Abstract
The goal of this study was to examine the effects of GRM1 blockade on melanoma anchorage independent growth and invasion. We performed colony and invasion assays using GRM1-expressing melanoma lines and the GRM1 negative UACC930 line. Using the glutamate-release inhibitor Riluzole or the noncompetitive GRM1 antagonist BAY36-7620 we were able to induced considerable inhibition of colony formation and invasion in GRM1-expressing melanoma lines. Neither pharmacological agent induced a significant reduction in colony formation or invasion in the GRM1 negative melanoma line, UACC930. Additionally we assessed the efficacy of these inhibitors to inhibit the growth of fresh melanoma tumor samples cultured on a 74μm nylon mesh. Both Riluzole and BAY36-7620 significantly inhibited tumor cell growth into the interstitial spaces of the mesh. When repeated with normal mole samples both inhibitors were much less effective in preventing the outgrowth of cells. These experiments show that a specific antagonist of GRM1 (BAY36-7620) or an inhibitor of glutamate release (Riluzole) can significantly suppress melanoma migration, invasion and colony formation as well as inhibit the proliferation of fresh melanoma cells. These findings added to our previous work, strengthen the case that GRM1 is a valid therapeutic target in patients with melanoma.
Keywords: Glutamate, Melanoma, Riluzole, Mitogen-Activated Protein Kinase Pathway, Cell Surface Receptors
Introduction
The incidence of melanoma continues to rise with almost 70,000 cases of invasive melanoma reported in the United States in 2008 (Jemal et al., 2008). When found early melanoma can usually be successfully treated with surgical excision (Dummer et al., 2009; Garbe and Eigentler, 2007). However, metastatic disease is refractory to current therapies and has a very poor prognosis (Bhatia et al., 2009; Mansfield and Markovic, 2009; Mouawad et al., 2009). Aberrant MAP kinase, as well as other specific signal transduction pathways, has been implicated in melanoma formation, local progression, and distant metastatic spread. Therapies targeting specific components of these pathways have been developed and many are currently in human trials. However, early results from the use of single agents targeting specific signaling molecules such as N-RAS, B-RAF, MEK, and others have been disappointing (Flaherty, 2008), though more specific inhibitors of mutated B-RAF have shown some success in early trials (Smalley et al., 2009). It is now generally believed that multiple pathways, many with redundant downstream effects, are dysregulated in melanoma and therapies that target multiple pathways at once will probably be needed if these therapies are going to be effective.
Recently, our group described a heretofore-unknown component of melanoma pathogenesis. A transgenic murine model of melanoma was developed by the ectopic expression of metabotropic glutamate receptor 1 (murine GRM1 or mGRM1) in melanocytes (Marin et al., 2006; Namkoong et al., 2007; Pollock et al., 2003). These mice spontaneously develop melanocytic lesions indistinguishable from human melanoma. We have expanded these original studies and have now shown that over 60% of human melanomas express the human form of this receptor, hGRM1, and that activation of this receptor results in activation of the MAPK and PI3K/AKT pathways in a B-RAF and N-RAS-independent fashion (Marin et al., 2006). In pre-clinical studies we have shown that the ectopic expression of mGRM1 in murine melanocytes is transforming and that inhibition of hGRM1 signaling in vitro and in vivo results in cell cycle arrest and subsequent apoptosis in human melanoma. We have now translated our findings into the clinic and have completed a Phase 0 trial of Riluzole, an inhibitor of glutamate signaling, in patients with stage III and IV melanoma. We found that administration of oral Riluzole resulted in suppression of MAPK and PI3K/AKT pathway signaling and involution of tumor in 34% of patients(Yip D, 2009). We also found an increase in the number of apoptotic cells in post-treatment tumor samples. We have now embarked on a Phase II therapeutic trial of single agent Riluzole in patients with advanced melanoma and we have continued our pre-clinical studies examining the consequences of Riluzole therapy on melanoma cells.
Our pre-clinical and early clinical experiments suggest that blockade of glutamate-signaling in GRM1-expressing melanoma cells results in suppression of signaling through the MAPK and PI3K/AKT pathways. However, we do not know if the therapeutic effects we have observed in pre-clinical and early clinical studies using GRM1 antagonists or Riluzole are the result of inhibition of MAPK and PI3K/AKT signaling. To help determine if inhibition of signaling through these pathways is responsible for the observed therapeutic effects, we investigated whether treatment with a non-competitive pharmacological antagonist of GRM1 signaling (BAY 36-7620) or the glutamate release inhibitor Riluzole could attenuate aspects of the transformed phenotype of human melanoma cells attributed to MAPK and PI3K/AKT activation (Hendrix et al., 2003; Hess et al., 2005). Additionally, the agents Riluzole and BAY 36-7620, were evaluated for their potential to inhibit the growth of fresh melanoma tumor samples in an in vitro organotypic culture system in anticipation of using this system to screen agents, either alone or in combination, that could be used in future in vivo experiments and clinical trials.
