Abstract
When the clinical decision to treat a critically ill patient with antibiotics has been made, one must attempt to identify the site of infection based on clinical signs and symptoms, laboratory or diagnostic radiology studies. Identification of site requires, examination of patient, inspection of all wounds, chest radiograph, and calculation of clinical pulmonary infection score if ventilated, obtaining blood cultures, urinalysis, and line change if clinical suspicion of central venous catheter (CVC) source. If it is impossible to identify site, obtain cultures from all accessible suspected sites and initiate empiric, broad spectrum antibiotics. If likely site can be identified answer these questions: Is intra-abdominal site suspected? Is pulmonary source of infection suspected? Is skin, skin structure or soft tissue site suspected? If yes, does the patient have clinical signs suspicion for necrotizing soft tissue infection (NSTI)? Is a CVC infection suspected? Risk factors for more complicated infections are discussed and specific antibiotic recommendations are provided for each type and severity of clinical infection. Decision to continue, discontinue and/or alter antibiotic/antimicrobial treatment should be based on the clinical response to treatment, diagnostic or interventional findings, and culture and sensitivity data, bearing in mind that not all patients with infections will have positive cultures because of limitations of specimen handling, microbiology laboratory variations, time between specimen acquisition and culture, or presence of effective antibiotics at the time that specimens were obtained. It should also be noted that not all patients with increased temperature/WBC have an infection. Discontinuation of antibiotics is appropriate if cultures and other diagnostic studies are negative.
Keywords: Sepsis, Antibiotic, Bacterial, Infection, Empiric treatment, Antimicrobial, Bacteria, Therapy, De-escalation
The goal of this clinical standard operating procedure (SOP) is to provide practical guidance with respect to appropriate choices of antibiotics in severely injured, critically ill patients. Even among seasoned surgical intensivists there are widely discordant views on selection of optimal antibiotics.1,2 This SOP is intended to assist clinicians making empiric antibiotic choices in critically ill patients after the initial resuscitation phase. This guideline should minimize the risk of both inadequate antimicrobial coverage and excessive, broad spectrum antibiotic administration. Excessively broad and/or prolonged antibiotics use has been associated with development of resistant pathogens.3 The primary factors governing antibiotic choice include the location/source of presumed infection (i.e., anatomic site or region), the duration of hospitalization (therefore likelihood of hospital acquired or resistant bacterial pathogens), and local antimicrobial resistant patterns (local “antibiogram”). Numerous studies have documented increasing prevalence of antimicrobial-resistant organisms4,5 within the hospitals, but these pathogens are also increasingly common “in the community.”6–8 The increasing frequency of antimicrobial resistance underscores the need to obtain culture specimens, ideally before initiation of any antimicrobials, whenever possible. Culture information will permit appropriate de-escalation of initial, frequently broad empiric therapy.
It is essential to recognize that antimicrobial agents cannot substitute for optimal clinical care. In the case of patients with severe injuries, infectious complications may indicate failure of initial operative source control9,10 or the onset of a postoperative complication.11,12 Although antibiotics are important adjuncts in caring for critically injured patients, surgeons caring for these patients must remain cognizant of the underlying posttraumatic pathophysiology and use appropriate diagnostic, interventional, and operative therapies. We appreciate that increasingly restrictive hospital formularies may necessitate use of equally efficacious alternative agents/classes. This SOP is based on (1) Fundamental principles of likely organism at specific sites. (2) Evidence-based guideline support of the recommended agents. (3) Current usage patterns of antibiotics. (4) Probable availability of the proposed agents. Clinicians must temper these suggestions with specific knowledge of bacterial pathogen isolation and antimicrobial susceptibility patterns in their institutions, along with patient-specific information (e.g., allergies).
PROTOCOL RATIONALE
The objective of this SOP is to provide recommendations to guide selection and administration of antibiotics/antimicrobials to patients who have sustained severe injury. Fortunately, there is a moderate amount of Level I evidence that pertains to antimicrobial choices for critically ill patients in general and a lesser amount of information that directly relates to trauma patients. Unfortunately, one problem with this plethora of information is the absence of practical guidance and recommendations to inform daily decision-making in the ICU. The guideline is constructed to classify infections into the broad classes that have been the subject of numerous prospective, randomized clinical trials. These primary classes are; intra-abdominal infection (IAI), several different types of pneumonia (ventilator associated pneumonia {VAP}, hospital acquired pneumonia {HAP}, health care associated pneumonia {HCAP}, and community acquired pneumonia {CAP}), skin and skin structure infections (SSSI, encompassing a broad range of infections-cellulitis to necrotizing fasciitis), and finally central venous catheter infections (CVCI).
