Abstract
Endurance exercise training increases basal active tone in coronary arteries and enhances myogenic tone in coronary arterioles of control animals. Paradoxically, exercise training has also been shown to augment nitric oxide production and nitric oxide-mediated relaxation in coronary arterioles. The purpose of the present study was to examine the effect of exercise training on basal active tone of arterioles (~150 µm ID) isolated from the collateral-dependent region of hearts exposed to chronic coronary occlusion. Ameroid occluders were surgically placed around the proximal left circumflex coronary artery of miniature swine. Arterioles were isolated from both the collateral-dependent and nonoccluded myocardial regions of sedentary (pen confined) and exercise-trained (treadmill run; 14 wk) pigs. Coronary tone was studied in isolated arterioles using microvessel myographs and standard isometric techniques. Exposure to nominally Ca2+-free external solution reduced resting tension in all arterioles; decreases were most profound (P < 0.05) in arterioles from the collateral-dependent region of exercise-trained animals. Furthermore, nitric oxide synthase (NOS) inhibition (Nω-nitro-l-arginine methylester; 100 µM) unmasked markedly increased nitric oxide-sensitive tone in arterioles from the collateral-dependent region of exercise-trained swine. Blockade of K+ channels revealed significantly enhanced K+ channel contribution to basal tone in collateral-dependent arterioles of exercise-trained pigs. Protein content of endothelial NOS (eNOS) and phosphorylated eNOS (pS1179), determined by immunoblot, was elevated in arterioles from exercise-trained animals with the greatest effect in collateral-dependent vasculature. Taken together, we demonstrate the interaction of opposing exercise training-enhanced arteriolar basal active tone, nitric oxide production, and K+ channel activity in chronic coronary occlusion, potentially enhancing the capacity to regulate blood flow to collateral-dependent myocardium.
Keywords: coronary artery disease, nitric oxide, potassium channel, endothelial nitric oxide synthase, porcine
Significant evidence indicates that endurance exercise training enhances blood flow capacity in both normal and diseased hearts (6, 21, 22, 24, 34). This effect is especially critical in diseased hearts, such as those with underlying progressive occlusion or stenosis of a coronary artery. Coronary collateral artery development after progressive chronic occlusion is often insufficient to restore adequate blood flow to the collateral-dependent myocardium during periods of increased metabolic demand (33). Importantly, endurance exercise training improves the perfusion deficit and contractile dysfunction of collateral-dependent myocardium (34). Chronic exercise training has also been established to reverse the impaired relaxation and enhanced contractile responses of coronary vasculature distal to chronic occlusion (8–10, 15), which may contribute to increased perfusion capacity of the myocardium at risk (34).
In coronary arteries and arterioles from normal hearts, exercise training augments nitric oxide-mediated relaxation responses (27), basal nitric oxide production (35), and endothelial nitric oxide synthase (eNOS) protein and mRNA expression (23, 35, 39). In addition, blockade of K+ channels increases resting tension to a greater extent in coronary arterial rings of exercise-trained compared with sedentary pigs, indicating that the K+ channel contribution to basal tone is significantly enhanced after exercise training (4). Paradoxically, studies have also reported increases in both basal tone and the myogenic responsiveness in coronary vasculature of exercise trained compared with sedentary animals (4, 26). In coronary arterioles isolated distal to chronic occlusion, exercise training increases nitric oxide-mediated relaxation and eNOS mRNA expression (10), a response similar to that observed in coronary arterioles of normal animals. However, the effect of exercise training on basal tone and the contribution of nitric oxide and K+ channels to resting tension have not been examined in arteries from diseased hearts. Therefore, the purpose of this study was to examine the effect of exercise training on basal active tone of coronary arterioles isolated from the collateral-dependent region of chronically occluded hearts and determine the role of nitric oxide and K+ channels in the maintenance of basal tone.
METHODS
Experimental animals and surgical instrumentation
All animal protocols were in accordance with “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” and approved by the Institutional Animal Care and Use Committee at Texas A&M University in accordance with Association for the Assessment and Accreditation of Laboratory Animal Care procedures. Furthermore, all methods conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (DHHS Publication NIH 85–23, Office of Science and Health Reports, Bethesda, MD). Adult female Yucatan miniature swine were surgically instrumented with ameroid constrictors around the proximal left circumflex coronary (LCX) artery as described previously (9, 10, 13–15). Animals were preanesthetized with glycopyrrolate (0.004 mg/kg im) and midazolam (0.5 mg/kg im). Anesthesia was induced with ketamine (20 mg/kg im) and maintained with 3% isoflurane and 97% O2 throughout aseptic surgery. Animals recovered from the surgery for 8 wk before the experimental protocols were initiated.
Exercise training
Ameroid-occluded animals were randomly assigned to either a sedentary (n = 46) or exercise training (n = 47) group. Exercise-trained pigs underwent a progressive treadmill exercise training program (5 days/wk for 14 wk) as described previously (9, 10, 15). Sedentary animals were confined to their pens. Pigs were fed to maintain a matched body mass throughout the study. Effectiveness of the exercise training program was determined by comparing heart-to-body weight ratio and skeletal muscle citrate synthase activity as previously described (9, 10, 15).
Preparation of coronary arterioles
After completion of the 14-wk exercise training protocol or sedentary confinement, animals were anesthetized with ketamine (30 mg/kg) and pentobarbital sodium (35 mg/kg). Hearts were removed, placed in Krebs bicarbonate buffer (0–4°C), and weighed. Visual inspection of the ameroid occluder during dissection of the LCX artery indicated 100% occlusion in all animals that had undergone surgery for this study. With the aid of a dissection microscope, size-matched arterioles (~150 µm inner diameter) were isolated from both the collateral-dependent LCX region (distal to occlusion) and the nonoccluded left anterior descending (LAD) artery region. Arterioles selected for study were isolated from the midmyocardium and typically second- or third-order branches arising from the main artery (LAD or LCX). Arteriolar segments were trimmed of fat and connective tissue, cut into rings, and measured with a calibrated Filar micrometer eyepiece (Hitschfel Instruments, St. Louis, MO) in a relaxed, unstretched state.
