Summary
We have investigated the effect of metformin on the mechanisms of angiogenesis. We show that metformin, particularly in the context of obesity, inhibits angiogenesis in vivo yet shows a contradictory effect on angiogenesis-related genes and proteins that involve AMPK.
Abstract
The biguanide metformin is used in type 2 diabetes management and has gained significant attention as a potential cancer preventive agent. Angioprevention represents a mechanism of chemoprevention, yet conflicting data concerning the antiangiogenic action of metformin have emerged. Here, we clarify some of the contradictory effects of metformin on endothelial cells and angiogenesis, using in vitro and in vivo assays combined with transcriptomic and protein array approaches. Metformin inhibits formation of capillary-like networks by endothelial cells; this effect is partially dependent on the energy sensor adenosine-monophosphate-activated protein kinase (AMPK) as shown by small interfering RNA knockdown. Gene expression profiling of human umbilical vein endothelial cells revealed a paradoxical modulation of several angiogenesis-associated genes and proteins by metformin, with short-term induction of vascular endothelial growth factor (VEGF), cyclooxygenase 2 and CXC chemokine receptor 4 at the messenger RNA level and downregulation of ADAMTS1. Antibody array analysis shows an essentially opposite regulation of numerous angiogenesis-associated proteins in endothelial and breast cancer cells including interleukin-8, angiogenin and TIMP-1, as well as selective regulation of angiopioetin-1, -2, endoglin and others. Endothelial cell production of the cytochrome P450 member CYP1B1 is upregulated by tumor cell supernatants in an AMPK-dependent manner, metformin blocks this effect. Metformin inhibits VEGF-dependent activation of extracellular signal-regulated kinase 1/2, and the inhibition of AMPK activity abrogates this event. Metformin hinders angiogenesis in matrigel pellets in vivo, prevents the microvessel density increase observed in obese mice on a high-fat diet, downregulating the number of white adipose tissue endothelial precursor cells. Our data show that metformin has an antiangiogenic activity in vitro and in vivo associated with a contradictory short-term enhancement of pro-angiogenic mediators, as well as with a differential regulation in endothelial and breast cancer cells.
Introduction
Metformin (dimethylbiguanide), a biguanide agent developed based on the observations of the hypoglycemic activity of the plant Galega Officinalis, is one of the most commonly prescribed antihyperglycemic drugs for the treatment of type 2 diabetes worldwide. It is a well-tolerated pharmaceutical with limited and transient side effects. During the last decade, a new interest in this compound arose from epidemiologic, preclinical and clinical studies suggesting that metformin may be effective in cancer prevention. In retrospective studies, individuals affected by type 2 diabetes receiving metformin, but not other antidiabetic drugs, showed a reduced incidence and improved survival for many common cancers (1,2), in particular breast and liver cancer, whereas it is still debated if metformin is advantageous for prostate cancer (2). Although type 2 diabetics not receiving metformin showed a higher incidence of several tumors than non-diabetics, the diabetic patients treated with metformin showed an incidence lower than healthy individuals (1). The potential anticancer activity is supported by published preclinical data showing an antiproliferative effect of metformin on several cultured cancer cell lines and in different animal models (3–5).
Although the molecular mechanisms by which metformin reduces cancer incidence and improves survival are still unclear, it may directly act on cancer cells, activating adenosine-monophosphate-activated protein kinase (AMPK) as a consequence of either mitochondrial activity inhibition leading to an increase in adenosine monophosphate levels or by activation of its upstream regulator liver kinase B1, a well-known tumor suppressor (4). AMPK activation by metformin diminishes p53 abundance and oxidative stress under high glucose conditions (6).
More recently, metformin has been suggested to affect angiogenic pathways (7–10). Angiogenesis, the formation of new blood vessels from pre-existing ones, is a crucial step in tumor growth, invasion and metastasis and represents a promising therapeutic target for cancer therapy (11). Neo-angiogenesis is primarily achieved through activation of proliferation, survival and migration of endothelial cells by a shift in the balance between angiogenesis stimulatory and inhibitory signals (11).
Angioprevention, which is chemoprevention directed at inhibition of tumor angiogenesis, is an important component in the overall preventive approach (10). Inflammatory mediators are targets in cancer prevention (12) and angioprevention (10). It is clear that early identification of individuals at cancer risk and adoption of adequate preventive measures are more effective in saving lives than intervention and treatment of end-stage disease (13). The effect of metformin on endothelial function could be related to the ability of this agent to prevent the angiogenic switch, reducing tumor incidence (10). Several studies have demonstrated that this agent is able to attenuate pro-angiogenic and inflammatory stimuli, such as tumor necrosis factor α (TNFα), nuclear factor-κB (NF-κB), plasminogen activator inhibitor-1 antigen and von Willebrand factor (14–16), to reduce soluble forms of the adhesion molecules ICAM-1, VCAM-1 and E-selectin (17–20), to decrease the expression of matrix metalloproteinases (MMPs) involved in new blood vessel formation (9). However, conflicting data concerning the antiangiogenic action of metformin are emerging with several reports indicating an angiogenic activity in tumor models (4,21,22). Further, in cardiovascular disease models, metformin has been associated with vascular endothelial growth factor (VEGF) upregulation and an increase in nitric oxide bioavailability (23,24), both of which have been linked with enhanced angiogenesis (11,25).
We therefore set out to (i) study the effects of metformin on endothelial cells in vitro and in vivo, (ii) evaluate the angiogenic mediators stimulated or repressed by metformin on endothelial cells through unbiased transcriptome and proteome approaches compared with cancer cells, (iii) investigate the role of AMPK in metformin-treated endothelial cells using small interfering RNA (siRNA) knockdown and (iv) examine the effects of metformin on endothelial cells in the context of a mouse model of obesity.
The effects of metformin on endothelial morphogenesis in matrigel in vitro were AMPK-dependent. To verify the role of the AMPK pathway downstream of metformin action, we used siRNAs targeting the AMPKα1 or α2 subunits. Endothelial production of the cytochrome P450 family member cytochrome P450 1B1 (CYP1B1) was upregulated by tumor cell supernatants, this effect was blocked by metformin and AMPK. Functional genomics analysis performed on endothelial cells derived from multiple donors showed that metformin treatment downregulates several angiogenesis-related genes in a contrasting time-dependent manner. Antibody arrays showed that metformin regulation of several angiogenic molecules in endothelial cells was opposite to that exerted on breast cancer cells. In vivo, metformin inhibited angiogenesis, prevented the obesity-associated increase in microvessel density (MVD) and reduced the levels of endothelial precursor cells in the adipose tissue of obese mice receiving a high-fat diet (HFD). Taken together, these data suggest that metformin may effectively target angiogenesis by AMPK-dependent and AMPK-independent mechanisms. This is particularly relevant in the context of obesity and obesity-associated inflammation and cancer.
Materials and methods
Reagents
Metformin (1,1-dimethylbiguanide hydrochloride) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were purchased from Sigma–Aldrich (St Louis, MO). 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine (compound C) was obtained from Calbiochem (Darmstadt, Germany). VEGF was obtained from Peprotech (Rocky Hill, NJ). The ON-TARGET plus SMART pool siRNAS targeting AMPKα1 (PRKAA1 L-005027-00) and α2 (PRKAA2 L-005361-00) and ON-TARGET plus Nontargeting Pool negative control siRNA (D-001810) were purchased from Dharmacon (Lafayette, CO). Lipofectamine RNAiMAX (catalog number 13778-075) was purchased from Invitrogen (Eugene, OR). Amicon Ultra-4 centrifugal Filter Units were purchased from Millipore Corporation (Billerica, MA).
Cell lines and culture conditions
Human umbilical vein endothelial (HUVE) cells were obtained from Promo Cell (Heidelberg, Germany) and cultured up to passage 8 in M199 medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum, 1% glutamine, fibroblast growth factors (1 μg acidic-fibroblast growth factor plus 1 μg basic-fibroblast growth factor/100 ml), epidermal growth factor (1 μg/100 ml), heparin (10 mg/100 ml) and hydrocortisone (0.1 mg/100 ml). Endothelial cells were derived from three different donors were seeded on plates coated with 0.1% gelatin. Prostate cancer cell lines (DU-145 and PC3) and breast cancer cell lines (MDA-MB-231 and MCF-7) were obtained from American-Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum.
