Abstract
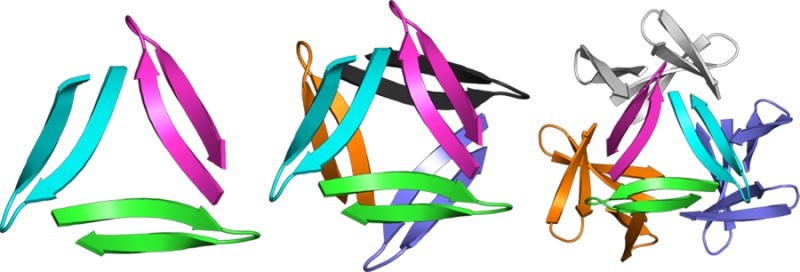
A peptide derived from Aβ17–36 crystallizes to form trimers that further associate to form higher-order oligomers. The trimers consist of three highly twisted β-hairpins in a triangular arrangement. Two trimers associate face-to-face in the crystal lattice to form a hexamer; four trimers in a tetrahedral arrangement about a central cavity form a dodecamer. These structures provide a working model for the structures of oligomers associated with neurodegeneration in Alzheimer’s disease.
Here we report the X-ray crystallographic structures of trimers and higher-order oligomeric assemblies of a peptide derived from the β-amyloid peptide (Aβ). Oligomers of Aβ are now thought to play a central role in neurodegeneration in Alzheimer’s disease.1 Selkoe et al. found that small Aβ oligomers disrupt long-term potentiation, with trimers showing the highest disruption.7 Ashe et al. found that a 56 kDa Aβ oligomer, termed Aβ*56, impairs memory.6 The oligomer appears to be a dodecamer composed of four trimers. Understanding the structures of these oligomers is essential to understanding their mechanism of action. Trimers are particularly enigmatic, because their structure cannot be explained by simply pairing monomers.
The hydrophobic central and C-terminal regions of Aβ are known to participate in aggregation to form fibrils and are likely involved in the aggregation of oligomers.13 Although many molecular details of the aggregation processes have yet to be elucidated, the formation of β-sheets appears to be involved. While the structures of the fibrils are relatively well understood, the structures of trimers and higher-order oligomers are not known.19
Peptide fragments derived from amyloidogenic peptides and proteins are valuable tools for studying the structures of amyloid fibrils and oligomers.22 In the current study, we set out to use a peptide fragment derived from both the central and C-terminal regions of Aβ to elucidate the structures of Aβ oligomers.15,16 We designed peptide 1a as a mimic of Aβ17–36 in which residues 17–23 (LVFFAED) and 30–36 (AIIGLMV) form the β-strands of a β-hairpin (Figure 1). In this structure, the δ-linked ornithine β-turn mimic connecting Asp23 and Ala30 promotes a β-hairpin structure and replaces residues 24–29 (VGSNKG). The δ-linked ornithine connecting Leu17 and Val36 forms a macrocycle to further enforce the β-hairpin structure. We incorporated an N-methyl group on Gly33 to help prevent uncontrolled aggregation through edge-to-edge H-bonding between β-sheets.31 We replaced Met35 with the hydrophilic isostere ornithine (α-linked) to enhance solubility and further prevent uncontrolled aggregation. We designed an analogue of peptide 1a containing 4-iodophenylalanine at the Phe19 position (peptide 1b) for crystallographic phase determination.
Figure 1.
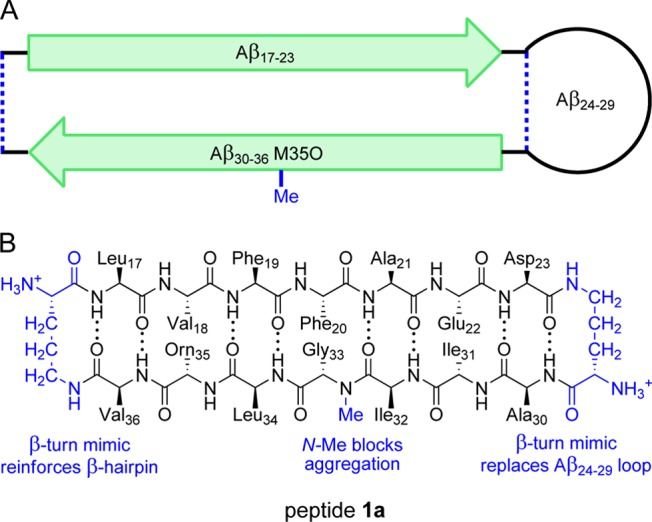
(A) Cartoon illustrating the design of peptide 1a and the envisioned relationship to Aβ17–36. (B) Chemical structure of 1a illustrating Aβ17–23, Aβ30–36 M35O, the N-methyl group, and the δ-linked ornithine β-turn mimic.
