Abstract
Vibrissal whisking is often employed to track facial nerve regeneration in rats; however, we have observed similar degrees of whisking recovery after facial nerve transection with or without repair. We hypothesized that the source of non-facial nerve-mediated whisker movement after chronic denervation was from autonomic, cholinergic axons traveling within the infraorbital branch of the trigeminal nerve (ION). Rats underwent unilateral facial nerve transection with repair (N=7) or resection without repair (N=11). Post-operative whisking amplitude was measured weekly across 10 weeks, and during intraoperative stimulation of the ION and facial nerves at ≥18 weeks. Whisking was also measured after subsequent ION transection (N=6) or pharmacologic blocking of the autonomic ganglia using hexamethonium (N=3), and after snout cooling intended to elicit a vasodilation reflex (N=3). Whisking recovered more quickly and with greater amplitude in rats that underwent facial nerve repair compared to resection (P<0.05), but individual rats overlapped in whisking amplitude across both groups. In the resected rats, non-facial-nerve mediated whisking was elicited by electrical stimulation of the ION, temporarily diminished following hexamethonium injection, abolished by transection of the ION, and rapidly and significantly (P<0.05) increased by snout cooling. Moreover, fibrillation-related whisker movements decreased in all rats during the initial recovery period (indicative of reinnervation), but re-appeared in the resected rats after undergoing ION transection (indicative of motor denervation). Cholinergic, parasympathetic axons traveling within the ION innervate whisker pad vasculature, and immunohistochemistry for vasoactive intestinal peptide revealed these axons branching extensively over whisker pad muscles and contacting neuromuscular junctions after facial nerve resection. This study provides the first behavioral and anatomical evidence of spontaneous autonomic innervation of skeletal muscle after motor nerve lesion, which not only has implications for interpreting facial nerve reinnervation results, but also calls into question whether autonomic-mediated innervation of striated muscle occurs naturally in other forms of neuropathy.
Keywords: autonomic, infraorbital nerve, motor, paralysis, parasympathetic, reinnervation
Facial paralysis is experienced by approximately 40,000 individuals in the United States each year (Jackson and von Doersten, 1999), and can lead to permanent disfigurement and functional loss despite advances in static and dynamic surgical intervention (Guntinas-Lichius et al., 2006). Efforts to improve management of facial nerve injury have turned to animal models for studying nerve coaptation techniques, grafting approaches, and interventions intended to enhance axonal regeneration. These interventions must be compared to appropriate controls, including the chronically denervated state, representing long-term failure for reinnervation to occur.
Our research team studies facial nerve recovery in the rat model, tracking whisking function over time after nerve injury and repair. Whisking is a highly quantifiable, dynamic behavior controlled primarily by the buccal and marginal mandibular branches of the facial nerve (Dorfl, 1985, Semba and Egger, 1986, Henstrom et al., 2012). In the course of comparing facial nerve main trunk transection and suture repair to a chronically denervated state, we discovered that whisker movements would re-appear approximately 30 days after facial nerve resection, and grow stronger over the ensuing weeks. Although maximal whisking amplitude was generally greater in rats who had undergone facial nerve repair, there was nevertheless overlap in whisker movement amplitude for individual animals between repaired and resected groups. A deeper understanding of the source and mechanism of this non-facial nerve-mediated whisker pad motor innervation is imperative to the validity of vibrissal function as a metric for rat facial nerve recovery.
The rodent whisker pad receives a dense motor and sensory nerve supply from the facial and trigeminal nerves, respectively (Figure 1) (Dorfl, 1985, Semba and Egger, 1986, Henstrom et al., 2012). Although the infraorbital division of the trigeminal nerve (ION) provides dense sensory innervation to the whisker pad (Dorfl, 1985), it is also known to contain postganglionic, parasympathetic, cholinergic axons (Wilke et al., 1992) that innervate blood vessels of the whisker pad (Fundin et al., 1997). We hypothesized that these parasympathetic, cholinergic fibers were the source of motor innervation causing whisker movement in rats with chronic facial nerve discontinuity. We tested this hypothesis in rats at 10 or more weeks after unilateral facial nerve resection by 1) electrically stimulating the ION intraoperatively and observing possible whisker movements, 2) transecting the ION and observing possible cessation of whisker movements, 3) administering the autonomic blocking drug hexamethonium to see if ION-based whisker movements were diminished, 4) cooling the snout to see if the vasodilation reflex would increase ION-based whisker movement, and 5) immunohistochemical identification of somatic and autonomic innervation of whisker pad intrinsic and extrinsic muscles.
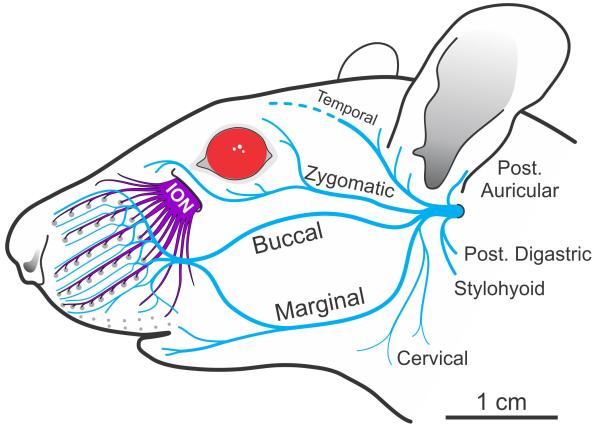
Figure 1.
Diagram of the rat facial nerve branches (in blue) beginning from the stylomastoid foramen, and infraorbital nerve (ION, in purple) beginning from the infraorbital fissure. Macro vibrissae follicles are represented by gray dots arranged in 5 major rows.
2. Experimental Procedures
2.1 Animals and Procedures
Eighteen female Wistar-Hannover rats (Charles River Laboratories, Wilmington, Massachusetts) weighing 250 to 300 grams were obtained in accordance with Massachusetts Eye and Ear Infirmary guidelines for animal care under an approved protocol. For all surgical procedures, rats were anesthetized with an intramuscular injection of ketamine hydrochloride (50 mg/kg) (Fort Dodge Animal Health, Fort Dodge, Iowa) and medetomidine hydrochloride (0.5 mg/kg) (Orion Corp., Espoo, Finland). For assessment of fibrillation-related whisker movements, rats were sedated with intramuscular injection of medetomidine hydrochloride (0.5 mg/kg), which was subsequently reversed with atipamezole hydrochloride (0.5 mg/kg) (Pfizer Inc., New York, NY). At the conclusion of the study, animals were euthanized according to NIH guidelines.
2.2 Head Restraint Device Implantation and Restraint Conditioning
Whisking assessment was performed under head and body restraint, requiring surgical implantation of a titanium head fixation device 5-6 weeks before testing (see (Hadlock et al., 2007) for details). Prior to restraint device implantation, rats were handled daily for 2-3 days in order to acclimate them to handling. After device implantation, rats were handled daily for two weeks, and then gradually conditioned to restraint over an additional two week period (see (Heaton et al., 2008) for a complete description of restraint training).
2.3 Facial Nerve Transection and Repair or Resection
All rats underwent left facial nerve (VII) transection at the main trunk distal to the stylomastoid foramen. The main trunk was exposed by removal of the parotid gland and divided with microsurgical scissors under an operating microscope (Leica Wild M65m, Wetzlar, Germany). In 7 rats, the transected facial nerve was repaired with two or three interrupted 10-0 nylon epineurial sutures (VII Repaired group). In 11 rats, the incision exposing the facial nerve was extended to the lateral border of the whisker pad, permitting extirpation of the buccal and marginal mandibular branches from the pes anserinus proximally to their distal convergence at the whisker pad (VII Resected group; see Figure 2). The proximal and distal nerve stumps were then sutured into silicone tubes sealed with cyanoacrylate to prevent axons from exiting or entering the cut nerve ends, respectively. Whisking assessment began one week after nerve manipulation and continued weekly for 10 weeks. The capped main trunk in rats undergoing nerve resection (VII Resected group; N=11) were exposed through a small incision once per month during the recovery period, and a Montgomery bipolar nerve stimulator (Boston Medical Products, Inc., Westborough, MA) was used to stimulate around the capped nerve in order to confirm that axons had not escaped encapsulation and re-innervated the whisker pad.
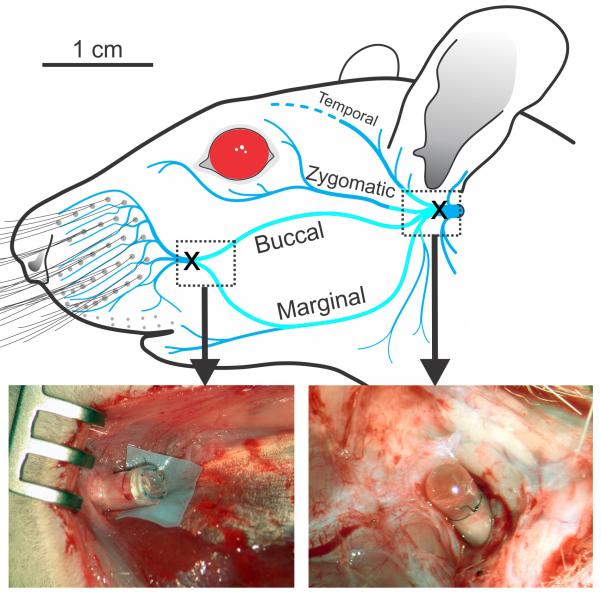
Figure 2.
