Abstract
Background
Recent studies suggest that statin use may improve outcome in critically ill patients. This has been attributed to the pleiomorphic effect and modulation of inflammatory mediators that occurs with statin use. We sought to determine whether preinjury statin (PIS) use was associated with improved outcome in severely injured blunt trauma patients.
Methods
Data were obtained from a multicenter prospective cohort study evaluating outcomes in blunt injured adults with hemorrhagic shock. Patients aged 55 years and older were analyzed. Those with isolated traumatic brain injury, cervical cord injury, and those who survived <24 hours were excluded. A propensity score predicting statin use was created using logistic regression. Cox proportional hazard regression was then used to evaluate the effects of PIS use on mortality and the development of multiple organ failure (MOF, multiple organ dysfunction syndrome >5 and nosocomial infection (NI) after adjusting for important injury characteristics and the propensity of taking PISs.
Results
Overall mortality and MOF rates for the study cohort (n = 295) were 21% and 50%, respectively. Over 24% of patients (n = 71) reported PIS use. Kaplan-Meier analysis revealed no difference in NI or mortality over time but did show a significant higher incidence of MOF in those with PIS use (p = 0.04). Regression analysis verified PIS was independently associated with an 80% higher risk of MOF (hazard ratio: 1.8; 95% confidence interval, 1.1–2.9) and was found to be one of the strongest independent risk factors for the development of MOF.
Conclusion
PIS use was independently associated with a higher risk of MOF postinjury. These results are contrary to previous analyses. The protective effect of statins may be lost in the severely injured, and modulation of the inflammatory response may result in higher morbidity. Further studies are required to better understand the impact and potential therapeutic utility of this commonly prescribed medication both before and after injury.
Keywords: Preinjury statin use, Multiple organ failure, Cox proportional hazard regression
Agrowing body of literature has emerged providing evidence for the beneficial effects attributable to 3-hydroxy-3-methyl-glut aryl-CoA reductase inhibitors (statins).1 Statins are emerging as one of the most frequently prescribed medications in the elderly population, and they have been shown to be effective in reducing low-density lipoprotein cholesterol levels, resulting in protection from cardiovascular morbidity.2 The use of prehospital statins has been shown to be associated with a reduction in mortality and morbidity in critically ill patients with sepsis.3-9 This has been hypothesized to result not from their cholesterol lowering properties but rather from a so-called “pleiotrophic modulation” of the inflammatory cascade.4,10 Statin use has been shown to bring about an attenuation of isoprenoid synthesis, which enhances endothelial function, provides antithrombotic effects, normalizes platelet aggregation, and results in a significant anti-inflammatory effect.10-12
Despite the high prevalence of statin use and the similarities between the inflammatory host response in septic and injured patients, there remains a paucity of literature, which characterizes the effects of prehospital statin use after injury. There exists only a single observational cohort study demonstrating an independent lower risk of mortality associated with preinjury statin (PIS) use after injury,13 whereas the proposed inflammatory modulating effects of PIS use and their association with the development of organ dysfunction and infection risk postinjury remains poorly characterized. We sought to characterize these relevant clinical outcomes and their association with PIS use in a large cohort of severely injured blunt trauma patients. Specifically, we sought to determine the effect of PIS use on multiple organ failure (MOF), the development of nosocomial infection (NI), as well as mortality after injury. We hypothesized that PIS use would be associated with a decreased risk of MOF, NI, and mortality in the severely injured trauma patient.
METHODS
Data were derived from the ongoing multicenter prospective cohort study known as the Inflammation and the Host Response to Injury Large Scale Collaborative Program (www.gluegrant.org) supported by the National Institute of General Medical Sciences, which is designed to characterize the genomic and proteomic response in injured patients at risk for MOF after traumatic injury and hemorrhagic shock.14 Standard operating procedures were developed and implemented across all institutional centers to minimize variation in postinjury care, including early goal directed resuscitation, strict glycemic control, venous thromboembolism prophylaxis, appropriate low tidal volume ventilation, ventilator-associated pneumonia management, and restrictive transfusion guidelines.14-20 Patients admitted to one of seven institutions, over a 4-year period (November 2003 to September 2007), were included in the analysis. Inclusion criteria for the overall cohort study included blunt mechanism of injury, presence of prehospital or emergency department systolic hypotension (<90 mm Hg) or an increased base deficit (≥6 meq/L), blood transfusion requirement within the first 12 hours, and any body region exclusive of the brain with an abbreviated injury score (AIS) ≥2, allowing exclusion of patients with isolated traumatic brain injury. Patients younger than 16 years or older than 90 years and those with cervical spinal cord injury were also excluded from enrolment. For the current analysis, only patients aged 55 years and older were included in the analysis because this represents the typical patient cohort who would be taking prehospital statins.21 Similarly we excluded those who did not survive beyond 24 hours from the time of injury as those who did not survive beyond this time point were considered inappropriate to estimate the attributable risks associated with statin use. Clinical data were entered and stored in TrialDb, a web-based data collection platform, by trained research nurses.22 All prehospital medications and comorbidities were prospectively recorded in the dataset either from the patient themselves or, in cases where the patient was unable to provide this information, the family was questioned. Integrity of the data were maintained through ongoing curation and external data review by an independent chart abstractor.
Although patients were admitted to the intensive care unit, multiple organ dysfunction scores for renal, hepatic, cardiovascular, metabolic, hematologic, respiratory, and neurologic systems were determined daily.23-25 All nosocomial infectious complications were monitored for and recorded (infection type, culture specimen source, and bacteriology). The diagnosis of MOF required a maximum Marshall Multiple Organ Dysfunction score >5, whereas diagnosis of NI required specific clinical criteria along with positive culture evidence. All time variables to the respective outcome event were determined from the day of initial injury, while the time to the first NI was used in those patients with multiple infections. Diagnosis of a ventilator-associated pneumonia required a quantitative culture threshold of ≥104 CFU/mL for broncho-alveolar lavage specimens.16 Diagnosis of catheter-related blood stream infections required positive peripheral cultures with the identical organism obtained from either a positive semiquantitative culture (>15 CFU/segment) or positive quantitative culture (>103 CFU/segment) from a catheter segment specimen. Urinary tract infections required >105 organisms/mL of urine.