Results
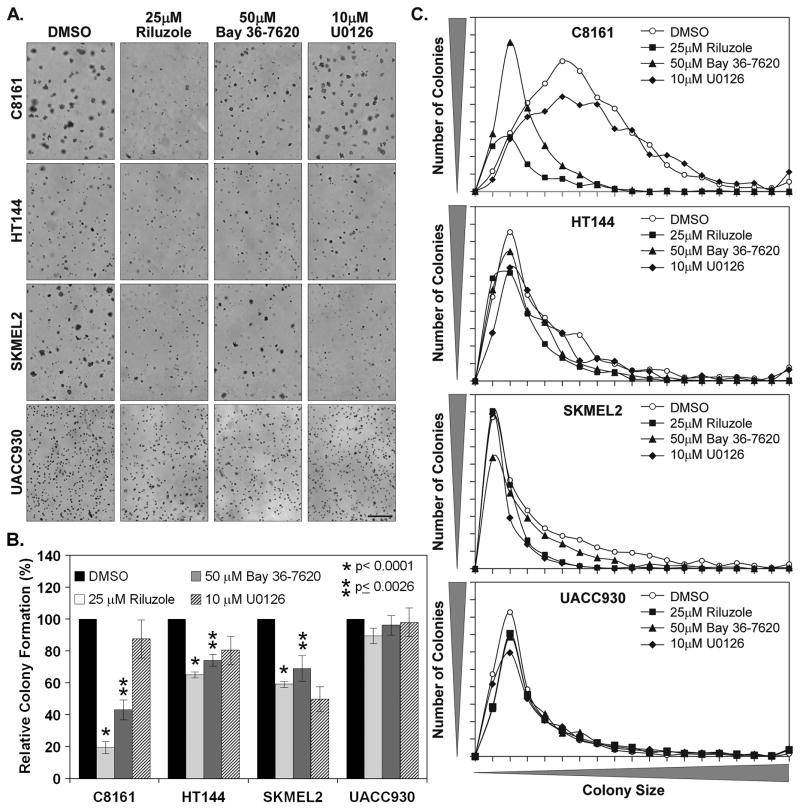
It was previously demonstrated that inhibition of GRM1 signaling with the glutamate release inhibitor Riluzole or the specific non-competitive GRM1 antagonist BAY 36-7620 resulted in decreased monolayer growth of human melanoma cell lines (Namkoong et al., 2007). To extend our understanding of the effects of GRM1 inhibition on melanocytic transformation we used these pharmacological agents to determine their effect on the anchorage independent growth of various human melanoma cell lines. The cell lines used were C8161(GRM1+, B-Raf/N-Ras WT), HT144(GRM1+, B-RafV600E), SKMEL2(GRM1+, N-RasQ61R) and UACC930(GRM1-, B-RafV600E) melanoma cell lines. These cell lines were chosen based on the GRM1, BRAF and NRAS status. Overall our results show that inhibition of GRM1 function significantly reduces colony formation in melanoma cells expressing GRM1 but not in GRM1-negative UACC930 cells (Fig. 1A). Treatment with 25μM Riluzole potently affected the colony formation of C8161 inducing an 80.5% inhibition (Fig. 1B). The inhibition was less pronounced in HT144 and SKMEL2 cells reducing the number of colonies 34.9% and 39.9%, respectively (Fig. 1B). Riluzole treatment had minimal effect on the GRM1-UACC930 cells reducing anchorage independent growth only 10.5%. The non-competitive GRM1 antagonist BAY 36-7620 produced slightly less but similar results, inducing 46.9%, 25.8% and 30.9% inhibition of colony formation in C8161, HT144 and SKMEL2 cells, respectively (Fig. 1B). BAY 36-7620 essentially had little to no effect on the UACC930 cells. Our positive control, the MEK inhibitor U0126, which has been shown to inhibit 3-dimensional growth and invasion in C8161 cells (Ge et al., 2002; Hess et al., 2005), proved to be least effective pharmacological inhibitor having only a dramatic effect on SKMEL2 colony formation.
Figure 1. Riluzole and Bay 36-7620 inhibit the anchorage independent growth of human melanoma cell lines.
Soft agar colony assays were performed with C8161 (5 × 104 cells), HT144 (1.5 × 104 cells), SKMEL2 (1.5 × 104 cells) and UACC930 (5 × 104 cells) in the presence of 25μM Riluzole, 50μM Bay 36-7620, 10μM U0126 or vehicle solvent (DMSO). Colony assays were fed with 200μL of 10% FBS RPMI containing the appropriate inhibitors every 48 hrs after the initiation of the assay. Photomicrographs of 3 representative fields were taken after 14 days or 28 days in the case of UACC930 (a.). The number of colonies (b.) and colony size (c.) for each photomicrograph was determined using Image J and the totals plotted. Histograms represent the average of 3 independent experiments with the exception of UACC930 (2 experiments). Manification bar = 500μm (a.)
In addition to decreasing the number of colonies formed, Riluzole and BAY 36-7620 had a significant effect on the size of the colonies that did form (Fig. 1C). This effect was most pronounced in C8161 as demonstrated by the colony size distribution curve (Fig. 1C). Treatment of the cells with Riluzole and BAY 36-7620 resulted in a size distribution curve with an early tight peak as opposed to untreated cells where there was a broad peak. The lower peak observed in Riluzole relative to BAY 36-7620 is reflective of the reduced number of colonies formed. The effect on the colony sizes in HT144 and SKMEL2 were less pronounced as the curve peaks are all at the same position. However, in HT144 there are fewer large colonies in both the Riluzole and BAY 36-7620 treated cells (Fig. 1C). While Riluzole also produced the same effect in SKMEL2 cells, BAY 36-7620 did not. U0126, which was effective in inhibiting SKMEL2 colony formation, also suppressed the formation of large colonies in these cells. Collectively our results show that Riluzole and BAY 36-7620 reduces both colony formation and colony size in C8161 cells. In HT144 and SKMEL2 cells Riluzole and BAY 36-7620 reduces colony numbers. While Riluzole prevented the formation of large colonies in both cell lines BAY 36-7620 was only effective in HT144 cells.