The synthesis and crystallization of peptides 1a and 1b were straightforward.32 The peptides were synthesized using Fmoc-based solid-phase peptide synthesis, solution-phase cyclization, and RP-HPLC purification. Initial crystallization conditions for peptide 1a were identified using the Hampton Research crystallization kits: Crystal Screen, Index, and PEG/Ion (288 experiments). Conditions with HEPES buffer and Jeffamine M-600 were selected and further optimized (0.1 M HEPES at pH 6.75 with 30% Jeffamine M-600) to give rapid (<24 h) formation of good crystals. Crystal diffraction data were collected at the Advanced Light Source at Lawrence Berkeley National Laboratory with a synchrotron source at 1.0 Å wavelength. Diffraction data were collected to 1.70 Å resolution. Data were scaled and merged using XDS, and phases were determined by isomorphous replacement of the Phe19 4-iodophenylalanine derivative 1b.33 The structure of peptide 1a was solved and refined in the R3 space group. The asymmetric unit contains 16 nearly identical monomers (rmsd ≈ 0.2 Å, Figure S3). Coordinates for hydrogens were generated by phenix.refine during refinement.34
The X-ray crystallographic structure reveals that peptide 1a folds to form a β-hairpin comprising two heptapeptide β-strands. Eight residues (Leu17, Phe19, Ala21, Asp23, Ala30, Ile32, Leu34, and Val36) make up one surface of the β-hairpin (the LFA face), and six residues (Val18, Phe20, Glu22, Ile31, Gly33, and Orn35) make up the other surface (the VF face). The β-hairpin has a strong twist in which each residue rotates in a right-handed fashion ∼25° along the β-strand axis.35 Contacts between the side chains of residues located diagonally across the β-sheet help stabilize the twisted β-hairpin (Phe19-Val36, Ala21-Leu34, and Asp23-Ile32; Figures 2A and S1).
Figure 2.
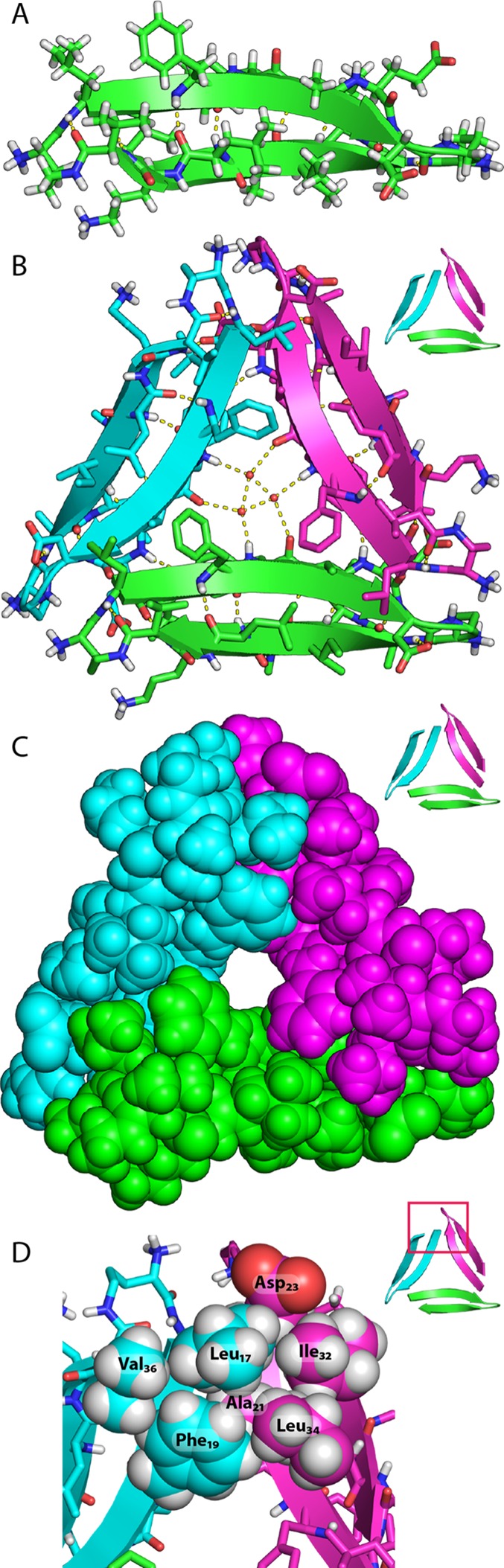
X-ray crystallographic structure of peptide 1a: (A) β-hairpin monomer; (B) trimer, cartoon and stick representation with ordered water; (C) trimer, space-filling representation; (D) detail of trimer interface illustrating the hydrophobic cluster formed by Leu17, Phe19, Ala21, Asp23, Ile32, Leu34, and Val36.
