Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder that is characterized by progressive memory loss. In contrast, accumulating evidence suggests a neuroprotective role of regular exercise in aging associated memory impairment. In this study, we investigated the ability of regular exercise to prevent impairments of short-term memory (STM) and early long-term potentiation (E-LTP) in area CA1 of the hippocampus in a rat model of AD ( i.c.v. infusion of 250 pmol/day Aβ1–42 peptides). We utilized behavioral assessment, in vivo electrophysiological recording, and immunoblotting in 4 groups of adult Wistar rats: control, treadmill exercise (Ex), β-amyloid-infused (Aβ), and amyloid-infused/treadmill exercised (Ex/Aβ). Our findings indicated that Aβ rats made significantly more errors in the radial arm water maze (RAWM) compared to all other groups and exhibited suppressed E-LTP in area CA1, which correlated with deleterious alterations in the levels of memory and E-LTP-related signaling molecules including calcineurin (PP2B), brain derived-neurotrophic factor (BDNF) and phosphorylated CaMKII (p-CaMKII). Compared to controls, Ex and Ex/Aβ rats showed a similar behavioral performance and a normal E-LTP with no detrimental changes in the levels of PP2B, BDNF, and p-CaMKII. We conclude that treadmill exercise maybe able to prevent cognitive impairment associated with AD pathology.
Keywords: Alzheimer’s disease; brain-derived neurotrophic factor (BDNF); calcineurin (PP2B); calcium-calmodulin dependent protein kinase II (CaMKII); exercise, learning and memory; synaptic plasticity
Introduction
Currently, the amyloid hypothesis holds strong support based on the fact that the prime neuropathological hallmarks of AD are extracellular deposits of amyloid plaques and intracellular formation of neurofibrillary tangles. Even though full-blown characteristics of AD have not yet been reproduced, various animal models of AD have shown important symptoms similar to the disease manifestation in humans. AD animals exhibit a marked impairment of learning and memory function as shown in various behavioral tasks such as Morris water maze (MWM), radial arm maze (RAM), and radial arm water maze (RAWM) [1–5]. In addition to cognitive deficits, aged transgenic AD mice also displayed non-cognitive disturbances (e.g. irritability) compared to their wild type counterparts [6]. Learning and memory impairment in AD animals correlates, at the cellular level, with an enhanced long-term depression (LTD) and a suppressed long-term potentiation (LTP) [1, 7].
The expression of LTP and LTD requires the entry of calcium into the postsynaptic cell. A high level of intracellular calcium induces LTP but only a modest rise in calcium can induce LTD. While the expression of LTP involves the action of kinases, phophastases are responsible for the expression of LTD. Two major phases of LTP have been identified; an early phase (E-) LTP, lasting up to 3 hours and the more durable late phase (L-) LTP, lasting more than 3 hours and even days. At the synapses, E-LTP and L-LTP are considered to be cellular analogues of short-term and long-term memory respectively. A single train of high frequency stimulation (HFS) induces E-LTP, a protein-independent process. The released glutamate activates post-synaptic glutamate receptors and with the post-synaptic membrane sufficiently depolarized, the Mg2+ blockage of the N-methyl-D-aspartate (NMDA) receptors is removed allowing the entry of Ca2+ into the cell. Calcium ion influx dissociates calmodulin from neurogranin and activates Ca2+/calmodulin-dependent protein kinase II (CaMKII), which autophosphorylates once activated. CaMKII serves as a critical molecule whose activation duration determines transiency or persistency of LTP [8].
Regular exercise is a non-pharmacological approach that ameliorates memory impairment secondary to brain injury, enhances cognitive function, and prevents memory decline in the aged brain [9, 10]. In addition to animal studies, epidemiological analysis in humans suggests that regular exercise can act as a preventive treatment against cognitive disorders (e.g. amnesia, dementia) with minimal cost and adverse effects [11]. The neuroprotective effects of exercise are thought to be mediated by various molecular mechanisms including upregulation of neurotrophins (e.g. BDNF) and other molecules associated with learning and memory function including CaMKII and calcineurin, which in turn enhance brain plasticity (LTP) and improve performance in memory tasks. Exercised animals performed better in the spatial memory tasks (e.g. MWM or RAWM) compared to sedentary animals as indicated by shorter swim paths or fewer errors to find the target platform [10, 12–14]. Similarly, exercise can also modify non-spatial memory as tested in the passive avoidance paradigm and object recognition tasks [9, 10, 12, 15–18]. Additionally, treadmill exercise is reported to restore memory impairment in rats treated with alcohol [19], streptozocin [20], reserpine [21], or sleep deprivation [22].
In this study, we investigated the effect of treadmill exercise on learning and short-term memory, E-LTP in area CA1 and related signaling molecules levels in a rat model of AD achieved by exogenous administration of amyloid peptides.
Materials and methods
Animals
Adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighed 176–200g at the beginning of all experiments. Upon arrival, rats were housed in a Plexiglas cage (4–6 rats/cage) in a climate-controlled room (25°C) on a 12hr/12hr light/dark cycle. Rats were provided with a regular rat chow diet and water ad libitum and allowed to acclimate in the new environment for a week. All experiments were conducted following the instructions from National Research Council’s Guide of The Care and Use of Laboratory Animals and with the approval of University of Houston Institutional Animal Care and Use Committee.
Exercise protocol
Rats ran on a leveled motorized treadmill (Columbus Instruments, Columbus, OH) on week days between 9:00 am and 4:00 pm for 4 weeks with a customized regimen as previously described by us [22]. Rats were familiarized with the treadmill environment before the exercise training commenced. Rats ran 2 sessions (15 minutes each) at a speed of 10 m/min (week 1 and week 2) followed by a gradual increase in exercise duration and intensity (3 sessions for week 3 and 4 sessions for week 4, 15 m/min). A 5 minute break was given between the sessions to reduce the risk of muscle fatigue. To encourage the rats to continue running, a mild foot shock (0.5mA) that caused a tingling sensation was delivered. The rats eventually learn to avoid this shock, which did not appear to be stressful.
