Structural Abstract
Purpose of review
The purpose is to discuss advances in the nutritional and pharmacological management of phenylketonuria (PKU).
Recent findings
Glycomacropeptide (GMP), a whey protein produced during cheese production, is a low-phe intact protein that represents a new dietary alternative to synthetic amino acids (AAs) for people with PKU. Skeletal fragility is a long-term complication of PKU that based on murine research, appears to result from both genetic and nutritional factors. Skeletal fragility in murine PKU is attenuated with the GMP diet, compared with an AA diet, allowing greater radial bone growth. Pharmacologic therapy with tetrahydrobiopterin (BH4), acting as a molecular chaperone for phenylalanine hydroxylase, increases tolerance to dietary phe in some individuals. Large neutral AAs (LNAA) inhibit phe transport across the intestinal mucosa and blood brain barrier; LNAA are most effective for individuals unable to comply with the low-phe diet.
Summary
Although a low-phe synthetic AA diet remains the mainstay of PKU management, new nutritional and pharmacological treatment options offer alternative approaches to maintain lifelong low phe concentrations. GMP medical foods provide an alternative to AA formula that may improve bone health, and BH4 permits some individuals with PKU to increase tolerance to dietary phe. Further research is needed to characterize the long-term efficacy of these new approaches for PKU management.
Keywords: phenylketonuria, glycomacropeptide, tetrahydrobiopterin, large neutral amino acids, bone biomechanical performance
Introduction
Phenyketonuria (PKU, OMIM 261600), an autosomal recessive disorder of phenylalanine (phe) metabolism, is the first human genetic disease to have effective programs for newborn screening and treatment. The average incidence of PKU in the United States is 1:12,707 live births with an estimated 14,988 individuals with PKU diagnosed from 1965–2010(1)**. PKU is caused by a deficiency of hepatic phenylalanine hydroxylase (PAH, EC 1.14.16.1) activity that catalyzes the conversion of phe to tyrosine in a reaction dependent on the PAH cofactor tetrahydrobiopterin (BH4; sapropterindihydrochloride), Figure 1(2). Tyrosine becomes an indispensable amino acid (AA) in PKU. Over 800 mutations in the PAH gene have been identified and most individuals with PKU are compound heterozygotes, www.pahdb.mcgill.ca. With normal intake of dietary protein, phe accumulates in the blood leading to toxic levels of phe in the brain and profound cognitive impairment. A lifelong low phe diet remains the mainstay of PKU management, reducing phe levels and protecting brain development. A comprehensive review indicates moderate evidence for a threshold effect of a phe level of >400 μmole/L associated with IQs of <85, Figure 2(3)**. Recommended treatment for individuals with PKU of all ages includes a low-phe diet with goal blood phe concentration between 120 to 360 μmole/L(4)**. The low-phe diet for individuals with classical PKU restricts protein intake from natural foods to 5–10 g protein per day (250–500 mg phe) and for nutritional adequacy requires a phe-free AA medical formula (24–32 oz per day) providing over 80% of protein and energy needs (5). Lifelong compliance with the diet is challenging and poor control of blood phe levels (6) results in neuropsychological deterioration and increased risk of congenital anomalies in children born to mothers with PKU (7)*. Although intellectual development is near normal with implementation of the low-phe diet shortly after birth, there is evidence of suboptimal health outcomes in PKU subjects treated with the AA diet including neurocognitive impairments such as poor executive function skills and psychiatric problems (8), skeletal fragility (9), and impaired renal function (10). Improved options for nutritional management and adjuvant therapy are needed to improve health outcomes for individuals with PKU. The purpose of this review is to discuss: advances in the nutritional management of PKU using glycomacropeptide (GMP), new evidence regarding the etiology of skeletal fragility in PKU, supplementation with large neutral amino acids (LNAA), and pharmacological treatment with the PAH cofactor BH4.