Those patients with and without PIS use were compared in a univariate fashion, and crude rates for mortality (in hospital), MOF, and the development of NI were then determined and compared. The time course of these outcomes of interest were then characterized and compared using Kaplan-Meier time-to-event analysis. Cox proportional hazard regression modeling was then used to characterize the independent risks of mortality, MOF, and NI associated with PIS use, after adjusting for important confounders. In attempts to adjust for the likelihood of taking statin medications, a propensity score was derived using logistic regression, and this propensity score was used as a covariate and adjusted for in the final Cox regression models. To determine the propensity score, preinjury characteristics including gender, body mass index, age, individual patient comorbidites (diabetes, hypertension, chronic obstructive pulmonary disease, congestive heart failure, liver disease, chronic renal insufficiency, previous myocardial infarction, current smoking history, previous coronary artery bypass or stenting, hypercholesterolemia or hyperlipidemia, and alcohol abuse), and prehospital medications (non-steroidal anti-inflammatory drugs, aspirin, other antiplatelet agents, corticosteroids, β-blockers, angiotensin receptor blockers, acetycholinesterase inhibitor, calcium channel blockers, other antihypertensives, diuretics, and coumadin) were placed into a logistic regression model with PIS used as the outcome variable. The probability (a decimal from 0 to 1) or propensity of PIS use was then determined for each patient based on these preinjury characteristics and other unmeasured or unknown characteristics associated with PIS use.
In addition to adjusting for the propensity for PIS use, other relevant confounders for the multivariate models were chosen to adjust for differences in injury characteristics, shock severity, operative and intensive care unit interventions, and early transfusion and crystalloid requirements. Confounders for the final Cox hazard regression model included PIS use propensity score, individual AISs (head, neck, abdomen, chest, extremity, skin), Acute Physiology and Chronic Health Evaluation (APACHE) II score, emergency department Glasgow Coma Score, presenting International Normalized Ratio (INR) and base deficit, blood, crystalloid, platelet and cryoprecipitate requirements (within 12 hours after injury), and the requirement of early operative intervention (exploratory laparotomy or thoracotomy or sternotomy). Clinically relevant interaction terms were tested and kept in the final model if statistically significant (p < 0.05).
All data were summarized as mean ± standard deviation, median (interquartile range [IQR]), or percentage. Student’s t or Mann-Whitney statistical tests were used to compare continuous variables, whereas χ2 or Fischer’s exact test were used for categorical variables. The institutional review board of each participating center approved the cohort study, whereas the institutional review board at the University of Pittsburgh Medical Center approved this current secondary analysis.
RESULTS
Of the 1,293 patients enrolled in the overall cohort study, 295 patients met our specified inclusion criteria and constituted the study population. (patients who did not survive beyond 24 hours, n = 21) In this study cohort, 24% of patients (n = 71) reported PIS use, similar to previous literature.13 The overall mortality for the study population was 21.7%, whereas the overall complication rates for MOF and NI, were 50.2% and 51.5%, respectively. This cohort of patients had a median age of 66 years (IQR, 58–76) and were significantly injured with a median injury severity score (ISS) of 29 (IQR, 21–41). An early (within 48 hours) exploratory laparotomy was required in 23.4% of patients, whereas 4.7% required an early thoracotomy or sternotomy. The median blood transfusion requirement in first 12 hours postinjury was 5.6 units (IQR, 3–10) units for the entire study cohort.
Those patients with PIS use as compared with those without PIS use were similar in demographics, injury and shock severity, early coagulopathy, the need for early operative intervention, and blood product and crystalloid resuscitation requirements. Those taking statins were, however, more commonly male patients (Table 1). As expected, those patients with PIS use had a significantly higher rate of cardiovascular comorbidities, a trend toward a higher rate of chronic obstructive pulmonary disease, were similar across preexisting renal disease, liver disease, and smoking history, yet had a trend toward a lower history of alcohol abuse relative to those without PIS use (Table 2). Predictably, those patients with PIS use were also more likely to be taking other preinjury medications concurrently, particularly those associated with cardiovascular disease management.
TABLE 1.
Univariate Comparison of Patient Demographics and Injury Characteristics Across Statin and Nonstatin Users
| No PIS Use (n = 224) |
PIS Use (n = 71) |
P | |
|---|---|---|---|
| Age (yr) | 66.6 ± 10 | 67.9 ± 11 | 0.357 |
| Gender (% male) | 58.8 | 72.1 | 0.030* |
| Initial base deficit (meq/L) | 7.8 ± 4 | 7.0 ± 3 | 0.133 |
| APACHE II score | 31.6 ± 7 | 29.7 ± 8 | 0.060 |
| Injury severity score | 31.9 ± 14 | 29.4 ± 14 | 0.203 |
| ED GCS | 9.3 ± 6 | 9.7 ± 6 | 0.622 |
| ED INR | 1.4 ± 0.6 | 1.5 ± 0.8 | 0.236 |
| ED or prehospital intubation (%) | 47.8 | 44.1 | 0.546 |
| Body mass index (kg/m2) | 29.1 ± 7 | 29.7 ± 7 | 0.538 |
| 12-h blood transfusion (per unit) | 8.4 ± 9 | 7.5 ± 8 | 0.460 |
| 12-h FFP transfusion (per unit) | 4.2 ± 6 | 3.2 ± 5 | 0.163 |
| 12-h cryoprecipitate (per unit) | 1.1 ± 3 | 0.5 ± 2 | 0.134 |
| 12-h platelet transfusion (per unit) | 0.6 ± 1 | 0.4 ± 1 | 0.106 |
| 12-h crystalloid (per L) | 10.7 ± 7 | 9.5 ± 6 | 0.162 |
| Early laparotomy (48 h) (%) | 24.1 | 21.1 | 0.605 |
| Early thoracotomy or sternotomy (48 h) (%) |
3.6 | 8.5 | 0.092 |
GCS, Glasgow Coma Score; FFP, fresh frozen plasma.
p value reaches statistical significance.
