Severely injured patients have marked metabolic derangements, generally characterized by increased substrate utilization and protein catabolism. Bench and clinical research have provided ample evidence supporting the notion that specialized nutritional support is beneficial and improves important clinical outcomes in the critically ill. However, marked differences exist in the application of the evidence to the care of these patients.1 The results of a recent survey of nutritional support practices in intensive care units (ICU) indicate that few patients receive more than 50% of estimated caloric or protein requirements in the initial 5 days in the ICU and that intake is quite variable from patient to patient.2 However, in the context of an established guideline or as part of a clinical trial, most patients can reach prescribed nutritional support targets and generally do so within the first 5 days to 7 days.3,4 This suggests that variability in achieving nutritional goals is often due to variation in practice.
PROTOCOL GOALS
As part of the Inflammation and the Host Response to Injury Large-Scale Collaborative Research Program, the participating investigators examined existing evidence for nutritional support in critically ill and injured patients and developed a standard operating procedure (SOP) to manage these patients. There are two parallel objectives of this SOP: (1) To optimize patient outcome through enhancing tolerance of enteral nutritional (EN) support and minimizing the complications associated both with “malnutrition” and with the delivery of nutritional support. (2) To generate guidelines that is based upon the best available evidence.
Our primary goal is to provide an approach to nutritional support that is based upon existing literature but also incorporates the cumulative experience of the Glue Grant investigators in this often debated and controversial area of critical care. We recognize that supportive data exist for many nutritional practices; however, the trauma surgeon should recognize that practices shown to be beneficial in animal models or in nontraumatized critically ill patients may not have the same effect in patients with severe injuries.
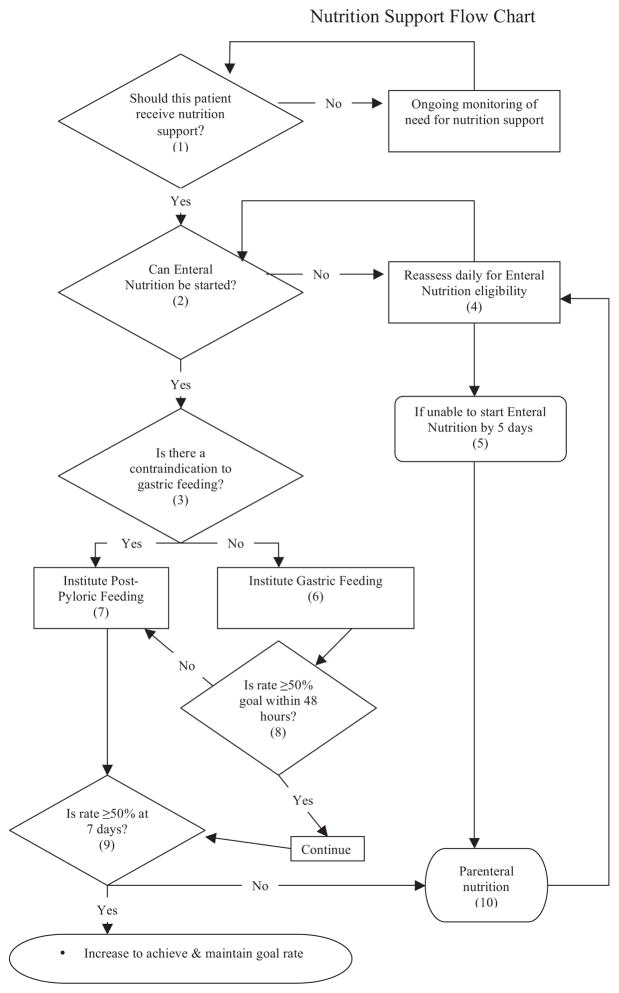
Herein, we present our SOP that addresses the following: (1) the selection of patients for nutritional support, (2) approach to initiation, (3) route of administration, (4) nutrient formulation, and (5) nutritional support monitoring. This set of guidelines is evidence-based where possible and devised for patients with severe multisystem injury, who have been resuscitated from marked physiologic derangements. This SOP also includes a Nutrition Support Flow Chart that provides a framework for managing nutritional support, with particular focus on the first postinjury week (Fig. 1).
Fig. 1.
Nutrition support flow chart.
GENERAL RATIONALE AND SELECTION OF PATIENTS FOR SPECIALIZED NUTRITIONAL SUPPORT
Most patients with severe injuries expected to survive beyond 24 hours to 48 hours after injury are likely to stay from days to weeks in the intensive care unit, and they remain at risk for nosocomial infections, acute respiratory distress syndrome, multiple organ dysfunction, and death.5 Although injury victims are not frequently malnourished before they are injured, all are at nutritional risk from the hypermetabolic response to injury and subsequent complications. Studies have demonstrated that injury victims benefit from an organized approach to nutritional support that incorporates early institution of enteral feeding. Based upon cumulative clinical evidence, we think that all injury victims expected to be unable to be extubated and begin oral intake within 72 hours of injury should receive specialized nutritional support. It is not our intention here to review all the biological and clinical evidence favoring our recommended approach to nutritional support. However, in the sections that follow, where important data exist to support our decisions, we review the biological rationale and clinical evidence that guide our approach.