This SOP focuses primarily on antimicrobial choices and specifically does not address the treatment of severe sepsis, controversial aspects of these treatments, or the role of adjunctive care modalities in patients with severe sepsis/septic shock. This issue has been addressed via the evidence-based efforts of the Surviving Sepsis Campaign13 and many of these recommendations have previously been incorporated into other Trauma SOPs published in the Journal of Trauma.14 The guideline also deemphasizes urinary tract infections, because virtually all critically ill trauma patients will require indwelling urinary drainage catheters and therefore the utility of urinary cultures and/or the significance of identification of pathogens in the urine is problematic. This SOP was developed and vetted by expert consensus to balance the potential detrimental effects of excessively broad or prolonged antimicrobial administration with the recognition that mortality is increased if the wrong initial empiric choices are made.3,15
CLINICAL DECISION TO TREAT WITH ANTIBIOTICS
The clinical decision-making process regarding whether a given patient needs to have antibiotics or other antimicrobials initiated, continued, altered, or stopped is one of the most frequent questions that arises in most ICUs. This question is particularly difficult to answer with certainty in many critically injured patients because the injuries alone can result in the systemic inflammatory response syndrome (SIRS).16–18 Furthermore, infectious complications are common in all ICU patients and especially common in patients with severe injuries. Trauma permits inoculation of bacteria into normally sterile sites, postinjury shock results in well-described systemic defects in host immunity, thereby increasing susceptibility to infection, and the need for PRBC transfusion has been associated with an increased risk of infections. It is often best to have a “high index of suspicion” for possible infectious complications in patients with new or persistent fevers, elevated WBC, or other signs or symptoms of infection.19,20 Such changes should prompt a rapid, thorough search for a possible source of infection, based on physical examination, laboratory findings, operative findings, central line status, and diagnostic radiology studies. Whenever possible culture specimens from suspected sites of infection should be obtained before initiation of antimicrobial agents, to minimize the risk of false-negative culture results,21 but in some instances (e.g., suspected intra-abdominal GI perforation) it may not be practical to delay antibiotic administration until after cultures can be obtained (in this case obtained at the time of laparotomy).
IDENTIFICATION OF SITE OF INFECTION
The first question that caregivers should be asking themselves when the decision to initiate antibiotic/antimicrobial therapy has been made is whether the site of likely infection can be identified? Frequently, the site of infection is readily apparent based on clinical signs and symptoms. Examples might include the new onset of peritonitis (likely IAI) or the development of discoloration, blistering and bullae in an extremity (severe SSSI). In other cases, the identification of the site may not be quite so obvious, but a high level of suspicion may be present based on the low likelihood of other sites (e.g., probable VAP in a patient who has been on mechanical ventilation for 10 days who now has a new infiltrate and thick, copious sputum). When a likely site can be identified, this information can be used to help inform the decision-making process, as described in the proposed algorithm (Fig. 1). It is critical that every wound be examined; plaster casts, or extensive dressings must be removed to permit full evaluation of all potential sources of infection. However, despite diligent diagnostic efforts, it is not infrequently the case that no such determination can be made initially. In such cases the evidence favors initiation of empiric broad spectrum antimicrobials directed against hospital acquired gram-positive and gram-negative aerobic and anaerobic pathogens. Appropriate empiric broad spectrum coverage generally requires combination antimicrobial therapy.22 Widely used empiric choices include, but are not limited to, piperacillin/tazobactam plus vancomycin, imipenem or meropenem plus vancomycin, or the combination of cefipime plus vancomycin plus metronidazole. In a penicillin/cephalosporin allergic patient an aminoglycoside or aztreonam (no anaerobic coverage with either) could be substituted to provide coverage of gram-negative aerobes. There is currently insufficient data to recommend empiric initial therapy with moxifloxacin23 or tigecycline,24 but these agents may also provide adequate coverage for aerobic and anaerobic pathogens in penicillin/cephalosporin allergic patients. Once culture and sensitivity information becomes available antimicrobial therapy can be appropriately adjusted.
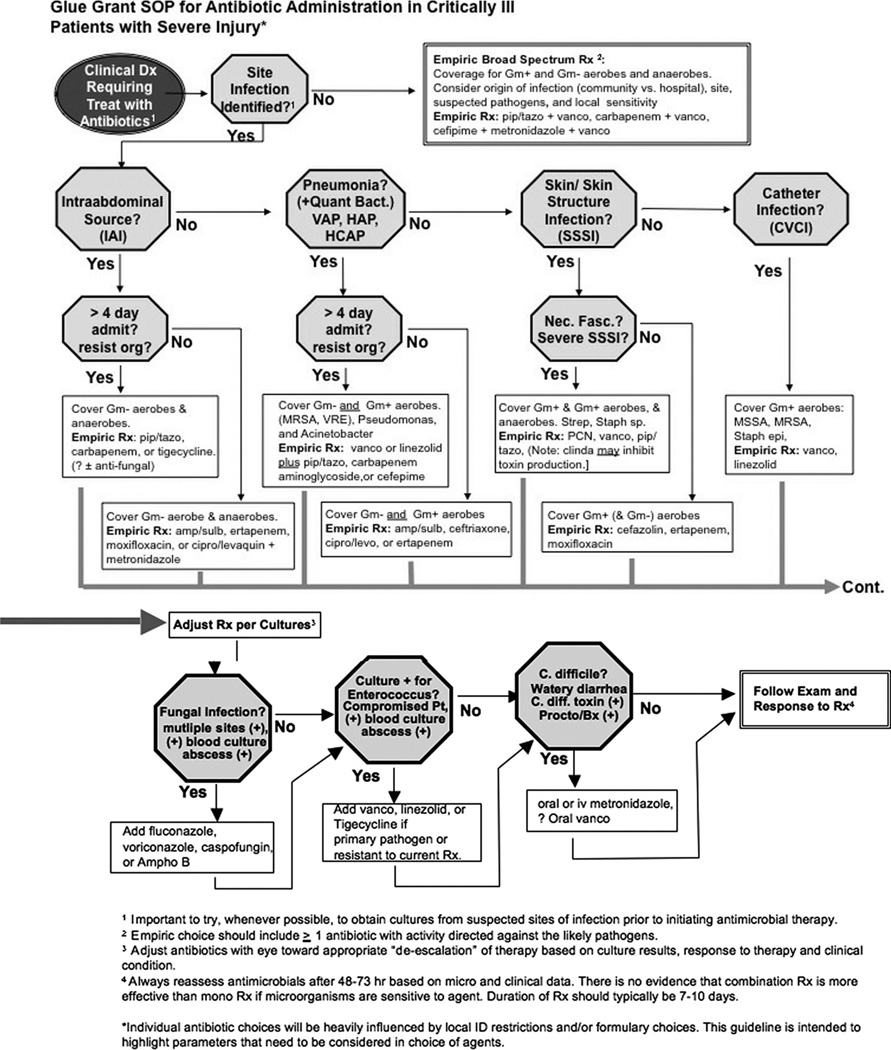
Fig. 1.
