Abstract
Background
Co-morbidity of schizophrenia (SZ) and metabolic problems such as diabetes mellitus (DM) has been suggested by many studies. Nonetheless, it is still debated whether DM affects cognitive dysfunction associated with SZ and how much treatment for DM is beneficial for cognitive functions in SZ. We addressed these questions by re-assessing the cognitive dataset from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study.
Methods
We identified 1289 SZ patients in which scores for several cognitive domains of verbal memory, vigilance, processing speed, reasoning, and working memory together with the composite score and metabolic characteristics (body mass index, dyslipidemia, hypertension, and DM) were available at baseline of the trial. We performed multiple linear regression analyses to assess the impact of DM on cognitive performance of SZ patients, controlling for a number of other confounding factors including obesity, hypertension, and dyslipidemia. We also conducted analyses of covariance to compare cognitive performance among SZ patients without DM and diabetic SZ sub-groups based on anti-diabetic drugs they were receiving at baseline of the trial.
Results
Co-morbid DM with SZ predicted worse overall cognitive performance and lower scores for three cognitive domains (vigilance, processing speed, and reasoning), but none of the other metabolic factors (i.e., obesity, hypertension and dyslipidemia) correlated with cognitive function in SZ. Furthermore, SZ patients with untreated DM showed poorer overall cognitive performance and a significantly lower score in the domain of vigilance compared with SZ patients without DM.
Conclusion
Our data suggest that DM negatively affects the overall cognitive function of SZ patients.
Keywords: diabetes, insulin resistance, cognitive function, metabolic syndrome, physical comorbidity, schizophrenia
1. Introduction
The prevalence of diabetes mellitus (DM) is high in patients with schizophrenia (SZ ) compared with normal controls, ranging from 8 to 22% (Chien et al., 2009; Cohen et al., 2006; Goff et al., 2005; Heald 2010; Okumura et al., 2010; Philippe et al., 2005; Suvisaari et al., 2008). This is partly due to side effects of long-term medications (Bergman and Ader 2005; Newcomer et al., 2002). However, recent studies have reported glucose intolerance even in recent-onset, drug-naïve SZ patients (Fernandez-Egea et al., 2009; Kirkpatrick et al., 2010; Ryan et al., 2003; Saddichha et al., 2008; Spelman et al., 2007). These results suggest that SZ is accompanied by intrinsic abnormalities that can underlie the metabolic disturbance associated with DM.
The pathophysiology of type 1 DM (destruction of beta cell) and type 2 DM (insulin resistance) are different. The positive association of insulin resistance or type 2 DM with SZ has been repeatedly reported (Cohen et al., 2006; Fernandez-Egea et al., 2009; Kirkpatrick et al., 2010; Ryan et al., 2003; Saddichha et al., 2008; Spelman et al., 2007; Suvisaari et al., 2008), whereas Juvonen et al. (2007) reported a negative association between SZ and type 1 DM (i.e., insulin deficiency).
Recently, a positive correlation of DM with cognitive impairment or dementia has been demonstrated (Reijmer et al., 2010; Strachan et al., 2011). Specifically in the general population, DM was associated with abnormal performance in the domains of memory, attention and psychomotor speed (reviewed by van den Berg et al., 2009). Nonetheless, it is not fully known to what extent and specific effects metabolic problems such as DM affect cognitive dysfunction associated with SZ, and if the treatment against DM has beneficial effects on cognitive functions in SZ. Of the very few studies so far published, Dickinson et al. (2008) reported cognitive deficits in SZ patients with DM, whereas Friedman et al. (2010) reported a negative relationship between cognitive ability and hypertension or obesity (body mass index, BMI) in SZ.
Here we assessed the relationship between DM and cognitive function in SZ patients to expand understanding of the impact of DM on cognition in SZ, by controlling for a number of other possible confounding factors including hypertension, dyslipidemia and BMI using the baseline dataset in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) SZ study (Keefe et al., 2003). We also compared the cognitive performances between SZ patients taking anti-diabetic drugs and those not taking anti-diabetic drugs to assess how anti-diabetic medication may influence cognitive performance in SZ.