Transfection of HUVE with AMPKα1- and AMPKα2-specific siRNA
Transient transfection of siRNA was performed using Lipofectamine RNAiMAX according to the manufacturer’s protocol. Briefly, on day 1, HUVE cells were seeded at a density of 1.2 × 105 per well in a six-well plate and pre-coated with 1% gelatin, in absence of antibiotics. The following day, cells were transfected with 10 nM siRNA for 6 h. Transfection efficiency was verified 24 and 48h later by western blotting. For the evaluation of HUVE cells ability to form capillary-like structures, 24 h after transfection, the cells were harvested, counted and the morphogenesis assay performed as described in Matrigel morphogenesis assay section. For western blotting analysis, transfected cells were treated with or without 10 mM metformin for additional 24 h and lysed with sample buffer as described.
Western blotting
HUVE cells were grown in complete medium and treated with increasing concentrations of metformin (0.01–100 mM). At the indicated time points, cells were collected by brief trypsinization and total lysates were prepared using cell lysis buffer (Cell Signaling Technology, Beverly, MA). Protein concentrations were evaluated by the DC Protein Assay (Bio-Rad, Hercules, CA). Equal amounts of proteins for each sample were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted onto polyvinylidene fluoride membranes (Amersham Biosciences, Otelfingen, CH). Following blocking with 5% non-fat milk powder (wt/vol) in Tris-buffered saline (10mM Tris–HCl, pH 7.5, 100mM NaCl, 0.1% Tween-20) for 1h at room temperature, membranes were incubated with primary antibodies directed against the following human antigens: AMPKα1, phospho-AMPKα (Thr172), β-actin, total and phospho-NF-κB, cyclin D1, glyceraldehyde-3-phosphate dehydrogenase, vinculin, CDK4, phospho-extracellular signal-regulated kinase (ERK1/2), caspase 3 (all purchased from Cell Signaling Technology, Danvers, MA), CYP1B1 (Abcam, Cambridge, UK), CXCR4 (Santa Cruz Biotechnology, Santa Cruz, CA), ADAM metallopeptidase with thrombospondin type 1 motif (ADAMTS1; Millipore Corporation), cyclooxygenase 2 (COX2; Cayman Chemical, Ann Arbor, MI) and VEGF (Calbiochem). The antibodies were diluted in 5% bovine serum albumin–Tris-buffered saline–0.1% Tween according to the manufacturer’s instructions. The bound antibodies were visualized by horseradish-peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence detection system from Amersham Biosciences (Pittsburg, PA).
For phospho-ERK1/2 expression, 2 × 105 HUVE cells were seeded on six-well plates in complete medium, then serum starved for 24 h. Cells were then stimulated with 10 mM metformin alone for 5, 15 and 30 min. Alternatively, cells were treated with 10 mM metformin in the presence or absence of compound C, a chemical inhibitor of AMPK, for 6 and 24 h; at this time point, 10 ng/ml of recombinant VEGF was added to the media for additional 15 min; cells were then washed and lysed for western blotting analysis.
Matrigel morphogenesis assay
Matrigel (300 μl, 10mg/ml) was polymerized on 24-well plates. About 5 × 104 HUVE cells transfected with AMPK or control siRNA were suspended in complete growth medium with or without increasing concentrations of metformin and seeded on the top of the matrigel. After 6 h of incubation at 37°C, the formation of capillary-like networks was examined under an inverted microscope (Zeiss, Oberkochen, Germany), equipped with charge-coupled device optics and a digital analysis system, and quantified using the Angiogenesis Analyzer toolset (26) for National Institutes of Health ImageJ software.
Microarray gene expression analysis
Total RNAs were isolated from cells derived from three independent donors and treated with metformin for 6 or 24 h and from untreated HUVE cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Details concerning quality control and statistical analysis are provided in the Supplementary Methods, available at Carcinogenesis Online. A list of angiogenesis-related genes was created using 2635 genes annotated under the term ‘angiogenesis’ by Gene Ontology (http://www.geneontology.org/). Statistically significant expression changes were determined using permutation tests (SAM, http://www-stat.stanford.edu/~tibs/SAM/). The delta value was set to return a median false significant number <1. Annotations were obtained through the DAVID database (http://david.abcc.ncifcrf.gov/).
Quantitative reverse transcription–PCR
Expression data validation was performed by quantitative real-time reverse transcription–PCR using RNA extracted from drug- or vehicle-treated cells as detailed in Supplementary Methods, available at Carcinogenesis Online. Expression data were normalized on the mean of the expression values for three housekeeping genes: glyceraldehyde-3-phosphate dehydrogenase, RNA polymerase II and glucose-6-phosphate dehydrogenase. Relative expression values with standard errors and statistical comparisons (unpaired two-tailed t-test) were obtained using Qgene software.
Human antibody array kit/proteome profiler
To analyze the expression profiles of angiogenesis-related proteins, we used the Proteome Profiler™ Human Antibody Array Kit (R&D Systems Ltd, Abingdon, UK), according to the manufacturer’s instructions. This kit uses an array of 55 antibodies directed to proteins involved in angiogenesis and invasiveness (the complete list of the antibodies spotted in the antibody array can be found at http://www.rndsystems.com/index.aspx), spotted onto a nitrocellulose membrane in a duplicate way. Briefly, HUVE cells and MCF-7 cells were seeded in complete medium in a 12-well flat bottom multiwell at a density of 2 × 105 cells per well. When cells were confluent, they were serum starved overnight. Medium was replaced with fresh complete medium containing 10 mM metformin. Supernatants were collected 24 h after stimulation and used for Human Array Kit/Proteome Profiler.
Supernatants from treated and untreated cells were centrifuged and mixed with 1.5 ml of biotinylated detection antibodies for 1h at room temperature. Membranes were then incubated with the sample/antibody mixtures overnight at 4°C on a rocking platform. Following a washing step to remove unbound material, streptavidin–horseradish and chemiluminescent detection reagents were added sequentially. Data were captured by exposure to Amersham Hyperfilm enhanced chemiluminescence. Arrays were scanned into a computer and optical density measurements were obtained with National Institutes of Health ImageJ software.
In vivo matrigel sponge angiogenesis assay
The in vivo angiogenesis assay was conducted as described previously (27). Briefly, unpolymerized liquid matrigel was mixed with a cocktail of pro-angiogenic factors (100ng/ml VEGF-A, 2ng/ml TNFα and 25 U/ml heparin), either alone or in combination with metformin. The mixture was brought to a final volume of 0.6 ml and injected subcutaneously into the flanks of 6- to 8-week-old C57/BL6 male mice [Charles River Laboratories, Calco (Lecco), Italy]. All animals were housed in a conventional animal facility with 12 h light/dark cycles and fed ad libitum. Manipulation of animals was in accordance with the Italian and European Community guidelines (D.L. 2711/92 No.116; 86/ 609/EEC Directive) and approved by the institutional ethics committee. Groups of 4–8 mice were used for each treatment. Four days after injection, the gels were recovered, minced and diluted in water to measure the hemoglobin content with a Drabkin Reagent Kit (Sigma).
Murine studies for obesity
Six-week-old C57 mice were bred and fed with HFD and control diet as described previously (29,30). Mice fed with normal diet (ND) or HFD and orally receiving metformin or vehicle (n = 6 per study arm) were subjected to the in vivo matrigel sponge angiogenesis assay as described and assessed and quantified for MVD by immunofluorescence staining for CD31+ vessels as described previously (28). After 30 days of HFD or control diet, mice received metformin (0.5mg metformin/ml in the drinking water, providing ~2mg metformin/mouse/day) or control vehicle for further 60 consecutive days (n = 10 per study arm). On day 90, mice were killed. Blood and visceral white adipose tissue (WAT) were collected for endothelial progenitor cells (EPCs) enumeration as described previously (29). CD45−Sca1+CD34+CD31+ EPCs were evaluated in the bone marrow, in the peripheral blood and in the WAT by six-color flow cytometry following an approach recently validated for the quantification of circulating EPCs and perivascular progenitors (29,30). The nuclear staining Syto16 was used to discriminate between DNA containing cells, platelets and cell debris. 7-Aminoactinomycin D was used to determine the viability status of the cells. The absolute count of viable DNA(Syto16)+CD45−Sca1+CD34+CD31+ EPCs was obtained using reference beads in Trucount tubes (BD).