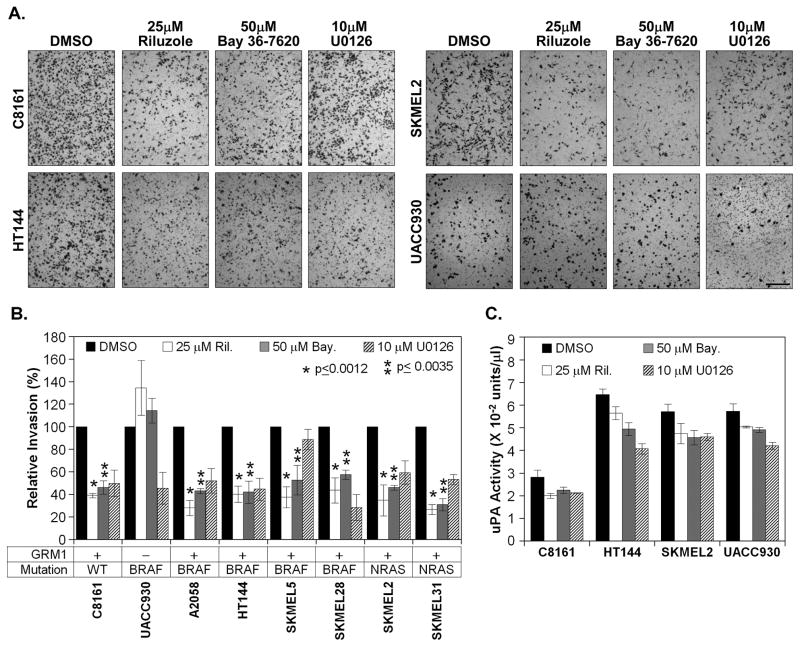
Another aspect of cellular transformation is the ability of cells to invade through an extracellular matrix and migrate. To determine if inhibition of GRM1 signaling affects the invasive potential of these melanoma cells an in vitro boyden chamber invasion assay was performed (Fig. 2A). Riluzole was quite effective in suppressing the invasive potential of C8161, HT144 and SKMEL2 reducing the numbers of invaded cells 60.9%, 59.7% and 65.2% (Fig 2B). BAY 36-7620 was just as effective, inducing 55.8%, 58.0% and 54.0% inhibition of invasion (Fig. 2B). Neither pharmacological agent inhibited UACC930 invasion, in fact both appeared to slightly increase invasion (Fig. 1B). These results were somewhat surprising since the levels of inhibition between the GRM1+ cell lines were very similar and inconsistent with the results observed in the colony formation of these cells where the BRAF and NRAS mutants were less affected by the treatments. While invasion and anchorage independent growth are different aspects of the transformed phenotype and thus may be differentially affected by GRM1 inhibition, they share many common pathways. To determine if this uniformity of inhibition was a unique phenomenon we examined the invasive potential of several other cell lines harboring BRAF and NRAS activating mutations when treated with Riluzole and BAY 36-7620. Similar levels of inhibition were observed in all the cell lines tested (Fig. 2B). With the exception of UACC930 Riluzole induced a 56.3% – 73.3% inhibition of invasion while BAY 36-7620 reduced invasion 42.4% – 68.9% in the cell lines tested. With the exception of SKMEL5, U0126 was effective in attenuating the invasive potential of all the cell lines including UACC930.
Figure 2. Glutamate blockade inhibits the invasive potential of human melanoma cell lines.
Boyden chamber invasion assays were performed with various human melanoma cell lines as described in the materials and methods in the presence of 25μM Riluzole, 50μM Bay 36-7620, 10μM U0126 or vehicle solvent (DMSO). 1 × 105 cells were used for the assays with the exception of C8161 and A2058 cell lines (2 × 104 cells). Cells were allowed to invade 18 hrs with the exception of UACC930 (48 hrs). For UACC930 a fluid renewal containing inhibitors was performed at 24hrs. Invaded cells were fixed, stained and 3 representative fields were photomicrograhed (A.). The relative levels of invasion for each cell line and treatment was determined with Image J and the results plotted (B.). The histogram represents the average of 3 independent experiments. For uPA activity assays (C.), 5 × 105 cells were plated into 4 parallel wells in a 6 well plate and allowed to recover O/N. They were then treated with the indicated inhibitors in serum free RPMI 16–40 media for 6 hrs, then replaced with fresh serum free media containing the inhibitors and returned to the incubator for an additional 12 hrs. Media was then collected and protein lysates prepared as indicated in the materials and methods. Using 20μg of total cell lysate, an invitro uPA activity assay was performed according to the manufacturer (C.). Manification bar = 500μm (A.)
Invasion is a multifaceted phenomenon in which both digestion of extracellular matrix proteins and cell motility, as well as other events, are involved. Using our panel of cell lines we next determined if inhibition of GRM1 signaling attenuates proteolytic breakdown of the extracellular matrix. We first assessed if our inhibitors had any effect on urokinase-type plasminogen activator (uPA) activity. uPA is secreted from the cells in an inactive form and binds its receptor, UPAR and becomes activated. We postulated that potentially activated uPA remains bound to UPAR and is not in the media. Under this assumption we performed uPA assays with total protein lysates isolated from cells treated with our inhibitors for 18hrs. Treatment of C8161 cells with Riluzole resulted in 27.5% decrease in activity. It was less effective in HT144 and SKMEL2, reducing activity 12.9% and 17.3%. BAY 36-7620 was equally effective across the 3 cell lines producing a 19.5% – 23.8% decrease in uPA activity. Riluzole and BAY 36-7620 were the least effective in UACC930 cells reducing activity 12.0% and 15.4% respectively. U0126 appeared to be the most effective giving a 24.3% – 37.5% reduction. Aside from uPA, matrix metalloproteases 2 (MMP2) has also been shown to be important in metastatic and invasive activity in melanoma, however neither Riluzole nor BAY 36-7620 appeared to affect MMP2 (Fig. S1).
