SUMMARY
Phosphoinositide 3-kinase (PI3K) activity is important for regulating cell growth, survival and motility. We report here the identification of bromodomain-containing protein 7 (BRD7) as a p85α-interacting protein that negatively regulates PI3K signaling. BRD7 binds to the inter-SH2 (iSH2) domain of p85 through an evolutionarily conserved region located at the C-terminus of BRD7. Via this interaction, BRD7 facilitates nuclear translocation of p85α. The BRD7-dependent depletion of p85 from the cytosol impairs formation of p85/p110 complexes in the cytosol, leading to a decrease in p110 proteins and in PI3K pathway signaling. In contrast, silencing of endogenous BRD7 expression by RNAi increases the steady state level of p110 proteins and enhances Akt phosphorylation after stimulation. These data suggest that BRD7 and p110 compete for the interaction to p85. The unbound p110 protein is unstable, leading to the attenuation of PI3K activity. Therefore, BRD7 functions as a potential tumor suppressor to regulate cell growth.
PI3K family members possess lipid kinase activity and phosphorylate the 3′-hydroxyl group of phosphatidylinositol and phosphoinositides. This activity is critical for cell proliferation and survival. Mutations in PI3K pathway are among the most frequent events in human cancers. There are three classes of PI3Ks categorized by their homology and substrate specificity (Fruman et al., 1998). Among these PI3Ks, class IA PI3K is the most common member implicated in cancer. Upon stimulation, growth factor receptors, antigen receptors, or adhesion receptors initiate tyrosine phosphorylation at sites that mediate binding and activation of class IA PI3Ks. The active PI3K then converts phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) to phosphatidylinositol-3,4,5-trisphosphate (PI-3,4,5-P3 or PIP3) and triggers a downstream signaling cascade, that includes activation of the protein-Ser/Thr kinase, Akt (Fruman et al., 1998).
Class IA PI3Ks are heterodimeric enzymes composed of a p85 family regulatory subunit (p85α, p85β and p55γ), and a p110 family catalytic subunit (p110α, p110β and p110δ). The interaction between p85 and p110 is important for the stability of p110 (Yu et al., 1998). There are five domains in p110 proteins: an N-terminal adaptor-binding domain (ABD) that mediates an essentially irreversible interaction with p85, a Ras binding domain, a C2 domain, a helical domain and a kinase catalytic domain. The p85α subunit contains an N-terminal Src homology-3 (SH3) domain, proline-rich sequences, and a break point cluster region homology (BH) domain, followed by two Src homology-2 (SH2) domains that bind to phosphorylated tyrosines and localize p110 to the plasma membrane where its substrate, PI-4,5-P2 resides. The iSH2 domain that separates the two SH2 domains forms a hairpin coiled coil that mediates binding to the ABD domain of p110 (Fruman, 2010).
The p85 family members play multiple roles in regulating the activity of the p110 catalytic subunit: 1) the tight binding of p85 to p110 prevents rapid denaturation and degradation of p110, insuring that very little p110 monomer is present in cells (Yu et al., 1998), 2) binding of p85 to p110 suppresses the catalytic activity of p110 in a manner that can be relieved through interaction of the p85 SH2 domains with Tyr-phosphorylated proteins (Fruman, 2010), 3) phosphorylation of p85 at various sites can lead to inhibition of PI3K activity (Comb et al., 2012; Fruman et al., 1998; Lee et al., 2011), and 4) when p85 is in excess of p110 it can compete for binding of the p85/p110 complex to Tyr-phosphorylated activators such as IRS1 (Luo et al., 2005) and can contribute to PTEN activation to turn off signaling (Chagpar et al., 2010; Rabinovsky et al., 2009). Mutations in p85α (usually in regions of the iSH2 domain outside the ABD binding region) are found frequently in endometrial cancers, glioblastomas, melanomas and colorectal cancers and have been shown to contribute to PI3K pathway signaling (Cheung et al., 2011; Jaiswal et al., 2009; Quayle et al., 2012). Therefore, the iSH2 domain of p85α plays an important role in regulating PI3K activity and downstream signaling.
BRD7 is a member of the family of bromodomain-containing proteins. It is a subunit of the PBAF complex (polybromo-associated BRG1-associated factor) (Kaeser et al., 2008). The mRNA levels of BRD7 are down-regulated in nasopharyngeal carcinoma and colorectal carcinoma (Wu et al., 2013; Zhou et al., 2004). BRD7 has been reported to interact with p53 and is required for p53-dependent replicative or oncogene-induced senescence (Burrows et al., 2010; Drost et al., 2010). Moreover, BRD7 has also been shown to regulate BRCA1-dependent transcription through its direct interaction with BRCA1 (Harte et al., 2010). These studies suggest BRD7 as a potential tumor suppressor.
Here we report that BRD7 interacts with the iSH2 domain of p85α but not with the p85/p110 heterodimer. We show that this interaction leads to the nuclear translocation of p85α. We also show that expressing BRD7 in cells reduces downstream PI3K signaling upon insulin or serum stimulation. This phenomenon is in part caused by the depletion of p85α from the cytosol and consequent reduction of p110α and p110β proteins in these cells. In contrast, knocking down endogenous BRD7 by RNAi increases the protein level of p110 subunits. These results indicate that BRD7 prevents p110 from binding p85α by recruiting p85α to the nucleus, leading to the degradation of unbound p110 and the attenuation of PI3K activity.