Statistical analysis
For comparisons between two data sets, two-tailed t-tests (GraphPad Prism software) were used, for multiple data sets one-way analysis of variance. Statistical analysis of the array data is provided in the Supplementary Methods, available at Carcinogenesis Online.
Results
Metformin downregulates endothelial cell network formation in vitro
Metformin has previously been shown to inhibit growth in several cancer cell lines (3). However, there are contrasting reports regarding the angiogenic activity and effects on angiogenesis mediators by metformin (7–9,22). When seeded on the basement membrane matrix matrigel in presence of pro-angiogenic stimuli, endothelial cells organize into capillary-like networks. Metformin significantly inhibited the formation of capillary-like structures in a dose-dependent manner (Figure 1A), as shown by the decrease in the number of segments and segments length (Figure 1B).
Fig. 1.
Metformin inhibits endothelial cell morphogenesis. HUVE cells were treated with metformin at the indicated concentrations and seeded on the top of the matrigel layer previously solidified on 24-well plates. The formation of capillary-like networks was documented by photography after 6h (×5 magnification). (A) Metformin (Metf) inhibited morphogenesis in a dose-dependent manner. (B) Quantification of capillary-like structures (both number of segments and segments length) using the Angiogenesis Analyzer ImageJ toolkit showed significant differences in both parameters, P-values are shown, C− is the negative control. (C) HUVE cells were transfected with an AMPKα1-specific (siRNAα1 or α1) control (siCTR) siRNA, cell lysates were harvested and analyzed by western blotting for AMPKα1 expression; AMPKα1 was successfully downregulated in HUVE transfected cells as compared with control. (D) Transfected HUVE cells were seeded on top of a matrigel layer in presence or absence of metformin and analyzed at 6h as in (A). Metformin-mediated inhibition of capillary-like structures formation was reverted when AMPKα1 expression was downregulated. (E) Quantification of capillary-like structures as described previously, P-values are shown.
AMPK plays important functions in endothelial cells, modulating the energy supply (31), protecting from apoptosis (32) and regulating inflammation and angiogenesis (33–35). Metformin activated AMPK in HUVE cells in a dose-dependent manner (Supplementary Figure 1A, available at Carcinogenesis Online). This induction was time-dependent, with a peak of activation after short times of exposure (5 min; Supplementary Figure 1B, available at Carcinogenesis Online). Metformin has also been reported to modulate NF-κB (16,36), a master regulator of inflammation (37). Metformin treatment of HUVE cells repressed phosphorylation of NF-κB (Supplementary Figure 1C, available at Carcinogenesis Online), although with slower kinetics than that exerted on AMPK, peaking after 60 min.
In order to evaluate AMPK involvement in HUVE cells ability to form capillary-like structures, we downregulated AMPK levels by transfecting endothelial cells with an AMPK-α1-specific-siRNA (Figure 1C). HUVE cells partially recovered their capacity to generate networks when AMPKα1 was downregulated by siRNA, suggesting that the inhibitory effect of metformin was in part mediated by activation of the AMPK pathway (Figure 1D and E). Similar results were observed using a siRNA targeting AMPKα2 (data not shown).
Metformin inhibits endothelial cell proliferation, invasion (Supplementary Figures 2 and 3, available at Carcinogenesis Online) and MMP expression (9). At concentrations between 1 and 10 mM, metformin exerted a cytostatic effect as evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Supplementary Figure 2A, available at Carcinogenesis Online), which is consistent with increased G0/G1 cell fraction (Supplementary Figure 2B, available at Carcinogenesis Online), in accordance with previously published data in cancer cell lines (38). Inhibition of endothelial cell proliferation was accompanied by a strong reduction of cyclin D1 messenger RNA (mRNA) and protein levels as early as 6 h after treatment (Supplementary Figure 2C, available at Carcinogenesis Online). Although p21 mRNA was only slightly affected by metformin, the cyclin kinase CDK4 was markedly downregulated (Supplementary Figure 2C, available at Carcinogenesis Online). In agreement with these data, the cytostatic effect of metformin on endothelial cells was not associated with induction of apoptosis at the doses used in the study, as shown by absence of caspase 3 activation (Supplementary Figure 2D, available at Carcinogenesis Online) and co-staining of HUVE cells with 7-aminoactinomycin D and annexin V (Supplementary Figure 2E, available at Carcinogenesis Online). Metformin-induced reduction of HUVE cell proliferation was associated with a significant reduction in invading cells even at a low dose (Supplementary Figure 3, available at Carcinogenesis Online). Taken together, these results indicate that metformin interferes with endothelial cell proliferation and invasion without exerting toxicity.
Gene and protein expression profiling in HUVEC treated with metformin
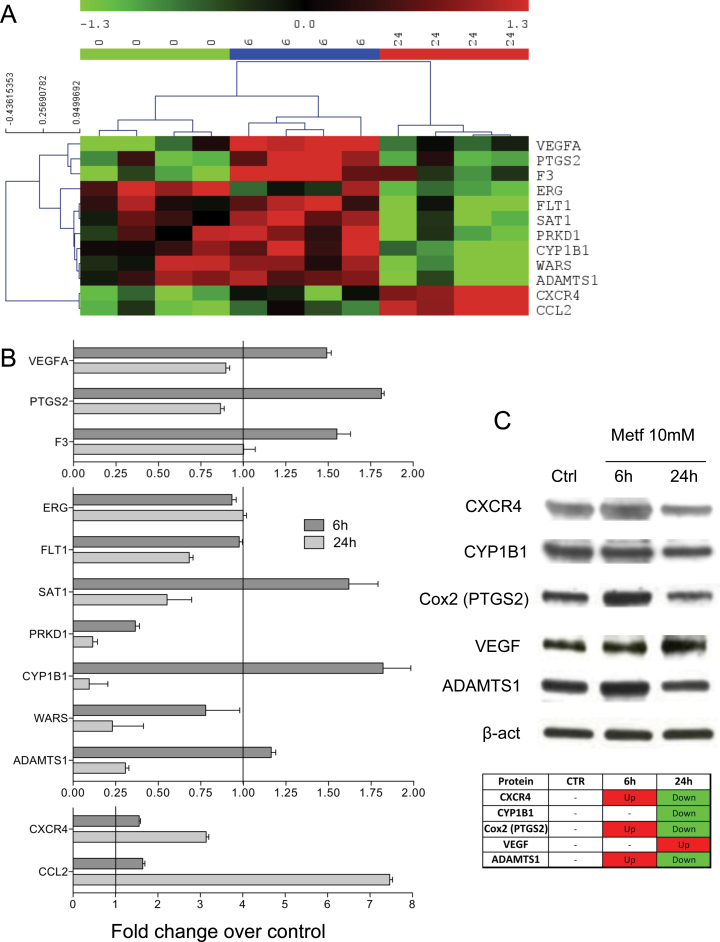
To investigate the effect of metformin on endothelial cells at the transcriptional level, we performed a gene expression profiling using Affymetrix Exon Arrays on HUVE cells from multiple donors treated with metformin or the vehicle alone. About 530 genes for which Affymetrix Exon 1.0 arrays contained probe sets were annotated in Gene Ontology under the term ‘angiogenesis’, and 301 of these were present in the data set after filtering for non-expressed and invariant genes. Twelve genes were found significantly differentially expressed in all donors after treatment with metformin for 6 or 24 h (Figure 2).
Fig. 2.
