Abstract
Bile acid composition in fasting duodenal bile was assessed at entry and at 2 years in patients with primary biliary cirrhosis (PBC) enrolled in a randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid (UDCA) (10–12 mg/kg/d) taken as a single bedtime dose. Specimens were analyzed by a high-pressure liquid chromatography method that had been validated against gas chromatography. Percent composition in bile (mean ± SD) for 98 patients at entry for cholic (CA), chenodeoxycholic (CDCA), deoxycholic (DCA), lithocholic (LCA), and ursodeoxycholic (UDCA) acids, respectively, were 57.4 ± 18.6, 31.5 ± 15.5, 8.0 ± 9.3, 0.3 ± 1.0, and 0.6 ± 0.9. Values for CA were increased, whereas those for CDCA, DCA, LCA, and UDCA were decreased when compared with values in normal persons. Bile acid composition of the major bile acids did not change after 2 years on placebo medication. By contrast, in patients receiving UDCA for 2 years, bile became enriched with UDCA on average to 40.1%, and significant decreases were noted for CA (to 32.2%) and CDCA (to 19.5%). No change in percent composition was observed for DCA and LCA. Percent composition at entry and changes in composition after 2 years on UDCA were similar in patients with varying severity of PBC. In patients whose bile was not enriched in UDCA (entry and placebo-treated specimens), CA, CDCA, DCA, and the small amount of UDCA found in some of these specimens were conjugated to a greater extent with glycine (52%–64%) than with taurine (36%–48%). Treatment with UDCA caused the proportion of all endogenous bile acids conjugated with glycine to increase to 69% to 78%, while the proportion conjugated with taurine (22%–31%) fell (P < .05). Administered UDCA was also conjugated predominantly with glycine (87%).
Despite extensive use of ursodeoxycholic acid (UDCA) in the therapy of primary biliary cirrhosis (PBC), relatively little information is available on the effect of UDCA on the composition of bile acids excreted in bile. Mazzella et al.1 presented data for 9 patients with histological stages I to III after 4 weeks of 600 mg/d. Van de Meeberg et al.2 in their report on cholestatic liver disease included data from 8 patients with PBC receiving 10 mg/kg/d for 3 months. Crosignani et al.3 provided their findings at 6 months for 10 patients (7 with stage IV histology, 1 each in stages I, II, and III) receiving 8 mg/kg/d (500 mg/d). Batta et al.4 reported their findings on 3 patients receiving 10 to 12 mg/kg/d (900 mg/d) for 6 months.
In the present report, data on bile acid composition in bile are provided for a much larger number of patients who were enrolled in a 2-year, randomized, double-blind, placebo-controlled trial of UDCA in PBC.5 Bile was analyzed in 98 patients obtained at the time of entry into the trial, and 2 years later from 51 patients who had been randomized to receive placebo medication and 55 patients receiving UDCA at bedtime at a dose of 10 to 12 mg/kg/d.
PATIENTS AND METHODS
A total of 151 patients with PBC defined by the criteria of: 1) a cholestatic liver disease of at least 6 months’ duration; 2) a serum alkaline phosphatase value at least 1.5 times the upper limit of normal; 3) a positive antimitochondrial antibody test; 4) exclusion of biliary obstruction by ultrasonography, computed tomography, or by endoscopic cholangiography; and 5) a liver biopsy specimen compatible with the diagnosis of PBC, were enrolled in a 2-year randomized, double-blind, controlled treatment trial. Seventy-seven patients received UDCA in a dose of 10 to 12 mg/kg/d at bedtime, and 74 received placebo medication. Patients were stratified into four groups on the basis of: 1) a serum bilirubin of < 2 mg/dL or ≥2 mg/dL; and 2) liver histology of either stages I and II or stages III and IV, as defined by Ludwig.6 Patients in stratum 1 had a serum bilirubin < 2 and stage I or II histology, stratum 2, had a bilirubin of < 2 and stage III or IV histology; stratum 3 had a bilirubin of ≥2 and stage I or II histology; stratum 4 had a bilirubin of ≥2 and stage III or IV histology.
Fasting duodenal bile specimens were obtained in the morning at entry and at 2 years at the time of upper endoscopy and after stimulation with intravenously administered cholecystokinin. Trial medication, i.e., UDCA or placebo, was not administered the evening before the 2-year endoscopy. Samples were stored frozen at −20°C until bile acid analysis. Bile acid composition was measured by a high-pressure liquid chromatography method,7 which had been validated against gas chromatography.8 For determination of the mode of conjugation of the four major biliary bile acids (cholic acid [CA], chenodeoxycholic acid [CDCA], deoxycholic acid [DCA], UDCA), chromatograms were selected that had excellent peak resolution of the corresponding glycine and taurine conjugates of those acids and in which unknown bile acids and non–bile acid components had distinctly different retention times. There were 61 such chromatograms: 12 from patients before treatment, 22 from patients who received placebo, and 27 from patients who had received UDCA. The mode of conjugation was expressed as percent conjugation with glycine, this unit being preferable to the glycine/taurine ratio.9 For evaluation of the effect of UDCA on the mode of conjugation, samples were assigned to two groups based on biliary bile acid composition: UDCA < 5%, and UDCA > 30%. The first group consisted of patients entering the trial as well as patients who had completed 2 years of therapy with placebo. One patient with 13% UDCA in biliary bile acids was excluded.