Results
BRD7 binds to p85α
In the database of the Alliance for Cell Signaling Data Center, the iSH2 domains of p85α and p85β were used as baits in yeast-two-hybrid screens in order to identify p85-binding proteins (AfCS ID: A001775 and A001776). Among the clones showing interaction with p85, BRD7 (PTP13IP) was shown as a potential p85-interacting protein, with twelve independent BRD7 clones identified from the p85α screen and nine independent BRD7 clones identified from the p85β screen (Table S1 and S2). We confirmed this interaction by showing that both GST-tagged full-length and NLS deleted (ΔNLS) BRD7 proteins pulled down p85α in HEK293T cells (Figure 1A). The yeast-two hybrid results suggest that p85α binds to the C-terminus of BRD7. Within the C-terminal region of BRD7 common to the clones that mediated interaction with p85α in the two-hybrid system, we identified residues 543 to 604 as highly conserved through evolution (Figure 1C) and we demonstrated that this region provided a minimal domain capable of binding to p85α (Figure 1B). The protein sequence of the C-terminus of BRD7 is highly conserved from humans to flies and is predicted to form two α helices (Figure 1C and Figure S1A). The fragment containing amino acid residues 543 to 624 (the 543-624 fragments) was not co-immunoprecipitated with p85α (Figure 1B), whereas the 543-614 fragment was able to interact with p85α (Figure S1B). The expression level of the 543-624 fragment was lower than those of other fragments in cells (Figure 1B), suggesting that the 543-624 fragment is structurally unstable. Compared to full-length BRD7, the 543-604 fragment was sufficient to bind to p85α (Figure S1C). Although the yeast-two hybrid results suggest that the 183-353 fragment of BRD7 might bind to p85β (Table S2), the N-terminus of BRD7 (1-478) does not interact with p85α (Figure S1C). Moreover, two segments within the iSH2 domain of p85α that span from amino acid residues 478 to 511 and from amino acid residues 534 to 563, respectively, are required for binding to BRD7 (Figure 1D). This region of p85 forms the hairpin coiled-coil that mediates interaction with the ABD domain of p110 (Huang et al., 2007; Mandelker et al., 2009) and is also highly conserved from humans to flies (Figure 1E), suggesting the interaction between BRD7 and p85α is conserved through evolution.
Figure 1. BRD7 binds to p85α.
(A) HEK293T cells were co-transfected with HA-tagged p85α and GST protein, GST-tagged wild-type BRD7 or GST-tagged NLS deleted BRD7 as indicated, followed by GST pull-down (GST-PD) and immunoblotting analysis with antibodies against GST or HA. (B) HEK293T cells were co-transfected with Flag-tagged p85α and various GST-tagged BRD7 fragments, followed by immunoprecipitation with anti-FLAG M2-agarose and immunoblot analysis with antibodies against GST or Flag. (C) Schematic illustration of domain structures of BRD7 (upper panel). The lower panel shows the alignment of the p85-binding region of BRD7 protein sequences from human (H. sapiens), mouse (M. musculus), cow (B. taurus), chicken (G. gallus), zebrafish (D. rerio), fruit fly (D. melanogaster) and worm (C. elegans). (D) GST-tagged BRD7 and various Flag-tagged p85α fragments were transfected into HEK293T cells and then subsequently analyzed by GST pull-down and immunoblot assay with anti-GST or Flag antibodies. (E) Schematic illustration of domain structures of p85α (upper panel). Alignment of the BRD7-binding region of p85α protein sequences (lower panel). See also Figure S1.
BRD7 induces nuclear translocation of p85α
BRD7 is predominately located in the nucleus due to the presence of an NLS, whereas in most cells p85α is mainly in the cytosol. To investigate whether the interaction between BRD7 and p85α would change the localization of BRD7 or p85α, we co-expressed GST-tagged BRD7 and GFP-fused p85α into COS7 cells and performed immunofluorescence analysis to monitor the localization of these two proteins. GFP-fused p85α was located primarily in the cytosol in the absence of BRD7, but was predominantly in the nucleus when co-expressed with BRD7 (Figure 2A). BRD7 with a deletion of the NLS was located in the cytosol, as expected. This mutant protein retained its ability to interact with p85α (Figure 1A) but failed to mediate translocation of p85α to the nucleus (Figure 2A). Moreover, mutant BRD7 lacking the p85-binding region (Δ543-604) or the N-terminal fragment of BRD7 (1-478) no longer induced the nuclear translocation of p85α (Figure 2A and Figure S1D). In addition, increasing the expression of BRD7 in HeLa cells increases the amount of p85α in the nucleus as judged by cell fractionation (Figure 2B). Using immunofluorescence, the endogenous p85α was also shown to be localized in the nucleus in BRD7 expressed COS7 cells (Figure 2C). The endogenous p85α immunofluorescence signals were reduced upon p85α RNAi treatment compared to the control RNAi treatment, indicating the specificity of the p85α antibody used in the assay (Figure S2A). Consistent with the previous results done with exogenous p85α, wild-type BRD7, but not the Δ543-604 mutant, binds to the endogenous p85α in HEK293T cells (Figure 2D). Thus, BRD7 forms a complex with p85α through its p85-binding domain and the nuclear localization of the BRD7-p85α complex is dependent on the NLS of BRD7. Moreover, the endogenous p85α was able to co-immunoprecipitate with the endogenous BRD7 in primary human mononuclear cells (Figure 2E). These data demonstrate that the interaction between BRD7 and p85α occurs in normal cells and is not due to an overexpression artifact. Further separating nuclear extracts into nucleoplasm and chromatin fractions showed that BRD7 and most of the nuclear p85α was associated with chromatin while a fraction of p85α was detected in nucleoplasm (Figure S2B). These results show that the BRD7-p85α complex is associated with chromatin.
