Abstract
Cocaine is a potent disruptor of photic and non-photic pathways for circadian entrainment of the master circadian clock of the suprachiasmatic nucleus (SCN). These actions of cocaine likely involve its modulation of molecular (clock gene) components for SCN clock timekeeping. At present, however, the physiological basis of such an interaction is unclear. To address this question, we compared photic and non-photic phase-resetting responses between wild-type (WT) and Per2 mutant mice expressing nonfunctional PER2 protein to systemic and intra-SCN cocaine administrations. In the systemic trials, cocaine was administered i.p. (20 mg/kg) either at midday or prior to a light pulse in the early night to assess its non-photic and photic behavioral phase-resetting actions, respectively. In the intra-SCN trial, cocaine was administered by reverse microdialysis at midday to determine if the SCN is a direct target for its non-photic phase-resetting action. Non-photic phase-advancing responses to i.p. cocaine at midday were significantly (~3.5-fold) greater in Per2 mutants than WTs. However, the phase-advancing action of intra-SCN cocaine perfusion at midday did not differ between genotypes. In the light pulse trial, Per2 mutants exhibited larger photic phase-delays than did WTs, and the attenuating action of cocaine on this response was proportionately larger than in WTs. These data indicate that the Per2 clock gene is a potent modulator of cocaine’s actions in the circadian system. With regard to non-photic phase-resetting, the SCN is confirmed as a direct target of cocaine action; however, Per2 modulation of this effect likely occurs outside of the SCN.
Keywords: cocaine, circadian, clock gene, suprachiasmatic nucleus, entrainment, mouse
1. INTRODUCTION
Cocaine abuse is associated with disturbances in the daily patterns of circadian-timed homeostatic functions, including those of the endocrine, autonomic, and immune systems [1, 2]. These actions indicate that cocaine may directly or indirectly disrupt circadian timing, which could contribute to the process of addiction. The basis for such effects of cocaine may be related to the reciprocal interaction between cocaine and circadian clock gene activity. Cocaine is known to affect central clock gene expression, and conversely, circadian clock genes (in particular Per2) regulate cocaine intake and reward. The Per2 clock gene is rhythmically expressed in the SCN, as well as in other regions, and stabilizes endogenous circadian pacemaker activity, as shown in studies from Per2 mutant mice that express inactive PER2 protein [3, 4]. Notably, Per2 mutant mice exhibit greater frequencies of drug (cocaine or ethanol) self-administration [5, 6], higher levels of voluntary drug intake [7, 8], and stronger conditioned and two-bottle free-choice preferences for drug solutions over water compared to wild-type mice [5, 6, 7, 8]. Behavioral analyses have shown that Per2 mutant mice also have differential locomotor reactions to acute and repeated presentations of rewards, including cocaine, compared to wild-type mice [5, 6, 9].
Recently, we have reported that acute and chronic modes of cocaine administration are highly disruptive to photic and non-photic circadian clock phase-resetting and entrainment [10, 11]. Acute cocaine treatment at midday promotes large non-photic phase-advance shifts of locomotor rhythms, and similar drug treatment at night blocks photic phase-delay shifts [10]. Notably, chronic forced and free-choice regimens of oral cocaine administration significantly alter the intrinsic free-running period of the circadian clock, an effect which persists for months after cocaine withdrawal [11]. We have also demonstrated that the SCN is a direct target of cocaine’s photic and non-photic phase-resetting action in vitro and that this action is mediated by its inhibition of the serotonin transporter [10, 12].
While the effects of the Per2 mutation on drug intake and reward are well documented, data are lacking on whether this mutation affects circadian clock photic and non-photic responses to cocaine. By inference, such action would earmark the Per2 gene as an important modulator of cocaine action in the circadian system. This information would significantly extend current knowledge of the genetics of drug addiction, particularly with regard to recent studies demonstrating that polymorphisms of Per2 in humans are strongly tied to increased risk for cocaine dependence [13].
2. METHODS
2.1. Animals
Male mice with a nonfunctional homolog of Per2 (Per2 mutant; B6.129S7-Per2tm1Brd/J) and wild-type (WT; B6(Cg)-Tyrc-2J/J) controls were raised from breeder pairs backcrossed on a C57BL/6 background (The Jackson Laboratory, Bar Harbor, ME, USA). Mice were 8–10 weeks old at the time of experimentation. Animals were singly housed in polycarbonate cages under a 12L:12D photocycle (270 lux; LD) in a temperature-controlled vivarium (23°C). Food and water were provided ad libitum. All experiments were performed using the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Kent State University Institutional Animal Care and Use Committee.