2. Methods
2.1. Study participants
The CATIE SZ study was primarily designed to compare the efficacy of atypical antipsychotics and one typical antipsychotic with a randomized clinical trial using a large sample of SZ patients at multiple (57) institutions. Patients between18–65 years who received a Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) diagnosis of SZ, based on the Structured Clinical Interview of the DSM-IV (SCID) were eligible to participate in this study. Patients were excluded if they 1) had a diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders, 2) had a history of severe adverse reaction to the antipsychotics used in the trial, 3) had had only one schizophrenic episode, 4) had a history of treatment resistance, 5) were pregnant or breast-feeding, or 6) had a severe medical condition. Although the baseline cohort consisted of 1460 individuals, laboratory data and neurocognitive measures required for our study were available for 1289 participants.
2.2. Assessments of diabetes
A number of laboratory evaluations, including serum lipids, serum glucose and hemoglobin A1c (HbA1c), were available at baseline of the CATIE trial phase 1. We used the American Diabetes Association (ADA) diagnostic criteria to define DM in our study sample (American Diabetes Association, 2011). A diagnosis of DM was defined as either (a) HbA1c ≥ 6.5% (b) serum glucose ≥ 126mg/dl at fasting state (i.e., ≥ 8 h since the last meal) or (c) use of any anti-diabetic medications. Fifty percent of the subjects included in this study donated blood in a fasting state at baseline. For those who donated their blood in non-fasting state, a glucose level of 200mg/dl or greater was used.
We also subdivided diabetic SZ patients into four groups as follows: (i) those who were not on anti-diabetic medications, (ii) those who received insulin or oral insulin secretion promoters (ISP) or both of these, (iii) those who received insulin resistance (IR) treatment agent(s) and (iv) those who had both types of medications (that is, insulin itself and/or ISP and IR treatment agents). Patients who received glimepiride were assigned to the group of “both types of medications” based on its multiple effects.
2.3. Other clinical assessments
The CATIE trial also assessed a number of clinical variables including demographic factors, years since first antipsychotic medication, antipsychotic medications at baseline, concomitant medications, co-morbid psychiatric illnesses, and psychopathology using the Positive and Negative Syndrome Scale (PANSS). The diagnosis of hypertension was based upon a systolic blood pressure (BP) of 140 mm Hg or greater, or diastolic BP of 90 mm Hg or greater, or use of any blood pressure lowering drugs. Blood pressure was determined as a single, seated value. Blood pressure lowering drugs included angiotensin-converting enzyme inhibitors, angiotensin II antagonists, antiadrenergic agents, beta-adrenergic blocking agents, diuretics, and selective calcium channel blockers. A diagnosis of dyslipidemia was based upon either (a) low serum HDL (< 40 mg/dl for male, < 50 mg/dl for female), (b) elevated fasting serum triglyceride (TG) (≥ 200 mg/dl), (c) elevated total cholesterol (≥ 240 mg/dl), or (d) use of any lipid lowering drugs. We excluded TG values from those subjects who donated the blood without fasting. Lipid lowering drugs consisted of statin, fibrate, nicotinic acid, and fish oil. Psychiatric co-morbidities were assessed through the SCID. The criteria for tardive dyskinesia (TD) used in this study were previously published (Miller et al., 2005). Briefly, patients who had at least one Abnormal Involuntary Movement scale (AIMS) item rated ≥ 3 (moderate) or at least two AIMS items rated ≥ 2 (mild) were considered as having a diagnosis of TD.
2.4. Neurocognitive Assessment
The rationale for the use of the neurocognive instruments, as well as their reliability and validity are described in detail in a previous publication which extensively focused on the methodology of the cognitive measurements used in the CATIE study (Keefe et al., 2003). Briefly, there were 24 individual scores from 11 neurocognitive tests that were grouped into five domain scores (processing speed, reasoning, verbal memory, working memory, and vigilance). Those domain summary scores were converted to standardized scores, and then a composite neurocognitive score (standardized average of the five domain scores) was calculated. We used the composite neurocognitive score and standardized domain summary scores for the statistical analyses. The cognitive tests, the description of each test and cognitive domains are summarized in Supplemental table 1 (taken from Keefe et al., 2006, with the publisher’s permission)
2.5. Statistical Analysis
Demographic and clinical variables were compared using a chi-square test and an independent two-sample t-test between SZ patients with and without DM.