INITIATION OF NUTRITIONAL SUPPORT
Timing of Initiation
Enteral support should be initiated as early as feasible which is typically between 24 hours and 48 hours postinjury. Feeding earlier than this is often precluded by operative procedures, continuing resuscitation and completion of the secondary survey, which often involves moving the patient from the ICU. Initial access is typically via a previously placed orogastric or nasogastric tube and gastric feeding (route of administration is discussed in more detail in the subsequent section) can be safely initiated early as long as the patient is carefully monitored for intolerance. When feasible, this tube can be replaced by a softer, 10 to 12 French silastic feeding tube. Feeding incompletely resuscitated patients enterally must be avoided. Splanchnic ischemia is common in severely injured patients and while we do not endorse gastric tonometry it is important to assure that usual indicators of shock (hypotension, metabolic acidosis, need for vasopressors, etc) have resolved before starting EN.
Initiation and Advancement
Gastric feeding is started with full-strength formula at 25 mL/h. There is no evidence to support dilution of enteral formula. Generally, continuous feeding is used as there is limited evidence to support intermittent bolus feeding.6 Advance gastric feeding by 25 mL/h every 8 hours to goal rate when there are no contraindications. The primary contraindication to advancement of EN rate is evidence of intolerance. This includes increasing abdominal distention, emesis, and evidence of aspiration of gastric contents or high-gastric residual volume as measured via a nasogastric tube. These complications are subsequently discussed in greater detail. For the few patients with gastrostomy tubes, the volume of drainage may not be an accurate reflection of gastric residual volume and other signs of intolerance should be carefully assessed. Gastric residual volume is assessed every 4 hours and if >300 mL, EN is held for 2 hours. With the feeding tube clamped, residual volume is again measured and feeding resumed at the previous rate if the volume has dropped below 300 mL. Gastric aspirates ≤300 mL are returned via the feeding tube whereas volumes >300 mL are not. Enteral feeds should be advanced with caution on patients receiving neuromuscular blockade or vasopressor agents. Use of these agents does not contraindicate enteral feeding. However, in patients requiring institution or increasing amount of vasopressor therapy for septic shock or other causes of hemodynamic deterioration, enteral support should be temporarily stopped until the patient has stabilized at which point enteral support is restarted and advanced accordingly. As an indicator of intolerance, gastric residual volume measurement is controversial. Recommended thresholds vary (200–400 mL), and it is unclear whether gastric residual volume even reflects feeding intolerance or risk for aspiration.7 In an effort to minimize cessation of enteral feeding, we have agreed to use >300 mL as an appropriate threshold above which intolerance likely exists and should be addressed.
There are circumstances when postpyloric EN support is indicated. First, when gastric feeding cannot be initiated within 48 hours, particularly in the case of large nasogastric tube output (>1,000 mL/d), a postpyloric feeding tube should be placed, either with fluoroscopic or endoscopic guidance. If possible, this tube should rest distal to the ligament of Treitz. Although it has not been proven that jejunal EN is better tolerated than duodenal infusion for patients intolerant of gastric nutrition, we recommend advancing the tube at least to the distal duodenum to minimize reflux into the stomach. Similarly, patients in whom gastric feeding has been initiated, but cannot be advanced to ≥50% of caloric goals within 48 hours, post-pyloric feeding should be attempted.
Incompletely resuscitated patients are likely to not tolerate gastric feeding, and should also not have direct small bowel feedings instituted, because of the risk of intestinal distention and the potential for small bowel necrosis. The possible causes for intolerance that would contraindicate EN regardless of administration site should be identified and treated (e.g., distal small bowel obstruction, severe ileus, and shock). Although bedside placement of postpyloric feeding tubes is possible, particularly when following a strict protocol, successful placement in the distal duodenum or jejunum is generally uncommon, and for this reason we recommend fluoroscopic or endoscopic guidance.8–10
In patients undergoing celiotomy for trauma, we recommend surgeon assistance with the passage of a nasoduodenal or nasojejunal feeding tube if circumstances permit. Advancing the tube to the distal duodenum or beyond the ligament of Treitz may reduce duodenogasrtic reflux and be more effective than simply advancing the tube to the first part of the duodenum. The principal contraindication to the placement of a distal duodenal or jejunal tube is ongoing instability requiring urgent transfer from the operating room. The feeding tube is passed orally in patients with severe facial, maxillary, and frontal basal skull injuries, which preclude safe nasal placement. Nasojejunal feeding tubes may be particularly beneficial in patients who have sustained gastric, pancreatic, or duodenal injuries and are ideally advanced past the location of the injury by the surgeon.