TABLE 2.
Unadjusted Univariate Comparison of Comorbidities and Preinjury Medications Across Statin and Nonstatin Users
| No PIS Use (n = 224) (%) |
PIS Use (n = 71) (%) |
P | |
|---|---|---|---|
| Comorbidities | |||
| Hypercholesterolemia or hyperlipidemia |
1.1 | 23.9 | <0.001* |
| Hypertension | 32.6 | 74.6 | <0.001* |
| CHF | 2.7 | 9.9 | 0.010* |
| Previous MI | 8.0 | 22.5 | <0.001* |
| Previous CABG or stenting | 0.7 | 14.1 | <0.001* |
| Diabetes | 14.3 | 36.6 | <0.001* |
| COPD | 6.7 | 14.1 | 0.051 |
| Chronic renal disease or insufficiency |
1.8 | 4.2 | 0.239 |
| Alcoholism | 10.7 | 4.2 | 0.098 |
| Liver disease | 2.2 | 2.8 | 0.778 |
| Smoking | 19.6 | 21.1 | 0.785 |
| Preinjury medications | |||
| ACE inhibitors | 5.4 | 21.1 | <0.001 |
| Angiotensin receptor blocker | 0.4 | 5.6 | 0.003 |
| ASA | 10.7 | 39.4 | <0.001 |
| β-Blockers | 10.3 | 47.9 | <0.001 |
| Diuretics | 3.1 | 9.9 | 0.020 |
| Plavix | 3.6 | 14.1 | <0.001 |
| Coumadin | 5.8 | 12.7 | 0.055 |
| Corticosteroids | 1.3 | 4.2 | 0.133 |
| NSAIDS | 4.9 | 7.0 | 0.490 |
| Calcium channel blockers | 2.2 | 2.8 | 0.779 |
| Other direct vasodilators | 0.9 | 2.8 | 0.222 |
CHF, congestive heart failure; MI, myocardial infarction; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; ACE, acetycholinesterase inhibitor; ASA, aspirin; NSAIDS, non-steroidal anti-inflammatory drugs.
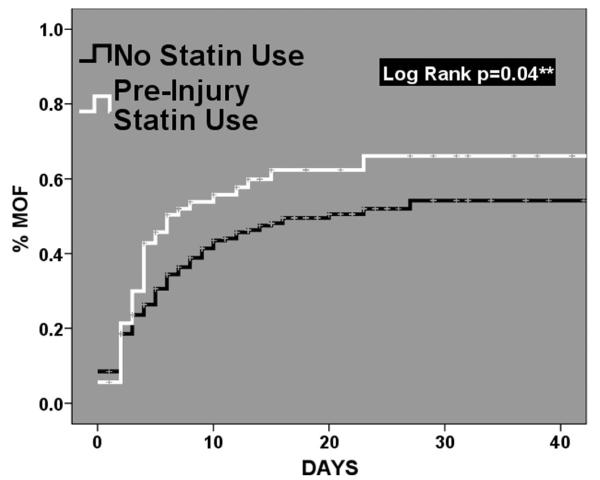
When our outcomes of interest were compared across groups through unadjusted univariate analysis, no significant differences were found; however, a clinical trend was evident with a higher rate of MOF in the PIS use group (Table 3). When we looked at these clinical outcomes over time using Kaplan-Meier time-to-event analysis, no significant differences were found for mortality and the development of NI (mortality: log rank p = 0.550, NI: log rank p = 0.362). Unexpectantly, those patients with PIS use did have a significantly higher incidence of MOF over time (log rank p = 0.04, Fig. 1). These time-to-event curves for MOF revealed a very early separation, within the first 4 days after injury.
TABLE 3.
Unadjusted Univariate Comparison of Outcomes Across Statin and Nonstatin Users
| NO PIS Use (n = 224) |
PIS Use (n = 71) |
P | |
|---|---|---|---|
| Nosocomial infection (%) | 52.9 | 47.1 | 0.399 |
| Multiple organ failure (%) | 47.3 | 59.2 | 0.082 |
| Mortality (%) | 21.0 | 23.9 | 0.598 |
| Ventilator days | 12.3 ± 12 | 10.7 ± 9 | 0.311 |
| ICU days | 15.9 ± 14 | 14.0 ± 11 | 0.309 |
| Hospital length of stay (d) | 22.6 ± 16 | 19.9 ± 13 | 0.220 |
ICU, intensive care unit.
Figure 1.
Kaplan-Meier time-to-event analysis for the development of multiple organ failure comparing those patient with and without preinjury statin use.