Diagram of the rat facial nerve showing where the nerve was unilaterally transected (X), and sutured into dead-end silicone tubes at the main trunk (right photo), as well as at the distal convergence of the buccal and marginal mandibular branches (left photo) for the VII Resected group (N=10). The region of nerve extirpated between the transection locations is shown as a lighter shade of blue.
Rats from the VII Resected group underwent ION exposure at ≥16 weeks after their initial surgery (N=11). The ION was transected just anterior to its exit point from the infraorbital fissure, and the distal fascicles were electrically stimulated while whisker movements were observed (N=11). Five rats from the VII Resected group were euthanized at the end of the ION transection surgery, and the remaining 6 were followed for an additional post-surgical period of at least one week to document spontaneous whisker movements.
2.4 Whisking Assessment
Horizontal whisker movement was assessed bilaterally during weekly, 5-minute recording sessions using non-contact laser micrometers (RX Series, MetraLight, Santa Mateo CA) while rats were under head and body restraint. The hardware and software used for monitoring whisker movement is described in detail by Heaton et al. (2008), and was adapted from the system employed by Bermejo and colleagues (Bermejo et al., 1998, Bermejo et al., 2002) through their generous assistance.
Rats typically sweep their prominent whiskers (vibrissae) in unison on each side of the face, so whisking ability can be assessed by tracking the movement of single right and left pad whiskers. To do this we accentuated the visibility of the first whisker of the middle row (i.e. whisker C1) on each side by threading them into lightweight (3-4 mg) polyimide tubes (SWPT-045, Small Parts, Inc.) to make them selectively visible to the laser micrometers (Bermejo et al., 1998, Bermejo et al., 2002, Heaton et al., 2008). Rats often whisk spontaneously, even while restrained, but we also attempted to elicit vigorous whisking using computer-controlled air valves to deliver 10-second scented air flows towards the snout at 3 random time points during each recording session (see method details in Heaton et al., 2008).
On any given post-operative week, rats were excluded from whisking assessment if they exhibited distress or discomfort when placed in restraint or during the 5 minute whisking recording period. This led to the exclusion of 5 out of 160 recording session data points during the 10 weeks of assessment (2 data points from rats in the VII Resected group and 3 from rats in the VII Repaired group). One rat in the VII Resected group experienced head implantation failure early in the postoperative period and was therefore excluded from quantitative whisking assessment, but was maintained for manipulations not requiring head restraint (i.e. fibrillation assessment, hexamethonium administration, snout cooling and intraoperative ION stimulation - see sections 2.5-2.7 below), and another rat from the VII Resected group was used for immunohistochemical axon staining 19 weeks after resection but did not contribute to the whisker movement data because it was studied in a later cohort.
The average amplitude of the C1 whisker movements from onset (retraction) to peak protraction was calculated for the largest 3 whisks recorded during each recording session from each side of the face (Hadlock et al., 2010). These maximal whisks were averaged within each rat, and compared for the left whisker pad across the recovery period using a mixed-factors analysis of variance (ANOVA) to evaluate the main effects of experimental group (VII Repaired versus VII Resected; between-subjects factor), week of recovery (within-subjects factor), and a possible interaction (alpha of P<0.05). Based on the outcome of omnibus (preliminary) main effects and interaction testing, post-hoc pair-wise comparisons between treatment groups were performed using one-tailed t-tests for particular weeks of recovery. Post-hoc testing was restricted to a few particular time-points of interest in order to minimize the number of pair-wise comparisons. This included the first week of apparent whisker movement recovery from each treatment group (weeks 3 and 4), the middle of the recovery period (weeks 6 and 7), and the last measured time point (week 10). A starting alpha level of P<0.05 was divided by the number of post-hoc tests between treatment groups (as a family of tests) to mitigate alpha inflation (i.e. alpha of P<0.05/5 = P<0.01). Post-hoc tests were also performed between weeks 3 vs. 4 and weeks 4 vs. 10 within the VII Resected group to determine whether the initial apparent recovery of whisker movement represented a true increase, and whether the initial whisker movement differed from the last time point in the tracked recovery (respectively), with an adjusted alpha level of P<0.025 for this second family of tests.
2.5 Fibrillation Assessment
Fibrillation contractions, which provide a means of documenting whisker pad denervation and subsequent reinnervation (Hadlock et al., 2005, Heaton and Kobler, 2005), were visually assessed from video recordings taken from directly above the head of sedated animals (Hadlock et al., 2005, Heaton and Kobler, 2005). A 10-second video file was created for each rat in the VII Resected group (N=10) every-other week from weeks 2 to 14 (or greater) of recovery, after sedating rats with medetomidine hydrochloride (0.5 mg/kg), using a digital video camera with a resolution of at least 720×480 pixels, with a data rate > 1900 kbps (Optura PI or VIXIA HF R200, Canon USA, Inc., Lake Success, NY). Six rats were also video recorded one or two weeks after ION transection survival surgery (at ≥17 weeks after facial nerve resection).
Video files were presented in random order to 3 observers (blind to experimental condition and naïve to the study hypotheses) via a custom-written graphic user interface (GUI) in Matlab software (The MathWorks Inc., Natick, MA). The software GUI provided instructions for how to rate fibrillation-related whisker movement, and presented 3 video clips representing none, moderate, and high amounts of movement (from rats selected from a different study), to familiarize raters with the range of motion they would observe in the rating task. The raters then judged the amount of fibrillation movement they observed in each randomly presented clip by sliding a marker on a linear continuum labeled “none” on the far left end, “moderate” in the middle, and “high” on the far right end, corresponding to a recorded value of 1-100 within the software. Each video clip was viewed at least twice, using methods detailed previously (Heaton and Kobler, 2005). The fibrillation-related movement rating for each clip was stored in computer memory after the clip’s first presentation, and the second time a given clip was viewed and rated (in random order), if the 2 ratings were within 20% of each other, the average value for the 2 ratings was taken as the final score for that clip. If the second rating was not within 20% of the first rating, then the second value became the new point of comparison for that clip, and the process continued until all consecutive judgments had a minimum amount of variability (i.e. ≤20%). Ratings were then exported to a Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA) for compiling rater scores.
2.6 Hexamethonium Effect on Whisker Movement
To test the hypothesis that non-facial-nerve derived whisking is mediated by post-ganglionic, parasympathetic axons (traveling within the ION), we injected 3 rats with the autonomic ganglia-blocking agent hexamethonium bromide (Paton and Zaimis, 1951) (H0879, Sigma-Aldrich Co. LLC., St. Louis, MO) dissolved in sterile physiological saline (300 mg/ml) at a dose of 7.4 to 30 mg/kg (IM) on two or more separate days. The head restraint implants of these rats would no longer reliably support rigid restraint at the time of hexamethonium injection, so rats were video recorded from a camera (VIXIA HF R200) positioned above their head while they were held in an experimenter’s hand resting on the surface of a laboratory table before and at multiple time points after drug injection. This enabled documentation of whisker movement bilaterally (with little head movement) for subsequent perceptual assessment by video clip observers. Ten second video segments were created for pre- and post-hexamethonium injection, and the clips were judged by 4 individuals blind to experimental conditions, using the same methods as described previously for fibrillation assessment. The GUI for this task provided example video clips representing no movement, moderate movement and the strongest ION-mediated movement anticipated from the left whisker pad (from video segments not also appearing in the test set).
All 3 rats were video recorded before injection and 20-30 minutes after injection of 30mg/kg hexamethonium bromide on two separate days. In addition, one rat was also video recorded 120 minutes after hexamethonium injection to demonstrate the return of ION-mediated movement. On a different day, one rat received a half dose (i.e. 15 mg/kg) and another rat received a quarter dose (i.e. 7.5 mg/kg) of hexamethonium; both rats were video recorded before and 20-30 minutes after injection for comparison with the full dose (30 mg/kg) condition.
2.7 Snout Cooling Effect on Whisker Movement
To test the possibility that snout cooling would trigger a vasodilation reflex and thereby potentiate ION-mediated whisker movement due to increased acetylcholine (ACh) release from parasympathetic axons, video recordings were obtained of whisker movements (as described in the prior section) in 3 rats before and during or immediately after a piece of ice was held against the snout and upper lip. The relative change in whisker amplitude (in degrees) was measured for the 3 largest whisks in the 10 seconds prior to cooling and the three largest whisks in the 10 seconds during or immediately after cooling. This was done by capturing video still frames (1920×1080 pixels, 30 frames per second, using Corel Video Studio Pro X3, Corel Corp., Ottawa, Ontario), and measuring the angle of whisker movement from maximal retraction to maximal protraction of a prominent row C whisker using Adobe Photoshop (Version CS5, Adobe Systems, Inc., San Jose, CA).
2.8 Immunohistochemical Identification of Somatic and Autonomic Axons
Axon staining was performed using antibodies for heavy weight neurofilament (anti-NF200) and vasoactive intestinal peptide (anti-VIP). Heavy weight neurofilament is found only in motor axons and large, myelinated sensory axons but not autonomic axons. VIP is found in essentially all postganglionic, parasympathetic neurons within the pterygopalatine ganglia (Lundberg et al., 1980, Lundberg, 1981, Kaji et al., 1988, Kaji et al., 1991) but not in motor axons or sensory fibers. These VIP-positive neurons are also believed to co-release ACh (Lundberg et al., 1980, Lundberg, 1981, Kaji et al., 1988, Lundberg et al., 1991).