Patients with open abdomens can be successfully fed enterally, generally via a nasojejunal feeding tube. Surgical jejunostomies, including needle catheter jejunostomies are not typically recommended as the primary method of access for enteral feeding. Specific situations in which they are indicated include complex pancreaticoduodenal or esophageal injuries where the need for prolonged artificial nutrition is predictable and gastric feeding contraindicated or unlikely to be successful. In these cases, direct jejunal access at the time of abdominal exploration is warranted. Studies have shown surgically placed jejunostomies generally to be safe and to facilitate reaching nutritional support targets in severely injured patients undergoing abdominal exploration.11 Surgical jejunostomies should be avoided in patients with open abdomens until final closure is obtained, at which point a surgical jejunostomy may be placed. They should not be used when the intestine is friable, inflamed, or edematous, and instead a nasojejunostomy can be placed if not already done at a prior exploration. Although potentially associated with fewer complications, needle catheter jejunostomies are generally of small caliber (8F), and therefore cannot be used to administer most medications and some enteral formula.12,13
Adjuvant Treatment to Facilitate Tolerance and Reduce Complications
Semirecumbent body position reduces the risk of nosocomial pneumonia, likely by minimizing aspiration events. This is particularly notable in patients receiving EN support.14 We recommend that the patient be treated with the head of the bed elevated to greater than 30 degrees as part of both our ventilator-associated pneumonia SOP and this SOP.15 Prokinetic agents (metoclopromide and erythromycin,) are not routinely administered and data supporting their effects are conflicting, without clear benefits on important clinical outcomes.16 These agents may be started once the decision to institute enteral feeding is made or at any point during the initial 48 hours, particularly if tolerance is in question (i.e. gastric aspirate >300 mL). However, we do not recommend delaying conversion from gastric to postpyloric feeding more than 48 hours to analyze whether the addition of a prokinetic agent is effective. Additional adjuncts, aimed at reducing the risk of ventilator-associated pneumonia, are important and are discussed in the previous article in this series that was cited above.
ROUTE OF ADMINISTRATION
Enteral Versus Parenteral Nutritional Support
The weight of evidence supports the use of EN support, initiated once the patient has been resuscitated from shock and as long as there are no contraindications (bowel discontinuity, for example). The generally accepted corollary is that the use of PN within the first postinjury week is not associated with improved outcomes when compared with EN support.17 However, PN will be necessary in the uncommon circumstances where attempts at EN fail or when EN is contraindicated. Prolonged starvation is detrimental in trauma patients, yet it is not certain at what point attempts at EN should yield to the use of PN. We recommend that PN be considered by the 5th postinjury day for patients whom have not received any enteral support, particularly in those where successful EN is unlikely by day 7. During the first 7 postinjury days, patients achieving 50% of estimated caloric needs with EN support do not seem to benefit from supplemental PN and may, in fact be harmed.* However, if patients have not been advanced beyond this 50% threshold by 7 days, consideration should be given to supplemental PN. The recommendations regarding when and in whom to begin PN are primarily based upon balancing the detrimental effects of longer periods of starvation with the potential complications associated with PN. Concerns during glucose administration and hyperglycemia are particularly relevant for patients receiving PN. Monitoring blood glucose and careful attention to infusion rates are of critical importance, as it is relatively easy to administer excessive glucose and calories intravenously in a short period of time. In a cohort of critically ill patients, we have demonstrated that, even short periods of excessive intravenous caloric intake often occurs and may lead directly to infectious morbidity.18
CALORIC REQUIREMENTS AND NUTRIENT FORMULATION
Estimating Initial Caloric and Nutrient Needs
For moderately to severely injured patients, energy requirements are generally 25–30 kcal/kg/d of body weight. We recommend this range as an initial target for enteral support. However, estimating the patient’s weight can be problematic if the usual body weight is not known. When the usual body weight is unknown, the dry weight is substituted and estimated by subtracting the resuscitation volume from the patient’s measured weight in the ICU. For obese patients (body mass index ≥30) an “adjusted weight” is substituted for estimating caloric and protein needs. † Although energy requirements may be slightly higher for patients with severe traumatic brain injury, the same initial estimates are used, regardless of specific anatomic injuries. Studies have examined the accuracy of the various equations in estimating energy expenditure relative to measurements by indirect calorimetry, with conflicting observations and recommendations. For nonobese patients, the Harris-Benedict equation, multiplied by a stress factor of 1.3–1.5 reasonably estimates energy expenditure and therefore, caloric needs in critically ill patients.19 Nevertheless, this and other estimates may be inaccurate and individual patients may benefit from indirect calorimetry as discussed subsequently. Assessing the patient’s preinjury nutritional and general health status is often difficult and frequently impossible, at least initially. However, some pieces of information may be useful in identifying preinjury malnutrition. A history of weight loss or of poor intake should be sought from the patient or a surrogate. Knowledge of a substantial recent weight loss or history, or a poor and limited dietary intake may lead the surgeon to be more aggressive with enteral and possibly even parenteral support. Perhaps, the most important cause of malnutrition in injury victims is substance abuse, particularly alcohol intake. It is likely that patients with chronic alcohol abuse are more often malnourished than others and they likely warrant particularly careful attention to nutritional therapy. The will be less able to tolerate unnecessary periods of reduced caloric intake. However, we do not endorse a necessarily more “aggressive” approach in terms of conversion or addition of PN, for example.