Statistical Methods
Means for each bile acid component (CA, CDCA, DCA, UDCA, lithocholic acid [LCA], sulfolithocholic acid [SLCA], and nonidentified peaks [NIP]) for patients with PBC were calculated and compared with normal controls using two-tailed t tests.10 MANOVA11 was performed to simultaneously examine the seven different acid components for the three strata of patients at entry; in addition, one-way ANOVA10 was also performed across the three strata for each acid component separately. To simultaneously examine the seven different acid components for the two drug groups at entry, a MANOVA was performed, followed by separate one-way ANOVAs for each acid component. Separate analyses for each type of bile acid component at 2 years versus entry bile acid was performed for the two drug groups (placebo and UDCA) using an independent sample t test (some of the patients did not have measurements for both entry and at 2 years). Finally, Pearson product-moment correlation coefficients10 were performed to examine the relationship between changes in the UDCA bile acid and the changes in the other six levels of bile acids from entry to 2 years for all patients treated with UDCA.
RESULTS
Patient Characteristics at Entry
Demographic, laboratory, and histological characteristics of the patients at entry were presented in detail in a previous publication.5 The patients were predominantly middle-aged, white women. The results of initial laboratory tests and histological staging were comparable in the patients randomized to receive either placebo or UDCA. Approximately 70% had an entry serum bilirubin of less than 2 mg/dL. One third demonstrated stage I,II histology; two thirds demonstrated stage III,IV histology.
Number of Bile Specimens Analyzed
Analyses were performed on 98 patients at entry, and on 106 at 2 years. The majority of the patients had paired entry and 2-year bile specimens (Table 1). The number of patients who had only an entry or 2-year specimen available for analysis are also indicated in Table 1. There were few patients in stratum 3. Bile acid data for patients in strata 3 and 4 were combined in the descriptions provided below.
Table 1.
Number of Bile Specimens Analyzed
| At Entry | At 2 Years | |||
|---|---|---|---|---|
| Placebo (n) | UDCA (n) | Placebo (n) | UDCA (n) | |
| Stratum 1 | ||||
| Paired | 11 | 15 | 11 | 15 |
| Unpaired | 4 | 4 | 4 | 8 |
| Total | 15 | 19 | 15 | 23 |
| Stratum 2 | ||||
| Paired | 18 | 14 | 18 | 14 |
| Unpaired | 3 | 4 | 9 | 4 |
| Total | 21 | 18 | 27 | 18 |
| Strata 3–4 | ||||
| Paired | 2 (1)* | 9 (2) | 2 (1) | 9 (2) |
| Unpaired | 6 | 8 (3) | 7 (1) | 5 |
| Total | 8 (1) | 17 (5) | 9 (2) | 14 (2) |
| All strata | ||||
| Paired | 31 | 38 | 31 | 38 |
| Unpaired | 13 | 16 | 20 | 17 |
| Total | 44 | 54 | 51 | 55 |
Number of patients in stratum 3 indicated in parentheses.
Bile Acid Composition at Entry
Comparison With Normal Values
Mean values for individual bile acids at entry in 98 patients with PBC (all strata) are compared with those obtained previously in gallbladder bile obtained from 14 normal control persons (see Rossi7) in Table 2. CA levels were higher than normal, whereas CDCA, DCA, UDCA, LCA, and bile acids in NIP were lower than normal. Values for SLCA were not significantly different from normal.
Table 2.
Bile Acid Composition in Bile at Entry % of Total Bile Acids
| PBC All Strata | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bile Acid |
Normal Persons7 | 95% Confidence Limits | Difference of Mean from Normal P < .05 |
|||||
| Mean | SD | Mean | SD | Lower | Upper | |||
| CA | 36.5 | 8.3 | 57.4 | 18.6 | 53.7 | 61.1 | Increased | |
| CDCA | 38.5 | 8.0 | 31.5 | 15.5 | 28.4 | 34.6 | Decreased | |
| DCA | 15.8 | 8.4 | 8.0 | 9.3 | 6.2 | 9.8 | Decreased | |
| UDCA | 2.1 | 1.4 | 0.6 | 0.9 | 0.4 | 0.8 | Decreased | |
| LCA | 0.7 | 0.4 | 0.3 | 1.0 | 0.1 | 0.5 | Decreased | |
| SLCA | 0.6 | 0.3 | 0.6 | 1.1 | 0.4 | 0.8 | Not significant | |
| NIP | 5.8 | 2.5 | 1.6 | 2.3 | 1.1 | 2.1 | Decreased | |
Mean values for the individual bile acids in the various strata (stratum 1, stratum 2, strata 3–4, data not shown), when compared with normal values yielded similar results, with the exception that LCA in stratum 1 was not significantly different from normal.
Comparison Among Patient Strata
A MANOVA comparing the three strata across the full set of bile acid components showed no significant difference between strata (Wilk’s Lambda, P = .15). DCA appeared to decrease and CA appeared to increase with worsening stages of PBC. Univariate ANOVA results for each bile acid component separately showed a significant result for DCA (11.1% for stratum 1, 8.1% for stratum 2, 3.5% for strata 3–4; P = .01), and nearly significant results for CA (53.1% for stratum 1, 57.0% for stratum 2, 64.0% for strata 3–4; P = .07) and for LCA (0.6% for stratum 1, 0.1% for stratum 2, 0.2% for strata 3–4; P = .08). However, the multivariate analysis indicates that these results may be spurious considering the intercorrelation among the seven components.
Comparison by Drug Assignment
A MANOVA comparing values in patients assigned to receive either placebo or UDCA showed no significant differences across the full set of bile acid components (Wilk’s Lambda, P = .61). The set of univariate ANOVA results for each bile acid component separately also showed no significant difference in results.