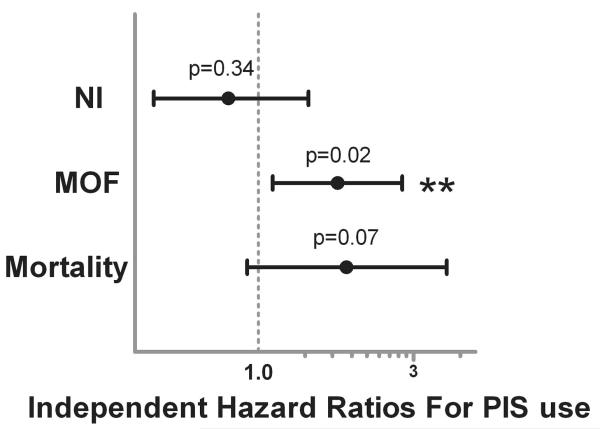
As univariate comparison did reveal significant differences between those who did and did not have PIS use, we attempted to address these differences in our multivariate Cox proportional hazard regression model by creating a propensity score predicting PIS use. We used logistic regression using patient demographics, comorbidities, and all other pre-injury medications as the model covariates, and PIS use as the outcome variable to create this probability score. Our propensity score was a good predictor of statin use, as demonstrated by an area under the curve of 0.89 through receiver operating characteristic analysis. In addition to important injury characteristics, shock severity, operative interventions, and resuscitation requirements, this propensity score was used as an individual covariate and adjusted for, in attempts to statistically control for these group differences. Our final multivariate Cox regression models revealed that PIS use was not significantly associated with the development of NI (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.5–1.4; p = 0.341, Fig. 2). Interestingly, PIS use was found to be associated with a significant higher independent risk of MOF (HR, 1.81; 95% CI, 1.1–2.9, p = 0.021), after controlling for important confounders and the propensity for PIS use. This HR may be interpreted as over an 80% higher independent risk of MOF associated with PIS use. Similar interpretation of the HR for mortality revealed almost a 2-fold higher independent risk of mortality associated with PIS use; however, in this model, PIS use did not reach statistical significance (HR, 1.98; 95% CI, 0.9–4.0, p = 0.072).
Figure 2.
Independent hazard ratios depicting the risk of developing nosocomial infection (NI), multiple organ failure (MOF), and mortality associated with preinjury statin (PIS) use through Cox proportional hazard regression analysis (**statistically significant). Additional covariates included in the regression model: propensity score for PIS use, individual maximum abbreviate injury scores (AIS, head, neck, chest, abdomen, extremity, skin), APACHE II score, ED Glasgow Coma Score, 12-hour blood, crystalloid, platelet, and cryoprecipitate requirements, presenting base deficit and ED coagulation status (INR), and the requirement of early operative intervention (exploratory laparotomy or thoracotomy or sternotomy).
Group stratification was unable to be performed due to limited power relative to our smaller sample size; however, we were able to characterize interactions between statin use and the injury severity and cardiac comorbidities of the cohort in our regression models. Specific interaction terms between PIS use and ISS and a composite cardiovascular comorbidity variable (history of 1 or more: hypertension, diabetes, previous myocardial infarction, congestive heart failure, previous bypass or stenting, hyperlipidemia) were created and evaluated in the Cox regression model. No significant interaction was found relative to injury severity (p = 0.217) or the cardiac comorbidity composite variable (p = 0.183). Interpretation of these interaction results suggest that the higher risk of MOF associated with PIS use was not significantly affected by varying injury severity or the presence or absence of cardiovascular comorbidities.
Finally, we wanted to determine the most robust independent risk factors for the development of MOF in this cohort of patients. We performed a backward stepwise Cox proportional hazard regression analysis with the same initial model covariates except that our propensity score was removed and replaced with all those covariates included in the initial propensity score determination. This would allow the effect of individual comorbidities, other individual preinjury medications including statins, along with all the injury, shock, and resuscitation characteristics to be analyzed relative to each other, to determine the strongest predictors of MOF. This analysis revealed that in this cohort of patients, PIS use was one of the four most significant, independent, predictors of the development of MOF, in addition to APACHE II score, chest AIS, and a patients 12-hour blood transfusion requirement (Table 4).
TABLE 4.
Backward Stepwise Cox Proportional Hazard Regression for Determination of the Strongest Independent Risk Factors for Multiple Organ Failure
| Hazard Ratio (95% CI) | P | |
|---|---|---|
| Preinjury statin use | 1.60 (1.10–2.33) | 0.014 |
| APACHE II score | 1.05 (1.03–1.09) | <0.001 |
| Maximum chest AIS | 1.15 (1.03–1.27) | 0.010 |
| 12-h blood transfusion requirement (per unit) |
1.03 (1.01–1.05) | 0.001 |
DISCUSSION
An expanding pool of literature has documented a benefit of prehospital statin use in the critically ill, with a proposed modulation of the inflammatory response as the mechanism responsible.3-9 Although the effects of other commonly prescribed medications, such as β-blockers and warfarin, have been adequately characterized in injured patients,26-31 a paucity of literature exists concerning the effects of statins after injury. Efron et al. have recently provided evidence that PIS use was independently associated with a 67% reduction in risk of in-hospital mortality, in an elderly cohort of 1,189 injured patients. When stratified by the presence or absence of cardiovascular comorbidities, statin use remained independently associated with a reduction in the risk of mortality only in those without cardiovascular comorbidities.13
The goal of this current analysis was to determine the impact of PIS use on the risk of MOF, NI, and mortality in a severely injured cohort of blunt trauma patients. Unexpectedly, our findings were in contrast to our stated hypothesis, and the previously published evidence analyzed in patients with sepsis and after injury. Our results suggest that PIS use was associated with an 80% higher independent risk of MOF with a trend toward a higher risk of mortality, whereas no significant association of PIS use and the development of NI was found. As there were significant differences in gender, comorbidities, and other prehospital medications across patients with and without PIS use, we attempted to statistically control for these group differences through a propensity score adjusted regression analysis. Perhaps the most intriguing results, however, were that relative to all documented patient comorbidities, prehospital medications, injury characteristics, markers of shock severity, resuscitation requirements, and other relevant confounders, PIS use was found to be one of the four strongest independent predictors of the development of MOF, equally robust as APACHE II score, chest AIS, and a patients early blood transfusion requirement, in this specific cohort of patients.