Whisker pad intrinsic and extrinsic muscles were harvested from the experimental (left) and control (right) sides of the face in one rat 19 weeks after facial nerve resection. Intrinsic muscle was represented by sling fibers obtained from the base of vibrissae along rows C and D (see Dorfl, 1982), and extrinsic muscles were represented by the levator labii superioris (LLS), and dilator naris muscles (DNM). Harvested muscles were immediately placed into a solution of 2% p-formaldehyde (PFA; EMS) in 0.1M phosphate buffer (PBS pH 7.4) for approximately 2 hours, and the sling muscles (left and right pairs) were dissected free from the follicles and further post-fixed (2%PFA) for approximately 2 additional hours. Samples were incubated overnight at 4°C (on a shaker) in blocking solution (StartingBlock, ThermoScientific, Waltham, MA) containing Triton X-100 (0.1%, Sigma), sodium azide (0.1%, Sigma) in PBS 0.1M pH7.4. The primary antibodies: chicken anti-NF200 (Sigma) and rabbit anti-VIP (AB982; Millipore Corp., Billerica, MA) were prepared in the blocking mixture, added to the sample, and incubated for 2 days at 1°C on a shaker. After washing with PBS 0.1M (3 times, each 15 min), samples were incubated overnight at 4°C (on a shaker) with secondary antibodies (Alexa 488 goat anti-chicken, Alexa 594 goat anti-rabbit) and Alexa 647-conjugated with α-bungarotoxin prepared in the same blocking solution. After several washes in PBS, the samples were incubated overnight with SlowFade Gold (Invitrogen Corp., Carlsbad, CA).
Immunostained samples were mounted on standard microscope glass slides in SlowFade Gold and compressed slightly between magnets for approximately 24 hr to enhance their optical accessibility. Samples were first screened for the location of neuromuscular junctions using a low power objective (10×, 0.45 NA) with a laser scanning confocal microscope (Zeiss 780, Carl Zeiss AG, Oberkochen, Germany) by excitation of 633 nm He-Ne laser. A 20×, 0.8 NA objective lens was then used to obtain confocal stacks in these regions. Alexa 488 (NF200), Alexa 594 (VIP), and Alexa 647 (α-bungarotoxin) labeling were then excited with 488, 594, and 647 nm wavelength lasers, respectively. Stacks were then processed with Zeiss ZEN software to created maximum projections.
3. Results
3.1 Electrical Stimulation of the ION and Facial Nerves
Electrical stimulation of tissues surrounding the capped proximal trunk of the transected facial nerve for the VII Resected group rats did not cause whisker movement during the periodic post-surgical test points (at weeks 4 and 8), or during any subsequent surgical manipulations (≥16 weeks after initial VII resection). Likewise, electrical stimulation of tissues surrounding the distal capped nerve ends also failed to elicit whisker movements, strongly suggesting that facial nerve axons had not regrown into the whisker pad.
In contrast, electrical stimulation of the ION at ≥16 weeks after initial facial nerve resection caused immediate, conspicuous whisker protraction in all rats (N=11) from the VII Resected group ipsilateral to their whisker pad denervation. Whisker protraction occurred roughly in rows, relating to the ION fascicular region being stimulated; stimulation of superomedial ION bundles resulted in upper whisker row protraction (rows A-C), while stimulation of inferolateral fascicles caused the lower rows to protract (rows D-E and labial follicles). This somatotopic relationship between stimulated fascicle location and corresponding row protraction was observed in all rats, and matches the general topography of whisker row sensory innervation by the ION (Dorfl, 1985). Stimulation of the contralateral, healthy whisker pad did not cause visible whisker movement (N=10). A video example of whisker movement during intraoperative ION stimulation on the VII Resected side is available as supplementary material through the publisher’s web site.
3.2 Whisking Recovery
The average amplitude of the 3 largest whisks across weekly, 5-minute recording sessions showed a different time course and magnitude of recovery for the two groups in this study (Figure 3). A mixed-factors ANOVA demonstrated a significant main effect for whisking amplitude, whereby the VII Repaired group recovered larger whisks than the VII Resected group (P<0.05, N= 7 and 10, respectively), and a significant main effect for week of recovery, with earlier weeks showing lower amplitude whisks than later weeks (P<0.05). There was also a significant interaction between experimental groups and week of recovery, where the VII Repaired group recovered whisks more rapidly than the VII Resected group (P<0.05). These significant omnibus main effects and interaction made it appropriate to conduct post-hoc, pair-wise comparisons.
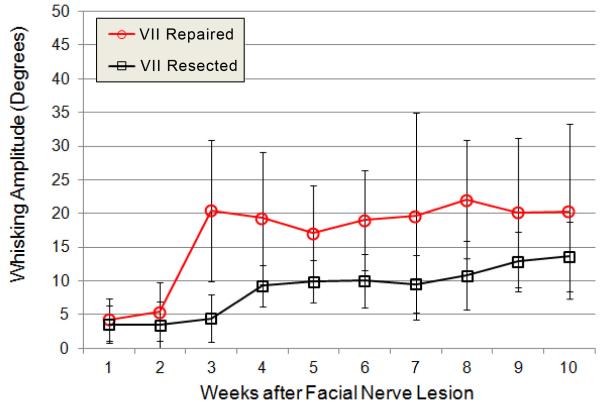
Figure 3.
Average whisking amplitude is shown for the 3 largest whisks across weeks 1-10 of recovery, ipsilateral to facial nerve transection and suture repair (VII Repaired group, open circles in red; N=9) versus ipsilateral to facial nerve resection (VII Resected group, open boxes in black, N=7). Group averages were statistically compared for weeks 3, 4, 6, 7, and 10, and significantly differed at weeks 3, 4, and 6, but not at weeks 7 and 10. Error bars are ± 1 standard deviation.
The VII Repaired group (N=7) had larger average maximal whisking amplitude than the VII Resected group (N=10) at the beginning of the whisking appearance (weeks 3 and 4, P=0.004 and P=0.0082 respectively), in the middle of the recovery period at week 6 but not at week 7 (P=0.0037 and P=0.0478, respectively), and not at week 10 (P=0.0984). The lack of significant differences between the groups towards the second half of the recovery period (adjusted alpha of P<0.01) is perhaps due to the apparent gradual increase in maximal whisking amplitude for the VII Resected group compared to a more steady amplitude for the VII Repaired group over those same weeks.
For the VII Resected group, whisking amplitude on week 4 of recovery was significantly larger than for week 3 (P=0.0045), consistent with the observation that recovery effectively began between those weeks for this group (Figure 3). However, week 4 was only borderline significantly different than week 10 for this group (P=0.0291), suggesting only a modest and/or inconsistent increase in ION-mediated whisker movement amplitude across the measured recovery period (adjusted alpha of P<0.025).
Unilateral ION transection at ≥16 weeks after ipsilateral facial nerve resection resulted in a complete and immediate cessation of visible or measurable whisker movements (N=6). Five of the six rats that were followed for a week or longer after ION transection had stable head implants, permitting rigid restraint for whisking quantification. These rats produced no measurable whisks (i.e. ≥3 degrees) on the combined ION and facial nerve lesioned side of the face.
3.3 Fibrillation-Related Whisker Movement
Visual assessment of fibrillation-related movements revealed persistent, random whisker movement in the first 4 weeks after facial nerve resection with rats under medetomidine sedation. The number of whiskers randomly moving and their relative fibrillation-related movement amplitude decreased steadily across the first 6 weeks, and remained weak or absent from week 8 onward across the recovery period (Figure 4). Within one or two weeks after unilateral ION transection (at ≥16 weeks after ipsilateral facial nerve resection; N=6), there was obvious re-appearance of fibrillation-related movements, similar to or greater than the degree of movement seen after initial facial nerve resection, suggesting that ION transection (re)denervated whisker pad muscle cells. There was an average correlation of 0.802 for observed fibrillation movement ratings among the 3 raters.
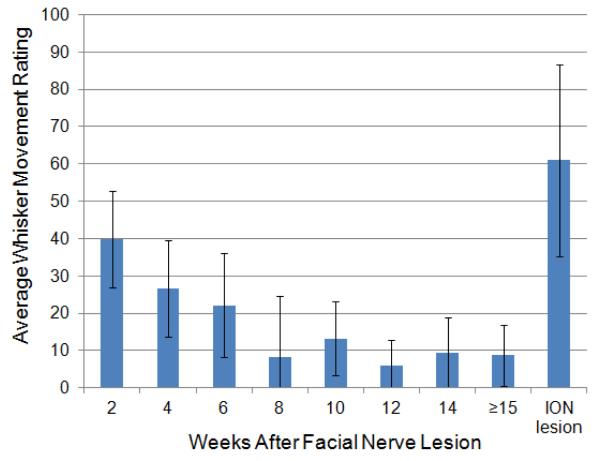
Figure 4.