Osmotic pump implantation
After two weeks of exercise, the rats underwent either a sham operation (control and exercise groups) or pump implantation (Aβ and Ex/Aβ groups). Rats in the Aβ and Ex/Aβ groups were implanted with mini-osmotic pumps (Alzet, Cupertino, CA) as previously described by us [1, 2, 23]. Amyloid peptide (Aβ1–42; AnaSpec Inc., San Jose, CA) solution was prepared following our previous protocols [23]. Briefly, the peptide was dissolved in a solution containing 64.9% distilled water, 35% acetonitrile, and 0.1% trifluoroacetate (TFA) to prevent peptide aggregation in the pump. One day before the implantation, the 14-day pumps were assembled, filled with Aβ1–42 solution (designed to deliver 250 pmol/day), and primed in isotonic (0.9%) saline at 37°C overnight. Rats were anesthetized with an i.p. injection of a cocktail mixture containing ketamine (75 mg/kg) and xylazine (2.5 mg/kg) (Webster Veterinary, Devens, MA). The implantation site was shaved and disinfected with isopropyl alcohol. Once positioned in the stereotaxic frame, the rat’s skull was exposed by a 2.5 cm midline sagittal incision starting behind the eyes. The infusion cannula was implanted into the right cerebral lateral ventricle (AP: −0.3, L: 1.2, V: 4) according to the atlas of Paxinos and Watson (1986). The cannula was initially held by drops of cyanoacrylic glue, connected to the pump and steadily fixed with dental cement. The pump was placed in a subdermal pocket in the back of the rat. The wound was then closed with wound clips, and tincture of iodine, diluted chlorohexidine, and triple antibiotic ointment were applied to prevent bacterial infections. Rats were monitored until full recovery.
Radial arm water maze (RAWM)
The RAWM is a hybrid of the Morris water maze and radial arm maze. It is a circular black water pool that consists of six swimming arms with an open central area. The experiments were done in a dimly lit room with visual cues placed on the surrounding walls. Each rat was randomly assigned a goal arm, with a hidden platform placed near its end. The behavioral testing protocol consisted of 12 learning trials followed by a short-term memory test (STM), which were administered 30 minutes after the 12th learning trial as previously described [22].
Electrophysiological experiment
Seventeen days after implantation surgery, in vivo electrophysiological recordings from area Cornu Ammonis 1 (CA1) of the hippocampus was accomplished as described [24, 25]. Briefly, rats were urethane-anesthetized (1.2 g/kg, i.p., Sigma Aldrich, USA) and two holes were stereotaxically drilled for placing the stimulating and recording electrodes. On the left side of the brain, a hole was drilled for placing the concentric bipolar stimulating electrode in area CA3 of the left hippocampus, which was positioned at 5° angle toward the midline (AP: −3, L: 3.5, V: 2.8). Similarly, a capillary glass (1–5 MΩ) recording electrode, filled with 2M NaCl solution, was inserted through the hole into area CA1 of the right hippocampus (AP: −3, L: 2, V: 2). Once the maximal response was attained, a 30 minute stabilization period was allowed without further stimulation. The test stimulus was adjusted to 30% of the maximal response. A baseline was established with test stimuli (1pulse/30s) to evoke population spikes (pspike) for 20 minutes. The magnitude of E-LTP, evoked by one train of HFS (8 pulses of 400 Hz every 10 seconds, repeated 8 times), is quantified by changes in the field excitatory post-synaptic potential (fEPSP) slope and pspike amplitude recorded for 1 hour following HFS. The fEPSP slope and pspike amplitude represent synaptic strength and the number of neurons reaching firing threshold respectively [24, 25].
Western blotting
The procedures were conducted as described [1, 22, 23]. At the end of the electrophysiological experiments, the brains were immediately removed and the CA1 area of the hippocampus was dissected out and cut into the septal (ventral) and temporal (dorsal) portions and stored at −80° C for later processing. Unstimulated samples were prepared from control rats that did not receive high frequency stimulation (HFS) while stimulated (S) samples were prepared from rats that received a single train of HFS to evoke E-LTP of the Schaffer collaterals pathway. As the septal side of the CA1 area received most of the stimulation, its value is expressed as ratio to that of the temporal side after normalization to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a loading control [22, 26]. The homogenates were prepared as described and proteins loaded (15 µg/well) on a high throughput E-PAGE 48 system. The proteins were transferred to a PVDF membrane on a dry blot system (Invitrogen Corp., Grand Island, NY). Detection of proteins was done using specific primary antibodies and subsequent conjugation with secondary horse-radish peroxidase antibodies. The protein bands were visualized using chemiluminescence reagents (Santa Cruz Biotechnology, Santa Cruz, CA) and quantified by densitometry using AlphaEase software.
The following antibodies were used: mouse monoclonal anti-p-CaMKII (1:500); rabbit polyclonal anti- t-CaMKII (1:1000); rabbit polyclonal anti-BDNF (1:500); rabbit polyclonal anti-PP2B (1:1000); rabbit polyclonal anti-GAPDH (1:1000); secondary anti-mouse/rabbit antibodies (1:5000). All antibodies were purchased from Santa Cruz Technology except GAPDH antibody, which was purchased from Cell Signaling Inc., Boston, MA.
Statistical analysis
Unpaired t-test was used to compare two groups while one-way analysis of variance (ANOVA) followed by Tukey post-hoc test was used to compare all groups. All statistical analyses were done with GraphPad Prism software. P<0.05 was considered statistically significant. Data were expressed as mean ± S.E.M.
Results
Exercise prevents AD-induced learning and short-term memory impairment
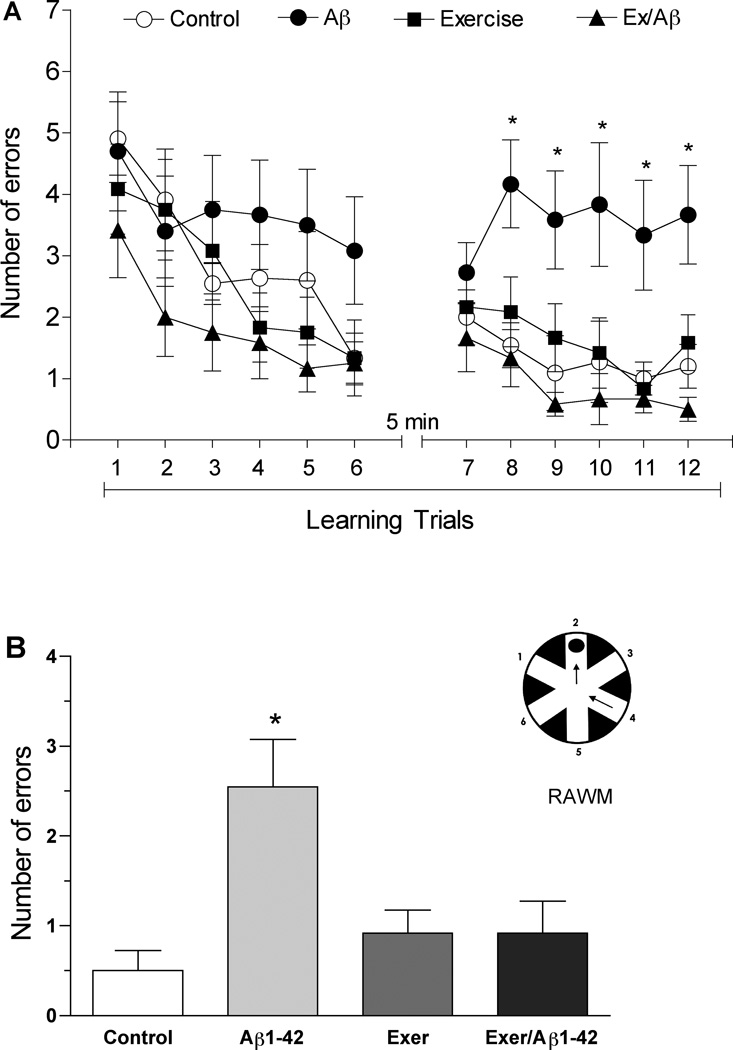
In the RAWM task, the number of errors made by rats in each learning trial or memory test was used as a quantitative measurement of learning and memory function. During the first six learning trials, rats in all groups made similar number of errors. However, during the second set of learning trials, the Aβ rats made significantly more errors than other groups (p= 0.01–0.05) (Figure 1A). In contrast, Ex/Aβ rats showed a learning ability similar to that of the control and exercised rats. These results suggest that amyloid infusion impairs the learning ability in rats whereas regular treadmill exercise prevents this impairment.