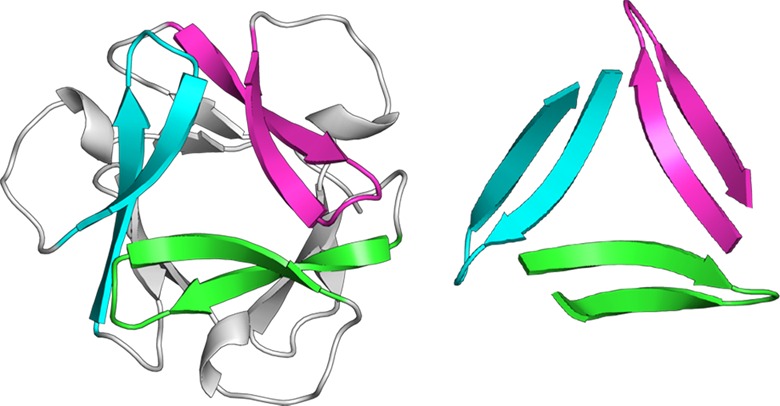
Three β-hairpins assemble in a triangular fashion and interlock to form a trimer, with each β-hairpin making up one edge of the equilateral triangle (Figure 2). The central Aβ strand (17–23) constitutes the inner edges of the equilateral triangle, and the C-terminal strand (30–36) constitutes the outer edges. A hole runs through the center of the triangle, and the NH and C=O groups of Phe20 line the hole. The side chains of Phe19 surround the front of the hole, and the side chains of Phe20 surround the back of the hole. Three ordered water molecules hydrogen bond to the NH and C=O groups of Phe20 and fill the hole in the center of the triangle (Figure 2B).36 The β-hairpins come together at the corners of the triangle and stabilize the trimer through both polar and nonpolar interactions. The main chains of Val18 and Glu22 H-bond at each corner to create a four-stranded β-sheet (Figure S2). The side chains of Leu17, Phe19, Ala21, Asp23, Ile32, Leu34, and Val36 form an extensive hydrophobic cluster at the corners where the β-hairpins meet. The side chain of Leu17 makes extensive contacts with the side chains of Ala21, Asp23, Ile32, and Leu34 of the adjacent β-hairpin and is buttressed by the side chains of Phe19 and Val36 (Figures 2D and S2).
The trimers assemble loosely in the crystal lattice to form hexamers and dodecamers (Figure 3). The hexamer consists of two trimers clasped through the VF faces. In the hexamer, the Ile31 residues of the two trimers H-bond through bridging waters. Loose contacts between Phe20 and Ile31 appear to further stabilize the hexamer. The dodecamer consists of four trimers in a tetrahedral arrangement around a central cavity, with the LFA faces lining the cavity. Hydrophobic contacts occur between the Leu17 residues of the trimers. Salt bridges between the Asp23 residues and the δ-linked ornithines preceding Leu17 further stabilize the dodecamer. The contacts stabilizing the hexamers and dodecamers appear to be in opposition, preventing either oligomer from packing tightly.
Figure 3.