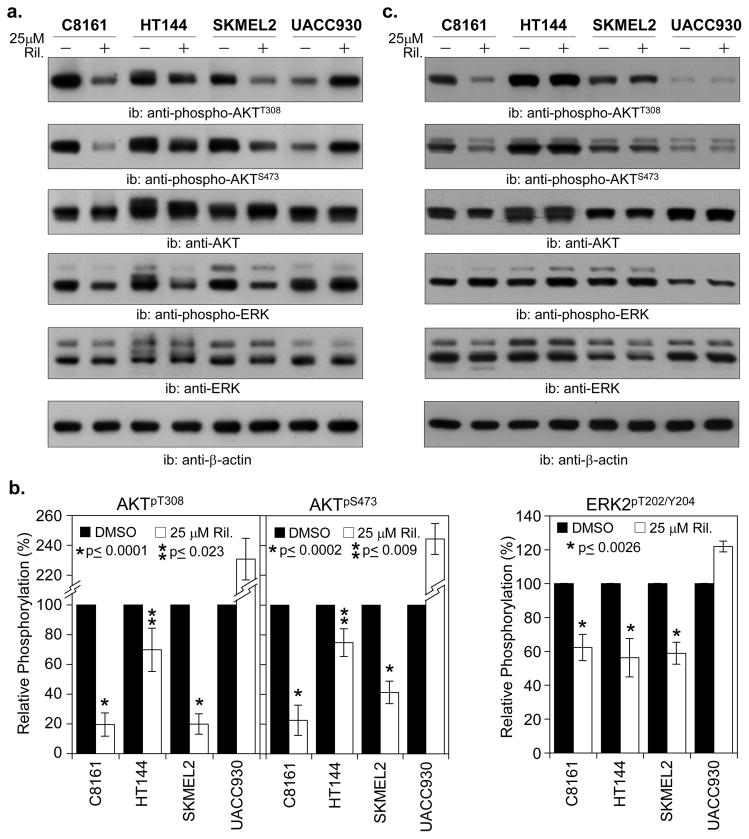
While our pharmacological agents did induce a slight inhibition of uPA activity, the levels of inhibition did not correlate with our invasion data. U0126, which was the least effective in inhibiting invasion, induced the greatest amount of inhibition of uPA activity. Clearly there was another component of invasion being impacted by the inhibitors. The MAP and PI3K/AKT pathways have been shown to be important in both anchorage independent growth and invasion (Hess et al., 2005; Meier et al., 2005). It has also been reported that GRM1 activation leads to upregulated signaling through these pathways (Marin et al., 2006). To assess the effects of GRM1 signaling inhibition on these pathways we performed western blot analysis. As Riluzole appears to be more effective than BAY 36-7620, particularly in the inhibition of colony formation we restricted our analysis to the effects of Riluzole. We performed a kinetic analysis where C8161 cells were treated with 25μM Riluzole and total protein lysates were prepared every 3 hrs post-treatment. These lysates were then western blotted for phospho-AKT and phospho-ERK to determine at which point after Riluzole treatment we would see downregulation of AKT and ERK1/2 phosphorylation (data not shown). It was determined that we see significant reduction of phosphorylation between 6 – 9 hrs. Using an 8 hr treatment time we proceeded to assess the effects of Riluzole in the cell lines (Fig. 3A, 3B).
Figure 3. Treatment of human melanoma cells with Riluzole inhibits AKT and ERK phosphorylation.
C8161, HT144, SKMEL2 and UACC930 human melanoma cell lines were plated at a density of 8 × 105 cell/60mm dish and allowed to recover O/N. The next day the cells were treated 25μM Riluzole or vehicle solvent (DMSO) for 8hrs after which total cell lysates (TCL) were prepared, protein levels determined and 10μg of TCL was resolve by SDS-PAGE (A.). Proteins were electroblotted to PVDF membranes and probed with the indicated primary and corresponding secondary antibodies. Chemiluminescent detection was performed via ECL-plus or ECL-Advance and exposure to X-ray film. The representative of 2 (UACC930) or 3 independent experiments is shown (A.). Densitometric analysis was performed using Image J to determine the protein phosphorylation and expression levels. The normalized protein phosphorylation levels were then plotted (B.) The histogram represents the average of 2 (UACC930) or 3 independent experiments. A parallel set of cells were treated as above, however total cell lysates (TCL) were prepared at 18 hrs post-treatment and processed as above. (C.).
C8161 and SKMEL2 cells, upon treatment with Riluzole for 8hrs exhibited an 80.4% and 80.1% reduction in pAKTT308 levels (Fig. 3A, 3B). It was much less effective in HT144 cells, only inducing a 30.2% decrease. In UACC930 cells the Riluzole treatment actually induced a greater than 2-fold increase in phosphorylation (Fig. 3A, 3B). Examination of the AKTS473 site showed that Riluzole was equally effective in inhibiting phosphorylation in C8161 cells but somewhat less effective in HT144 and SKMEL2 cells, 77.5% vs. 25.3% and 59.7% (Fig. 3A, 3B). As with AKTT308, it appears that Riluzole induced greater than a 2-fold increase in phosphorylation in UACC930 cells. A similar pattern was observed when we assessed the phospho-ERK1/2 levels. There was a 37.8%, 14.9% and 41.1% decreases in phosphorylation in C8161, HT144 and SKMEL2 cells respectively. As with phospho-AKT, Riluzole appears to have the opposite effect on UACC930 cells, inducing 21.9% increase in phosphorylation of ERK1/2.