Arteriolar rings were mounted in specialized isometric microvessel myographs (Living Systems Instrumentation) as used previously (31, 32), which allowed for direct determination of vessel wall force while internal circumference was controlled (28). The arteriolar rings were threaded onto two tungsten wires (20-µm diameter); one wire was attached to a force transducer and the other to a digitalized micrometer to allow precise changes in circumferential length of the vessel. Initially, arterioles were stretched using the micrometer to yield a baseline tension of ~0.1– 0.2 mN/mm. Vessels were superfused continuously with aerated (95% O2-5% CO2) Krebs bicarbonate buffer, warmed to 37°C, and allowed to equilibrate for 30 min. After the initial equilibration, arteriolar rings were progressively stretched to the maximum of the length-active tension relationship (Lmax) in increments equal to 10% of the initial vessel outer diameter. After each stretch, contraction was elicited with exposure to high KCl (40 mM), and Lmax was defined as the circumferential length at which developed tension was <5% greater than the developed tension produced at the previous length. Arteriolar rings were allowed to equilibrate for 45–60 min at Lmax before subsequent evaluation of pharmacological responsiveness.
Immunoblots
Arterioles (~150 µm diameter; ~6–8 mm length) were isolated from both the nonoccluded (LAD) and collateral-dependent (LCX) myocardial regions, quick-frozen, and stored at −80°C for later immunoblot analysis of eNOS and phosphorylated eNOS (p-eNOS; pS1179). Arterioles were homogenized in 25 µl of 2 × sample buffer (126 mM Tris-Cl, pH 6.8; 12.6% glycerol; 1.44 M 2-mercaptoethanol; 0.004% bromophenol blue; 5% SDS) by using a pestle homogenizer. Arteriole lysate (5 µg total protein) was subjected to SDS-PAGE (9–16.5% gradient gel) and transferred to polyvinylidene difluoride membrane. Membranes were cut and blocked for 2–4 h at 25°C in Tris-buffered saline containing 5% nonfat dry milk (Carnation) and 0.1% Tween-20, and incubated with primary eNOS or p-eNOS (top portion of membrane) and GAPDH (bottom portion of membrane) antibodies overnight at 4°C. Primary antibody dilutions were as follows: eNOS, 1:1,250; p-eNOS, 1:750; and GAPDH, 1:1,000 in blocking buffer. After washing, membranes were incubated with the appropriate horseradish peroxidase-conjugated species-specific anti-IgG (1:50,000 –1:100,000, depending on primary antibody) for 2 h at 25°C. Peroxidase activity was detected using SuperSignal West Dura Substrate (Pierce). Normalization for loading differences was accomplished using ratios of the densitometry signals for proteins of interest to GAPDH. Previous studies have documented no effect of chronic occlusion or exercise training on GAPDH expression (10, 39). Scanning densitometry (Scion Image software) was used to quantify signal density from luminograms.
Solutions and drugs
Krebs bicarbonate buffer contained (in mM) 131.5 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 13.5 NaHCO3, and 0.025 EDTA. High-KCl solutions were produced by equimolar substitution of KCl for NaCl. These solutions were aerated with 95% O2-5% CO2 and maintained at 37°C. Drugs were obtained from Sigma Chemical unless otherwise noted. Endothelin- 1 was purchased from Peninsula Laboratories. Primary monoclonal antibodies were obtained from BD Transduction Laboratories (eNOS and p-eNOS) and Advanced Immunochemicals (GAPDH).
Data analysis
Developed tension (T) was calculated as the millinewtons of force generated (F) per axial vessel length (g; in mm), where T = F/2 g (28). Relaxation responses to nitroprusside are presented as the percentage decrease in tension at each nitroprusside concentration relative to the endothelin-stimulated preconstriction [(CT0 − CT)/DT0] × 100, where CT0 is total contractile tension (endothelin-stimulated plus basal tone) and DT0 is the developed tension produced by endothelin. Body weight, heart-to-body weight, and citrate synthase values were analyzed using one-way ANOVA. Dimensional characteristics of coronary arteriolar rings, developed tension, and immunoblot data were compared using two-way ANOVA. Concentration-response relationships were analyzed using two-way ANOVA with repeated measures. Bonferroni correction for multiple comparisons was used when a main effect was identified by ANOVA. For all analyses, a P value ≤0.05 was considered significant. Data are presented as means ± SE, and n values in parentheses reflect the number of animals studied. When more than one coronary arteriole from the collateral-dependent or nonoccluded regions of a given animal was used in identical protocols, the responses from these rings were averaged before data analyses were conducted.
RESULTS
Efficacy of the exercise training program
Effectiveness of the 14-wk exercise training program was demonstrated by significant increases in skeletal muscle oxidative enzyme capacity and an increased heart-to-body weight ratio in exercise-trained compared with sedentary animals. Citrate synthase activity increased significantly in the deltoid muscle (26.1 ± 1.3 vs. 21.9 ± 1.0 µmol·min−1 ·g−1), and the anterior (16.0 ± 0.7 vs. 13.4 ± 0.8 µmol·min−1 ·g−1), medial (31.2 ± 2.0 vs. 22.1 ± 1.2 µmol·min−1 ·g−1) and long (22.8 ± 1.5 vs. 17.3 ± 1.1 µmol·min−1 ·g−1) heads of the triceps brachii muscle in exercise-trained (n = 44) compared with sedentary (n = 40) pigs, respectively. Although body weight did not differ between sedentary and exercise-trained animals at termination (39.7 ± 0.9 vs. 38.2 ± 0.8 kg, respectively), heart-to-body weight ratio was significantly greater from exercise-trained (n = 47) compared with sedentary (n = 43) pigs (6.0 ± 0.1 vs. 4.9 ± 0.1 g/kg, respectively).