To evaluate the impact of clinical variables including DM on cognitive functioning, multiple linear regression analyses were conducted with the neurocognitive composite score and the standardized domain summary scores as the dependent variable. First, all variables (i.e., demographic variables, antipsychotic medications at baseline, PANSS negative score, the use of concomitant psychotropic medications, the use of lipid lowering and hypotensive medications, the use of anti-diabetic drugs, co-morbid psychiatric illnesses, the diagnosis of tardive dyskinesia, the diagnosis of hypertension, the diagnosis of dyslipidemia, and the diagnosis of DM) were entered as independent variables into each model. Then in order to apply the most parsimonious model, the variable for which the p-value was the largest and exceeding 0.05 was excluded from the model at each step, retaining in the equation age, gender, race, ethnicity, years of education, years since first antipsychotic medication, the diagnosis of hypertension, the diagnosis of dyslipidemia, and the diagnosis of DM. We retained years since first antipsychotic medication in each equation since greater exposure to antipsychotic medications could have been associated with the likelihood of developing DM. The backward selection procedure ended when there was no variable left for which p-value exceeded 0.05. This combination of variables was retained in the final model.
The effects of type of anti-diabetic treatment on neurocognitive composite and standardized cognitive domain scores were analyzed using analysis of covariance (ANCOVA) controlling for age, gender, race, ethnicity, education, years since first antipsychotic medication, the severity of negative symptoms, the use of lipid lowering drugs and the use of concomitant psychotropic medications (i.e., two or more antipsychotics, antidepressants, anticholinergics, antiepileptics, and anxioletics), and past history of major depressive disorder. Covariates were chosen based on the results of regression analyses described below. To prevent possible type I errors, Bonferroni corrections were used to follow up the pair-wise comparisons.
All statistical analyses were conducted using SPSS version 19.
3. Results
We identified 161 patients with diabetes (12.5%). Diabetic SZ patients were significantly older than SZ patients without diabetes (t = −6.9, p < 0.001) and had longer years since first antipsychotic medication use (t = −5.8, p <0.001). While there was no difference in the severity of negative symptoms between patients with and without diabetes, diabetic SZ patients had a slightly lower overall PANSS score (t = 2.99, p = 0.003). Patients with DM were more likely to have greater BMI (t = −10.1, p < 0.001), the diagnoses of hypertension (χ2 = 31.0, p < 0.001), dyslipidemia (χ2 =31.7, p < 0.001) and tardive dyskinesia (χ2 = 5.4, p = 0.029), and more likely to receive antiepileptics (χ2 = 5.7, p = 0.021), blood pressure lowering drugs (χ2 = 76.6, p < 0.001) and lipid lowering drugs (χ2 = 60.8, p < 0.001) (Table 1). Anti-diabetic medications taken at baseline are grouped as follows: ISP (Glibenclamide, Glipirizide, Tolazamide, and Nateglinide); IR (Metformin, Pioglitazone, Rosiglitazone); and Glimepride, which has multiple effects. Most persons with diabetes were taking no anti-diabetic medication (Table 2a). In this study, HbA1c level was available from 977 participants (76%) while serum glucose level was obtained from all patients. Most of the criteria for diabetes were met via the HbA1c level, in combination with other criteria (Table 2b).
Table 1.