Bile Acid Composition at 2 Years; Comparison With Entry Values
Placebo
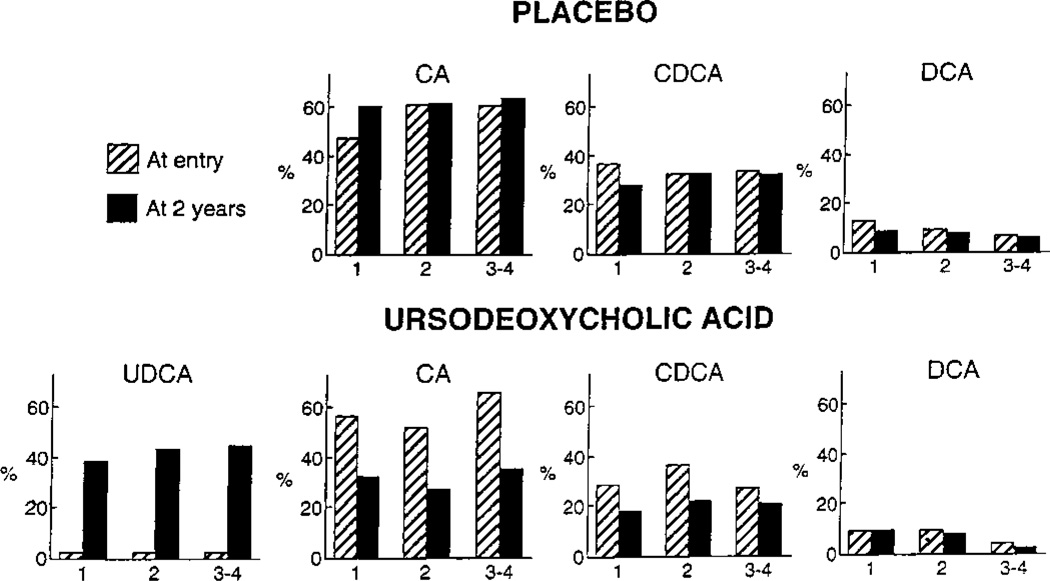
No significant changes in bile acid composition were noted for CA, CDCA, DCA, UDCA or LCA. SLCA decreased from 0.8% to 0.3% (P = .03), and NIP increased marginally from 1.4% to 2.7% (P = .06) (Table 3).
Table 3.
Comparison of Bile Acid Composition at Baseline and After 2 Years on Placebo or UDCA
| Bile Acid |
Time | % of Bile Acids in Bile | |
|---|---|---|---|
| Placebo | UDCA | ||
| CA | Entry | ||
| Mean | 56.0 | 58.6 | |
| SD | 19.1 | 18.2 | |
| 2 years | |||
| Mean | 61.4 | 32.2 | |
| SD | 16.5 | 13.8 | |
| P | NS | <.001 | |
| CDCA | Entry | ||
| Mean | 32.6 | 30.6 | |
| SD | 15.5 | 15.7 | |
| 2 years | |||
| Mean | 28.9 | 19.5 | |
| SD | 12.6 | 9.3 | |
| P | NS | <.001 | |
| DCA | Entry | ||
| Mean | 8.1 | 7.9 | |
| SD | 9.0 | 9.6 | |
| 2 years | |||
| Mean | 6.0 | 6.7 | |
| SD | 7.0 | 6.6 | |
| P | NS | NS | |
| UDCA | Entry | ||
| Mean | 0.6 | 0.6 | |
| SD | 0.9 | 1.0 | |
| 2 years | |||
| Mean | 0.4 | 40.1 | |
| SD | 0.6 | 15.8 | |
| P | NS | <.001 | |
| LCA | Entry | ||
| Mean | 0.4 | 0.2 | |
| SD | 1.3 | 0.7 | |
| 2 years | |||
| Mean | 0.2 | 0.3 | |
| SD | 0.6 | 1.0 | |
| P | NS | NS | |
| SLCA | Entry | ||
| Mean | 0.8 | 0.4 | |
| SD | 1.3 | 0.9 | |
| 2 years | |||
| Mean | 0.3 | 0.6 | |
| SD | 0.9 | 1.1 | |
| P | .03 | NS | |
| NIP | Entry | ||
| Mean | 1.4 | 1.8 | |
| SD | 2.5 | 2.2 | |
| 2 years | |||
| Mean | 2.7 | 0.9 | |
| SD | 3.8 | 1.5 | |
| P | .06 | .012 | |
UDCA
UDCA became the major bile acid in bile, accounting for 40.1% of the bile acids detected. This was accompanied by statistically significant decreases in CA, CDCA, and NIP. No change in percent composition was noted for DCA, LCA, or SLCA (Table 3). Alterations in composition of the major bile acids in bile were comparable for the various strata (see Fig. 1).
Fig. 1.
Changes in bile acid composition for the major bile acids in bile according to stratum (1, 2, 3–4) after 2 years of treatment with placebo or UDCA.
