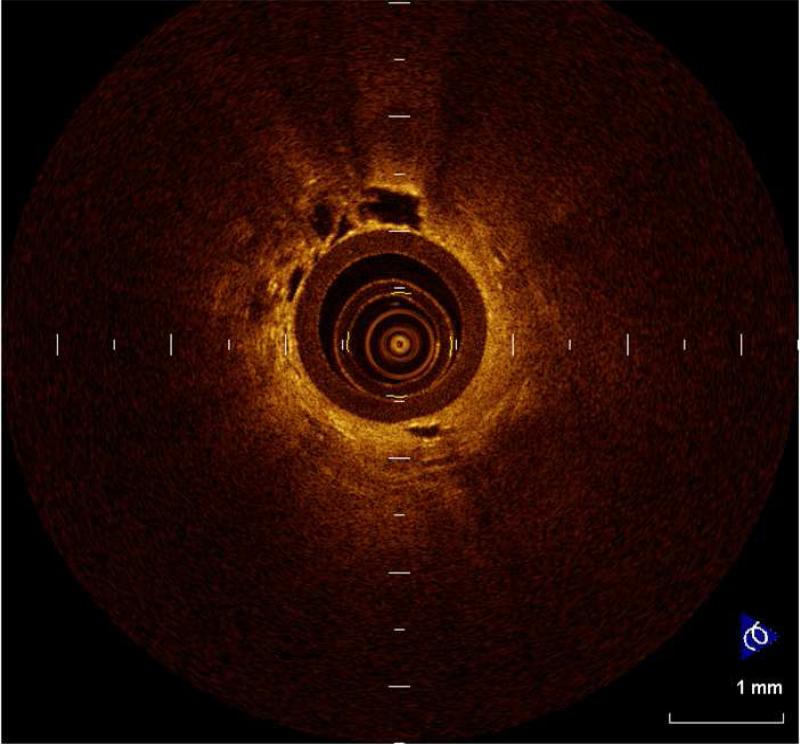
Figure 4.
Figure 4 provides a view using OCT. At the center can be seen the fiberoptic probe. Adjacent to the outer surface of the probe are alveolar ducts and alveoli. Slide courtesy of Dr. Anthony Lee of the British Columbia Cancer Research Centre.
While there are definite niches and limitations to the imaging modalities presented in this chapter, each offers unique strengths and in aggregate has provided new and exciting insight into the potential pathogenesis and physiology of COPD. The greatest contribution to imaging has been its ability to facilitate in-vivo investigation in both small cohorts and population based investigation. It will be some time before MRI, PET, OCT, or even quantitative CT scanning becomes part of standard clinical practice but they have already become the foundation for most clinical trials. It is clear that spirometric measures of lung function alone do not suffice for disease classification in smokers. They are too insensitive to disease heterogeneity and are only weakly correlated to both the symptoms and functional capacity experienced by patients with COPD. The future of investigation and clinical care lies in a combination of clinical characterization and image based assessments of lung structure.
Key points.
The advent of radiological techniques capable of providing detailed information about the structure of the anatomical elements contained in the thoracic cage has enhanced the capacity of health care providers of enhancing routine medical care.
New generation computerized tomography with visualization of lung parenchyma, airways and vessels has helped clarify the association between radiological changes and clinical phenotypes.
In chronic obstructive pulmonary disease (COPD), radiological phenotyping has been instrumental in the development of therapies such as surgical and bronchoscopic lung volume reduction.
Expansion of the knowledge of COPD using integration of the data obtained through imaging and function will likely help plan therapeutic trials such as regenerative therapy.