2.2. Behavioral Measures
General circadian locomotor activity was recorded using overhead infrared motion detectors interfaced with a computerized data acquisition system (Clocklab; Coulbourn Instruments, Whitehall, PA, USA). Data were collected in 10 min bins. Activity onset associated with lights-off under LD (designated as zeitgeber time [ZT] 12) was defined by the initial 10 min period that: 1) coincided with an intensity of activity that exceeded 10% of the maximum rate for the day; 2) preceded by at least 4 hr of activity quiescence; and 3) followed by at least 60 min of sustained activity. Following release to constant darkness (DD) to examine phase-shifts using a modified Aschoff type II procedure [14] and rhythm period in response to drug administration and genotype, activity onset was used as the reference point for the beginning of the subjective night (designated as circadian time [CT] 12). Phase-shifts were calculated from the 1) back extrapolation of the least squares line through activity onsets on days 3–10 after treatment; and 2) extrapolation of the least squares line calculated from activity onset data collected for a minimum of 7 days prior to treatment. Assessments of changes in activity (duration and intensity) after i.p. and intra-SCN drug treatment were undertaken using data exported from a Clocklab data acquisition system. A count represented an individual event registered by an overhead infrared sensor. Activity duration represented the length of increased activity bouts (relative to pre-treatment level) immediately following treatment at midday. Each bout was defined as the sum of activity events collected in each 10 min bin.
2.3. Drug Administration
In the systemic cocaine trials, mice received an intraperitoneal (i.p.) injection of physiological saline or cocaine hydrochloride (20 mg/kg; Sigma-Aldrich Corp St. Louis, MO, USA) dissolved in physiological saline. In the intra-SCN trial, artificial cerebrospinal fluid (ACSF) or cocaine hydrochloride (0.5 mM) dissolved in ACSF was perfused at a flow rate of 1.0 μl/min through a microdialysis probe (see below) targeting the lateral margin of the suprachiasmatic nucleus (SCN; anteroposterior, −0.46 mm from bregma; lateral, +0.20 mm from midline; horizontal, 5.50 mm from dura). Concentrically designed microdialysis probes were constructed from a 26-gauge stainless steel outer cannula into which was inserted 32-gauge fused silica tubing. Hemicellulose dialysis tubing (230 μm outer diameter; 12 kDa MW cutoff; SpectraPore, Fisher Scientific) was affixed to the outer cannula with epoxy glue. The active dialysis length was 1.0 mm. Theoretical probe efficiency was estimated in vitro by measuring the yield of cocaine from probes immersed in a 0.5 mM cocaine solution at 37°C. Probe efficiency averaged ~23%. Based on in vitro probe efficiency of 23%, this provided a theoretical cocaine concentration of ~ 115 μM outside the probe. The microdialysis probe was inserted three days prior to intra-SCN drug administration which is sufficient for blood brain barrier repair and return of locomotor activity rhythms to baseline levels [see ref. 10]. For probe implantation, animals were anesthetized with pentobarbital sodium (Nembutal; 35 mg/kg) and pretreated with Marcaine (0.25% bupivicaine; 0.05 ml) in the scalp area and atropine subcutaneously (0.09%; 0.10 ml) to reduce localized pain and respiratory occlusion, respectively. Probes were secured to the skull with dental cement and stainless steel screws. Surgery was undertaken at the beginning of the light phase under LD. Mice were returned to their home cages following surgery. Following experimentation, microdialysis probe placement was verified from 60 μm-thick cryostat sections stained with cresyl violet.
2.4. Experimental Protocols
In both experiments, general locomotor activity rhythms of WT and Per2 mutant mice were monitored for 2 wk under LD prior to experimentation to establish baseline conditions.
2.4.1
Experiments 1 and 2 compared non-photic phase-advancing responses of Per2 mutant and WT mice to i.p. and intra-SCN administration of cocaine at ZT 6 (n=6/group), respectively. For the latter trial, animals were surgically outfitted with a microdialysis probe stereotaxically aimed at the lateral margin of the SCN three days prior to experimentation as described above. On the day of experimentation, microdialysis probes were continuously perfused with ACSF alone or ACSF + cocaine from a syringe pump (n=4/group). Continuous 80 min perfusion of ACSF or ACSF + cocaine extended from ZT 6–7.3. In both trials, animals were released into DD at the released to DD for 2 wk to assess phase-shifting responses using the Aschoff type II procedure.
