Abstract
Angiotensin converting enzyme 2, (ACE2), is a key enzyme in the metabolism of angiotensin II. 1-[[2-(dimetilamino)ethyl]amino]-4-(hidroximetil)-7-[[(4-metilfenil)sulfonil]oxi]-9H-xantona-9 (XNT)and Diminazene (DIZE)have been reported to exert various organ-protective effects that have been attributed to activation of ACE2. To test the effect of these compounds we studied Ang II degradation in vivo and in vitro as well as their effect on ACE2 activity in vivo and in vitro. In a model of Ang II induced acute hypertension, blood pressure recovery was markedly enhanced by XNT (slope with XNT -3.26±0.2 vs.-1.6±0.2 mmHg/min without XNT, p<0.01). After Ang II infusion, neither plasma nor kidney ACE2 activity was affected by XNT. Plasma Ang II and Ang (1-7) levels also were not significantly affected by XNT. The blood pressure lowering effect of XNT seen in WT animals was also observed in ACE2 KO mice (slope with XNT -3.09±0.30 mmHg/min vs. -1.28±0.22 mmHg/min without XNT, p<0.001). These findings show that the blood pressure lowering effect of XNT in Ang II induced hypertension cannot be due to activation of ACE2. In vitro and ex vivo experiments in both mice and rat kidney confirmed a lack of enhancement of ACE2 enzymatic activity by XNT and DIZE. Moreover, Ang II degradation in vitro and ex vivo was unaffected by XNT and DIZE. We conclude that the biologic effects of these compounds are ACE2 independent and should not be attributed to activation of this enzyme.
Keywords: ACE2 activators, Renin-Angiotensin System, XNT, Diminazene, hypertension
Introduction
Since the discovery of Angiotensin converting enzyme 2 (ACE2) in 20001, 2 our understanding of the renin-angiotensin system (RAS) has been greatly expanded and an important role for the angiotensin-converting enzyme 2 – Ang (1-7) – Mas-receptor axis within the overall renin-angiotens in system is now generally accepted3-5. This system operates as an antagonist to the system's pressor branch (ACE-Ang II-AT2R) and appears to exert primarily organ-protective effects3-21. Thus, this axis is a promising target for new therapeutic strategies to treat hypertension5, 14, 17, 19 cardiovascular10, 15 and kidney disease11, 13, 18, 21. The monocarboxypeptidase ACE2 itself is a promising therapeutic target as it efficiently degrades angiotensin (Ang) II, a pressor peptide, to form the septapeptide Ang (1-7)2-4. The latter peptide has vasodilatory, antioxidative, antithrombotic and antifibrotic effects that have been reported to be due to activation of the Mas receptor3, 12, 16.
In the mouse, administration of human recombinant (r)ACE219 and murine recombinant ACE220 have been shown to effectively lower plasma Ang II with the attendant formation of Ang (1-7). The development of compounds that activate ACE2 could be useful for potential therapeutic use, particularly in the chronic setting, where the intravenous administration of recombinant ACE2 might not be practical. In 2008 the group led by Raizada22 introduced two compounds as ACE2 activators: 1-[[2-(dimetilamino)ethyl]amino]-4-(hidroximetil)-7-[[(4-metilfenil)sulfonil]oxi]-9H-xantona-9 (XNT) and resorcinolnaphthalein. These compounds were identified out of a library consisting of ≈ 140,000 compounds from the National Cancer Institute Developmental Therapeutics Program via structure based virtual screening using the DOCKv5.2 package22. XNT was prioritized over resorcinolnaphthalein for research use due to significantly favorable solubility properties22. Three years later Diminazene (DIZE)23, an agent already established in veterinary medicine, e.g. as a treatment option against trypanosomiasis24, was identified via virtual screening as an ACE2 activator. In subsequent years, XNT and DIZE have been used successfully to treat a vast array of conditions such as hypertension22, 25, pulmonary hypertension26-28, cardiac and renal fibrosis22 and glaucoma29 in experimental rat or mice models. The premise of the therapeutic benefit has been attributed to ACE2 activation and the conversion of Ang II to Ang (1-7)23, 24, 26-36. However, in none of these studies it was demonstrated that ACE2 activation had taken place by demonstrating the enhanced conversion of Ang II to Ang (1-7). Moreover, the effect on ACE2 activity was generally not reported in vivo22, 23, 25-35. We examined the effect of XNT on Ang II induced hypertension in an ACE2 KO line and found that this compound was effective in lowering blood pressure, thereby indicating that its action is ACE2-independent. We therefore designed further studies to comprehensively evaluate the effect of XNT and DIZE on ACE2 activity and angiotensin II in vitro, in vivo and ex vivo.
Methods
Animal models
Male wild-type (WT) and ACE2-deficient mice (ACE2KO; breeding pairs donated by Drs. S. Gurley and T. Coffman, Duke University, Durham, NC) on C57BL6 and FVB genetic background were used to identify the pattern of potential XNT and rACE2-induced changes in blood pressure, Ang peptides and enzyme activities.