Acknowledgments
Dr. Washko was supported by grants K23 HL089353 and an award from the Parker B. Francis Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Gold executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, Zhang Y, Leader JK, Gur D, Greenspan SL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 183:885–890. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snider GL. Emphysema: The first two centuries--and beyond. A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992;146:1334–1344. doi: 10.1164/ajrccm/146.5_Pt_1.1334. [DOI] [PubMed] [Google Scholar]
- 5.Webb WR. Thin-section ct of the secondary pulmonary lobule: Anatomy and the image--the 2004 fleischner lecture. Radiology. 2006;239:322–338. doi: 10.1148/radiol.2392041968. [DOI] [PubMed] [Google Scholar]
- 6.Sutinen S, Christoforidis AJ, Klugh GA, Pratt PC. Roentgenologic criteria for the recognition of nonsymptomatic pulmonary emphysema. Correlation between roentgenologic findings and pulmonary pathology. Am Rev Respir Dis. 1965;91:69–76. doi: 10.1164/arrd.1965.91.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Nicklaus TM, Stowell DW, Christiansen WR, Renzetti AD., Jr The accuracy of the roentgenologic diagnosis of chronic pulmonary emphysema. Am Rev Respir Dis. 1966;93:889–899. doi: 10.1164/arrd.1966.93.6.889. [DOI] [PubMed] [Google Scholar]
- 8.Foster WL, Jr., Pratt PC, Roggli VL, Godwin JD, Halvorsen RA, Jr., Putman CE. Centrilobular emphysema: Ct-pathologic correlation. Radiology. 1986;159:27–32. doi: 10.1148/radiology.159.1.3952318. [DOI] [PubMed] [Google Scholar]
- 9.Bergin C, Muller N, Nichols DM, Lillington G, Hogg JC, Mullen B, Grymaloski MR, Osborne S, Pare PD. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133:541–546. doi: 10.1164/arrd.1986.133.4.541. [DOI] [PubMed] [Google Scholar]
- 10.Hruban RH, Meziane MA, Zerhouni EA, Khouri NF, Fishman EK, Wheeler PS, Dumler JS, Hutchins GM. High resolution computed tomography of inflation-fixed lungs. Pathologic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis. 1987;136:935–940. doi: 10.1164/ajrccm/136.4.935. [DOI] [PubMed] [Google Scholar]
- 11.Hayhurst MD, MacNee W, Flenley DC, Wright D, McLean A, Lamb D, Wightman AJ, Best J. Diagnosis of pulmonary emphysema by computerised tomography. Lancet. 1984;2:320–322. doi: 10.1016/s0140-6736(84)92689-8. [DOI] [PubMed] [Google Scholar]
- 12.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 13.Kinsella M, Muller NL, Abboud RT, Morrison NJ, DyBuncio A. Quantitation of emphysema by computed tomography using a “Density mask” Program and correlation with pulmonary function tests. Chest. 1990;97:315–321. doi: 10.1378/chest.97.2.315. [DOI] [PubMed] [Google Scholar]
- 14.Stern EJ, Frank MS. Ct of the lung in patients with pulmonary emphysema: Diagnosis, quantification, and correlation with pathologic and physiologic findings. AJR Am J Roentgenol. 1994;162:791–798. doi: 10.2214/ajr.162.4.8140992. [DOI] [PubMed] [Google Scholar]
- 15.Heremans A, Verschakelen JA, Van fraeyenhoven L, Demedts M. Measurement of lung density by means of quantitative ct scanning. A study of correlations with pulmonary function tests. Chest. 1992;102:805–811. doi: 10.1378/chest.102.3.805. [DOI] [PubMed] [Google Scholar]
- 16.Gould GA, Redpath AT, Ryan M, Warren PM, Best JJ, Flenley DC, MacNee W. Lung ct density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J. 1991;4:141–146. [PubMed] [Google Scholar]
- 17.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 18.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 19.Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, Silverman EK. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest. 2007;131:424–431. doi: 10.1378/chest.06-1040. [DOI] [PubMed] [Google Scholar]
- 20.Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, Hoffman EA, Reilly JJ. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. Copd. 2008;5:177–186. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 21.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 22.Uppaluri R, Mitsa T, Sonka M, Hoffman EA, McLennan G. Quantification of pulmonary emphysema from lung computed tomography images. Am J Respir Crit Care Med. 1997;156:248–254. doi: 10.1164/ajrccm.156.1.9606093. [DOI] [PubMed] [Google Scholar]
- 23.Uppaluri R, Hoffman EA, Sonka M, Hartley PG, Hunninghake GW, McLennan G. Computer recognition of regional lung disease patterns. Am J Respir Crit Care Med. 1999;160:648–654. doi: 10.1164/ajrccm.160.2.9804094. [DOI] [PubMed] [Google Scholar]
- 24.Chabat F, Yang GZ, Hansell DM. Obstructive lung diseases: Texture classification for differentiation at ct. Radiology. 2003;228:871–877. doi: 10.1148/radiol.2283020505. [DOI] [PubMed] [Google Scholar]