Figure 1.
Radial arm water maze (RAWM) performance in sedentary or exercised rats with and without Aβ42 infusion. The Aβ group revealed impairment in the learning trials (A) and short-term memory test (B). Rats in the Aβgroup made more errors compared to other groups during the last 5 learning trials. Ex/Aβ performed similarly as the control and exercised rats. Note that exercised rats showed no significant difference from controls. (*) denotes significant difference from all other groups (p<0.05, 10–12 rats/group).
In the STM test, administered 30 minutes after the last learning trial, the Aβ rats made significantly higher number of errors than all other groups (p= 0.01) (control: 0.5 ± 0.224, Ex: 0.917 ± 0.26, Aβ: 2.545 ± 0.529, Ex/Aβ: 0.917 ± 0.358) (Figure 1B). Four weeks of treadmill exercise reduced the number of errors that Aβ rats made, which was not different from those of control and exercise rats. It is noteworthy that our exercise regimen in normal rats did not enhance performance as both the sedentary control and exercised rats performed similarly in this behavioral paradigm. Hence, at the behavioral level, regular exercise prevented the learning and memory deficits in this AD model.
AD-induced suppression of E-LTP in area CA1 is prevented by regular exercise
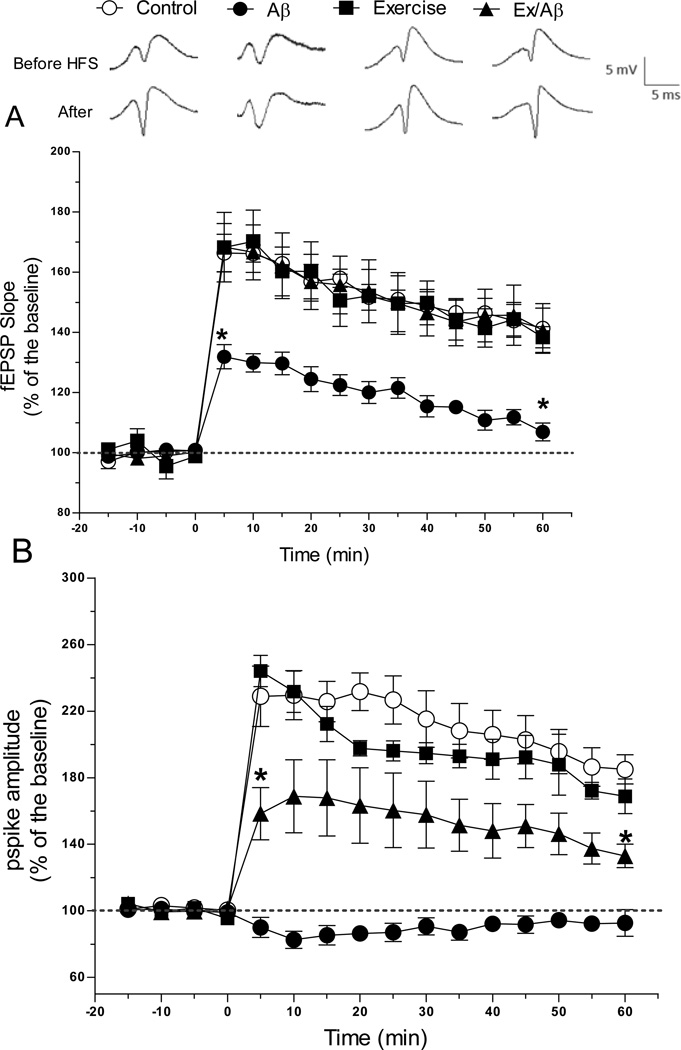
Long-term potentiation (LTP) measurement in our AD rat model was used to assess changes at the synapses. Compared to baseline, the fEPSP slope from the Aβ rat group was significantly lower than that of the other groups (p= 0.001–0.05) (control: 141.29% ± 8.24, Aβ: 106.93% ± 2.99, Ex: 138.36% ± 3.5, Ex/Aβ: 140.7% ± 7.36) but not significantly different from the baseline (Figure 2A). This result indicates impaired synaptic plasticity in this AD rat model. Interestingly, one hour after HFS, the fEPSP slope of Aβ rats was back to the baseline value. In contrast, the fEPSP slope of Ex/Aβ rats was similar to that of control rats throughout the recording period of one hour. Thus, treadmill exercise prevented AD-induced synaptic inhibition in Aβ rats. Additionally, 1 hour after HFS the pspike amplitude of Aβ rats (92.81% ± 7.99) was markedly lower than those of the other groups (p= 0.001–0.05) (control: 184.94% ± 8.79, Ex: 168.79% ± 10.35, Ex/Aβ: 132.98 ± 7.03) (Figure 2B). Together, these results indicate that 4 weeks of treadmill exercise prevents the AD-induced E-LTP suppression.
Figure 2.
Hippocampal early phase LTP (E-LTP) measured as increases in the slope of the fEPSP (A) and pspike amplitude (B) in area CA1 was evoked by HFS (applied at time zero) of the Schaffer collateral synapses in anesthetized rats. In rats with Aβ1–42 infusion (Aβ), E- LTP was significantly more impaired than other groups. Ex/Aβ rats exhibited a similar fEPSP slope compared to that of control and exercised rats. Additionally, the pspike amplitude of Ex/Aβ rats was markedly different from those of Aβ rats. Each point is the mean ± SEM of 5–6 rats. Points between the two asterisks (*) indicate significant difference from all groups.