Demographic and clinical variables of the study’s sample
| Variables | Without diabetes N=1128 | With diabetes N=161 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | Statistics | p value | |
| Age (years) | 39.6 | 11.1 | 45.9 | 8.7 | t=−6.93 | <0.001 |
| Patient’s education (years) | 12.2 | 2.2 | 12.0 | 2.0 | NS | NS |
| Years since first antipsychotic medication | 13.6 | 10.5 | 18.8 | 10.1 | t=−5.80 | <0.001 |
| PANSS total score | 75.7 | 17.7 | 71.4 | 15.2 | t=2.99 | 0.003 |
| PANSS negative score | 20.0 | 6.4 | 19.3 | 6.0 | NS | NS |
| Body mass index | 29.0 | 6.5 | 34.8 | 7.9 | t=−10.10 | <0.001 |
| HbA1c (%)a | 5.4 | 0.4 | 7.6 | 2.0 | t=−29.40 | <0.001 |
| N | % | N | % | |||
|
| ||||||
| Gender (Male) | 862 | 76 | 111 | 69 | X2=4.25 | 0.05 |
| Race (White) | 717 | 64 | 80 | 50 | X2=11.50 | 0.001 |
| Hispanic | 129 | 11 | 15 | 9 | NS | NS |
| Hypertension | 370 | 33 | 89 | 55 | X2=31.04 | <0.001 |
| Dyslipidemia | 689 | 61 | 135 | 84 | X2=31.68 | <0.001 |
| Antipsychotic medication at baseline | ||||||
| Olanzapine monotherapy | 252 | 22 | 46 | 29 | NS | NS |
| Quetiapine monotherapy | 73 | 7 | 10 | 6 | NS | NS |
| Risperidone monotherapy | 215 | 19 | 28 | 17 | NS | NS |
| Other monotherapy | 185 | 16 | 24 | 15 | NS | NS |
| Two or more antipsychotics | 103 | 9 | 14 | 9 | NS | NS |
| Other concomitant medications | ||||||
| Antidepressants | 345 | 31 | 57 | 35 | NS | NS |
| Anticholinergic agents | 192 | 17 | 28 | 17 | NS | NS |
| Antiepileptics | 169 | 15 | 36 | 22 | X2=5.73 | p=0.021 |
| Anxiolytics | 130 | 12 | 25 | 16 | NS | NS |
| Blood pressure lowering drugs | 139 | 12 | 63 | 39 | X2=76.62 | p<0.001 |
| Lipid lowering drugs | 62 | 6 | 37 | 23 | X2=60.75 | p<0.001 |
| Other psychiatric diagnosis in past 1 month | ||||||
| Major depression | 126 | 11 | 16 | 10 | NS | NS |
| Alcohol dependence or alcohol abuse | 93 | 8 | 8 | 5 | NS | NS |
| Drug dependence or drug abuse | 137 | 12 | 12 | 8 | NS | NS |
| Tardive dyskinesia | 171 | 15 | 36 | 22 | X2=5.42 | 0.029 |
| Donated blood at fasting state | 569 | 50 | 79 | 49 | NS | NS |
Available from 977 patients.
Table 2.
| Table 2a Receiving
anti-diabetic medications at baseline | |
|---|---|
| ISPa or insulin or both (%) | 26 (16) |
| IRb treatment agents (%) | 37 (23) |
| Both insulin/ISP and IR treatment agents (%) | 33 (21) |
| No anti-diabetic drug (%) | 65 (40) |
| Total | 161 (100) |
| Table 2b. Criteria for diabetes
met by each patient | |
|---|---|
| HbA1c ≥ 6.5% (%) | 26 (16) |
| Glucose ≥ 126mg/dlc (%) | 20 (12) |
| Any anti-diabetic drug(s) (%) | 29 (18) |
| HbA1c ≥ 6.5% and glucose ≥ 126mg/dl (%) | 19 (12) |
| HbA1c ≥ 6.5% and any anti-diabetic drug(s) (%) | 32 (20) |
| Glucose ≥ 126mg/dl and any anti-diabetic drug(s) (%) | 14 (9) |
| HbA1c ≥ 6.5%, glucose ≥ 126mg/dl and any anti-diabetic drug(s) (%) | 21 (13) |
| Total | 161 (100) |
Insulin secretion promoter
Insulin resistane
Glucose ≥ 200mg/dl was used if unfasted
In the backward linear regression analysis controlling for age, gender, ethnicity, race, education, years since first antipsychotic medication and other independent variables which survived after the backward variable selection, the diagnosis of DM predicted poorer score of the cognitive composite [standardized coefficients (beta) = −0.080, p = 0.002], and of the cognitive domains of vigilance (beta = −0.10, p = 0.001), processing speed (beta = −0.081, p = 0.002), and reasoning (beta = −0.060, p = 0.027). Other aspects of the metabolic syndrome (i.e., BMI, hypertension and dyslipidemia) were not significant predictors for the composite score or any of the individual cognitive domains (Table 3)
Table 3.