- 25.Sluimer IC, van Waes PF, Viergever MA, van Ginneken B. Computer-aided diagnosis in high resolution ct of the lungs. Med Phys. 2003;30:3081–3090. doi: 10.1118/1.1624771. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen L, Shaker SB, de Bruijne M. Quantitative analysis of pulmonary emphysema using local binary patterns. IEEE Trans Med Imaging. 29:559–569. doi: 10.1109/TMI.2009.2038575. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Sonka M, McLennan G, Guo J, Hoffman EA. Mdct-based 3-d texture classification of emphysema and early smoking related lung pathologies. IEEE Trans Med Imaging. 2006;25:464–475. doi: 10.1109/TMI.2006.870889. [DOI] [PubMed] [Google Scholar]
- 28.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: The multi-ethnic study of atherosclerosis (mesa)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washko GRHG, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, San Jose Estepar R, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D'Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO. Lung volumes and emphysema in smokers with interstital lung abnormalites. NEJM. 2011 doi: 10.1056/NEJMoa1007285. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: Over a 4-year follow-up. Respir Med. 104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 32.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 35.Washko GR, Dransfield MT, Estepar RS, Diaz A, Matsuoka S, Yamashiro T, Hatabu H, Silverman EK, Bailey WC, Reilly JJ. Airway wall attenuation: A biomarker of airway disease in subjects with copd. J Appl Physiol. 2009;107:185–191. doi: 10.1152/japplphysiol.00216.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogg JC, McDonough JE, Sanchez PG, Cooper JD, Coxson HO, Elliott WM, Naiman D, Pochettino M, Horng D, Gefter WB, et al. Micro-computed tomography measurements of peripheral lung pathology in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:546–549. doi: 10.1513/pats.200905-029DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz AA, Valim C, Yamashiro T, Estepar RS, Ross JC, Matsuoka S, Bartholmai B, Hatabu H, Silverman EK, Washko GR. Airway count and emphysema assessed by chest ct imaging predicts clinical outcome in smokers. Chest. 138:880–887. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coxson HO, Rogers RM, Whittall KP, D'Yachkova Y, Pare PD, Sciurba FC, Hogg JC. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med. 1999;159:851–856. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 39.Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:549–555. doi: 10.1513/pats.200709-148ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falk JA, Kadiev S, Criner GJ, Scharf SM, Minai OA, Diaz P. Cardiac disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:543–548. doi: 10.1513/pats.200708-142ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in copd. Eur Respir J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 42.Hasleton PS, Heath D, Brewer DB. Hypertensive pulmonary vascular disease in states of chronic hypoxia. J Pathol Bacteriol. 1968;95:431–440. doi: 10.1002/path.1700950213. [DOI] [PubMed] [Google Scholar]
- 43.Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, Wiggs BR, Rodriguez-Roisin R. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149:423–429. doi: 10.1164/ajrccm.149.2.8306040. [DOI] [PubMed] [Google Scholar]
- 44.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild copd. Am J Physiol. 1998;274:L908–913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 45.Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: Relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis. 1980;122:273–278. doi: 10.1164/arrd.1980.122.2.273. [DOI] [PubMed] [Google Scholar]
- 46.Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, Silverman EK, Patz S, Hatabu H. Quantitative ct measurement of cross-sectional area of small pulmonary vessel in copd: Correlations with emphysema and airflow limitation. Acad Radiol. 17:93–99. doi: 10.1016/j.acra.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka S, Washko GR, Yamashiro T, Estepar RS, Diaz A, Silverman EK, Hoffman E, Fessler HE, Criner GJ, Marchetti N, et al. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 181:218–225. doi: 10.1164/rccm.200908-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuoka S, Yamashiro T, Diaz A, Estepar RS, Ross JC, Silverman EK, Kobayashi Y, Dransfield MT, Bartholmai BJ, Hatabu H, et al. The relationship between small pulmonary vascular alteration and aortic atherosclerosis in chronic obstructive pulmonary disease: Quantitative ct analysis. Acad Radiol. 18:40–46. doi: 10.1016/j.acra.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci U S A. 107:7485–7490. doi: 10.1073/pnas.0913880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA, et al. Chronic obstructive pulmonary disease exacerbations in the copdgene study: Associated radiologic phenotypes. Radiology. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 52.Smith-Bindman R. Is computed tomography safe? N Engl J Med. 363:1–4. doi: 10.1056/NEJMp1002530. [DOI] [PubMed] [Google Scholar]