Levesl of p-CaMKII during E-LTP
Calcium-calmodulin dependent protein kinase II (CaMKII) is considered to be an “inducible molecular switch” for the expression of LTP [27]. Various studies have demonstrated that CaMKII plays a critical role in the hippocampus dependent short-term memory and E-LTP [28–30]. Inhibition of CaMKII results in impaired spatial learning and memory accompanied by failed LTP induction [31–33]. In contrast, increased CaMKII activity leads to better hippocampus dependent memory performance, which correlates with an enhanced LTP [34–36].
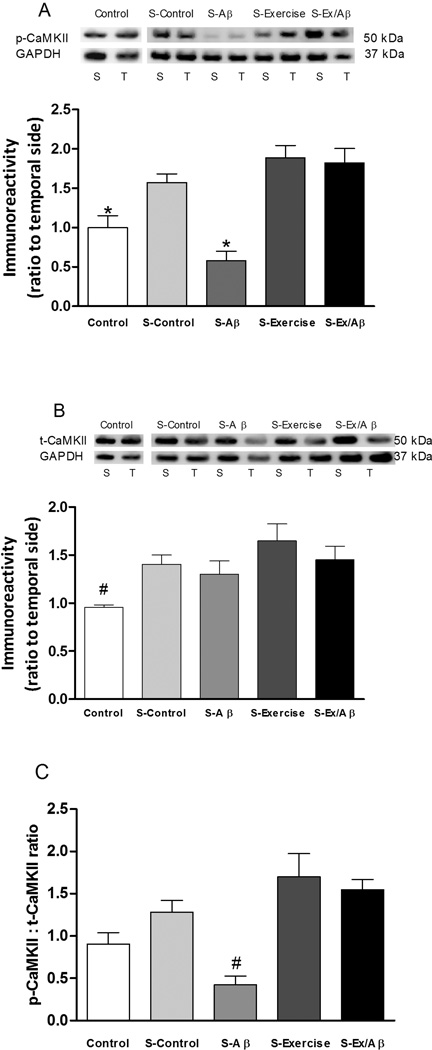
The levels of phosphorylated (p)-CaMKII in unstimulated control (1.0 ± 0.152) were statistically different compared to those of stimulated (S-) Control, S-Ex, and S-Ex/Aβ groups (S-Control: 1.575 ± 0.152, S-Ex: 1.889 ± 0.153, S-Ex/Aβ: 1.825 ± 0.18, p= 0.01–0.05) but were not significantly different from those of S-Aβ rats (0.578 ± 0.122) (Figure 3A). Total (t)-CaMKII levels were similar across all stimulated groups but markedly higher than those of unstimulated control (unstimulated control: 0.956 ± 0.028, S-Control: 1.404 ± 0.096, S-Aβ: 1.301 ± 0.138, S-Ex: 1.648 ± 0.175, S-Ex/Aβ: 1.453 ± 0.137, p= 0.05 (Figure 3B). This result indicates that CaMKII phosphorylation is inhibited in Aβ rats, which is prevented by treadmill exercise. Furthermore, this conclusion is supported by the finding that the ratio of p-CaMKII:t-CaMKII was markedly lower in S-Aβ rats compared to all other stimulated groups and even the unstimulated control (p=0.05–0.001, Figure 3C). These data suggest that although the total pool of CaMKII is unaltered, phosphorylation of this protein is negatively affected and that this effect is prevented by 4 weeks of treadmill exercise.
Figure 3.
Levels of phosphorylated (p)-CaMKII (A), total (t)-CaMKII (B), and the p-CaMKII:t-CaMKII ratio (C) in area CA1 after E-LTP induction. S: septal portion, T: temporal portion of CA1 area. Compared to the unstimulated control group, HFS increased the level of p-CaMKII in all groups except the Aβ rats and significantly increased the levels of t-CaMKII in all groups. Thus, after HFS, the pCaMKII : t-CaMKII ratio was significantly lowered in Aβ rats compared to all other groups including unstimulated control rats. Regular exercise prevented AD-induced reduction in p-CaMKII level. (*) indicates statistically different from stimulated (S)-Control, S-Exercise, and S-Ex/Aβ, p= 0.001–0.05. (#) indicates significantly different from all other groups, p = 0.01–0.05. Values are mean ± S.E.M., n = 4–6 rats/group. Insets are representative western blots.
Upregulation of calcineurin during E-LTP is inhibited by exercise
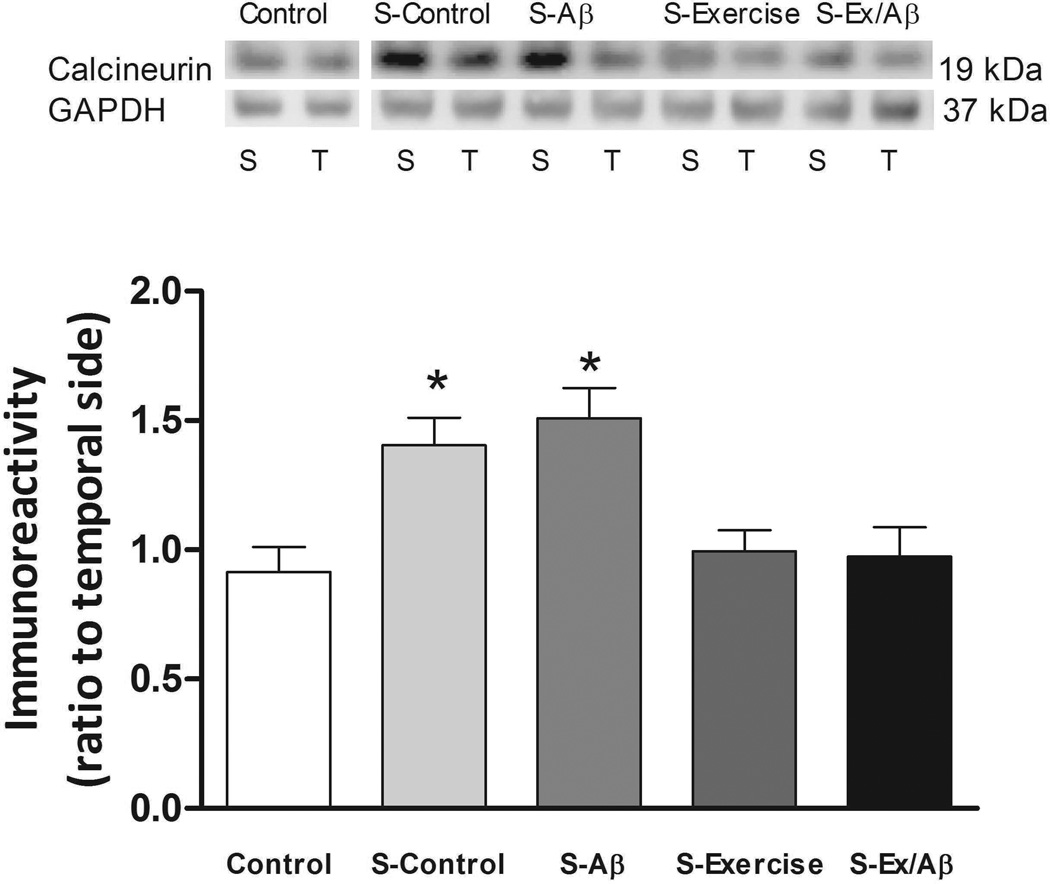
Calcineurin (PP2B) is a protein phosphatase that is important for dephosphorylation of CaMKII. One hour after HFS application, the level of calcineurin in stimulated (S)-Control (1.404 ± 0.107) and S-Aβ (1.51 ± 0.117) rats increased significantly compared to unstimulated control (0.913 ±0.098) (p= 0.05). In contrast, the levels of calcineurin after HFS in S-Ex (0.995 ± 0.08) and S-Ex/Aβ (0.974 ±0.113) rats were similar to those in unstimulated controls suggesting that exercise prevents upregulation of PP2B (Figure 4).