Impact of BMI, diabetes, hypertension and dyslipidemia in the multiple linear regression analyses with backward variable selectiona
| Dependent variable | Independent variables | Unadjusted coefficients | Beta* | t value | p value |
|---|---|---|---|---|---|
| Neurocognitive compositeb | BMI | 0.00 | 0.002 | 0.08 | 0.936 |
| Diabetes | −0.22 | −0.080 | −3.08 | 0.002 | |
| Hypertension | 0.01 | 0.008 | 0.31 | 0.756 | |
| Dyslipidemia | 0.01 | 0.005 | 0.20 | 0.843 | |
|
| |||||
| Verbal memoryc | BMI | 0.00 | 0.011 | 0.38 | 0.707 |
| Diabetes | −0.15 | −0.052 | −1.83 | 0.068 | |
| Hypertension | −0.01 | −0.003 | −0.10 | 0.919 | |
| Dyslipidemia | 0.00 | 0.001 | 0.02 | 0.985 | |
|
| |||||
| Vigilanced | BMI | 0.00 | 0.015 | 0.49 | 0.626 |
| Diabetes | −0.30 | −0.100 | −3.34 | 0.001 | |
| Hypertension | 0.10 | 0.050 | 1.72 | 0.086 | |
| Dyslipidemia | 0.02 | 0.011 | 0.35 | 0.723 | |
|
| |||||
| Processing speede | BMI | 0.00 | −0.012 | −0.45 | 0.653 |
| Diabetes | −0.25 | −0.081 | −3.13 | 0.002 | |
| Hypertension | 0.01 | 0.004 | 0.16 | 0.875 | |
| Dyslipidemia | −0.05 | −0.025 | −0.97 | 0.334 | |
|
| |||||
| Reasoningf | BMI | 0.00 | 0.010 | 0.34 | 0.733 |
| Diabetes | −0.18 | −0.060 | −2.22 | 0.027 | |
| Hypertension | −0.03 | −0.015 | −0.55 | 0.583 | |
| Dyslipidemia | 0.06 | 0.028 | 1.02 | 0.308 | |
|
| |||||
| Working memoryg | BMI | 0.00 | −0.007 | −0.25 | 0.803 |
| Diabetes | −0.09 | −0.033 | −1.20 | 0.229 | |
| Hypertension | 0.01 | 0.005 | 0.17 | 0.864 | |
| Dyslipidemia | 0.03 | 0.015 | 0.55 | 0.584 | |
Adjusted for age, gender, race, ethnicity, education, years since first antipsychotic medication and other variables survived after the backward variable selection
Adjusted r2=0.302, F=36.59, p<0.001
Adjusted r2=0.149, F=17.58, p<0.001
Adjusted r2=0.149, F=14.18, p<0.001
Adjusted r2=0.307, F=37.50, p<0.001
Adjusted r2=0.222, F=26.19, p<0.001
Adjusted r2=0.193, F=22.06, p<0.001
Standardized coefficients
Besides the variables retained in each equation (i.e., age, gender, ethnicity, race, education, years since first antipsychotic medication, BMI, the diagnosis of DM, the diagnosis of hypertension and the diagnosis of dyslipidemia), several parameters predicted poorer multiple cognitive domains or the cognitive composite score including PANSS negative symptoms score (composite score and all individual cognitive domains), anticholinergic medications (composite score, vigilance, processing speed, reasoning and working memory), antiepileptics (composite score, vigilance, processing speed and working memory), anxiolytics (composite score, processing speed and reasoning), and the use of two or more antipsychotics (vigilance and working memory). The use of lipid lowering drugs predicted better scores of composite, verbal memory, vigilance and processing speed (Supplemental Table 2).
ANCOVAs controlling for a number of possible confounders revealed significant main effects of the composite score (F = 3.125, p = 0.014), vigilance (F = 3.062, p = 0.016) and processing speed (F = 3.092, p = 0.015) for groups based on receiving anti-diabetic drugs. Post hoc tests demonstrated that SZ patients with untreated DM had lower scores of the composite score (p = 0.019), and vigilance (p = 0.038) compared with SZ patients without DM. Post hoc tests showed that there was no significant difference in cognitive performance among the 4 DM groups (Table 4, Figure 1).
Table 4.
Comparison of standardized neurocognitive scores between SZ without diabetes and diabetic groups categorized based on anti-diabetic medicationsa
| Domains | Type of anti-diabetic treatment | Mean score | SD | Main effect for groups
|
|
|---|---|---|---|---|---|
| F | p | ||||
| Overall | Without diabetes (N=1128) | 0.01 | 0.93 | 3.125 | 0.014 |
| IR treatment agents (N=26) | −0.43 | 0.71 | |||
| Insulin or ISP or both (N=37) | −0.42 | 0.78 | |||
| Insulin/ISP and IR treatment agents (N=33) | −0.16 | 0.84 | |||
| No anti-diabetic drug (N=65) | −0.47 | 0.96 | |||
|
| |||||
