Abstract
There has been a conceptual shift in toxicological studies from describing what happens to explaining how the adverse outcome occurs, thereby enabling a deeper and improved understanding of how biomolecular and mechanistic profiling can inform hazard identification and improve risk assessment. Compared to traditional toxicology methods, which have a heavy reliance on animals, new approaches to generate toxicological data are becoming available for the safety assessment of chemicals, including high-throughput and high-content screening (HTS, HCS). With the emergence of nanotechnology, the exponential increase in the total number of engineered nanomaterials (ENMs) in research, development, and commercialization requires a robust scientific approach to screen ENM safety in humans and the environment rapidly and efficiently. Spurred by the developments in chemical testing, a promising new toxicological paradigm for ENMs is to use alternative test strategies (ATS), which reduce reliance on animal testing through the use of in vitro and in silico methods such as HTS, HCS, and computational modeling. Furthermore, this allows for the comparative analysis of large numbers of ENMs simultaneously and for hazard assessment at various stages of the product development process and overall life cycle. Using carbon nanotubes as a case study, a workshop bringing together national and international leaders from government, industry, and academia was convened at the University of California, Los Angeles to discuss the utility of ATS for decision-making analyses of ENMs. After lively discussions, a short list of generally shared viewpoints on this topic was generated, including a general view that ATS approaches for ENMs can significantly benefit chemical safety analysis.
New approaches and technologies for the evaluation of chemical safety are necessary to keep current with the ever-increasing pace of innovation. Currently, toxicologists are exploring the use of approaches that involve transitioning from animal models to newly developed high-throughput screening (HTS) analyses. This alteration would increase not only the number of chemicals that can be assessed simultaneously, but also speed data acquisition (testing, data generation), which can be used to explore the effectiveness of computational data analyses further. In addition, these new approaches hold the potential promise of greater accuracy in the prediction of human health effects versus currently performed descriptive animal studies. With the emergence of nanotechnology and the rapid increase in the number and diversity of novel engineered nanomaterials (ENMs) in research, development, and the commercial value chain, a robust new toxicological approach is required to reduce the reliance on primary animal testing. Such approaches should include the consideration of alternative test strategies (ATS) such as in vitro and in silico approaches that can be performed by high-content screening (HCS) and HTS methods.1 This strategy will enable comparative analyses of large numbers of existing and newly introduced ENMs. Such approaches could be implemented during product development to understand better the structural and functional determinants of toxicity that could be applied in lead-molecule selection to result in the development of safer and more sustainable products. These approaches could also be applied for the comparative assessment of nanomaterials that could be used to support materials grouping for purposes of establishing and supporting read-across for hazard characterization and risk assessment. Although ATS has the potential to eliminate animal testing in the distant future, focused animal testing, including for toxicity and bio-distribution (dosimetry) is currently still required to validate, to verify, or to bridge the in vitro testing and to develop exposure-dose-response extrapolation for hazard analysis.
The need to define and to assess groups of ENMs provides an opportunity to utilize ATS with the goal of evaluating mechanistic biological outcomes to assess material safety from the perspective of specific material physicochemical properties. Target endpoints should include susceptible homeostasis mechanisms and pathways of toxicity that are known to contribute to disease. A pathway of toxicity is a cellular response pathway that, when perturbed, would be expected to result in an adverse health effect or outcome.2 In the case of chemicals, “mechanism of action” (MOA), is frequently used as an alternative or parallel concept to “pathway of toxicity” (Figure 1). Mechanism of action embodies a wider concept, however, which includes pathways of toxicity but could also be a biomolecular event (such as binding to DNA or interfering with enzyme activity) leading to an adverse outcome without necessarily engaging a pathway. Collectively, the use of pathway of toxicity or MOA as the basis for performing toxicological analysis (including by HTS or HCS assays) is also known as a mechanistic toxicological approach, which includes the goal of establishing structure–activity relationships (SARs), which are useful for predicting the likelihood of adverse effects in animals and humans.3 If the comparative in vitro analysis (and accompanying SARs) anticipates adverse in vivo outcomes, especially when establishing a quantitative relationship between in vitro mechanisms and the pathogenesis of disease, then the approach has been called a predictive toxicological platform.3 Although the development of this approach is still evolving, the data and information generated by ATS could be used to prioritize ENMs to test and develop toxicological endpoints to measure, to expedite test planning, and to improve the ENM testing efficiency. Moreover, a predictive toxicological approach is useful for setting testing priorities for traditional toxicology approaches toward hazard assessment (e.g., inhalation studies) and for introducing mechanistic interpretations of in vivo toxicological data. This is analogous to the use of MOA categories for chemical testing.
FIGURE 1.
Key Definitions
Progress in the use of HTS or HCS for a number of ENM compositions (such as carbon nanotubes, CNTs, and metal oxides, MOx) has enabled materials grouping by pathways of toxicity, hazard potential, and/or SARs.4,5 Whereas SARs for ENMs are potentially more complex due to the contribution of physical and chemical characteristics to nanomaterial toxicity, the ATS approaches for ENMs have begun to evolve for a quantitative correlation of specific material properties to a mechanistic outcome. Based on the success of early attempts at materials grouping, this approach can be implemented to facilitate testing of large batches of previously unexplored materials. The data may then support grouping of specific materials such that follow-up in vivo testing may be done for select members of the group as opposed to testing of individual substances. The value of an established predictive toxicological paradigm is that the bulk of the discovery and hazard ranking can be carried out in vitro, and are therefore useful for planning and prioritizing more complex and costly in vivo testing.6 It is envisaged that the predictive, high-throughput, and computational tools for ENMs could enhance the decision-making process being developed for chemicals as envisioned in the National Research Council’s 21st-century approach to toxicology.2
A two-day roundtable workshop was convened in January 2013 at the California NanoSystems Institute of the University of California, Los Angeles (UCLA), hosted by the University of California Center for the Environmental Implications of Nanotechnology as well as the UCLA Center for Nanobiology and Predictive Toxicology. This meeting brought together national and international leaders from government, industry, and academia to discuss the utility of ATS for decision-making analysis of the safety of ENMs and chemicals. During this workshop, CNT safety assessment was used as a case study to illustrate how a predictive toxicological approach can be implemented for hazard ranking. While it was recognized that using ATS data for regulatory purposes will require wider acceptance and rigorous validation, there was lively and extensive discussion about the appropriate place and utility of ATS for safety assessment of ENMs. What follows are the highlights of the discussion, which led to the formulation of abbreviated consensus statements that the majority of participants could support regardless of the diversity of opinions (“consensus” here refers to a general sense of agreement, not necessarily unanimous approval). In order to facilitate the understanding of this discussion, Figure 1 lists a set of definitions, which, although not universally accepted, may help the reader to understand the ensuing discussion better.