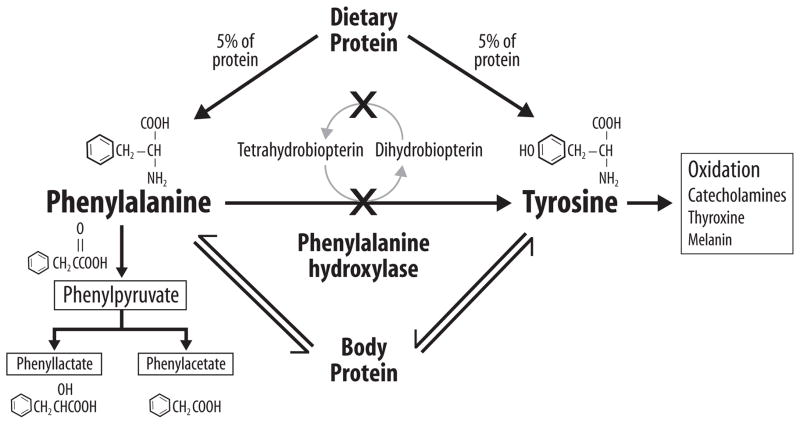
Figure 1.
Phenylalanine (phe) metabolism in phenylketonuria (PKU). As indicated by the “X”, PKU results from mutations (over 800 have been identified) that affect the hepatic phe hydroxylase (PAH) enzyme needed for the hydroxylation of the indispensable amino acid phe to tyrosine. PKU may also result from mutations in genes involved in recycling the essential PAH cofactor tetrahydrobiopterin. Due to these mutations which reduce the conversion of phe to tyrosine, phe accumulates in blood and is transaminated and decarboxylated into many compounds which appear in blood and urine; three of the compounds which are measured clinically are shown. Tyrosine, a precursor for multiple biological products, becomes an indispensable AA and must be provided by the diet for those with PKU. Under physiological conditions PAH catalyzes about 75% of the phe input from the diet and protein catabolism.
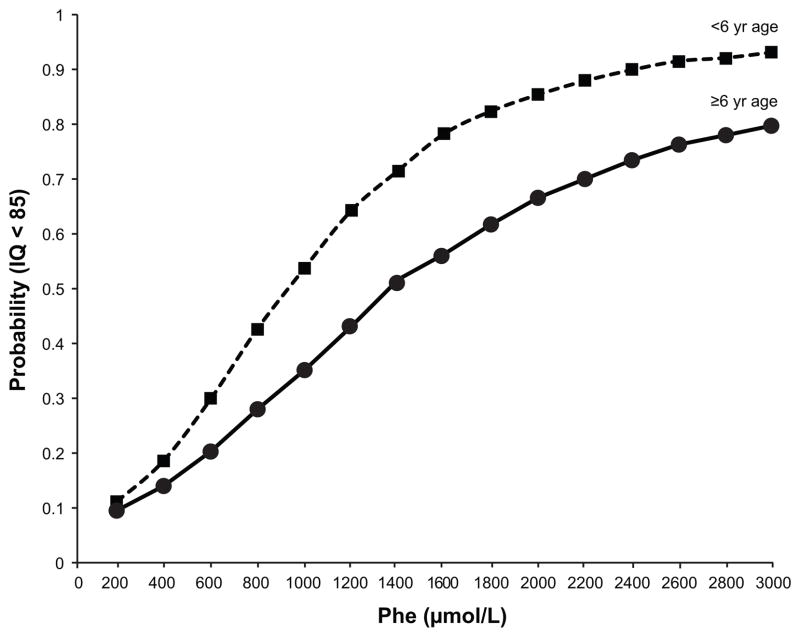
Figure 2.
Probability of intelligence quotient (IQ) <85 at varying blood phenylalanine (phe) levels and phe measurement times. Blood phe levels were historical, that is measured more than one year prior to IQ testing, in children before age 6 or at or after age 6. There is moderate evidence for a threshold effect of a phe level of 400 μmole/L associated with IQs of <85. The probability of having an IQ <85 does not increase considerably above a blood phe level of 2,000 μmole/L. Adapted with permission from [3].
Glycomacropeptide provides a source of low-phe intact protein for PKU
GMP occurs naturally in bovine milk within the whey fraction, and is the only known dietary protein that contains no phe. Thus, GMP provides a source of low-phe intact protein that is an alternative to synthetic AAs in the PKU diet. GMP is a polar glycophosphopeptide comprised of 64 AAs whose unique AA profile includes an absence of the aromatic amino acids, phe, tryptophan and tyrosine, and higher concentrations of isoleucine and threonine than those found in other dietary proteins (11). Commercial GMP is a by-product of cheese production and is used as a food ingredient for a variety of applications (11). Highly-purified GMP containing less than 2.0 mg phe per gram of protein is required for formulation of GMP medical foods for PKU. Sensory studies in individuals with PKU indicate that GMP medical foods are acceptable alternatives to AA medical foods and, in general, improve taste and variety in the PKU diet (7, 12).