Figure 2. BRD7 induces the nuclear translocation of p85α.
(A) COS7 cells were transfected with GFP-p85 and GST-tagged wild-type or mutant (ΔNLS or Δ543-604) BRD7. Anti-GST antibody was used to detect BRD7 in the immunofluorescence analysis. Cells were stained with DAPI and imaged with a fluorescent microscope. (B) Crude lysates from HeLa cells transfected with Flag-tagged p85α and increasing amounts of myc-tagged BRD7 (0, 1, 2 or 4 μg) were fractionated into cytosolic and nuclear fractions as described in Experimental Procedures. Proteins in these fractions were analyzed by immunoblotting with antibodies against p85α, myc, lamin B1 and α-tubulin. (C) COS7 cells were transfected with mCherry-BRD7 and then stained with anti-p85α antibody and DAPI. The localization of p85α and BRD7 were analyzed by immunofluorescence. (D) HEK293T cells were transfected with GST protein alone, GST-tagged wild-type or Δ543-604 BRD7, followed by GST pull-down and immunoblotting analysis with antibodies against p85α or GST. (E) Endogenous BRD7 was immunoprecipitated with anti-BRD7 antibodies from mononuclear cell lysates. Rabbit IgG was used as a control. Cell lysates and immunoprecipitates were analyzed by immunoblotting with antibodies against p85α and BRD7. (F) CHO-K1 cells were co-transfected with GFP-p85α and HA-tagged p110α together with GST-tagged wild-type or ΔNLS BRD7. The cells were immunostained with anti-GST and anti-HA antibodies, and imaged with a fluorescent microscope. (G) Cell lysates from HeLa cells transfected with GST-tagged BRD7 and HA-tagged p85α were separated by Superdex 200 using the FPLC system. Every other fractions were subjected to immunoblotting analysis with antibodies against GST, HA, p110α, p110β or ARID2. The fractions contained 670 or 200 kDa proteins were indicated. (H) Fraction 14 from (G) was subjected to GST-pull down assay (GSTPD) and immunoprecipitation (IP) using rabbit IgG, anti-p85α, p110α or p110β antibodies. Samples were further analyzed by immunoblotting using antibodies against GST, p85α, p110α, p110β and ARID2. See also Figure S2 and S3.
BRD7 does not mediate translocation of p110 subunits or PTEN to the nucleus
Since p85α can exist as a monomer (or homodimer) or in a heterodimeric complex with p110, we examined whether p110α is present in the BRD7-p85α complex. BRD7, p85α and p110α were co-expressed in CHO-K1 cells, followed by immunoprecipitation of either BRD7 or p85 or p110. By comparing the ratio of p110α to p85α in the various immunoprecipitates, it was clear that anti-p85α and anti-p110α precipitates had significantly more p110α than the BRD7 precipitate, when normalized to the amount of to p85α in the same precipitate (Figure S2C). Thus, consistent with the observation that BRD7 binds to the same region of p85 that mediates the interaction with p110, BRD7 preferentially binds to the fraction of p85α that is not in a complex with p110α. Also consistent with this observation we did not observe a concentration of p110α in the nucleus of cells overexpressing BRD7 under conditions where p85α is almost entirely in the nucleus (Figure 2F). The protein levels of p110α and p110β in nucleoplasm and chromatin fractions were barely detected in BRD7 overexpressed cells (Figure S2B). As expected, both p85α and p110α were in the cytosol of cells overexpressing ΔNLS BRD7.
We further investigated the ability of BRD7 to bind to p85α and to the p85α/p110α complex using purified proteins. Recombinant BRD7 was incubated with the p85α/p110α complex or p85α in in vitro binding assay at 5:1 or 10:1 ratio (Figure S2D and S2E). BRD7 was able to interact with recombinant p85α in vitro. However, BRD7 could not bind to p85α in PI3K complex even though the amount of BRD7 was in excess of that of p110α in the assay. These data are consistent with the in vivo data and indicate that BRD7 binds to free p85α rather than the p85α/p110α complex. Taken together, p110α does not significantly bind to the BRD7-p85α complex and therefore p110α does not translocate into nucleus with BRD7.