Coronary arteriole dimensions and characteristics
No significant differences in dimensional characteristics were observed between nonoccluded LAD and collateral-dependent LCX arteriolar rings from either sedentary or exercise-trained animals (Table 1). Basal tension of rings after stretch to Lmax was not different between groups.
Table 1.
Dimensional characteristics of coronary arteriolar rings in evaluation of basal active tone
| Outer Diameter, µm |
Inner Diameter, µm |
Wall Thickness, µm |
Axial Length, mm |
RT Lmax, mN/mm |
|
|---|---|---|---|---|---|
| Sed LAD (n = 44) | 250±9 | 153±7 | 49±2 | 1.54±0.01 | 0.88±0.04 |
| Sed LCX (n = 44) | 245±7 | 151±6 | 47±1 | 1.54±0.01 | 0.93±0.05 |
| Ex LAD (n = 47) | 243±6 | 145±5 | 48±1 | 1.54±0.01 | 0.86±0.04 |
| Ex LCX (n = 47) | 250±6 | 152±5 | 48±1 | 1.54±0.01 | 1.01±0.05 |
Values are means ± SE; n = no. of animals studied. Sed, sedentary; Ex, exercise trained; LAD, left anterior descending coronary arteriolar ring; LCX, left circumflex coronary arteriolar ring; RT Lmax, resting tension (RT) where maximal active tension (Lmax) to KCl-induced depolarization is developed. No significant differences exist.
Effects of occlusion and exercise training on basal active tone
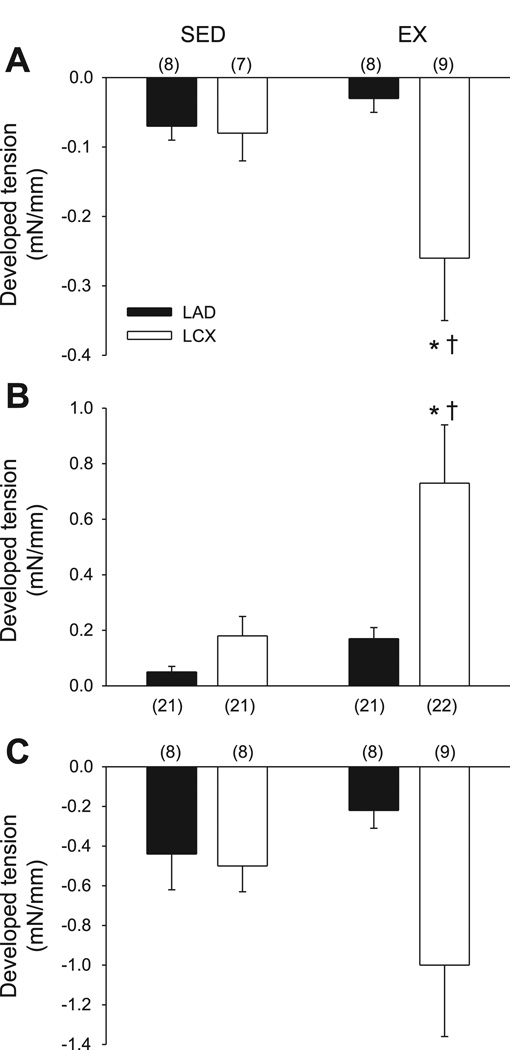
Exposure to nominally Ca2+-free external solution reduced resting tension in coronary arterioles from the nonoccluded and collateral-dependent regions of both sedentary and exercise-trained animals; however, the decrease in resting tension was significantly greater in the collateral-dependent arteriolar rings of exercise-trained animals compared with rings from the other treatment groups (Fig. 1A).
Fig. 1.
Effect of chronic occlusion and exercise training on Ca2+-dependent basal active tone. A: decline in resting tension induced by changing from Ca2+-containing buffer to nominally Ca2+-free buffer in arterioles isolated from collateral-dependent and nonoccluded regions of chronically occluded hearts of sedentary (Sed) and exercise-trained (Ex) animals. LAD, left anterior descending coronary artery. LCX, left circumflex coronary artery. B: increase in resting tension of arterioles in response to nitric oxide synthase (NOS) inhibition [Nω-nitro-l-arginine methyl ester (l-NAME); 100 µM]. C: decline in tension in response to nominally Ca2+-free solution after NOS. Values are means ± SE of no. of animals in parentheses. *Significantly different from sedentary LCX. †Significantly different from exercise-trained LAD.
Incubation of coronary arterioles with the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) (100 µM; Fig. 1B) increased resting tension in all treatment groups; however, arterioles from the collateral-dependent region of exercise-trained animals displayed a significantly greater increase in tension than rings from the other treatment groups.
Exposure to nominally Ca2+-free external solution after pretreatment with the NOS inhibitor l-NAME (100 µM) reduced resting tension in coronary arterioles from the nonoccluded and collateral-dependent regions of both sedentary and exercise-trained animals; the decrease in tension tended (P = 0.08) to be greater in the collateral-dependent arterisoles of exercise-trained animals compared with those from the other treatment groups (Fig. 1C). This finding suggests that nitric oxide inhibits the development of Ca2+-dependent basal active tone in coronary arterioles isolated from both nonoccluded and collateral-dependent myocardium with a tendency for the greatest effect in collateral-dependent arterioles of exercise-trained animals.