Correlation of Changes in UDCA With Changes in Other Bile Acid Components
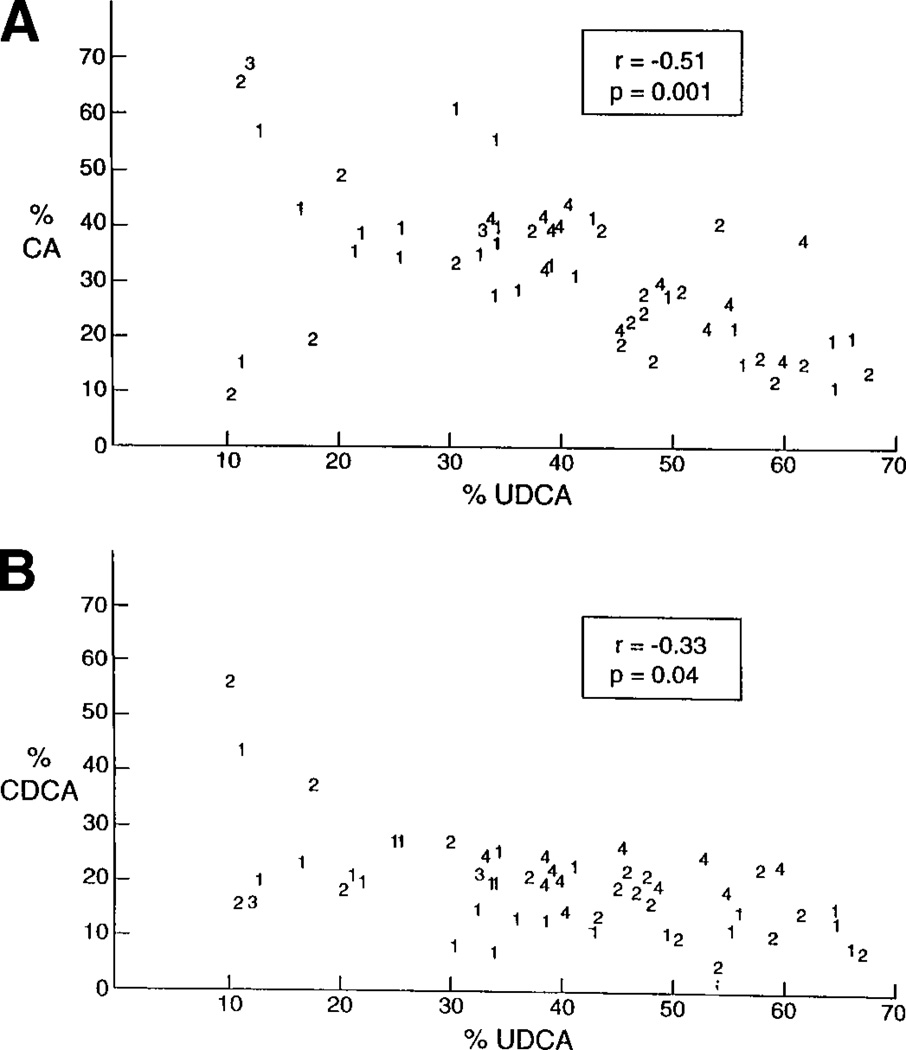
Significant negative correlations were found between UDCA and CA (r = −.51, P = .001) (Fig. 2A), and CDCA (r = −.33, P = .04) (Fig. 2B). Thus, as UDCA increased, CA and CDCA decreased. Correlations between UDCA with changes in other bile acid components were not significant statistically.
Fig. 2.
Comparison of percent CA (A), and percent CDCA (B) with percent UDCA in bile after 2 years of treatment with UDCA. The numbers indicate the stratum from which each bile specimen was obtained.
Mode of Conjugation
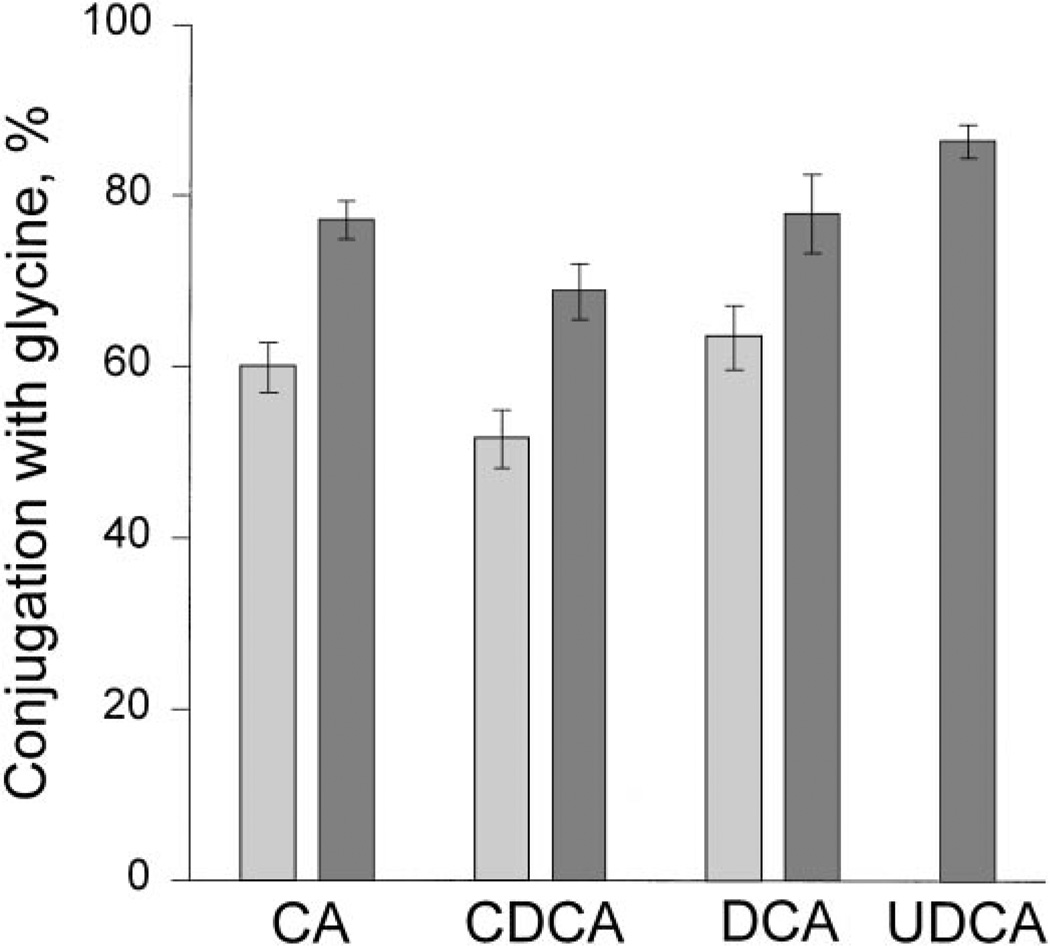
In patients whose bile was not enriched in UDCA, the three major endogenous bile acids (CA, CDCA, DCA) and UDCA were conjugated to a greater extent with glycine (52%–64%) than with taurine (36%–48%), but there was considerable between-patient variation. Treatment with UDCA caused the proportion of all endogenous bile acids conjugated with glycine to increase to 69% to 78% with a reciprocal decrease in the proportion conjugated with taurine (22%–31%; P < .05). Administered UDCA was also conjugated predominantly with glycine (87%). The proportion of individual bile acids conjugated with glycine is illustrated in Fig. 3. In subjects whose bile was enriched in UDCA, the preferential conjugation with glycine of UDCA was not influenced by the degree of UDCA enrichment in biliary bile acids (data not shown). For all endogenous bile acids, the percent conjugation with glycine was fairly similar in a given patient (for CA vs. CDCA: r=.78; for CA vs. DCA: r=.67; and for CDCA vs. DCA: r = .55 [data not shown]). The correlation coefficients were significant (P < .002).
Fig. 3.
Mode of conjugation, expressed as percent conjugation with glycine (mean ± SE), for endogenous bile acids (CA, CDCA, DCA), and UDCA in biliary bile acids of PBC patients on entry or after treatment with placebo (light stippling) or after treatment with UDCA (dark stippling). Patients who were pretreatment or who had been treated with placebo had < 5% UDCA in biliary bile acids; those treated with UDCA had > 30% UDCA in biliary bile acids. Treatment with UDCA caused the proportion of bile acids conjugated with glycine to increase (P < .05). ( ), UDCA < 5%; (
), UDCA < 5%; ( ), UDCA > 30%.
), UDCA > 30%.
DISCUSSION