To provide a complete source of protein for individuals with PKU, GMP is supplemented with arginine, histidine, leucine, tyrosine and tryptophan (13). Studies in the murine model of PKU (Pahenu2) demonstrate that GMP supplemented with limiting indispensable AAs provides an adequate source of protein that supports growth and accretion of lean body mass with the benefit of reduced body fat mass and an apparent increase in fat oxidation (14, 15). The anti-obesity effects of GMP are relevant, as females with PKU have a greater incidence of overweight and obesity compared with U.S. children (16).
Evidence from studies in murine and human PKU indicates that GMP is a more physiologic source of low-phe dietary protein compared with synthetic AAs (7, 12). The salient feature is that GMP medical foods provide predominantly intact protein (less than 25% supplemental AAs) compared with AA medical foods which provide 100% free AAs and a typical Western diet which provides less than 10% free AAs. Greater protein synthesis and N retention are observed with ingestion of intact protein compared with a mixture of single AA. Therefore, GMP consumption mimicks ingestion of intact proteins, due to slower absorption and decreased hepatic degradation of dietary intact protein compared with free AAs (17). Similar benefits were observed in an inpatient study of 11 PKU subjects. The GMP diet showed significantly higher postprandial plasma AA concentrations and a trend for higher insulin concentration, suggesting slower AA absorption after a meal, and significantly lower blood urea concentration, suggesting decreased ureagenesis, compared with the AA diet (13). Postprandial plasma phe concentrations were not different with the AA and GMP diets, but fasting phe levels tended to be lower with the GMP diet, suggesting less daily variation in phe concentration. Evidence also suggests that GMP promotes satiety in those with PKU, based on greater subjective feelings of fullness and decreased postprandial plasma ghrelin concentration (18). In summary, GMP might improve dietary compliance and reduce phe levels (12). Further studies in PKU subjects are underway to evaluate metabolic and nutritional features of the low-phe GMP and AA diets (www.clinicaltrials.gov NCT 01428258).
Skeletal fragility is a chronic, poorly understood complication of PKU
Skeletal fragility is a chronic complication of treated PKU. Compared to siblings and age- and gender-matched controls, patients with PKU have lower bone mineral density (BMD) (9) and lower bone mineral content, suggesting a higher lifetime fracture risk. In the only study to evaluate fracture rates, individuals with PKU had higher fracture rates after age 8 compared to sibling controls, suggesting that fractures could relate to higher phe levels (19). However, the pathophysiology of skeletal fragility in PKU is unknown. Cross sectional studies show no consistent relationships between BMD and blood phe levels (9), vitamin D levels (9, 20), nutrient intake (20, 21), and physical activity (22). Longitudinal studies are needed to determine the pathogenesis of the PKU skeletal phenotype, permitting clinicians to recommend appropriate therapy to help individuals with PKU to achieve and maintain optimal bone biomechanical performance and prevent fractures.
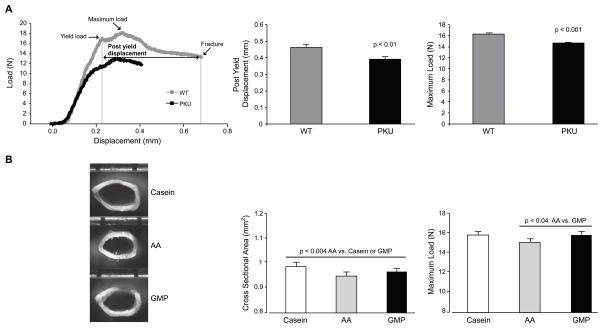
A critical question is whether the skeletal fragility in PKU is inherent to the genotype and/or its management with the low-phe, synthetic AA diet. This question cannot be addressed in humans, since the low-phe AA diet must be followed for life to prevent neurologic sequelae including cognitive impairment. Evidence from a long-term factorial experiment in wild type and Pahenu2 mice answered this question – both the PKU genotype and the AA diet contribute to the PKU bone phenotype (23)**. Regardless of diet and sex, PKU mice showed global deficits in BMD and bone biomechanical performance resulting in brittle, weak femora compared to wild type littermates, Figure 3. It is important to note that the bone deficit in the PKU mice is global. Stiffness, strength, and brittleness are all adversely affected by the disease. The increased bone brittleness in murine PKU strongly suggests that the disease results in the production of abnormal bone matrix protein as well as abnormal mineralization. Moreover, the AA diet resulted in smaller femora with reduced cross-sectional area and reduced strength, reflected in reduced maximal load tolerated before fracture, in both PKU and wild type mice compared with the GMP diet (23).
