Abstract
We have developed a thiol-modified nanoporous silica material (SH-SAMMS) as an oral therapy for the prevention and treatment of heavy metal poisoning. SH-SAMMS has been reported to be highly efficient at capturing heavy metals in biological fluids and water. Herein, SH-SAMMS was examined for efficacy and safety in both in vitro and in vivo animal models for the oral detoxification of heavy metals. In simulated gastrointestinal fluids, SH-SAMMS had a very high affinity (Kd) for methyl mercury (MeHg(I)), inorganic mercury (Hg(II)), lead (Pb(II)), and cadmium (Cd(II)) and was superior to other SAMMS with carboxylic acid or phosphonic acid ligands or commercially available metal chelating sorbents. SH-SAMMS also effectively removed Hg from biologically digested fish tissue with no effect on most nutritional minerals found in fish. SH-SAMMS could hold Hg(II) and MeHg(I) tightly inside the nanosize pores, thus preventing bacteria from converting them to more absorbable forms. Rats fed a diet containing MeHg(I), Cd(II), and Pb(II) and SH-SAMMS for 2 weeks had blood Hg levels significantly lower than rats fed the metal-rich diet only. Upon cessation of the metal-rich diet, continued administration of SH-SAMMS for 2 weeks facilitated faster and more extensive clearance of Hg than in animals not continued on oral SH-SAMMS. Rats receiving SH-SAMMS also suffered less weight loss as a result of the metal exposure. Retention of Hg and Cd in major organs was lowest in rats fed with SH-SAMMS throughout the entire four weeks. The reduction of blood Pb by SH-SAMMS was significant. SH-SAMMS was safe to intestinal epithelium model (Caco-2) and common intestinal bacteria (Escherichia coli). Altogether, it has great potential as a new oral drug for the treatment of heavy metal poisoning. This new application is enabled by the installation of tailored interfacial chemistry upon nontoxic nanoporous materials.
Keywords: cadmium, chelation therapy, detoxification, mercury, lead, thiol, nanoporous, mesoporous silica
Introduction
Heavy metal exposure is a growing health problem worldwide. For example, in 2010, the United Nations Environment Programme (UNEP) reported that nearly two kilotons of mercury were released into air and one kiloton into water, largely from artisanal gold mining and the burning of fossil fuels. In the environment, bacteria convert Hg to methyl mercury (MeHg(I)), which is ultimately consumed by humans via fish and shellfish. A second major source of Hg exposure is silver–mercury amalgam used in dental restorations. Amalgam has been phased out in several European countries but is still widely used in the USA and many other countries. A recent risk analysis of data from several studies including 3800 subjects in US National Health and Nutrition Examination (NHANES)1 revealed that amalgam dental restorations (50% metallic mercury) were a major source of Hg exposure in the US. The several studies considered in the risk analysis showed that Hg content in urine, feces, exhaled breath, saliva, blood, and kidney, liver, brain, and pituitary gland increases with increasing amalgam number. In the most conservative risk scenario,1 67.2 million Americans would exceed the Hg reference exposure level established by the US Environmental Protection Agency (0.5 to 1 μg/day/filled tooth). In a separate analysis of NHANES data, Laks2 reported that blood inorganic Hg levels in 6174 U.S. women increased sharply with age over the last two decades. In other studies, Hg has been observed to increase in amniotic fluid, placenta, fetal tissues, and breast milk with increasing maternal amalgams3 placing the developing infants and neonates at an increased risk of Hg exposure. In addition to its dental, mining, and other industrial uses, Hg figures in traditional religious rituals and remedies throughout the world.4
Elemental liquid mercury (Hg(0)) easily vaporizes and is inhaled and oxidized by heme peroxidases in the blood to reactive inorganic Hg(II), which spontaneously combines with biochemical thiols such as cysteine and glutathione as well as cysteinyl residues at key sites in enzymes and other proteins.5,6 Once in circulation, Hg(II)-thiol conjugates partition into all tissues and those which go to the liver are susceptible to enterohepatic recirculation. Hg-resistant bacteria in the gastrointestinal tract may also convert Hg(II)-thiols into volatile, membrane permeable Hg vapor, capable of transiting back into circulation.7−9 In addition, oral and intestinal bacteria can use amalgam-derived Hg to form methyl mercury, which is readily absorbed by the oral epithelium.10 These bacterial transformations and other reports on the Hg enterohepatic cycle make clear that the effective elimination of Hg in any form from the body requires keeping it away from bacteria while getting it through the gastrointestinal tract, which is the route for >90% of Hg ingested or inhaled.6
Apart from treating acute Hg exposure with chelating agents developed decades ago, such as Dimercaprol (British anti-Lewisite or BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and 2,3-dimercaptosuccinic acid (DMSA or succimer), medical practice has no formally adopted practicum for subacute, chronic (e.g., occupational or iatrogenic) metal exposure. Thus, persons who consider themselves to suffer chronic metal exposure often use commercial naturopathic or homeopathic remedies advertised for removal of harmful metals. These include N-acetylcysteine (NAC), glutathione (GSH), selenocysteine, zinc, charcoal, zeolite, EDTA, and alginate. However, none of these materials have FDA approval and there is limited or no peer-reviewed scientific evidence to validate marketing claims that they are effective and safe for mercury detoxification.