SOP for antimicrobial choices. See text for full discussion. Once the clinical decision to treat with antibiotics has been made, antibiotic choices are governed by ability or lack of ability to identify likely origin of infection. Specific empiric acceptable antibiotic choices are proposed for broad coverage when site cannot be identified and for treatment of suspected intra-abdominal, pulmonary, skin and skin structure, and central venous catheter infections. The clinical response, in conjunction with culture information (when available) can permit reliable adjustments to or discontinuation of antibiotics. Susceptibility patterns or pathogenic organisms and formulary availability of antimicrobial agents varies widely and therefore appropriate implementation of the principles outlined above will vary from center to center.
INTRA-ABDOMINAL INFECTION
Antibiotic choices for IAIs are based upon the likely pathogens responsible for these infections. With the exception of some cases of primary spontaneous bacterial peritonitis, generally seen only in patients with advanced cirrhosis or patients undergoing chronic peritoneal dialysis, all other IAIs are polymicrobial. When investigational laboratory microbiologic culture methods are used an average of 3 to 5 aerobic species and 5 to 9 anearobic bacterial species are identified. Using the specimen processing and microbiology techniques available in most hospitals it is more typical for 1 to 2 aerobic and at most a single anaerobic organism to be identified. Nonetheless, the importance of obtaining intraoperative (procedural) microbiologic specimens has recently assumed more importance because of the increasing frequency of antibiotic-resistant bacterial isolates.
Intra-abdominal infections can best be understood in terms of the respective, frequently synergistic, contributions of the aerobic and anaerobic gram-negative pathogens present within the gut, that are released into the peritoneal cavity during secondary (and even “tertiary”) peritonitis. Prototypically Escherichia coli is representative of the aerobic gram-negative Enterobacteriaceae and B. fragilis sp. are indicative of the gram-negative anaerobes present in the gut. Experimental model systems have clarified that the gram-negative aerobes are associated with systemic bacteremia and lethality, whereas cell surface components of gram-negative anaerobes are associated with development of abscesses.25 Antimicrobial agents or combinations that fail to target both types of organisms have much lower clinical cure rates than therapy that covers both classes of bacteria.
It is particularly important for surgeons to recognize that antibiotics alone are seldom sufficient to eradicate IAIs. Surgical source control (drainage, resection, patching, repair, etc.) of the underlying pathological findings responsible for the IAI, must be addressed.10 In many instances empiric antibiotics choices must be made before the surgical intervention and the fact that patients have circulating systemic antibiotics at the time that specimens may be obtained further decreases the yield of those cultures. When infection is encountered during the surgical intervention, it is a basic precept that broad coverage (targeting aerobic and anaerobic gram-negative bacteria) should be continued, even if cultures return “negative.” However, if the cultures identify unsuspected organisms, or bacteria that are resistant to the empiric antibiotics chosen, then it is reasonable to appropriately adjust the antimicrobial coverage.26
IAI Empiric Choices
Evidence-based guidelines regarding antibiotic choices in IAI have been published by the Surgical Infection Society (SIS) in 200227 and by the Infectious Disease Society of America (IDSA) in 2003.28 The antibiotic choices shown in Figure 1 represent tangible, appropriate choices based on those guidelines. These two organizations are currently jointly updating these guidelines to incorporate changes in flora and the availability of newer antimicrobial agents. Both guidelines stratified IAI depending upon whether the infections were lower-risk (SIS), community-acquired (IDSA) or higher-risk (SIS), nosocomial/hospital associated (IDSA). This stratification recognized that the pathogenic organisms responsible, as well as their sensitivity to antimicrobial agents, would differ markedly within these strata. The SOP follows this stratification scheme with different antibiotics depending upon whether IAI are severe. Antibiotic recommendations for uncomplicated infections reflect the lower likelihood of resistant organisms and/or the absence of comorbidities. More complicated infections should be treated initially with broad-spectrum agents, possibly with the addition of an antifungal agent as well. If there is persistent evidence of infection after 5 to 7 days of therapy appropriate diagnostic investigation is warranted, with investigation for potential other sites of infection.