To further analyze the composition of the cellular BRD7-p85α complex, cell lysates from BRD7 and p85α transfected HeLa cells were separated by gel filtration using FPLC. BRD7 was found in high molecular weight complexes (> 670kDa) while p110α and p110β were present in lower molecular weight fractions (Figure 2G). A majority of p85α proteins co-migrated with either BRD7 or p110α/β. A small amount of p110α and p110β were found in the same fraction containing BRD7 and p85α (Fraction 14). In this fraction, p110α or p110β formed complexes with p85α, but not with BRD7 (Figure 2H). These results are consistent with the in vitro data (Figure S2D and S2E) and indicate that BRD7 interacts free p85α, but not with p85/p110 complexes. Consistent with published results, BRD7 also formed a complex with ARID2, another subunit of PBAF complex. However, ARID2 did not co-immunoprecipitated with p85α, indicating that the BRD7/p85α complex and BRD7/ARID2 complex are distinct (Figure 2H).
Recent studies have shown that p85 interacts with PTEN and this interaction is important for regulation of PTEN activity (Chagpar et al., 2010; Cheung et al., 2011; Rabinovsky et al., 2009). To determine if the BRD7-p85 complex enhances the nuclear localization of PTEN, we performed immunofluorescence and subcellular fractionation to detect the presence of nuclear PTEN. Consistent with previous studies, we observe a basal level of nuclear PTEN in COS7 cells without expressing any exogenous proteins. The immunofluorescence signals from nuclear PTEN were not increased in cells overexpressing p85α alone, BRD7 alone or p85α together with BRD7 (Figure S3A). Subcellular fractionation experiment showed similar results: overexpressing BRD7 in HeLa cells did not increase the level of PTEN in the nuclear fraction (Figure S3B). Therefore, BRD7 does not appear to play a role in PTEN translocation into the nucleus.
BRD7 decreases the level of p110 proteins and attenuates PI3K signaling
Considering the primary function of p85α is to regulate PI3K activity, we further investigated the role of BRD7 in the PI3K signaling pathway. An antibody specifically detecting Akt phosphorylation at Ser-473 was used to monitor activation of the PI3K pathway. Stimulation of HeLa cells with insulin or serum after overnight starvation resulted in an increase in Akt phosphorylation, and of interest, this phosphorylation was decreased in cells overexpressing BRD7 (Figure 3A). Furthermore, the protein levels of p110α and p110β were decreased in parallel with the increase in BRD7 protein (Figure 3B). The p85α and β actin protein levels remained unaffected in the presence of BRD7, indicating BRD7 does not change the stability of p85 or general protein stability. These results suggest that the decline of PI3K activity after stimulation is, at least in part, due to the reduction of p110α and p110β in BRD7 expressed cells.
Figure 3. BRD7 reduces PI3K signaling and p110 expression.
(A) HeLa cells transfected with myc-tagged BRD7 or a control plasmid were under serum starvation overnight and then stimulated with 100μM insulin (I) or 15% serum in DMEM (S). Cell lysates were separated by SDS-PAGE and analyzed with antibodies against phospho-Akt (S473), total Akt, and myc. (B) HeLa cells transfected with increasing amount of myc-tagged BRD7 and immunoblotted with antibodies against p110α, p110β, p85α, myc and β-actin. (C) Stable cells expressing V5-tagged wild-type or ΔNLS BRD7 were confirmed by immunoblotting with anti-V5 antibody. The amount of β-actin was used as a loading control (lower panel). Stable cells were stimulated with insulin or serum, followed by immunoblot analysis as metioned in (A). (D) HeLa cells expressing wild-type or ΔNLS BRD7 were analyzed as indicated in (B). (E and F) HeLa cells were transfected with the indicated amount of BRD7 DNA. Total RNAs were extracted from those cells and analyzed with real-time RT-PCR using primers specifically designed for detecting p110α, p110β, p85α, BRD7 and GAPDH. The mRNA level of p110α, p110β, p85α (E), or BRD7 (F) was normalized with the level of the housekeeping gene, GAPDH. ns: non-significant difference. The values are the average of triplicates ± SD.
Stable cell lines constitutively expressing wild-type or ΔNLS mutant BRD7 were derived from HeLa cells and used for evaluating the effect of BRD7 on the PI3K pathway (Figure 3C). While wild-type BRD7 suppressed Akt activation, the ΔNLS mutant BRD7 had little effect even though expressed at a higher level (Figure 3C). Consistent with these results, wild-type BRD7 lowered p110α and p110β levels while ΔNLS mutant BRD7 did not (Figure 3D). These results suggest that although the ΔNLS mutant BRD7 still binds to monomeric p85α, the nuclear translocation of the BRD7-p85α complex plays a critical role in preventing p85α from stabilizing p110 proteins. These results are consistent with previous studies indicating that once the p85/p110 complex forms, it is essentially irreversible (Carpenter et al., 1990; Fruman et al., 1998; Fry et al., 1992). Thus, due to the higher affinity of p110 for p85, BRD7 must remove p85 from the cytosol to be effective in preventing formation of the complex.
BRD7 contains a bromodomain, which can potentially recognize acetylated lysine residues such as those on histones. Bromodomain-containing proteins have important functions in gene transcription (Filippakopoulos and Knapp, 2012). Therefore, we examined whether BRD7 reduced the expression of p110α and p110β at the transcriptional level. HeLa cells were transfected with various amounts of BRD7 DNA and then total RNA from those cells was extracted for real-time RT-PCR. Unlike the protein levels, the mRNA levels of p110α and p110β exhibited statistically non-significant difference between control and BRD7 overexpressed cells (Figure 3E and 3F). Thus, the reduction of p110α and p110β expression by BRD7 does not occur at the transcriptional stage.