Smooth muscle responsiveness to nitroprusside
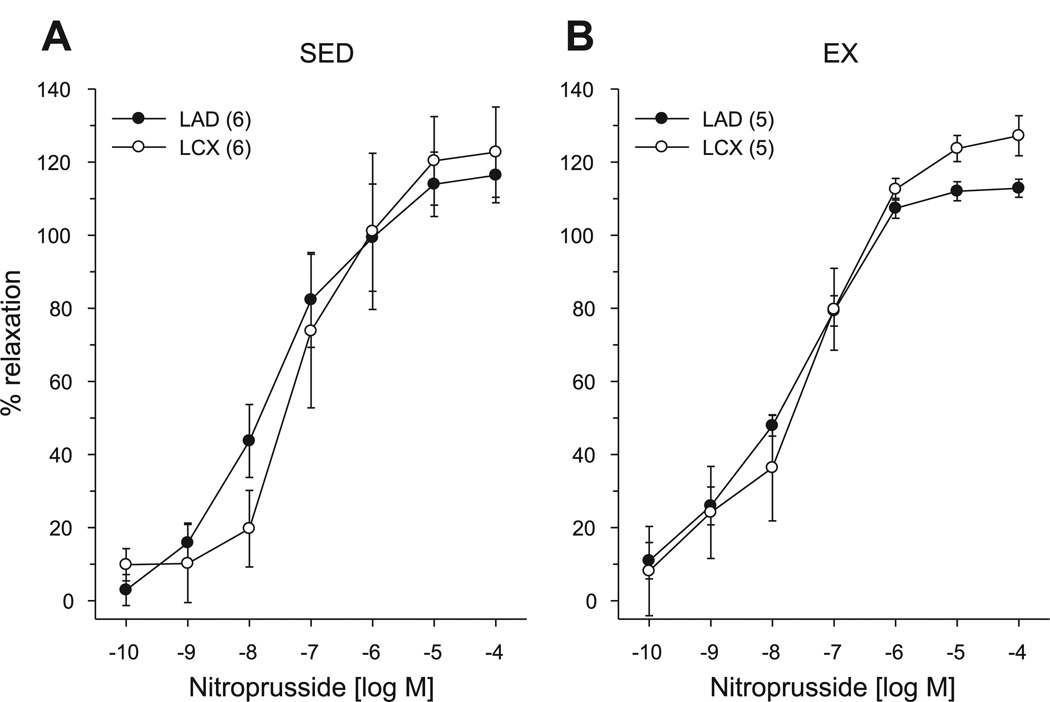
To examine the effects of chronic occlusion and exercise training on coronary smooth muscle responsiveness to nitric oxide, we evaluated the response of arteriolar rings to the endothelium-independent nitric oxide donor nitroprusside. After preconstriction with endothelin-1 (2 nM), concentration-dependent relaxation responses to nitroprusside were not significantly different in arterioles isolated from regions distal to the collateral-dependent LCX compared with the nonoccluded LAD in either sedentary (Fig. 2A) or exercise-trained pigs (Fig. 2B).
Fig. 2.
Effect of chronic occlusion and exercise training on relaxation responses to the nitric oxide donor nitroprusside. Nitroprusside-induced relaxation was not significantly different in arterioles isolated from collateral-dependent compared with nonoccluded regions of either sedentary (A) or exercise-trained (B) animals. Values are means ± SE of no. of animals in parentheses.
K+ channel contribution to resting tone in occlusion and exercise training
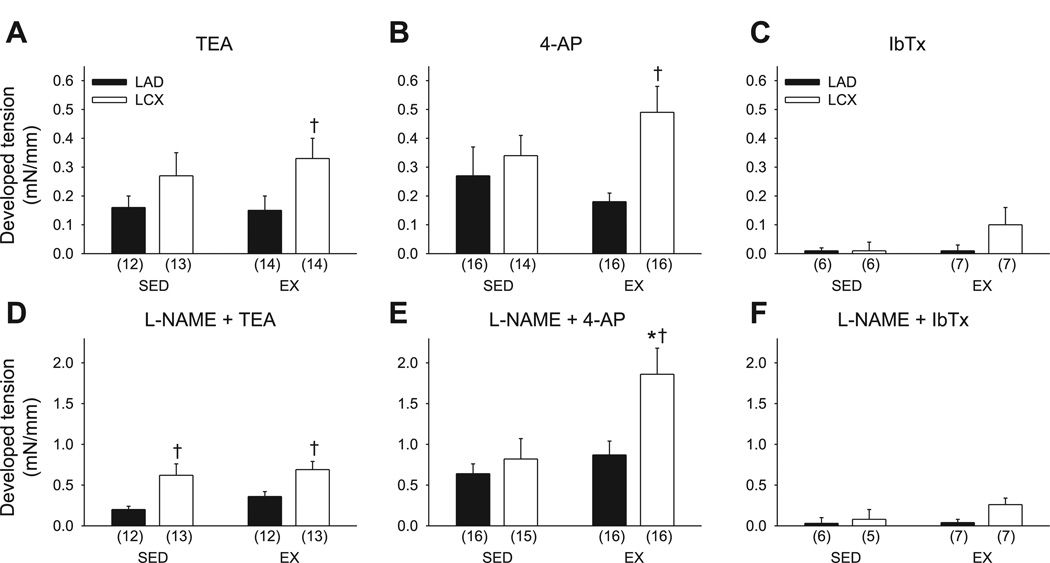
We also examined the effect of the K+ channel blockers tetraethylammonium (TEA; 1 mM), 4-aminopyridine (4-AP; 1 mM), and iberiotoxin (IbTx; 100 nM) on resting tension. The nonselective K+ channel blocker TEA increased basal tension in all vessel groups, with the most profound effect in arterioles of the collateral-dependent region of exercise-trained animals (Fig. 3A). Similarly, the classic voltage-dependent K+ (Kv) channel blocker 4-AP increased resting tension in all vessel groups, again with the greatest effect in arterioles of the collateral-dependent region of exercise trained animals (Fig. 3B). In contrast, the selective large-conductance Ca2+-dependent K+ (BKCa) channel blocker IbTx (Fig. 3C) produced minimal change in basal tension in any vessel group. In additional studies, we examined the effect of TEA, 4-AP, and IbTx on resting tension in the presence of NOS inhibition. These data demonstrate that both TEA and 4-AP more markedly increased basal tension in the presence (Fig. 3, D and E) compared with the absence (Fig. 3, A and B) of NOS inhibition. Furthermore, TEA increased resting tension to a significantly greater extent in arterioles from the collateral-dependent region of both sedentary and exercise-trained animals compared with rings from the nonoccluded region. On the other hand, 4-AP increased basal tension to a significantly greater extent only in arterioles from the collateral-dependent region of exercise-trained animals. Similar to that observed in the absence of NOS inhibition, IbTx again produced minimal change in resting tension in the presence of nitric oxide inhibition, with no significant differences between treatment groups.