| Verbal memory | Without diabetes (N=1128) | 0.01 | 1.00 | 1.863 | 0.115 |
| IR treatment agents (N=26) | −0.39 | 0.82 | |||
| Insulin or ISP or both (N=37) | −0.05 | 0.90 | |||
| Insulin/ISP and IR treatment agents (N=33) | 0.03 | 0.84 | |||
| No anti-diabetic drug (N=65) | −0.29 | 1.10 | |||
|
| |||||
| Vigilance | Without diabetes (N=1039) | 0.09 | 1.00 | 3.062 | 0.016 |
| IR treatment agents (N=22) | −0.24 | 0.73 | |||
| Insulin or ISP or both (N=30) | −0.37 | 0.73 | |||
| Insulin/ISP and IR treatment agents (N=29) | −0.03 | 0.84 | |||
| No anti-diabetic drug (N=59) | −0.39 | 0.86 | |||
|
| |||||
| Processing speed | Without diabetes (N=1128) | 0.06 | 1.05 | 3.092 | 0.015 |
| IR treatment agents (N=26) | −0.50 | 0.93 | |||
| Insulin or ISP or both (N=37) | −0.51 | 0.93 | |||
| Insulin/ISP and IR treatment agents (N=33) | −0.29 | 0.85 | |||
| No anti-diabetic drug (N=65) | −0.41 | 1.10 | |||
|
| |||||
| Reasoning | Without diabetes (N=1127) | 0.06 | 0.99 | 1.470 | 0.209 |
| IR treatment agents (N=26) | −0.40 | 0.83 | |||
| Insulin or ISP or both (N=37) | −0.33 | 0.80 | |||
| Insulin/ISP and IR treatment agents (N=33) | −0.25 | 0.92 | |||
| No anti-diabetic drug (N=65) | −0.41 | 1.05 | |||
|
| |||||
| Working memory | Without diabetes (N=1128) | 0.02 | 0.96 | 1.126 | 0.343 |
| IR treatment agents (N=26) | −0.16 | 0.95 | |||
| Insulin or ISP or both (N=37) | −0.38 | 0.81 | |||
| Insulin/ISP and IR treatment agents (N=33) | −0.03 | 1.01 | |||
| No anti-diabetic drug (N=64) | −0.35 | 0.99 | |||
Analysis of covariance adjusted for age, gender, race, education, the severity of negative symptoms, years since first antipsychotic medication, the use of antidepressants/anticholinergics/antiepileptics/anxioletics/lipid lowering drugs, psychiatric polpharmacy and major depressive disorder in the past month.
Figure 1.
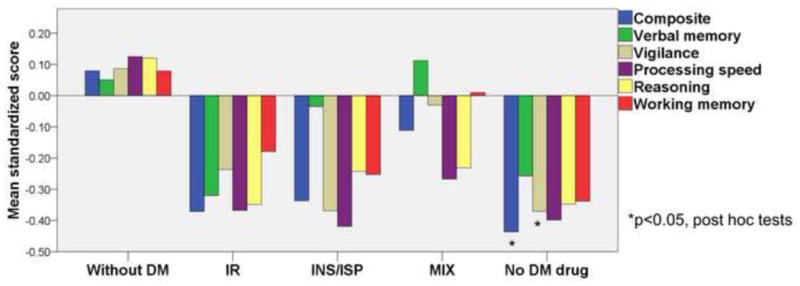
Comparison of mean standardized scores of neurocognitive composite and five neurocognitive domains (verbal memory, vigilance, processing speed, reasoning and working memory) among schizophrenia patients without diabetes and diabetic schizophrenia patients divided based on receiving anti-diabetic drugs. Positive value indicates better cognitive performance. DM, diabetes mellitus; IR, insulin resistance treatment agents; INS, insulin; ISP, insulin secretion promoter; MIX, receiving both IR and INS/ISP. *p<0.05, results of post hoc tests compared with schizophrenia patients without diabetes.
4. Discussion
Our results demonstrate a substantial impact of comorbid DM on global cognitive function in SZ patients. These cognitive impairments are unlikely to be the consequences of other possible confounding demographic and clinical factors since we adjusted them in the regression models.
As far as we are aware, this is the first study which examined the association of all components of the metabolic syndrome (i.e., obesity, dyslipidemia, hypertension and DM) with cognitive ability in SZ. Recently Friedman et al. (2010) found negative impacts of hypertension and obesity on cognition in SZ. Their conclusion may look different from ours. This discrepancy might be explained by differences in the methodologies. For example, the study by Friedman et al. (2010), excluded subjects with substance abuse disorder or poorly controlled physical illness (e.g., DM and hypertension), who were still eligible for the CATIE study.
In this study, there were cognitive deficits in the domains of vigilance, processing speed and reasoning among persons with SZ who also had DM, as compared with those persons with SZ who did not have DM. But there were no differences in verbal/working memory. DM is also associated with cognitive deficits in the domains of memory (including verbal and working memory), attention (vigilance), processing speed in the general population (reviewed by van den Berg et al., 2009). Therefore, the association of cognition with DM may be different in persons with SZ than in general population.