PULMONARY INFECTION (VAP, HAP, HCAP, AND CAP)
If IAI is considered clinically unlikely the next important cause to consider is the possibility of a pulmonary source. Pulmonary infection should always be entertained in surgery patients generally, but particularly in critically ill surgical patients requiring mechanical ventilation or others in the ICU.14,29 The nomenclature of pulmonary infections sometimes reads a bit like an “alphabet soup,” but this taxonomy again recognizes important differences in etiologic pathogens and thereby aids in determining optimal empiric antimicrobial therapy. Surgeons will encounter ventilator-associated pneumonia (VAP) or hospital acquired pneumonia (HAP) most frequently. The other two common abbreviations, CAP and HCAP, refer to community-acquired pneumonia and health care associated pneumonia, respectively. In contrast to IAI, most pulmonary infections are monomicrobial. Therefore, an important aspect of diagnosis and treatment is to reliably analyze three things: (1) Does the patient have pneumonia? (2) What organism (rarely more than one) is responsible? (3) Is the organism causing the pneumonia susceptible to the antimicrobial agents being given?
Recommendations of empiric antibiotics for suspected pneumonia reflect the experiences with the various types of pneumonia. In terms of trauma and surgical patients in the hospital or ICU setting, it is important to recognize that a number of conditions can cause fever, leukocytosis, and pulmonary infiltrates in addition to pneumonia. For example, hormonal and stress responses to surgical interventions typically cause elevations of WBC for several days postoperatively in the absence of infection. Atelectasis is a very frequent cause of low-grade postoperative fever and may also cause chest radiographic changes that can be difficult to distinguish from pneumonic infiltrates. Several clinical scoring systems, such as the clinical pulmonary infection score (CPIS) have been developed to assist clinicians with identification of patients in whom SIRS criteria are likely to be due to pneumonia.30 We have previously published a trauma SOP14 that addresses this conundrum and encourages use of “invasive” diagnostic techniques (e.g., bronchoscopic or non-bronchoscopic bronchoalveolar lavage with quantitative microbiology) to help to distinguish endotracheal colonization from infection. Patients with severe injuries are at higher risk for more complex pulmonary or extrapulmonary chest infections and hemothoraces. Thus failure to improve within 72 hours of initiation of antimicrobial therapy should prompt CT imaging of the chest to exclude complications that require definitive source control in conjunction with antimicrobial therapy.
VAP/HAP Empiric Choices
Both gram-positive (especially Staphylococcus aureus and Streptococci) and gram-negative aerobic (Enterobacteriaceae, including Pseudomonas sp. and Acinetobacter sp.) bacteria are responsible for VAP and HAP. Anaerobic organisms are rarely primary pathogens, although this should not be surprising in light of the generally high PAO2 within the lung. Overall there is nearly an even split between gram-positive and gram-negative organisms, so empiric therapy needs to be directed against both classes of organisms until culture data are available to permit informed de-escalation of treatment. The attributable mortality form pneumonia is on the order of 20% and numerous studies have demonstrated that mortality is significantly higher if the initial antimicrobial therapy does not target the pathogen.15,30,31 Thus, current, evidence-based recommendations emphasize initial broad empiric choices based upon knowledge of the institutional or community resistance patterns, with equally aggressive de-escalation and elimination of unnecessary antibiotics based on culture (ideally quantitative) information when available. The SOP divides the timeframe after admission for development of pneumonia into early (≤ 4 days) and late (>4 days). Early infections can generally be treated with single agents, as shown in Figure 1. Late infections, which reflect the epidemiology of hospital acquired pulmonary infections, require empiric coverage of both gram-positive and gram-negative bacteria and takes into account the high likelihood of antibiotic resistance encountered with these pathogens. It is possible to treat patients aggressively for the “worst case scenario” and still be a responsible steward of antibiotic sensitivity if these principles are followed.
Several other clinical questions frequently arise in terms of treating documented pulmonary infections. First, since Pseudomonas is a frequent isolate and may be resistant to some treatments, should “combination-therapy” (i.e., two antimicrobial agents with activity against Pseudomonas aeruginosa) be routinely employed? The consensus of the expert panel and the ATS-ACCP report was that combination therapy should be limited to patients likely to be infected with multidrug resistant pathogens.32 This recommendation must be tempered with clinical knowledge of antibiotic sensitivity patterns in the surgeon’s ICU, as well as recognition of available formulary choices, patient allergies, etc. In most cases, appropriate empiric therapy (with a single agent) likely to be active against Pseudomonal isolates from the institution should be sufficient. A second question that often arises is what duration of antibiotic treatment is needed? For example, is 7–8 days of treatment sufficient for VAP/HAP, or do patients require 14–15 days? This question is equally relevant to all surgical infections, but studies have shown equivalent responses with either 8 days or 15 days of treatment,33 so our recommendation is that for most patients 7 to 8 days of antibiotics is sufficient, although studies are ongoing to analyze whether even shorter durations of treatment are adequate. Finally, there is ongoing controversy regarding the appropriate antibiotic for empiric coverage of gram-positive organisms. Historically, vancomycin has been considered the agent of choice for VAP/HAP, but recent data suggests that other agents (for example, linezolid) may have better clinical outcomes than vancomycin.34 In addition, some institutions have witnessed more frequent pulmonary infections because of VRE.35,36 At the present time, empiric coverage with either vancomycin or linezolid is considered appropriate.