2.4.2
Experiment 3 compared the action of i.p. administration of cocaine delivered 15 min prior to a phase-delaying 30 min light pulse (25 lux) or no light pulse beginning at ZT 16 (n=6/group) between Per2 mutant and WT mice. Immediately after the light pulse, the mice were released to DD to assess phase-shifting.
2.5. Statistical Analyses
Univariate ANOVAs and subsequent Student Newman-Keuls post-hoc mean comparison tests were used for treatment (cocaine or saline) and genotype (WT or Per2 mutant) statistical comparisons of phase-resetting responses to cocaine administration. All statistical analyses were completed with SPSS 19.0 (Chicago, IL). The significance level was set at p<0.05, in all cases.
3. RESULTS
3.1. Experiments 1 and 2
Per2 mutation and cocaine-induced phase-resetting
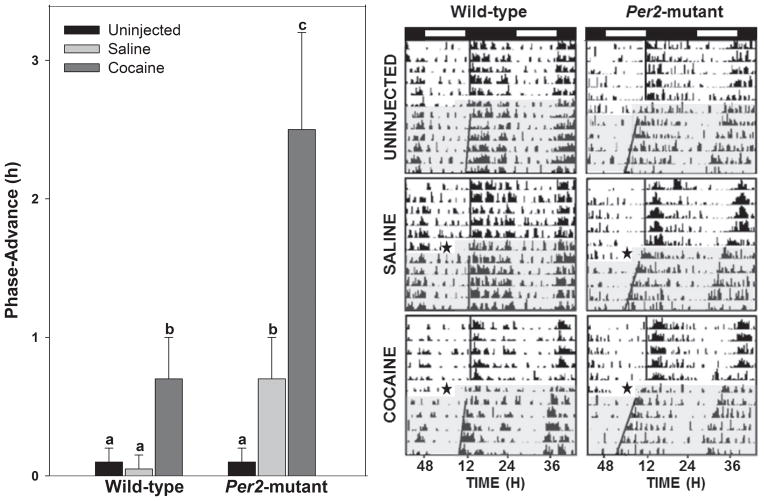
Phase-advances of locomotor activity rhythms were significantly larger for both WT and Per2 mutant mice treated with i.p. cocaine compared with saline or no injection (F2,30 = 9.8; p<0.01; Fig. 1). There was a main effect for genotype, such that Per2 mutants treated with cocaine had significantly (~3.5 fold) larger phase-advance shifts compared with WTs (2.5±0.7 h vs. 0.7±0.3 h; F1,30 = 16.5; p<0.01). There was also a significant interaction for genotype and treatment (F1,30 = 10.6; p<0.01). Analyses of rhythm period under free-running (DD) conditions revealed a main effect for genotype (Per2 mutant: 23.2±0.3 h; WT: 23.9±0.1 h; F1,30 = 15.5; p<0.01), but not for treatment (p>0.05). Within-genotype analyses revealed no differences in behavioral activity measures between i.p. cocaine and saline groups in Per2 mutants (activity duration: 144±55 min vs. 115±38 min, respectively; total activity counts: 243±95 vs. 496±221, respectively; Student’s t test for both measures; p>0.05 for both). In contrast, WTs were more behaviorally reactive to i.p. cocaine compared with saline (activity duration: 210±30 min vs. 90±37 min, respectively; total activity counts: 432±60 vs. 179±84, respectively; Student’s t test for both measures; p<0.05 for both; Fig. 2).
Figure 1.
Per2 mutation enhances the phase-advancing response to acute i.p. administration of cocaine at midday (ZT 6). Left panel: graphic representation of the phase-resetting responses to cocaine and control treatments. Bars represent means±SEM. Bars with different letters are significantly different within and between strains (p<0.05). Right panel: representative double-plotted actograms of general locomotor activity showing phase-advancing responses to the cocaine and control treatments at ZT 6 (closed star designates time of treatments). Light and dark phases of the LD cycle are represented above the actograms by the open and filled horizontal spaces, respectively. The shaded portion designates constant darkness following drug treatment (star).