The bile acid pool becomes significantly enriched with UDCA in patients with PBC who take this compound. This is substantiated by measurements of the biliary composition of UDCA reported in the studies containing sufficient data for all of the major bile acids (see Table 4). UDCA comprised 32% to 40% of the bile acids in bile with doses up to 10 to 12 mg/kg/d and with treatment encompassing periods of 4 weeks to 2 years. The range of values was broad and was not accounted for by the different doses of UDCA administered. In the treatment trial of Lindor et al.,12 patients with PBC receiving an even higher dose of 13 to 15 mg/kg/d in divided doses with meals and at bedtime achieved a mean UDCA level in bile at 2 years of 39.5%. The range of values varied from barely detectable to over 70%.
Table 4.
Effect of UDCA on Bile Acid Composition in Bile
| Mazzella et al.1 | Crosignani et al.2 | Current Study | Lindor et al.16 | |
|---|---|---|---|---|
| UDCA dose | 600 mg/d | 500 mg/d 8 mg/kg/d | 10–12 mg/kg/d bedtime dose | 13–15 mg/kg/d, divided dose with meals and at bedtime |
| Duration | 4 wk | 6 mo | 2 yr | 2 yr |
| n | 9 | 10 | 55 | 55 |
| Bile Acid Composition in Bile (percent of total) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | 2 Years | P | Entry | 2 Years | P | Entry | 2 Years | P | Entry | 2 Years | P | |
| UDCA | Trace | 34.2 | <.001 | 4.5 | 31.9 | <.001 | 0.6 | 40.1 | <.001 | 1.0 | 44.0 | <.0001 |
| CA | 48.4 | 35.1 | <.001 | 57.9 | 30.8 | <.005 | 58.6 | 32.2 | <.001 | 54.0 | 28.0 | <.0001 |
| CDCA | 31.2 | 21.3 | <.001 | 29.5 | 26.2 | NS | 30.6 | 19.5 | <.001 | 32.0 | 18.0 | <.002 |
| DCA | 16.5 | 6.8 | <.001 | 8.1 | 11.1 | NS | 7.9 | 6.7 | NS | 10.0 | 10.0 | NS |
| LCA | 2.5 | 2.5 | NS | 0.8 | 3.0 | <.05 | 0.2 | 0.3 | NS | 0.2 | 0.8 | .009 |
The factors responsible for the great intersubject variation in enrichment in biliary bile acids have yet to be elucidated. In each of the above reports, values were obtained from a single specimen of bile obtained after a period of UDCA. The extent to which biliary enrichment with UDCA varies from day to day in a given patient is unknown. Enrichment in biliary bile acids by an administered bile acid depends on multiple factors. These include compliance, fraction absorbed (bioavailability), conservation of the administered bile acid and its metabolites, and the effect of the administered bile acid on endogenous bile acid metabolism.13 In the present study, we identified several patients whose biliary bile became enriched with CDCA during therapy with UDCA (see Fig. 2), presumably because of bacterial conversion of administered UDCA to CDCA.14,15 Such enrichment in CDCA should decrease the enrichment in UDCA. Our own data, presented here (see Fig. 1) and elsewhere for our UDCA results alone,5 indicate that the extent of enrichment with UDCA is comparable in patients in the different strata. Thus, severity of liver disease does not appear to be a major determinant of the extent of enrichment of bile with UDCA.