SKIN, SKIN STRUCTURE, OR SOFT TISSUE INFECTION
“Occult” skin and skin structure infections (SSSI) are rare in most clinical circumstances. Identification of such infections is partly a matter of elimination, but unfortunately the worst types of SSSI may not be obvious on physical examination. It can be difficult to appreciate the extent or depth of many SSSI and complicated SSSI often require aggressive surgical debridement. There should be a very high level of suspicion for severe SSSI if patients have high WBC, pain “out of proportion to examination,” or skin changes such as bullae or blisters. Complicated SSSI are differentiated from “simple” infections by virtue of the fact that they require a surgical intervention. Although antibiotics are important adjuncts to management, the cornerstone of treatment for complicated SSSI is early, aggressive, definitive surgical debridement.
SSSI Empiric Choices
Empiric antimicrobial choices for these infections are based upon the most likely pathogens.6,37,38 Figure 1 shows an approach that stratifies SSSI into uncomplicated and necrotizing soft tissue infections (NSTI) (including myonecrosis, necrotizing fasciitis, etc.). Uncomplicated SSSI are typically caused by aerobic gram-positive organisms (frequently gram-negative in diabetic patients), whereas the most common pathogens associated with severe SSSI include: Streptococcus species, Staphylococcal species, polymicrobial synergistic infections with gram-negative Enterobacteriaceae along with anaerobic species, and finally Clostridium perfringens. Gram’s stain of purulent material often provides important information regarding the microbiology of these infections. For example, a Gram’s stain showing large gram-positive rods would be highly suggestive of C. perfringens infection, the presence of gram-positive cocci would suggest either a Staph. or Strep. infection, and mixed flora would be seen with a mixed infection. In each case, the Gram’s stain could assist in refining antibiotic choices early. Empiric therapy needs to cover gram-positive and gram-negative aerobes, along with the most feared gram-positive anaerobic organisms, C. perfringens. Appropriate antibiotic recommendations for uncomplicated and complicated/severe infections are summarized in Figure 1. Vancomycin or linezolid are reasonable empiric choices to cover the gram-positive pathogens (including MRSA) and piperacillin-tazobactam (or aztreonam in penicillin allergic patients) is a good choice to cover gram-negative aerobes and anaerobes. An important consideration with many SSSI is the contribution of bacterial toxins to the pathogenesis of clinical illness. For this reason, a protein synthesis inhibitor such as linezolid or high dose clindamycin may also be included. If an SSSI infection arises in the hospital or if the patients present from an “outside” health care facility, it is much more likely that resistant pathogens are present.
PRESUMED CENTRAL VENOUS CATHETER INFECTION
The algorithm appropriately suggests that clinicians consider abdominal, pulmonary, and skin sources before entertaining a diagnosis of intravenous catheter (typically central venous catheters, CVC) infections. The criteria promulgated by the CDC emphasize that CVC infections (CVCI) are frequently a diagnosis of exclusion, although CVC are the most frequent cause of positive blood cultures in the ICU. It is axiomatic that critically ill patients all have indwelling invasive lines. In the setting of possible infection, it is important to carefully inspect all intravenous catheters for local signs of infection (i.e., erythema, purulence). However, absence of external signs of a central line infection does not exclude contamination of the intravascular portion of the catheter. If signs are present, the catheters should be removed and the catheter tip and/or subcutaneous segment sent for semiquantitative culture.39 Arterial lines, with the possible exception of those in the groin, are an infrequent site of CVCI. In many instances indwelling CVCs suspected of possibly being infected will be exchanged over a guide-wire (using full barrier precautions, see below) and the tip sent for semiquantitative culture. Growth of greater than 15 cfu/cm of catheter length is consistent with infection. Catheter tips should not simply be inoculated into broth culture, since this will result in identification of a high number of false positives.
Gram-positive bacteria, most commonly coagulase negative Staphylococci, and fungi, particularly Candida species, are the most likely pathogens associated with CVCI. Isolation of the same organism from the catheter tip and the bloodstream is definitive proof of CVCI. If a culture-positive, infected catheter had been exchanged over a guidewire most clinicians would remove the offending catheter and perform a new insertion, preferably with a catheter-free interval, if possible. Appropriate empiric choices to cover possible CVCI include vancomycin or linezolid. De-escalation and/or tailoring of antimicrobial therapy may be performed once culture results are available. Addition of an antifungal agent, such as fluconazole, would be appropriate while awaiting culture results in patients who have received prior systemic antibiotics, prolonged hospitalization, or if immunosuppressed.
It has become clear that relatively simple methods will dramatically reduce the incidence of CVCI in ICU patients.40 Use of sterile technique with full barrier precautions (i.e., gloves, gown, cap, mask, full body drape, etc.) significantly reduced the incidence of CVCI. In addition, antibiotic/antimicrobial impregnated CVC, although more expensive, have been shown to decrease infection rate. Finally, elimination of CVC whenever possible (in many cases using PICC lines or the equivalent as an alternative) reduce the incidence of CVCI in the ICU.39
ASSESSING RESPONSE TO THERAPY, INTERPRETING CULTURE RESULTS, DURATION OF THERAPY
Culture specimens should be obtained from all possibly infected sites whenever possible. The algorithm and current clinical practices emphasize initial broad spectrum coverage to insure that the worst likely pathogens are addressed from the outset. Gram’s stain of initial specimens can be very helpful in terms of identifying the presence of infection and the type of organisms present. Numerous studies attest to the fact that mortality and morbidity are decreased when appropriate antibiotics are chosen initially. However, it is critically important to assess the clinical response of the patient to this initial treatment and to appropriately adjust antimicrobial therapy in light of any culture results (both positive and negative results). If the patient has/had an infection at the time antimicrobials were initiated, it is reasonable to expect that there will be a demonstrable clinical response within 48 to 72 hours. When ICU “bad bugs” (e.g., MRSA, Acinetobacter, etc.) are associated with clinical infection, it is very unusual to have negative cultures at 48 to 72 hours. The absence of clinical improvement in vital signs, chemistry, WBC, etc. raises the possibility that a noninfectious cause is responsible. When lack of clinical improvement on antibiotics is also associated with negative cultures, strong consideration should be given to stopping antibiotics.