In Vivo Studies
To study the acute effect of XNT on systolic blood pressure (SBP) and Ang II degradation, mice were anesthetized with an IP ketamine injection (200 mg/kg of body weight), as previously described. Thirty minutes before anesthesia, WT mice were pretreated with an IP injection of either sterile vehicle (VHC) or XNT (18 mg/kg). Immediately after inducing anesthesia, mice were placed on a heating platform for 10 minutes. Systolic blood pressure (SBP) was measured noninvasively every 30 seconds for a period of 25 minutes by determining the tail blood volume with a volume-pressure recording sensor and an occlusion tail-cuff using a computerized system (CODA System, Kent Scientific). This volume-pressure recording system has been validated and provides a high correlation with telemetry and direct arterial blood pressure measurements36. After 5 minutes of baseline SBP recording, acute hypertension in anesthetized mice was induced with an IP bolus of Ang II (0.2 mg/kg) and the SBP was monitored for the remaining 20 minutes. In additional experiments, Ang II (0.2 mg/kg) was infused together with an ACE2 inhibitor (MLN-4760, Millennium Pharmaceuticals; 1 mg/kg) after XNT was infused 30 minutes earlier, as described above. In separate experiments, ACE2KO mice, pretreated with vehicle or XNT, were injected with Ang II (0.2 mg/kg), the same way as described above for the wild-type mice.
ACE2 activity
Kidney and serum ACE2 activity were determined following incubation with the intramolecularly quenched synthetic ACE2-specific substrate Mca-APK-Dnp (Anaspec). The measurements were performed in black microtiter plates with a total volume of 100 ul as described previously in detail20, 37.
To study the effect of XNT and DIZE on enzyme activity of purified ACE2, these compounds were added in quadruplicate at 10ˆ-4 to 10ˆ-10 M (end concentrations) to the black microtiter plate wells containing mouse recombinant ACE220 (200ng/ml). Furthermore, XNT and DIZE were added in duplicate at the same concentrations to human recombinant ACE2 (100 ng/ml; R&D Systems). Kinetic curves were followed for a period of 1 hour. Quadruplicate wells containing assay buffer alone constituted a reference control.
Effect of XNT and DIZE on enzyme activity of recombinant Aminopeptidase A
The in vitro effect of XNT and DIZE on enzyme activity of human recombinant aminopeptidase A (APA) (R&D Systems) was studied using synthetic specific, substrate H-Glu-AMC, (Bachem Americas). Measurements were performed in black microtiter plates with a total volume of 100ul. XNT and DIZE were added in concentrations from 10ˆ-4 to 10ˆ-10 to buffer (50 mM/l, 150mM/l NaCl, 0.025mM ZnCl2, HEPES 0.5% Triton-X-100, pH 7.4) and the recombinant protein. Fluorescence was measured continuously for 1 h in 26 cycles using an FLX800 microplate fluorescence reader (BIOTEK Instruments Inc., Winooski, VT, USA) at 380 nm excitation and 460 nm emission wavelength for APA activity.
Measurements of Plasma Ang II and Ang-(1-7)
Please see Online Supplement.
Measurements of Ang II degradation in vitro
Ang II was incubated at 37°C with either 200ng/mL or 800ng/ml of recombinant ACE2 in the presence of XNT or DIZE for a period of 4 hours. Samples were collected at 0.5, 1, 2 and 4 hours and diluted in EDTA-containing II EIA Buffer (SPIBio, Cayman Chemical, Ann Arbor, MI) to stop the reaction. The samples were stored at −80°C. The quantity of Ang II in the samples were determined via enzyme immunoassay kit (SPIBio, Cayman Chemical, Ann Arbor, MI), as per the manufacturer's instructions.
Mass spectrometry
Please see Online Supplement
Results
Effect of XNT on hypertension acutely induced by Ang II infusion in WT mice
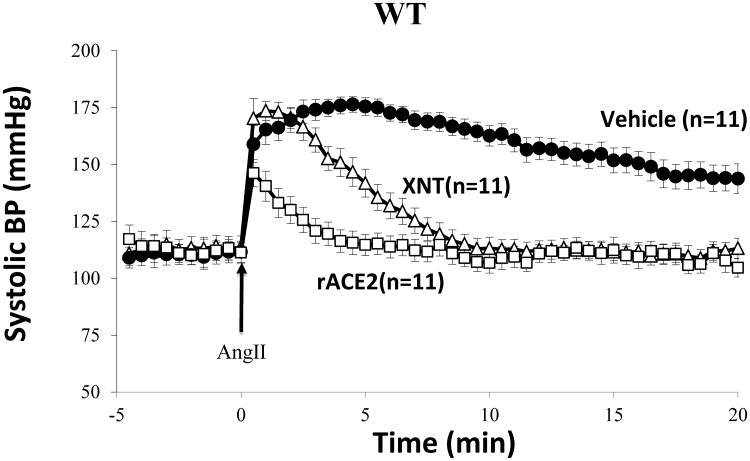
The effect of XNT on blood pressure was examined in studies in C57BL6 WT mice following a bolus of Ang II to induce acute hypertension. Blood pressure was monitored continuously for a period of at least 25 minutes every 30 seconds (figure 1). In wild-type mice that were pretreated with XNT 30 minutes before Ang II infusion (n=11), baseline SBP was not significantly different from mice pretreated with vehicle (n=11) (116±3.8 versus 111±4.4mmHg, respectively). Administration of a bolus of Ang II, to PBS-pretreated mice resulted in a rapid increase in SBP in both groups. The recovery in the XNT-group was markedly faster than in PBS-pretreated controls (slope -3.26±0.2 vs. -1.6±0.2 mmHg/min, p<0.01) (Figure 1). The difference in blood pressure between the 2 groups persisted throughout the continuous monitoring for 20 minutes (Figure 1). At 5 minutes the BP has nearly completely normalized in the XNT group whereas in the vehicle treated group it had only started to decline.