Summary of the Moderated Discussions at the Workshop
Any national or international framework considering ATS needs to develop a transparent participatory process that broadly engages a multi-stakeholder community in order to be credible. A robust validation or evaluation process will be required to implement these strategies for regulatory decision-making. For this to happen, the traditional risk assessment framework will need to evolve to take ATS and predictive toxicology data into consideration, including adjusting conventional risk assessment to incorporate new scientific approaches in a weight of evidence analysis. The adoption and validation of new testing approaches has traditionally been a slow process because of the cautious and practical hurdles that emerge once we deviate from standard protocols and established case histories. Thus, the evaluation and/or validation process could be time-consuming, especially when deciding if ATS or new assays are ready for implementation. Nonetheless, given the recent and rapid emergence of the field and the numerous structural variations, nanotechnology environmental, health, and safety (nano-EHS) research provides an ideal opportunity to consider the use of ATS and other innovative scientific approaches for hazard ranking, material grouping, computational modeling, and adapting new risk assessment approaches that can also be useful for chemicals. Implementing ATS will require an iterative approach with transparency and continuous communication among stakeholders throughout the deliberative process. The use of ATS for regulatory decision-making also needs to consider the statutory authorities and legal requirements of the respective government agencies.
Grouping of ENMs according to the impact of physicochemical properties on early biomolecular and cellular events that are useful for hazard ranking, read-across, and establishing SAR methodologies can be used for qualitative hazard assessment, e.g., control banding. Moreover, participants generally agreed on the utility of tiered testing,7,8 which, along with the use of exposure-dose-response extrapolation, could lead to quantitative risk estimation.9 Such approaches can be integrated by comparing ENMs to benchmark materials, which serve as traditional examples of risk assessment.9 Quantitative doses in vitro could also be projected against real-life exposures.10 A later section will discuss in more detail the use of dose-response extrapolations, using the example of expressing ENM mass (or surface area) per unit of cellular surface in the tissue culture dish as a basis for comparison with the same dose metric in the animal and human lungs.
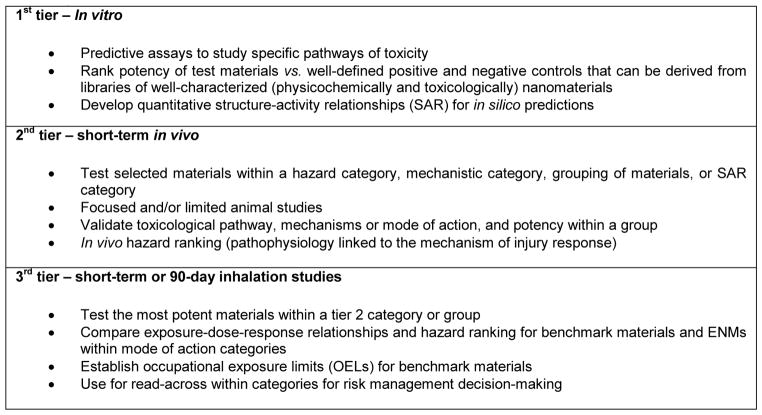
For inhalable ENMs, such as CNTs and MOx nanoparticles, it would be particularly helpful if predictive toxicological methods were implemented to reconcile exposure-dose-response extrapolation to lung burdens with repeat-dose in vivo inhalation studies in rodents. One proposal discussed in the workshop was for a tiered testing approach in which predictive toxicological modeling in in vitro cell models would be used to select (or to prioritize) materials for short-term inhalation,11 bolus instillation, or aspiration studies in rodents, which, in turn, would serve as the basis for setting 90-day inhalation study requirements and for evaluating and validating the initial hazard ranking and establishing exposure-dose-response extrapolations (Figure 2).7–9 Such an approach would enable stepwise investigation of a large number of materials, which could be compared, grouped, and prioritized when moving from the tissue culture dish to short-term in vivo assays and, ultimately, to the long-term inhalation exposures in vivo. A tiered strategy is also appropriate as an initial step in a mechanistic screening approach to assess how selective a chemical or an ENM may be in terms of the MOA or a pathway of toxicity. The development of material grouping and SAR analysis can also assist in early decision-making about identification of viable product candidates and the development of safety controls by industry as an integral component of new product development. In addition, it is important to consider that industry is a diverse sector, in which small companies can benefit from an ATS approach rather than having to begin with a costly inhalation study. This view, however, has to be balanced by the prudence expressed by some industry attendants, who cautioned that a change in regulatory protocols and procedures to accommodate ATS could be costly and time-consuming. Therefore, the strategy needs to be flexible to allow for innovative and diverse applications of such an approach to be explored.
FIGURE 2.
Tiered Approach Using Predictive Toxicological Modeling for Hazard Ranking and Risk Assessment7–9
The development of predictive toxicological approaches for ENMs, premised on mechanistic approaches, HTS, or HCS, presents an example of how ATS could assist or improve chemical toxicity screening. Pathways of toxicity or MOAs are likely to emerge from the linkage that is being developed between phenotypic signatures and HTS/HCS results by ToxCast™ and Tox-21, as well as using “omics” approaches and pharmaceutical HTS procedures for small molecules.1,7 Importantly, data were also provided to show that in the first phase of chemical toxicity screening (~300 substances, mostly pesticide active ingredients) by ToxCast™, a large percentage of these non-nano chemicals did not exhibit a clearly defined MOA. Within a relatively narrow concentration range, multiple molecular targets were activated or inhibited by some chemicals, suggesting that these chemicals may act through multiple and potentially non-specific MOAs. It was therefore not possible to develop a predictive model for phenotype changes using a variety of statistical model development methods. Instead, combinations of statistical and biological relevance for model building are needed. With these steps and sometimes also data from ToxCast™ Phase II screening, various predictive models for phenotypic changes12–14 and biological perturbations15,16 have been developed. In contrast to traditional chemicals, for ENMs studied in our laboratories to date, the emergence of predictive paradigms could consistently be traced to at least one pathway of toxicity that links cellular responses to testable in vivo outcomes.3,6,17–19 Studies undertaken at UCLA and in other laboratories demonstrate that the utility of pathways of toxicity, derived from the pathophysiology of disease or from omics approaches, can assist in the development of predictive toxicological approaches for CNTs and MOx nanoparticles.18–20 The HTS and HCS methodologies developed at UCLA have enabled the screening of 24 ENMs in one assay as well as the hazard ranking and grouping of ENMs and SAR development of several categories of materials.21 This, in turn, has allowed prioritization and focused use of animal studies.21
An ATS approach can provide sufficient data to reduce animal use by prioritizing testing at each of the incremental assessment stages described above. Using CNTs as a case study for discussion purposes, an example was presented of how a predictive toxicological approach can be used to screen multiple CNTs according to a pro-inflammatory response in cells, which provides hazard ranking to prioritize in vivo animal testing of inflammation-mediated endpoints and conditions.17–20 This predictive paradigm requires good material characterization but allows potential CNT hazard to be compared according to the contribution of wall number, hydrophobicity, state of dispersion, type of surface functionalization, surface coating, and amounts and types of impurities. Experimental studies have shown that CNT exposure by inhalation can lead to a variety of deleterious biological outcomes, including granulomatous inflammation in the lungs, pulmonary fibrosis, oxidative stress, coronary artery dysfunction, DNA fragmentation/mutation, and disruption of the mitotic spindle (leading to errors in chromosome number).17–20,22–30 Currently, the most pressing concern from an occupational perspective (e.g., manufacturing and processing plants) has been CNT’s potential to induce lung toxicity.31 Although not a single incident of lung injury has been reported in humans from exposure to CNTs, the possibility that CNTs could act as poorly soluble, high-surface-area particles or as “fiber-like substances” that induce chronic lung inflammation, fibrosis, and mesothelial injury has raised concerns that exposed workers may develop lung diseases. Consequently, the focus on occupational lung disease has dominated CNT safety efforts in humans, and 90-day inhalation studies in rodents have been considered the appropriate safety assessment standard during the notification of this new class of substances under the Toxic Substances Control Act (TSCA).32,33