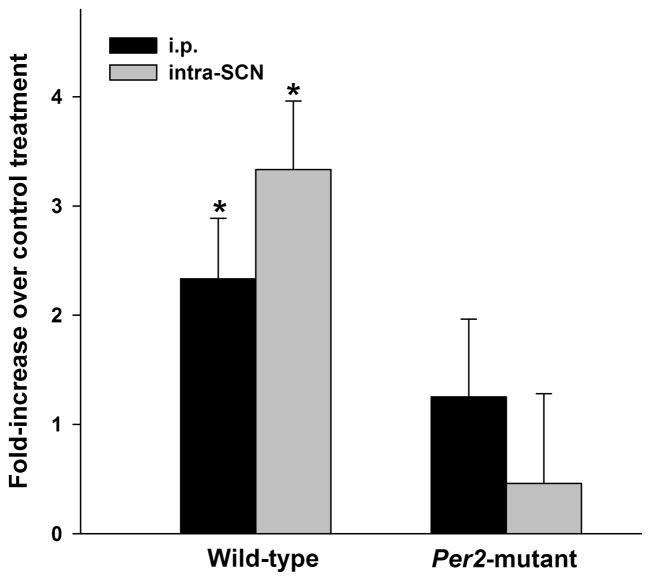
Figure 2.
Cocaine treatment enhances locomotor activity intensity in wild-type but not Per2 mutant mice. Bars represent means±SEM of fold-increases in activity duration immediately following systemic (i.p) or central (intra-SCN) cocaine compared with control (saline of ACSF) treatment. Asterisks annotate within-genotype statistical differences (p<0.05).
Localized reverse-microdialysis perfusion of the SCN region with cocaine dissolved in ACSF (~115 μM estimated tissue concentration) at ZT 6 resulted in larger phase-advances of locomotor activity rhythms compared with ACSF alone in WT and Per2 mutant mice (F1,12= 9.1, p<0.01; Fig. 3). There was no main effect for genotype (F1,12= 0.9; p>0.05). Phase-advances averaged 4.1±1.2 h in WTs and 2.2±0.7 h for Per2 mutants perfused with cocaine. Control perfusion with ACSF alone caused shifts of only 0.3±0.3 h for WTs and 0.3±0.5 h for Per2 mutants. There was also no significant interaction for genotype and treatment (F1,12 = 2.0; p>0.05). Analyses of rhythm period under free-running conditions revealed a main effect for genotype (F1,12 = 21.5; p<0.01), but not for treatment (p>0.05). Within-genotype analyses revealed no differences in behavioral activity measures between Per2 mutants perfused with cocaine dissolved in ACSF or ACSF alone (Student’s t test; p>0.05). In contrast, WTs were more behaviorally reactive to continuous perfusion of cocaine dissolved in ACSF compared to ACSF alone in the SCN, with both activity duration and intensity increasing by 3.3- and 3.5-fold, respectively (p<0.01; Fig. 2).
Figure 3.
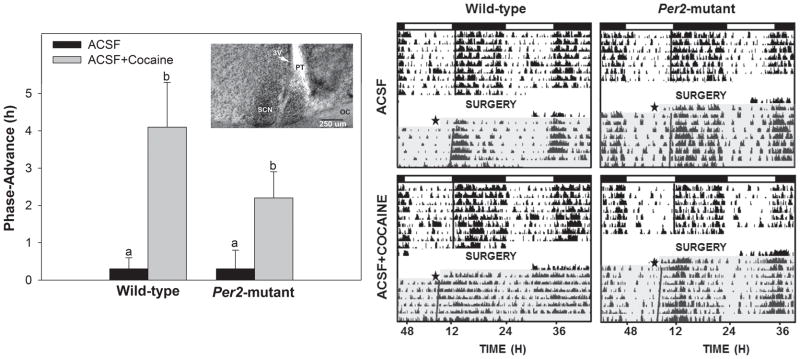
Direct reverse-microdialysis perfusion of the SCN with cocaine at midday (ZT 6) phase-advances the daily locomotor activity rhythm in Per2 mutant and WT mice. Left panel: graphic representation of the phase-resetting responses to cocaine and control treatments. Bars represent means±SEM. Bars with different letters are significantly different within and between strains (p<0.05). The insert is a photomicrograph showing a microdialysis probe tract (PT) targeted to the lateral margin the SCN. OC, optic chiasm; 3V, third ventricle. Right panel: representative double-plotted actograms of general locomotor activity showing phase-advancing responses to cocaine treatment (closed star designates onset of treatment). The light and dark phases of the LD cycle are represented above the actograms by the open and filled horizontal spaces, respectively. The 3-day absence of actographic data represents the post-operative recovery period. The shaded portion designates constant darkness following drug treatment (star).