Figure 3.
PKU genotype decreases femoral strength (maximum load) and ductility (post yield displacement) compared with WT genotype (A). Provision of dietary protein from AAs decreases femoral size (cross sectional area) and strength (maximum load) in both PKU and WT mice compared with casein and GMP diets (B). Schematic of a load displacement curve generated from the three-point bending test is shown in A and representative photographs of femoral cross-sectional geometry in PKU mice fed casein, AA and GMP diets is shown in B. Adapted with permission from [23].
The observation of smaller and weaker femora in both wild type and PKU mice fed an AA diet strongly suggests that the AA diet prevents optimal bone development. Radial bone growth is a homeostatically regulated process that is believed to maintain strain, or fractional changes in bone length that result from mechanical loading, within a narrow range over the course of usual activities (24, 25). This model successfully accounts for the anabolic effects of skeletal loading, the catabolic effects of skeletal unloading, and the growth bone response to disorders in which the tissue-level mechanical performance of bone is impaired. Moreover, bone size is a major determinant of bone strength (24, 25), so failure to achieve normal bone size increases the risk of subsequent fracture. There are several possible explanations for the detrimental effects of the AA diet on bone. Impaired biosynthesis of the bone matrix protein collagen due to poor retention and subtle deficiencies of AAs required for collagen synthesis, such as glycine, proline and lysine, may occur. Cross link formation, maturation and chemistry may be altered, thus reducing the tensile strength of the matrix (26). Alterations in substrate utilization for energy balance (27); impaired renal function (10) leading to alterations in vitamin D metabolism and calcium balance; and provision of a high dietary acid load with the AA diet.
Manz et al. recognized metabolic acidosis as a complication of the synthetic AA diet in 1977 (28). Surprisingly, no investigators have subsequently assessed whether the acidic AA diet contributes to PKU-related osteoporosis. In an inpatient metabolic study, the AA diet introduced a greater dietary potential renal acid load and evoked adaptations consistent with metabolic acidosis based on reduced serum bicarbonate levels, compared with the GMP diet (13). In humans, a high dietary acid load adversely affects skeletal health due to bone buffering of H+ ions, evoking increased bone resorption and renal calcium excretion with lower BMD (29, 30). Support for the acid load hypothesis comes from a recent study of 67 individuals with PKU, which reported hypercalciuria in nearly one-fourth of subjects (10). In a randomized, placebo controlled trial of 201 older adults without PKU (31), neutralizing dietary acid load with potassium citrate for 24 months increased areal spine BMD and increased trabecular density in the extremities. Additional research is needed to evaluate whether the high dietary acid load conferred by the AA diet contributes to skeletal fragility by increasing bone resorption and renal calcium excretion in people with PKU.
Among young adults with PKU treated since birth with an AA diet, increasing dietary protein intake was associated with decreasing glomerular filtration rate(r=−0.41, p=0.003) (10). Reduced renal function in PKU could decrease renal 1,25 (OH)2D synthesis and reduce fractional calcium absorption, leading to a decline in BMD. Taken together, the AA diet provides a high dietary acid load and might also impair renal function (32) resulting in multiple negative effects on bone health. The pathophysiology of skeletal fragility in PKU is an important topic for future investigation.
Supplementation with large neutral amino acids (LNAA) may competitively inhibit phe transport
LNAA are dietary supplements prescribed for adults with PKU who cannot adhere to a low-phe diet (33). The rationale is that LNAA may decrease concentrations of phe in blood and brain by competitive inhibition of phe transportacross the intestinal mucosa and blood brain barrier by the four L system transporters (LAT 1–4) (34)*. LNAA transported by these carriers are all indispensable for synthesis of proteins in PKU and include branched chain (valine, leucine, and isoleucine), aromatic (phe, tyrosine and tryptophan) and other AAs including histidine, threonine and methionine (35)*. The LAT-1 transporter expressed in brain has a high affinity for phe, and this in combination with 2–10 fold higher concentrations of phe relative to other LNAAs in blood, results in excess transport of phe into brain which is generally considered neurotoxic (8). Moreover, concentrations of the non-phe LNAAs are decreased, reducing synthesis of essential brain proteins such as the neurotransmitters dopamine and serotonin, due to limited availability of their respective tyrosine and tryptophan precursors (36). Specific supplementation with tyrosine and tryptophan improved metabolism of dopamine and serotonin in PKU patients, however later studies with larger doses of tyrosine and tryptophan did not show positive results (33). Moreover, evidence that LNAA supplementation increases melatonin, a serotonin metabolite, suggests that levels of melatonin in blood and urine may serve as an accessible biomarker reflecting brain serotonin synthesis in subjects with PKU (35). Additional surrogate biomarkers reflecting the metabolic effects of brain phe are needed to complement blood phe levels, thereby assisting with assessment of metabolic control of PKU.