Fig. 3.
Effect of chronic occlusion and exercise training on K+ channel contribution to basal active tone. A: blockade of tetraethylammonium (TEA)-sensitive K+ channels increased resting tension in arterioles isolated from collateral-dependent and nonoccluded regions of chronically occluded hearts of sedentary and exercise-trained animals, with the most profound effect in arterioles of collateral-dependent region of exercise-trained pigs. B: blockade of 4-aminopyridine (4-AP)-sensitive K+ channels increased resting tension in arterioles isolated from collateral-dependent and nonoccluded regions of chronically occluded hearts of sedentary and exercise-trained animals, with the most profound effect in arterioles of collateral-dependent region of exercise-trained pigs. C: blockade of iberiotoxin (IbTx)-sensitive K+ channels had a negligible effect on resting tension in arterioles isolated from collateral-dependent and nonoccluded regions of chronically occluded hearts of sedentary and exercise-trained animals. D: increase in basal tension of arterioles in response to TEA after NOS inhibition (l-NAME; 100 µM) was significantly elevated in collateral-dependent compared with nonoccluded arterioles from both sedentary and exercise-trained pigs. E: increase in basal tension of arterioles in response to 4-AP after NOS inhibition was significantly increased in collateral-dependent arterioles of exercise-trained pigs. F: increase in basal tension of arterioles in response to IbTx after NOS inhibition was negligible in all treatment groups. Values are means ± SE of no. of animals in parentheses. *Significantly different from sedentary LCX. †Significantly different from respective LAD.
Immunoblots for eNOS and p-eNOS (pS1179)
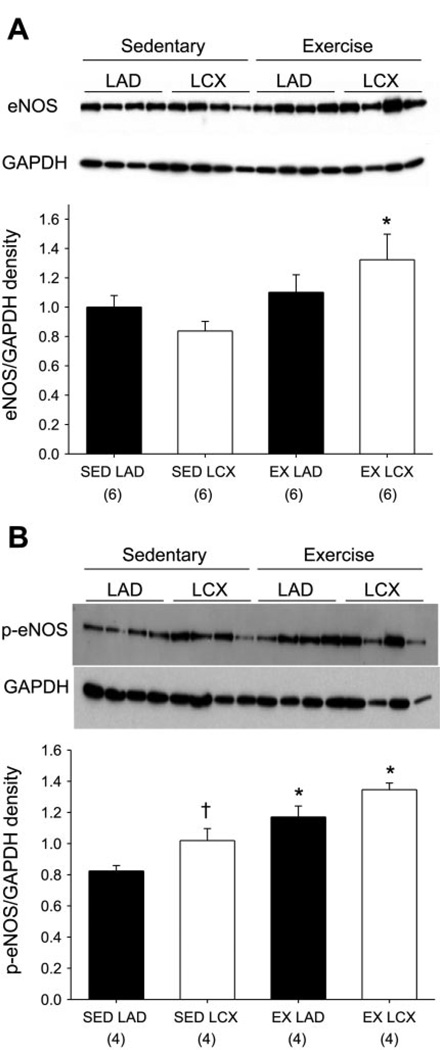
Determination of protein levels by immunoblot revealed that exercise training significantly increased eNOS protein content in coronary arterioles of the collateral-dependent region (Fig. 4A). The protein content of p-eNOS (pS1179) was increased by both occlusion and exercise training alone; furthermore, these effects appeared additive as p-eNOS protein levels were significantly greater in the collateral-dependent arteriole of exercise-trained animals compared with those from the other treatment groups (Fig. 4B).
Fig. 4.
Effect of chronic occlusion and exercise training on endothelial NOS (eNOS) and phosphorylated eNOS (p-eNOS; pS1179) protein levels as determined by immunoblot. A: protein levels for eNOS. B: protein levels for p-eNOS (pS1179). Protein of interest was quantified by densitometry analysis, normalized to GAPDH, and expressed relative to sedentary LAD density. Values are means ± SE of no. of animals in parentheses. *Significantly different from respective sedentary arterioles. †Significantly different from respective LAD arterioles.