Protein needs are initially estimated at 1.5 g to 2.0 g of protein/kg/d.20 This is despite relatively little evidence regarding the optimal protein delivery in these patients. As with caloric needs, protein requirements are at the upper end of this range for patients with traumatic brain injury. Achieving positive nitrogen balance varies according to the phase of injury and is unlikely in the initial week after injury. Therefore, attempts to match nitrogen losses are not endorsed and may be harmful; particularly if large volumes of enteral formula or if supplemental TPN are used. Provision of large protein loads to elderly or to those with chronic hepatic, renal, or pulmonary disease are considered to be deleterious.
If it is determined that PN is required, caloric and nutrient needs are analyzed by the same formula and methods as for EN. However, parenteral lipid intake is limited to1 g/kg/d (this is typically less than 30% of total kcal). Calories from propofol and all dextrose infusions are included in the calculation of caloric intake, as these are often sources of “occult” and potentially excessive calories. Provision of excess calories to trauma patients may induce hyperglycemia, excess CO2 production, fluid/electrolyte abnormalities, lipogenesis, and hepatic steatosis. Furthermore, excess parenteral calories have been linked to the development of bloodstream infections (bacteremia and catheter-related infections) in patients receiving PN.18
Nutrient Formulation and Supplementation
The choice of nutritional formula and additional supplementation are left to the discretion of the surgical team and, in part, depends upon the products available at each institution. However, we recommend the use of standard, high-protein enteral formula of which there are a variety of products available. Typically, we recommend standard high-protein 1 kcal/mL formula in most circumstances. However, in circumstances when limiting volumes are considered important (following large volume resuscitations, open abdomens, and ongoing diuresis) higher caloric density (1.5–2.0 kcal/mL) formulas may be used to facilitate caloric intake in smaller volumes.
A number of specific nutrients, including glutamine, arginine, ribonucleic acids, and omega-3 fatty acids have been studied as additives, either individually or together, to standard nutritional formula for their proposed beneficial effects on outcomes in both surgical and critically ill patients. Although beneficial in animal models of injury and infection, the effects of these “immune enhanced” products in severely injured humans are uncertain.21,22 The balance of evidence indicates that the combined supplementation of EN formula with arginine, omega-3 fatty acids, and glutamine does not reduce mortality, infections, or organ failure in critically ill patients. This contrasts with the demonstrated reduction in infectious complications observed when immune-enhanced supplements are used in elective surgical patients.23 Our rationale for not extrapolating from experimental models and elective patients to critically ill trauma patients perhaps warrants explanation. Although such supplementation no doubt affects biochemical measures of immunity and inflammation, their clinical effects are uncertain and heterogeneous. This is due, in part to the fact that immune system activation differs between patient groups (elective surgery versus critically ill trauma patients) and also varies within patients over time. The pharmacological effects of immune-enhanced EN therefore, likely interact with the immune status of the critically ill trauma patient in ways that are difficult to predict, but may depend upon whether the patient is in the early postinjury period or has developed a nosocomial infection. Therefore, although beneficial in some patients, in others, supplementation with these agents is seemingly detrimental.21 One possible biological reason relates to the role of arginine in increasing nitric oxide (NO) production. It is posited that increased NO production, while beneficially influencing innate immune function and infectious outcomes in elective surgery patients, is detrimental in critically ill patients with sepsis.24 We therefore, do not advocate the use of commercially available immune-enhanced enteral supplements that contain arginine. However, other individual agents may be beneficial and warrant comment.