In cancer cell systems there are feedback mechanisms that often diminish the negative effects induced by inhibitors resulting in a reactivation of the affected signal transduction pathways. To determine if the observed Riluzole downregulation of AKT and ERK signaling is affected by these feedback loops we repeated the western analysis in cells with an extended Riluzole treatment. When C8161 cells were treated with 25μM Riluzole for 18hrs there was still a dramatic decrease in both pAKTT308 and pAKTS473 when compared to DMSO treated cells (Fig. 3C). However AKT and ERK1/2 phosphorylation levels returned to untreated levels in HT144 and SKMEL2 cell treated with Riluzole for 18hrs. The reduced phosphorylation of ERK1/2 also returned to steady state levels in all the cell lines treated with Riluzole at the 18-hour time point. For UACC930 cells the induction of AKT and ERK1/2 phosphorylation observed in the 8 hour treatment was no longer visible in the 18 hour treated cells. This data suggest that over time feedback mechanisms in these cells can reduce the AKT and ERK inhibition induced by Riluzole. Additionally the presence of a BRAFV600E mutation and PTEN truncation in HT144 and NRASQ61R mutation in SKMEL2 cells may attenuate the inhibition induced by Riluzole
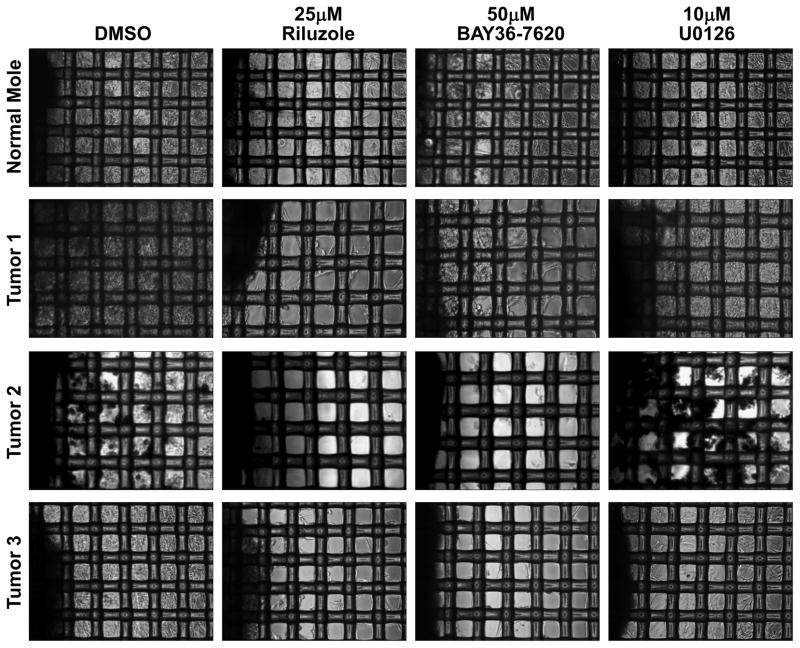
One of the issues of using cancer cell lines for the study of pharmacological agents is that cell lines potentially may have acquired mutations that were not originally present in the tumor of origin. Additionally, cell lines do not replicate the three dimensional microenvironment present in tumors. This is likely why successful inhibition in a cell culture system often does not translate into the clinic. To address this issue, we tested the ability of Riluzole and BAY 36-7620 to inhibit the growth of melanoma tumors. Growing fresh melanoma tumors can usually be done in a simple explant assay where the tumors are placed on a tissue culture dish and outgrowth of cells from the tumor can be observed. The issue with this method is that normal cells from residual stromal tissue can also grow under these conditions. To circumvent this issue we performed our fresh tumor growth assays using an organotypic culture method. In this procedure the tumor tissue is implanted on a 74μm nylon mesh and growth of cells into the interstitial spaces of the mesh is observed. Tumor cells can grow into the interstitial spaces of the mesh but normal cells cannot due to a lack of anchorage. The tumor samples were carefully dissected to remove any stromal tissue and then implanted onto Corning Netwells that contain a 74μm nylon mesh and cultured in the presence of Riluzole, BAY 36-7620, U0126 and vehicle solvent (DMSO). In vehicle treated tumors, cells readily grew out from the implanted tumor mass into the interstitial spaces of the mesh (Fig. 4). Treatment with 25μM Riluzole completely abrogated this growth. Use of 50μM BAY 36-7620 also produced the same effect. U0126 did not prevent the outgrowth of cells from the tumor mass at all. All three tumor samples were shown to be GRM1 expressers by immunohistochemical staining (data not shown). When the treatments were repeated with samples of a normal mole we observed that although Riluzole and BAY 36-7620 did decrease the growth of cells into the interstitial spaces of the mesh, it was not to the degree exhibited in the tumor samples.
Figure 4. Riluzole and Bay 36-7620 inhibit the growth of fresh melanoma tumors.
Freshly harvested melanoma tissues were used in an organotypic growth assay as indicated in the materials and methods. 24 hrs after implanting the tumor tissue on the mesh the samples were treated with 25μM Riluzole, 50μM Bay 36-7620, 10μM U0126 or vehicle solvent (DMSO). Media was replenished every 48 hrs. Cells were allowed to grow out from the tumor sample for a period of 14 days after which photomicrographs were taken. Original tumor sample is on the left of each photomicrograph.
Discussion
Our group has established that the majority of human melanomas ectopically express GRM1 and that this cell surface receptor is functional with its activation resulting in activation of the MAPK and PI3K/AKT pathways (Marin et al., 2006; Namkoong et al., 2007; Shin et al., 2008a; Shin et al., 2008b). We have now also shown that inhibition of GRM1 activation results in a decrease in melanoma cell migration and invasion and a decrease in proliferation in vitro, phenotypic characteristics that have been attributed to MAPK and PI3K/AKT signaling in human melanoma (Hendrix et al., 2003; Hess et al., 2005). Lack of effect of Riluzole and BAY 36-7620 on the GRM1 negative UACC930 cell line demonstrates these agents likely function at least in part by suppressing signaling through this receptor. We have also shown that treatment of melanoma cells cultured in soft agar with Riluzole or BAY 36-7620 suppresses colony formation and decreases colony size in GRM1 positive cell lines. Finally, we have now shown that we can suppress proliferation of fresh melanoma samples by the addition of Riluzole or BAY 36-7620 to the culture media in an organotypic culture model. These findings suggest that GRM1 is a potential therapeutic target for the treatment of patients with melanoma and that suppression of MAPK and PI3K/AKT signaling is likely responsible for at least some of the effects seen when the GRM1 signaling pathway is inhibited. These results will be important as we design Riluzole-based combination therapies that complement the effects of Riluzole on human melanoma cells.