Relatively little information is available about the effects of divided doses taken with meals versus a single daily bedtime dose of UDCA on the extent of biliary enrichment. In a group of 27 patients with cholestatic liver disease (19 primary sclerosing cholangitis and 8 PBC) who received 10 mg/kg/d for 3 months, mean enrichment of UDCA in bile was 40.1% in 13 patients receiving a single bedtime dose, and 40.8% in 14 patients receiving three divided doses with meals.2 In our PBC patients, a single bedtime dose of 10 to 12 mg/kg/d for 2 years yielded a mean UDCA value in bile of approximately 40%. This compares with a mean value of 39.5% for the PBC patients of Lindor et al.,12 who ingested 13 to 15 mg/kg/d in divided doses with meals and at bedtime for 2 years. Thus, it appears that a single bedtime dose of UDCA yields similar biliary enrichment with UDCA as multiple divided doses. Intuitively, a single-dose regimen should improve compliance with intake of UDCA.
The relative proportions of the other major bile acids in bile also changed with ingestion of UDCA. Since submission of our manuscript, Lindor et al.16 have also reported on the effect of UDCA on biliary bile acid composition, and their findings are incorporated in Table 4. CA fell significantly in all four studies. CDCA fell in three of the four. DCA decreased in one, and LCA increased in two of the four. Although LCA increased in our patients, the change was not statistically significant. Our data obtained at 2 years and presented in Fig. 2 indicate that there is an inverse relationship between the degree of enrichment of UDCA in bile and the fall in CA and CDCA.
Assessments of bile acid composition in serum by others1,3,16–19 also revealed decreases in CA (all 6 reports), in CDCA (5 of the 6 reports), and lesser falls in DCA (5 of the 6 reports) during treatment with UDCA. In serum as in bile, the relative changes in bile acid composition were comparable in patients with milder and more severe forms of PBC.
Our study also indicates that in PBC patients, treatment with UDCA caused the proportion of endogenous bile acids conjugated with glycine to increase with the result that endogenous bile acids became conjugated mostly with glycine. Administered UDCA was also conjugated predominantly with glycine. This finding was also observed by Crosignani et al. in their analysis of biliary bile acids in 10 PBC patients, but not commented upon.3 We explain this finding as follows: The steady-state mode of conjugation depends on the partition of bile acid conjugation between glycine and taurine in the hepatocyte, and the relative susceptibility of glycine or taurine conjugates to bacterial deconjugation during enterohepatic cycling.20 In healthy subjects, the majority of bile acid conjugation involves reconjugation of unconjugated bile acids formed in the small intestine by bacterial deconjugation of previously secreted bile acids.21 Such reconjugation should occur in the periportal hepatocytes, whereas bile acid synthesis is considered to occur largely in pericentral hepatocytes.22,23 Administered UDCA also is likely to be conjugated predominantly in the periportal hepatocytes, and there is evidence that UDCA conjugates are extensively deconjugated during enterohepatic cycling.24,25 Therefore, periportal hepatocytes must conjugate not only newly ingested UDCA, but also unconjugated UDCA as well as unconjugated endogenous bile acids originating from bacterial deconjugation of secreted bile acids and subsequent absorption of the unconjugated steroid moiety. We postulate that the large flux of unconjugated UDCA through the periportal hepatocyte induces transient depletion of its taurine stores, which, in turn, should result in preferential conjugation of UDCA as well as endogenous bile acids with glycine.26 A similar mode of conjugation of primary bile acids was not unanticipated, because such has been observed previously on a small number of bile samples from healthy subjects7 as well as bile samples from many vertebrates.27
A large body of evidence indicates that certain bile acids can induce cholestasis and liver cell necrosis when administered intravenously or intraduodenally to intact animals,28–33 and can decrease bile flow in the isolated perfused rat liver.34,35 Toxicity has been shown to correlate with increasing hydrophobicity of the bile acids as measured by reverse-phase high-pressure liquid chromatography.36,37 UDCA in its conjugated forms usually is able to prevent or minimize both cholestasis and liver cell injury in these experimental models,30–33 even when the absolute load of the toxic bile acid presented to the liver is not lowered. This hepatoprotective effect can occur very rapidly and need not require changes in pool size of the toxic bile acid. Hepatoprotection may involve inhibition of transport of endogenous hepatotoxic bile acids such as CDCA (as its glycine or taurine conjugate) into hepatocyte organelles.38
CDCA and DCA feeding to humans can induce liver injury, and toxicity has been attributed to the fed bile acid.8,39,40 Little information is available about the effects of feeding CA to PBC patients. When Guldutuna et al.41 administered CA to their patients with PBC, worsening of liver test results was observed in the second and third months of treatment; but CA administration to PBC patients or healthy subjects causes biliary bile acids to become enriched in DCA, its 7-deoxy metabolite.
The changes in biliary bile acid composition1,3,16 (also presented in this article) observed during treatment of patients with PBC with UDCA support the hepatoprotective mechanism proposed for UDCA. Thus, the composition of biliary bile acids becomes less hydrophobic, and presumably, the intrahepatic composition does as well. Moreover, the ratio of UDCA to the other bile acids increases significantly. While considerable attention has been directed toward the potential hepatotoxicity of the dihydroxy bile acids, CDCA and DCA, the impressive decreases in CA observed in the present and other reports in bile and in serum1,3,16–19 that accompany improvement in laboratory tests during UDCA therapy raise the possibility that CA also contributes to ongoing hepatic injury in PBC. While CA is much less toxic than CDCA and DCA, CA can induce cholestasis and liver cell injury29–33,35 that is protected against by UDCA.