On the basis of the location of the first unsaturated bond relative to the methyl terminus, omega-3 polyunsaturated fatty acids (PUFA) differ from omega-6 PUFA. The relative intake of these two PUFAs analyzes the fatty acid composition of immune cell membranes and thus, the profile of arachadonic acid metabolites released in response to inflammatory activation of phospholipases. Omega-3 PUFA supplementation leads to partial replacement of arachadonic acid in cell membranes with eicosapentanoic and docosahexaenoic acids and altered inflammatory responses.25 Furthermore, omega-3 PUFAs may influence inflammation by altering TNF-α and IL-1β responses by mechanisms independent of the effects on cell membrane composition.26 This may be mediated via a stabilizing effect on NFκB/IκB complexes. Only small clinical trials have examined whether omega-3 PUFA supplementation (in conjunction with antioxidants) influence outcomes in critically ill patients. For patients with ARDS, omega-3 supplementation was associated with improved oxygenation, but no clear benefit in clinical outcomes.27
Glutamine is the most abundant free amino acid in humans, functions as precursor to protein synthesis and as a nitrogen source for arginine, nucleotide synthesis, and glutathione production. Under stress conditions, glutamine becomes an essential amino acid with its depletion leading to lack of substrate in proliferating tissues. This may have profound effects on the integrity of the intestinal mucosal barrier and on immune function in critically ill patients.28 However, beneficial effects of enteral supplementation with glutamine on important clinical outcomes are unproven.
Oxidative stress is central to the pathophysiology of injury, inflammation, and critical illness. Reactive oxygen species (ROS) are generated after injury and have a role in cell signaling, cell protection, and pathogen destruction. However, these ROS can damage intra and extracellular constituents leading to tissue injury and organ failure. Although homeostasis exists between ROS and endogenous antioxidants in healthy individuals, this balance is disturbed in critically ill and injured patients. As indicated in the preceding paragraph, glutamine supplementation may directly enhance antioxidant capacity. Other agents are available for administration as anti-oxidant supplements. Selenium, Vitamins A, C, and E when given enterally or parenterally can restore circulating anti-oxidant capacity. Furthermore, the administration of these anti-oxidants, individually or in combination may reduce the risk of multiple organ failure and mortality in critically ill patients.29,30 We recommend the following: (A) Vitamin C, 100 mg IV Q8H. (B) Selenium 400 μg every day. (C) Vitamin E 1500 IU Q12H. Each are given for 7 days or until the patient is discharged from the ICU. For the first 2 days, Vitamin C and selenium are given intravenously and enterally thereafter. Vitamin E is given enterally for the entire 7 days.
In summary, we recommend enteral support with a high-protein polymeric formula. Arginine supplementation should be avoided, particularly in patients with sepsis. Supplementation with omega-3 PUFA, glutamine, and antioxidants (selenium, etc.) offers potential benefit, and their use is left to individual surgeon discretion.
MONITORING NUTRITIONAL SUPPORT
These guidelines recommend protein and calorie quantities typically required by the severely injured patient and are based on formulas that approximate initial requirements. In general, monitoring includes bedside assessment of the patient tolerance; particularly to EN support, daily evaluation by the dietitian to ensure nutritional targets are being met or approached, and biochemical monitoring to measure the effects of support on indices of nutritional status. The effects of massive resuscitation and marked fluid shifts during the initial postinjury week generally preclude the use of serum markers and body weight as indicators of both prior nutritional status and response to nutritional therapy during the initial postinjury week.
It is also prudent to objectively measure individual patient responses to nutritional therapy. We recognize that no single test accurately characterizes an individual’s response to treatment and furthermore, that there is no evidence that adjustments in nutritional support, based upon monitoring affects outcome. However, in patients receiving nutritional support for 7 days or more, analyzing whether caloric and protein needs are being appropriately matched by intake may be beneficial as the patient moves from a catabolic to anabolic phase of recovery. This shift occurs at differing times after injury and may not be static, with patients moving back and forth from a catabolic to an anabolic phase as complications develop and resolve. Once the patient enters an anabolic state, sufficient substrate is needed to rebuild proteins, heal wounds, and restore muscle mass.
Monitoring GI Tolerance
High-gastric residual volumes have often been considered a marker for gastric intolerance of EN, reflux, and bronchopulmonary aspiration of GI contents. However, intolerance may manifest in a variety of ways, including poor gastric emptying, abdominal distention, abdominal tenderness, and diarrhea. These manifestations may reflect gastric ileus, infectious colitis, or more ominously, intestinal ischemia and necrosis.
Aspiration is a major complication of EN support. Its occurrence and consequences may negate the overall benefits of enterally based nutrition. Unfortunately, there are no specific predictors of aspiration risk and there is little agreement on a particular gastric residual volume that reflects intolerance, portends aspiration, and mandates cessation of enteral feeding. Therefore, careful and ongoing clinical assessment is necessary to avoid complications of enteral feeding. Changes in the clinical examination, including sudden increases in nasogastric output or gastric residual volume, new abdominal tenderness or distention require cessation of enteral feeding and mandate careful evaluation. This may avoid overt episodes of aspiration. Our approach to monitoring and management of gastric residual volumes is detailed previously but warrants re-emphasis. When feeding into the stomach, gastric aspirates are obtained every 4 hours to 6 hours and a residual volume of >300 mL mandates cessation of feeding with reassessment of residual volume 2 hours later.