Work undertaken at UCLA and other laboratories has demonstrated that triggering lysosomal damage, followed by the accompanying pro-inflammatory effects in macrophages and cooperative interaction with epithelial cells (that produce pro-fibrotic growth factors) consistently predict pro-inflammatory and pro-fibrotic outcomes in the murine lung.17–20,34–36 While much of this work has focused on multi-walled CNTs, it has also been demonstrated that cellular studies can predict the pulmonary fibrosis potential of specific CNT surface functionalization and surface coatings17,37 as well as titanium dioxide nanoparticles.38 It will now be interesting to see how these results compare to inhalation studies. The predictive approach for short-term oropharyngeal or intratracheal instillation studies can also be applied to future studies to compare the results of the predictive paradigm to data from three historical sub-chronic studies22–24 and one chronic (2 year) inhalation assessment (study ongoing, results unpublished). This could assist in validating the predictive paradigm. Importantly, data provided by the National Institute for Occupational Safety and Health (NIOSH) has shown that oropharyngeal instillation of CNTs yielded the same qualitative inflammatory and fibrotic lung responses in mice as seen in a short-term inhalation study of the same CNT material. The quantitative lung responses were approximately four times greater in the inhalation exposure at an estimated equivalent deposited mass lung dose of CNTs in mice.25,26 A possible explanation for the greater potency by mass of inhaled CNTs is that the inhaled material was more highly dispersed and consequently had a greater surface area available for biological interaction.27 These findings suggest that similar bolus doses would not over-predict the inhalation response.
Although the workshop focused on ATS for CNTs, these results could be generalized to other ENMs, where a predictive approach could be equally helpful for regulatory decision-making as well as for safer-by-design support. Data were also presented to show that it is possible to develop a predictive toxicological paradigm for MOx nanoparticles,21,39 which are used in cosmetics, sunscreens, catalysts, textiles, and solar batteries. Metal oxide testing in this platform was executed in an automated robotic facility, similar to the automated approaches used by ToxCast™ or in the pharmaceutical industry to screen potential drug candidates for toxicity.21 The MOx platform is premised on a homeostatic-regulated oxidative stress pathway in which cellular HCS could be used to predict the ability of MOx nanoparticles to generate oxidant injury that translates into the generation of acute inflammation in the rodent lungs.21 This example illustrates how careful selection of a set of cellular responses can help to predict in vivo outcomes if the pathway of toxicity is aligned with the pathogenesis of disease. Earlier studies showed good correlations between the oxidative stress response in vitro or in cell-free systems and the acute pulmonary inflammation in vivo, especially when the doses were normalized as the mass or surface area particle dose per surface area of cells both in vitro and in vivo,40–43 and the steepest parts of the dose-response curves were compared.41 The acute pulmonary inflammation observed in this experimental model is analogous to the acute neutrophilic inflammation that occurs in the lungs of workers exposed to certain airborne particles and fumes, for example, welding fumes that can cause a condition known as metal fume fever.44,45 Finally, the predictive platform for MOx-induced oxidative stress and inflammation could be shown to reflect the semiconductor properties and dissolution of specific materials, which could serve as the basis for in silico toxicological modeling in the future.
Workshop participants identified a number of challenges to the acceptance of ATS. For example, the application of predictive toxicological approaches for a chronic disease process is a challenge for cellular HTS because cultured cells currently do not have the capacity to express the chronology of a chronic disease process at the organ or systemic level.46 While this is an acknowledged shortcoming of in vitro tests, it was conveyed that the cellular pro-inflammatory and pro-fibrotic effects of some ENMs such as CNTs actually do reflect similar acute pathophysiologic effects in the lungs, except that at the organ level these responses can be cooperative and progressive, ultimately leading to a chronic pathology in the lungs.17–20 This is similar to the well known practice of using biomarkers of disease in clinical medicine to assess chronic disease processes in which the particular biomarkers reflect intermediary pathophysiological events that evolve over time to a chronic disease process. Nonetheless, the concern remains that for a number of chronic diseases, complex interactions between different target cell populations may evolve slowly over time, often after significant latent or subclinical periods, and that this sequence cannot be captured by an in vitro assay. Another concern was that it may be difficult to differentiate between end-points that reflect or lead to adverse outcomes and events that are non-adverse.47 An example includes a perturbation of homeostatic processes, which in some instances may trigger an adaptive response, while under other circumstances may lead to a toxicological outcome. Thus, not every biological perturbation necessarily reflects toxicity, and it is also important that MOAs may change in relation to the dose of the toxic substance.47 Participants also pointed out that current in vitro screening assays have not been proven to be useful in detecting chronic conditions such as immune-mediated diseases or in determining whether ENMs are involved in multistage carcinogenesis. It is important to identify how ATS assays may be used and what adverse health effects they can predict. For example, pulmonary inflammation may be a poor predictor of the chronic effects of CNT exposure because as the inflammation resolves, the fibrosis persists or progresses.25,27,41 This illustrates that the ATS assays must be validated for their utility in predicting specific adverse effects in vivo. An in vitro assay is promising for its ability to predict pulmonary fibrosis tests for lysosomal disruption and inflammasome activation in alveolar macrophages, as discussed above.17–20,34–38
We have briefly touched on the importance of in vitro to in vivo dose-response extrapolation as an important exercise to align ATS and predictive toxicological approaches with hazard assessment and a tiered approach to nanomaterial safety assessment.10 Often, orders of magnitude higher doses are administered in vitro and in vivo compared to real-life exposures, which results in physiological cellular defenses being overwhelmed; therefore, underlying mechanisms are different from those induced by more realistic exposures.47 This is especially true if the dose rate is extraordinarily high, for example, large mass dose bolus deliveries in vivo. One main difference between in vitro and in vivo exposures is that in vitro test systems typically do not contain mechanisms that are important for clearance of biopersistent ENMs and this limitation makes it difficult to mimic longer-term in vivo (inhalation) exposures. However, in vitro studies may be better equipped to predict adverse effects, e.g., to highly reactive substances, when the adverse effects occur within 24 h.