Metformin modulates several angiogenesis-related genes. Microarray analysis was performed on total RNAs isolated from HUVE cells from three different donors that were treated with either vehicle alone (CTR) or 6 or 24h with 10mM metformin (Metf). Data from the scans were normalized and analyzed for relative intensity of regulation of genes with metformin treatment. (A) Hierarchical clustering of human endothelial cells from the three different donors shows clear division into control, 6 and 24h metformin treatment, genes showing statistically significant regulation in all donors are shown. The genes are as follows: VEGF-A; PTGS2 (COX2); F3 (coagulation factor III, thromboplastin); ERG (ETS-related gene); FLT1 (VEGF receptor 1); SAT1 (spermidine/spermine N1-acetyltransferase 1); PRKD1 (protein kinase D1) CYP1B1 (cytochrome P450, family 1, subfamily B, polypeptide 1); WARS (tryptophanyl-tRNA synthetase); ADAMTS1; CXCR4; and CCL2 (CC chemokine ligand 2). (B) Real-time PCR validation of gene expression of the statistically significant angiogenesis-related genes up or downregulated by metformin in microarrays. Relative expression values are indicated as fold change over untreated HUVE cells (=1) assessed after normalization on glyceraldehyde 3-phosphate dehydrogenase, RNA polymerase II and glucose-6-phosphate dehydrogenase expression data obtained from reactions run in parallel. All amplifications were performed in triplicate. (C) Analysis of CXCR4, CYP1B1, COX2, VEGF-A and ADAMTS1 protein expression on HUVE cells, by western blotting. A table summarizing protein changes relative to control (Ctrl) is also shown (upregulated proteins are shown in red; downregulated proteins are shown in green). β-Actin was used as loading control.
Interestingly, 6 h of treatment led to a significant, albeit transient, induction of VEGF-A, PTGS2 (the gene encoding for COX2), F3 (coagulation factor III, thromboplastin) and ADAMTS1 expression levels, indicating a transient pro-angiogenic activity of metformin (Figure 2A). After 24 h of treatment, these genes returned to control levels or were downregulated as compared with control (Figure 2A). Validation of the array data by quantitative reverse transcription–PCR showed the same modulation (Figure 2B).
FLT1 (VEGF receptor 1), SAT1 (spermidine/spermine N1-acetyltransferase 1), PRKD1 (protein kinase D1) and WARS (tryptophanyl-tRNA synthetase) were downregulated after 24 h of metformin treatment as compared with untreated cells or cells treated for 6 h (Figure 2A and B). ADAMTS1, an antiangiogenic protease, was strongly downregulated after 24 h, confirming a paradoxical effect of metformin on angiogenesis. The downregulation of the VEGF-A receptor FLT1 and of PRKD1, a kinase activated by VEGF-A (39) and a stimulator of hypoxia-inducible factor 2α(40), could contribute to the antiangiogenic effect. Another gene strongly downmodulated by metformin after 24 h both at mRNA and protein levels was the cytochrome P450 family 1 member CYP1B1.
We found a induction at the mRNA level of the chemokine CCL2 and the CXC chemokine receptor 4 (CXCR4, Figure 2B), both of which are considered pro-angiogenic stimuli. Previous studies also reported an induction of CCL2 in vivo by metformin (8). However, a more recent publication (24) reported that metformin was able to contrast the increased levels of CCL2 in a rat type 2 diabetes model, in particular on a HFD. CXCR4 mediates the response to CXCL12, which is considered to be a key mechanism for mobilization of endothelial precursor cells from the bone marrow to the periphery (41).
In order to further explore gene and protein expression, we performed western blotting on proteins obtained from HUVE cells treated with or without 10 mM metformin for 6 and 24 h. We observed increased protein expression of COX2 and ADAMTS1 protein at 6 h, followed by a decrease at 24 h, confirming the gene expression pattern (Figure 2C). CCL2 was poorly expressed at the protein level (Supplementary Figure 4, available at Carcinogenesis Online) and was not further investigated. We also confirmed CYP1B1 downregulation by metformin at 24 h (Figure 2B and C), as observed in the gene expression profiling. Interestingly, VEGF protein was still upregulated at 24 h, thus highlighting the controversial effect of metformin on HUVE cells (Figure 2C).
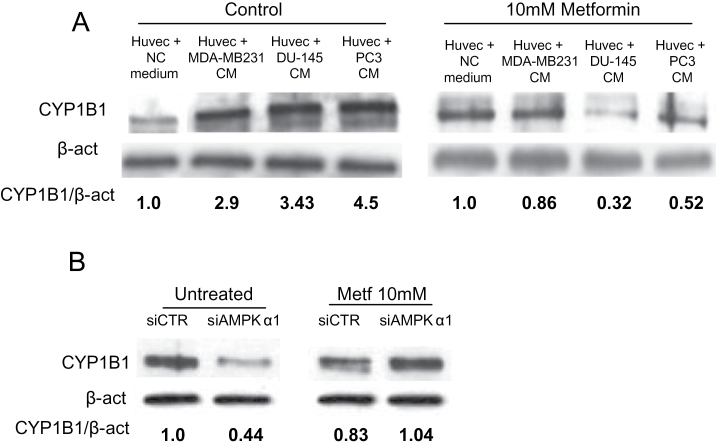
CYP1B1 levels is upregulated in HUVE cells by tumor cell conditioned medium and inhibited by metformin in a AMPK-dependent manner
CYP1B1 deficiency in endothelial cells has been reported to inhibit capillary morphogenesis in matrigel, while inducing expression of antiangiogenic factors, suggesting that CYP1B1 is pro-angiogenic (42,43). In order to evaluate if tumor cells from prostate cancer (DU-145 and PC3) and breast cancer (MDA-MB-231), which are sensitive to metformin treatment (3–5), are able to modulate CYP1B1 expression in endothelial cells, we cultured HUVE cells with or without tumor cell supernatants for 24 h in presence or absence of metformin. We observed a marked increase in CYP1B1 levels in HUVE cells cultured with tumor cell supernatants (Figure 3A). The addition of metformin to the medium strongly inhibited this effect (Figure 3A). Because AMPK is activated by metformin, we evaluated the role of AMPK in CYP1B1 regulation. AMPK levels were downregulated by transfecting endothelial cells with an AMPK-α1-specific siRNA compared to a control siRNA; CYP1B1 expression was then measured by western blotting. We observed a significant downregulation of CYP1B1 expression in AMPKα1 siRNA-treated cells as compared with control siRNA (Figure 3B). Metformin also reduced CYP1B1 protein levels in control cells, as shown previously (Figure 2C). AMPKα1 siRNA reverted the effect of metformin (Figure 3B) suggesting that CYP1B1 expression in endothelial cells is AMPK-dependent and is regulated by an AMPK-dependent pathway by metformin.
Fig. 3.
Tumor cell supernatants upregulate CYP1B1 expression in HUVE cells, metformin abrogates this effect. HUVE cells were incubated with serum-free unconditioned medium (NC) or conditioned supernatants (conditioned medium, CM) from tumor cells (MDA-MB-231 for breast cancer; PC3 and DU-145 for prostate cancer), all harvested after 24h. (A) Western blotting shows a significant upregulation of CYP1B1 in HUVE cells cultured in presence of conditioned supernatants as compared with those treated with unconditioned medium. Addition of metformin significantly abrogates this effect. (B) HUVE cells were transfected with an AMPKα-specific or control (SiCTR) siRNAs, treated with or without 10mM metformin, lysed and analyzed by western blotting for CYP1B1 expression. CYP1B1 is significantly downregulated in AMPKα siRNA-transfected cells as compared with control siRNA. Metformin upregulates CYP1B1 levels in AMPKα siRNA-transfected HUVE cells, suggesting AMPK-dependent CYP1B1 expression in these cells. β-Actin was used as a loading control. The values in the western blot panels represented the ratio of the indicated protein and actin.