Average fibrillation-related whisker movement ratings ipsilateral to facial nerve resection across the post-surgical period. Ratings were made on a continuous scale of whisker movement ranging from “none” (score 0), to “moderate” (score 50) to “high” (score 100). The visible appearance of fibrillation-related movements decreased over time (N=10), but increased again within one or two weeks after ION transection, as shown in the far right data point (N=6).
A video example of fibrillation-related whisker movements observed at one week after facial nerve resection, their disappearance by 20 weeks after facial nerve resection, and the re-appearance of fibrillation-related movements at 21 weeks (1 week after ION transection) is available as supplementary material to this article.
3.4 Hexamethonium Injection Effects on Whisker Movement
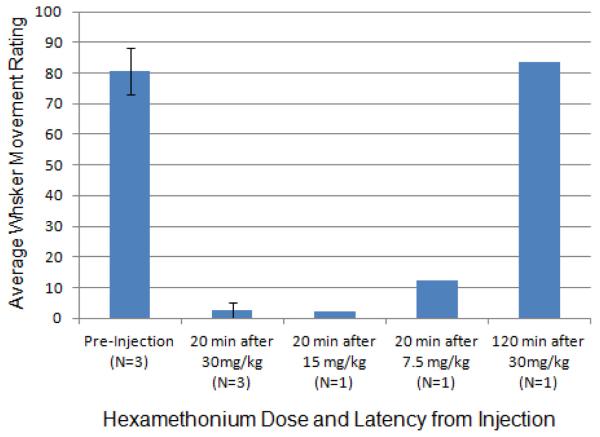
Visual assessment of whisker movements ≥16 weeks after facial nerve resection revealed consistent, low-amplitude, synchronous twitches (or whisks) of most macro vibrissae ipsilateral to facial nerve manipulation (N=11). These movements were substantially or entirely eliminated within 20 minutes after 30 mg/kg IM injection of hexamethonium (N=3) based on observer ratings of pre- versus post-injection video recordings using a visual analog scale ranging from “no movement” to “strong movement” (Figure 5). A recording made 120 minutes after drug injection (N=1) revealed a return of movement, indicating that autonomic ganglia blocking of ION-mediated whisker movement lasts less than two hours for a 30 mg/kg dose in adult female rats. Moreover, suppression of ION-mediated movement 20 minutes after only 15 mg/kg (N=1) suggests that this smaller dose may provide adequate autonomic suppression, but the persistence of very slight movement at 20 minutes after 7.5 mg/kg indicates that this lowest tested dose fails to block the pterygopalatine ganglion supplying autonomic axons to the whisker pad through the ION. Average correlation among the 4 visual raters of video recorded whisker movement was 0.92. A video example of whisker movement at rest, 20 minutes after an intramuscular injection of 30 mg/kg hexamethonium, and 120 minutes after injection is available as supplementary material to this article.
Figure 5.
Average whisker movement ratings from video clips of rats before hexamethonium injection, 20 minutes after injection of three different doses (30, 15, and 7.5 mg/kg), and 120 minutes after the largest dose (30 mg/kg). Ratings were made on a continuous scale ranging from “no movement” (score 0), to “moderate movement” (score 50), to “strong movement” (score 100). Hexamethonium substantially reduced or completely blocked observable movement after the two larger doses, but incompletely blocked ION-mediated movement at the smallest dose. Movement began to return within 2 hours of drug delivery, as shown for the largest dose. The number of rats contributing to each data point is listed in the bar labels. Error bars are ± 1 standard deviation.
3.5 Snout Cooling Effects on Whisker Movement
Within only a few seconds of ice contact with the snout, an observable increase in ION-mediated whisker movement amplitude and frequency was appreciated. Average amplitude for the 3 largest whisker movements at ≥16 weeks after facial nerve resection in the 10 seconds prior to snout cooling compared to the 3 largest whisks in the 10 seconds during or immediately after cooling (N=3) showed an approximate doubling of whisking amplitude associated with cooling (Figure 6). A video example of ION-mediated whisker movement increasing in amplitude and frequency with brief ice cube contact with the snout is available as supplementary material to this article.
Figure 6.
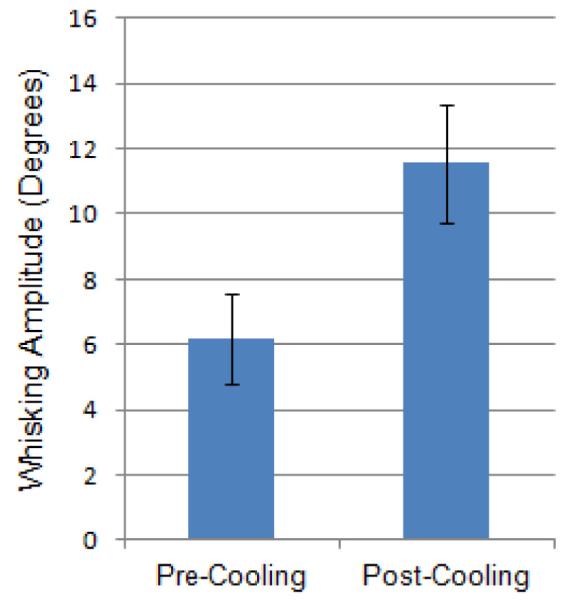
Average whisking amplitude (in degrees) for the 3 largest whisks 10 seconds before versus 10 seconds during or immediately after brief snout cooling with an ice cube (N=3). Error bars are ± 1 standard deviation.
3.6 Immunohistochemical Identification of Somatic and Autonomic Axons
Neuromuscular junctions were identifiable in muscles from both the experimental and control sides of the face by immunoreactivity to -bungarotoxin (Figures 7-9, in red). Endplates were normal in appearance on the control side (i.e. flattened, oblong regions), but were relatively swollen with somewhat fragmented staining on the facial nerve-resected side. Heavy weight neurofilament staining (using NF200 antibodies) revealed large caliber somatic motor fibers contacting endplates in the sling, LLS and DNM muscles on the control side, but there was a complete absence of these fibers at 19 weeks after facial nerve resection in all 3 muscles on the experimental side (Figures 7-9, in yellow). Some thin NF200-positive sensory axons were found in both the experimental and control muscles in the vicinity of the neuromuscular junctions, but without a particular relationship with the endplates (Figures 7-9, in yellow).
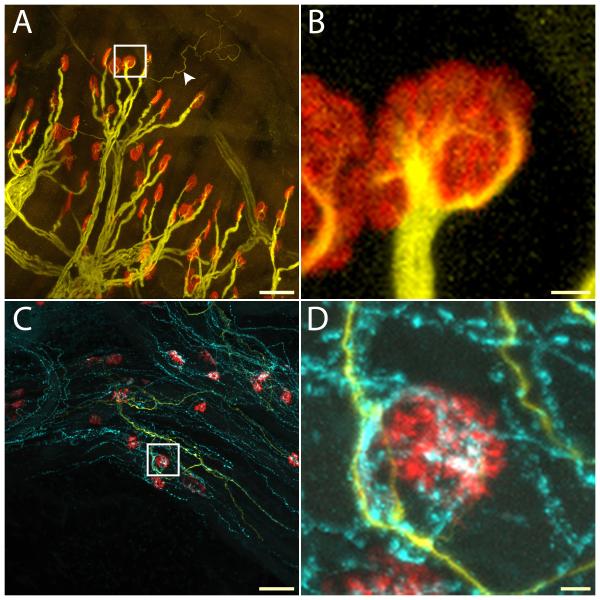
Figure 7.
NF200-positive (yellow) and VIP-positive axons (cyan) in sling muscle. Neuromuscular junctions were labeled with α-bungarotoxin (red). In the control side, motor axons containing thick heavy weight neurofilament (NF200) bundles showed normal arborization patterns (A), with neurofilament staining of finer branches entering the neuromuscular junction (B, from bounded area in A). Occasionally, thin NF200-positive sensory axons could be seen (A, arrow head). No VIP-positive parasympathetic axons were present near neuromuscular junctions. On the experimental side, no motor axons with thick neurofilament could be seen, while sensory axons with thin NF200 labeling and numerous VIP-positive branches were readily present (C). Many junctions were un-innervated after facial nerve resection. However, scattered neuromuscular junction showed strong co-localization with VIP-positive nerve terminals, suggesting innervation by parasympathetic axons (D, from bounded area in C). Scale bar: A, C: 50 μm. B, D: 5 μm.
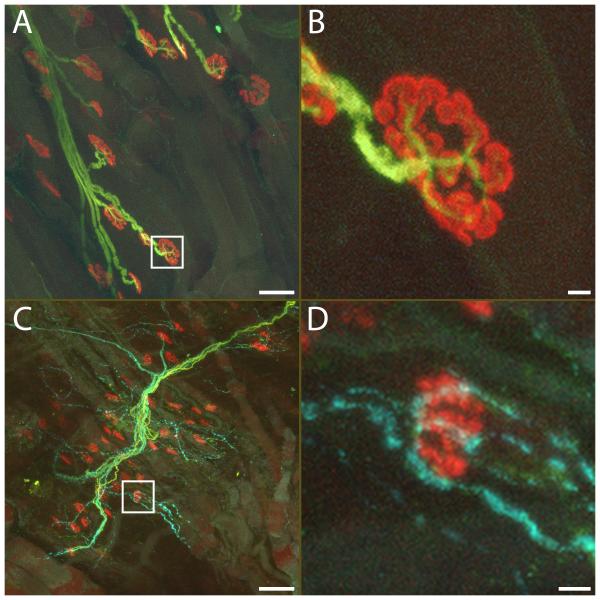
Figure 9.