Riluzole (2-amino-6-trifluoromethoxybenzothiazole) is the only FDA appoved GRM1 blocking agent and is used to slow the progression of disease in patients with amyotropic lateral sclerosis (Gordon, 2005; Miller et al., 2003). Riluzole is a potent inhibitor of glutamate release from neurons, likely disrupting the autocrine loops responsible for the excitotoxicity thought to be the cause of ALS, but the exact mechanism of Riluzole’s action is unknown (Miller, 2003; Miller et al., 2003; Noh et al., 2000; Swash, 2005). We were interested in Riluzole because it has a well-characterized toxicity profile (Le Liboux et al., 1999) and could be quickly introduced into clinical trials if it was shown effective in inhibiting melanoma proliferation in vitro and in murine models of human melanoma. We have previously published our pre-clinical studies of Riluzole and we have now reported the results of a Phase 0 clinical trial of oral Riluzole in patients with advanced melanoma (Yip D, 2009). We have shown that Riluzole inhibits signaling through the MAPK and PI3K/AKT pathways in post-treatment tumor samples compared to pre-treatment samples in a significant proportion of the patients receiving the drug. Surprisingly, we also found that 34% of patients on this trial had significant clinical responses to the administration of just 2 weeks of oral Riluzole. We have begun a Phase II therapeutic trial of Riluzole in patients with advanced melanoma and we have continued to explore the effects of this agent on melanoma cells. The current study demonstrates that one effect Riluzole has on human melanoma cells is to inhibit migration and invasion of melanoma cells and to inhibit melanoma proliferation in vitro. It is less clear if inhibition of signaling through GRM1 has an effect on the release of uPA by these cells, with only a modest change in uPA release detected in our pre-clinical experiments.
It is also important to note that there are eight metabotropic glutamate receptors that have been described in the CNS of mammals (Chong et al., 2003; Fagni et al., 2004; Simeone et al., 2004). All of these GRM receptors use glutamate as their natural ligand and inhibition of glutamate release by agents such as Riluzole could conceivably affect signaling through all eight of these receptors if present on melanoma cells. We have checked for the presence of GRM2-8 in melanoma cell lines and tumor samples (unpublished data) and found GRM6 and GRM8 to be expressed by the majority of the cell lines and tissue examined. This includes UACC930 cells that we use for controls in these experiments because this cell line does not express GRM1. We are continuing to examine the role of the other GRMs in melanoma pathogenesis and progression but it does appear that GRM1 is necessary for much of the effects seen with Riluzole and BAY 36-7620 treatment given the lack of effects on UACC930 cells.
It is also significant that we found that Riluzole can inhibit signaling through the MAPK and PI3K/AKT pathways in melanoma cells in vitro when the cells are treated for 8 hours but that this is effect is reversed by 18 hours of treatment. While the reactivation of the MAP kinase pathway was observed in all the GRM1+ cell lines the rephosphorylation of AKT was only observed in the HT144 and SKMEL2 melanoma cell lines harboring activating mutations in B-RAF and N-RAS respectively. It should be noted that HT144 with a PTEN truncation has hyper-activation of the PI3 kinase pathway. In C8161 cells, which are WT for PTEN, a rephosphorylation of AKT was not observed Potentially the constitutive activation of the PI3 kinase pathway in HT144, via a PTEN inactivation, and SKMEL2, via N-RAS activation, are responsible for transient nature of the inhibitory effects of Riluzole in these cell lines. It is also possible that feedback loops activated by the inhibition of these pathways by Riluzole result in re-phosphorylation of AKT and ERK, reactivating these pathways. We are currently investigating both possibilities in order to gain further insight into the inhibitory effects of Riluzole.
In our anchorage independent growth assays Riluzole displayed less inhibition in the HT144 and SKMEL2 lines than in the C8161 cell line. There was also a rephosphorylation of AKT in these lines but not in C8161. This implies that the reactivation of the PI3 kinase signaling pathway may be responsible for the decreased efficacy of Riluzole in these lines. If this is the case expression of a constitutively active PI3 kinase p110 (cap110) in C8161 cell would attenuate the inhibitory effects of Riluzole. Alternatively direct inhibition of signaling molecules of the PI3 kinase pathway in HT144 and SKMEL2 may increase the efficacy of Riluzole in these cells. Investigations into expression of cap110 in C8161 and combinations of Riluzole with inhibitors to PI3 kinase pathway signaling molecules in pre-clinical experiments are currently ongoing.
It is important to note that despite the reactivation of AKT and ERK signaling in HT144 and SKMEL2 cells there was still significant decreases in both invasion and colony formation. This suggests that while both ERK and PI3 kinase signaling contribute to melanocytic transformation, other factors are involved. GRM1 is primarily coupled to the Gq family of proteins and stimulate phosphoinositide hydrolysis where phospholipase Cβ (PLCβ converts phosphatidylinositol (PI) into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 induces the release of intracellular calcium and DAG activates PKC. PKC activates multiple downstream effectors including phospholipase A2 (PLA2), phospholipase D (PLD) and ERK. Gs proteins have also been shown to be coupled to GRM1, resulting in the activation adenylate cyclase (AC) and protein kinase A (PKA). GRM1 is also known to signal through the HOMER family of scaffolding proteins to induce PI3 kinase activity. The contribution of GRM1 induction of these other pathways to the development of melanoma and the effect of Riluzole on these pathways is currently unknown and need to be further investigated.
Our results with the GRM1-cell line, UACC930, were somewhat mixed in that Riluzole had essentially no effect on colony formation but appeared to induce increase invasion. Additionally there appeared to be a transient increase in AKT and ERK1/2 phosphorylation. Our UACC930 colony assays required 21 days for the growth of colonies whereas the invasion assays were completed in 48 hrs. Given the transient nature of the induction of AKT and ERK1/2 phosphorylation it is likely that the nature of the experiments played an important role in the outcome. In the short invasion assays the initial induction of PI3 and MAP kinase signaling may have caused an increase in invasion. In the colony assay, due to the longer duration, the initial induction of AKT and ERK1/2 phosphorylation does not impact the formation of colonies significantly since by 18 hrs phosphorylation has returned to steady state levels. Given that the goal of this study is to evaluate the therapeutic potential of Riluzole it is important that any adverse outcomes be investigated. It is critical to repeat these experiments in other GRM1-cell lines with differential PI3 kinase and MAP kinase activation states. We have only been able to locate 1 other GRM1-melanoma cell line, however this cell line is not suitable for our experiments as they grow extremely slowly and do not form colonies in soft agar. We will continue in our attempts to locate GRM1-cell lines as well as explore the possibility of using primary melanoma cultures to resolve the issue.