Metformin-modulated expression of angiogenesis-associated factors in HUVE and tumor cells
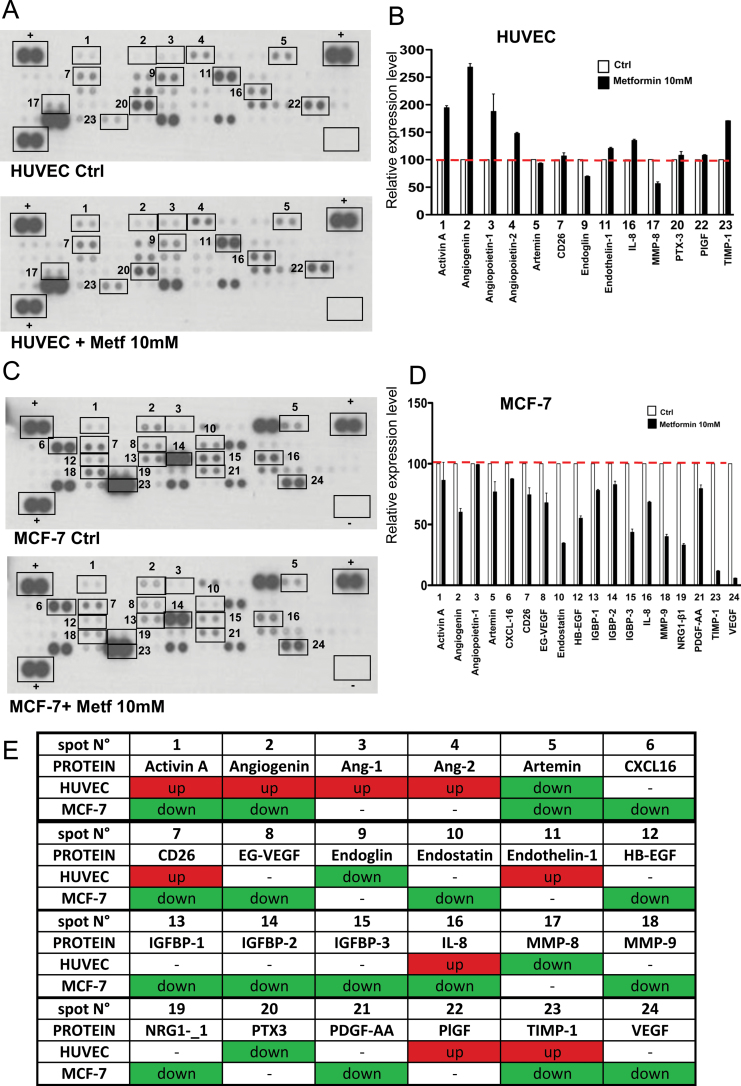
To further investigate the effect of metformin on angiogenesis-related proteins, with either pro- or anti-angiogenic function in our system, we collected supernatants from HUVE and tumor cells (MCF-7) treated or untreated with 10 mM metformin and analyzed them through a proteome profiler human angiogenesis antibody array (Figure 4). We observed both upregulation (angiopoietin-1, angiopoietin-2, angiogenin, Il-8, endothelin-1) and downregulation (MMP-8) of known pro-angiogenic factors in HUVE cells treated with metformin as compared with control. On the other hand, antiangiogenic factors such as activin-A and TIMP-1 are upregulated by metformin, whereas others (e.g. endoglin) are downmodulated (Figure 4A and B). In tumor cells, the expression patterns are differentially regulated with downregulation of both pro- and anti-angiogenic factors (Figure 4C and D). VEGF-C is produced in higher amounts by tumor cells as compared with endothelial cells. The effects of metformin on release of certain angiogenic factors seem to be often the opposite on the two types of cells (Figure 4E). This suggests that metformin exerts contrasting effects on endothelial and tumor cells, which might explain the apparent paradoxic effects of metformin on angiogenic properties reported in the literature.
Fig. 4.
Expression of angiogenesis-related proteins in HUVE and breast cancer cells treated with metformin. HUVE and MCF-7 (breast cancer) cells were treated with vehicle or 10mM metformin for 24h. Cell culture medium were collected, centrifuged and applied to a human angiogenesis antibody array as described in Materials and methods section. Control proteins are located in duplicate in three corners of the arrays, whereas negative control is indicated as an empty box (lower right corner) in the array. The experiment was repeated in duplicate cell lysates. Modulated proteins in treated cells as compared with control are highlighted with squares and indicated by numbers. Each spot is spotted on the array membrane in duplicate. (A) Metformin modulates 13 angiogenesis-related proteins in HUVE cells. (B) Relative amounts of proteins in selected spots indicated in (A) showing mean and standard error of the three experiments in duplicate. (C) In MCF-7, metformin downregulates 19 angiogenesis protein with either antiangiogenic or pro-angiogenic function. (D) Relative amounts of proteins in selected spots indicated in (B) showing mean and standard error of the duplicate. (E) When we compare the modulation of several factors by metformin in the two cell types, we observed different effects. The phenomenon is summarized in the table: in green, downregulated protein; in red: upregulated protein; in white: no significant modulation.
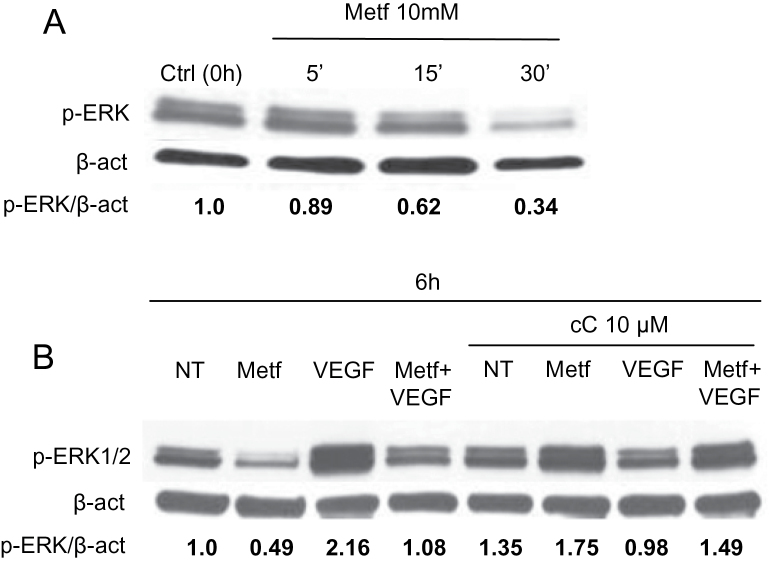
Metformin inhibits VEGF-induced ERK1/2 activation in a AMPK-dependent manner
Serum from women suffering from polycystic ovary syndrome treated with metformin decreases NF-κB and ERK1/2 activation in human microvascular endothelial cells (7,44). Reduced activation of ERK1/2 was observed in HUVE cells treated with metformin (Figure 5A). VEGF is a potent inducer of endothelial cell migration through ERK activation (10). When HUVE cells were treated with or without metformin in presence or absence of VEGF, we observed a VEGF-induced ERK1/2 phosphorylation, which was abrogated in the presence of metformin (Figure 5B). In order to analyze AMPK involvement in this mechanism, we treated cells with compound C and observed a reversion of both metformin and VEGF-mediated ERK1/2 phosphorylation effects (Figure 5B). These data suggest that metformin abrogates ERK activation both in absence and presence of VEGF and that AMPK is directly involved in this effect.
Fig. 5.
Metformin inhibits VEGF-induced ERK1/2 activation in an AMPK-dependent manner. HUVE cells were plated onto six-well plates, treated with or without VEGF in presence or absence of 10mM metformin and/or 10 µM compound C and analyzed at different time points by western blotting. ERK1/2 phosphorylation was measured at 5, 15, 30min or 6h after treatment. (A) ERK1/2 activation was reduced in metformin-treated cells, starting at 15min after metformin addition to the medium. (B) Metformin inhibited phosphorylation of ERK1/2 and inhibited VEGF-induced ERK1/2 phosphorylation. When compound C (cC) was added to the medium, the effects of metformin inhibition and induction of phosphorylation by VEGF alone were counteracted suggesting an involvement of AMPK in ERK1/2 signaling. The experiments were performed twice in triplicate with similar results. The values in the western blot panels represented the ratio of the indicated protein and actin; β-actin was used as loading control.