There are several limitations that should be taken into account. First, we lack a normal reference group. Second, we lack the evaluation of sub-group of DM (i.e., type 1 and type 2) though the majority of diabetic patients in this study are considered to have type 2 DM rather than type 1 DM. Third, as our study design is cross-sectional, prospective studies will be needed to assess whether DM contributes to cognitive exacerbation overtime or treating DM could reverse cognitive deficits in SZ. Fourth, we were unable to exclude potential confounding by several factors which may have exacerbated glucose intolerance such as atypical antipsychotics (Guo et al., 2006; Lambert et al., 2006), lifestyle (Henderson et al., 2006), genetic vulnerability (Lin and Shuldiner 2010), or factors that may have affected cognitive function including smoking (George et al., 2002; Levin et al., 1996; Sacco et al., 2005; Smith et al., 2006) and age of onset of SZ (Rajji et al., 2009). Fifth, since HbA1c level was not available from 24% of the subjects, we might have underestimated the prevalence of DM.
SZ patients are more likely to have physical diseases such as DM which are undiagnosed possibly because the psychosis dominates the clinical presentation, and this may be also due to stigma and the lack of social skills (Fagiolini and Goracci 2009). They are less likely to receive sufficient treatment or adequate monitoring of DM and related co-morbidities (Fagiolini and Goracci 2009). Co-morbid DM in SZ may affect cognitive performance which may affect the treatment adherence, which may further worsen DM.
Supplementary Material
Acknowledgments
Role of the funding source
This study is supported by NIH grants (MH 53188 to WWE, P50MH094268, P20 MH084018, RO1 MH069853, RO1 MH092443, R21 MH085226, RC1 MH088753. to AS. R01 MH078175-04A1, 4R33 MH079017-03 to NGC) and grants from NARSAD, SR/RUSK, Maryland Stem to AS, Stanley to AS and NGC.
This study was based on results from the Clinical Antipsychotic Trials of Intervention Effectiveness study, supported with Federal funds from the NIMH (MH90001).
Footnotes
Contributors
Authors YT, NGC, AS and WWE designed the study and wrote the protocol. Author YT performed statistical analyses. Author YT wrote the first draft of the manuscript. Authors NGC, AS and WWE contributed to editing the final manuscript. All authors have approved the final manuscript.
Conflict of interest
None.
References
- American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Ader M. Atypical antipsychotics and glucose homeostasis. The Journal of Clinical Psychiatry. 2005;66(4):504–514. doi: 10.4088/jcp.v66n0414. [DOI] [PubMed] [Google Scholar]
- Chien IC, Hsu JH, Lin CH, Bih SH, Chou YJ, Chou P. Prevalence of diabetes in patients with schizophrenia in taiwan: A population-based national health insurance study. Schizophrenia Research. 2009;111(1–3):17–22. doi: 10.1016/j.schres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Cohen D, Stolk RP, Grobbee DE, Gispen-de Wied CC. Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders. Diabetes Care. 2006;29(4):786–791. doi: 10.2337/diacare.29.04.06.dc05-1261. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Gold JM, Dickerson FB, Medoff D, Dixon LB. Evidence of exacerbated cognitive deficits in schizophrenia patients with comorbid diabetes. Psychosomatics. 2008;49(2):123–131. doi: 10.1176/appi.psy.49.2.123. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. The Journal of Clinical Psychiatry. 2009;70(Suppl 3):22–29. doi: 10.4088/JCP.7075su1c.04. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada E, Justicia A, Esmatjes E, Garcia-Rizo C, Kirkpatrick B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. The British Journal of Psychiatry: The Journal of Mental Science. 2009;194(5):434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Wallenstein S, Moshier E, Parrella M, White L, Bowler S, Gottlieb S, Harvey PD, McGinn TG, Flanagan L, et al. The effects of hypertension and body mass index on cognition in schizophrenia. The American Journal of Psychiatry. 2010;167(10):1232–1239. doi: 10.1176/appi.ajp.2010.09091328. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Research. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Guo JJ, Keck PE, Jr, Corey-Lisle PK, Li H, Jiang D, Jang R, L’Italien GJ. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case-control study. The Journal of Clinical Psychiatry. 2006;67(7):1055–1061. doi: 10.4088/jcp.v67n0707. [DOI] [PubMed] [Google Scholar]