Possible explanations for these divergent results may stem in part from differences in the cohort of patients analyzed. The Glue Grant cohort represents a severely injured group of patients as compared with the patient population analyzed by Efron et al. (mean ISS 31 vs. 19). It is known that severity of illness may alter any beneficial effects attributable to statin use,32,33 with similar findings found for other pre-hospital medications.34 It may be that the protective effects associated with statin use in other critically ill populations are overwhelmed in the midst of the robust inflammatory response after severe injury. If not more important, it has been shown that prehospital comorbidities play a significant role in determining outcomes after traumatic injury.35-40 In the analysis by Efron et al., when patients were stratified by the presence or absence of prehospital cardiovascular disease, the beneficial effects associated with statins on mortality was found only in those without a history of cardiovascular comorbidity.13 In the severely injured trauma patient, the detrimental effects associated with prehospital comorbidities may be accentuated. In our analysis, interestingly, the association between PIS use and MOF was not effected by changes in injury severity or by the presence or absence of cardiovascular comorbidities, based on interpretation of the interaction terms tested in our model.
As those patients with PIS use were shown to have a higher incidence of comorbidities and prehospital medications, it may be that PIS use is simply a marker for preexisting cardiovascular disease and an independent risk factor for organ dysfunction and poor outcome. Arguments against this “statin” confounding effect come from the fact that in our backward stepwise regression model, PIS use was found to be one of the four strongest independent predictors of the development of MOF. If PIS use was simply a proxy for preexisting cardiovascular disease, these other preexisting comorbidities and prehospital medications would likely have been found to be significant determinants of MOF from our modeling techniques.
Perhaps the most intriguing potential explanation derives from one of the limitations of this analysis. It was unknown whether or not prehospital medications were continued after injury because this information was not recorded in the dataset. Based on the severity of illness, the relatively high incidence of MOF, and the lack of an intravenous statin alternative, it can be assumed that a majority of oral prehospital medications were not resumed in the immediate peri-injury period. A “rebound” effect from statin withdrawal has been previously described.41-44 Statin withdrawal after chronic, prehospital use has been shown to result in increased mortality rate after acute coronary syndrome,41 increased myonecrosis after vascular surgery,42 and an increased risk of perioperative cardiac events after discontinuation.43 Multiple studies have demonstrated an acute benefit from statin use derived not from lipid lowering properties but rather from reduced plateilation, and lower levels of C-reactive protein.45-50 Although the full, cholesterol lowering properties of statins are observed with use over time, the administration of lipid-lowering agents in the acute postoperative period has been shown to dramatically reduce in-hospital mortality in patients undergoing noncardiac surgery. They demonstrated a dramatically increased risk of mortality when statins were started after postoperative day 3 or withheld completely.51 This may represent the “window” for the development of withdrawal from statin therapy. In smaller studies designed with the intention to uncover the mechanism behind statin withdrawal, levels of the inflammatory markers interleukin-6 and C-reactive protein increased 3 days after the withdrawal of statin therapy.52,53 In addition, in a small group of patients randomized to statin therapy versus placebo for 5 weeks, flow-mediated vasodilation was shown to improve with statin therapy; however, this improvement deteriorated to baseline 24 hours after discontinuation of statin therapy.54 These previous findings support the observation of an early window for withdrawal from statins. Importantly, our Kaplan-Meier time-to-event analysis revealed that the curves for MOF began to separate within the first 72 hours (Fig. 1), suggesting that our finding of increased risk of MOF and a trend toward increased risk of mortality may not necessarily be an effect of PIS use, but rather the sequelae of withdrawal after chronic therapy.
As a final possible explanation, it may be that in severely injured trauma patients, the beneficial attributes associated with prehospital statin use are altered, by a yet to be determined mechanism. The strength of the associations found in our multivariate regression analysis, with all attempts to adjust for confounder factors, and the additional confirmation of PIS use as one of the four most robust risk factors for the development of MOF, offer justification for this possibility. It may be that there exists specific cohorts of critically ill patients where statins are not beneficial or are possibly deleterious. Obviously, further higher level analyses are required to further expand our current understanding of this matter.
Our analysis does have several potential limitations. First, this study is a secondary analysis of a prospective cohort study investigating the genomic and proteomic response after severe injury and hemorrhagic shock, and data were not recorded to answer our specific hypothesis. As compared with previous analyses, this analysis was limited by a smaller sample size, which reduced the power to reveal other significant associations. With a larger elderly cohort, the results described here may have been altered or even possibly made more robust. Importantly, the patients in the study cohort were severely injured blunt trauma patient, all of whom were older than 55 years. Thus, the results of this analysis may not be generalized to all trauma patients, or even to all trauma patients taking statins, because the degree and mechanism of injury may have contributed to the outcome in our study. Although the comorbidities for cardiovascular disease were recorded in the data set individually, they were too infrequent when analyzed individually to properly adjust for them. Because of this, we combined these under the heading of “cardiovascular disease” in our final regression models, thus we cannot rule out that one or more of these in isolation may alter the conclusions formulated in this manuscript. The use of a propensity score in this study, which attempted to serve as a “balancing score” to control for known and other unknown confounding variables, was only able to be used as an individual covariate in our final Cox regression model. Propensity scores derived from larger sized cohorts or datasets can be used to match patients with identical scores, allowing a quasi-randomized analysis. Because of our relative smaller sample size, this type of analysis was unable to be performed and does represent a limitation of this analysis Finally, the possibility exists that unknown or unmeasured confounding variables may be responsible for the associations described and the conclusions formulated.
In conclusion, in a cohort of severely injured, elderly, trauma patients, those who were taking PISs were found to have over an 80% higher independent risk for the development of MOF, and PIS use was determined to be one of the four most strongest independent predictors of this outcome. A trend was also observed toward increased mortality, although this association did not reach statistical significance. This finding is contrary to previous literature, which has looked at other cohorts of critically ill patients and also previous work after injury. Further research on the effects of statin use in the setting of traumatic injury is needed, with specific focus on the timing of reinitiation of therapy after injury, and the resultant effects on the inflammatory cascade, to better understand the impact and potential therapeutic utility of this commonly prescribed medication after injury.