Reducing BRD7 enhances PI3K signaling
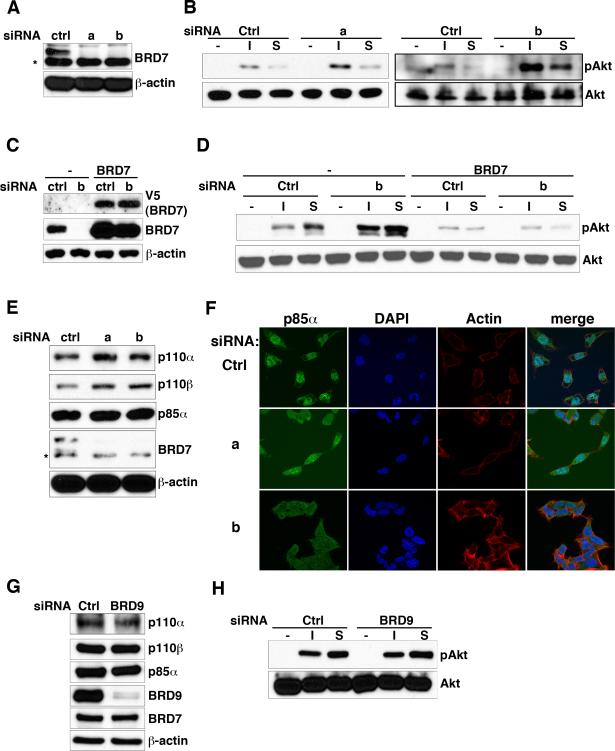
To determine if reducing endogenous BRD7 has an effect on PI3K signaling, we employed RNAi to silence the expression of BRD7 in HeLa cells. Two different pairs of siRNA oligos targeting BRD7 were transfected into HeLa cells and were shown to efficiently knock down BRD7 protein expression (Figure 4A). Akt phosphorylation was observed in non-targeting control siRNA transfected cells after stimulated with insulin or serum for 30 minutes. In contrast to the results from BRD7 overexpression experiments, both pairs of siRNA oligos against BRD7 enhanced Akt phosphorylation upon stimulation compared to the control siRNA treated cells (Figure 4B). These results show that in the absence of BRD7, the PI3K pathway is more readily activated.
Figure 4. BRD7 destabilizes p110 and negatively regulates PI3K signaling.
(A) HeLa cells were transfected with two different pairs of siRNA oligos (a and b) against BRD7 or a control oligo. High-salt cell lysates were immunoblotted with antibodies against BRD7 (for RNAi efficiency) or β-actin (for loading control). An asterisk (*) indicates a non-specific band detected by anti-BRD7 antibody. (B) Cells from (A) were serum-starved for overnight and subsequently treated with 100μM insulin (I) or 15% serum in DMEM (S). Cell lysates were analyzed by immunoblotting with an antibody against phospho-Akt (S473) or total Akt. (C) BRD7 siRNA-resistant cells and the parental HeLa cells were transfected with control siRNA or siRNA against BRD7 (oligos b) and then immunoblotted with anti-V5, BRD7 or β-actin. (D) Cells from (C) were analyzed as described in (B). (E) NCI-H520 cells were transfected with control siRNA and siRNA oligos against BRD7 (a and b). Cell lysates were immunoblotted with antibodies against p110α, p110β, p85α, BRD7 or β-actin. An asterisk (*) indicates a non-specific band detected by anti-BRD7 antibody. (F) Cells from (E) were examined with immunofluorescence using anti-p85α antibody, followed by Alexa fluor 568 phalloidin and DAPI staining and imaged with a fluorescent microscope. (G) HeLa cells were transfected with siRNA oligos against BRD9 or a control oligo. High-salt cell lysates from those cells were immunoblotted with antibodies against p110α, p110β, p85α, BRD9, BRD7 or β-actin. (H) Cells from (G) were serum-starved for overnight and then treated with 100μM insulin (I) or 15% serum in DMEM (S). Cell lysates were analyzed by immunoblotting with an antibody against phospho-Akt (S473) or total Akt. See also Figure S4.
In order to confirm that the results from BRD7 knock-down experiments are not due to off-target effects, we generated cells stably expressing BRD7 with silent mutations circumventing the siRNA (Figure 4C). Expressing this mutant BRD7 blocked the ability of the siRNA to enhance Akt activation by insulin or serum and also suppressed basal insulin and serum stimulation due to the expression of mutant BRD7 being higher than endogenous BRD7 (Figure 4D).
Reducing BRD7 increases the level of p110 proteins and decreases the fraction of p85α in the nucleus
The Cancer Cell Line Encyclopedia (CCLE) project (Barretina et al., 2012) identified the lung carcinoma cell line, NCI-H520 as a cell line expressing high levels of BRD7 mRNA. Consistent with the other studies presented above, knocking down BRD7 with two distinct pairs of siRNA oligos increased the levels of p110 proteins in these cells, without affecting p85α expression (Figure 4E). Taken together, p110 protein levels are inversely correlated with BRD7 protein levels in cells (Figure 3B and 4E).