NF200-positive (yellow) and VIP-positive axons (cyan) in the levator labii superioris muscle (LLS). Neuromuscular junctions were labeled with α-bungarotoxin (red). In the control side, motor axons containing thick heavy weight neurofilament (NF200) bundles showed normal arborization patterns (A), with neurofilament staining of finer branches entering the neuromuscular junction (B, from bounded area in A). No VIP-positive parasympathetic axons were present. On the experimental side (C, D), no motor axons with thick neurofilament could be seen, while sensory axons with thin NF200 labeling and numerous VIP-positive branches were present. A significant number of the junctions were un-innervated after facial nerve resection. However, scattered neuromuscular junctions showed co-localization with VIP-positive nerve terminals, suggesting innervation by parasympathetic axons (D, from bounded area in C). Scale bar: A, C: 50 μm. B, D: 5 μm.
VIP-positive axons were observed on blood vessels in and around muscles on the control side of the face, but were not found near the neuromuscular junctions or contacting the surface of muscle cells. In contrast, VIP-positive axons (Figures 7-9, in cyan) were found to have extensively grown across the muscle surface on the facial-nerve-resected side, with a greater apparent degree of surface contact in sling muscle (Fig 7) compared to the DNM (Fig 8) or LLS (Fig 9). Although there were some endplates in all 3 muscles without VIP-positive neural contact on the experimental side, many endplates did have VIP-positive axons in close proximity, suggesting that they were the source of parasympathetic input driving contraction.
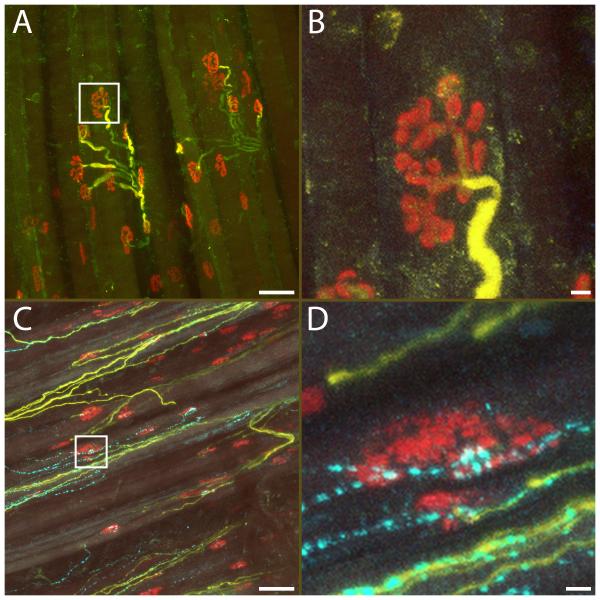
Figure 8.
NF200-positive (yellow) and VIP-positive axons (cyan) in the dilator naris muscle (DNM). Neuromuscular junctions were labeled with -bungarotoxin (red). In the control side, motor axons containing thick heavy weight neurofilament (NF200) bundles showed normal arborization patterns (A), with neurofilament staining of finer branches entering the neuromuscular junction (B, from bounded area in A). No VIP-positive parasympathetic axons were present. On the experimental side (C), no motor axons with thick neurofilament could be seen, while sensory axons with thin NF200 labeling and numerous VIP-positive branches were present. A significant number of the junctions were un-innervated after facial nerve resection. However, scattered neuromuscular junctions showed co-localization with VIP-positive nerve terminals, suggesting innervation by parasympathetic axons (D, from bounded area in C). Scale bar: A, C: 50 μm. B, D: 5 μm.
4. Discussion
Rat whisking is a dynamic and highly quantifiable behavior studied in many laboratories as a method of assessing facial nerve function. Whisking occurs primarily in an anterior-posterior direction (i.e. horizontal plane), through the interaction of intrinsic and extrinsic whisker pad muscles (Dorfl, 1982, Hill et al., 2008). Protraction is achieved mostly through the contraction of small “sling” muscles wrapping around the base of each macro vibrissa follicle (i.e. like a sling), whereas retraction occurs through an interaction of passive tissue recoil and extrinsic whisker pad muscle contraction (Berg and Kleinfeld, 2003, Hill et al., 2008). The sling muscles are extremely small and difficult to study, prompting many groups to study the extrinsic pad muscles such as the levator labii superioris (LLS) (Guntinas-Lichius et al., 2005, Pavlov et al., 2008) and dilator naris muscle (DNM) (Jergovic et al., 2001) in order to examine whisker pad innervation.
The sling muscles and DNM receive their motor supply from the buccal and marginal mandibular branches of the ipsilateral facial nerve (Dorfl, 1985, Semba and Egger, 1986, Henstrom et al., 2012), and cutting these branches immediately eliminates whisker protraction (Semba and Egger, 1986, Henstrom et al., 2012). The return of whisker movement in the weeks and months after facial nerve injury has logically been attributed to facial nerve regeneration, but the present study demonstrates that whisker protraction can occur in the absence of facial nerve input, which must be considered when determining the neural source of low-amplitude whisking after facial nerve manipulation.
Skeletal muscle undergoes predictable changes in response to denervation, including 1) oscillation in sodium conductance leading to spontaneous contraction of individual muscle cells in a process known as fibrillation (Purves and Sakmann, 1974), and 2) synthesis of ligand receptors along the muscle length, causing hypersensitivity to ACh (Axelsson and Thesleff, 1959, Cangiano, 1985) and broadening potential locations for neural contact beyond the original endplate region. Systemic delivery of ACh can reveal this hypersensitivity by causing a slow, sustained contraction of denervated muscle (Radmanovic and Torres, 1972, Cangiano, 1985). Slow contraction of denervated muscle can also be elicited by local release of ACh through stimulation of postganglionic, autonomic, cholinergic axons innervating a denervated muscles’ blood vessels. Such contraction is known as the Philipeaux-Vulpian phenomenon (or the Vulpian-Heidenhain-Sherrington phenomenon), which was described over 150 years ago (Philipeaux & Vulpian, 1863 and Heidenhain, 1883, as cited by Dale and Gaddum, 1930). For example, within a week or two after hypoglossal nerve transection, the denervated tongue of cat (Dale and Gaddum, 1930, Bulbring and Burn, 1936, Lewy et al., 1938, Radmanovic and Torres, 1972) and dog (Dale and Gaddum, 1930, Bulbring and Burn, 1936) will begin slowly contracting a few seconds after the initiation of repetitive lingual nerve stimulation, and begin relaxing on a similarly slow time-scale once stimulation ends. This has been attributed to stimulation of cholinergic, postganglionic axons traveling to the tongue vasculature via the lingual nerve, causing a local liberation of ACh (to which the hypersensitive muscle is responding) rather than direct synaptic contact between skeletal muscle cells and autonomic fibers (Hinsey, 1927, Hinsey and Gasser, 1930).
In addition to the tongue, other muscles of the head are also known to contain vascular beds regulated in part by parasympathetic innervation, including those of the lips (Euler and Gaddum, 1931, Kaji et al., 1988) and whisker pad (Wilke et al., 1992, Fundin et al., 1997). These postganglionic, parasympathetic axons are unmyelinated, wrapping around vessels with a varicosed (beaded) appearance along their length, providing numerous en passant synapses (Kilbinger, 1988) for the diffuse release of nitric oxide, vasoactive intestinal peptide and ACh onto vascular smooth muscle and endothelial cells (Lombard and Cowley, 2012). Metabotropic reception of these transmitters causes vasodilation for thermoregulation (Fundin et al., 1997) and blood flow control (see review by Campbell, 1970).
The extensive innervation of whisker pad vasculature is completely eliminated by transection of the IONs, but is not appreciably altered by loss of sympathetic supply through removal of the superior cervical ganglia (Rice et al., 1993, Waite and Li, 1993, Fundin et al., 1997), indicating that vascular autonomic innervation originates from parasympathetic neurons located within the pterygopalatine ganglia. Electrical stimulation of the cut distal portion of the ION (in an otherwise intact whisker pad) does not reliably cause whisker movement (present report and Mameli et al., 2008), and when it does occur, is likely due to current spread into adjacent facial nerve branches. However, in this study, we found that ION stimulation caused robust whisker protraction in all rats having undergone facial nerve resection 4 months previously. Given that sensory axons are incapable of functionally innervating muscle cells (Langley and Anderson, 1904, Gutmann, 1945), we suggest that these contractions stem from autonomic innervation of denervated whisker pad sling muscles. In addition, we suggest that parasympathetic axons formed direct (junctional) synaptic contacts with sling muscles in our rats rather than contractions stemming from hypersensitivity to locally liberated ACh from parasympathetic fibers (i.e. not the Philipeaux-Vulpian phenomenon) based on our immunohistochemical identification of VIP-positive (autonomic) axons on otherwise denervated sling muscle fibers, in addition to multiple functional observations discussed below.