The availability of a predictive in vivo particle deposition model for rodents and humans (e.g., the multipath particle deposition model [MPPD])48 or the in vitro deposition model (in vitro sedimentation, diffusion, and dosimetry model [ISDD])49 could be used jointly for predictive toxicological approaches of respiratory tract effects. For example, using the results of a multi-dose ENM in vitro study looking at the induction of oxidative stress in rat alveolar epithelial cells could enable the use of the ISDD model as a first step in determining the amount of particles coming into contact with the cell.49 This information could then be used to derive the biggest response per unit of particle dose (number, surface area, mass) on the in vitro dose-response curve.41 This dose, expressed per cell surface area (e.g., cm2 of the ENM per cm2 of cell surface),40–43 can then be used as input for the in vivo MPPD model to estimate the inhaled concentration that is required to achieve a comparable in vivo dose per cm2 alveolar surface area (e.g., after a 6 or 8 h inhalation exposure in animals or humans).10 If longer term, repeat exposures are used for comparative analysis, ENM clearance and retention has to be taken into account. The in vivo response to an equivalent in vitro dose can then be used to compare the extent of agreement to validate the conceptual approach. Conversely, if the in vivo lung burden for a known occupational exposure is calculated, the equivalent in vitro cell dose can be obtained to perform an in vivo–in vitro comparison.10 However, the role of dose rate on the predictability of in vitro (or short-term in vivo) assays has yet to be evaluated.50
Consensus Statement
While diversity of opinions and perspectives were expressed at the workshop, there was general agreement about the utility of ATS approaches from the following perspectives:
The use of ATS to investigate ENM hazard and prioritize ENMs for additional toxicity testing, risk assessment and product development are generally accepted goals. However, the use of ATS in lieu of in vivo testing for regulatory risk assessment or management purposes is not yet at the level of general acceptance.
Any framework that includes ATS for regulatory purposes needs to be developed using a transparent, participatory process that engages a broad stakeholder community and should be premised on scientifically and legally robust validation processes. The use of ATS for regulatory decision-making requires further discussion about the state of the science and applicable regulatory frameworks. Further discussion is needed to identify the opportunities of how and where to introduce new scientific platforms for screening and hazard identification, which can then be used for further deliberation about regulatory decision-making.
The development of predictive toxicological approaches for ENMs, which are executed by expedited and validated HCS and HTS assays, present a good opportunity to inform ATS use for chemicals. Predictive toxicology approaches for CNT safety assessment is potentially helpful for hazard ranking; prioritizing animal experiments; and grouping of materials by hazard category, pathways of toxicity, and discoverable SARs and potency. This approach could also be useful for the screening of chemicals that have been shown to exhibit a specific MOA. The advances made for ENMs could assist the comprehensive Tox-21 initiative, which is collecting molecular and phenotypic signatures of toxicity that can be used to identify additional mechanistic pathways on which to base predictive toxicological testing. Predictive testing should ultimately be based on selective animal studies that implement realistic and natural scenarios of occupational and/or environmental exposure.
The use and understanding of pathways of toxicity, establishment of SARs, grouping of nanomaterials, and decision-making tools for ENM safety assessment, once validated, could assist regulatory decision-making; adaptation of current regulatory processes; and methods such as alternatives analysis, control banding, and occupational risk assessment procedures such as establishing initial recommended exposure limits. There is, however, a need to consider the respective statutory authorities and obligations of each government agency.
Alternative test strategies and the establishment of predictive toxicological paradigms for CNTs and MOx could be used to establish hazard categories and material grouping as a first tier of testing, which can be then used to prioritize nanomaterials for further, more costly animal studies. Prioritizing the material selections and forming material categories will reduce the number of animals required for toxicological testing. Short-term animal studies and in vivo hazard ranking at the second tier can be used to plan more expensive and longer term third tier inhalation studies for more quantitative or comprehensive risk assessments. This framework can assist in occupational and regulatory decision-making.
The development of hazard ranking, material grouping, and SARs can become integral parts of new product development and assist industry in developing safer ENMs.
It is important to consider dose-response extrapolation and exposure scenarios in linking mechanistic and predictive toxicological assessment in cells to dose metrics that can be used for understanding the correlation to in vivo exposure-dose-response relationships, including how those relate to real-life exposures. It is also important to consider the uptake, distribution, and clearance of ENMs and chemicals in understanding dose-response relationships.
What Does the Future Hold?
A critical question raised in the discussions was: how can the concepts included in the consensus statement be further developed to allow ATS to be more broadly considered for risk management and in policy frameworks? This consideration could begin by interpreting the definition of “risk assessment”. In its 2009 Science and Decisions report,51 the National Research Council noted that risk assessment is a broad concept embracing more than just traditional quantitative risk assessment methodologies. Thus, risk assessment can include more streamlined approaches such as control banding and safer-by-design procedures. Predictive in vitro assays and SARs could provide relevant information for the spectrum of evidence used in traditional risk assessment, particularly in scoping and problem definition. Further exploration is needed before ATS could become a cornerstone of quantitative risk assessment, particularly from the perspective of dose-response considerations in traditional risk assessment methods.
Alternative test strategies for nanomaterials present opportunities for more timely and transparent hazard communication. Although animal studies can provide a wealth of information, including molecular and phenotypic changes, MOAs, metabolism, and biodistribution, such studies can be resource (time, money, labor) intensive. For ethical and many other reasons, it is also not practical to test all chemicals to which human beings might be exposed in whole animal studies. In contrast, most ATS techniques generate quantitative data in a relatively short time, and these data, along with their analysis process (often done with computer programs), can be easily made publicly available via the Internet and other means. It is useful to have rapidly available initial data for hazard assessment in otherwise data-poor situations in such emergency situations as the Deepwater Horizon oil spill.52 Alternative test strategies can be mechanistically oriented and therefore provide a dimension of “how” that can supplement the description of a disease outcome. The use of ATS to provide hazard classification could therefore provide a more transparent and reproducible approach to risk assessment. It should be noted that expertise is required to analyze and to interpret ATS data, and expert judgment may be needed, as in animal studies. The communication challenge for ATS (particularly the in vitro cell-based or biochemical cell-free assay results, compared to whole animal study results) may be in making the results, interpretation, and uncertainties clear and easy to understand for the intended audience(s). Nevertheless, ATS, such as HTS and SARs, can provide important and timely information about hazard and categorization of materials based upon potency and severity,9,53 but ATS still has limitations with regard to quantitative risk characterization. In particular, validation of the HTS data and SAR-based models with animal or epidemiology data will increase their utility in hazard communication.