Alternatively, other patients may have a dramatic clinical response, but all cultures return negative. Under such circumstances, it would be reasonable to continue antibiotics, recognizing the vagaries of clinical microbiology laboratories and the frequent situation wherein cultures were obtained after antibiotics had been started (for example, operative culture specimens). Hopefully, the majority of patients with suspected infection will have one or more positive cultures from the infected site. When possible, antimicrobial therapy should be tailored to cover the species identified with the most targeted, least toxic, least expensive antimicrobial agent. Not infrequently, resistant organisms or organisms that were not covered by the initial broad empiric therapy will be identified. In these cases, alternative or additional agents may be needed to optimize treatment.
INFECTIONS AT OTHER SITES, FUNGAL INFECTIONS, ANTIBIOTIC ASSOCIATED DIARRHEA
Depending upon the cause of the traumatic injury other sites and organ systems should also be considered. For example, empyema is common after chest trauma, especially if hemothorax is incompletely evacuated. Urinary tract infections (UTIs) are frequent in ICU patients and quantitative culture of urine from indwelling catheters should be performed if infection is suspected. Because most antibiotics discussed above are at least partially eliminated by the kidney and antibiotic concentrations are very high in urine, most UTIs will be treated with the regimens used for other sites. Central nervous system (CNS) infections can occur with prolonged ICP monitoring, as well as with facial or head injuries. Such infections are beyond the scope of this SOP, but can be particularly problematic because many antimicrobial agents do not cross the blood-brain barrier.
Exposure to antibiotics and the immunosuppressive effects of injury and transfusion predispose the ICU patient population to a high risk of superinfection with fungal organisms, Clostridium difficile associated diarrhea (CDAD), and vancomycin-resistant Enterococci (VRE). Most trauma patients requiring more than a 48 hours ICU stay are at increased risk for fungal colonization (most often C. albicans)41 and consideration should be given to empiric antifungal prophylaxis/treatment with fluconazole.42 Over-use of anti-fungal agents has resulted in the increasingly frequent identification of nonalbicans Candida sp., along with other more serious fungal pathogens, such as Aspergillus sp.43 Watery diarrhea is the clinical hallmark of C. difficile-associated diarrhea, although significant disease may be present without diarrhea. C. difficile is generally treated with intravenous metronidazole (same luminal drug levels with oral or intravenous treatment) or oral/rectal vancomycin.44 C. difficile is rare in the absence of prior antibiotic exposure, but should be considered in all hospitalized patients (it is becoming endemic in health care facilities) or in any patient with prior exposure to any antibiotic, even a single dose.
SOP SUMMARY
Identify the critically ill patient in whom clinical decision to treat with antibiotics has been made. If possible, identify the likely site of infection. If it is impossible to identify site, obtain cultures from all accessible suspected sites and initiate empiric, broad-spectrum antibiotics. If likely site can be identified follow guideline questions: Is intra-abdominal, pulmonary, skin structure or soft tissue site suspected, or is the clinical suspicion that the patient has central venous catheter infection? Acceptable empiric suggestions for likely pathogens associated with infections at each location are provided. All decisions to continue, discontinue, and/or alter antibiotic/antimicrobial treatment should be based on the clinical response to treatment, diagnostic or interventional findings, and culture and sensitivity data. Bear in mind that not all patients with infections will have positive cultures because of limitations of specimen handling, microbiology laboratory variations, time between specimen acquisition and culture, or presence of effective antibiotics at the time that specimens were obtained. Conversely, not all patients with a fever and leukocytosis harbor an infection.
SOP DETAILS
Identify the critically ill patient in whom the clinical decision to treat with antibiotics has been made.
- Attempt to identify the site of infection based on clinical signs and symptoms, laboratory or diagnostic radiology studies.
- Examine the patient.
- Inspect all wounds.
- If ventilated, obtain chest radiograph, calculate clinical pulmonary infection score.
- Obtain blood cultures.
- Urinalysis.
- Line change if clinical suspicion of CVC.
If impossible to identify site, obtain cultures from all accessible suspected sites and initiate empiric, broad spectrum antibiotics.
- If likely site can be identified follow guideline questions:
- Is intra-abdominal site suspected? If yes, has patient had prolonged (>4 days) hospitalization? Acceptable empiric suggestions for community acquired/less serious infections or hospital acquired/more serious and/or resistant infections are provided.
- Is pulmonary source of infection suspected? If yes, has patient had prolonged (>4 days) hospitalization? Acceptable empiric suggestions for community acquired/ less serious infections or hospital acquired/more serious and/or resistant infections are provided. [Note quantitative microbiology and “invasive” diagnostic techniques have superior accuracy for diagnosis of pneumonia in critically ill patients.