The principal difference between the Philipeaux-Vulpian phenomenon and the contractions we elicited by ION stimulation is the latency of motor response in relation to electrical stimulation. Hypersensitive muscle responds relatively slowly to rising (and falling) ACh levels brought about by local, diffuse release and circulation of ACh from parasympathetic innervation of glands or vasculature. In sharp contrast, whiskers protracted immediately upon ION stimulation in our facial nerve resected rats, and likewise returned immediately to their resting position once stimulation ended. This pattern of movement was demonstrated repeatedly in each chronically denervated rat, and was visually indistinguishable from the timing of whisker movement we observe when stimulating the facial nerve in intact rats. This rapid response to ION stimulation implies direct, functional synaptic contact between autonomic fibers and skeletal muscle cells, which is not thought to occur (for review see Buckley and Caulfield, 1992) and has not been reported previously. This would be consistent with the close physical approximation we observed between VIP-positive axons and neuromuscular endplates on many muscle cells 19 weeks after facial nerve resection.
Another indication that autonomic fibers of the ION directly innervated whisker sling muscle cells in our facial nerve resected rats was the post-surgical time-course of whisker movement appearance, combined with the somewhat simultaneous disappearance of fibrillation-related movements. Muscle begins to fibrillate within a few days after denervation (Heaton and Kobler, 2005) and likewise becomes hypersensitive to autonomic release of ACh with a week (Radmanovic and Torres, 1972, Cangiano, 1985). However, clear whisker protraction did not appear until approximately 4 weeks after denervation, and grew stronger over time while fibrillation faded. This observation suggests that parasympathetic innervation of sling muscle vasculature sprouted in response to sling fiber denervation over several weeks, and eventually formed junctional synaptic contacts with sling fibers that then generated periodic, twitch-like contractions when parasympathetic fibers released ACh, while at the same time reducing or eliminating extrajunctional receptors (that had previously produced fibrillation). The re-appearance of fibrillation-related movement in whiskers within a week after transection of the ION (and parasympathetic supply) suggests that the resulting loss of junctional synapses caused the re-expression of extrajunctional receptors on sling muscle cells (or some other source of membrane instability), just as would be expected after loss of somatic motor innervation.
In addition to the facial nerve motor supply, extrinsic whisker pad muscles have also been reported to receive neural input from the mediodorsal part of the hypoglossal nucleus via the ION (Mameli et al., 2006). This input may be cholinergic, and has been suggested to serve a proprioceptive role (Mameli et al., 2006, Mameli et al., 2008), although through an unknown mechanism, given the absence of neuromuscular spindles in the whisker pad (Bowden and Mahran, 1956, Rice et al., 1986). It is possible that these hypoglossal fibers sprouted collateral branches from their extrinsic targets and grew into the sling muscles, generating whisker protraction and diminishing fibrillation in our facial nerve resected rats. However, that would not explain why ION-mediated whisker movements were greatly diminished (or eliminated) by the autonomic ganglion blocking drug hexamethonium. Moreover, our facial nerve-resected rats demonstrated significantly increased ION-mediated whisker movement from snout cooling, which is more consistent with an autonomic vasodilation reflex than with a hypoglossal proprioceptive input. Lastly, these hypoglossal fibers were not found in any of the 3 whisker pad muscles examined using immunohistochemical fiber staining 19 weeks after facial nerve resection.
Distal branches of the facial and trigeminal nerves are known to communicate (merge) in humans (Baumel, 1974, Tohma et al., 2004, Diamond et al., 2011, Yang et al., 2013) and other mammals (Bowden and Mahran, 1960), raising the question of whether motor axons may have diverged from the facial nerve main trunk proximal to the point of transection and joined the ION to reach the whisker pad. These facial axons could then have sprouted after main trunk lesion, and have been the source of ION-mediated whisker movement found in this study. However, we find this possibility highly unlikely given that essentially all 25 macrovibrissae were observed to move both spontaneously and during electrical stimulation of the ION, which would require widespread sprouting throughout all of the whisker pad rows and columns by these ION-accompanying facial motor axons, yet none was observed in our immunohistochemical staining of pad muscle innervation. Moreover, retrograde axonal tracer applied to cut branches of the ION at the point where they enter vibrissae rows did not label neurons within the facial motor nuclei (Mameli et al., 2006). Additional retrograde pathway tracing studies are needed to rule-out potential sources of neural input (aside from the pterygopalatine ganglia) after chronic facial nerve discontinuity, but the present findings and prior pathway tracing (Mameli et al., 2006) indicate that facial motor neurons are not the source of whisker pad innervation after facial nerve resection.
A facilitating role for autonomic axons in motor recovery of regenerating nerves has been suggested by the mathematical modeling work of Medinaceli and Rawlings (de Medinaceli and Rawlings, 1987). They followed the conventional notion that axons of one genus (i.e. sensory, motor or autonomic) could not form connections with neural targets normally innervated by another genus, so the potential role of autonomic input to skeletal muscle was perhaps underrepresented in their model. Reports have long shown that the central end of autonomic sensory neurons release ACh and are capable of innervating denervated skeletal muscle when experimentally directed to do so (Langley and Anderson, 1904, Vera and Luco, 1967, Landmesser, 1971, Bennett et al., 1973, Coget and Rousseau, 1983, Delorme et al., 1997), as can the peripheral end of preganglionic, parasympathetic efferent axons (Flumerfelt et al., 1986). However, this is the first report of electrically stimulated twitch-like skeletal muscle contractions (and loss of muscle fibrillation) due to putative postganglionic autonomic synapse formation with skeletal muscle. Autonomic endings have been shown (using electron microscopy) to form synapses with vacated neuromuscular junctions in chronically denervated cat laryngeal muscles after recurrent laryngeal nerve (RLN) injury (Nomoto et al., 1991, Nomoto et al., 1993a, Nomoto et al., 1993b), but attempted electrical stimulation of these autonomic fibers was unable to produce laryngeal muscle contraction (Nomoto et al., 1991). Investigators have suggested that spontaneous autonomic reinnervation of laryngeal muscles may explain why substantial atrophy does not always occur after long-term (i.e. >20 months) denervation in cat (Nomoto et al., 1991, Nomoto et al., 1993a, Nomoto et al., 1993b) and rat (Miyamaru et al., 2008) models. Autonomic synapse formation may also explain why many cases of vocal fold paralysis show a return of electromyographic activity and resting tone months after RLN palsy, while still lacking meaningful vocal fold abduction or adduction (defined as laryngeal synkinetic reinnervation by some authors – for review see Crumley, 1989).
Autonomic reinnervation after various cranial nerve lesions may be naturally occurring in an occult manner, but is revealed in the rat whisker pad due to the amplification of very small muscle forces at the follicle base (via sling muscles) into observable whisker movements. Likewise, fibrillation-related fiber contraction, which occurs in a visually undetectable manner in most body locations, can be seen under certain circumstances such as through the thin mucosa of the tongue and when amplified by the whiskers (Heaton and Kobler, 2005). Lastly, the possibility of autonomic reinnervation should be considered in instances when sensory neural input is implicated in preventing atrophy of a denervated muscle through a process called “sensory protection” (Hynes et al., 1997, Papakonstantinou et al., 2002, Bain et al., 2008, Elsohemy et al., 2009, Zuijdendorp et al., 2010), since most, if not all, sensory nerves contain autonomic axons.
5. Conclusion
We have demonstrated whisker movements in rats with chronic facial nerve discontinuity, and provide functional and anatomical evidence that non-facial-nerve mediated whisking stems from reinnervation of intrinsic whisker pad sling muscles by postganglionic, parasympathetic axons traveling within the ION from the pterygopalatine ganglia. This conclusion is based upon the identified growth of VIP-positive axons into whisker pad muscles after facial nerve resection, and the effect of electrical stimulation and transection of the ION on non-facial nerve-mediated whisking, as well as selective pharmacologic blockage, and autonomic (vasodilation) reflex elicitation of such whisking. This represents the first reported instance of functional synapse formation of postganglionic, autonomic fibers with skeletal muscle. Our discovery has implications not only for interpreting facial muscle reinnervation results, but also raises the question of whether autonomic-mediated innervation of striated muscle occurs spontaneously in other forms of neuropathy, possibly underlying phenomena such as sensory protection of denervated muscle and contributing to some instances of synkinetic reinnervation of the face and larynx.
Supplementary Material
Intraoperative ION stimulation. Windows Media File format (.wmv), with audio narration. Nerve stimulation on the operated side of the face is shown for a rat 9 months after ipsilateral (left) facial nerve resection. The tissues surrounding the capped distal stump of the facial nerve were stimulated, producing no whisker movement (whiskers not shown). The infraorbital nerve (ION) was exposed proximal to the whisker pad, and stimulation generated movement in rows of whiskers closest to the particular ION fascicles being stimulated. Lightweight tubes are shown on a prominent whisker of each of the 5 main rows to emphasize whisker movement. Stimulation was 2-3 V, 60 Hz AC, using a bipolar Montgomery Nerve Stimulator (via forceps electrode).
Fibrillation movements. Windows Media File format (.wmv), without audio. One week after left facial nerve resection, ipsilateral fibrillation-related movements are observable in a rat under sedation. Within 8 weeks, fibrillation movements are typically minimal or absent, as shown here at 20 weeks just prior to ION transection. One week after ION transection (at week 21), a return of fibrillation-related movements is shown, indicating that the ION had provided motor innervation prior to transection.