Figure 1.
Systolic blood pressure (SBP) was recorded at 30-second intervals 5 minutes before an IP bolus of Ang II (0.2 mg/kg; arrow; time point, 0 minutes) and 20 minutes thereafter under light anesthesia. WT mice received PBS (vehicle) or XNT (18 mg/kg) in a single IP injection 30 min prior to Ang II infusion. A group of mice received mouse recombinant ACE2 (1.0 mg/kg) via IP bolus 2 hours before blood pressure measurement to achieve a large increase in serum ACE2 activity.
Effect of XNT on serum and kidney ACE2 activity and Ang II and Ang (1-7) levels after Ang II infusion
In a separate group of experiments, a different set of WT mice were treated exactly the same way as described in figure 1 but with the purpose of euthanizing them at 5 minutes following the Ang II bolus. This time point was chosen because previous work by our lab revealed a marked fall in Ang II induced hypertension as a result of rapid degradation of this peptide.
Serum and kidney ACE2 activity in mice infused with XNT or control was measured 5 minutes following an Ang II bolus. At the time of sacrifice, animals had been exposed to XNT for a total of 35 minutes. Serum ACE2 activity is very low in mice, whereas in the kidney ACE2 activity is very high1, 11. As shown in figure 2 there were no significant differences in either kidney (upper panel) or serum (lower panel) ACE2 activity between XNT-treated and control animals. In fact, a slight decrease was noted after XNT infusion in both serum and kidney enzyme activity although this effect was not statistically significant.
Figure 2.
Kidney (upper panel) and serum ACE2 activity (lower panel) 5 minutes after administration of Ang II bolus to WT mice pretreated with either vehicle (VHC; n=7) or XNT (n=7) in a similar manner as described in Figure 1.
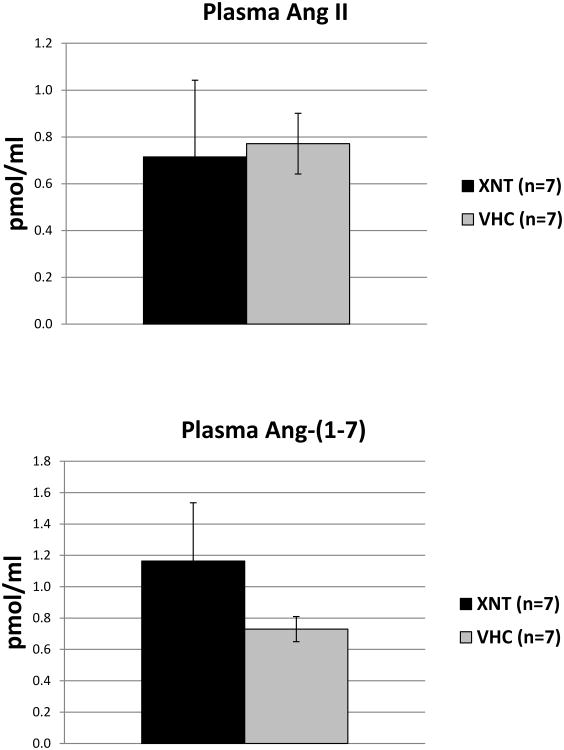
In the same mice the levels of plasma Ang II, measured five min after Ang II-infusion, were not significantly different between vehicle and XNT group (0.771±0.129 vs. 0.715±0.326 pmol/ml, respectively). Likewise, the plasma levels of Ang (1-7) were not significantly different between controls and XNT treated mice (0.729±0.080 vs. 1.163.1±0.371 pmol/ml, respectively) (figure 3).
Figure 3.
Plasma angiotensin (Ang) II (upper panel) and Ang (1-7) (lower panel) measured 5 minutes after IP bolus of Ang II in XNT or vehicle pre-treated WT mice (same as in Figure 2).