Although there are a variety of regulatory contexts in which ATS could potentially be applied, the occupational setting is quite important given the high potential for exposure. Because ATS can provide useful data to help fill knowledge gaps about the potential hazardous properties of ENMs, there is growing interest in the use of ATS to guide workplace exposure control decisions. Wherever ENMs are produced or used, be it in laboratories, pilot facilities, manufacturing, or application of products, NIOSH needs information to develop guidance for risk management recommendations on engineering controls and other workplace practices to minimize the risk of adverse health effects in workers. In the absence of sufficient health and adverse outcome data in humans, animal inhalation studies have typically been used to evaluate the exposure-dose-response relationship of an airborne hazardous substance to identify an exposure concentration that does not cause adverse effects or only a low level of effect.31,54 Alternative test strategies that rely on predictive in vitro assays and limited bolus exposure studies in animals (instead of inhalation exposure) could assist NIOSH, the Occupational Safety and Health Administration (OSHA), as well as occupational safety and health regulatory agencies outside the U.S. to develop occupational safety and health recommendations for ENMs. One example is the use of a tiered approach (Figure 2), wherein dose-response extrapolations in validated assays between the tissue culture dish and the lungs (rodents and humans) could be used to supplement the existing scientific literature of inhaled particles and fibers in order to develop occupational health and safety recommendations for airborne ENMs.28,43
In such an approach, hazard ranking information for ENMs (e.g., from tiers I and II in Figure 2), in conjunction with quantitative risk estimates for benchmark materials based on tier III data (new or existing), could be used to categorize ENMs into occupational exposure bands (OEBs) for linkage with control banding schemes.9 This approach would also be used to evaluate and to validate the applicability to ENMs of the existing control bands, which are order-of-magnitude exposures based on the performance of certain engineering controls (e.g., as used in the pharmaceutical industry).55–57 The ideal benchmark materials (see definition, Figure 1) would be well-characterized substances within given MOA categories, for which health hazards are well known and quantitative risk estimates could be or have been developed.9,58 Possible benchmark materials to evaluate inhalation hazards may include fine crystalline silica, asbestos, and ultrafine titanium dioxide and/or carbon black.8 Comparative potency analyses from validated ATS assays would be used to categorize the ENMs and to assign initial occupational exposure limits (OELs) or OEBs,9,58 e.g., using a parallelogram approach59 as used for pharmaceutical intermediates.60 These comparative analyses would examine the biological responses at estimated equivalent doses, for example, based on the particle mass (or surface area) dose per cell surface area in culture, which reflects the lung burden per alveolar epithelial surface area in animal studies and/or predicted worker airborne exposure.10,28,41,43 Among the most promising in vitro assays that have been developed are assessment of reactive oxygen species generation and the production of inflammatory mediators that predict acute pulmonary responses in rodents.21,41,43 The National Institute for Occupational Safety and Health could also use ATS to identify biomarkers from toxicological studies for use as markers of exposure and/or early biological effects and for subsequent use in monitoring of worker populations as a secondary health protection measure.61,62 Finally, the occupational hazards of ENMs could be mitigated by implementing safer-by-design principles for the physical and chemical properties of the materials produced, and ATS can play an important role in their development.
A key challenge to utilizing ATS data is the development and application of validation criteria, which include reliability, relevance, and reproducibility of an assay (Figure 1). The validation process would also typically include evaluation of variability within an assay and across laboratories for selected assays and reference particles.63 Given that some ATS assays are only performed in one laboratory (e.g., one-of-a-kind robot system, patented process), evaluation in multiple laboratories may not be possible. Instead, validation for ATS must be flexible in order to enable fit-for-purpose development of new methods, for example, as part of a tiered toxicology testing framework.7 Past validation efforts that involved the use of expensive and time-consuming cross-laboratory validation procedures may be too restrictive for HTS methods used in prioritization procedures (e.g., in tier 1 assessments).7,64 In vitro data used in tiered testing would enable the selection of priority materials (e.g., highest toxicity within a category) in the in vitro assays to go forward in focused or targeted animal studies (Figure 2).
The validation process should also be based on relevant information necessary to make informed decisions about the predictability of the assay within a material domain, and with respect to its dosimetric and mechanistic relevance. Selection of relevant dose metrics and dose levels in vitro and in vivo remains a challenge to such analyses, but biologically based and quantitative dose-response extrapolation approaches have been suggested.10,41,43,49,50 To facilitate the validation and use of ATS data in hazard ranking and risk assessments, standard sets of particle descriptors, dose metrics, and response parameters are needed to compare MOA and dose-response relationships within and across studies.9
Anticipating the emergence and potential impacts of ENMs and observing the interest in the stakeholder community to work collaboratively provides an unprecedented opportunity to incorporate recent advances into the decision-making framework for evaluating these materials’ environmental, health, and safety impacts throughout the product lifecycle.65 The U.S. Environmental Protection Agency (U.S. EPA) supports and is funding the development of systems to evaluate the environmental health and safety of ENMs. In light of recent advances in toxicology, biology, chemistry, and bioinformatics, the development of approaches that take advantage of these innovations are of significant interest to the U.S. EPA. In this context, ATS can be used as a means for establishing various categories of ENMs and evaluative tools to be used for risk assessment and green or benign design. More broadly, ATS could enhance and inform decision-making, help reduce regulatory uncertainty, and contribute to the knowledge base of regulatory processes.
Nanotechnologies still represent emerging technologies. There are two implications that follow from this. First, the relatively low penetration of the technology into the marketplace to date provides both industry and regulators with more degrees of freedom to introduce and to approve the use of ENMs in commerce than exist with a large number of entrenched chemicals and materials. These degrees of freedom can enable robust dialogues among stakeholders around which nanoscale properties provide beneficial performance characteristics, while minimizing potential adverse impacts to humans and the environment. Second, although the U.S. EPA has almost a decade of experience in evaluating new ENMs, the sample sizes of ENMs reviewed under the TSCA new chemicals program is still too small to adopt read-across and SAR approaches broadly for premanufacturing notification decisions on new ENMs. The U.S. EPA’s recent discussions with companies that have submitted or plan to submit TSCA premanufacture notices indicate an agreement on the importance of identifying inherent material properties. Those properties relate to the behavior of nanoscale particles in the environment, allowing the creation of more robust databases to support decision-making for safe development, manufacture, and use of ENMs. There is also agreement that an important contributor to this database will come from data generated by ATS, including in vitro and in silico approaches.