Perhaps, the most devastating complication of EN support is intestinal infarction and necrosis. Although rare (<1% of patients fed enterally), it carries a substantial mortality and unfortunately, seems to have no early and specific signs.31 Intestinal infarction typically occurs in patients fed in the jejunum, but is also reported in patients with duodenal feeding tubes. Both nasoenteric and surgical jejunostomies have been linked to this complication. Most cases seem not to be a consequence of feeding incompletely resuscitated patients early after injury as this complication is typically recognized days to weeks later. In all reported cases and series, abdominal distention, diarrhea, leukocytosis, and additional features of the systemic inflammatory response are often present but are not particularly helpful in making the diagnosis. Although abdominal distention and tenderness may direct clinicians toward the appropriate diagnosis, they are not an early signs. If this diagnosis is suspected, enteral feeding must be stopped. Patients with peritonitis should be surgically explored. Otherwise, the diagnosis may be made by plain abdominal radiographs (pneumoperitoneum or pneumatosis) or abdominal computed tomography.31,32
Diarrhea is the most commonly reported gastrointestinal side effect in patients receiving EN. It may be a complication of EN or as a result of another pathophysiological process such as Clostridium difficile colitis. Furthermore, as indicated above, diarrhea may portend a serious catastrophe such as intestinal infarction. We recommend a systematic approach, when a patient develops diarrhea (defined as >500 mL stool/d or >3 stools/d for 2 consecutive days) that includes assessment for infectious colitis, fecal impaction, and potential medication related causes. In addition to identifying a cause, associated fluid and electrolyte abnormalities should be treated. The addition of nondigestible fiber (water soluble fiber such as pectin, for example) may be effective in preventing diarrhea in critically ill patients receiving EN.
Biochemical Monitoring
Biochemical monitoring of response to nutritional support can be performed although no evidence exists that such practice improves outcomes. Serum transthyretin (prealbumin) can be measured after 7 days to analyze whether protein support is sufficient or should be adjusted. Alternatives include transferrin and retinol binding protein. Serum albumin is a poor indicator of nutritional status and is not used to analyze the adequacy of nutritional support. Measurements of these serum constituents can be supplemented by obtaining a 24-hour urine sample for determination of nitrogen balance either by measuring total urinary nitrogen (TUN) or urea urinary nitrogen (UUN). Because urinary nitrogen losses are initially high during the initial postinjury week, we do not obtain urine nitrogen measurements during the first week and therefore, do not adjust protein intake in an effort to match nitrogen losses. In addition, nitrogen balance is generally not influenced by the amount of nonprotein caloric intake during the first postinjury week, therefore, we do not attempt to stem nitrogen loss by increasing overall caloric intake.33 Nitrogen balance studies may be helpful after this first week, when nitrogen losses have generally decreased, and positive nitrogen balance may be reasonably achieved with supplemental protein intake. However, urine nitrogen measurements do not reflect protein catabolism in patients with acute renal failure nor are they helpful in patients with spinal cord injuries, who have large, obligatory nitrogen losses as a result of skeletal muscle breakdown consequent to denervation. Finally, large upward adjustments of protein intake should be avoided in patients with chronic hepatic or renal failure.
Contrasting a general stabilization and reduction in urinary nitrogen loss during the first postinjury week, energy expenditure often increases and peaks in the most severely injured patients during the second week.34 Indirect calorimetry can also be obtained where the facilities are available and no contraindications exist. Its use may be helpful under some circumstances, such as in patients who are markedly overweight or underweight, those in whom baseline weight, if measured, was inaccurate as a result of massive volume resuscitation, or in patients with severe traumatic brain injury. Indirect calorimetry is technically difficult to perform and many factors may contribute to its inaccuracy. These are patient related (fever and marked agitation during the procedure) and system related (air leaks, high-FIO2), but if performed correctly and under appropriate circumstances, indirect calorimetry may be the best means of assessing the adequacy of nutritional support. Serum albumin and prealbumin, urinary nitrogen measurements and indirect calorimetry are not obtained in the first postinjury week and then only obtained in patients still requiring nutritional support and in whom adjustments are being considered.
NUTRITIONAL FLOW CHART LEGEND
Nutritional support should be considered necessary in any patient not expected to tolerate oral intake within 72 hours of injury. Furthermore, patients who otherwise would be able to eat and drink but will require frequent interruptions for operative procedures should be considered for enteral feeding.
Inability to obtain enteral access and contraindications to enteral feeding require frequent reassessment. Shock, ongoing massive resuscitation, and intestinal discontinuity are the primary early contraindications to enteral feeding.
Although most patients can be started with gastric feeding, primary, and distal access is indicated in cases of esophageal, gastric or duodenal repairs or with large volume nasogastric drainage (>1,000 mL/24 hours).