Effect of XNT+MLN4760 on hypertension acutely induced by Ang II infusion in WT mice
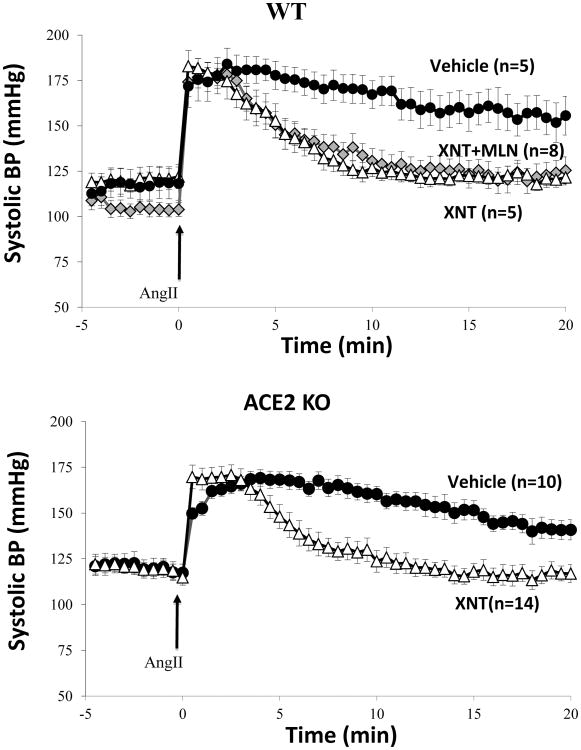
In a different set of experiments, C57BL6 wild-type mice received either vehicle (PBS) (n=5) or XNT (n=5) as in the experiments described in figure 1. Concurrently another group was given XNT+MLN-4760 (n=8) (Figure 4, upper panel), to establish whether the effect of XNT on blood pressure could be prevented by a specific ACE2 inhibitor, MLN-4760. The BP recovery was faster in XNT pretreated animals than in vehicle controls (slope -3.12±0.4 versus -1.47±0.5mmHg/min). In XNT+MLN-4760 treated mice the recovery was not significantly different from XNT treated animals (-2.88±0.4 versus -3.12±0.4mmHg/min, respectively). Both the XNT treated group and the XNT+MLN4760 treated group had a much faster recovery than controls (p<0.05), suggesting that XNT accelerates BP recovery by a mechanism that is ACE2-independent.
Figure 4.
Effect of ACE2 inhibition on XNT-mediated blood pressure recovery from Ang II-induced acute hypertension. WT mice received Vehicle (n=5) or XNT (18 mg/kg; n=5) in a single IP injection 30 min before anesthesia. A group of XNT infused mice received a specific ACE2 inhibitor, MLN-4760 (1.0 mg/kg), (XNT+MLN; n=8) (upper panel). SBP was recorded at 30-second intervals 5 minutes before an IP bolus of Ang II (0.2 mg/kg; arrow; time point, 0 minutes) (upper panel).
ACE2KO mice received Vehicle (n=10; filled circles) or XNT (n=14; open triangles) in a single IP injection 30 min before anesthesia (lower panel). SBP was recorded continuously before and after an IP bolus of Ang II (0.2 mg/kg; arrow; time point, 0 minutes).
Effect of XNT on hypertension acutely induced by Ang II infusion in ACE2KO mice
The studies in the WT mice using the ACE2 inhibitor MLN-4760 clearly suggested that the effect of XNT on blood pressure was ACE2 independent. To verify this finding, the effect of XNT on Ang II-induced hypertension was examined in ACE2-deficient mice (ACE2KO) using the same infusion protocol as in wild-type mice.
Baseline SBP was not different between XNT-(n=14) and vehicle-infused (n=11) animals (120.7±3.9 versus 122.2±4.8mmHg, respectively). The recovery in the XNT group was faster than in PBS pretreated controls (slope -3.09±0.30 mmHg/min in XNT group versus -1.28±0.22 mmHg/min in controls p<0.001) (figure 4, lower panel). Moreover, the difference in BP persisted between the 2 groups throughout the 20 minutes of continuous monitoring (figure 4, lower panel). SBP in the XNT infused mice almost normalized within 5 minutes after the Ang II bolus, while the BP of vehicle infused mice had only begun do decline. These findings were essentially the same as those seen in WT mice (compare figures 1 and 4, upper panel) and prompted us to conduct further experiments in vitro and ex vivo to examine ACE2 activity and Ang levels.
In vitro effect of XNT and DIZE on rACE2-mediated Ang II degradation
In order to investigate the effect of XNT and DIZE on Ang II dissipation in vitro, these compounds were added to mouse recombinant ACE2 in the presence of Ang II. Recombinant ACE2 was added at 800ng/ml and at a lower concentration (200ng/ml) to evaluate whether or not the effects of these compounds were dependent on ACE2 concentration. This is relevant because plasma ACE2 levels are relatively low1, 11. As depicted in figure 5, Ang II dissipation was rACE2 dose dependent. The rACE2-mediated Ang II hydrolysis was enhanced by neither XNT nor DIZE. Without recombinant ACE2, there was no detectable Ang II degradation either with or without XNT or DIZE, (data not shown).
Figure 5.
In vitro Ang II dissipation by recombinant murine ACE2 is not affected by the presence of XNT or DIZE. Both compounds were incubated with two different concentrations of recombinant ACE2 (200ng/ml and 800ng/ml) in the presence of its substrate Ang II for 4 hours. Samples were taken at 0, 0.5, 1, 2 and 4 hours and subsequently frozen at -80°C. Ang II levels were measured at each time point via EIA
In vitro Effect of XNT and DIZE on ACE2 activity
To evaluate the effect of XNT and DIZE on ACE2 activity, fluorescence formation from hydrolysis of the ACE2-specific fluorogenic substrate, Mca-APK-Dnp, was monitored continuously in the presence of mouse rACE2. Several concentrations of XNT (10ˆ-10 to 10ˆ5) had no significant effect on Mca-APK-Dnp-mediated fluorescence formation conferred by recombinant mouse ACE2 in a time dependent manner (figure 6, upper left panel). At very high concentrations (10ˆ-4) XNT reduced fluorescence and therefore ACE2 activity to 51.6% of control.