Figure 4.
Levels of calcineurin in area CA1 after E-LTP induction. S: septal portion, T: temporal portion of CA1 area. HFS application increased the expression of calcineurin in S-Control and S-Aβ rats which were significant higher than those of S-Exercise, S-Ex/Aβ, and unstimulated control rats. (*) indicates significantly different from unstimulated control, S-Exercise, and S-Ex/Aβ, p =0.05. Values are mean ± S.E.M., n = 4–6 rats/group. Insets are representative western blots.
Levels of BDNF in exercised rats during E-TLP
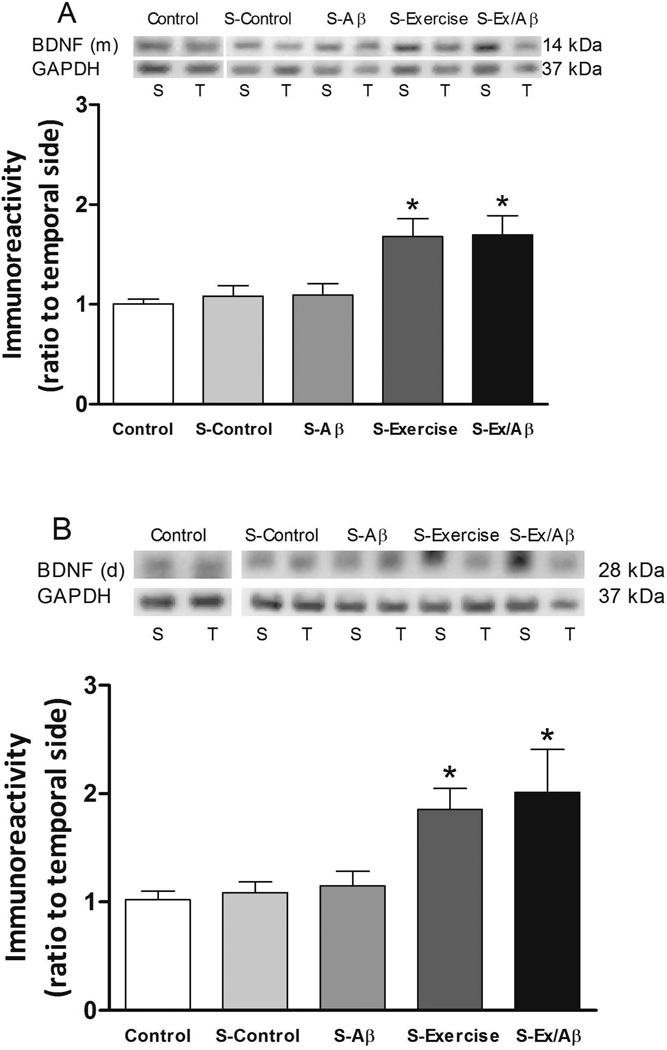
Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor critical for neurogenesis, cognition, and synaptic plasticity [37–41]. BDNF can exist in both monomeric and homodimeric forms in cells [42]. The present protocol of high frequency stimulation of the Schaffer collateral synapses did not appreciably increase the levels of BDNF in S-Control (monomer level: 1.084 ± 0.103, dimer level: 1.087 ± 0.098) and S-Aβ rats (monomer level: 1.093 ± 0.116, dimer level: 1.149 ± 0.134). However, treadmill exercise significantly increased both BDNF monomer (Figure 5A) and dimer protein (Figure 5B) levels in exercised animals including S-Ex (monomer level: 1.679 ± 0.179, dimer level: 1.854 ± 0.195) and S-Ex/Aβ rats (monomer level: 1.696 ± 0.193, dimer level: 2.012 ± 0.398) compared to unstimulated control rats (monomer level: 1.005 ± 0.049, dimer level: 1.149 ± 0.134) (p= 0.05–0.01). Thus, in our rat model of AD, treadmill exercise markedly up-regulated both forms of BDNF, even in the presence of Aβ (Figure 5).
Figure 5.
Monomeric (m) (A, 14 kDa) and dimeric (d) (B, 28KDa) BDNF in area CA1 after HFS induction. S: septal portion, T: temporal portion of CA1 area. HFS did not increase the level of BDNF monomers or dimers in S-Control and S-Aβ rats but significantly elevated those of S-Exercise and S-Ex/Aβ rats. (*) indicates significantly different from unstimulated control, S-Control, and S-Aβ, p = 0.01 – 0.05. Values are mean ± S.E.M., n = 4–6 rats/group. Insets are representative western blots.
Discussion
This study investigated the effect of 4 weeks of treadmill exercise on learning and memory and synaptic plasticity in a rat model of AD. Our findings consistently indicated a neuroprotective effect of exercise in this AD model. The Aβ rats exhibited severely impaired learning and short-term memory, suppressed E-LTP in area CA1, and curtailed CaMKII phosphorylation. In contrast, the moderate treadmill exercise regimen used in our AD-model proved to be beneficial in AD-like pathology by preventing learning and memory impairment and suppression of E-LTP of area CA1 pyramidal cells, which correlated with a reduction in calcineurin increase and exercise-induced elevation in BDNF and p-CaMKII levels.
Important cognitive impairment and neuropathological changes associated with AD have been recapitulated in different animal models ranging from the fruit fly to non-human primates. Among these, rodent transgenic and non-transgenic models have provided useful insights into the understanding of the pathogenesis as well as the therapeutic approaches for AD. In this study, we used a rat model of AD generated by i.c.v. infusion of Aβ1–42 peptides for 2 weeks as a model of sporadic AD that accounts for the majority of AD cases.