The concept of competitive inhibition of phe transport underlying supplementation with LNAAs, is being applied to non-physiological AAs. Studies in Pahenu2 mice provide support for the use of a variety of non-physiological AAs to act as competitive inhibitors of brain AA transporters resulting in reduced brain phe concentrations with minimal impact on other downstream intermediates(34).
Limited evidence supports the efficacy of LNAA supplementation to reduce blood phe levels and improve cognitive outcomes. Three short-term studies addressed the effects of LNAA in a total of 47 participants using dosages ranging from 250 mg to 1 g LNAA/kg/day (3). One study noted negative nitrogen balance in 4 subjects taking LNAAs (0.8 g/kg/day) and consuming natural protein (0.6 g/kg/day) without AA formula (37). Individuals with phe levels >1,000 μmoles/L showed 25–39% decreases in plasma phe levels, and one randomized controlled study reported a positive effect of LNAA on executive functions (38). In summary, LNAA supplementation, either alone or in combination with a low-phe diet, has potential to improve health outcomes for individuals unable to follow the low-phe diet. However, current evidence for the efficacy of LNAA supplementation cannot be considered as more than proof of concept; additional research is needed to demonstrate that LNAAs provide a viable approach for reducing phe levels or increasing dietary phe tolerance (3), without adversely affecting renal and bone health.
Pharmacologic treatment with tetrahydrobiopterin acting as a molecular chaperone increases dietary phe tolerance for some with PKU
Tetrahydrobiopterin (BH4) is an essential cofactor for nitric oxide synthases and three aromatic AA hydroxylase enzymes, PAH, tyrosine hydroxylase, and tryptophan hydroxylase. In about 20%–30% of PKU patients, phe levels may be controlled through pharmacologic treatment with BH4 (39)*. Sapropterindihydrochloride (Kuvan®), the synthetic form of BH4, was approved for the treatment of patients with BH4-responsive PKU in combination with diet by the US Food and Drug Administration in 2007 and the European Medicines Agency in 2008(40). In general, patients with the milder PKU phenotypes have a higher response rate to BH4 than those with low dietary phe tolerance. Patient genotypes can help predict BH4 responsiveness. The BIOPKU database lists PAH mutations and patients/genotypes and their reported response to BH4 (www.biopku.org). An oral challenge with BH4 (20 mg/kg/day for 2 days or 1–3 weeks) can identify those with BH4-responsive PKU; a 30% decrease in blood phe level compared to the basal value is a positive response (39).
For a patient to respond to BH4, sufficient residual liver PAH protein must be present to interact with BH4. Clinical BH4 responsiveness is usually determined by the presence of a single BH4-responsive mutation with at least 25% residual PAH activity; although there are exceptions depending on interallelic complementation within the genotype (39). BH4 increases residual PAH activity by acting as a molecular chaperone to stabilize partially misfolded PAH proteins. BH4 also affects PAH enzyme kinetics as illustrated by different responses to BH4 depending on baseline phe concentration and the BH4 dose administered in patients with the same genotype (41).
BH4 therapy significantly reduced phe levels compared to placebo in two randomized controlled trials and two open label trials, without significant adverse effects (3). In positive-responders baseline phe tolerance may increase 2-4 fold permitting an additional 400-1,000 mg of dietary phe each day, and allowing for the addition of 2-3 servings of typical bread and cereal products in place of low protein products. However, few individuals with PKU can achieve a completely liberalized diet, and low-phe medical foods are almost always required for nutritional adequacy. A retrospective study of 147 patients with both mild and classic PKU treated with BH4 and followed for over 10 years indicated that 47% of patients reported improvement in dietary adherence and 63% reported improved adherence to treatment (40). The potential effects of BH4 on long term health outcomes such as cognition, executive function and quality of life have not been established; studies are ongoing. Overall, the strength of the evidence for a large, positive effect of BH4 on reducing phe levels and improving metabolic control over the short term in some PKU groups is moderate(3).