- Is skin, skin structure, or soft tissue site suspected? If yes, does the patient have clinical signs suspicion for necrotizing soft tissue infection (NSTI)? Acceptable empiric suggestions for less serious SSSI or severe NSTI are provided.
- Is a central venous catheter infection suspected? Acceptable empiric suggestions for likely pathogens for CVC infections are provided.
Decision to continue, discontinue, and/or alter antibiotic/antimicrobial treatment should be based on the clinical response to treatment, diagnostic or interventional findings, and culture and sensitivity data. Bear in mind that not all patients with infections will have positive cultures because of limitations of specimen handling, microbiology laboratory variations, time between specimen acquisition and culture, or presence of effective antibiotics at the time that specimens were obtained.
Not all patients with increased temperature/WBC have an infection. Discontinuation of antibiotics is appropriate if cultures, diagnostic studies are negative.
ACKNOWLEDGMENT
Additional participating investigators in “The Inflammation and the Host Response to Injury Collaborative Research Program”, include: Henry V. Baker, PhD, Ulysses G. J. Balis, MD, Timothy R. Billiar, MD, Bernard H. Brownstein, PhD, Steven E. Calvano, PhD, David G. Camp II, PhD, Irshad H. Chaudry, PhD, J. Perren Cobb, MD, Ronald W. Davis, PhD, Asit K. De, PhD, Celeste C. Finnerty, PhD, Bradley Freeman, MD, Richard L. Gamelli, MD, Nicole S. Gibran, MD, Douglas L. Hayden, MA, Laura Hennessay, RN, David N. Herndon, MD, Marc G. Jeschke, MD, PhD, Matthew B. Klein, MD, James A. Lederer, PhD, Stephen F. Lowry, MD, John A. Mannick, MD, Philip H. Manson, PhD, Grace P. McDonald- Smith, MEd, Carol L. Miller-Graziano, PhD, Michael Mindrinos, PhD, Lyle L. Moldawer, PhD, Grant E. O’Keefe, MD, MPH, Laurence G. Rahme, PhD, Daniel G. Remick, Jr., MD, David Schoenfeld, PhD, Geoffrey M. Silver, MD, Richard D. Smith, PhD, John D. Storey, PhD, Robert Tibshirani, PhD, Ronald G. Tompkins, MD, ScD, Mehmet Toner, PhD, H. Shaw Warren, MD, Rebbecca P. Wilson, BA, Wenzhong Xiao, PhD
Supported by Large-Scale Collaborative Project Award (U54-GM62119) from The National Institute of General Medical Sciences, National Institutes of Health.
REFERENCES
- 1.Ortiz R, Lee K. Nosocomial infections in neurocritical care. Curr Neurol Neurosci Rep. 2006;6:525–530. doi: 10.1007/s11910-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 2.Jaeger M, Maier D, Kern WV, Sudkamp NP. Antibiotics in trauma and orthopedic surgery—a primer of evidence-based recommendations. Injury. 2006;37(Suppl 2):S74–S80. doi: 10.1016/j.injury.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee I, Iredell JR, Woods M, Lipman J. The implications of enterococci for the intensive care unit. Crit Care Resusc. 2007;9:69–75. [PubMed] [Google Scholar]
- 5.Merlino JI, Yowler CJ, Malangoni MA. Nosocomial infections adversely affect the outcomes of patients with serious intraabdominal infections. Surg Infect (Larchmt) 2004;5:21–27. doi: 10.1089/109629604773860273. [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Kuti JL, Nicolau DP. Antimicrobial management of complicated skin and skin structure infections in the era of emerging resistance. Surg Infect (Larchmt) 2005;6:283–295. doi: 10.1089/sur.2005.6.283. [DOI] [PubMed] [Google Scholar]
- 7.Mylotte JM, Goodnough S, Tayara A. Antibiotic-resistant organisms among long-term care facility residents on admission to an inpatient geriatrics unit: retrospective and prospective surveillance. Am J Infect Control. 2001;29:139–144. doi: 10.1067/mic.2001.114225. [DOI] [PubMed] [Google Scholar]
- 8.Rohrborn A, Wacha H, Schoffel U, et al. Coverage of enterococci in community acquired secondary peritonitis: results of a randomized trial. Surg Infect. 2000;1:95–107. doi: 10.1089/109629600321137. [DOI] [PubMed] [Google Scholar]
- 9.Bohnen JM, Marshall JC, Fry DE, Johnson SB, Solomkin JS. Clinical and scientific importance of source control in abdominal infections: summary of a symposium. Can J Surg. 1999;42:122–126. [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JC, Maier RV, Jimenez M, Dellinger EP. Source control in the management of severe sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32(11 Suppl):S513–S526. doi: 10.1097/01.ccm.0000143119.41916.5d. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC. Intra-abdominal infections. Microbes Infect. 2004;6:1015–1025. doi: 10.1016/j.micinf.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Danielson D, West MA. Recent developments in clinical management of surgical sepsis. Curr Opin Crit Care. 2001;7:367–370. doi: 10.1097/00075198-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 14.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core–standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 15.Kollef MH. Treatment of ventilator-associated pneumonia: get it right from the start. Crit Care Med. 2003;31:969–970. doi: 10.1097/01.CCM.0000055381.70829.94. [DOI] [PubMed] [Google Scholar]