It is thought that cleavage of amyloid precursor protein (APP) by a family of secretases leads to the production of Aβ1–40, Aβ1–42, and other peptide fragments. Even though Aβ1–40 is considered to be the most abundant among APP cleavage products, Aβ1–42 is thought to trigger the amyloidogenic cascade that leads to AD [43]. Under physiological conditions, extracellular amyloid proteins can be taken up by microglia or degraded by neprilysin shortly after their production [44]. Failure of adequate Aβ clearance consequently favors amyloid accumulation and extracellular plaque deposition [45, 46]. The exact mechanism by which amyloid plaque formation leads to AD is unclear. However, it is known that amyloid aggregation lead to neurotoxicity often associated with oxidative stress, calcium dysregulation via amyloid channel formation and glutamate receptors, metabolic off-balance, and deleterious alterations in intracellular transduction pathways [47, 48]. In addition, Aβ aggregates facilitate hyperphosphorylation of tau via modulation of kinases that phosphorylate this protein [49, 50]34].
In agreement with previous findings, our data showed that in the RAWM, the performance of Aβ rats is severely impaired [1, 2]. During behavioral assessment, we also observed that the Aβ rats spent significantly more time in the central area of the pool and appeared disoriented. At the end of each learning trial, these rats simply sat on the platform without showing any interest in exploring the surroundings, which was not observed in rats of the other groups. In contrast, the performance of Ex/Aβ rats in the RAWM was similar to that of the control and exercise rats.
In experimental setting, LTP is believed to be the closest cellular correlate of learning and memory and thus used as a valuable tool for evaluating therapeutic treatments for disorders of the central nervous system (CNS). Amyloid oligomers can inhibit LTP both in vitro and in vivo in various hippocampal areas involved in the learning and memory processes [1, 2, 7, 51–54]. Previous findings from this lab have shown that amyloid infusion can impair synaptic plasticity both in the hippocampus and the sympathetic ganglia [1, 7, 55]. In the present study, E-LTP in area CA1 of Aβ rats was markedly suppressed as shown by impaired fEPSP slope and pspike amplitude. The mechanism of AD-induced LTP suppression remains unknown. However, it has been postulated that cellular ionic dysregulation may contribute to AD pathology by embedding amyloid oligomers into the membrane to form amyloid channels [48, 56, 57], which in turn directly or indirectly alter membrane ion channel expression and activity [58–62]. Regular exercise is known to produce a positive effect on cognition and synaptic plasticity. For example, treadmill exercise increases LTP expression measured as increases in fEPSP slope and pspike amplitude in the dentate gyrus (DG) both in vivo and in vitro [63, 64]. The ability of exercise to enhance synaptic plasticity is thought to be mediated by lowering LTP threshold for induction [64]. Additionally, treadmill exercise can also prevent learning and memory impairment in rats that are acutely sleep-deprived, diabetic, or alcohol intoxicated [20, 22, 65].
How does muscle movement initiate these protective effects in the CNS? This is an intriguing question that has, as yet, no definitive answer, even though evidence for “cross-talk” between the skeletal muscles and CNS exists. For example, as running velocity increases, the discharge frequency of hippocampal CA1 pyramidal cells and interneurons increases [66]. Studies proposed that muscle can secrete numerous humoral factors that exert a protective effect on the brain [43]. Even though the exact molecular mechanism responsible for the cross-talk between the skeletal muscles and CNS remains to be elucidated, experimental data support two possible mechanisms. First, events associated with energy balance play an important role in CNS function. For example, exercise up-regulates hippocampal expression of the mitochondrial molecule, uncoupling protein 2 (UCP2), which in turn protects neuronal mitochondria from oxidative stress, enhances ATP production, and regulates normal calcium level [67]. In addition, UCP2 can also modulate BDNF signaling and its downstream mediators such as CREB and CaMKII [68]. Second, interleukin-6 (IL-6), an immunomodulatory cytokine may be responsible for the crosstalk between the periphery and CNS. During exercise training, IL-6 increase is linked to reduced level of glycogen [69], which in turn can positively regulate glucose homeostasis in the brain.
It is well-documented that regular exercise can modulate the expression of several cognition-related molecules (e.g. BDNF, CaMKII) whose functions are severely affected by AD pathology. For example, during LTP induction the level of p-CaMKII is reduced due to AD-induced impairment of CaMKII phosphorylation [1, 55, 70]. In normal rats, HFS results in an increase in the levels of both phosphorylated and total CaMKII. Our data revealed that HFS failed to appreciably increase the level of p-CaMKII in Aβ rats and this change was prevented by exercise even though the total-CaMKII level is unchanged across all groups after HFS. This finding indicates that the disease mainly targets the phosphorylation process enabled by CaMKII, which is a critical step for LTP induction. In addition to LTP inhibition, amyloid exposure can result in up-regulation of phosphatases that regulate CaMKII activation [53, 62, 71, 72]. Calcineurin (PP2B) is considered a gating mechanism of LTP as it inactivates kinases. In normal rats, HFS is expected to increase the level of PP2B, which is thought to prevent LTP saturation. Our data showed that LTP induction significantly elevated the levels of PP2B in stimulated control and Aβ rats but did not affect those of exercised rats. Together, these findings demonstrate that exercise exerts a beneficial effect on memory and LTP possibly by restoring normal phosphatase-kinase balance.
It is not coincidental that the level of BDNF, a potent mediator of synaptic plasticity and memory, is highly elevated during exercise training [73–75]. In addition to its central production and release, BDNF can be produced from peripheral non-neuronal tissues and cross the blood brain barrier via a “high capacity, saturable transport system” [76, 77]. In brain tissues, BDNF exists in both monomeric and dimeric forms, which exert maximal effect on neuronal survival at saturated concentration as tested in dorsal root ganglion neurons [78]. BDNF can act pre- or post-synaptically to modulate its own signaling or other pathways including CaMKIV and CREB, that are important in the learning and memory process [79–81]. In addition to its neurotrophic effect, BDNF also possesses metabotrophic properties. BDNF up-regulates the expression of energy-related molecules including AMP-activated protein kinase (AMPK), ubiquitous mitochondrial creatine kinase (uMtCK) and UCP2 [82–84]. Thus, it is reasonable to suggest that depletion/disruption of BDNF expression/activity would significantly alter the action of these metabolic factors and eventually disrupt learning and memory function. The present findings show that the levels of monomeric and dimeric BDNF after HFS are significantly elevated in exercised rats compared to the sedentary group. The inability of HFS to increase BDNF level in S-Control rats suggests that, perhaps the stimulation protocol we used is not strong enough to cause a significant change in BDNF protein levels. However, HFS and exercise training seem to act in concert to increase the BDNF levels well beyond the normal levels in both normal-exercised and Aβ/exercised rats. This increase in BDNF may lead to improved performance in the RAWM and preservation of E-LTP in Ex/Aβ rats. In contrast, disruption of BDNF or its receptor (TrkB) impairs spatial memory and suppresses LTP expression [85, 86]. The fact that these cognitive deficits can be restored by exogenous administration of BDNF suggests that BDNF plays an integral role in synaptic plasticity and memory [87–89].