DISCUSSION
In the present study, we demonstrate for the first time that collateral-dependent coronary arterioles of exercise-trained animals display significantly enhanced Ca2+-dependent basal active tone compared with arterioles from nonoccluded regions of exercise-trained pigs and arterioles from the collateral-dependent and nonoccluded regions of sedentary animals. These data indicate that together, chronic occlusion and exercise training produce a profound increase in Ca2+-dependent basal active tone, despite a negligible effect of either occlusion or exercise training alone. We also demonstrate that arterioles from the collateral-dependent region of exercise-trained animals display significantly enhanced constriction in response to NOS inhibition (l-NAME) compared with arterioles from the other treatment groups. The increased response to NOS inhibition in the collateral-dependent arterioles of exercise-trained animals suggests that enhanced nitric oxide activity may mask the increased Ca2+-dependent tone observed in these arterioles under basal conditions. Indeed, taken together with our findings of increased eNOS and p-eNOS protein levels in the collateral-dependent coronary arterioles after exercise training, our data suggest that the enhanced constrictor response to NOS inhibition with occlusion and exercise training may be attributable to increased nitric oxide production.
Previous studies have documented increased eNOS protein and mRNA expression with endurance exercise training in arteries and arterioles from control and diseased hearts (10, 11, 23, 35, 39). In our model of chronic occlusion, Griffin et al. (10) previously reported that chronic occlusion decreased eNOS mRNA expression in small arterioles (~100 µm ID) isolated from the collateral-dependent myocardium compared with that of the nonoccluded region. Furthermore, exercise training reversed the reduced eNOS mRNA expression observed in arterioles isolated distal to chronic occlusion (10). Similarly, in the present study, eNOS protein levels in sedentary pigs tended to be decreased in arterioles from the collateral-dependent myocardium compared with the nonoccluded region, and exercise training significantly enhanced eNOS protein levels in vessels from the collateral-dependent region. Interestingly, we also demonstrate a tendency for enhanced constriction to NOS inhibition in the collateral-dependent region of sedentary animals despite a slightly reduced eNOS protein level. This finding may be attributable to the increased p-eNOS protein levels in arterioles from the collateral-dependent myocardium and subsequent increased nitric oxide production via the phosphorylation of eNOS.
In an elegant clinical study, Hambrecht and colleagues (11) exercise-trained candidates for coronary artery bypass grafting for 4 wk before bypass surgery. Subsequent ex vivo evaluation of a sample of the left internal mammary artery taken at surgery revealed improved endothelium-dependent relaxation and increases in eNOS protein and mRNA expression as well as enhanced phosphorylation of eNOS at S1177 (human equivalent of porcine S1179) in patients that were exercise trained compared with sedentary counterparts (11). These findings support our observation that exercise training enhances eNOS and p-eNOS protein levels in the underlying setting of coronary artery disease. Control subjects were not included in these studies (11); thus whether coronary artery disease altered eNOS and p-eNOS protein levels compared with arteries from control subjects was not determined.
Our finding that the relaxation response to the nitric oxide donor nitroprusside was similar in arterioles of all treatment groups indicates that the smooth muscle responsiveness to nitric oxide and the downstream protein kinase G-dependent pathway are not altered by occlusion or exercise training in arterioles. These findings are in agreement with previous findings in arterioles of both normal and diseased hearts (10, 27) and provide evidence that the increase in resting tension in response to NOS inhibition (l-NAME) in arterioles from the collateral-dependent region of both sedentary and exercise-trained animals may be attributed to enhanced nitric oxide bioavailability rather than increased responsiveness of the vascular smooth muscle of these arterioles to nitric oxide.
Previous studies have reported that BKCa and Kv channel inhibition enhances resting vascular tone (5, 19, 36). These findings suggest that basal K+ channel activity counteracts the development of tone, most likely via membrane hyperpolarization and subsequent inhibition of Ca2+ influx through voltage-dependent Ca2+ channels (30). At concentrations of 1 mM, TEA is generally accepted to block primarily BKCa channels. However, we have previously documented that 1 mM TEA markedly inhibits Kv channels in coronary arterioles (12). Similarly, in the present study, while TEA substantially increased resting tone in coronary arterioles from all treatment groups, the selective BKCa channel inhibitor IbTx had a negligible effect on basal tone, suggesting that TEA exerted its effect via Kv channels. Taken together, our data suggest that Kv channels contribute substantially to basal tone in coronary arterioles of nonoccluded and occluded regions in both sedentary and exercise-trained pigs (Fig. 3, A and B). Interestingly, the contractile response to Kv channel blockade was significantly greater in arterioles isolated from the collateral-dependent region of exercise-trained animals, suggesting that Kv channels contribute to the regulation of resting tension to a greater extent in these arterioles. These data are the first to demonstrate increased Kv channel activity under basal conditions in vasculature of the collateral-dependent region of exercise-trained pigs. In contrast to a previous observation that endurance exercise training increases the K+ channel contribution to basal tone (4), we found that basal tone in arterioles from the nonoccluded region was not altered by exercise training. Furthermore, endurance exercise training has previously been reported to enhance both nitroprusside-sensitive basal tone and myogenic responsiveness in the coronary vasculature of control animals (4, 26). Our observation that basal active tone in arterioles from the nonoccluded region remained unaffected by exercise training suggests that chronic occlusion may produce global coronary adaptations that differ from those observed in the normal heart, a finding similar to that observed previously (17).