The subcellular localization of endogenous p85α in NCI-H520 cells was monitored by confocal immunofluorescence microscope (Figure 4F). Alexa Fluor 568 phalloidin was used to stain F-actin to outline the cells. Using DAPI as a nuclear marker, a nuclear population of endogenous p85α was observed in control NCI-H520 cells. The p85α staining was more diffuse and more cytosolic in NCI-H520 cells transfected with either one of the two different BRD7 siRNAs. These data show that BRD7 is involved in determining the nuclear localization of endogenous p85α.
BRD9 is another bromodomain-containing protein that belongs to the same subgroup as BRD7. The siRNA against BRD9 was used to specifically knock down the expression of BRD9 (Figure 4G). In contrast to the results with BRD7 knock-down, knocking down BRD9 did not increase p110α/β levels nor PI3K activity (Figure 4G and 4H). These results suggest that the ability of BRD7 to regulate PI3K pathway is not a common feature shared within the bromodomain-containing protein family.
BRD7 has been reported to function as a p53 regulator (Burrows et al., 2010; Drost et al., 2010). BRD7 interacts with p53 and p300 and is recruited to the promoter regions of p53 target genes to mediate p53-dependent oncogenic-induced senescence. Our findings presented in this study indicate that BRD7 can serve as a tumor suppressor not only by promoting p53 function, but also by reducing the survival signaling mediated by PI3K/Akt activation. Crosstalk between p85α and p53 has been previously observed. Independent of PI3K, p85α is involved in p53-mediated apoptosis under oxidative stress (Yin et al., 1998). Moreover, p85 has been reported to bind to p300, leading to the recruitment of p53-p300 complex to the promoter regions in response to UVB irradiation (Song et al., 2011). Therefore, we further tested the role of p85 in BRD7 mediated p53-target gene induction using wild-type, p85α knockout (KO), p85β KO and p85α/β KO MEFs (Figure S4A). Overexpressing BRD7 promoted p21 mRNA production in wild-type MEFs in agreement with a previous report (Burrows et al., 2010) (Figure S4B). BRD7 was able to induce p21 in the cells lacking of p85α and/or p85β, indicating p85 is dispensable for the function of BRD7 on regulating p53. More importantly, our data show that the BRD7-p85α complex is distinct from PBAF complex, suggesting a unique role of BRD7-p85α complex in nucleus (Figure 2H).
Discussion
In this report, we have demonstrated that BRD7 binds to the iSH2 domain of p85α, the same region involved in interacting with p110. BRD7 forms a complex with p85α but not with p110α and induces the nuclear translocation of p85α through its NLS. High levels of BRD7 sequester p85 away from p110 and result in reduced p110 levels, presumably due to the well-known instability of p110 monomers. This decrease in p110 proteins results in attenuation of PI3K signaling. In contrast, knocking down BRD7 allows p85α to accumulate in the cytosol and stabilize p110 levels and thereby enhance PI3K signaling.
The ability of BRD7 to effectively compete with p110 for binding to p85 and thereby reduce p110 levels appears to require that BRD7 translocate p85 into the nucleus. Previous studies showed that once p85 and p110 form a complex it is essentially irreversible (Carpenter et al., 1990; Fruman et al., 1998; Fry et al., 1992). The in vitro binding experiments indicated that BRD7 binds to free p85α that is not in the PI3K complex (Figure S2D and S2E). Thus, it is likely that BRD7 does not dissociate p110 from p85, but reduces the level of p85 in the cytosol available to bind to newly-synthesized p110. Monomeric p110s are unstable and degraded quickly (Yu et al., 1998) and accordingly, PI3K signaling is suppressed under the influence of BRD7. BRD7 is down-regulated in nasopharyngeal carcinoma and colorectal carcinoma (Wu et al., 2013; Zhou et al., 2004) and constitutively activated PI3K is found in many tumors (Fruman, 2010). Our results suggest that rather than bringing p85α into nucleus upon stimulation, BRD7 consistently sequesters p85α into nucleus to attenuate PI3K signaling and maintains the homeostasis of cell growth. Therefore, we propose here that, in addition to its role in the PBAF complex and in p53 signaling, BRD7 functions as a direct regulator of PI3K signaling.
Although the primary location of the p85/p110 PI3K complex is in the cytosol, several studies have shown that PI3K translocates into nucleus upon the stimulation of nerve growth factor, interleukin 1, insulin-like growth factor I or platelet-derived growth factor (Bavelloni et al., 1999; Martelli et al., 2000; Neri et al., 1994). However, the function of the nuclear PI3K is not well defined. There is evidence showing the existence of p85 monomer that is not bound to p110 (Fruman, 2010). The p85 protein has different functions depending on its associated proteins. The 85/p110 complex phosphorylates the 3’-OH group of the inositol ring of phosphatidylinositides, while recent studies have indicated that when p85 binds to PTEN, it enhances PTEN-dependent dephosphorylation of PIP3 (Chagpar et al., 2010; Rabinovsky et al., 2009). p85 was also reported to facilitate XBP-1 transport into the nucleus under ER stress (Park et al., 2010). Therefore, p85α appears to have a variety of distinct functions beyond regulating PI3K activity. The role of the BRD7-p85α complex in the nucleus needs to be further investigated, but the fact that the p85 interacting region of BRD7 is conserved from humans to flies argues that this complex is likely to be conserved through evolution and thus fundamental to the function of both proteins. Identifying other potential components in the BRD7-p85α complex may help uncover the physiological function of this nuclear complex.