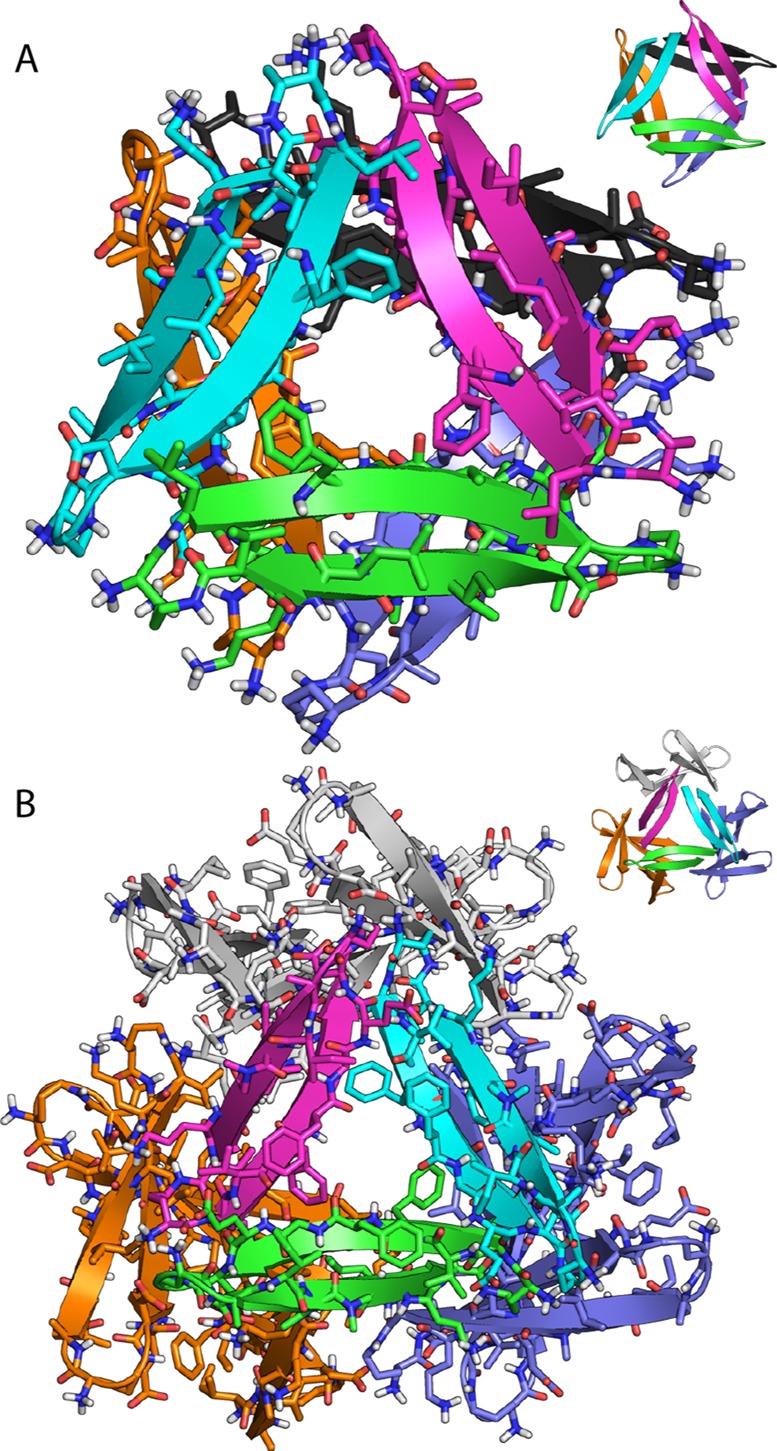
(A) Hexamer and (B) dodecamer observed in the X-ray crystallographic structure of peptide 1a.