Metformin inhibits angiogenesis in matrigel pellets in vivo
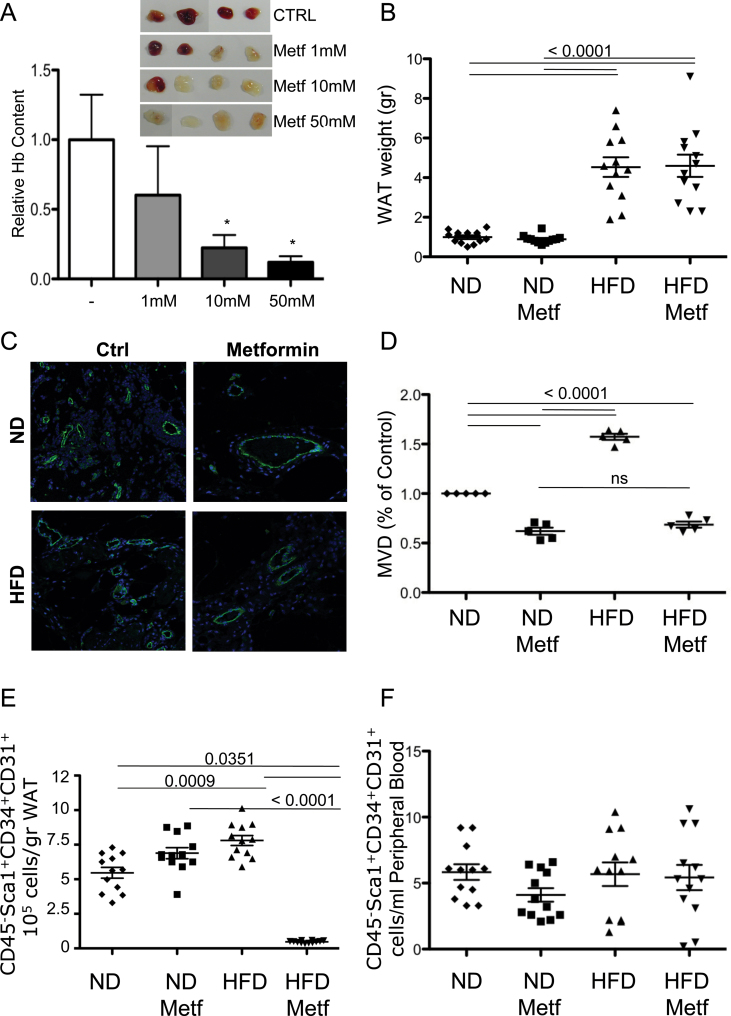
In order to evaluate the effect of metformin on angiogenesis in vivo, we performed the matrigel sponge assay in C57Bl6 mice (27). A cocktail of pro-angiogenic factors (VEGF, TNFα and heparin) promoted a vascularization of the matrigel sponges, which was clearly detectable at 4 days postimplantation (Figure 6A). Visual inspection of the pellets indicated that addition of metformin inhibited the vascularization of the matrigel sponges (Figure 6A). Quantification using hemoglobin as a marker for vascularization indicated a significant, dose-dependent inhibition (P = 0.0296 for 50 mM, P = 0.0456 for 10 mM, Mann–Whitney) (Figure 6A). Staining for CD31, a marker of endothelial cells, showed a reduction in vessel number (Figure 6C and D), consistent with previous reports (8).
Fig. 6.
Metformin inhibits angiogenesis in vivo in normal and obese mice. A cocktail of pro-angiogenic factors (VEGF-A, TNFα and heparin), either alone (Ctrl) or in combination with different concentrations of metformin as indicated was added into matrigel and injected subcutaneously into C57/BL6 mice. (A) Measurement of the hemoglobin content of the matrigel sponges (*P < 0.05, Mann–Whitney). Inset: matrigel pellets photographed 4 days postinjection. Six-week-old C57 mice were fed with either a control ND or a HFD. After 30 days of the respective diet, mice then received the same diet with metformin (0.5mg metformin/ml drinking water, leading to 2mg metformin/mouse/day) or without (control vehicle in drinking water) for further 60 consecutive days (n = 10 per study arm). On day 90, mice were killed. (B) Measurement of the weight of the WAT shows that the mice on the HFD were clearly obese (P < 0.0001, Mann–Whitney). Mice fed with ND or HFD treated with metformin or vehicle were injected with matrigel as described. (C) Immunofluorescence analysis of vascularization by staining matrigel plug sections with anti-CD31 antibody for endothelial cells in mice either on ND or HFD, with or without oral metformin. (D) Quantification of MVD in the sponges based on CD31 staining (shown as CD31-positive cells/field normalized to control). HFD increased the number of CD31-positive cells as compared with ND, whereas metformin abrogated this effect. (E and F) Blood and visceral WAT were collected for the enumeration of CD45−Sca1+CD34+CD31+ progenitor cells by flow cytometry as described previously (29). (E) Obesity was associated with higher numbers of CD45−Sca1+CD34+CD31+ progenitor cells in the WAT (P = 0.0009, Mann–Whitney). Metformin treatment of the obese mice drastically lowered the CD45−Sca1+CD34+CD31+ progenitor cells in the WAT (P < 0.0001, Mann–Whitney), yet had no effect on the numbers of these cells in mice on a ND. (F) Obesity and/or metformin treatment did not significantly alter the number of CD45−Sca1+CD34+CD31+ progenitor cells in the peripheral blood.
We then examined the effect of metformin in the context of obesity using a murine HFD model because most diabetic patients receiving metformin are obese. The metformin dosage used in the murine obesity model (0.5mg metformin/ml drinking water, leading to ~2mg metformin/mouse/day) was chosen after several studies (data not shown) as the one that was biologically active over WAT progenitor cell number and viability and that was associated with no toxicity and no significant changes in mouse weight, and circulating levels of glucose, cholesterol and triglycerides. Mice on a HFD showed significantly increased WAT as compared with mice on a control diet, regardless of whether they received metformin or control vehicle (P < 0.0001, Mann–Whitney) (Figure 6B). Metformin had no significant effect on obesity as determined by WAT content (Figure 6B). As expected (28), mice on the HFD had significantly increased the number of newly created CD31+ vessels (MVD) in the matrigel plugs (Figure 6C and D). In mice receiving metformin, the number of newly created CD31+ vessels was significantly reduced when compared with control mice for both mice on the ND and HFD (Figure 6C and D).
Metformin decreases the number of adipose tissue progenitor endothelial cells in vivo only in obese mice
As previously reported (29), CD45−Sca1+CD34+CD31+ EPCs were significantly increased in mice that received HFD compared with mice fed with control diet (Figure 6E, P = 0.0351, Mann–Whitney). In mice that received control diet, the administration of metformin did not significantly change the number of CD45−Sca1+CD34+CD31+ EPCs in the WAT. However, in obese mice on the HFD, the administration of metformin drastically decreased the number of CD45−Sca1+CD34+CD31+ EPCs (P < 0.0001, Mann–Whitney; Figure 6E). In contrast, in the peripheral blood, HFD or control diet and the administration of metformin or vehicle control did not significantly change the number of circulating CD45−Sca1+CD34+CD31+ EPCs (Figure 6F). Taken together, these data show that metformin has a specific effect on EPCs in the activated adipose tissue of obese animals.
Discussion
Angiogenesis is a critical step to sustain neoplastic proliferation and metastasis. To date, the research in the field of tumor angiogenesis has provided the foundation for a radical change in the management and treatment of human cancers and many antiangiogenic drugs have been approved in clinical practice (11). The tumor microenvironment and angiogenesis can be a target for therapy, chemoprevention and angioprevention (10,13). Many phytochemicals are cancer preventive agents, most of which show angioprevention activity (10,13).
The antidiabetic drug metformin is used by >100 million people with type 2 diabetes worldwide. Metformin activates AMPK (36), acting as a hypoglycemic agent, and inhibits NF-κB (16,36). Epidemiological studies have indicated that metformin is associated with a reduction in incidence of several tumors, thus it is recognized as a potential cancer preventive agent (1,2,10). Several case-control and observational cohort clinical studies have reported that systemic treatment of diabetic patients with metformin significantly decreased the risk of certain tumors, including liver, colorectal, pancreatic, breast and ovarian cancer (2,3). These epidemiological observations provided a strong biological rationale for investigating metformin as an antitumor and chemopreventive agent in basic and translational research. As key step in tumor progression, angiogenesis has been investigated as a putative target of metformin. Experimental data have demonstrated an indirect antiangiogenic effect of metformin by modulating different mediators governing the angiogenesis process (7,14,45,46), suggesting that metformin could exert its anticancer effect, at least in part, through an antiangiogenic effect. However, pro-angiogenic effects associated with upregulation of VEGF have also been reported for breast cancer (4) and melanoma (22). Endothelial and cardiacprotective effects have been reported for metformin, particularly in the context of diabetes and obesity (23,24). The precise mechanisms, as well as the regulatory components for the antiangiogenic or pro-angiogenic properties of metformin, still remain unclear and further investigation is needed.