Hexamethonium effects on ION-based whisker movement. Windows Media File format (.wmv), without audio. Whisker movements are shown in a hand-held rat at 7 months after left facial nerve resection, revealing periodic ION-based movement of the left whiskers. This movement was greatly diminished or eliminated within 20 minutes after intramuscular injection of 30 mg/kg hexamethonium, and returned within 120 minutes after injection.
Vasodilation reflex increases ION-mediated whisker movement. Windows Media File format (.wmv), without audio. A hand-held rat at 30 weeks after left facial nerve resection is shown producing natural whisking on the right side and ION-mediated, low-amplitude whisking on the left side. Soon after ice cube application to its snout and upper lip, the ION-based movements increase in frequency and amplitude, likely due to an autonomic vasodilation reflex causing increased acetylcholine (ACh) release.
Highlights.
Denervated rat whisker pad muscles spontaneously acquire autonomic innervation.
Parasympathetic axons to whisker pad muscles travel in the infraorbital nerve.
Parasympathetic contraction of skeletal muscle can be blocked by hexamethonium.
Eliciting a vasodilation reflex potentiates autonomic-mediated muscle contraction.
This is the first report of spontaneous autonomic reinnervation of skeletal muscle.
Acknowledgments
This work was supported by R01NS071067 to T. A. Hadlock and J. T. Heaton. The authors thank Jeff Lichtman, M.D., Ph.D. for the generous use of his immunohistochemistry lab, and James B. Kobler, Ph.D. for his insightful suggestions regarding the methods used to demonstrate autonomic neural control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axelsson J, Thesleff S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959;147:178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JR, Hason Y, Veltri K, Fahnestock M, Quartly C. Clinical application of sensory protection of denervated muscle. J Neurosurg. 2008;109:955–961. doi: 10.3171/JNS/2008/109/11/0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumel JJ. Trigeminal-facial nerve communications. Their function in facial muscle innervation and reinnervation. Arch Otolaryngol. 1974;99:34–44. doi: 10.1001/archotol.1974.00780030038007. [DOI] [PubMed] [Google Scholar]
- Bennett MR, McLachlan EM, Taylor RS. The formation of synapses in mammalian striated muscle reinnervated with autonomic preganglionic nerves. J Physiol (Lond) 1973;233:501–517. doi: 10.1113/jphysiol.1973.sp010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking--I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:341–346. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- Bowden RE, Mahran ZY. The functional significance of the pattern of innervation of the muscle quadratus labii superioris of the rabbit, cat and rat. J Anat. 1956;90:217–227. [PMC free article] [PubMed] [Google Scholar]
- Bowden RE, Mahran ZY. Experimental and histological studies of the extrapetrous portion of the facial nerve and its communications with the trigeminal nerve in the rabbit. J Anat. 1960;94:375–386. [PMC free article] [PubMed] [Google Scholar]
- Buckley NJ, Caulfield M. Transmission: Acetylcholine. In: Burnstock G, Hoyle CHV, editors. Autonomic Neuroeffector Mechanisms. Vol. 1. Harwood Academic Publishers; Philadelphia: 1992. pp. 257–322. [Google Scholar]
- Bulbring E, Burn JH. The Sherrington Phenomenon. The Journal of Physiology. 1936;86:61–76. doi: 10.1113/jphysiol.1936.sp003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Autonomic nervous supply to effector tissues. In: Bulbring E, et al., editors. Smooth Muscle. Edward Arnold; London: 1970. pp. 451–495. [Google Scholar]
- Cangiano A. Denervation supersensitivity as a model for the neural control of muscle. Neuroscience. 1985;14:963–971. doi: 10.1016/0306-4522(85)90268-4. [DOI] [PubMed] [Google Scholar]
- Coget J, Rousseau JP. Reinnervation of striated muscle by peripheral vagal fibres cut above or below the nodose ganglion in the cat and rabbit. J Physiol (Lond) 1983;335:481–493. doi: 10.1113/jphysiol.1983.sp014545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumley RL. Laryngeal synkinesis: its significance to the laryngologist. Ann Otol Rhinol Laryngol. 1989;98:87–92. doi: 10.1177/000348948909800201. [DOI] [PubMed] [Google Scholar]
- Dale HH, Gaddum JH. Reactions of denervated voluntary muscle, and their bearing on the mode of action of parasympathetic and related nerves. J Physiol. 1930;70:109–144. doi: 10.1113/jphysiol.1930.sp002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medinaceli L, Rawlings RR. Is it possible to predict the outcome of peripheral nerve injuries? A probability model based on prospects for regenerating neurites. Biosystems. 1987;20:243–258. doi: 10.1016/0303-2647(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Delorme P, Rousseau A, Bernard J, Leek BF, Rousseau JP. Ultrastructural changes in the nerve fiber population of anastomosed vagal and spinal accessory nerves in the sheep. Anat Rec. 1997;248:129–136. doi: 10.1002/(SICI)1097-0185(199705)248:1<129::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Diamond M, Wartmann CT, Tubbs RS, Shoja MM, Cohen-Gadol AA, Loukas M. Peripheral facial nerve communications and their clinical implications. Clin Anat. 2011;24:10–18. doi: 10.1002/ca.21072. [DOI] [PubMed] [Google Scholar]
- Dorfl J. The musculature of the mystacial vibrissae of the white mouse. J Anat. 1982;135:147–154. [PMC free article] [PubMed] [Google Scholar]
- Dorfl J. The innervation of the mystacial region of the white mouse: A topographical study. J Anat. 1985;142:173–184. [PMC free article] [PubMed] [Google Scholar]
- Elsohemy A, Butler R, Bain JR, Fahnestock M. Sensory protection of rat muscle spindles following peripheral nerve injury and reinnervation. Plast Reconstr Surg. 2009;124:1860–1868. doi: 10.1097/PRS.0b013e3181bcee47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler US, Gaddum JH. Pseudomotor contractures after degeneration of the facial nerve. The Journal of Physiology. 1931;73:54–66. doi: 10.1113/jphysiol.1931.sp002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flumerfelt BA, Kiernan JA, Krcek JP, Sholdice J. Reinnervation of skeletal muscle in the tongue by preganglionic parasympathetic vagal neurons. J Anat. 1986;146:117–130. [PMC free article] [PubMed] [Google Scholar]
- Fundin BT, Pfaller K, Rice FL. Different distributions of the sensory and autonomic innervation among the microvasculature of the rat mystacial pad. J Comp Neurol. 1997;389:545–568. [PubMed] [Google Scholar]
- Guntinas-Lichius O, Irintchev A, Streppel M, Lenzen M, Grosheva M, Wewetzer K, Neiss WF, Angelov DN. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci. 2005;21:391–402. doi: 10.1111/j.1460-9568.2005.03877.x. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Streppel M, Stennert E. Postoperative functional evaluation of different reanimation techniques for facial nerve repair. Am J Surg. 2006;191:61–67. doi: 10.1016/j.amjsurg.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Gutmann E. The reinnervation of muscle by sensory nerve fibers. Journal of Anatomy. 1945;79:1–8. [PMC free article] [PubMed] [Google Scholar]
- Hadlock T, Kowaleski J, Mackinnon S, Heaton JT. A novel method of head fixation for the study of rodent facial function. Exp Neurol. 2007;205:279–282. doi: 10.1016/j.expneurol.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock T, Lindsay R, Edwards C, Smitson C, Weinberg J, Knox C, Heaton JT. The effect of electrical and mechanical stimulation on the regenerating rodent facial nerve. Laryngoscope. 2010;120:1094–1102. doi: 10.1002/lary.20903. [DOI] [PubMed] [Google Scholar]
- Hadlock TA, Heaton J, Cheney M, Mackinnon SE. Functional recovery after facial and sciatic nerve crush injury in the rat. Arch Facial Plast Surg. 2005;7:17–20. doi: 10.1001/archfaci.7.1.17. [DOI] [PubMed] [Google Scholar]
- Heaton JT, Kobler JB. Use of muscle fibrillation for tracking nerve regeneration. Muscle Nerve. 2005;31:235–241. doi: 10.1002/mus.20257. [DOI] [PubMed] [Google Scholar]
- Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171:197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstrom D, Hadlock T, Lindsay R, Knox CJ, Malo J, Vakharia KT, Heaton JT. The convergence of facial nerve branches providing whisker pad motor supply in rats: implications for facial reanimation study. Muscle Nerve. 2012;45:692–697. doi: 10.1002/mus.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsey JC. Some observations on the innervation of skeletal muscle of the cat. Journal of Comparative Neurology. 1927;44:87–195. [Google Scholar]
- Hinsey JC, Gasser HS. The component of the dorsal root mediating vasodilation and Sherrington contracture. American Journal of Physiology. 1930;92:679–689. [Google Scholar]
- Hynes NM, Bain JR, Thoma A, Veltri K, Maguire JA. Preservation of denervated muscle by sensory protection in rats. J Reconstr Microsurg. 1997;13:337–343. doi: 10.1055/s-2007-1006413. [DOI] [PubMed] [Google Scholar]