Carbon nanotubes, because of their potentially broad applications in products as well as the ability to produce them in many forms with different physicochemical and material properties, offer a promising starting point for building a robust regulatory science database that incorporates data generation by ATS. Complementing animal data with in vitro and in silico information will not only enable better-informed decisions on CNT safety, but can also bring new testing approaches into the broader chemical decision context.
The need for new testing approaches is particularly evident as the emergence of ENMs plays a central role in challenging historical risk assessment paradigms. Not only can nanotechnology be a driving force for new and better products, but it can also play a role in transitioning cutting-edge testing approaches from pharmaceutical development and academic sciences to private- and public-sector decision makers.
Footnotes
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the view or policies of the NIOSH, U.S. EPA, or California Department of Toxic Substances Control. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- 1.Available from: http://www.epa.gov/ncct/toxcast/.
- 2.Toxicity Testing in the 21st Century: A Vision and a Strategy. The National Academies Press; 2007. [Google Scholar]
- 3.Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, Zhang H. Nanomaterial Toxicity Testing in the 21st Century: Use of a Predictive Toxicological Approach and High-Throughput Screening. Accounts of Chemical Research. 2012;46:607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu R, Rallo R, George S, Ji Z, Nair S, Nel A, Cohen Y. Classification NanoSAR Development for Cytotoxicity of Metal Oxide Nanoparticles. Small. 2011;7:1118–1126. doi: 10.1002/smll.201002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu R, Rallo R, Weissleder R, Tassa C, Shaw S, Cohen Y. Nano-SAR Development for Bioactivity of Nanoparticles with Considerations of Decision Boundaries. Small. 2013;9:1842–1852. doi: 10.1002/smll.201201903. [DOI] [PubMed] [Google Scholar]
- 6.Meng H, Xia T, George S, Nel AE. A Predictive Toxicological Paradigm for the Safety Assessment of Nanomaterials. ACS Nano. 2009;3:1620–1627. doi: 10.1021/nn9005973. [DOI] [PubMed] [Google Scholar]
- 7.Cote I, Anastas PT, Birnbaum LS, Clark RM, Dix DJ, Edwards SW, Preuss PW. Advancing the Next Generation of Health Risk Assessment. Environ Health Perspect. 2012;120:1499–1502. doi: 10.1289/ehp.1104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, et al. Principles for Characterizing the Potential Human Health Effects from Exposure to Nanomaterials: Elements of a Screening Strategy. Part Fibre Toxicol. 2005;2:1–35. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuempel ED, Castranova V, Geraci CL, Schulte PA. Development of Risk-Based Nanomaterial Groups for Occupational Exposure Control. J Nanopart Res. 2012;14:1–15. doi: 10.1007/s11051-012-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangwal S, Brown JS, Wang A, Houck KA, Dix DJ, Kavlock RJ, Hubal EA. Informing Selection of Nanomaterial Concentrations for ToxCast In Vitro Testing Based on Occupational Exposure Potential. Environ Health Perspect. 2011;119:1539–1546. doi: 10.1289/ehp.1103750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein CL, Wiench K, Wiemann M, Ma-Hock L, van Ravenzwaay B, Landsiedel R. Hazard Identification of Inhaled Nanomaterials: Making Use of Short-Term Inhalation Studies. Arch Toxicol. 2012;86:1137–1151. doi: 10.1007/s00204-012-0834-2. [DOI] [PubMed] [Google Scholar]
- 12.Martin MT, Knudsen TB, Reif DM, Houck KA, Judson RS, Kavlock RJ, Dix DJ. Predictive Model of Rat Reproductive Toxicity from ToxCast High Throughput Screening. Biol Reprod. 2011;85:327–339. doi: 10.1095/biolreprod.111.090977. [DOI] [PubMed] [Google Scholar]
- 13.Sipes NS, Martin MT, Reif DM, Kleinstreuer NC, Judson RS, Singh AV, Chandler KJ, Dix DJ, Kavlock RJ, Knudsen TB. Predictive Models of Prenatal Developmental Toxicity from ToxCast High-Throughput Screening Data. Toxicol Sci. 2011;124:109–127. doi: 10.1093/toxsci/kfr220. [DOI] [PubMed] [Google Scholar]
- 14.Kleinstreuer NC, Dix DJ, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Paul KB, Reif DM, Crofton KM, Hamilton K, et al. In Vitro Perturbations of Targets in Cancer Hallmark Processes Predict Rodent Chemical Carcinogenesis. Toxicol Sci. 2013;131:40–55. doi: 10.1093/toxsci/kfs285. [DOI] [PubMed] [Google Scholar]
- 15.Rotroff DM, Dix DJ, Houck KA, Knudsen TB, Martin MT, McLaurin KW, Reif DM, Crofton KM, Singh AV, Xia M, et al. Using In Vitro High Throughput Screening Assays to Identify Potential Endocrine-Disrupting Chemicals. Environ Health Perspect. 2013;121:7–14. doi: 10.1289/ehp.1205065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinstreuer NC, Judson RS, Reif DM, Sipes NS, Singh AV, Chandler KJ, Dewoskin R, Dix DJ, Kavlock RJ, Knudsen TB. Environmental Impact on Vascular Development Predicted by High-Throughput Screening. Environ Health Perspect. 2011;119:1596–1603. doi: 10.1289/ehp.1103412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, Lin S, Meng H, Liao YP, Wang M, et al. Surface Charge and Cellular Processing of Covalently Functionalized Multiwall Carbon Nanotubes Determine Pulmonary Toxicity. ACS Nano. 2013;7:2352–2368. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Xia T, Ntim SA, Ji ZX, George S, Meng H, Zhang H, Castranova V, Mitra S, Nel AE. Quantitative Techniques for Assessing and Controlling the Dispersion and Biological Effects of Multiwalled Carbon Nanotubes in Mammalian Tissue Culture Cells. ACS Nano. 2010;4:7241–7252. doi: 10.1021/nn102112b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Xia T, Ntim SA, Ji Z, Lin S, Meng H, Chung C, George S, Zhang H, Wang M, et al. Dispersal State of Multi-Walled Carbon Nanotubes Elicits Pro-Fibrogenic Cellular Responses that Correlate with Fibrogenesis Biomarkers and Fibrosis in the Murine Lung. ACS Nano. 2011;5:9772–9787. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Xia T, Duch MC, Ji Z, Zhang H, Li R, Sun B, Lin S, Meng H, Liao YP, et al. Pluronic F108 Coating Decreases the Lung Fibrosis Potential of Multiwall Carbon Nanotubes by Reducing Lysosomal Injury. Nano Lett. 2012;12:3050–3061. doi: 10.1021/nl300895y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Ji Z, Xia T, Meng H, Low-Kam C, Liu R, Pokhrel S, Lin S, Wang X, Liao YP, et al. Use of Metal Oxide Nanoparticle Band Gap to Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. ACS Nano. 2012;6:4349–4368. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauluhn J. Subchronic 13-Week Inhalation Exposure of Rats to Multi-Walled Carbon Nanotubes: Toxic Effects are Determined by Density of Agglomerate Structures, not Fibrillar Structures. Toxicol, Sci. 2010;113:226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- 23.Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, Van Ravenzwaay B, Landsiedel R. Inhalation Toxicity of Multiwall Carbon Nanotubes in Rats Exposed for 3 Months. Toxicol Sci. 2009;112:468–481. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- 24.Delorme MP, Muro Y, Arai T, Banas DA, Frame SR, Reed KL, Warheit DB. Nine-Day Inhalation Toxicity Study with a Vapor Grown Carbon Nanofiber in Rats. Toxicol Sci. 2012;128:449–460. doi: 10.1093/toxsci/kfs172. [DOI] [PubMed] [Google Scholar]
- 25.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual Inflammatory and Fibrogenic Pulmonary Responses to Single-Walled Carbon Nanotubes in Mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 26.Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, et al. Inhalation vs. Aspiration of Single-Walled Carbon Nanotubes in C57BL/6 Mice: Inflammation, Fibrosis, Oxidative Stress, and Mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercer R, Scabilloni J, Wang L, Kisin E, Murray AD, Shvedova AA, Castranova V. Alteration of Deposition Pattern and Pulmonary Response as a Result of Improved Dispersion of Aspirated Single-Walled Carbon Nanotubes in a Mouse Model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Mercer RR, Rojanasakul Y, Qiu A, Lu Y, Scabilloni JF, Wu N, Castranova V. Direct Fibrogenic eEfects of Dispersed Single-Walled Carbon Nanotubes on Human Lung Fibroblasts. J Toxicol Environ Health A. 2010;73:410–422. doi: 10.1080/15287390903486550. [DOI] [PubMed] [Google Scholar]
- 29.Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, Lowry DT, Murray AR, Kisin ER, Friend S, et al. Induction of Aneuploidy by Single-Walled Carbon Nanotubes. Environ Mol Mutagen. 2009;50:708–717. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- 30.Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, Benkovic SA, Lowry DT, Murray AR, Kisin ER, et al. Single-Walled Carbon Nanotube-Induced Mitotic Disruption. Mutat Res. 2012;745:28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.C.f.D.C.a.P. Department of Health and Human Services, National Occupational Safety and Health, editor. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers. 2013. [Google Scholar]
- 32.Warshaw J. The Trend Towards Implementing the Precautionary Principle in U.S. Regulation of Nanomaterials. Dose-Response. 2012;10:384–396. doi: 10.2203/dose-response.10-030.Warshaw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. E.P.A AGENCY, editor. Consent Order and Determinations Supporting Consent Order. Aug 11, 2008. [Google Scholar]
- 34.Hamilton RF, Buford M, Xiang C, Wu N, Holian A. NLRP3 Inflammasome Activation in Murine Alveolar Macrophages and Related Lung Pathology is Associated with MWCNT Nickle Contamination. Inhal Toxicol. 2012;24:995–1008. doi: 10.3109/08958378.2012.745633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter DW, Wu N, Hubbs AF, Mercer RR, Funk K, Meng F, Li J, Wolfarth MG, Batteli L, Friend S, et al. Differential Mouse Pulmonary Dose and Time Course Responses to Titanium Dioxide Nanospheres and Nanobelts. Toxicol Sci. 2013;131:179–193. doi: 10.1093/toxsci/kfs261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sager TM, Wolfarth MW, Andrew M, Hubbs A, Friend S, Chen TH, Porter DW, Wu N, Yang F, Hamilton RF, et al. Effect of Multi-Walled Carbon Nanotube Surface Modification on Bioactivity in the C57BL/6 Mouse Model. Nanotoxicology. 2013 doi: 10.3109/17435390.2013.779757. http://dx.doi.org/10.3109/17435390.2013.779757. [DOI] [PMC free article] [PubMed]
- 37.Hamilton RF, Xiang C, Li M, Ka I, Yang F, Ma D, Porter DW, Wu N, Holian A. Purification and Sidewall Functionalization of Multiwalled Carbon Nanotubes and Resulting Bioactivity in Two Macrophage Models. Inhal Toxicol. 2013;25:199–210. doi: 10.3109/08958378.2013.775197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle Length-Dependent Titanium Dioxide Nanomaterials Toxicity and Bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landsiedel R, Ma-Hock L, Kroll A, Hahn D, Schnekenburger J, Wiench K, Wohlleben W. Testing Metal-Oxide Nanomaterials for Human Safety. Adv Mater. 2010;22:2601–2627. doi: 10.1002/adma.200902658. [DOI] [PubMed] [Google Scholar]
- 40.Duffin R, Tran L, Brown D, Stone V, Donaldson K. Proinflammogenic Effects of Low-Toxicity and Metal Nanoparticles In Vivo and In Vitro: Highlighting the Role of Particle Surface Area and Surface Reactivity. Inhal Toxicol. 2007;19:849–856. doi: 10.1080/08958370701479323. [DOI] [PubMed] [Google Scholar]
- 41.Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, et al. Concept of Assessing Nanoparticle Hazards Considering Nanoparticle Dosemetric and Chemical/Biological Response Metrics. J Toxicol Environ Health A. 2010;73:445–461. doi: 10.1080/15287390903489422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K. The Pro-Inflammatory Effects of Low-Toxicity Low Solubility Particles, Nanoparticles and Fine Particles, on Epithelial Cells In Vitro: The Role of Surface Area. Occup Environ Med. 2007;64:609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donaldson K, Borm PJ, Oberdorster G, Pinkerton KE, Stone V, Tran CL. Concordance Between In Vitro and In Vivo Dosimetry in the Proinflammatory Effects of Low-Toxicity, Low-Solubility Particles: The Key Role of the Proximal Alveolar Region. Inhal Toxicol. 2008;20:53–62. doi: 10.1080/08958370701758742. [DOI] [PubMed] [Google Scholar]