Continue with attempts to address the reasons for the inability to start enteral support. This includes resuscitation from shock, re-establishing intestinal continuity, and potentially obtaining enteral access distal to the duodenum. Obtaining access via either a nasojejunal tube at the time of abdominal exploration may avoid later difficulties with gastric feeding. A gastrostomy or jejunostomy should be considered in patients predicted to need long-term access (patients with severe traumatic brain injuries, for example).
After 5 days, if enteral support has not been started or has been unsuccessful, parenteral nutritional support should be started.
Gastric feeding is started at 25 mL/hr and residual volume measured every 4 hours. The rate is advanced by 25 mL/hr every 8 hours until the goal rate in reached, in the absence of intolerance.
Distal duodenal or jejunal feeding is indicated when gastric feeding is contraindicated or not tolerated. Intolerance exists when ≥50% of the goal rate has not been achieved by 48 hours.
Generally, patients able to tolerate 50% of calculated goal rate by 48 hours will tolerate continued rate advancement over the subsequent week.
By 7 days to 10 days after injury, many patients have reached an anabolic state, with greater protein and possibly caloric requirements. Both of which may be inaccurately estimated. At this point, it is appropriate to consider direct measurements of nitrogen loss and caloric expenditure to facilitate adjustments of caloric and nitrogen intake.
The role and potential benefits of total or supplemental PN are not certain. Parenteral nutrition is recommended when enteral support cannot be started by day 5 or cannot be advanced to support caloric and protein requirements after day 7.
Acknowledgments
Additional participating investigators in the Large Scale Collaborative Research Agreement entitled, “Inflammation and the Host Response to Injury” include Henry V. Baker, PhD, Timothy R. Billiar, MD, Bernard H. Brownstein, PhD, Steven E. Calvano, PhD, Irshad H. Chaudry, PhD, J. Perren Cobb, MD, Chuck Cooper, MS, Ronald W. Davis, PhD, Adrian Fay, PhD, Robert J. Feezor, MD, Richard L. Gamelli, MD, Nicole S. Gibran, MD, Doug Hayden, MS, David N. Herndon, MD, Jureta W. Horton, PhD, John Lee Hunt, MD, Krzysztof Laudanski, MD, MA, James A. Lederer, PhD, Tanya Logvinenko, PhD, John A. Mannick, MD, Carol L. Miller-Graziano, PhD, Michael Mindrinos, PhD, Lyle L. Moldawer, PhD, Laurence G. Rahme, PhD, Daniel G. Remick, Jr., MD, David Schoenfeld, PhD, Robert L. Sheridan, MD, Geoffrey M. Silver, MD, Richard D. Smith, PhD, Scott Somers, PhD, Ronald G. Tompkins, MD, ScD, Mehmet Toner, PhD, H. Shaw Warren, MD, Steven E. Wolf, MD, Wenzhong Xiao, PhD, Martin Yarmush, MD, PhD, and Vernon R. Young, PhD, ScD.
Supported, in part, by a Large-Scale Collaborative Project Award (U54-GM62119) from The National Institute of General Medical Sciences, National Institutes of Health.
Footnotes
Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. In Press Corrected Proof, Available online June 25, 2008. Matthew J. Sena, Garth H. Utter, Joseph Cuschieri, Ronald V. Maier, Ronald G. Tompkins, Brian G. Harbrecht, Ernest E. Moore, Grant E. O’Keefe. Journal of the American College of Surgeons (http://www.journalacs.org/inpress).
Adjusted weight = (usual [or dry] weight + predicted weight)/2. Predicted weight (men) = 50 + 0.91(ht in cm—152.4). Predicted weight (women) = 45.5 + 0.91(ht in cm—152.4). All in kilograms.