To test whether trimer formation results from N-methylation of Gly33, we prepared and studied peptide 2a, in which Phe20 is N-methylated
and Gly33 is not. Peptide 2a crystallized
in conditions similar to those for peptide 1a. Phases
were determined by isomorphous replacement of the Phe19 4-iodophenylalanine derivative 2b. The
structure of peptide 2a was solved and refined in the P3221 space group and contains 12 nearly identical
monomers (rmsd ≈ 0.3 Å, Figure S4).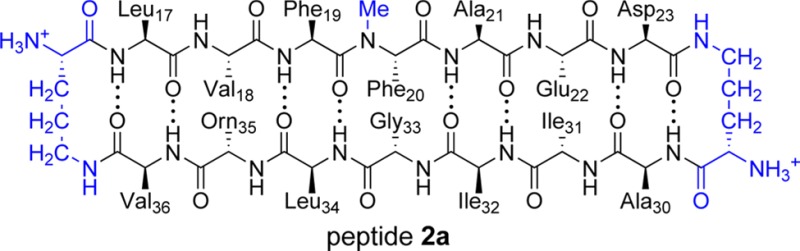
The X-ray crystallographic structure of peptide 2a is nearly identical to that of peptide 1a. Peptide 2a crystallizes as a β-hairpin that assembles to form trimers, which further form hexamers and dodecamers. Moving the N-methyl group from Gly33 to Phe20 does not significantly alter the structures of the oligomers. The central Aβ strand (17–23) still forms the inner edges of the trimer, and the C-terminal strand (30–36) still forms the outer edges. In the X-ray crystallographic structure of peptide 2a, the N-methyl groups from Phe20 replace the three ordered waters that fill the hole in the center of the triangle (Figure 4). While N-methylation of either Phe20 or Gly33 is necessary to prevent aggregation, it does not dictate the formation of the trimer, hexamer, or dodecamer.
Figure 4.
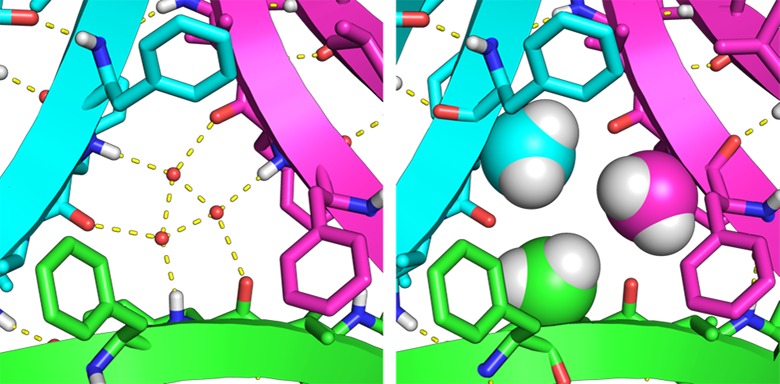
Detail of the X-ray crystallographic structure of the trimers formed by peptides 1a (left) and 2a (right). The N-methyl groups of 2a take the place of the ordered water molecules in 1a.
The trimer formed by peptides 1a and 2a is unlike the structures of fibrils or oligomers previously observed for amyloidogenic peptides or proteins. A similar triangular assembly of three β-hairpins occurs in actinohivin, a 114-amino-acid lectin that binds HIV gp120.37 Actinohivin contains three nearly identical sequences in tandem that form the three β-hairpins and fold into a triangular assembly (Figure 5, PDB 3A07). Each β-hairpin comprises two pentapeptide β-strands connected by a turn of three residues. Although these β-strands are smaller than those of peptide 1a, they occupy similar positions to residues 17–23 and 30–36 of 1a in the trimer. Actinohivin binds to mannose-containing glycans on gp120 with high affinity through trivalent interactions of the concave surfaces formed by the three near-repeats. This mode of interaction suggests that trimers of Aβ may bind to molecules on the surface of neurons through trivalent interactions of three β-hairpins formed by Aβ17–36.
Figure 5.
Cartoon representation showing the structural similarities of actinohivin (left) and peptide 1a trimer (right).
We modeled a trimer of Ac-Aβ17–36-NHMe β-hairpins to generate a working model of a trimer of Aβ. We used the crystallographic coordinates of peptide 1a to generate residues 17–23 (LVFFAED) and 30–36 (AIIGLMV) of the trimer and added loops comprising residues 24–29 (VGSNKG). We performed replica-exchange molecular dynamics (REMD) in NAMD using the CHARMM22 force field with generalized Born implicit solvent to generate realistic conformations of the loops.39 Figure 6 illustrates 20 low-energy structures from the simulation. These structures provide a working model in which residues 24–29 act as a loop connecting the 17–23 and 30–36 β-strands. The concave surfaces formed by the loops and the twisted β-strands might serve as binding sites in multivalent biological interactions.