Acknowledgment
The authors are indebted to Renate Davis for preparation of the manuscript and for valuable administrative support. Joseph H. Steinbach, Ph.D., provided aid in data analysis and graphics.
Supported in part by a research grant from Ciba-Geigy; NIH General Clinical Research Center grants to UT Southwestern (M01-RR00633), Yale (MO1-RR00125), Medical College of Virginia of Virginia Commonwealth University (MO1-RR00065), Washington University, St. Louis, (MO1-RR00036), NIH grants Yale (P30-DK34989), University of California San Diego (DK-21506), Institutional Funds: Thomas Jefferson-Louis A. Rosen Fund for Liver Research; Nebraska - Clinical Research Funds of University of Nebraska Medical Center.
Abbreviations
- UDCA
ursodeoxycholic acid
- PBC
primary biliary cirrhosis
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- LCA
lithocholic acid
- SLCA
sulfolithocholic acid
- NIP
nonidentified peaks
REFERENCES
- 1.Mazzella G, Parini P, Bazzoli F, Villanova N, Festi D, Aldini R, Roda A, et al. Ursodeoxycholic acid administration on bile acid metabolism in patients with early stages of primary biliary cirrhosis. Dig Dis Sci. 1993;38:896–902. doi: 10.1007/BF01295917. [DOI] [PubMed] [Google Scholar]
- 2.Van de Meeberg PC, Wolfhagen FHJ, Van Berge-Henegowoen G-P, Salemans JMJI, Tangerman A, Van Buuren HR, Van Hattum J, et al. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. J Hepatol. 1996;25:887–894. doi: 10.1016/s0168-8278(96)80293-5. [DOI] [PubMed] [Google Scholar]
- 3.Crosignani A, Podda M, Battezzati PM, Bertolini E, Zuin M, Watson D, Setchell K. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991;14:1000–1007. [PubMed] [Google Scholar]
- 4.Batta AK, Salen G, Mirchandani R, Tint GS, Shefer S, Batta M, Abroon J, et al. Effect of long-term treatment with ursodiol on clinical and biochemical features and biliary bile acid metabolism in patients with primary biliary cirrhosis. Am J Gastroenterol. 1993;88:691–700. [PubMed] [Google Scholar]
- 5.Combes B, Carithers RL, Jr, Maddrey WC, Lin D, McDonald MF, Wheeler DE, Eigenbrodt EH, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–766. [PubMed] [Google Scholar]
- 6.Ludwig J, Dickson ER, McDonald GS. Staging of chronic non-suppurative destructive cholangitis (syndrome of primary biliary cirrhosis) Virchows Arch. 1978;379:103–112. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 7.Rossi SS, Converse JL, Hofmann AF. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J Lipid Res. 1987;28:589–595. [PubMed] [Google Scholar]
- 8.Fisher RL, Hofmann AF, Converse JL, Rossi SS, Lan S-P. Lack of a relationship between hepatotoxicity and lithocholic acid sulfation during chenodiol therapy in the National Cooperative Gallstone Study. Hepatology. 1991;14:454–463. [PubMed] [Google Scholar]
- 9.Hoffman NE, Hofmann AF. Metabolism of steroid and amino acid moieties of conjugated bile acids in man. V. Equations for the perturbed enterohepatic circulation and their application. Gastroenterology. 1977;72:141–148. [PubMed] [Google Scholar]
- 10.Zar JH. Biostatistical Analysis. 3rd ed. Upper Saddle River, NJ: Prentice-Hall, Inc.; 1996. [Google Scholar]
- 11.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 3rd ed. New York, New York: Harper Collins Publishers, Inc.; 1996. [Google Scholar]
- 12.Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol. 1994;29(Suppl 204):1–15. doi: 10.3109/00365529409103618. [DOI] [PubMed] [Google Scholar]
- 14.Fedorowski T, Salen G, Tint GS, Mosbach E. Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology. 1979;77:1068–1073. [PubMed] [Google Scholar]
- 15.Hirano S, Masuda N, Oda H. In vitro transformation of chenodeoxycholic acid and ursodeoxycholic acid by lumen intestinal flora, with particular reference to the mutual conversion between the two bile acids. J Lipid Res. 1981;22:735–743. [PubMed] [Google Scholar]
- 16.Lindor DK, Lacerda MA, Jorgensen RA, DeSotel CK, Batta AK, Salen G, Dickson ER, et al. Relationship between biliary and serum bile acids and response to ursodeoxycholic acid in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998;93:1498–1504. doi: 10.1111/j.1572-0241.1998.00470.x. [DOI] [PubMed] [Google Scholar]
- 17.Leuschner U, Fischer H, Kurtz W, Guldutuna S, Hubner K, Hellstern A, Gatzen M, et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology. 1989;97:1268–1274. doi: 10.1016/0016-5085(89)91698-3. [DOI] [PubMed] [Google Scholar]
- 18.Stiehl A, Rudolph G, Raedsch R, Moller B, Hopf U, Lotterer E, Bircher J, et al. Ursodeoxycholic acid-induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12:492–497. doi: 10.1002/hep.1840120308. [DOI] [PubMed] [Google Scholar]