Figure 6.
The effect of XNT (upper panel) and DIZE (lower panel) at concentrations from 10-10 M to 10-4 M on enzymatic activity of mouse recombinant (r) ACE2. Each data point represents mean ± SE of quadruplicate wells (n=4). Wells containing rACE2 and assay buffer constituted a reference control.
DIZE, at the same concentrations as XNT, also had no significant effect on Mca-APK-Dnp hydrolysis by mouse rACE2 (figure 6, lower left panel). At the highest concentration (10ˆ-4) of DIZE the fluorescence formation was reduced by 74.5%.
To examine potential differences between human and mouse ACE2, the same experiments were performed with human recombinant ACE2. Addition of XNT had no significant effect on enzyme activity human rACE2 (figure 6, upper right panel). DIZE also had no stimulatory effect on fluorescence formation mediated by human rACE2 and, at the highest concentration, inhibited substrate fluorescence to 43.6% of control (figure 6, lower right panel).
Effect of XNT and DIZE on mouse and rat kidney ACE2 activity ex vivo
In kidney lysates from WT mice (n=4) and ACE2KO mice (n=4) ACE2 activity was measured for one hour when different concentrations of XNT and DIZE (10ˆ-4 to 10ˆ-10) were added ex vivo. MLN-4760, used as a specificity control, nearly completely inhibited ACE2 activity (figure7). XNT at various concentrations had no stimulatory effect on ACE2 substrate Mca-APK-Dnp-derived fluorescence formation which increased linearly over time as a result of exposure to ACE2 from WT kidneys. In fact, at the highest concentration XNT reduced the fluorescence by 27.1% (figure 7, upper left panel). DIZE at various concentrations also had no stimulatory effect on the Mca-APK-Dnp-mediated fluorescence formation and at the highest concentration had a marked inhibitory effect, reducing fluorescence by 76.8% (figure 7, lower left panel), which is consistent with the in vitro studies using recombinant ACE2.
Figure 7.
The effect of XNT (upper panels) and DIZE (lower panels), at concentrations 10-10 M to 10-4 M on enzymatic activity of endogenous ACE2 in kidney cortex lysates from WT mice (n=4; 1ug total protein/well) and rats (n=4; 10ug total protein/well). Each data point represents mean ± SE for kidney lysates from 4 mice and from 4 rats (n=4).
The same experiment was conducted in rat kidney lysates. XNT, at concentrations of 10ˆ-5 to 10ˆ-10, did not alter fluorescence formation significantly. At the highest concentration (10ˆ-4) XNT decreased fluorescence by 31.2% (figure 7, upper right panel). Addition of the highest concentration of DIZE caused an even stronger decline (figure 7, lower right panel), reducing fluorescence by 41.8%, while lower concentrations (10ˆ-5 to 10ˆ-10) had no significant effect.
Angiotensin peptides ex vivo after addition of XNT and recombinant murine ACE2
To further verify our findings in vivo and in vitro we used a recently described ex vivo assay that permits highly sensitive measurement of multiple angiotensin peptides concurrently in response to enzymes that affect Ang II metabolism and their inhibitors20,38.
Angiotensin peptide analysis revealed no shift in distribution of angiotensin-peptides between samples from XNT- and vehicle (VHC)-group (figure 8). By contrast, rACE2, used as a positive control, induced the expected shift towards the Ang (1-7) formation and subsequent accumulation of its metabolite Ang (1-5) (figure 8).
Figure 8.
Plasma Ang peptides measured by LC-MS/MS in a plasma ex vivo assay, after recombinant renin was added to isolated blood plasma (XNT (black bars) PBS (white bars) and recombinant ACE2 (grey bars)) on the levels of. After 10 minutes of incubation at 37°C, in the presence of XNT, PBS or rACE2, protease inhibitor solution was added to stop the reaction. Recombinant ACE2 but not XNT had a marked effect on Ang II, Ang (1-7) and Ang (1-5).
In vitro effect of XNT and DIZE on enzymatic activity of Aminopeptidase A
A potential effect of XNT on Aminopeptidase A, an enzyme capable of degrading Ang II to Ang (2-8) by cleaving the N-terminal amino acid aspartate, was also investigated. Various concentrations of XNT had no stimulatory effect on APA substrate H-Glu-AMC-derived fluorescence generation which increased linearly over time as a result of exposure to recombinant APA. In fact, at the highest concentrations XNT reduced the fluorescence formation by 43.6% (figure 9, upper panel). DIZE at various concentrations also had no effect on the fluorescence of H-Glu-AMC substrate and at high concentrations had a marked inhibitory effect, reducing fluorescence by 71.5% (figure 9, lower panel).
Figure 9.
The effect of XNT (upper panel) and DIZE (lower panel) at concentrations from 10-10 M to 10-4 M on enzymatic activity of recombinant (r) Aminopeptidase A (APA). Each data point represents mean ± SE of quadruplicate wells (n=4). Wells containing rAPA and assay buffer constituted a reference control.