Figure 6.
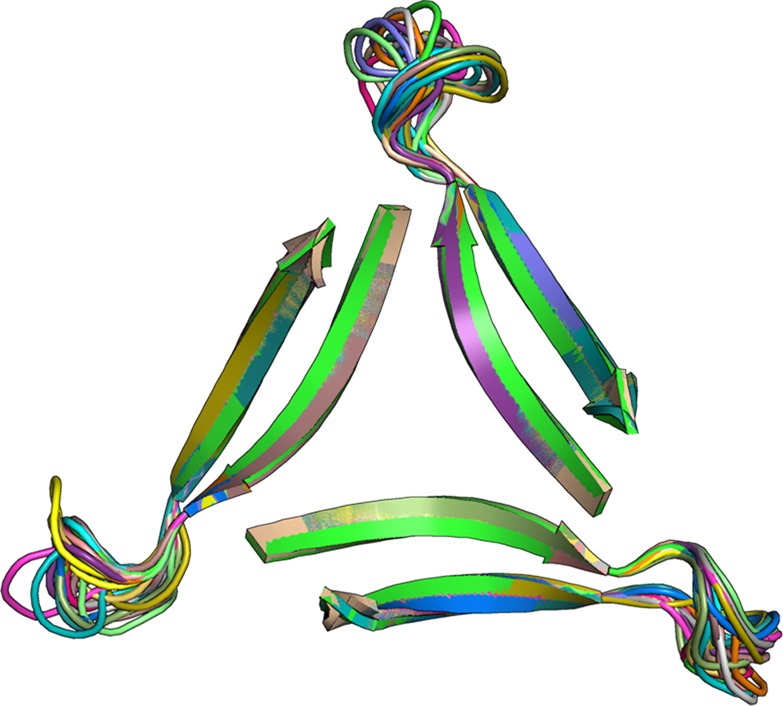
Cartoon representation of 20 low-energy structures for Ac-Aβ17–36-NHMe generated by REMD.
The X-ray crystallographic structures of the trimers and higher-order oligomeric assemblies formed by peptides 1a and 2a provide working models for the structures of the trimers and higher-order oligomers of Aβ that are important in neurodegeneration. In this model, Aβ associates to form trimers comprising three β-hairpins in a triangular arrangement. The trimers are stabilized through interactions among the central region of Aβ17–23 (LVFFAED). These interactions are buttressed by hydrophobic interactions with the hydrophobic C-terminal region of Aβ—either Aβ30–36 (AIIGLMV) or another segment of the extensive hydrophobic C-terminal sequence. Two trimers can stack to form hexamers. While the hexamers formed by peptides 1a and 2a associate along the VF face, there is little distinction between the residues of the VF face and the LFA face, and stacking might occur through interactions among either face. Although the dodecamer in Figure 3b might explain the structure of Aβ*56, an assembly consisting of a stack of four trimers is also possible and may provide greater contact and stabilization. These structures and ideas serve as a starting point for developing and testing hypotheses about the structures and mechanism of action of amyloid oligomers.
Acknowledgments
We thank Biopeptek for generously providing a sample of peptide 1a;32 the ALS/BCSB for data collection and processing; Profs. Tom Poulos and Celia Goulding and Drs. Huiying Li and Nicholas Chim for helpful advice on X-ray crystallography; Prof. Ioan Andricioaei and Gavin Bascom for help with REMD simulations; and the National Institutes of Health for funding (Grant 1R01GM097562). H.L. thanks Allergan and UCI for support.