We confirmed that metformin inhibited endothelial cell proliferation modulating the expression of cell cycle regulatory genes rather than exerting apoptotic effects. The antiproliferative effect of metformin has been associated to cyclin D1 downregulation in several tumor cell lines derived from prostate, breast, ovarian, renal carcinoma and gastric cancer (3), data consistent with our observations in endothelial cells. The effect of metformin on endothelial cell proliferation was time-dependent and associated with an increase of p21 expression after 24 h of treatment, without activation of the apoptotic cascade (Supplementary Figure 2C).
AMPK activation has been demonstrated to exert a potent antiproliferative effect in transformed tumor cells (3) and we questioned whether this might be associated with inhibition of angiogenesis. Matrigel morphogenesis of HUVE cells was inhibited by metformin and this effect could be abolished by transfecting endothelial cells with siRNA to AMPK (Figure 1), confirming that this is a further mechanism by which metformin could block endothelial cell invasion in addition to its ability to reduce expression of MMP-2 and MMP-9 (9). Previous studies have suggested that AMPK activation can enhance angiogenesis (33–35) supporting the hypothesis of context-dependent effects of AMPK.
Gene expression analysis showed a complex pattern of regulation of angiogenesis-associated genes by metformin, which may explain the contrasting reports regarding the pro- and anti-angiogenic activity of metformin. There was a transient induction of VEGF-A mRNA after 6 h of treatment that is consistent with previous reports of enhanced VEGF and pro-angiogenic activity (4,21–23). Similar to endothelial cells, metformin has also been found to induce endothelial nitric oxide synthase and VEGF expression in cardiomyocytes (23,24). The early pro-angiogenic effect could also be associated with a rapid induction of inflammatory response mediators such as COX2 and CXCR4 (Figure 2).
Other genes downmodulated by metformin include ADAMTS1 and the cytochrome P450 family 1 member CYP1B1. ADAMTS1 is a metalloproteinase with antiangiogenic activity. ADAMTS1 gene transfer inhibited angiogenesis in vitro and in vivo, probably the result of induction of endothelial cell apoptosis (47). CYP1 is one of the xenobiotic-metabolizing enzyme families, orthologous forms of CYP1B1 are found in a number of vertebrates. Human CYP1B1 has been reported to metabolize a number of endogenous compounds and is also involved in estrogen metabolism. There are numerous reports on CYP1B1 overexpression in tumor cells (48,49), yet there are few studies on endothelial cell CYP1B1. P450 (CYP) enzymes utilize endogenous substrates, such as arachidonic acid, to generate intracellular second messengers with important roles in vascular function (50). Recent studies indicate that CYP1B1 is expressed in retinal endothelial cells and plays a key role in regulating endothelial cells adhesion and migration in vitro and angiogenesis in vivo (42,43). CYP1B1 deficiency in mice resulted in attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy (42,43). Here, we demonstrate that metformin downregulates CYP1B1. The effect after 24 h is strong and confirmed by quantitative PCR and western blotting. Interestingly, exposure to cancer cell conditioned medium increased endothelial CYP1B1 levels, this was prevented by metformin (Figure 3). Endothelial cells respond to a variety of factors released by tumor cells in the surrounding microenvironment including VEGF, platelet-derived growth factor and fibroblast growth factor (51). By producing these factors, tumor cells promote angiogenesis, which in turn provides nutrients and oxygen to the tumor bulk. Given CYP1B1-mediated promotion of endothelial cell morphogenesis in vitro and angiogenesis in vivo (42), cancer cells may promote endothelial cell functions by upregulating CYP1B1. This is the first report showing that tumor cell conditioned medium increased CYP1B1 expression in endothelial cells. Exposure of endothelial cells to other chemopreventive agents, such as phytochemicals, for instance resveratrol (52), also decreases CYP1B1 expression. CYP1B1 has also been suggested to be involved in generating metabolites of phytochemicals that are toxic to tumor cells (48). We postulate that reduction of CYP1B1 could be a leading mechanism of the antiangiogenic effects of metformin.
A paradoxic effect of metformin on angiogenesis has been shown in different cellular contexts (4,22). Analyzing secreted angiogenesis-related proteins in both endothelial and tumor cells, we observed two major aspects of this contradiction: on one side, metformin modulates both pro- and anti-angiogenic factors in endothelial cells with no apparent advantage of one over the other. On the other hand, metformin differently modulates these proteins in endothelial and breast cancer cells. TIMP-1 and interleukin-8 are two representative examples, the first exerting antiangiogenic activity (53) and the latter being a potent pro-angiogenic factor (10). Metformin upregulated both proteins in endothelial cells and downmodulated both in tumor cells, a clear example of contradictory metformin effects. Interestingly, VEGF, a major promoter of angiogenesis, was almost absent in treated endothelial cell supernatants while markedly secreted by tumor cells. These findings suggest that metformin acts through a cell-specific mechanism. In addition, we speculate that because AMPK is a metabolic sensor differently regulated in tumor and normal cells, the opposite effects of metformin on angiogenesis-related proteins in normal and tumor cells might be at least partially dependent on AMPK levels and activation.
Endothelial migration is promoted by VEGF through ERK activation (10). Metformin has been shown to inhibit ERK activation (54), consistent with its antiangiogenic function and our data (Figure 5). The array data in endothelial cells suggest that metformin has both pro- and anti-angiogenic activity; functionally, the antiangiogenic effect prevails. The transient upregulation of VEGF induced by metformin that we observed at the mRNA and protein level, and addition of exogenous VEGF, are probably not sufficient to restore VEGF-induced ERK phosphorylation in the presence of metformin. This effect is at least in part AMPK-dependent, since inhibition of AMPK activation by compound C reverted this effect (Figure 5). Previous findings indicate the existence of an AMPK-independent reduction of phospho-ERK levels in cancer cells (54), our data on endothelial cells suggest an AMPK-dependent control of metformin downstream signaling, including the ERK pathway.
Metformin is widely used for treatment of type 2 diabetic patients, 80% of whom are obese, and the epidemiological data on the cancer prevention effects of metformin come from this patient group, which have a baseline high cancer incidence (1,2). In agreement with a previous publication (8), metformin treatment attenuated vascularization in matrigel sponges implanted in mice (Figure 6). Obese mice showed even greater MVD in the matrigel sponges compared to controls (again in agreement with previous reports) (28). Metformin reverted the effect of obesity on MVD (Figure 6C–D), suggesting that increased angiogenesis may be part of the higher cancer risk in type 2 diabetics and that angioprevention might be particularly effective in obese individuals. The increase in angiogenesis in obese animals may in part be due to higher release of endothelial precursors from the WAT. In obesity, inflammation is initiated by a local hypoxia to increase angiogenesis and improve adipose tissue blood supply (55). It has also been suggested that macrophages and pro-inflammatory cytokines are essential for adipose remodeling and adipocyte differentiation. In mice receiving a HFD, metformin might induce apoptosis of CD45−Sca1+CD34+CD31+ EPCs, differentiate them toward another type of specialized cell or mobilize them to other tissues. Studies to validate these hypotheses are ongoing.
Our data assessing the effects of metformin on endothelial cells find paradoxical effects, as antiangiogenesis is associated with transient induction of pro-angiogenic genes and long-term induction of inflammatory mediators. These observations have important implications for the investigation and treatment of vascular dysfunctions by metformin, not only in cancer patients but also in metabolic and cardiovascular diseases. Further, we show that metformin, inhibits precursor endothelial cells only in obese animals on a fat diet, suggesting that metformin may be most effective as a cancer prevention agent in the context of obesity.
Supplementary material
Supplementary Methods and Figures 1–4 can be found at http://carcin.oxfordjournals.org/
Funding
Associazione Italiana per la Ricerca sul Cancro; Italian Ministry of Health Grande Progetto Strategico; Ministero dell’ Istruzione dell’ Università e della Ricerca Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (2010NECHBX_003). Fodanzione Italiana per la Ricerca sul Cancro fellowship (to A.B.); Fondazione Veronesi (A.R.C.).