Discussion
Amplification of ACE2 enzymatic activity has substantial therapeutic potential, as this enzyme effectively degrades Ang II to Ang (1-7) and therefore could provide another approach to modify the RAS in addition to well established drugs such as ACE-inhibitors and AT-1R antagonists6, 11, 20, 39. Promising advances in this field were made after XNT and DIZE were identified via structure-based virtual screening as potential ACE2 activators22, 23. In subsequent studies it was not directly examined whether the observed biologic actions of these compounds were associated with an increase in ACE2 activity and an enhanced conversion of Ang II to Ang (1-7)26-35, 40. Rather it was assumed that the presumed mechanism of action was due to activation of ACE2 based on the original reports by Hernandez Prada et al22 and Kulemina et al23.
In this present study, we investigated whether or not XNT and DIZE are effective activators of ACE2. Moreover, we used a model of acute Ang II-induced hypertension as a paradigm to investigate the previously reported effect of XNT on blood pressure22. In these acute studies, XNT markedly accelerated the recovery from Ang II-induced hypertension in a manner that resembled the effect of recombinant ACE2 but to a lesser extent (figure 1). The dose of XNT (18mg/kg) was in the same range as the dose that has proven to be the most potent in previous studies (10mg/kg)22. Plasma ACE2 activity after Ang II infusion, however, remained low in both XNT- and vehicle infused mice. Moreover, following the infusion of Ang II the plasma levels of Ang II, the substrate of ACE2 and those of Ang (1-7), the peptide generated by the cleavage of Ang II by ACE2, were not affected in the presence of XNT. These findings altogether suggested that the effect of XNT on Ang II induced hypertension could not be due to activation of ACE2. This possibility was substantiated in experiments in the ACE2 KO where XNT also elicited enhanced recovery from Ang II induced hypertension in a manner similar to the recovery in WT mice (compare fig. 1 and 4A). The blood pressure lowering effect of XNT in the ACE2KO therefore provides irrefutable evidence that this action of XNT cannot be due to ACE2 activation. It was recently reported, however, that XNT and DIZE may cause a significant increase in ACE2 mRNA30, 35. This would suggest that these compounds upregulate the ACE2 gene expression which could contribute to their mechanism of action. We think that such an effect would not be able to explain the rapid effect of XNT on blood pressure that we found in wild type mice after Ang II infusion. Moreover, an increase in mRNA for ACE2 cannot happen in the ACE2KO. Since XNT markedly reduced Ang II dependent hypertension in this model, we conclude that this action of XNT is ACE2 independent. Recent reports have suggested that XNT induces vascular relaxation via an Ang (1-7) independent mechanism as administration of a Mas receptor antagonist did not alter the vasorelaxant responses to XNT32.
We also consider the possibility that XNT could trigger Ang II dissipation by a mechanism that could be ACE2 independent. For this purpose mass spectrometry was used to see if XNT had an effect on Ang II dissipation in an ex vivo plasma model20. Murine rACE2, used as a positive control, nearly completely diminished Ang II and increased Ang (1-7) and subsequently Ang (1-5). XNT on the other hand had no effect whatsoever as Ang II, Ang (1-7) and Ang (1-5) levels were not altered as compared to control levels. We confirmed these findings through in vitro experiments, where incubation of Ang II in the presence of recombinant ACE2 did not result in accelerated Ang II dissipation by either XNT or DIZE.
Additionally we evaluated whether addition of different concentrations (10ˆ-4 to 10ˆ-10) of XNT or DIZE ex vivo can alter ACE2 activity in the kidney, an organ with very high levels of ACE2 expression and activity21. To address potential species differences in Ang II metabolism we conducted these ex vivo experiments in both mouse and rat kidneys. Both molecules had no activating impact on endogenous mouse and rat ACE2 enzymatic activity at any concentration applied (figure 7). In fact ACE2 enzyme activity was inhibited by both compounds at the highest concentration. The inhibitory effect was more prominent after DIZE addition. We also examined in vitro the effect of these compounds on Aminopeptidase A in order to rule out that XNT triggers alternative pathways of Ang II dissipation. Neither XNT nor DIZE were capable of enhancing Aminopetidase A activity. Instead at the highest concentration (10ˆ-4) of XNT and DIZE Aminopeptidase A activity was inhibited substantially. This inhibitory effect of both XNT and DIZE at very high concentrations likely reflects a non-specific effect and does not seem to be relevant to the in vivo situation, where such high concentrations would never be achieved.
In vitro experiments to evaluate whether or not XNT or DIZE can alter enzyme activity of highly purified mouse and human recombinant ACE2 revealed no stimulatory effect. In fact, at a very high concentration both XNT and DIZE inhibited ACE2 activity which is consistent with our ex vivo studies on kidney ACE2 activity (figure 7). These findings are in sharp contrast with an earlier study showing about a two fold increase in ACE2 activity by XNT using a similar in vitro approach22. We cannot find an explanation for the discrepancy between our in vitro studies and the report by Hernandez Prada et al22. In both studies human rACE2 was used to examine the effect of XNT in vitro. Overall our data shows that there is no stimulatory effect of XNT on ACE2 activity ex vivo in plasma or kidney tissue. In vivo XNT also has no effect on plasma or kidney ACE2 activity. Moreover, the lack of a significant difference in Ang II and Ang (1-7) plasma levels after XNT further negates any significant stimulatory effect of this compound on ACE2 activity. Finally, there was no effect of XNT on Ang II peptides measured using a very sensitive ex vivo plasma system which is in sharp contrast to the profound effect of murine ACE2, which rapidly dissipated Ang II through formation of Ang (1-7) and subsequent degradation to Ang (1-5) (figure 8).