We also examined the response of these arterioles to K+ channel blockers after pretreatment with l-NAME (100 µM) and revealed that, in the presence of NOS inhibition, the contractile response to K+ channel blockade was enhanced compared with the response in the absence of NOS inhibition. These data suggest an increase in K+ channel activity secondary to NOS inhibition or the removal of an inhibitory action of nitric oxide on K+ channel activity. Previous studies have documented nitric oxide-mediated stimulation of K+ channel activity (2, 20, 29, 38), suggesting an inhibitory action of nitric oxide on K+ channel activity in our studies is unlikely. Nitric oxide has also been reported to inhibit Ca2+ channels (16); thus NOS inhibition would remove an inhibitory effect on Ca2+ channels. Subsequent membrane depolarization in response to K+ channel inhibition may result in increased Ca2+ influx and enhanced vascular smooth muscle contraction (30), such as the increased contraction observed in response to K+ channel blockade after NOS inhibition in our studies. However, the mechanism by which NOS inhibition increases Kv channel activity in our preparation remains unknown.
Although the observed enhanced basal active tone, nitric oxide production, and K+ channel activity are paradoxical, these adaptations may provide a basis for enhanced flow reserve and vasodilatory capacity in the collateral-dependent region after exercise training. We speculate that chronic, intermittent increases in coronary blood flow that are associated with exercise training generate physical forces, including shear stress and distension, at the vascular wall that may contribute to the adaptations observed in these studies. Cyclic strain and shear stress have both been documented to increase eNOS expression in cultured endothelial cells (1, 37). Furthermore, previous studies have revealed increased voltage-gated Ca2+ channel current (3) and increased basal (4) and myogenic tone (26) in coronary arteries and arterioles of exercise-trained compared with sedentary swine, suggesting that the increase in Ca2+-dependent basal active tone may be attributable to increased Ca2+ influx via voltage-gated Ca2+ channels in the collateral-dependent arterioles of exercise-trained animals. Although coronary arterioles from the nonoccluded region of exercise-trained swine in our model did not display increased Ca2+-dependent tone as expected based on these previous studies, the global response to exercise training may differ in ischemic compared with control hearts as observed previously (17). The adaptations observed in our studies may be most profound in the collateral-dependent region because of coupling of physical forces with intermittent ischemia during each bout of exercise. Indeed, exercise-induced myocardial ischemia persists in this model despite improvements in myocardial perfusion and contractile function after chronic exercise training (34).
Limitations
We recognize the difficulties of extrapolating in vitro findings to the intact heart; however, we examine the intrinsic properties of coronary smooth muscle and endothelium using in vitro approaches because the intricate relationships of numerous mechanisms that control coronary vasomotor tone make in vivo measures difficult to interpret. However, direct correlation of our findings to in vivo responses remains unknown. Furthermore, the adaptations observed in our study may be time dependent in that with longer-term exercise training and potentially further increases in collateral development, structural adaptations may occur in the collateral-dependent vasculature that reduce the stimuli for the adaptations we observe after 14 wk of exercise training. For example, mechanical stimuli (shear stress or distension) may be reduced by structural changes in the collateral-dependent vasculature, reducing the stimuli for increases in eNOS expression in these arterioles. Thus after longer-term exercise training, adaptations in the coronary circulation may be more structural in nature than functional.
Clinical implications
Our studies demonstrate increased eNOS and p-eNOS protein levels and increased nitric oxide bioactivity under basal conditions in the collateral-dependent region of exercise-trained animals. Taken together with previous findings from our group that agonist-mediated, nitric oxide-dependent relaxation is enhanced in collateral-dependent arterioles of exercise-trained animals (8, 10), the current studies support the concept that exercise training enhances the regulatory role for nitric oxide in coronary microvascular function in the underlying setting of coronary artery disease. Similarly, recent clinical studies have revealed that chronic exercise training in coronary artery disease patients leads to improved endothelium-dependent vasodilatation that is associated with increased eNOS and p-eNOS protein levels (11). We speculate that the effects of exercise training observed in our studies contribute to improved perfusion and enhanced vasodilator reserve of the collateral-dependent myocardium. Exercise may provide persistent ischemic and mechanical stimuli that elicit continued development of the collateral circulation that may not be present under resting conditions.
The observed increase in Ca2+-dependent basal active tone suggests that Ca2+ channel blockade may specifically enhance blood flow into the collateral-dependent region of exercise-trained animals. However, the effectiveness of Ca2+ channel antagonists in chronic coronary artery occlusion/stenosis depends in part on the degree of collateralization (18). Previous studies of patients with stenosed or obstructed coronary arteries have indicated that those individuals with relatively greater collateral blood flow develop significant increases in ischemic episodes after Ca2+ channel antagonist administration, whereas patients with poor or no collateral flow showed a reduction in ischemic episodes with Ca2+ channel blockade (7). These data suggest that because the reduction in coronary resistance with Ca2+ channel antagonism is typically greatest in the normally perfused (nonoccluded) region, flow to the collateral vasculature is reduced and myocardial ischemia is exacerbated in the collateral-dependent region. Thus the increased ischemic activity in patients with relatively higher collateralization may result from a coronary steal phenomenon (7, 18). In the porcine model of chronic coronary occlusion, the improvements in perfusion and contractile function of the collateral-dependent myocardium observed after chronic exercise training have been attributed to increased collateral development (25, 34). Thus Ca2+ channel antagonism in our model of chronic occlusion may similarly exacerbate myocardial ischemia in the collateral-dependent region of exercise-trained animals. However, the effect of Ca2+ channel blockade in this model has not been evaluated.
In conclusion, our data demonstrate that coronary arterioles isolated from the collateral-dependent region of exercise-trained pigs display a significantly increased basal active tone that is Ca2+ dependent. Furthermore, these arterioles also demonstrate increased Kv channel activity and enhanced nitric oxide production under basal conditions. Importantly, enhanced nitric oxide production is likely attributable, at least partially, to increased eNOS protein levels and increased phosphorylation of eNOS protein. Although paradoxical, the enhanced resting tension, nitric oxide production, and K+ channel activity in coronary arterioles isolated from the collateral-dependent region of exercise-trained animals potentially provide a greater intrinsic capacity of local vascular control mechanisms to regulate blood flow to collateral-dependent myocardium and subsequently contribute to the improved perfusion observed after exercise training.