Supporting Information Available
Details of the synthesis and crystallization of peptides 1a, 1b, 2a, and 2b; X-ray diffraction data collection, processing, and refinement; and modeling of Ac-Aβ17–36-NHMe. Crystallographic data in CIF format. Coordinates for models of Ac-Aβ17–36-NHMe in PDB format. This material is available free of charge via the Internet at http://pubs.acs.org. Crystallographic coordinates of 1a, 1b, 2a, and 2b were deposited into the Protein Data Bank with PDB codes 4NTR, 4NTP, 4NW9, and 4NW8.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Lambert M. P.; Barlow A. K.; Chromy B. A.; Edwards C.; Freed R.; Liosatos M.; Morgan T. E.; Rozovsky I.; Trommer B.; Viola K. L.; Wals P.; Zhang C.; Finch C. E.; Krafft G. A.; Klein W. L. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 6448. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Selkoe D. J. Science 2002, 298, 789. [DOI] [PubMed] [Google Scholar]; c Nagele R. G.; D’Andrea M. R.; Anderson W. J.; Wang H. Y. Neuroscience 2002, 110, 199. [DOI] [PubMed] [Google Scholar]; d Walsh D. M.; Klyubin I.; Fadeeva J. V.; Cullen W. K.; Anwyl R.; Wolfe M. S.; Rowan M. J.; Selkoe D. J. Nature 2002, 416, 535. [DOI] [PubMed] [Google Scholar]; e Kayed R.; Head E.; Thompson J. L.; McIntire T. M.; Milton S. C.; Cotman C. W.; Glabe C. G. Science 2003, 300, 486. [DOI] [PubMed] [Google Scholar]; f Lesné S.; Koh M. T.; Kotilinek L.; Kayed R.; Glabe C. G.; Yang A.; Gallagher M.; Ashe K. H. Nature 2006, 440, 352. [DOI] [PubMed] [Google Scholar]; g Townsend M.; Shankar G. M.; Mehta T.; Walsh D. M.; Selkoe D. J. J. Physiol. 2006, 572, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Haass C.; Selkoe D. J. Nat. Rev. Mol. Cell. Biol. 2007, 8, 101. [DOI] [PubMed] [Google Scholar]; i Shankar G. M.; Li S. M.; Mehta T. H.; Garcia-Munoz A.; Shepardson N. E.; Smith I.; Brett F. M.; Farrell M. A.; Rowan M. J.; Lemere C. A.; Regan C. M.; Walsh D. M.; Sabatini B. L.; Selkoe D. J. Nat. Med. 2008, 14, 837. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhao W.-Q.; De Felice F. G.; Fernandez S.; Chen H.; Lambert M. P.; Quon M. J.; Krafft G. A.; Klein W. L. FASEB J. 2008, 22, 246. [DOI] [PubMed] [Google Scholar]; k Querfurth H. W.; LaFerla F. M. N. Engl. J. Med. 2010, 362, 329. [DOI] [PubMed] [Google Scholar]; l Fändrich M. J. Mol. Biol. 2012, 421, 427. [DOI] [PubMed] [Google Scholar]
- a Thirumalai D.; Klimov D. K.; Dima R. I. Curr. Opin. Struct. Biol. 2003, 13, 146. [DOI] [PubMed] [Google Scholar]; b Liu R.; McAllister C.; Lyubchenko Y.; Sierks M. R. J. Neurosci. Res. 2004, 75, 162. [DOI] [PubMed] [Google Scholar]; c Hoyer W.; Gronwall C.; Jonsson A.; Stahl S.; Härd T. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 5099. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sandberg A.; Luheshi L. M.; Söllvander S.; Pereira de Barros T.; Macao B.; Knowles T. P.; Biverstål H.; Lendel C.; Ekholm-Petterson F.; Dubnovitsky A.; Lannfelt L.; Dobson C. M.; Härd T. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 15595. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yu L.; Edalji R.; Harlan J. E.; Holzman T. F.; Lopez A. P.; Labkovsky B.; Hillen H.; Barghorn S.; Ebert U.; Richardson P. L.; Miesbauer L.; Solomon L.; Bartley D.; Walter K.; Johnson R. W.; Hajduk P. J.; Olejniczak E. T. Biochemistry 2009, 48, 1870. [DOI] [PubMed] [Google Scholar]; f Cerf E.; Sarroukh R.; Tamamizu-Kato S.; Breydo L.; Derclaye S.; Dufrêne Y. F.; Narayanaswami V.; Goormaghtigh E.; Ruysschaert J. M.; Raussens V. Biochem. J. 2009, 421, 415. [DOI] [PubMed] [Google Scholar]