Conclusions
A low-phe diet remains the cornerstone of PKU management. GMP medical foods improve taste and variety and provide a more physiologically normalized source of intact protein that might improve bone health compared with synthetic AAs. Adjuvant pharmacologic therapy with BH4 allows some individuals with PKU to liberalize their diet. Long-term outcome studies assessing efficacy and safety of BH4, LNAA supplementation, and GMP medical foods are needed. Emerging therapeutic approaches are currently in preclinical and clinical trials including PAH enzyme substitution with phe ammonia lyase joined with polyethylene glycol (PEG-PAL) (39), hepatocyte repopulation, and gene therapy. The future holds promise for new options to allow PKU patients to liberalize their diet and still improve lifelong metabolic control.
Key Points.
Phenylketonuria (PKU) results from mutations in the phenylalanine hydroxylase (PAH) gene that in concert with the essential cofactor tetrahydrobiopterin (BH4) converts phenylalanine (phe) to tyrosine.
A low-phe diet that restricts intake of natural protein and requires a phe-free amino acid (AA) medical formula is the mainstay of PKU management, reducing phe levels and preventing cognitive impairment.
Food products made with glycomacropeptide (GMP), a low-phe whey protein, provide an alternative to AA formula for the nutritional management of PKU.
Skeletal fragility has emerged as a poorly understood, but common complication of treated PKU that shows no consistent association with blood phe levels.
New adjunct therapies for PKU include drug treatment with the cofactor BH4, to enhance residual PAH activity, and supplementation with large neutral amino acids to decrease phe levels by competing with phe for shared AA transporters.
Acknowledgments
No financial support provided.
Footnotes
Conflicts of Interest: DMN is a co-inventor on U.S. Patent Application US-2010-0317597, entitled “Glycomacropeptide (GMP) medical foods for nutritional management of phenylketonuria (PKU) and other metabolic disorders,” which is held by the Wisconsin Alumni Research Foundation and licensed to Cambrooke Foods, LLC. A percentage of all royalty payments is awarded to the inventors. RDB has no conflicts of interest to declare. KEH is a consultant to Deltanoid Pharmaceuticals and Takeda Pharmaceuticals.
References
- 1**.Berry SA, Brown C, Grant M, et al. Newborn screening 50 years later: access issues faced by adults with PKU. Genet Med. 2013 Mar 7; doi: 10.1038/gim.2013.10. This review summarizes the prevalence of PKU and individual patient, social and economic factors preventing more than 70% of adults PKU patients in the United States from accessing treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life. 2013 Apr;65(4):341–9. doi: 10.1002/iub.1150. [DOI] [PubMed] [Google Scholar]
- 3**.Lindegren M, Krishnaswami S, Fonnesbeck C, et al. Adjuvant treatment for phenylketonuria (PKU). Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; Feb, 2012. Review No.56. AHRQ Publication No 12-EHC035-EF. Systematic review of evidence on adjuvant treatment of PKU, including sapropterin dihydrochloride (BH4) and large neutral amino acid supplementation, conducted by the Vanderbilt Evidence-Based Practice Center under contract to the Agency for Healthcare Research and Quality and presented at the NIH Scientific Review of Evidence for PKU conference held February 2012. [PubMed] [Google Scholar]
- 4**.Howell R. PKU treatment guidelines. Genetics in Medicine. 2013 To be published summer 2013 Recommendations for the treatment of PKU based in part on a comparative effectiveness review conducted by the Vanderbilt Evidence-Based Practice Center under contract to the Agency for Healthcare Research and Quality and presented at the NIH Scientific Review of Evidence for PKU conference held February 2012. [Google Scholar]