Biographies
DISCUSSION
Dr. David Efron (Baltimore, Maryland): I would like to thank the Program Committee for the opportunity to discuss this work. I would also like to thank the authors for a well written and constructed manuscript and Dr. Neal on a very nice job.
While there is an accumulating body of data analyzing the effect of premorbid statin use on the outcomes in critically ill patients, this is only the second effort to assess this specifically in trauma patients. Previously, we have shown that pre-injury statin use was associated with significant reduction in the risk of in-hospital mortality. The authors of the current study demonstrate no difference in mortality, despite a trend, a worse trend, and an association of pre-injury statin use with an increased risk of multiple organ failure.
Two robust databases and two very different results and so where does that leave us? Statins, are they a magic bullet or are they a dud? I submit neither right now. At first glance, these appear to be conflicting results. However, there’s some significant differences between the studies, highlighted nicely in the manuscript.
Unlike the previous analysis of the NSCOT dataset, this analysis of the Glue Grant dataset examines markedly more severely injured patients and benefits from more detailed data on hospital course and individual treatment parameters.
My first question stems from this. Overall, there was a 50 percent rate of multiple organ failure in the patients studied and pre-injury statin use was associated with 80 percent higher risk of multiple organ failure. Were the authors able to analyze individual organ dysfunction scores for potential association with pre-injury statin use? That is, what organs failed and to what extent?
My second question, perhaps more difficult to address, is regarding the construction of a propensity variable for the prediction of statin use. Any propensity score relies on the available variables and is most effective in larger datasets. Previous works have used social parameters, such as health habits, to most accurately identify people likely to be prescribed statins. Many patients are prescribed statins for hypercholesterolemia alone, in the absence of other comorbidities. Indeed, this was the group that derived the benefit in the previous study.
On the other hand, in this study, comorbidity-focused variables were used to construct the propensity model. Given the relatively small total number of patients and the disease-focused variables that were used to construct the score, is the current propensity analysis robust enough to include it as a covariate or does it serve more like a marker for comorbidity? Are statins a marker for sicker patients?
I suspect that the combination of severe injury and heavy burden of comorbidities in the statin users here conspire to eclipse any potential benefit and even suggest harm. Thank you again for the privilege of discussing the paper.
Dr. Matthew D. Neal (Pittsburgh, Pennsylvania): Thank you very much, Dr. Efron, for your comments and for your questions. As I understand the first question, particularly in looking at multiple organ failure, and the question was regarding whether or not we looked at individual systems and we did analyze individual systems in multiple organ failure and did not find one particular system that predominated over any other as being a sort of harbinger for the multiple organ failure.
In order to define multiple organ failure, as I mentioned, we used the Maximum Organ Dysfunction Score of greater than five and in the Glue Grant process, we’re reassessing multiple organ failure on a daily basis and we’ve found that it really developed within days two to four, but there wasn’t one particular system that stood out on our analysis.
The second question, regarding the propensity score, I think it’s particularly valid. In our particular model, in order to determine whether or not propensity score was adequate, we used a ROC curve and the ROC curve – The area under the curve was actually 0.89 and so we felt that for our particular study and for our cohort of patients that the propensity score was valid.
Now, it certainly is a valid point that propensity may not be applicable to all statin users, but particularly for this cohort, based on that area under the curve, it seemed like it was adequate for this assessment.
Dr. Richard Dutton (Baltimore, Maryland): USA Today says that eleven million more patients should get statins, you’ll be happy to know. I have a couple of questions. First, on the propensity scoring, any kind of data like that is going to be dependent on what you feed into the top of it. There are a couple of variables, in particular, I would be interested if you looked at. Obesity was not on your list of comorbidities and socioeconomic status or race was not included.
Then from the perioperative surgical literature, statins are generally considered beneficial, but there is a clearly observed negative effect if you stop them abruptly at the time of surgery and I think the best explanation for your data is that these are patients who were on statins when they were abruptly stopped, causing a change in the anti-inflammatory response. Can you comment on that?
Dr. Matthew D. Neal (Pittsburgh, Pennsylvania): I appreciate the insightful questions and also the opportunity to talk about the statin withdrawal, which we had delineated a bit in our manuscript. To answer your first question, I apologize if I didn’t make it clear, but actually as part of constructing our propensity score, we did use BMI as one of the demographics. I didn’t specifically state it as obesity, but it is there as part of BMI. We did not look at socioeconomic status. It would be of particular interest, especially given our trauma population.
To the issue of withdrawal from statin therapy, one of the big limitations of our study, as I mentioned, was that we don’t have information about continuation of outpatient medical therapy on an inpatient basis. I can tell you anecdotally, from caring for these folks who are severely injured and in the trauma ICU, that absent an intravenous form of statin – We’re not crushing statins and giving it to them right away, post-op day one down the tube, but maybe we should be.
As you mentioned, there is a strong amount of data suggesting that there is a withdrawal effect associated with cessation of statin use and of particular interest, the basic science data that looks at levels of IL-6 and CRP and a number of other inflammatory markers in withdrawal of statins show the effect really starts to peak somewhere around seventy-two hours, which maybe not coincidentally, corresponds with our separation of the curves of multiple organ failure.
It very well could be the case that cessation of statin therapy is what’s at play here. The other studies in the literature looking at statins and sepsis and Dr. Efron’s paper looking at another dataset, again, all suffered from the same limitation as ours, that we did not have data on continuation. I think it’s of particular interest and one of the things that I think that I should come out of this paper and this body of work, is to go back to the laboratory to look at what the mechanism is here and statin effect on the inflammatory cascade and whether withdrawal is at play.