- a Lührs T.; Ritter C.; Adrian M.; Riek-Loher D.; Bohrmann B.; Döbeli H.; Schubert D.; Riek R. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 17342. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Petkova A. T.; Yau W.-M.; Tycko R. Biochemistry 2006, 45, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lu J.-X.; Qiang W.; Yau W.-M.; Schwieters C. D.; Meredith S. C.; Tycko R. Cell 2013, 154, 1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nelson R.; Sawaya M. R.; Balbirnie M.; Madsen A. Ø.; Riekel C.; Grothe R.; Eisenberg D. Nature 2005, 435, 773. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sawaya M. R.; Sambashivan S.; Nelson R.; Ivanova M. I.; Sievers S. A.; Apostol M. I.; Thompson M. J.; Balbirnie M.; Wiltzius J. J.; McFarlane H. T.; Madsen A. Ø.; Riekel C.; Eisenberg D. Nature 2007, 447, 453. [DOI] [PubMed] [Google Scholar]; c Laganowsky A.; Liu C.; Sawaya M. R.; Whitelegge J. P.; Park J.; Zhao M.; Pensalfini A.; Soriaga A. B.; Landau M.; Teng P. K.; Cascio D.; Glabe C.; Eisenberg D. Science 2012, 335, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu C.; Sawaya M. R.; Cheng P.-N.; Zheng J.; Nowick J. S.; Eisenberg D. J. Am. Chem. Soc. 2011, 133, 6736. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu C.; Zhao M.; Jiang L.; Cheng P.-N.; Park J.; Sawaya M. R.; Pensalfini A.; Gou D.; Berk A. J.; Glabe C. G.; Nowick J. S.; Eisenberg D. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 20913. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Cheng P.-N.; Pham J. D.; Nowick J. S. J. Am. Chem. Soc. 2013, 135, 5477. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Pham J. D.; Chim N.; Goulding C. W.; Nowick J. S. J. Am. Chem. Soc. 2013, 135, 12460. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Streltsov V. A.; Varghese J. N.; Masters C. L.; Nuttall S. D. J. Neurosci. 2011, 31, 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Apostol M. I.; Perry K.; Surewicz W. K. J. Am. Chem. Soc. 2013, 135, 10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R.; Chen K. H.; Manuel G.; Nowick J. S. Eur. J. Org. Chem. 2013, 3523. [Google Scholar]
- Peptides 1 and 2 were synthesized in house using the PS3 automated peptide synthesizer (Protein Technologies, Inc.). Biopeptek also synthesized an authentic sample of 1a to corroborate the reproducibility of this synthesis.
- Kabsch W. Acta Crystallogr., D: Biol. Crystallogr. 2010, 66, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. D.; Afonine P. V.; Bunkoczi G.; Chen V. B.; Davis I. W.; Echols N.; Headd J. J.; Hung L. W.; Kapral G. J.; Grosse-Kunstleve R. W.; McCoy A. J.; Moriarty N. W.; Oeffner R.; Read R. J.; Richardson D. C.; Richardson J. S.; Terwilliger T. C.; Zwart P. H. Acta Crystallogr., D: Biol. Crystallogr. 2010, 66, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisted two-stranded β-hairpins — termed β-ribbons — are common in protein structure. For details, see:Richardson J. S. Adv. Protein Chem. 1981, 34, 167. [DOI] [PubMed] [Google Scholar]; See also http://kinemage.biochem.duke.edu/teaching/anatax/.
- The three ordered waters are in unusually close contact, with an average distance of ∼2.2 Å between oxygen atoms.
- a Tanaka H.; Chiba H.; Inokoshi J.; Kuno A.; Sugai T.; Takahashi A.; Ito Y.; Tsunoda M.; Suzuki K.; Takénaka A.; Sekiguchi T.; Umeyama H.; Hirabayashi J.; O̅mura S. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 15633. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Suzuki K.; Tsunoda M.; Hoque M. M.; Zhang F.; Jiang J.; Zhang X.; Ohbayashi N.; Tanaka H.; Takénaka A. Acta Crystallogr., D: Biol. Crystallogr. 2013, 69, 1818. [DOI] [PubMed] [Google Scholar]
- a Sugita Y.; Okamoto Y. Chem. Phys. Lett. 1999, 314, 141. [Google Scholar]; b Phillips J. C.; Braun R.; Wang W.; Gumbart J.; Tajkhorshid E.; Villa E.; Chipot C.; Skeel R. D.; Kalé L.; Schulten K. J. Comput. Chem. 2005, 26, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