3.2. Experiment 3
Per2 mutation and cocaine attenuation of photic phase-resetting
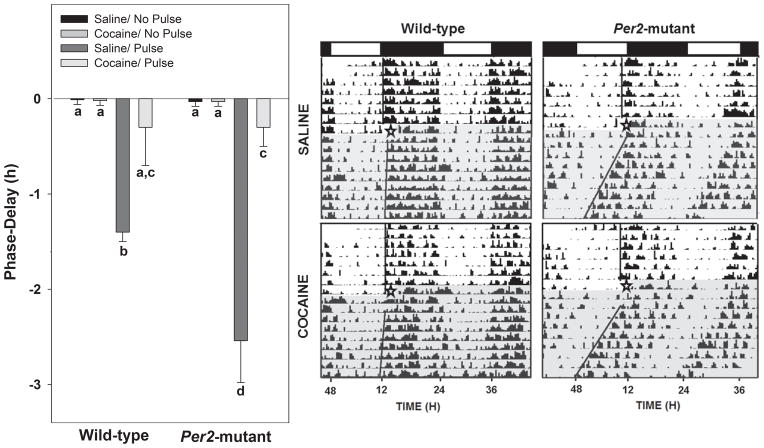
Photic phase-delays of locomotor activity were significantly attenuated by cocaine in WT and Per2 mutant mice compared to saline controls (F1,20= 34.3; p<0.01; Fig. 4). Although there was no effect of genotype on the magnitude of cocaine attenuated shifts (WT = −0.3±0.4 h, Per2-mutants = −0.3±0.2 h; F1,20= 1.6; p>0.05), the percent cocaine inhibition vs. saline was significantly greater for the Per2 mutant compared to WT mice (Per2 mutants = 88.9±0.7%, WT = 75.6±3.0%; F1,20= 18.0; p<0.01). There was also a significant interaction for genotype and treatment (F1,20 = 13.7; p<0.01). There was little phase-delaying response to cocaine or saline prior to sham (no light pulse) stimulation.
Figure 4.
Acute i.p. administration of cocaine attenuates the phase-delaying response to photic stimulation during early night (ZT 16) in Per2 mutant and WT mice. Left panel: graphic representation of the phase-delaying responses to light following cocaine and control treatments. Bars represent means±SEM. Bars with different letters are significantly different within and between strains (p<0.05). Right panel: representative double-plotted actograms of general locomotor activity showing phase-resetting responses to light following cocaine and control treatments (open star designates onset of treatment). The light and dark phases of the LD cycle are represented above the actograms by the open and filled horizontal spaces, respectively. The shaded portion designates constant darkness following drug treatment (star).
4.0 DISCUSSION
The circadian clock gene, Per2, is rhythmically expressed in the SCN and other areas, and is necessary for stabilizing endogenous circadian pacemaker activity as revealed by studies from Per2 mutant mice that express inactive PER2 protein [3]. In addition to its circadian function, Per2 is also strongly implicated in cocaine-seeking behavior in rodents [6] and cocaine dependence in humans [13]. This circadian clock gene-drug interaction appears to be reciprocal, as cocaine treatment alters clock gene expression in brain regions involved in drug-seeking and reward [15, 16]. Also, it has been reported that acute systemic cocaine treatment significantly impacts non-photic and photic pathways for clock entrainment [10], and chronic cocaine intake causes long-term alterations in the intrinsic free-running circadian period [11]. The neurophysiological basis of these drug effects on circadian entrainment and period is not clear, but it is likely that SCN clock genes are ultimately involved. To shed light on this question, we assessed the circadian effects of acute cocaine treatment in Per2 mutant mice. It was found that both non-photic and photic phase-resetting responses to acute systemic cocaine were enhanced in Per2 mutant vs. WT mice. Specifically, the non-photic phase-advancing action of cocaine at midday was significantly (3.5-fold) greater in the Per2 mutants. Interestingly, the phase-advancing response to intra-SCN cocaine application was similar between the two genotypes, suggesting that the enhanced shifting response of the Per2 mutants to systemic cocaine involves an extra-SCN mechanism. The Per2 mutants exhibited larger nighttime photic phase-delays than did WTs, and the attenuating action of cocaine on this response was proportionately greater than that seen in WTs. By inference, these data reveal that normal Per2 gene expression is a negative modulator of cocaine’s effects in the circadian system. This function may have direct relevance to reports that polymorphisms of this gene in humans are associated with increased risks for cocaine dependence [13].