The Western blot experiments were carried out in a standard monolayer culture environment and so might not recapitulate what happens in vivo. Indeed, in patients on our Phase 0 trial of Riluzole we found a profound decrease in AKTT308 and pERK1/2 in post-treatment tumors as compared to pre-treatment tumors in patients who clinically responded to Riluzole administration. We are currently adapting an anchorage-independent culture system for the recovery of colonies to use in biochemical analysis to test whether this phenomenon occurs when melanoma cells are grown in anchorage-independent colonies. If we find that an anchorage-independent culture system better mimics observed in vivo effects of Riluzole on melanoma cells we will use this system to screen future compounds and combination therapies prior to in vivo experiments. It is also possible that feedback reactivation of the PI3K/AKT or MAPK pathways could result in resistance to Riluzole-based therapies and we are currently examining therapies that combine Riluzole and other agents that affect signaling through the MAPK and PI3K/AKT pathways. We are also continuing our studies into GRM1 signaling in melanoma and the mechanism of action or Riluzole by investigating other pathways stimulated by GRM1 or inhibited by Riluzole.
Our group first described the ectopic expression of GRM1 in human cancer and that these receptors function and are likely important in the melanomagenesis. We have translated these findings into the clinic in the form of Phase 0 and Phase II trials of the glutamate signaling inhibitory agent Riluzole in patients with advanced melanoma and have now taken our clinical and pre-clinical findings back to the laboratory. We now report that the inhibition of signaling through the MAPK and PI3K/AKT pathways seen in pre-clinical and clinical studies does indeed translate into decreased migration, invasion, and proliferation in melanoma cell lines and tissues that express GRM1, but not in those that do not. We will use this new data to begin to design combinatorial therapies that target GRM1 signaling and signaling through other pathways known to be important for the pathogenesis of melanoma.
Materials and Methods
Reagents
Riluzole, BAY 36-7620 and U0126 were purchased from Tocris Biosciences (Cat #s 0768, 2501 and 1144 respectively). Anti-phospho-ERK (#9010), total ERK (#9102), phospho-AKT473 (#4051), phospho-AKT308 (#2965) and total AKT # (9272) were purchased from Cell Signaling Technologies (Danvers, MA, USA). Goat anti-rabbit IgG-HRP was purchased from Sigma (St. Louis, MO, USA) (A0545).
Cell Culture
A2058, A375 and C8161 melanoma cell lines were grown in Dulbecco’s Modification of Eagle’s Media (D-MEM) (Mediatech, 10-013-CV) containing 10% FBS. SKMEL2, SKMEL5, SKMEL28 and SKMEL31 melanoma cell lines were grown in MEM (ATCC, 30-2003) containing 10% FBS. HT144 cells were grown in McCoys5A (Invitrogen, Carlsbad, CA, USA [16600]) containing 10% FBS and UACC930 cell were grown in RPMI1640 (Invitrogen, Carlsbad, CA, USA [11875]) containing 10% FBS. All cell lines were maintained in a humidified incubator at 37°C with 5% CO2.
Invasion Assays
Invasion assays were performed using a standard Boyden chamber according to established protocols (Cheng et al., 2007). Briefly 8.0 mm cell culture inserts in their 24-well companion plates (BD-Falcon, Franklin Lakes, NJ, USA [3097 and 3504, respectively]) were coated with 100μl of 0.5 mg/ml Matrigel in 0.1% BSA DMEM and allowed to gel for 2 hr at 37°C. The gel was then hydrated for 30 minutes with 400 μl 0.1% BSA in DMEM. The media was then aspirated carefully and 2 × 104 – 1 × 105 cells in 500 ml of 0.1% BSA DMEM containing the inhibitors were added to the insert and placed in a 37°C incubator for 2 hrs. 750 ml of 10% FCS DMEM containing the inhibitors was added to the companion plate to act as a chemoattractant and cell were allowed to invade 18 – 48 hrs. For 48 hr invasion assays fluid renewal of both the upper and lower chambers occurred at the 24 hr mark. Cell in the chamber were then removed using cotton swabs and the invaded cells were fixed and stained with a 0.1% crystal violet, 1% formalin, and 20% ethanol solution for 30 minutes. The inserts were then washed with PBS 2X and photomicrographs of 3 representative fields taken. Using Image J invasion levels was determined by analyzing the surface area occupied by the cells in each of the representative photomicrographs and averaging. Invasion activity was expressed as levels relative to vehicle control and histograms represent the average of 3 independent experiments.
Soft Agar Colony Assays
Cells were suspended in 600 μl of RPMI-1640 containing 10% FBS, 10% MCDB 153 and 0.35% agar. This is then overlayed onto 1ml of RPMI-1640 containing 10% FBS, 10% MCDB 153 and 0.75% agar in 1 well of a 12-well tissue culture plate. The pharmacological agents were incorporated into both the layers. Colonies were allowed to form for 14 – 21 days at which point 3 representative photomicrographs were taken.
Colony numbers and sizes were analyzed using Image J software. Briefly photomicrographs were analyzed to determine the number of colonies and pixel area of each colony. The number of colonies from 3 representative photomicrographs were totaled and colony forming potential relative to DMSO treated cells determined. Colony pixel areas for all treatments for each cell line was used to determine the minimum, maximum and standard deviation values. These values were used to determine a colony size range which was divided into 16 subranges for sorting of the colony sizes. Numbers of colonies for each subrange was plotted for a size distribution curve. Histograms represent the average of 3 independent experiments.