- Jackson CG, von Doersten PG. The facial nerve. Current trends in diagnosis, treatment, and rehabilitation. Med Clin North Am. 1999;83:179–195. doi: 10.1016/s0025-7125(05)70096-1. [DOI] [PubMed] [Google Scholar]
- Jergovic D, Stal P, Lidman D, Lindvall B, Hildebrand C. Changes in a rat facial muscle after facial nerve injury and repair. Muscle Nerve. 2001;24:1202–1212. doi: 10.1002/mus.1133. [DOI] [PubMed] [Google Scholar]
- Kaji A, Maeda T, Watanabe S. Parasympathetic innervation of cutaneous blood vessels examined by retrograde tracing in the rat lower lip. J Auton Nerv Syst. 1991;32:153–158. doi: 10.1016/0165-1838(91)90065-b. [DOI] [PubMed] [Google Scholar]
- Kaji A, Shigematsu H, Fujita K, Maeda T, Watanabe S. Parasympathetic innervation of cutaneous blood vessels by vasoactive intestinal polypeptide-immunoreactive and acetylcholinesterase-positive nerves: histochemical and experimental study on rat lower lip. Neuroscience. 1988;25:353–362. doi: 10.1016/0306-4522(88)90031-0. [DOI] [PubMed] [Google Scholar]
- Kilbinger H. The autonomic cholinergic neuroeffector junction. In: Whittaker VP, editor. Handbook of experimental pharmacology. Vol. 86. Springer Verlag; Berlin and Heidelberg: 1988. pp. 581–595. [Google Scholar]
- Landmesser L. Contractile and electrical responses of vagus-innervated frog sartorius muscles. J Physiol. 1971;213:707–725. doi: 10.1113/jphysiol.1971.sp009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JN, Anderson HK. The union of different kinds of nerve fibres. Journal of Physiology. 1904;31:365–391. doi: 10.1113/jphysiol.1904.sp001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy FH, Groff RA, Grant FC. Autonomic innervation of the face: II. An experimental study. Archives of Neurology and Psychiarty. 1938;39:1238–1249. [Google Scholar]
- Lombard JH, Cowley AWJ. Neural Control of Blood Vessels. In: Robertson D, et al., editors. Primer on the Autonomic Nervous System. Academic Press; Boston: 2012. pp. 187–191. [Google Scholar]
- Lundberg JM. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Lundberg JM, Anggard A, Fahrenkrug J, Hokfelt T, Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980;77:1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Lacroix JS, Pernow J. Release of vasoactive peptides from autonomic and sensory nerves. Blood Vessels. 1991;28:27–34. doi: 10.1159/000158840. [DOI] [PubMed] [Google Scholar]
- Mameli O, Pellitteri R, Russo A, Stanzani S, Caria MA, De Riu PL. Role of the trigeminal nerve in regrowth of hypoglossal motoneurons after hypoglossal-facial anastomosis. Acta Otolaryngol. 2006;126:1334–1338. doi: 10.1080/00016480600801332. [DOI] [PubMed] [Google Scholar]
- Mameli O, Stanzani S, Russo A, Romeo R, Pellitteri R, Spatuzza M, Caria MA, De Riu PL. Hypoglossal nuclei participation in rat mystacial pad control. Pflugers Arch. 2008;456:1189–1198. doi: 10.1007/s00424-008-0472-y. [DOI] [PubMed] [Google Scholar]
- Miyamaru S, Kumai Y, Ito T, Yumoto E. Effects of long-term denervation on the rat thyroarytenoid muscle. Laryngoscope. 2008;118:1318–1323. doi: 10.1097/MLG.0b013e31816f693f. [DOI] [PubMed] [Google Scholar]
- Nomoto M, Yoshihara T, Kanda T. Persistent adrenergic reinnervation of previously denervated muscle in cat. J Electron Microsc (Tokyo) 1993a;42:236–239. [PubMed] [Google Scholar]
- Nomoto M, Yoshihara T, Kanda T, Kaneko T. Synapse formation by autonomic nerves in the previously denervated neuromuscular junctions of the feline intrinsic laryngeal muscles. Brain Res. 1991;539:276–286. doi: 10.1016/0006-8993(91)91632-b. [DOI] [PubMed] [Google Scholar]
- Nomoto M, Yoshihara T, Kanda T, Konno A, Kaneko T. Misdirected reinnervation in the feline intrinsic laryngeal muscles after long-term denervation. Acta Otolaryngol Suppl. 1993b;506:71–74. doi: 10.3109/00016489309130245. [DOI] [PubMed] [Google Scholar]
- Papakonstantinou KC, Kamin E, Terzis JK. Muscle preservation by prolonged sensory protection. J Reconstr Microsurg. 18:173–182. doi: 10.1055/s-2002-28469. discussion 183-174.2002. [DOI] [PubMed] [Google Scholar]
- Paton WD, Zaimis EJ. Paralysis of autonomic ganglia by methonium salts. Br J Pharmacol Chemother. 1951;6:155–168. doi: 10.1111/j.1476-5381.1951.tb00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov SP, Grosheva M, Streppel M, Guntinas-Lichius O, Irintchev A, Skouras E, Angelova SK, Kuerten S, Sinis N, Dunlop SA, Angelov DN. Manually-stimulated recovery of motor function after facial nerve injury requires intact sensory input. Exp Neurol. 2008;211:292–300. doi: 10.1016/j.expneurol.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Purves D, Sakmann B. Membrane properties underlying spontaneous activity of denervated muscle fibres. J Physiol. 1974;239:125–153. doi: 10.1113/jphysiol.1974.sp010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanovic B, Torres SH. The effect of bradykinin on denervated tongue. Br J Pharmacol. 1972;46:676–687. doi: 10.1111/j.1476-5381.1972.tb06892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Kinnman E, Aldskogius H, Johansson O, Arvidsson J. The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J Comp Neurol. 1993;337:366–385. doi: 10.1002/cne.903370303. [DOI] [PubMed] [Google Scholar]
- Rice FL, Mance A, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J Comp Neurol. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Semba K, Egger MD. The facial “motor” nerve of the rat: control of vibrissal movement and examination of motor and sensory components. J Comp Neurol. 1986;247:144–158. doi: 10.1002/cne.902470203. [DOI] [PubMed] [Google Scholar]
- Tohma A, Mine K, Tamatsu Y, Shimada K. Communication between the buccal nerve (V) and facial nerve (VII) in the human face. Ann Anat. 2004;186:173–178. doi: 10.1016/S0940-9602(04)80036-0. [DOI] [PubMed] [Google Scholar]
- Vera CL, Luco JV. Reinnervation of smooth and striated muscle by sensory nerve fibers. J Neurophysiol. 1967;30:620–627. doi: 10.1152/jn.1967.30.3.620. [DOI] [PubMed] [Google Scholar]
- Waite PM, Li L. Unmyelinated innervation of sinus hair follicles in rats. Anat Embryol (Berl) 1993;188:457–465. doi: 10.1007/BF00190140. [DOI] [PubMed] [Google Scholar]
- Wilke RA, Riley DA, Sanger JR. Histochemical discrimination of fibers in regenerating rat infraorbital nerve. Microsurgery. 1992;13:39–44. doi: 10.1002/micr.1920130110. [DOI] [PubMed] [Google Scholar]
- Yang HM, Won SY, Kim HJ, Hu KS. Sihler staining study of anastomosis between the facial and trigeminal nerves in the ocular area and its clinical implications. Muscle Nerve. 2013;48:545–550. doi: 10.1002/mus.23875. [DOI] [PubMed] [Google Scholar]
- Zuijdendorp HM, Tra WM, van Neck JW, Mollis L, Coert JH. Delay of denervation atrophy by sensory protection in an end-to-side neurorrhaphy model: a pilot study. J Plast Reconstr Aesthet Surg. 2010;63:1949–1952. doi: 10.1016/j.bjps.2010.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative ION stimulation. Windows Media File format (.wmv), with audio narration. Nerve stimulation on the operated side of the face is shown for a rat 9 months after ipsilateral (left) facial nerve resection. The tissues surrounding the capped distal stump of the facial nerve were stimulated, producing no whisker movement (whiskers not shown). The infraorbital nerve (ION) was exposed proximal to the whisker pad, and stimulation generated movement in rows of whiskers closest to the particular ION fascicles being stimulated. Lightweight tubes are shown on a prominent whisker of each of the 5 main rows to emphasize whisker movement. Stimulation was 2-3 V, 60 Hz AC, using a bipolar Montgomery Nerve Stimulator (via forceps electrode).
Fibrillation movements. Windows Media File format (.wmv), without audio. One week after left facial nerve resection, ipsilateral fibrillation-related movements are observable in a rat under sedation. Within 8 weeks, fibrillation movements are typically minimal or absent, as shown here at 20 weeks just prior to ION transection. One week after ION transection (at week 21), a return of fibrillation-related movements is shown, indicating that the ION had provided motor innervation prior to transection.
Hexamethonium effects on ION-based whisker movement. Windows Media File format (.wmv), without audio. Whisker movements are shown in a hand-held rat at 7 months after left facial nerve resection, revealing periodic ION-based movement of the left whiskers. This movement was greatly diminished or eliminated within 20 minutes after intramuscular injection of 30 mg/kg hexamethonium, and returned within 120 minutes after injection.
Vasodilation reflex increases ION-mediated whisker movement. Windows Media File format (.wmv), without audio. A hand-held rat at 30 weeks after left facial nerve resection is shown producing natural whisking on the right side and ION-mediated, low-amplitude whisking on the left side. Soon after ice cube application to its snout and upper lip, the ION-based movements increase in frequency and amplitude, likely due to an autonomic vasodilation reflex causing increased acetylcholine (ACh) release.