In summary, our behavioral, electrophysiological and molecular findings from rat model of AD-like pathology strongly support the proposition that regular physical exercise may be beneficial in alleviating/preventing neurodegenerative disorders including AD.
Acknowledgements
This study was supported by SGP grants from the University of Houston (KA) and the National Institutes of Health (1R15AG039008; JLE) and (NIH R15G103327, SS).
Footnotes
The authors disclose no conflict of biomedical or financial interest.
References
- 1.Srivareerat M, et al. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer's disease. Biol Psychiatry. 2009;65(11):918–926. doi: 10.1016/j.biopsych.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Srivareerat M, et al. Chronic nicotine restores normal Abeta levels and prevents short-term memory and E-LTP impairment in Abeta rat model of Alzheimer's disease. Neurobiol Aging. 2011;32(5):834–844. doi: 10.1016/j.neurobiolaging.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Xiong H, et al. Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer's disease. Neurosci Bull. 2011;27(4):221–232. doi: 10.1007/s12264-011-1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malm T, Koistinaho J, Kanninen K. Utilization of APPswe/PS1dE9 Transgenic Mice in Research of Alzheimer's Disease: Focus on Gene Therapy and Cell-Based Therapy Applications. Int J Alzheimers Dis. 2011;2011:517160. doi: 10.4061/2011/517160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkadhi KA, Srivareerat M, Tran TT. Intensification of long-term memory deficit by chronic stress and prevention by nicotine in a rat model of Alzheimer's disease. Mol Cell Neurosci. 2010;45(3):289–296. doi: 10.1016/j.mcn.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Walker JM, et al. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer's disease. Behav Brain Res. 2011;222(1):169–175. doi: 10.1016/j.bbr.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Alkadhi KA AK, Srivareerat M, Tran TT. Chronic psychosocial stress exacerbates impairment of synaptic plasticity in β-amyloid rat model of Alzheimer's disease: prevention by nicotine. Curr Alzheimer Res. 2011;8(7):718–731. doi: 10.2174/156720511797633188. [DOI] [PubMed] [Google Scholar]
- 8.Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57(2):113–119. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Kim SE KI, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45(5):357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2007;184(2):124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofi F, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 12.Grace L, et al. Effect of exercise on learning and memory in a rat model of developmental stress. Metab Brain Dis. 2009;24(4):643–657. doi: 10.1007/s11011-009-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khabour OF, et al. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus. 2010;20(5):637–645. doi: 10.1002/hipo.20657. [DOI] [PubMed] [Google Scholar]
- 14.Luo CX JJ, Zhou QG, Zhu XJ, Wang W, Zhang ZJ, Han X, Zhu DY. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85(8):1637–1646. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- 15.Radak Z, et al. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49(4):387–392. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Albeck DS, et al. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168(2):345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Alaei H, et al. Treadmill running reverses retention deficit induced by morphine. Eur J Pharmacol. 2006;536(1–2):138–141. doi: 10.1016/j.ejphar.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins ME, Bucci DJ. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behav Neurosci. 2010;124(6):868–872. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helfer JL, et al. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res. 2009;1294:1–11. doi: 10.1016/j.brainres.2009.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisi P, et al. Effects of treadmill running on spatial learning and memory in streptozotocin-induced diabetic rats. Neurosci Lett. 2009;455(2):79–83. doi: 10.1016/j.neulet.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Aguiar AS, Jr, et al. Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Res Bull. 2009;79(6):452–457. doi: 10.1016/j.brainresbull.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Zagaar M, et al. The beneficial effects of regular exercise on cognition in REM sleep deprivation: behavioral, electrophysiological and molecular evidence. Neurobiol Dis. 2012;45(3):1153–1162. doi: 10.1016/j.nbd.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Tran TT, Srivareerat M, Alkadhi KA. Chronic psychosocial stress triggers cognitive impairment in a novel at-risk model of Alzheimer's disease. Neurobiol Dis. 2010;37(3):756–763. doi: 10.1016/j.nbd.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Alzoubi KH, Aleisa AM, Alkadhi KA. Nicotine prevents disruption of the late phase LTP-related molecular cascade in adult-onset hypothyroidism. Hippocampus. 2007;17(8):654–664. doi: 10.1002/hipo.20306. [DOI] [PubMed] [Google Scholar]
- 25.Alzoubi KH, Alkadhi KA. A critical role of CREB in the impairment of late-phase LTP by adult onset hypothyroidism. Exp Neurol. 2007;203(1):63–71. doi: 10.1016/j.expneurol.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Papatheodoropoulos C, Kostopoulos G. Dorsal-ventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neurosci Lett. 2000;286(1):57–60. doi: 10.1016/s0304-3940(00)01084-3. [DOI] [PubMed] [Google Scholar]
- 27.Hedou G, Mansuy IM. Inducible molecular switches for the study of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):797–804. doi: 10.1098/rstb.2002.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukunaga K, Miyamoto E. A working model of CaM kinase II activity in hippocampal long-term potentiation and memory. Neurosci Res. 2000;38(1):3–17. doi: 10.1016/s0168-0102(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 29.Malenka RC, et al. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- 30.Aleisa AM, et al. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol Dis. 2006;22(3):453–462. doi: 10.1016/j.nbd.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Silva AJ, et al. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 32.Silva AJ, et al. Alpha calcium/calmodulin kinase II mutant mice: deficient long-term potentiation and impaired spatial learning. Cold Spring Harb Symp Quant Biol. 1992;57:527–539. doi: 10.1101/sqb.1992.057.01.058. [DOI] [PubMed] [Google Scholar]
- 33.Wang JH KP. The balance between postsynaptic Ca(2+)-dependent protein kinase and phosphatase activities controlling synaptic strength. Learn Mem. 1996;3(2–3):170–181. doi: 10.1101/lm.3.2-3.170. [DOI] [PubMed] [Google Scholar]
- 34.Moriguchi S SN, Han F, Yeh JZ, Narahashi T, Fukunaga K. Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus. 2009;19(9):845–854. doi: 10.1002/hipo.20572. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi E, Niimi K, Itakura C. Enhanced CaMKII activity and spatial cognitive function in SAMP6 mice. Behav Neurosci. 2009;123(3):527–532. doi: 10.1037/a0015119. [DOI] [PubMed] [Google Scholar]
- 36.Oomura Y, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27(11):2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24(7):1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 39.Tyler WJ, et al. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol. 2006;574(Pt 3):787–803. doi: 10.1113/jphysiol.2006.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267(5204):1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 41.Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol Paris. 1995;89(1):11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- 42.Shen J, Maruyama IN. Brain-derived neurotrophic factor receptor TrkB exists as a preformed dimer in living cells. J Mol Signal. 2012;7(1):2. doi: 10.1186/1750-2187-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 44.Gotz J, et al. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9(7):664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 45.Wetzel R. Kinetics and thermodynamics of amyloid fibril assembly. Acc Chem Res. 2006;39(9):671–679. doi: 10.1021/ar050069h. [DOI] [PubMed] [Google Scholar]