References
- 1.Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in canadian ICUs–opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27:74–83. doi: 10.1177/014860710302700174. [DOI] [PubMed] [Google Scholar]
- 2.Improving the Practice of Nutrition Therapy in the Critically Ill. An International Quality Improvement Project. 2007 [PMC free article] [PubMed] [Google Scholar]
- 3.Braga M, Gianotti L, Vignali A, Cestari A, Bisagni P, Di CV. Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med. 1998;26:24–30. doi: 10.1097/00003246-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kozar RA, McQuiggan MM, Moore EE, Kudsk KA, Jurkovich GJ, Moore FA. Postinjury enteral tolerance is reliably achieved by a standardized protocol. J Surg Res. 2002;104:70–75. doi: 10.1006/jsre.2002.6409. [DOI] [PubMed] [Google Scholar]
- 5.O’Keefe GE, Hybki DL, Munford RS. The G–>A Single Nucleotide Polymorphism at the -308 position in the tumor necrosis factor-alpha promoter increases the risk for severe sepsis after trauma. J Trauma. 2002;52:817–826. doi: 10.1097/00005373-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 6.MacLeod JB, Lefton J, Houghton D, et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57–61. doi: 10.1097/01.ta.0000249294.58703.11. [DOI] [PubMed] [Google Scholar]
- 7.McClave SA, Lukan JK, Stefater JA, et al. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33:324–330. doi: 10.1097/01.ccm.0000153413.46627.3a. [DOI] [PubMed] [Google Scholar]
- 8.Ott L, Annis K, Hatton J, McClain M, Young B. Postpyloric enteral feeding costs for patients with severe head injury: blind placement, endoscopy, and PEG/J versus TPN. J Neurotrauma. 1999;16:233–242. doi: 10.1089/neu.1999.16.233. [DOI] [PubMed] [Google Scholar]
- 9.Kortbeek JB, Haigh PI, Doig C. Duodenal versus gastric feeding in ventilated blunt trauma patients: a randomized controlled trial. J Trauma. 1999;46:992–996. doi: 10.1097/00005373-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nicholas JM, Cornelius MW, Tchorz KM, et al. A two institution experience with 226 endoscopically placed jejunal feeding tubes in critically ill surgical patients. Am J Surg. 2003;186:583–590. doi: 10.1016/j.amjsurg.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma–a prospective, randomized study. J Trauma. 1986;26:874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Eddy VA, Snell JE, Morris JA., Jr Analysis of complications and long-term outcome of trauma patients with needle catheter jejunostomy. Am Surg. 1996;62:40–44. [PubMed] [Google Scholar]
- 13.Holmes JH, Brundage SI, Yuen P, Hall RA, Maier RV, Jurkovich GJ. Complications of surgical feeding jejunostomy in trauma patients. J Trauma. 1999;47:1009–1012. doi: 10.1097/00005373-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Orozco-Levi M, Torres A, Ferrer M, et al. de la Bellacasa JP, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1387–1390. doi: 10.1164/ajrccm.152.4.7551400. [DOI] [PubMed] [Google Scholar]
- 15.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core–standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. [DOI] [PubMed] [Google Scholar]
- 16.Berne JD, Norwood SH, McAuley CE, et al. Erythromycin reduces delayed gastric emptying in critically ill trauma patients: a randomized, controlled trial. J Trauma. 2002;53:422–425. doi: 10.1097/00005373-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dissanaike S, Shelton M, Warner K, O’Keefe GE. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit Care. 2007;11:R114. doi: 10.1186/cc6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander E, Susla GM, Burstein AH, Brown DT, Ognibene FP. Retrospective evaluation of commonly used equations to predict energy expenditure in mechanically ventilated, critically ill patients. Pharmacotherapy. 2004;24:1659–1667. doi: 10.1592/phco.24.17.1659.52342. [DOI] [PubMed] [Google Scholar]
- 20.Larsson J, Lennmarken C, Martensson J, Sandstedt S, Vinnars E. Nitrogen requirements in severely injured patients. Br J Surg. 1990;77:413–416. doi: 10.1002/bjs.1800770418. [DOI] [PubMed] [Google Scholar]
- 21.Mendez C, Jurkovich GJ, Garcia I, Davis D, Parker A, Maier RV. Effects of an immune-enhancing diet in critically injured patients. J Trauma. 1997;42:933–940. doi: 10.1097/00005373-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg. 1996;224:531–540. doi: 10.1097/00000658-199610000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–953. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 24.Suchner U, Heyland DK, Peter K. Immune-modulatory actions of arginine in the critically ill. Br J Nutr. 2002;87 (Suppl 1):S121–S132. doi: 10.1079/bjn2001465. [DOI] [PubMed] [Google Scholar]
- 25.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans. 2005;33(Pt 2):423–427. doi: 10.1042/BST0330423. [DOI] [PubMed] [Google Scholar]
- 27.Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 28.Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 2001;27:84–90. doi: 10.1007/s001340000703. [DOI] [PubMed] [Google Scholar]
- 29.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 31.Marvin RG, McKinley BA, McQuiggan M, Moore FA. Nonocclusive bowel necrosis occurring in critically ill trauma patients receiving enteral nutrition manifests no reliable clinical signs for early detection. Am J Surg. 2000;179:7–12. doi: 10.1016/s0002-9610(99)00261-5. [DOI] [PubMed] [Google Scholar]
- 32.Thaler K, Garreau J, Hansen PD. Non-occlusive small bowel necrosis during enteral feeding after pancreaticoduodenectomy. Dig Surg. 2005;22:375–377. doi: 10.1159/000090997. [DOI] [PubMed] [Google Scholar]
- 33.Iapichino G, Radrizzani D, Solca M, et al. The main determinants of nitrogen balance during total parenteral nutrition in critically ill injured patients. Intensive Care Med. 1984;10:251–254. doi: 10.1007/BF00256262. [DOI] [PubMed] [Google Scholar]
- 34.Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med. 1999;27:1295–1302. doi: 10.1097/00003246-199907000-00015. [DOI] [PubMed] [Google Scholar]