- Heald A. Physical health in schizophrenia: A challenge for antipsychotic therapy. European Psychiatry: The Journal of the Association of European Psychiatrists. 2010;25(Suppl 2):S6–11. doi: 10.1016/S0924-9338(10)71700-4. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Borba CP, Daley TB, Boxill R, Nguyen DD, Culhane MA, Louie P, Cather C, Eden Evins A, Freudenreich O, et al. Dietary intake profile of patients with schizophrenia. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists. 2006;18(2):99–105. doi: 10.1080/10401230600614538. [DOI] [PubMed] [Google Scholar]
- Juvonen H, Reunanen A, Haukka J, Muhonen M, Suvisaari J, Arajarvi R, Partonen T, Lonnqvist J. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Archives of General Psychiatry. 2007;64(8):894–899. doi: 10.1001/archpsyc.64.8.894. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Mohs RC, Bilder RM, Harvey PD, Green MF, Meltzer HY, Gold JM, Sano M. Neurocognitive assessment in the clinical antipsychotic trials of intervention effectiveness (CATIE) project schizophrenia trial: Development, methodology, and rationale. Schizophrenia Bulletin. 2003;29(1):45–55. doi: 10.1093/oxfordjournals.schbul.a006990. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, Miller DD, Canive JM, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31(9):2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ, Garcia-Rizo C, Fernandez-Egea E, Bernardo M. Is abnormal glucose tolerance in antipsychotic-naive patients with nonaffective psychosis confounded by poor health habits? Schizophrenia Bulletin. 2010 doi: 10.1093/schbul/sbq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert BL, Cunningham FE, Miller DR, Dalack GW, Hur K. Diabetes risk associated with use of olanzapine, quetiapine, and risperidone in veterans health administration patients with schizophrenia. American Journal of Epidemiology. 2006;164(7):672–681. doi: 10.1093/aje/kwj289. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 1996;15(5):429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Lin PI, Shuldiner AR. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophrenia Research. 2010;123(2–3):234–243. doi: 10.1016/j.schres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Miller DD, McEvoy JP, Davis SM, Caroff SN, Saltz BL, Chakos MH, Swartz MS, Keefe RS, Rosenheck RA, Stroup TS, et al. Clinical correlates of tardive dyskinesia in schizophrenia: Baseline data from the CATIE schizophrenia trial. Schizophrenia Research. 2005;80(1):33–43. doi: 10.1016/j.schres.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP, Selke G. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Archives of General Psychiatry. 2002;59(4):337–345. doi: 10.1001/archpsyc.59.4.337. [DOI] [PubMed] [Google Scholar]
- Okumura Y, Ito H, Kobayashi M, Mayahara K, Matsumoto Y, Hirakawa J. Prevalence of diabetes and antipsychotic prescription patterns in patients with schizophrenia: A nationwide retrospective cohort study. Schizophrenia Research. 2010;119(1–3):145–152. doi: 10.1016/j.schres.2010.02.1061. [DOI] [PubMed] [Google Scholar]
- Philippe A, Vaiva G, Casadebaig F. Data on diabetes from the french cohort study in schizophrenia. European Psychiatry: The Journal of the Association of European Psychiatrists. 2005;20(Suppl 4):S340–4. doi: 10.1016/s0924-9338(05)80188-9. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: Meta-analysis. The British Journal of Psychiatry: The Journal of Mental Science. 2009;195(4):286–293. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes/metabolism Research and Reviews. 2010;26(7):507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. The American Journal of Psychiatry. 2003;160(2):284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: Involvement of nicotinic receptor mechanisms. Archives of General Psychiatry. 2005;62(6):649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Saddichha S, Manjunatha N, Ameen S, Akhtar S. Diabetes and schizophrenia - effect of disease or drug? results from a randomized, double-blind, controlled prospective study in first-episode schizophrenia. Acta Psychiatrica Scandinavica. 2008;117(5):342–347. doi: 10.1111/j.1600-0447.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, Khan A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31(3):637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabetic Medicine: A Journal of the British Diabetic Association. 2007;24(5):481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nature Reviews Endocrinology. 2011;7(2):108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- Suvisaari J, Perala J, Saarni SI, Harkanen T, Pirkola S, Joukamaa M, Koskinen S, Lonnqvist J, Reunanen A. Type 2 diabetes among persons with schizophrenia and other psychotic disorders in a general population survey. European Archives of Psychiatry and Clinical Neuroscience. 2008;258(3):129–136. doi: 10.1007/s00406-007-0762-y. [DOI] [PubMed] [Google Scholar]
- van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica Et Biophysica Acta. 2009;1792(5):470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