- 53.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de Gonzalez A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petty TL. Are copd and lung cancer two manifestations of the same disease? Chest. 2005;128:1895–1897. doi: 10.1378/chest.128.4.1895. [DOI] [PubMed] [Google Scholar]
- 55.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose ct of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 57.International Early Lung Cancer Action Program I. Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage i lung cancer detected on ct screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 58.Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, Passera E, Angeli E, Chiarenza M, Aranzulla G, et al. A randomized study of lung cancer screening with spiral computed tomography: Three-year results from the dante trial. Am J Respir Crit Care Med. 2009;180:445–453. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 59.Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, Ascher S, Bailey W, Brewer B, Church T, et al. Final results of the lung screening study, a randomized feasibility study of spiral ct versus chest x-ray screening for lung cancer. Lung Cancer. 2005;47:9–15. doi: 10.1016/j.lungcan.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, Prorok PC. Lung cancer mortality in the mayo lung project: Impact of extended follow-up. J Natl Cancer Inst. 2000;92:1308–1316. doi: 10.1093/jnci/92.16.1308. [DOI] [PubMed] [Google Scholar]
- 61.National Lung Screening Trial Research T. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, et al. Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 63.Sone S, Takashima S, Li F, Yang Z, Honda T, Maruyama Y, Hasegawa M, Yamanda T, Kubo K, Hanamura K, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351:1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 64.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, Luketich JD, Siegfried JM. The pittsburgh lung screening study (pluss): Outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178:956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Happer W. Spin exchange, past, present and future. Ann Phys Fr. 1985;10:645–657. [Google Scholar]
- 66.Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. Quantification of lung microstructure with hyperpolarized 3he diffusion mri. J Appl Physiol. 2009;107:1258–1265. doi: 10.1152/japplphysiol.00386.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patz SMI, Hrovat MI, Dabaghyan M, Washko GR, Hatabu H, Butler JP. Diffusion of hyperpolarized 129xe in the lung: A simplified model of 129xe septal uptake and experimental results. New Journal of Physics. 2011;13:2–18. [Google Scholar]
- 68.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized 129xe mri. Proc Natl Acad Sci U S A. 2006;103:18278–18283. doi: 10.1073/pnas.0608458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patz S, Hersman FW, Muradian I, Hrovat MI, Ruset IC, Ketel S, Jacobson F, Topulos GP, Hatabu H, Butler JP. Hyperpolarized (129)xe mri: A viable functional lung imaging modality? Eur J Radiol. 2007;64:335–344. doi: 10.1016/j.ejrad.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patz S, Muradian I, Hrovat MI, Ruset IC, Topulos G, Covrig SD, Frederick E, Hatabu H, Hersman FW, Butler JP. Human pulmonary imaging and spectroscopy with hyperpolarized 129xe at 0.2t. Acad Radiol. 2008;15:713–727. doi: 10.1016/j.acra.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruppert K, Mata JF, Brookeman JR, Hagspiel KD, Mugler JP., 3rd Exploring lung function with hyperpolarized (129)xe nuclear magnetic resonance. Magn Reson Med. 2004;51:676–687. doi: 10.1002/mrm.10736. [DOI] [PubMed] [Google Scholar]
- 72.Mansson S, Wolber J, Driehuys B, Wollmer P, Golman K. Characterization of diffusing capacity and perfusion of the rat lung in a lipopolysaccaride disease model using hyperpolarized 129xe. Magn Reson Med. 2003;50:1170–1179. doi: 10.1002/mrm.10649. [DOI] [PubMed] [Google Scholar]
- 73.Deninger AJ, Eberle B, Bermuth J, Escat B, Markstaller K, Schmiedeskamp J, Schreiber WG, Surkau R, Otten E, Kauczor HU. Assessment of a single-acquisition imaging sequence for oxygen-sensitive (3)he-mri. Magn Reson Med. 2002;47:105–114. doi: 10.1002/mrm.10032. [DOI] [PubMed] [Google Scholar]
- 74.Deninger AJ, Mansson S, Petersson JS, Pettersson G, Magnusson P, Svensson J, Fridlund B, Hansson G, Erjefeldt I, Wollmer P, et al. Quantitative measurement of regional lung ventilation using 3he mri. Magn Reson Med. 2002;48:223–232. doi: 10.1002/mrm.10206. [DOI] [PubMed] [Google Scholar]