Finally, it should be pointed out that previous studies from our lab and other laboratories have shown that in some tissues, especially in the liver, p85 isoforms are in large excess over p110 and play a significant role in suppressing insulin signaling, in part by competing with the p85/p110 complex for binding to IRS proteins and also by enhancing PTEN activation (Luo et al., 2005; Taniguchi et al., 2007; Taniguchi et al., 2006; Taniguchi et al., 2010; Ueki et al., 2003). Thus, it is likely that in some tissues, BRD7-mediated translocation of a subfraction (but not all) of the p85 protein to the nucleus could enhance PI3K signaling. Further studies will be necessary to elucidate the full complexity of the role of BRD7 in PI3K and p53 signaling.
Experimental Procedures
Cell lysates
Cells were lysed in lysis buffer (20 mM Tris, pH7.5, 150 mM NaCl and 0.5% NP-40) and incubated on ice for 10 min. Cell lysates were collected after centrifugation at 13,000 × g for 5 min. For high-salt lysates, cells were lysed in high-salt lysis buffer (20 mM Tris, pH7.5, 500 mM NaCl and 0.5% NP-40), followed by sonication for 20 sec and prepared as indicated above.
To prepare cell lysates for immunoprecipitation from mononuclear cells, cells were lysed in lysis buffer (10mM Hepes, pH7.4, 10mM KCl and 0.05% NP-40) on ice for 10 min and then were centrifuged at 13,000 × g for 5 min. The pellet was resuspended in low salt buffer (10mM Tris-HCL, pH7.4, 2mM MgCl2 and 1% Triton-X 100) and then incubated on ice for 15 min. After centrifugation at 13,000 × g for 10 min, the supernatant was collected and used for immunoprecipitation.
Immunofluorescence
Cells cultured on glass coverslips were washed in PBS three times, and then fixed in 4% Formaldehyde for 15 min. After washing three times with PBS for 5 min, cells were blocked in the blocking solution (1% BSA and 0.2% Triton X-100 in PBS) for 1 hr. Cells were incubated with primary antibodies according to manufacturer's instructions for overnight. Coverslips were washed three times with PBS for 5 min before being incubated with fluorescence conjugated secondary antibodies for 2 hr. In some experiments, the samples were washed with PBS and stained with Alexa fluor 568 phalloidin for 15 min. Coverslips were washed three times with PBS for 5 min and mounted with mounting medium containing DAPI (Vector Laboratories).
Supplementary Material
Highlights.
BRD7 interacts with the iSH2 domain of p85alpha.
BRD7 induces the nuclear translocation of p85alpha.
BRD7-p85alpha complex leads to a decrease in p110 proteins and in PI3K signaling
BRD7 prevents p110 from binding p85alpha by recruiting p85alpha to the nucleus
Acknowledgments
We thank Lily Wang for critical reading of the manuscript. This research was supported by National Institutes of Health Grant R01-GM041890. Y.-H.C was supported by the American Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional experimental procedures are available in the Supplemental Experimental Procedures.
References
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelloni A, Santi S, Sirri A, Riccio M, Faenza I, Zini N, Cecchi S, Ferri A, Auron P, Maraldi NM, et al. Phosphatidylinositol 3-kinase translocation to the nucleus is induced by interleukin 1 and prevented by mutation of interleukin 1 receptor in human osteosarcoma Saos-2 cells. Journal of cell science. 1999;112(Pt 5):631–640. doi: 10.1242/jcs.112.5.631. [DOI] [PubMed] [Google Scholar]
- Burrows AE, Smogorzewska A, Elledge SJ. Polybromo-associated BRG1- associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14280–14285. doi: 10.1073/pnas.1009559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC. Purification and characterization of phosphoinositide 3-kinase from rat liver. The Journal of biological chemistry. 1990;265:19704–19711. [PubMed] [Google Scholar]
- Chagpar RB, Links PH, Pastor MC, Furber LA, Hawrysh AD, Chamberlain MD, Anderson DH. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5471–5476. doi: 10.1073/pnas.0908899107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, Lu Y, Stemke-Hale K, Dyer MD, Zhang F, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer discovery. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb WC, Hutti JE, Cogswell P, Cantley LC, Baldwin AS. p85alpha SH2 domain phosphorylation by IKK promotes feedback inhibition of PI3K and Akt in response to cellular starvation. Molecular cell. 2012;45:719–730. doi: 10.1016/j.molcel.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost J, Mantovani F, Tocco F, Elkon R, Comel A, Holstege H, Kerkhoven R, Jonkers J, Voorhoeve PM, Agami R, et al. BRD7 is a candidate tumour suppressor gene required for p53 function. Nature cell biology. 2010;12:380–389. doi: 10.1038/ncb2038. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Knapp S. The bromodomain interaction module. FEBS letters. 2012;586:2692–2704. doi: 10.1016/j.febslet.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Fruman DA. Regulatory subunits of class IA PI3K. Current topics in microbiology and immunology. 2010;346:225–244. doi: 10.1007/82_2010_39. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annual review of biochemistry. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Fry MJ, Panayotou G, Dhand R, Ruiz-Larrea F, Gout I, Nguyen O, Courtneidge SA, Waterfield MD. Purification and characterization of a phosphatidylinositol 3-kinase complex from bovine brain by using phosphopeptide affinity columns. The Biochemical journal. 1992;288(Pt 2):383–393. doi: 10.1042/bj2880383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MT, O'Brien GJ, Ryan NM, Gorski JJ, Savage KI, Crawford NT, Mullan PB, Harkin DP. BRD7, a subunit of SWI/SNF complexes, binds directly to BRCA1 and regulates BRCA1-dependent transcription. Cancer research. 2010;70:2538–2547. doi: 10.1158/0008-5472.CAN-09-2089. [DOI] [PubMed] [Google Scholar]