- 19.Poupon RE, Chretien Y, Poupon R, Paumgartner G. Serum bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid therapy. Hepatology. 1993;17:599–604. doi: 10.1002/hep.1840170412. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman NE, Hofmann AF. Metabolism of steroid and amino acid moieties of conjugated bile acids in man. IV. Description and validation of a multicompartmental model. Gastroenterology. 1974;67:887–897. [PubMed] [Google Scholar]
- 21.Hofmann AF. Enterohepatic circulation of bile acids. In: Schultz SG, editor. Handbook of Physiology: The Gastrointestinal System. Volume III. Bethesda, MD: 1989. pp. 567–596. Am Physiol Soc. [Google Scholar]
- 22.Twisk J, Hoekman MRM, Mager WH, Moorman AFM, deBoer PAJ, Scheja L, Princen HMG, et al. Heterogeneous expression of cholesterol 7-alpha-hydroxylase and sterol 27-hydroxylase genes in the rat liver lobulus. J Clin Invest. 1995;95:1235–1243. doi: 10.1172/JCI117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massimi M, Lear SR, Huling SL, Jones AL, Erickson SK. Cholesterol 7-alpha-hydroxylase (CYP7A): pattern of messenger RNA expression during rat liver development. Hepatology. 1998;28:1064–1072. doi: 10.1002/hep.510280422. [DOI] [PubMed] [Google Scholar]
- 24.Batta AK, Salen G, Shefer S, Tint GS, Dayal B. The effect of tauroursode-oxycholic acid and taurine supplementation on biliary bile acid composition. Hepatology. 1982;2:811–816. doi: 10.1002/hep.1840020612. [DOI] [PubMed] [Google Scholar]
- 25.Hardison WG, Grundy SM. Effect of ursodeoxycholate and its taurine conjugate on bile acid synthesis and cholesterol absorption. Gastroenterology. 1984;87:130–135. [PubMed] [Google Scholar]
- 26.Hardison WG. Hepatic taurine concentration and dietary taurine as regulators of bile acid conjugation with taurine. Gastroenterology. 1978;75:71–75. [PubMed] [Google Scholar]
- 27.Hagey LR, Gavrilkina MA, Hofmann AF. Age-related changes in the biliary bile acid composition of bovids. Can J Zool. 1997;75:1193–1201. [Google Scholar]
- 28.Javitt NB, Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid secretion. J Clin Invest. 1968;47:1002–1014. doi: 10.1172/JCI105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drew R, Priestly GB. Choleretic and cholestatic effects of infused bile salts in the rat. Experientia. 1978;35:809–811. doi: 10.1007/BF01968265. [DOI] [PubMed] [Google Scholar]
- 30.Kitani K, Kanai S. Tauroursodeoxycholate prevents taurocholate induced cholestasis. Life Sci. 1982;30:515–523. doi: 10.1016/0024-3205(82)90264-8. [DOI] [PubMed] [Google Scholar]
- 31.Kanai S, Kitani K. Glycoursodeoxycholate is as effective as taurodeoxycholate in preventing the taurocholate-induced cholestasis in the rat. Res Commun Chem Pathol Pharmacol. 1983;42:423–430. [PubMed] [Google Scholar]
- 32.Kitani K, Ohta M, Kenai S. Tauroursodeoxycholate prevents biliary protein excretion induced by other bile salts in the rat. Am J Physiol. 1985;248:G407–G417. doi: 10.1152/ajpgi.1985.248.4.G407. [DOI] [PubMed] [Google Scholar]
- 33.Heuman DM, Mills AS, McCall JI, Hylemon PB, Pandak WM, Vlahcevic ZR. Conjugates of ursodeoxycholate protect against cholestasis and hepatocellular necrosis caused by more hydrophobic bile salts: in vivo studies in the rat. Gastroenterology. 1991;100:203–211. doi: 10.1016/0016-5085(91)90602-h. [DOI] [PubMed] [Google Scholar]
- 34.Fisher MM, Magnuson R, Miyai K. Bile acid metabolism in mammals. I Bile acid-induced intrahepatic cholestasis. Lab Invest. 1971;25:88–91. [PubMed] [Google Scholar]
- 35.Herz R, Paumgartner G, Preisig R. Inhibition of bile formation by high doses of taurocholate in the perfused rat liver. Scand J Gastroenterol. 1975;11:741–746. [PubMed] [Google Scholar]
- 36.Armstrong MJ, Carey MC. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J Lipid Res. 1982;23:70–80. [PubMed] [Google Scholar]
- 37.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 38.Rodrigues CMP, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochrondrial membrane perturbation. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher RL, Anderson DW, Boyer JL, Ishak K, Klatskin G, Lachin JM, Phillips MJ. A prospective morphologic evaluation of hepatic toxicity of chenodeoxycholic acid in patients with cholelithiasis: the National Cooperative Gallstone Study. Hepatology. 1982;2:187–201. doi: 10.1002/hep.1840020202. [DOI] [PubMed] [Google Scholar]
- 40.LaRusso NF, Szczepanik PA, Hofmann AF. Effect of deoxycholic acid ingestion on bile acid metabolism and biliary lipid secretion in normal subjects. Gastroenterology. 1977;72:132–140. [PubMed] [Google Scholar]
- 41.Guldutuna S, Leuschner M, Wunderlich N, Nickel A, Bhatti S, Hubner K, Leuschner U. Cholic acid and ursodeoxycholic acid therapy in primary biliary cirrhosis. Eur J Clin Pharmacol. 1993;45:221–225. doi: 10.1007/BF00315387. [DOI] [PubMed] [Google Scholar]