4.1. Cocaine and Non-Photic Phase-Resetting
The present demonstration that cocaine has a phase-advancing action when applied at midday is consistent with a previous report on this effect as shown in vivo and in vitro [10]. This action is comparable to those of other non-photic stimuli, such as wheel running, sleep deprivation, and serotonin agonist (8-OH-DPAT) administration [17, 18]. Also in line with being a non-photic stimulus, cocaine’s shifting effect is phase-dependent, as it does not cause phase-shifts at early or late night [present results; 10]. Like the non-photic stimuli listed above, we hypothesize that the phase-resetting effect of cocaine in the absence of nonfunctional Per2 is mediated through its stimulation of SCN serotonergic activity. This contention is based upon several lines of converging evidence, including observations that treatment with the 5-HT receptor antagonist, metergoline, blocks the phase-resetting effect of cocaine in vivo and in vitro, and serotonin desensitizing pretreatment with the 5-HT receptor agonist, 8-OH-DPAT blocks this cocaine effect in vitro [10]. The neurochemical basis of cocaine’s stimulatory effect on serotonergic transmission in the SCN is likely through its inhibition of 5-HT transporters (SERT), resulting in increased extracellular levels of 5-HT. Within the striatum, the ability of cocaine to bind to serotonin transporters is evidenced by the total displacement of the cocaine analog (125I-RTI-55) by the selective 5-HT transport blocker, citalopram [19]. Also, brain microdialysis measurements have shown that cocaine markedly increases the extracellular concentration of serotonin [20, 21]. Notably, our recent finding that transgenic mice with a cocaine-insensitive SERT [22] fail to express cocaine-induced phase shifts in vitro strongly supports the SERT inhibition hypothesis [12].
The reason for the heightened phase-advancing response of the Per2 mutants to systemic treatment is not clear but warrants further investigation in other Per2 transgenic (conditional knockdown and overexpression) lines; however it could relate to demonstrations that exposure to non-photic stimuli, including wheel-running, NPY and 8-OH-DPAT administration suppresses Per2 expression [23, 24]. Thus, for the Per2 mutants, permanently inactivated PER could theoretically render them more responsive to the phase-resetting action of many non-photic stimuli. We also have shown that exposure to constant light, a procedure that reduces Per2 mRNA expression [25], greatly increases the phase-resetting actions of 8-OH-DPAT and other non-photic stimuli at midday [17, 26]. An important additional aspect related to the genotype difference in cocaine-induced phase-resetting is that, while our intra-SCN perfusion and in vitro data [10] confirm that the SCN is directly responsive to this drug action, the Per2 mutants’ shifting response to intra-SCN cocaine administration was not statistically different from that of the WT mice (or to their shifting response to systemic cocaine). In contrast, WT mice exhibited a ~4-fold increase in their shifting response to the intra-SCN vs. systemic cocaine treatment. The similarity of SCN-targeted phase-resetting responses between the Per2 mutant and WT mice raises the possibility that, since their SCN’s appear to be equally responsive to cocaine, the increased Per2 mutant shifting response to systemic cocaine may be due to slower peripheral clearance of the drug compared to WTs, or possibly, Per2 actions outside of the SCN. Comparisons of the pharmacokinetic profile of cocaine activity and in vitro shifting responses between both genotypes will be necessary to approach this question.
Two other points germane the cocaine phase-resetting effect should be considered. First, cocaine enhances extracellular dopamine (DA) levels by inhibiting DA transporters, and this could indirectly stimulate locomotor activity, resulting in a phase-resetting response. Although DA is important for fetal SCN clock entrainment, it is most likely not involved in the cocaine phase-resetting action, as the adult SCN does not respond to DA agonist stimulation [27]. Moreover, in vitro co-treatment of the SCN with the dopamine antagonist, fluphenazine, does not block cocaine-induced phase shifts. Second, the phase-resetting response to cocaine could be mediated by behavioral arousal. This is unlikely however, since the present analyses of locomotor activity intensity and duration after systemic drug treatment revealed that only WTs were responsive to the locomotor-stimulating effects of cocaine (relative to saline treatment). A reduction in locomotor responsiveness to acute cocaine administration in Per2-mutant compared with wild-type mice has also been reported in a previous study [5]. In sum, the amplified phase-advancing response to systemic cocaine in Per2 mutant mice does not correlate with increases in activity duration or intensity.
4.2. Cocaine and Photic Phase-Resetting
Another potentially disruptive effect of cocaine observed here is its attenuation of photic phase-delay shifts during the night. This finding concurs with previous demonstrations of this effect as shown in vivo and in vitro [10]. Such an effect is significant, because the SCN circadian clock synchronizes with the external environment largely through photic information conveyed from the retina to the SCN [28, 29, 30]. Impairment of this entrainment process by cocaine could theoretically cause marked disturbances in the timing of behavioral and other circadian clock-regulated functions. The mechanism underlying cocaine attenuation of photic phase-resetting may again be based on its enhancement of increased 5-HT signaling since 5-HT is a potent negative regulator of photic signaling in the SCN [30, 31, 32], and 5-HT antagonist treatment blocks cocaine’s inhibition of photic (glutamate-mediated) shifts in vitro [10].