Protein Extraction and Western Blotting
Total cell lysates were prepared using a modified NP-40 extraction buffer (25mM Tris-HCl pH 7.4, 150mm NaCl, 1mM EDTA, 1mM DTT, 0.5% NP-40, 10% glycerol, complete protease inhibitor tablets (Roche, Indianapolis, IN, USA [1836153]), 1mM phenyl methyl sulfonyl fluoride (PMSF), 50mM β-glycerol phosphate, 10mM p-nitrophenyl phosphate, 2.5mM sodium pyrophosphate, 100nM okadaic acid, 10mM Sodium Fluoride, 1mM Sodium Orthovanadate and 10μM phenylarsine oxide). Protein amounts will be determined by Bradford Assay and routinely 10μg of total cell lysates was resolved on 10% SDS-PAGE gels, proteins transferred onto 0.2μm PVDF membrane. The membrane was then blocked and probed with antibodies according to a standard western blotting protocol. After primary antibody binding, membranes were incubated with the corresponding horseradish peroxidase (HRP) conjugated secondary antibody. Detection of the proteins was accomplished via enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA [RPN2132]) and exposure to X-ray film.
uPA Activity Assay and Gelatin Zymography
uPA activity assays were performed according to the manufacturers suggested protocol (Chemicon, Billerica, MA, USA [ECM600]). 20 μg of total cell lysate was used to perform the assay. Assays were performed in duplicate and values averaged. Results are from 3 independent experiments. Zymogram gels were purchased from Invitrogen (EC61752) and used according to manufacturer’s protocol.
Organotypic Tumor Culture
Freshly harvested melanoma tumor samples were obtained through the Tissue Retrieval Service, a shared resource core of the Cancer Institute of New Jersey, in an IRB-approved and HIPPA-compliant fashion. Samples were first carefully dissected to remove any non-tumor tissue. The tumor samples were then sliced using stacked razor blades to obtain slices of uniform thickness (~ 0.5mm). From these slices 2mm punches of tissue were taken and treated with dispase for 1 minute. The samples were then implanted into Corning/Costar Netwells containing a 74 mm nylon mesh in 12 well plates. 1 ml of 10% FBS RPMI 1640 was added to each well and the plates incubated at 37°C for 24 hours. Media was then replaced with 1 ml of 10% RPMI 1640 containing either DMSO, Riluzole (25μm), BAY 36-7620 (50μM), or U0126 (10μM) and the plates incubated 7 – 14 days. Fluid renewal occurred every 2 days. At the initial implantation and at each fluid renewal 20 μl of media was directly added to the tumor tissue to keep it sufficiently humidified. Phase contrast photomicrographs were taken at the end of the assay.
Supplementary Material
Acknowledgments
Supported by National Institute of Health grant R01CA124975 (Goydos) and a Grant from the Benjamin Foundation
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488–496. [PMC free article] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- Dummer R, Hauschild A, Pentheroudakis G. Cutaneous malignant melanoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):129–131. doi: 10.1093/annonc/mdp152. [DOI] [PubMed] [Google Scholar]
- Fagni L, Ango F, Perroy J, Bockaert J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin Cell Dev Biol. 2004;15:289–298. doi: 10.1016/j.semcdb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Flaherty KT. The future of tyrosine kinase inhibitors: single agent or combination? Curr Oncol Rep. 2008;10:264–270. doi: 10.1007/s11912-008-0040-9. [DOI] [PubMed] [Google Scholar]
- Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res. 2007;17:117–127. doi: 10.1097/CMR.0b013e328042bb36. [DOI] [PubMed] [Google Scholar]
- Ge X, Fu YM, Meadows GG. U0126, a mitogen-activated protein kinase kinase inhibitor, inhibits the invasion of human A375 melanoma cells. Cancer Lett. 2002;179:133–140. doi: 10.1016/s0304-3835(02)00004-6. [DOI] [PubMed] [Google Scholar]
- Gordon PH. Advances in clinical trials for amyotrophic lateral sclerosis. Curr Neurol Neurosci Rep. 2005;5:48–54. doi: 10.1007/s11910-005-0023-2. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hess AR, Postovit LM, Margaryan NV, Seftor EA, Schneider GB, Seftor RE, et al. Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Res. 2005;65:9851–9860. doi: 10.1158/0008-5472.CAN-05-2172. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Le Liboux A, Cachia JP, Kirkesseli S, Gautier JY, Guimart C, Montay G, et al. A comparison of the pharmacokinetics and tolerability of riluzole after repeat dose administration in healthy elderly and young volunteers. J Clin Pharmacol. 1999;39:480–486. [PubMed] [Google Scholar]
- Mansfield AS, Markovic SN. Novel therapeutics for the treatment of metastatic melanoma. Future Oncol. 2009;5:543–557. doi: 10.2217/fon.09.15. [DOI] [PubMed] [Google Scholar]
- Marin YE, Namkoong J, Cohen-Solal K, Shin SS, Martino JJ, Oka M, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 2005;10:2986–3001. doi: 10.2741/1755. [DOI] [PubMed] [Google Scholar]
- Miller R. Riluzole for ALS: what is the evidence? Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:135. [PubMed] [Google Scholar]
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:191–206. [PubMed] [Google Scholar]
- Mouawad R, Sebert M, Michels J, Bloch J, Spano JP, Khayat D. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Namkoong J, Shin SS, Lee HJ, Marin YE, Wall BA, Goydos JS, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Shin SS, Martino JJ, Chen S. Metabotropic glutamate receptors (mGlus) and cellular transformation. Neuropharmacology. 2008a;55:396–402. doi: 10.1016/j.neuropharm.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 2008b;21:368–378. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol. 2004;19:343–360. doi: 10.1177/088307380401900507. discussion 361. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Nathanson KL, Flaherty KT. Genetic subgrouping of melanoma reveals new opportunities for targeted therapy. Cancer Res. 2009;69:3241–3244. doi: 10.1158/0008-5472.CAN-08-4305. [DOI] [PubMed] [Google Scholar]
- Swash M. New ideas for therapy in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:3–4. doi: 10.1080/14660820510035379. [DOI] [PubMed] [Google Scholar]
- Yip DLM, Chan JL-K, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS. A Phase 0 Trial of Riluzole in Patients with Resectable Stage III and IV Melanoma. Clin Cancer Res. 2009;15:3896–3902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.