Supplementary Material
Acknowledgements
We thank Alessandra Panvini Rosati for assistance and Dr Paola Corradino for bibliographic searches.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ADAMTS1
ADAM metallopeptidase with thrombospondin type 1 motif
- AMPK
adenosine-monophosphate-activated protein kinase
- COX2
cyclooxygenase 2
- CXCR4
CXC chemokine receptor 4
- CYP1B1
cytochrome P450 1B1
- EPCs
endothelial progenitor cells
- ERK
extracellular signal-regulated kinase
- HFD
high-fat diet
- HUVECs
human umbilical vein endothelial cells
- MMP
matrix metalloproteinase
- mRNA
messenger RNA
- MVD
microvessel density
- ND
normal diet
- NF-κB
nuclear factor-κB
- siRNA
small interfering RNA
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
- WAT
white adipose tissue.
References
- 1. Evans J.M., et al. (2005). Metformin and reduced risk of cancer in diabetic patients. BMJ, 330, 1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang P., et al. (2013). Association of metformin use with cancer incidence and mortality:a meta-analysis. Cancer Epidemiol., 37, 207–218 [DOI] [PubMed] [Google Scholar]
- 3. Ben Sahra I., et al. (2008). The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene, 27, 3576–3586 [DOI] [PubMed] [Google Scholar]
- 4. Phoenix K.N., et al. (2009). Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res. Treat., 113, 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akinyeke T., et al. (2013) Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis, 34, 2823–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eid A.A., et al. (2010) AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem., 285, 37503–37512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan B.K., et al. (2009). Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc. Res., 83, 566–574 [DOI] [PubMed] [Google Scholar]
- 8. Xavier D.O., et al. (2010). Metformin inhibits inflammatory angiogenesis in a murine sponge model. Biomed. Pharmacother., 64, 220–225 [DOI] [PubMed] [Google Scholar]
- 9. Esfahanian N., et al. (2012). Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol. Med. Rep., 5, 1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albini A., et al. (2012). Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol., 9, 498–509 [DOI] [PubMed] [Google Scholar]
- 11. Chung A.S., et al. (2010). Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer, 10, 505–514 [DOI] [PubMed] [Google Scholar]
- 12. Mann J.R., et al. (2005). Mechanisms of disease:inflammatory mediators and cancer prevention. Nat. Clin. Pract. Oncol., 2, 202–210 [DOI] [PubMed] [Google Scholar]
- 13. Albini A., et al. (2007). The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer, 7, 139–147 [DOI] [PubMed] [Google Scholar]
- 14. Ersoy C., et al. (2008). The effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patients. Diabetes Res. Clin. Pract., 81, 56–60 [DOI] [PubMed] [Google Scholar]
- 15. Lund S.S., et al. (2008). Impact of metformin versus repaglinide on non-glycaemic cardiovascular risk markers related to inflammation and endothelial dysfunction in non-obese patients with type 2 diabetes. Eur. J. Endocrinol., 158, 631–641 [DOI] [PubMed] [Google Scholar]
- 16. Hirsch H.A., et al. (2013). Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl Acad. Sci. USA, 110, 972–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caballero A.E., et al. (2004). The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo-controlled, randomized clinical trial. J. Clin. Endocrinol. Metab., 89, 3943–3948 [DOI] [PubMed] [Google Scholar]
- 18. Abbasi F., et al. (2004). Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism., 53, 159–164 [DOI] [PubMed] [Google Scholar]
- 19. Cacicedo J.M., et al. (2004). AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun., 324, 1204–1209 [DOI] [PubMed] [Google Scholar]
- 20. De Jager J., et al. (2005). Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J. Intern. Med., 257, 100–109 [DOI] [PubMed] [Google Scholar]
- 21. Hadad S.M., et al. (2009). Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res. Treat., 114, 391. [DOI] [PubMed] [Google Scholar]
- 22. Martin M.J., et al. (2012). Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov., 2, 344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cittadini A., et al. (2012). Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes, 61, 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sena C.M., et al. (2011). Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol., 163, 424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ziche M., et al. (2009). Molecular regulation of tumour angiogenesis by nitric oxide. Eur. Cytokine Netw., 20, 164–170 [DOI] [PubMed] [Google Scholar]
- 26. Carpentier G. (2012). Contribution:angiogenesis analyzer. ImageJ News, 5 October 2012. http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ (21 October 2013, date last accessed). [Google Scholar]
- 27. Passaniti A., et al. (1992). A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Invest., 67, 519–528 [PubMed] [Google Scholar]
- 28. Balwierz A., et al. (2009). Angiogenesis in the New Zealand obese mouse model fed with high fat diet. Lipids Health Dis., 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin-Padura I., et al. (2012). The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res., 72, 325–334 [DOI] [PubMed] [Google Scholar]
- 30. Mancuso P., et al. (2011). Circulating perivascular progenitors: a target of PDGFR inhibition. Int. J. Cancer, 129, 1344–1350 [DOI] [PubMed] [Google Scholar]
- 31. Dagher Z., et al. (1999). The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun., 265, 112–115 [DOI] [PubMed] [Google Scholar]
- 32. Ido Y., et al. (2002). Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes, 51, 159–167 [DOI] [PubMed] [Google Scholar]
- 33. Nagata D., et al. (2003) AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem., 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 34. Ouchi N., et al. (2004) Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem., 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shibata R., et al. (2004) Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J. Biol. Chem., 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 36. Hattori Y., et al. (2006). Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension, 47, 1183–1188 [DOI] [PubMed] [Google Scholar]
- 37. Baud V., et al. (2009) Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov., 8, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jalving M., et al. (2010). Metformin: taking away the candy for cancer? Eur. J. Cancer, 46, 2369–2380 [DOI] [PubMed] [Google Scholar]
- 39. Wong C., et al. (2005) Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J. Biol. Chem., 280, 33262–33269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. To K.K., et al. (2006). The phosphorylation status of PAS-B distinguishes HIF-1alpha from HIF-2alpha in NBS1 repression. EMBO J., 25, 4784–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teicher B.A., et al. (2010). CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res., 16, 2927–2931 [DOI] [PubMed] [Google Scholar]
- 42. Tang Y., et al. (2010). CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am. J. Physiol. Cell Physiol., 298, C665–C678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang Y., et al. (2009). CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood, 113, 744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palomba S., et al. (2013). Effects of metformin in women with polycystic ovary syndrome treated with gonadotrophins for in vitro fertilisation and intracytoplasmic sperm injection cycles: a systematic review and meta-analysis of randomised controlled trials. BJOG, 120, 267–276 [DOI] [PubMed] [Google Scholar]
- 45. Rattan R., et al. (2011). Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo . Neoplasia, 13, 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao H., et al. (2012). Luteinizing hormone facilitates angiogenesis in ovarian epithelial tumor cells and metformin inhibits the effect through the mTOR signaling pathway. Oncol. Rep., 27, 1873–1878 [DOI] [PubMed] [Google Scholar]
- 47. Obika M., et al. (2012). Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis. Cancer Sci., 103, 1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ware W.R. (2009). Nutrition and the prevention and treatment of cancer: association of cytochrome P450 CYP1B1 with the role of fruit and fruit extracts. Integr. Cancer Ther., 8, 22–28 [DOI] [PubMed] [Google Scholar]
- 49. Luby T.M. (2008). Targeting cytochrome P450 CYP1B1 with a therapeutic cancer vaccine. Expert Rev. Vaccines, 7, 995–1003 [DOI] [PubMed] [Google Scholar]
- 50. Conway D.E., et al. (2009). Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc. Res., 81, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weis S.M., et al. (2011). Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med., 17, 1359–1370 [DOI] [PubMed] [Google Scholar]
- 52. Mikstacka R., et al. (2007). Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol. Nutr. Food Res., 51, 517–524 [DOI] [PubMed] [Google Scholar]
- 53. Guedez L., et al. (2001). Tissue inhibitor of metalloproteinase-1 alters the tumorigenicity of Burkitt’s lymphoma via divergent effects on tumor growth and angiogenesis. Am. J. Pathol., 158, 1207–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gou S., et al. (2013). Low concentrations of metformin selectively inhibit CD133⁺ cell proliferation in pancreatic cancer and have anticancer action. PLoS One, 8, e63969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ye J., et al. (2013). Inflammation during obesity is not all bad: evidence from animal and human studies. Am. J. Physiol. Endocrinol. Metab., 304, E466–E477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.