In conclusion, the present studies show a complete lack of stimulatory effect of XNT and DIZE on ACE2 activity. The reported biologic effects of these agents22, 23, 25-33 are therefore the result of mechanisms of action that remain to be elucidated and should not be attributed to ACE2 activation. ACE2 remains a promising therapeutic target and it is of the utmost importance to continue the search for compounds that activate this enzyme.
Perspectives
ACE2 activators could provide a promising treatment option for a vast array of pathological conditions such as hypertension, pulmonary hypertension, cardiovascular and diabetic kidney disease. To date only two compounds, XNT and DIZE, have been used as ACE2 activators with remarkable positive 22, 26, 29-31, 33. The present study shows that XNT is very effective in the treatment of acute Ang II induced hypertension in WT mice, even when ACE2 is inhibited pharmacologically, and in mice with genetic ablation of ACE2. The latter finding demonstrates that the mechanism of action of XNT is ACE2 independent. Our findings further show that XNT and DIZE are not activators of ACE2 enzymatic activity ex vivo and in vitro. Given the importance of ACE2 as a therapeutic target, renewed efforts should be made to search for compounds capable of amplifying ACE2 activity, particularly at the tissue level where this enzyme is present in substantial amounts. Plasma levels of this enzyme are low and therapeutic approaches may be much more effective by the direct administration of recombinant ACE2.
Supplementary Material
Novelty and Significance.
What is new
This study reports that the compounds used as ACE2 activators, XNT and DIZE, lack a stimulatory effect on ACE2 activity ex vivo, in vivo and in vitro.
It was shown for the first time that XNT exerts its hypotensive effects via an ACE2- and Ang II independent pathway.
What is relevant
Given the potential importance of ACE2 as a therapeutic target, there is a need to identify compounds that are capable of activating ACE2.
Summary
Reported ACE2 activator XNT greatly attenuated Ang II- induced acute hypertension not only in wilt type mice but also in an ACE2KO line, demonstrating an ACE2 independent mechanism of action. In further ex vivo and in vitro studies neither XNT nor DIZE were capable of increasing ACE2 enzymatic activity. Both compounds had no effect on Ang II dissipation or Ang (1-7) formation.
Acknowledgments
We thank Dr. Mohan K. Raizada for his generosity in supplying us with both XNT and DIZE.
Sources of Funding: DB has active grant support from the National Institute of Diabetes and Digestive Kidney Diseases (Grant 1R01DK080089–01A2), the Juvenile Diabetes Research Foundation and a gift to Northwestern University by the Feinberg Foundation.
Footnotes
Disclosures: None
References
- 1.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ace2) converts angiotensin i to angiotensin 1-9. Circulation Research. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 2.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 3.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 4.Harrison-Bernard LM, Chappell MC. Unraveling the glomerular ras: One peptidase at a time. Am J Physiol Renal Physiol. 2012;303:F373–374. doi: 10.1152/ajprenal.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell MC. Comprehensive physiology. John Wiley & Sons, Inc.; 2012. Nonclassical renin-angiotensin system and renal function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingelfinger JR. Ace2: A new target for prevention of diabetic nephropathy? J Am Soc Nephrol. 2006;17:2957–2959. doi: 10.1681/ASN.2006090986. [DOI] [PubMed] [Google Scholar]
- 8.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ace2) in sars coronavirus-induced lung injury. Nature medicine. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ace 2 and decreased ace protein in renal tubules from diabetic mice: A renoprotective combination? Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- 10.Alghamri MS, Weir NM, Anstadt MP, Elased KM, Gurley SB, Morris M. Enhanced angiotensin ii-induced cardiac and aortic remodeling in ace2 knockout mice. Journal of Cardiovascular Pharmacology and Therapeutics. 2013;18:138–151. doi: 10.1177/1074248412460124. [DOI] [PubMed] [Google Scholar]
- 11.Batlle D, Wysocki J, Soler MJ, Ranganath K. Angiotensin-converting enzyme 2: Enhancing the degradation of angiotensin ii as a potential therapy for diabetic nephropathy. Kidney Int. 2012;81:520–528. doi: 10.1038/ki.2011.381. [DOI] [PubMed] [Google Scholar]
- 12.Benter IF, Yousif MHM, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of nadph oxidase and renal vascular dysfunction in diabetic hypertensive rats. American Journal of Nephrology. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 13.Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ace2 and angiotensin-(1-7) in a mouse model of early chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1523–1532. doi: 10.1152/ajprenal.00426.2009. [DOI] [PubMed] [Google Scholar]