- 5.Camp KM, Lloyd-Puryear MA, Huntington KL. Nutritional treatment for inborn errors of metabolism: indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol Genet Metab. 2012 Sep;107(1–2):3–9. doi: 10.1016/j.ymgme.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartnett C, Salvarinova-Zivkovic R, Yap-Todos E, et al. Long-term outcomes of blood phenylalanine concentrations in children with classical phenylketonuria. Mol Genet Metab. 2013 Apr;108(4):255–8. doi: 10.1016/j.ymgme.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 7*.Van Calcar SC, Ney DM. Food Products Made with Glycomacropeptide, a Low-Phenylalanine Whey Protein, Provide a New Alternative to Amino Acid-Based Medical Foods for Nutrition Management of Phenylketonuria. Journal of the Academy of Nutrition and Dietetics. 2012;112:1201–10. doi: 10.1016/j.jand.2012.05.004. Review of evidence and future research needs for utilization of GMP medical foods in the PKU diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antenor-Dorsey JA, Hershey T, Rutlin J, et al. White matter integrity and executive abilities in individuals with phenylketonuria. Mol Genet Metab. 2013 Jun;109(2):125–31. doi: 10.1016/j.ymgme.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groot MJ, Hoeksma M, van Rijn M, et al. Relationships between lumbar bone mineral density and biochemical parameters in phenylketonuria patients. Mol Genet Metab. 2012 Apr;105(4):566–70. doi: 10.1016/j.ymgme.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Hennermann JB, Roloff S, Gellermann J, et al. Chronic kidney disease in adolescent and adult patients with phenylketonuria. J Inherit Metab Dis. 2012 Nov 9; doi: 10.1007/s10545-012-9548-0. [DOI] [PubMed] [Google Scholar]
- 11.Brody EP. Biological activities of bovine glycomacropeptide. Br J Nutr. 2000 Nov;84( Suppl 1):S39–46. doi: 10.1017/s0007114500002233. [DOI] [PubMed] [Google Scholar]
- 12.Ney DM, Gleason ST, van Calcar SC, et al. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis. 2009;32(1):32–9. doi: 10.1007/s10545-008-0952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Calcar SC, Macleod EL, Gleason ST, et al. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am J Clin Nutr. 2009;89(4):1068–77. doi: 10.3945/ajcn.2008.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solverson P, Murali SG, Brinkman AS, et al. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am J Physiol Endocrinol Metab. 2012 Apr;302(7):E885–95. doi: 10.1152/ajpendo.00647.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu SP, Mao XY, Cheng X, Chen B. Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague-Dawley rats. Food Chem Toxicol. 2013 Jun;56:1–7. doi: 10.1016/j.fct.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Burrage LC, McConnell J, Haesler R, et al. High prevalence of overweight and obesity in females with phenylketonuria. Mol Genet Metab. 2012 Sep;107(1–2):43–8. doi: 10.1016/j.ymgme.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Dangin M, Boirie Y, Garcia-Rodenas C, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001 Feb;280(2):E340–8. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 18.Macleod EL, Clayton MK, van Calcar SC, Ney DM. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol Genet Metab. 2011 Apr 14; doi: 10.1016/j.ymgme.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greeves LG, Carson DJ, Magee A, Patterson CC. Fractures and phenylketonuria. Acta Paediatr. 1997 Mar;86(3):242–4. doi: 10.1111/j.1651-2227.1997.tb08882.x. [DOI] [PubMed] [Google Scholar]
- 20.Modan-Moses D, Vered I, Schwartz G, et al. Peak bone mass in patients with phenylketonuria. J Inherit Metab Dis. 2007 Apr;30(2):202–8. doi: 10.1007/s10545-007-0462-9. [DOI] [PubMed] [Google Scholar]
- 21.McMurry MP, Chan GM, Leonard CO, Ernst SL. Bone mineral status in children with phenylketonuria--relationship to nutritional intake and phenylalanine control. Am J Clin Nutr. 1992 May;55(5):997–1004. doi: 10.1093/ajcn/55.5.997. [DOI] [PubMed] [Google Scholar]