Dr. Gregory Jurkovich (Seattle, Washington): This was a very nice presentation. Maybe you said this and maybe I just missed it, but were all these patients hypotensive to be enrolled? Did they all have a blood pressure of less than ninety as one of the entry criteria? That’s just one question, but two points that I want to make though about the presentation might be the following.
One, have you or could you sort out the definition of multiple organ failure? Is it renal failure? Is it acute lung injury or which part of MOF are they more likely to get?
Secondly, it might helpful – I know the numbers are small, but it might be helpful to have gender-specific Cox-Regression Survival Analysis Curves. There’s such a dramatic difference in your univariate analysis between gender roles that instead of trying to adjust for it, it may simply be better just to have a male versus a female survival analysis curve, to see how that comes into play in this analysis. Thanks for the presentation.
Dr. Matthew D. Neal (Pittsburgh, Pennsylvania): To answer your question, hypotension and systolic blood pressure of less than ninety was part of the inclusion criteria for these patients. With regards to the MOF, in looking at individual organ systems, we did not find particularly one system that stood out. It wasn’t cardiovascular or it wasn’t acute lung injury. The numbers across the board were relatively similar and so there wasn’t one particular system that stood out as being the offender.
I certainly appreciate the suggestion regarding the gender and agree that with the dramatic difference in univariate analysis that it’s worthy of us taking a look in a regression model.
Dr. Karen Zink (Portland, Oregon): That was a very good talk and my question is, do you think it’s the statin that makes the difference or do you think that genetic and patient factors that make patients more likely to have high cholesterol may also be more likely to have them have a higher proinflammatory state and are there any patients that have high cholesterol that were not on statins that you would be able to determine their outcomes?
Dr. Matthew D. Neal (Pittsburgh, Pennsylvania): That’s an interesting question, because there certainly is data in the critical care and trauma literature suggesting that cholesterol levels influence outcomes and as part of this dataset, we don’t actually have cholesterol levels.
We also, as I mentioned, don’t have the indication for why the statin was started and how long they had been on it and whether there was any variability in their baseline cholesterol levels and so I can’t necessarily answer that question.
There has been, as previously delineated in Dr. Efron’s paper, what’s been described as a healthy user effect, whereas those patients who were on statins may represent patients who have better access to care and are more likely to be prescribed a statin because they have somebody being a little bit more vigilant over their care.
In his paper, when they separated out cardiovascular morbidity, the findings for increased mortality held up in those patients who did not have cardiovascular morbidity and a suggestion that maybe the healthy user effect was at play there. I don’t think that necessarily is applicable to this data, because we’re sort of on the opposite end of the spectrum, showing essentially a negative outcome there.
The healthy user effect doesn’t really necessarily correspond and so I think access to care and pre-hospital treatment of cholesterol in that regard is not necessarily influential, but certainly I would love to have the numbers as to what their cholesterol levels were and whether that came into play.
Footnotes
Presented at the 22nd Annual Meeting of the Eastern Association for the Surgery of Trauma, January 13-17, 2008, Lake Buena Vista, Florida.
Supported by funding from the National Institutes of Health (NIH NIGMS U54 GM062119-1 and NIH KL2 RR024154-03).
References
- 1.Shepherd J. Who should receive a statin these days? Lessons from recent clinical trials. J Intern Med. 2006;260:305–319. doi: 10.1111/j.1365-2796.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams TM, Harken AH. Statins for surgical patients. Ann Surg. 2008;247:30–37. doi: 10.1097/SLA.0b013e3181492c0d. [DOI] [PubMed] [Google Scholar]
- 3.Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 4.Hackam DG, Mamdani M, Li P, Redelmeier DA. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367:413–418. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- 5.Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 6.Kruger PS. Statins: the next anti-endotoxin. Crit Care Resusc. 2006;8:223–226. [PubMed] [Google Scholar]
- 7.Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005;6:82. doi: 10.1186/1465-9921-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomsen RW, Hundborg HH, Johnsen SP, et al. Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med. 2006;34:1080–1086. doi: 10.1097/01.CCM.0000207345.92928.E4. [DOI] [PubMed] [Google Scholar]
- 10.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis. 2006;6:242–248. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 11.Daumerie G, Fleisher LA. Perioperative beta-blocker and statin therapy. Curr Opin Anaesthesiol. 2008;21:60–65. doi: 10.1097/ACO.0b013e3282f35ea5. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efron DT, Sorock G, Haut ER, et al. Preinjury statin use is associated with improved in-hospital survival in elderly trauma patients. J Trauma. 2008;64:66–73. doi: 10.1097/TA.0b013e31815b842a. discussion 73-74. [DOI] [PubMed] [Google Scholar]
- 14.Maier RV, Bankey P, McKinley B, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core—standard operating procedures for clinical care. Fore-ward. J Trauma. 2005;59:762–763. [PubMed] [Google Scholar]
- 15.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core—standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61:82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 16.Minei JP, Nathens AB, West M, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 17.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005;59:764–769. [PubMed] [Google Scholar]