- 75.Mansson S, Deninger AJ, Magnusson P, Pettersson G, Olsson LE, Hansson G, Wollmer P, Golman K. 3he mri-based assessment of posture-dependent regional ventilation gradients in rats. J Appl Physiol. 2005;98:2259–2267. doi: 10.1152/japplphysiol.00245.2004. [DOI] [PubMed] [Google Scholar]
- 76.de Lange EE, Mugler JP, 3rd, Brookeman JR, Knight-Scott J, Truwit JD, Teates CD, Daniel TM, Bogorad PL, Cates GD. Lung air spaces: Mr imaging evaluation with hyperpolarized 3he gas. Radiology. 1999;210:851–857. doi: 10.1148/radiology.210.3.r99fe08851. [DOI] [PubMed] [Google Scholar]
- 77.Kauczor HU, Hofmann D, Kreitner KF, Nilgens H, Surkau R, Heil W, Potthast A, Knopp MV, Otten EW, Thelen M. Normal and abnormal pulmonary ventilation: Visualization at hyperpolarized he-3 mr imaging. Radiology. 1996;201:564–568. doi: 10.1148/radiology.201.2.8888259. [DOI] [PubMed] [Google Scholar]
- 78.MacFall JR, Charles HC, Black RD, Middleton H, Swartz JC, Saam B, Driehuys B, Erickson C, Happer W, Cates GD, et al. Human lung air spaces: Potential for mr imaging with hyperpolarized he-3. Radiology. 1996;200:553–558. doi: 10.1148/radiology.200.2.8685356. [DOI] [PubMed] [Google Scholar]
- 79.Woodhouse N, Wild JM, Paley MN, Fichele S, Said Z, Swift AJ, van Beek EJ. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging. 2005;21:365–369. doi: 10.1002/jmri.20290. [DOI] [PubMed] [Google Scholar]
- 80.McLure LE, Peacock AJ. Cardiac magnetic resonance imaging for the assessment of the heart and pulmonary circulation in pulmonary hypertension. Eur Respir J. 2009;33:1454–1466. doi: 10.1183/09031936.00139907. [DOI] [PubMed] [Google Scholar]
- 81.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–228. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 82.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 83.Benza R, Biederman R, Murali S, Gupta H. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;52:1683–1692. doi: 10.1016/j.jacc.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 84.Bogren HG, Klipstein RH, Mohiaddin RH, Firmin DN, Underwood SR, Rees RS, Longmore DB. Pulmonary artery distensibility and blood flow patterns: A magnetic resonance study of normal subjects and of patients with pulmonary arterial hypertension. Am Heart J. 1989;118:990–999. doi: 10.1016/0002-8703(89)90235-4. [DOI] [PubMed] [Google Scholar]
- 85.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 86.Jardim C, Rochitte CE, Humbert M, Rubenfeld G, Jasinowodolinski D, Carvalho CR, Souza R. Pulmonary artery distensibility in pulmonary arterial hypertension: An mri pilot study. Eur Respir J. 2007;29:476–481. doi: 10.1183/09031936.00016806. [DOI] [PubMed] [Google Scholar]
- 87.Amundsen T, Kvaerness J, Jones RA, Waage A, Bjermer L, Nilsen G, Haraldseth O. Pulmonary embolism: Detection with mr perfusion imaging of lung--a feasibility study. Radiology. 1997;203:181–185. doi: 10.1148/radiology.203.1.9122390. [DOI] [PubMed] [Google Scholar]
- 88.Hatabu H, Gaa J, Kim D, Li W, Prasad PV, Edelman RR. Pulmonary perfusion: Qualitative assessment with dynamic contrast-enhanced mri using ultra-short te and inversion recovery turbo flash. Magn Reson Med. 1996;36:503–508. doi: 10.1002/mrm.1910360402. [DOI] [PubMed] [Google Scholar]
- 89.Hatabu H, Gaa J, Kim D, Li W, Prasad PV, Edelman RR. Pulmonary perfusion and angiography: Evaluation with breath-hold enhanced three-dimensional fast imaging steady-state precession mr imaging with short tr and te. AJR Am J Roentgenol. 1996;167:653–655. doi: 10.2214/ajr.167.3.8751673. [DOI] [PubMed] [Google Scholar]
- 90.Berthezene Y, Croisille P, Wiart M, Howarth N, Houzard C, Faure O, Douek P, Amiel M, Revel D. Prospective comparison of mr lung perfusion and lung scintigraphy. J Magn Reson Imaging. 1999;9:61–68. doi: 10.1002/(sici)1522-2586(199901)9:1<61::aid-jmri8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 91.Kirby M, Mathew L, Wheatley A, Santyr GE, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: Longitudinal hyperpolarized (3)he mr imaging. Radiology. 256:280–289. doi: 10.1148/radiol.10091937. [DOI] [PubMed] [Google Scholar]
- 92.Vidal Melo MF, Winkler T, Harris RS, Musch G, Greene RE, Venegas JG. Spatial heterogeneity of lung perfusion assessed with (13)n pet as a vascular biomarker in chronic obstructive pulmonary disease. J Nucl Med. 51:57–65. doi: 10.2967/jnumed.109.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coxson HO, Quiney B, Sin DD, Xing L, McWilliams AM, Mayo JR, Lam S. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med. 2008;177:1201–1206. doi: 10.1164/rccm.200712-1776OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:588–597. doi: 10.1164/rccm.200901-0159PP. [DOI] [PubMed] [Google Scholar]