- 16.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 17.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25:1789–1795. doi: 10.1097/00003246-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Sprung CL, Sakr Y, Vincent JL, et al. An evaluation of systemic inflammatory response syndrome signs in the Sepsis Occurrence in Acutely Ill Patients (SOAP) study. Intensive Care Med. 2006;32:421–427. doi: 10.1007/s00134-005-0039-8. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J, Brun-Buisson C, Torres A, Jorgensen J. Diagnosis of infection in sepsis: an evidence-based review. Crit Care Med. 2004;32(11 Suppl):S466–S494. doi: 10.1097/01.ccm.0000145917.89975.f5. [DOI] [PubMed] [Google Scholar]
- 20.McGilvray ID, Rotstein OD. Management of infection in the surgical patient: an update. Surg Technol Int. 2003;11:39–43. [PubMed] [Google Scholar]
- 21.Nathens AB. Relevance and utility of peritoneal cultures in patients with peritonitis. Surg Infect (Larchmt) 2001;2:153–160. doi: 10.1089/109629601750469474. discussion 160–162. [DOI] [PubMed] [Google Scholar]
- 22.Bochud PY, Bonten M, Marchetti O, Calandra T. Antimicrobial therapy for patients with severe sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32(11 Suppl):S495–S512. doi: 10.1097/01.ccm.0000143118.41100.14. [DOI] [PubMed] [Google Scholar]
- 23.Malangoni MA, Song J, Herrington J, Choudhri S, Pertel P. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann Surg. 2006;244:204–211. doi: 10.1097/01.sla.0000230024.84190.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford PA, Weaver-Sands DT, Petersen PJ. In vitro activity of tigecycline against isolates from patients enrolled in phase 3 clinical trials of treatment for complicated skin and skin-structure infections and complicated intra-abdominal infections. Clin Infect Dis. 2005;41(Suppl 5):S315–S332. doi: 10.1086/431673. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett JG, Onderdonk AB, Louie T, et al. A review. Lessons from an animal model of intra-abdominal sepsis. Arch Surg. 1978;113:853–857. doi: 10.1001/archsurg.1978.01370190075013. [DOI] [PubMed] [Google Scholar]
- 26.Mosdell DM, Morris DM, Voltura A, et al. Antibiotic treatment for surgical peritonitis. Ann Surg. 1991;214:543–549. doi: 10.1097/00000658-199111000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazuski JE, Sawyer RG, Nathens AB, et al. The Surgical Infection Society Guidelines on Antimicrobial Therapy for Intra-Abdominal Infections: an executive summary. Surg Infect. 2002;3:161–173. doi: 10.1089/109629602761624171. [DOI] [PubMed] [Google Scholar]
- 28.Solomkin JS, Mazuski JE, Baron EJ, et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis. 2003;37:997–1005. doi: 10.1086/378702. [DOI] [PubMed] [Google Scholar]
- 29.Smith RL, II, Sawyer RG, Pruett TL. Hospital-acquired infections in the surgical intensive care: epidemiology and prevention. Zentralbl Chir. 2003;128:1047–1061. doi: 10.1055/s-2003-44848. [DOI] [PubMed] [Google Scholar]
- 30.Shorr AF, Kollef MH. Ventilator-associated pneumonia: insights from recent clinical trials. Chest. 2005;128(5) Suppl 2:583S–591S. doi: 10.1378/chest.128.5_suppl_2.583S. [DOI] [PubMed] [Google Scholar]
- 31.Niederman MS. De-escalation therapy in ventilator-associated pneumonia. Curr Opin Crit Care. 2006;12:452–457. doi: 10.1097/01.ccx.0000244126.84989.a2. [DOI] [PubMed] [Google Scholar]
- 32.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 33.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 34.Wunderink RG, Rello J, Cammarata SK, Cross-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. [PubMed] [Google Scholar]
- 35.National Nosocomial Infections Surveillance (NNIS) System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 to June 2002, issued August 2002. Am J Infect Control. 2002;30:458–475. doi: 10.1067/mic.2002.130032. [DOI] [PubMed] [Google Scholar]
- 36.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Fung HB, Chang JY, Kuczynski S. A practical guide to the treatment of complicated skin and soft tissue infections. Drugs. 2003;63:1459–1480. doi: 10.2165/00003495-200363140-00003. [DOI] [PubMed] [Google Scholar]
- 38.DiNubile MJ, Lipsky BA. Complicated infections of skin and skin structures: when the infection is more than skin deep. J Antimicrob Chemother. 2004;53(Suppl 2):ii37–ii50. doi: 10.1093/jac/dkh202. [DOI] [PubMed] [Google Scholar]
- 39.Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 40.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–2020. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 41.Pelz RK, Lipsett PA, Swoboda SM, Diener-West M, Hammond JM, Hendrix CW. The diagnostic value of fungal surveillance cultures in critically ill patients. Surg Infect. 2000;1:273–281. doi: 10.1089/109629600750067200. [DOI] [PubMed] [Google Scholar]
- 42.Cruciani M, de Lalla F, Mengoli C. Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intensive Care Med. 2005;31:1479–1487. doi: 10.1007/s00134-005-2794-y. [DOI] [PubMed] [Google Scholar]
- 43.Pelz RK, Hendrix CW, Swoboda SM, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg. 2001;233:542–548. doi: 10.1097/00000658-200104000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624–631. doi: 10.1001/archsurg.142.7.624. discussion 631. [DOI] [PubMed] [Google Scholar]