- 46.Zerovnik E. Amyloid-fibril formation. Proposed mechanisms and relevance to conformational disease. Eur J Biochem. 2002;269(14):3362–3371. doi: 10.1046/j.1432-1033.2002.03024.x. [DOI] [PubMed] [Google Scholar]
- 47.Di Carlo M. Beta amyloid peptide: from different aggregation forms to the activation of different biochemical pathways. Eur Biophys J. 2010;39(6):877–888. doi: 10.1007/s00249-009-0439-8. [DOI] [PubMed] [Google Scholar]
- 48.Capone R JH, Kotler SA, Connelly L, Teran Arce F, Ramachandran S, Kagan BL, Nussinov R, Lal R. All-d-Enantiomer of β-Amyloid Peptide Forms Ion Channels in Lipid Bilayers. J Chem Theory Comput. 2012;8(3):1143–1152. doi: 10.1021/ct200885r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3(5):437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- 50.Lloret A, et al. Amyloid-beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer's disease. J Alzheimers Dis. 2011;27(4):701–709. doi: 10.3233/JAD-2011-110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ondrejcak T WQ, Kew JN, Virley DJ, Upton N, Anwyl R, Rowan MJ. Activation of α7 nicotinic acetylcholine receptors persistently enhances hippocampal synaptic transmission and prevents Aβ-mediated inhibition of LTP in the rat hippocampus. Eur J Pharmacol. 2012;677(1–3):63–70. doi: 10.1016/j.ejphar.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Barry AE KI, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. Alzheimer's disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31(20):7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo J WD, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Aβ(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat Neurosci. 2011;14(5):545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 54.Ma T, et al. Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci. 2011;31(15):5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alzoubi KH, et al. Impaired neural transmission and synaptic plasticity in superior cervical ganglia from beta-amyloid rat model of Alzheimer's disease. Curr Alzheimer Res. 2011;8(4):377–384. doi: 10.2174/156720511795745311. [DOI] [PubMed] [Google Scholar]
- 56.Capone R JH, Kotler SA, Kagan BL, Nussinov R, Lal R. Probing structural features of Alzheimer's amyloid-β pores in bilayers using site-specific amino acid substitutions. Biochemistry. 2012;51(3):776–785. doi: 10.1021/bi2017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connelly L, et al. Atomic force microscopy and MD simulations reveal pore-like structures of all-D-enantiomer of Alzheimer's beta-amyloid peptide: relevance to the ion channel mechanism of AD pathology. J Phys Chem B. 2012;116(5):1728–1735. doi: 10.1021/jp2108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demuro A SM, Parker I. Single-channel Ca(2+) imaging implicates Aβ1-42 amyloid pores in Alzheimer's disease pathology. J Cell Biol. 2011;195(3):515–524. doi: 10.1083/jcb.201104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S RH. Effects of amyloid-β peptides on voltage-gated L-type Ca(V)1.2 and Ca(V)1.3 Ca(2+) channels. Mol Cells. 2011;32(3):289–294. doi: 10.1007/s10059-011-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mezler M BS, Schoemaker H, Gross G, Nimmrich V. A β-amyloid oligomer directly modulates P/Q-type calcium currents in Xenopus oocytes. Br J Pharmacol. 2012;165(5):1572–1583. doi: 10.1111/j.1476-5381.2011.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewachter I, et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging. 2009;30(2):241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Calon F, et al. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005;22(3):617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 63.O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176(2):362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Sim YJ, et al. Effect of postnatal treadmill exercise on c-Fos expression in the hippocampus of rat pups born from the alcohol-intoxicated mothers. Brain Dev. 2008;30(2):118–125. doi: 10.1016/j.braindev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Czurko A, et al. Sustained activation of hippocampal pyramidal cells by 'space clamping' in a running wheel. Eur J Neurosci. 1999;11(1):344–352. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- 67.Vaynman S, et al. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139(4):1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 68.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Steensberg A, et al. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537(Pt 2):633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao D, Watson JB, Xie CW. Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol. 2004;92(5):2853–2858. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- 71.Knobloch M, et al. Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci. 2007;27(29):7648–7653. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen QS, et al. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77(3):354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- 73.Castren E, et al. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4(7):895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Silhol M, et al. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience. 2007;146(3):962–973. doi: 10.1016/j.neuroscience.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Kesslak JP, et al. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112(4):1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- 76.Pan W, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 77.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61(5):533–541. [PubMed] [Google Scholar]
- 78.Kolbeck R, Jungbluth S, Barde YA. Characterisation of neurotrophin dimers and monomers. Eur J Biochem. 1994;225(3):995–1003. doi: 10.1111/j.1432-1033.1994.0995b.x. [DOI] [PubMed] [Google Scholar]
- 79.Spencer TK, Mellado W, Filbin MT. BDNF activates CaMKIV and PKA in parallel to block MAG-mediated inhibition of neurite outgrowth. Mol Cell Neurosci. 2008;38(1):110–116. doi: 10.1016/j.mcn.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams CM, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45(3):295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Cassilhas RC, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28(11):2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaldakov G. The metabotrophic NGF and BDNF: an emerging concept. Arch Ital Biol. 2011;149(2):257–263. doi: 10.4449/aib.v149i2.1366. [DOI] [PubMed] [Google Scholar]
- 84.Pedersen BK, et al. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94(12):1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- 85.Hennigan A, et al. Deficits in LTP and recognition memory in the genetically hypertensive rat are associated with decreased expression of neurotrophic factors and their receptors in the dentate gyrus. Behav Brain Res. 2009;197(2):371–377. doi: 10.1016/j.bbr.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J, Zhang F, Zhang Y. Corticosterone inhibits generation of long-term potentiation in rat hippocampal slice: involvement of brain-derived neurotrophic factor. Brain Res. 2000;885(2):182–191. doi: 10.1016/s0006-8993(00)02934-6. [DOI] [PubMed] [Google Scholar]
- 87.Kline DD, et al. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci. 2010;30(15):5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rex CS, et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96(2):677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaw KN, Commins S, O'Mara SM. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broad-spectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. Eur J Neurosci. 2003;17(11):2438–2446. doi: 10.1046/j.1460-9568.2003.02643.x. [DOI] [PubMed] [Google Scholar]