- 22.Miras A, Boveda MD, Leis MR, et al. Risk factors for developing mineral bone disease in phenylketonuric patients. Mol Genet Metab. 2013 Mar;108(3):149–54. doi: 10.1016/j.ymgme.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 23**.Solverson P, Murali SG, Litscher SJ, et al. Low bone strength is a manifestation of phenylketonuria in mice and is attenuated by a glycomacropeptide diet PloS ONE. 2012;7(9):e45165. doi: 10.1371/journal.pone.0045165. This study characterizes the PKU bone phenotype and the contributions of genotype and dietary protein source to the bone phenotype in Pahenu2 mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost HM. From Wolff’s law to the Utah paradigm: Insights about bone physiology and its clinical applications. Anatomical Record. 2001 Apr 1;262(4):398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 25.McBride SH, Silva MJ. Adaptive and injury response of bone to mechanical loading. BoneKEy Rep. 2012;1:192. doi: 10.1038/bonekey.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010 Feb;21(2):195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 27.Duque G, Li W, Vidal C, et al. Pharmacological inhibition of PPARgamma increases osteoblastogenesis and bone mass in male C57BL/6 mice. J Bone Miner Res. 2013 Mar;28(3):639–48. doi: 10.1002/jbmr.1782. [DOI] [PubMed] [Google Scholar]
- 28.Manz F, Schmidt H, Scharer K, Bickel H. Acid-base status in dietary treatment of phenylketonuria. Pediatr Res. 1977 Oct;11(10 Pt 2):1084–7. [PubMed] [Google Scholar]
- 29.Adeva MM, Souto G. Diet-induced metabolic acidosis. Clin Nutr. 2011 Aug;30(4):416–21. doi: 10.1016/j.clnu.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Bushinsky DA. Net calcium efflux from live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol. 1989 May;256(5 Pt 2):F836–42. doi: 10.1152/ajprenal.1989.256.5.F836. [DOI] [PubMed] [Google Scholar]
- 31.Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013 Jan;98(1):207–17. doi: 10.1210/jc.2012-3099. [DOI] [PubMed] [Google Scholar]
- 32.Ney DM. Does the PKU diet contribute to impaired renal function? J Inherit Metab Dis. 2013 May 8; doi: 10.1007/s10545-013-9615-1. [DOI] [PubMed] [Google Scholar]
- 33.van Spronsen FJ, de Groot MJ, Hoeksma M, et al. Large neutral amino acids in the treatment of PKU: from theory to practice. J Inherit Metab Dis. 2010 Dec;33(6):671–6. doi: 10.1007/s10545-010-9216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Vogel KR, Arning E, Wasek BL, et al. Non-physiological amino acid (NPAA) therapy targeting brain phenylalanine reduction: pilot studies in PAH (ENU2) mice. J Inherit Metab Dis. 2013 May;36(3):513–23. doi: 10.1007/s10545-012-9524-8. Studies in Pahenu2 mice provide proof-of-principle for the use of non-physiological amino acids to act as competitive inhibitors of brain amino acid tranporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Yano S, Moseley K, Azen C. Large neutral amino acid supplementation increases melatonin synthesis in phenylketonuria: a new biomarker. J Pediatr. 2012 May;162(5):999–1003. doi: 10.1016/j.jpeds.2012.10.015. This study demonstrates that levels of melatonin in blood and urine are an accessible biomarker reflecting brain serotonin synthesis in subjects with PKU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walterfang M, Bonnot O, Mocellin R, Velakoulis D. The neuropsychiatry of inborn errors of metabolism. J Inherit Metab Dis. 2013 May 23; doi: 10.1007/s10545-013-9618-y. [DOI] [PubMed] [Google Scholar]
- 37.Dotremont H, Francois B, Diels M, Gillis P. Nutritional value of essential amino acids in the treatment of adults with phenylketonuria. J Inherit Metab Dis. 1995;18(2):127–30. doi: 10.1007/BF00711746. [DOI] [PubMed] [Google Scholar]
- 38.Schindeler S, Ghosh-Jerath S, Thompson S, et al. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab. 2007 May;91(1):48–54. doi: 10.1016/j.ymgme.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39*.Heintz C, Cotton RG, Blau N. Tetrahydrobiopterin, its Mode of Action on Phenylalanine Hydroxylase, and Importance of Genotypes for Pharmacological Therapy of Phenylketonuria. Hum Mutat. 2013 Apr 4; doi: 10.1002/humu.22320. Detailed review of the phenylalanine hydroxylase system and the genetic, molecular and metabolic basis for response to tetrahydrobiopterin therapy in PKU. [DOI] [PubMed] [Google Scholar]
- 40.Keil S, Anjema K, van Spronsen FJ, et al. Long-term Follow-up and Outcome of Phenylketonuria Patients on Sapropterin: A Retrospective Study. Pediatrics. 2013 Jun;131(6):e1881–8. doi: 10.1542/peds.2012-3291. [DOI] [PubMed] [Google Scholar]
- 41.Staudigl M, Gersting SW, Danecka MK, et al. The interplay between genotype, metabolic state and cofactor treatment governs phenylalanine hydroxylase function and drug response. Hum Mol Genet. 2011 Jul 1;20(13):2628–41. doi: 10.1093/hmg/ddr165. [DOI] [PubMed] [Google Scholar]