REFERENCES
- 1.Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 3.Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol. 1998;275:H2159–H2169. doi: 10.1152/ajpheart.1998.275.6.H2159. [DOI] [PubMed] [Google Scholar]
- 4.Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+ channel contribution to regulation of coronary arterial tone. J Appl Physiol. 1998;84:1225–1233. doi: 10.1152/jappl.1998.84.4.1225. [DOI] [PubMed] [Google Scholar]
- 5.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 6.DiCarlo SE, Blair RW, Bishop VS, Stone HL. Daily exercise enhances coronary resistance vessel sensitivity to pharmacological activation. J Appl Physiol. 1989;66:421–428. doi: 10.1152/jappl.1989.66.1.421. [DOI] [PubMed] [Google Scholar]
- 7.Egstrup K, Andersen PE., Jr Transient myocardial ischemia during nifedipine therapy in stable angina pectoris, and its relation to coronary collateral flow and comparison with metoprolol. Am J Cardiol. 1993;71:177–183. doi: 10.1016/0002-9149(93)90735-u. [DOI] [PubMed] [Google Scholar]
- 8.Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation. 2004;109:664–670. doi: 10.1161/01.CIR.0000112580.31594.F9. [DOI] [PubMed] [Google Scholar]
- 9.Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol. 1999;87:1948–1956. doi: 10.1152/jappl.1999.87.5.1948. [DOI] [PubMed] [Google Scholar]
- 10.Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation. 2001;104:1393–1398. doi: 10.1161/hc3601.094274. [DOI] [PubMed] [Google Scholar]
- 11.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 12.Heaps CL, Bowles DK. Gender-specific K+-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol. 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- 13.Heaps CL, Parker JL, Sturek M, Bowles DK. Altered calcium sensitivity contributes to enhanced contractility of collateral-dependent coronary arteries. J Appl Physiol. 2004;97:310–316. doi: 10.1152/japplphysiol.01400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Sarcoplasmic reticulum Ca2+ uptake is impaired in coronary smooth muscle distal to coronary occlusion. Am J Physiol Heart Circ Physiol. 2001;281:H223–H231. doi: 10.1152/ajpheart.2001.281.1.H223. [DOI] [PubMed] [Google Scholar]
- 15.Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol. 2000;278:H1984–H1992. doi: 10.1152/ajpheart.2000.278.6.H1984. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa T, Hume JR, Keef KD. Regulation of calcium channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res. 1993;73:1128–1137. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- 17.Jones JJ, Dietz NJ, Heaps CL, Parker JL, Sturek M. Calcium buffering in coronary smooth muscle after chronic occlusion and exercise training. Cardiovasc Res. 2001;51:359–367. doi: 10.1016/s0008-6363(01)00305-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann PA, Mandinov L, Seiler C, Hess OM. Impact of exercise-induced coronary vasomotion on anti-ischemic therapy. Coron Artery Dis. 2000;11:363–369. doi: 10.1097/00019501-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 20.Koh SD, Campbell JD, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J Physiol. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laughlin MH. Effects of exercise training on coronary transport capacity. J Appl Physiol. 1985;58:468–476. doi: 10.1152/jappl.1985.58.2.468. [DOI] [PubMed] [Google Scholar]
- 22.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol. 1989;67:1140–1149. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin MH, Tomanek RJ. Myocardial capillarity and maximal capillary diffusion capacity in exercise-trained dogs. J Appl Physiol. 1987;63:1481–1486. doi: 10.1152/jappl.1987.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 25.McKirnan MD, Bloor CM. Clinical significance of coronary vascular adaptations to exercise training. Med Sci Sports Exerc. 1994;26:1262–1268. [PubMed] [Google Scholar]
- 26.Muller JM, Myers PR, Laughlin MH. Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol. 1993;75:2677–2682. doi: 10.1152/jappl.1993.75.6.2677. [DOI] [PubMed] [Google Scholar]
- 27.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- 28.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 31.Parker JL, Mattox ML, Laughlin MH. Contractile responsiveness of coronary arteries from exercise-trained rats. J Appl Physiol. 1997;83:434–443. doi: 10.1152/jappl.1997.83.2.434. [DOI] [PubMed] [Google Scholar]
- 32.Rapps JA, Jones AW, Sturek M, Magliola L, Parker JL. Mechanisms of altered contractile responses to vasopressin and endothelin in canine coronary collateral arteries. Circulation. 1997;95:231–239. doi: 10.1161/01.cir.95.1.231. [DOI] [PubMed] [Google Scholar]
- 33.Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol Heart Circ Physiol. 1987;253:H1279–H1288. doi: 10.1152/ajpheart.1987.253.5.H1279. [DOI] [PubMed] [Google Scholar]
- 34.Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effects of chronic exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation. 1990;82:1778–1789. doi: 10.1161/01.cir.82.5.1778. [DOI] [PubMed] [Google Scholar]
- 35.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu S, Yokoshiki H, Sperelakis N, Paul RJ. Role of voltage-dependent and Ca2+-activated K+ channels on the regulation of isometric force in porcine coronary artery. J Vasc Res. 2000;37:16–25. doi: 10.1159/000025709. [DOI] [PubMed] [Google Scholar]
- 37.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol Cell Physiol. 1995;269:C1371–C1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 38.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca2+-dependent K+ channels. Circ Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 39.Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol Heart Circ Physiol. 1997;273:H1–H5. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]