- 44.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh J, Zink J, Nel AE. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia T, Zhao Y, Sager T, George S, Pokhrel S, Li N, Schoenfeld D, Meng H, Lin S, Wang X, et al. Decreased Dissolution of ZnO by Iron Doping Yields Nanoparticles with Reduced Toxicity in the Rodent Lung and Zebrafish Embryos. ACS Nano. 2011;5:1223–1235. doi: 10.1021/nn1028482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leist M, Hartung T. Inflammatory Findings on Species Extrapolations: Humans are Definitely No 70-kg Mice. Arch Toxicol. 2013;87:563–567. doi: 10.1007/s00204-013-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slikker W, Andersen ME, Bogdanffy MS, Bus JS, Cohen SD, Conolly RB, David RM, Doerrer NG, Dorman DC, Gaylor DW, et al. Dose-Dependent Transitions in Mechanisms of Toxicity. Toxicol Appl Pharmacol. 2004;201:203–225. doi: 10.1016/j.taap.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 48.ARA. Multiple-Path Particle Deposition (MPPD 21): A Model for Human and Rat Airway Particle Dosimetry. Raleigh, N.C: Applied Research Associates, Inc; 2011. [Google Scholar]
- 49.Hinderliter PM, Minard KR, Orr G, Chrisler WB, Thrall BD, Pounds JG, Teeguarden JG. ISDD: A Computational Model of Particle Sedimentation, Diffusion and Target Cell Dosimetry for In Vitro Toxicity Studies. Part Fibre Toxicol. 2010;7:36. doi: 10.1186/1743-8977-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oberdorster G. Nanotoxicology: In Vitro–In Vivo Dosimetry. Environ Health Perspect. 2012;120:A13. doi: 10.1289/ehp.1104320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Science and Decisions: Advancing Risk Assessment. The National Academies Press; 2009. [PubMed] [Google Scholar]
- 52.Judson RS, Martin M, Reif D, Houck K, Knudsen T, Rotroff D, Xia M, Sakamuru S, Huang R, Shinn P, et al. Analysis of Eight Oil Spill Dispersants Using Rapid, In Vitro Tests for Endocrine and Other Biological Activity. Environ Sci Technol. 2010;44:5979–5985. doi: 10.1021/es102150z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quintero FA, Patel SJ, Munoz F, Mannan MS. Review of Existing QSAR/QSPR Models Developed for Properties Used in Hazardous Chemicals Classification System. Ind Eng Chem Res. 2012;51:16101–16115. [Google Scholar]
- 54.C.f.D.C.a.P. Department of Health and Human Services, National Occupational Safety and Health, editor. Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide. Cincinnati, Ohio: 2011. [Google Scholar]
- 55.Naumann BD, Sargent EV, Starkman BS, Fraser WJ, Becker GT, Kirk GD. Performance-Based Exposure Control Limits for Pharmaceutical Active Ingredients. Am Ind Hyg Assoc J. 1996;57:33–42. doi: 10.1080/15428119691015197. [DOI] [PubMed] [Google Scholar]
- 56.Ader AW, Farris JP, Ku RH. Occupational Health Categorization and Compound Handling Practice Systems—Roots, Application and Future. Chem Health Safety. 2005 Jul-Aug;:20–26. [Google Scholar]
- 57.Zalk DM, Nelson DI. History and Evolution of Control Banding: Review. J Occup Environ Hyg. 2008;5:330–346. doi: 10.1080/15459620801997916. [DOI] [PubMed] [Google Scholar]
- 58.Kuempel ED, Geraci CL, Schulte PA. Risk Assessment Approaches and Research Needs for Nanoparticles: An Examination of Data and Information from Current Studies. In: Simeonova PON, Luster M, editors. Nanotechnology: Toxicological Issues and Environmental Safety; Proceedings of the NATO Advanced Research Workshop on Nanotechnology: Toxicological Issues and Environmental Safety; Varna, Bulgaria. August 12–17, 2006; New York: Springer; 2007. pp. 119–145. [Google Scholar]
- 59.Sobels FH. Some Problems Associated with the Testing for Environmental Mutagens and a Perspective for Studies in “Comparative Mutagenesis”. Mutat Res/Envir Muta. 1977;46:245–260. doi: 10.1016/0165-1161(77)90001-2. [DOI] [PubMed] [Google Scholar]
- 60.Maier MS. Setting Occupational Exposure Limits for Unstudied Pharmaceutical Intermediates Using an In Vitro Parallelogram Approach. Toxicol Mech Methods. 2011;21:76–85. doi: 10.3109/15376511003638280. [DOI] [PubMed] [Google Scholar]
- 61.Erdely A, Liston A, Salmen-Muniz R, Hulderman T, Young SH, Zeidler-Erdely P, Castranova V, Simeonova P. Identification of Systemic Markers from a Pulmonary Carbon Nanotube Exposure. J Occup Environ Med. 2011;53:S80–86. doi: 10.1097/JOM.0b013e31821ad724. [DOI] [PubMed] [Google Scholar]
- 62.Schubauer-Berigan MK, Dahm MM, Yenchen MS. Engineered Carbonaceous Nanomaterials Manufacturers in the United States: Workforce Size, Characteristics, and Feasibility of Epidemiologic Studies. J Occup Environ Med. 2011;53:S62–67. doi: 10.1097/JOM.0b013e31821b1e2c. [DOI] [PubMed] [Google Scholar]
- 63.Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdorster G, Harkema JR, et al. Interlaboratory Evaluation of Rodent Pulmonary Responses to Engineered Nanomaterials: The NIEHS Nano Go Consortium. Environ Health Perspect. 2013;121:676–682. doi: 10.1289/ehp.1205693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Judson R, Kavlock R, Martin M, Reif D, Houck K, Knudsen T, Richard A, Tice RR, Whelan M, Xia M, et al. Perspectives on Validation of High-Throughput Assays Supporting 21st Century Toxicity Testing. Altex. 2013;30:51–56. doi: 10.14573/altex.2013.1.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials. The National Academies Press; 2012. [PubMed] [Google Scholar]
- 66.(ISO), I.O.f.S. ISO Guide 35:2006: Reference Materials-General and Statistical Principles for Certification. International Organization for Standardization; Geneva, Switzerland: 2006. [Google Scholar]
- 67.U.S. Environmental Protection Agency, editor. Guidelines for Carcinogen Risk Assessment. Washington, D.C: 2005. [Google Scholar]
- 68.Schulte PA, Murashov V, Zumwalde R, Kuempel ED, Geraci CL. Occupational Exposure Limits for Nanomaterials: State of the Art. J Nanopart Res. 2010;12:1971–1987. [Google Scholar]
- 69.C.o.C. Academies, editor. Expert Panel on the Integrated Testing of Pesticides, Integrating Emerging Technologies into Chemical Safety Assessment. Council of Canadian Academies; Ottawa, Canada: 2012. [Google Scholar]