- 18.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61:436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 19.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-orientedresearch core-standard operating procedures for clinical care. VI. Blood glucose control in the critically ill trauma patient. J Trauma. 2007;63:703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 20.Cuschieri J, Freeman B, O’Keefe G, et al. Inflammation and the host response to injury a large-scale collaborative project: patient-oriented research core standard operating procedure for clinical care X. Guidelines for venous thromboembolism prophylaxis in the trauma patient. J Trauma. 2008;65:944–950. doi: 10.1097/TA.0b013e3181826df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 22.Brandt CA, Deshpande AM, Lu C, et al. TrialDB: a web-based Clinical Study Data Management System. AMIA Annu Symp Proc. 2003:794. [PMC free article] [PubMed] [Google Scholar]
- 23.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. Eur J Surg Suppl. 1999;584:62–67. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Arbabi S, Campion EM, Hemmila MR, et al. Beta-blocker use is associated with improved outcomes in adult trauma patients. J Trauma. 2007;62:56–61. doi: 10.1097/TA.0b013e31802d972b. discussion 61-62. [DOI] [PubMed] [Google Scholar]
- 27.Cotton BA, Snodgrass KB, Fleming SB, et al. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62:26–33. doi: 10.1097/TA.0b013e31802d02d0. discussion 33-35. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy DM, Cipolle MD, Pasquale MD, Wasser T. Impact of preinjury warfarin use in elderly trauma patients. J Trauma. 2000;48:451–453. doi: 10.1097/00005373-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Mina AA, Bair HA, Howells GA, Bendick PJ. Complications of preinjury warfarin use in the trauma patient. J Trauma. 2003;54:842–847. doi: 10.1097/01.TA.0000063271.05829.15. [DOI] [PubMed] [Google Scholar]
- 30.Neideen T, Lam M, Brasel KJ. Preinjury beta blockers are associated with increased mortality in geriatric trauma patients. J Trauma. 2008;65:1016–1020. doi: 10.1097/TA.0b013e3181897eac. [DOI] [PubMed] [Google Scholar]
- 31.Riordan WP, Jr, Cotton BA, Norris PR, Waitman LR, Jenkins JM, Morris JA., Jr Beta-blocker exposure in patients with severe traumatic brain injury (TBI) and cardiac uncoupling. J Trauma. 2007;63:503–510. doi: 10.1097/TA.0b013e3181271c34. discussion 510-501. [DOI] [PubMed] [Google Scholar]
- 32.Lee TH. Reducing cardiac risk in noncardiac surgery. N Engl J Med. 1999;341:1838–1840. doi: 10.1056/NEJM199912093412410. [DOI] [PubMed] [Google Scholar]
- 33.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 34.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 35.Hollis S, Lecky F, Yates DW, Woodford M. The effect of pre-existing medical conditions and age on mortality after injury. J Trauma. 2006;61:1255–1260. doi: 10.1097/01.ta.0000243889.07090.da. [DOI] [PubMed] [Google Scholar]
- 36.McGwin G, Jr, MacLennan PA, Fife JB, Davis GG, Rue LW., III Preexisting conditions and mortality in older trauma patients. J Trauma. 2004;56:1291–1296. doi: 10.1097/01.ta.0000089354.02065.d0. [DOI] [PubMed] [Google Scholar]
- 37.Milzman DP, Boulanger BR, Rodriguez A, Soderstrom CA, Mitchell KA, Magnant CM. Pre-existing disease in trauma patients: a predictor of fate independent of age and injury severity score. J Trauma. 1992;32:236–243. discussion 243-244. [PubMed] [Google Scholar]
- 38.Morris JA, Jr, MacKenzie EJ, Damiano AM, Bass SM. Mortality in trauma patients: the interaction between host factors and severity. J Trauma. 1990;30:1476–1482. [PubMed] [Google Scholar]
- 39.Morris JA, Jr, MacKenzie EJ, Edelstein SL. The effect of preexisting conditions on mortality in trauma patients. JAMA. 1990;263:1942–1946. [PubMed] [Google Scholar]
- 40.Stevenson J. When the trauma patient is elderly. J Perianesth Nurs. 2004;19:392–400. doi: 10.1016/j.jopan.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105:1446–1452. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 42.Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104:1326–1333. doi: 10.1213/01.ane.0000263029.72643.10. table of contents. [DOI] [PubMed] [Google Scholar]
- 43.Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100:316–320. doi: 10.1016/j.amjcard.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 44.Spencer FA, Allegrone J, Goldberg RJ, et al. Association of statin therapy with outcomes of acute coronary syndromes: the GRACE study. Ann Intern Med. 2004;140:857–866. doi: 10.7326/0003-4819-140-11-200406010-00006. [DOI] [PubMed] [Google Scholar]
- 45.Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999;99:3227–3233. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 46.Huggins GS, Pasternak RC, Alpert NM, Fischman AJ, Gewirtz H. Effects of short-term treatment of hyperlipidemia on coronary vasodilator function and myocardial perfusion in regions having substantial impairment of baseline dilator reverse. Circulation. 1998;98:1291–1296. doi: 10.1161/01.cir.98.13.1291. [DOI] [PubMed] [Google Scholar]
- 47.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 48.Osamah H, Mira R, Sorina S, Shlomo K, Michael A. Reduced platelet aggregation after fluvastatin therapy is associated with altered platelet lipid composition and drug binding to the platelets. Br J Clin Pharmacol. 1997;44:77–83. doi: 10.1046/j.1365-2125.1997.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridker PM. Inflammation, infection, and cardiovascular risk: how good is the clinical evidence? Circulation. 1998;97:1671–1674. doi: 10.1161/01.cir.97.17.1671. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 51.Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291:2092–2099. doi: 10.1001/jama.291.17.2092. [DOI] [PubMed] [Google Scholar]
- 52.Li JJ, Li YS, Chen J, Yang JQ. Rebound phenomenon of inflammatory response may be a major mechanism responsible for increased cardiovascular events after abrupt cessation of statin therapy. Med Hypotheses. 2006;66:1199–1204. doi: 10.1016/j.mehy.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 53.Li JJ, Li YS, Chu JM, et al. Changes of plasma inflammatory markers after withdrawal of statin therapy in patients with hyperlipidemia. Clin Chim Acta. 2006;366:269–273. doi: 10.1016/j.cca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Westphal S, Abletshauser C, Luley C. Fluvastatin treatment and withdrawal: effects on endothelial function. Angiology. 2008;59:613–618. doi: 10.1177/0003319708316005. [DOI] [PubMed] [Google Scholar]