In addition to the enhanced cocaine-induced non-photic phase-advances in Per2 mutants, there was a marked attenuation of cocaine-induced photic phase-resetting in these animals. Specifically, the extent of this attenuation was proportionately greater (90%) for the Per2 mutants compared to WT mice (75%). This result suggests that a normal physiologic level of Per2 expression negatively modulates cocaine’s suppression of photic signaling in the SCN. The basis of the enhanced cocaine suppression of Per2 mutant photic response is unknown, but is possibly related to an increase in serotonergic signaling and/or slower rate of clearance of cocaine compared to WT levels as posited above. We also observed that photic stimulation during early night (ZT 16) increased phase-delaying responses to i.p. saline in Per2 mutants compared with WTs. Per2 mutant mice also have significantly shorter intrinsic rhythms of locomotor activity compared to WTs [present analyses, 33], hypothetically requiring that Per2 mutant mice have larger phase-delaying responses in order to re- entrain to LD.
In conclusion, there is growing evidence for a reciprocal linkage between neural pathways regulating drug intake/reward and molecular systems for circadian timing. Our present findings that: 1) cocaine significantly alters non-photic and photic circadian entrainment; and 2) these effects are modulated by the Per2 clock gene lend support to this linkage. Our results also have translational value, since tandem repeat polymorphisms of PER2 are strongly linked to increased risks for cocaine dependence [13] and substance abuse is thought to exacerbate drug-induced disruption to mammalian circadian timing systems [34]. The pervasive effects of cocaine on circadian timing compounded by perturbed Per2 expression could amplify misalignment of behavioral, physiological and endocrine rhythms associated with cocaine consumption, thus contributing to the process of addiction.
HIGHLIGHTS.
Cocaine acts in the SCN clock as a non-photic circadian entrainment stimulus
Mice with non-functional Per2 are hypersensitive to non-photic actions of cocaine
Cocaine blocks the circadian phase-delaying action of light at night
Cocaine attenuation of photic phase-delays is heightened in Per2 mutant mice
Per2 is a potent (negative) modulator of cocaine’s actions in the circadian system
References
- 1.Oxenkrug GF, Dragovic LJ, Marks BH, Yuwiler A. Effect of cocaine on rat pineal melatonin synthesis in vivo and in vitro. Psychiatry Research. 1990;34:185–91. doi: 10.1016/0165-1781(90)90018-z. [DOI] [PubMed] [Google Scholar]
- 2.Giorgetti M, Zhdanova IV. Chronic cocaine treatment induces dysregulation in the circadian pattern of rats’ feeding behavior. Brain Research. 2000;877:170–175. doi: 10.1016/s0006-8993(00)02671-8. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. Journal of Biological Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 4.Ripperger JA, Albrecht U. The circadian clock component PERIOD2: from molecular to cerebral functions. Progress in Brain Research. 2012;199:233–45. doi: 10.1016/B978-0-444-59427-3.00014-9. [DOI] [PubMed] [Google Scholar]
- 5.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Neuroscience. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 7.Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiology International. 2011a;28:664–72. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brager AJ, Prosser RA, Glass JD. Acamprosate-responsive brain sites for suppression of ethanol intake and preference. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2011b;301:R1032–43. doi: 10.1152/ajpregu.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feillet CA, Ripperger JA, Magnone MC, Bulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Current Biology. 2006;16:2016–22. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 10.Glass JD, Brager AJ, Stowie AC, Prosser RA. Cocaine modulates pathways for photic and nonphotic entrainment of the mammalian SCN circadian clock. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2012;302:R740–50. doi: 10.1152/ajpregu.00602.2011. [DOI] [PubMed] [Google Scholar]
- 11.Stowie AC, Amicarelli MJ, Prosser RA, Glass JD. Effects of chronic oral cocaine self-administration and withdrawal on circadian regulation. Annual Society of Neuroscience Meeting; 2012; p. Abstr# 94.20. [Google Scholar]
- 12.Prosser RA, Blakely RD, Nackenoff AG, Glass JD. In vitro cocaine fails to phase-shift the mammalian circadian clock knock-in mice expression a serotonin transporter lacking high-affinity cocaine recognition. Annual Society of Neuroscience Meeting; 2012; p. Abstr# 94.06. [Google Scholar]