- Huang CH, Mandelker D, Schmidt-Kittler O, Samuels Y, Velculescu VE, Kinzler KW, Vogelstein B, Gabelli SB, Amzel LM. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science. 2007;318:1744–1748. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring P, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser MD, Aslanian A, Dong MQ, Yates JR, 3rd, Emerson BM. BRD7, a novel PBAF-specific SWI/SNF subunit, is required for target gene activation and repression in embryonic stem cells. The Journal of biological chemistry. 2008;283:32254–32263. doi: 10.1074/jbc.M806061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Chiu YH, Asara J, Cantley LC. Inhibition of PI3K binding to activators by serine phosphorylation of PI3K regulatory subunit p85alpha Src homology-2 domains. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14157–14162. doi: 10.1073/pnas.1107747108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. The Journal of cell biology. 2005;170:455–464. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelker D, Gabelli SB, Schmidt-Kittler O, Zhu J, Cheong I, Huang CH, Kinzler KW, Vogelstein B, Amzel LM. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16996–17001. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Borgatti P, Bortul R, Manfredini M, Massari L, Capitani S, Neri LM. Phosphatidylinositol 3-kinase translocates to the nucleus of osteoblast-like MC3T3-E1 cells in response to insulin-like growth factor I and platelet-derived growth factor but not to the proapoptotic cytokine tumor necrosis factor alpha. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:1716–1730. doi: 10.1359/jbmr.2000.15.9.1716. [DOI] [PubMed] [Google Scholar]
- Neri LM, Milani D, Bertolaso L, Stroscio M, Bertagnolo V, Capitani S. Nuclear translocation of phosphatidylinositol 3-kinase in rat pheochromocytoma PC 12 cells after treatment with nerve growth factor. Cell Mol Biol (Noisy-le-grand) 1994;40:619–626. [PubMed] [Google Scholar]
- Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nature medicine. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle SN, Lee JY, Cheung LW, Ding L, Wiedemeyer R, Dewan RW, Huang-Hobbs E, Zhuang L, Wilson RK, Ligon KL, et al. Somatic mutations of PIK3R1 promote gliomagenesis. PloS one. 2012;7:e49466. doi: 10.1371/journal.pone.0049466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, Sellers WR. p85 Associates with unphosphorylated PTEN and the PTEN-associated complex. Molecular and cellular biology. 2009;29:5377–5388. doi: 10.1128/MCB.01649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Gao M, Dong W, Hu M, Li J, Shi X, Hao Y, Li Y, Huang C. p85alpha mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene. 2011;30:1360–1371. doi: 10.1038/onc.2010.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Aleman JO, Ueki K, Luo J, Asano T, Kaneto H, Stephanopoulos G, Cantley LC, Kahn CR. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Molecular and cellular biology. 2007;27:2830–2840. doi: 10.1128/MCB.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Tran TT, Kondo T, Luo J, Ueki K, Cantley LC, Kahn CR. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Winnay J, Kondo T, Bronson RT, Guimaraes AR, Aleman JO, Luo J, Stephanopoulos G, Weissleder R, Cantley LC, et al. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer research. 2010;70:5305–5315. doi: 10.1158/0008-5472.CAN-09-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Fruman DA, Yballe CM, Fasshauer M, Klein J, Asano T, Cantley LC, Kahn CR. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. The Journal of biological chemistry. 2003;278:48453–48466. doi: 10.1074/jbc.M305602200. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Hu KS, Chen DL, Zeng ZL, Luo HY, Wang F, Wang DS, Wang ZQ, He F, Xu RH. Prognostic relevance of BRD7 expression in colorectal carcinoma. European journal of clinical investigation. 2013;43:131–140. doi: 10.1111/eci.12024. [DOI] [PubMed] [Google Scholar]
- Yin Y, Terauchi Y, Solomon GG, Aizawa S, Rangarajan PN, Yazaki Y, Kadowaki T, Barrett JC. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature. 1998;391:707–710. doi: 10.1038/35648. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Molecular and cellular biology. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ma J, Zhang BC, Li XL, Shen SR, Zhu SG, Xiong W, Liu HY, Huang H, Zhou M, et al. BRD7, a novel bromodomain gene, inhibits G1-S progression by transcriptionally regulating some important molecules involved in ras/MEK/ERK and Rb/E2F pathways. Journal of cellular physiology. 2004;200:89–98. doi: 10.1002/jcp.20013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.