- 14.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ace2-null mice. The Journal of clinical investigation. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circulation Journal. 2013;77:301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- 16.Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1–7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H1341–H1352. doi: 10.1152/ajpheart.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RA, Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of shrsp rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52:967–973. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- 18.Tikellis C, Bialkowski K, Pete J, Sheehy K, Su Q, Johnston C, Cooper ME, Thomas MC. Ace2 deficiency modifies renoprotection afforded by ace inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 19.Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin ii with recombinant angiotensin-converting enzyme 2: Prevention of angiotensin ii-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye M, Wysocki J, Gonzalez-Pacheco FR, Salem M, Evora K, Garcia-Halpin L, Poglitsch M, Schuster M, Batlle D. Murine recombinant angiotensin-converting enzyme 2: Effect on angiotensin ii-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60:730–740. doi: 10.1161/HYPERTENSIONAHA.112.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 23.Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. Journal of biomolecular screening. 2011;16:878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- 24.Kuriakose S, Muleme HM, Onyilagha C, Singh R, Jia P, Uzonna JE. Diminazene aceturate (berenil) modulates the host cellular and inflammatory responses to trypanosoma congolense infection. PloS one. 2012;7:e48696. doi: 10.1371/journal.pone.0048696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins Lima A, Xavier CH, Ferreira AJ, Raizada MK, Wallukat G, Santos RAS, Fontes MAP. Activation of angiotensin-converting enzyme 2/angiotensin-(1-7)/mas axis attenuates the cardiac reactivity to acute emotional stress. American Journal of Physiology - Heart and Circulatory Physiology. 2013;305:H1057–1067. doi: 10.1152/ajpheart.00433.2013. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. American journal of respiratory and critical care medicine. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigatto K, Casali KR, Shenoy V, Katovich MJ, Raizada MK. Diminazene aceturate improves autonomic modulation in pulmonary hypertension. European journal of pharmacology. 2013;713:89–93. doi: 10.1016/j.ejphar.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenoy V, Gjymishka A, Jarajapu YP, Qi Y, Afzal A, Rigatto K, Ferreira AJ, Fraga-Silva RA, Kearns P, Douglas JY, Agarwal D, Mubarak KK, Bradford C, Kennedy WR, Jun JY, Rathinasabapathy A, Bruce E, Gupta D, Cardounel AJ, Mocco J, Patel JM, Francis J, Grant MB, Katovich MJ, Raizada MK. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. American journal of respiratory and critical care medicine. 2013;187:648–657. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foureaux G, Nogueira JC, Nogueira BS, Fulgêncio GO, Menezes GB, Fernandes SOA, Cardoso VN, Fernandes RS, Oliveira GP, Franca JR, Faraco AAG, Raizada MK, Ferreira AJ. Antiglaucomatous effects of the activation of intrinsic angiotensin-converting enzyme 2. Investigative Ophthalmology & Visual Science. 2013;54:4296–4306. doi: 10.1167/iovs.12-11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi Y, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, Song C, Pepine CJ, Katovich MJ, Raizada MK. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62:746–752. doi: 10.1161/HYPERTENSIONAHA.113.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira AJ, Shenoy V, Qi Y, Fraga-Silva RA, Santos RA, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol. 2011;96:287–294. doi: 10.1113/expphysiol.2010.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraga-Silva RA, Costa-Fraga FP, Murca TM, Moraes PL, Martins Lima A, Lautner RQ, Castro CH, Soares CM, Borges CL, Nadu AP, Oliveira ML, Shenoy V, Katovich MJ, Santos RA, Raizada MK, Ferreira AJ. Angiotensin-converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61:1233–1238. doi: 10.1161/HYPERTENSIONAHA.111.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraga-Silva RA, Sorg BS, Wankhede M, Dedeugd C, Jun JY, Baker MB, Li Y, Castellano RK, Katovich MJ, Raizada MK, Ferreira AJ. Ace2 activation promotes antithrombotic activity. Molecular medicine. 2010;16:210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murca TM, Almeida TC, Raizada MK, Ferreira AJ. Chronic activation of endogenous angiotensin-converting enzyme 2 protects diabetic rats from cardiovascular autonomic dysfunction. Exp Physiol. 2012;97:699–709. doi: 10.1113/expphysiol.2011.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murca TM, Moraes PL, Capuruco CA, Santos SH, Melo MB, Santos RA, Shenoy V, Katovich MJ, Raizada MK, Ferreira AJ. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regulatory peptides. 2012;177:107–115. doi: 10.1016/j.regpep.2012.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng M, Whitesall S, Zhang Y, Beibel M, Alecy LD, DiPetrillo K. Validation of volume–pressure recording tail-cuff blood pressure measurements. American Journal of Hypertension. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 37.Wysocki J, Garcia-Halpin L, Ye M, Maier C, Sowers K, Burns KD, Batlle D. Regulation of urinary ace2 in diabetic mice. Am J Physiol Renal Physiol. 2013;305:F600–611. doi: 10.1152/ajprenal.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poglitsch M, Domenig O, Schwager C, Stranner S, Peball B, Janzek E, Wagner B, Jungwirth H, Loibner H, Schuster M. Recombinant expression and characterization of human and murine ace2: Species-specific activation of the alternative renin-angiotensin-system. Int J Hypertens. 2012;2012:428950. doi: 10.1155/2012/428950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raij L. The pathophysiologic basis for blocking the renin-angiotensin system in hypertensive patients with renal disease. American journal of hypertension. 2005;18:95S–99S. doi: 10.1016/j.amjhyper.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Shenoy V, Qi Y, Katovich MJ, Raizada MK. Ace2, a promising therapeutic target for pulmonary hypertension. Curr Opin Pharmacol. 2011;11:150–155. doi: 10.1016/j.coph.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.