- 13.Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, Maloney T, Wong C, Volkow ND. Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Translational Psychiatry. 2012;2:e86. doi: 10.1038/tp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian Clocks. Amsterdam: North-Holland; 1965. pp. 95–111. [Google Scholar]
- 15.Lynch WL, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: A focus on circadian genes. Brain Research. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcón E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2008;56 (Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoch ME, Gobes SM, Pavlovska I, Su C, Mistlberger RE, Glass JD. Short-term exposure to constant light promotes strong circadian phase-resetting responses to non-photic stimuli in Syrian hamsters. European Journal of Neuroscience. 2004;19:2779–2790. doi: 10.1111/j.0953-816X.2004.03371.x. [DOI] [PubMed] [Google Scholar]
- 18.Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and serotonergic regulation of circadian rhythms. Biological Rhythm Research. 2000;31:240–283. [Google Scholar]
- 19.Rothman RB, Cadet JL, Akunne HC, Silverthorn ML, Baumann MH, Carroll FI, Rice KC, de Costa BR, Partilla JS, Wang JB. Studies of the biogenic amine transporters. IV. Demonstration of a multiplicity of binding sites in rat caudate membranes for the cocaine analog [125]RTI-55. Journal of Pharmacology and Experimental Therapeutics. 1994;270:296–309. [PubMed] [Google Scholar]
- 20.Andrews CM, Hank F, Kung HF, Lucki I. The 5-HT1A receptor modulates the effects of cocaine on extracellular serotonin and dopamine levels in the nucleus accumbens. European Journal of Pharmacology. 2005;508:123–130. doi: 10.1016/j.ejphar.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Essman WD, Singh A, Lucki I. Serotonergic properties of cocaine: Effects on a 5-HT2 receptor-mediated behavior and on extracellular concentrations of serotonin and dopamine. Pharmacology Biochemistry and Behavior. 1994;49:107–113. doi: 10.1016/0091-3057(94)90463-4. [DOI] [PubMed] [Google Scholar]
- 22.Thompson BJ, Jessen T, Henry LK, Field JR, Gamble KL, Gresch PJ, Carneiro AM, Horton RE, Chisnell PJ, Belova Y, McMahon DG, Daws LC, Blakely RD. Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. Proceedings of the National Academy of Sciences. 2011;108:3785–90. doi: 10.1073/pnas.1011920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya Tm, Okamura H, Shibata S. Non-photic entrainment by 5-HT 1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. Journal of Neuroscience. 2000;20:5867–73. doi: 10.1523/JNEUROSCI.20-15-05867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proceedings of the National Academy of Science. 1999;96:15211–15116. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudo M, Sassahara K, Moriya T, Akiyama M, Hamada Tm, Shibata S. Constant light housing attenuates circadian rhythms of mPer2 mRNA and mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience. 2003;121:493–499. doi: 10.1016/s0306-4522(03)00457-3. [DOI] [PubMed] [Google Scholar]
- 26.Duncan M, Franklin KM, Davis VA, Grossman GH, Knoch ME, Glass JD. Short-term constant light potentiation of large-magnitude circadian phase shifts induced by 8-OH-DPAT: effects on serotonin receptors and gene expression in the hamster suprachiasmatic nucleus. European Jouranl of Neuroscience. 2005;22:2306–2314. doi: 10.1111/j.1460-9568.2005.04399.x. [DOI] [PubMed] [Google Scholar]
- 27.Duffield GE, Hastings MH, Ebling FJ. Investigation into the regulation of the circadian system by dopamine and melatonin in the adult Siberian hamster (Phodopus sungorus) Journal of Neuroendocrinology. 1998;10:871–884. doi: 10.1046/j.1365-2826.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Research. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 29.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. Journal of Comparative Neurology. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 30.Ying S-W, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Research. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]
- 31.Selim M, Glass JD, Hauser UE, Rea MA. Serotonergic inhibition of light-induced Fos protein expression and extracellular glutamate in the suprachiasmatic nuclei. Brain Research. 1993;621:181–188. doi: 10.1016/0006-8993(93)90105-v. [DOI] [PubMed] [Google Scholar]
- 32.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. Journal of Neuroscience. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinlachner S, Jaconmeier B, Scherbarth F, Dernbach H, Kruse F, Albrecht U. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. Journal of Biological Rhythms. 2002;17:202–209. doi: 10.1177/074873040201700303. [DOI] [PubMed] [Google Scholar]
- 34.Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Medicine Reviews. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]