Our objective is to develop a new oral treatment for heavy metal poisoning based on thiol-modified nanoporous silica (SH-SAMMS) with a specific focus upon Hg. Self-assembled monolayers on mesoporous supports (SAMMS) are hybrid materials generated by functionalizing mesoporous silica (SiO2) by covalently binding organic moieties to the silica surface and then cross-linking the organics to create a “dense molecular rug”. SAMMS are highly efficient sorbents with superior properties over conventional sorbents. Their multiligand chelation ability enhances their binding affinity and stability. The high surface area of the porous silica substrate (∼1000 m2/g) and the monolayer self-assembly technique achieves functional group density up to 10-fold higher than simpler methods11−13 and consequent high metal loading capacity. SAMMS’ rigid, open channel structure is ideal for mass transport of metal ions. We have successfully tailored the interfacial chemistry on the SAMMS to be selective for toxic heavy metals,12−14 transition metals,15,16 lanthanides and actinides,17−22 oxometallic anions,23,24 and cesium and thallium.25,26 By exploiting nontoxic nanoporous materials with a well-designed surface chemistry, we aim to achieve an oral therapy that is safe and effective for daily, long-term use in chronic heavy metal exposure, as well as enhancing the effectiveness of conventional chelating agents in acute heavy metal poisoning. This could have a high impact to public health worldwide. The principle focus of this study is Hg, but it will be shown that SH-SAMMS also has therapeutic potential for lead, cadmium, and arsenic exposure.
Our previous report in this ACS AMI journal12 suggests that SH-SAMMS possesses many desired characteristics for oral mercury detoxification. The linear rigid channels of the mesoporous silica make the thiol sites readily available to metal ions. More than 99% of Hg in simulated gastric fluid (SGF, pH 1.1) was removed in 3 min, just as observed with other metals, Cd(II) in simulated intestinal fluid (SIF, pH 6.8)12 and Pb(II) in natural waters.14 In contrast, swellable polymer ion exchange resins, such as GT-73, took much longer (∼120 min) to reach steady-state sorption.14 Fast binding kinetics is advantageous for rapid capture of toxic metals in the GI tract to minimize reabsorption back to the body. The extent of Hg capture on SH-SAMMS was stable over a 24-h exposure period tested, indicating no significant leaching of Hg from the sorbent nor degradation of the SH-SAMMS in SGF. The affinity of SH-SAMMS for Cd(II), Pb(II), and Hg(II) in gastrointestinal fluids with a pH range of 1–8, chosen to cover physiological pH’s along the gastrointestinal tract, has been reported.12 SH-SAMMS’ affinity for Hg(II) was very high (1 × 106 fold higher by weight of Hg on SH-SAMMS than in the supernatant solution) across the whole pH range, while those for Cd(II) and Pb(II) was high at pH > 5.5. The SH-SAMMS’ affinity for the metal ions was not significantly affected by increased ionic strength from 0.001 to 0.1 M. When exposing SH-SAMMS bound with Cd(II), Pb(II), and Hg(II) to Caco-2 cells (possessing many properties of the intestinal epithelium), there was no leachate of the four metal ions from SH-SAMMS across the Caco-2 monolayer and no decrease in trans-epithelial electrical resistance (TEER) across the exposed cell monolayers, thus SH-SAMMS did not damage the cell monolayer. There was no cellular uptake of SH-SAMMS having particle size greater than 5 μm.12
Herein, we extend the work to include other essential in vitro and in vivo studies to show the great potential of SH-SAMMS for detoxification of heavy metals in humans. The sorbent material can be simply administered orally and the material properties are engineered for the therapy needs. This novel application of the SAMMS materials is enabled by the installation of tailored interfacial chemistry upon nontoxic nanoporous materials.
Experimental Section
Sorbent Materials
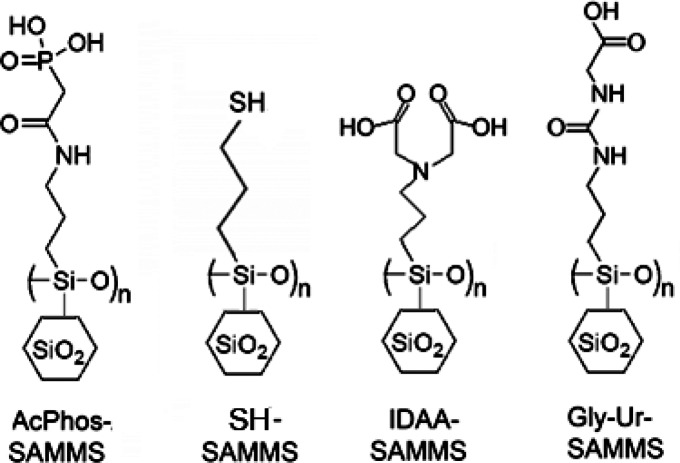
Synthesis and characterization of the SAMMS materials have been described elsewhere, including SH-SAMMS,13,27 AcPhos-SAMMS,28 Gly-Ur-SAMMS,22 and IDAA-SAMMS.16 Their chemical structures are shown in Figure 1. The SH-SAMMS was synthesized from MCM-41 with a pore size of 5.0 nm and a Brunauer–Emmett–Teller (BET) surface area of 870 m2/g. After thiol functionalization, the material had a pore size of 3.8 nm, a BET surface area of 438 m2/g, and a silane population of 3.9 thiol silanes/nm2 (determined using thermogravimetric analyzer, NETZCH STA 409 C/CD TGA). This high level of coverage forces the propyl chains into an upright posture, and forces the thiols up to the monolayer/solution interface. Additional characterization (e.g., 13C and 29Si NMR and TEM) of SH-SAMMS has been previously reported.13
Figure 1.
Surface chemistries of various SAMMS materials explored for toxic metal capture.
Test Matrices
Batch metal sorption experiments were performed with artificial gastric and intestinal fluids. The simulated gastric fluid (SGF)29 and simulated intestinal fluid (SIF)30 were prepared daily following the recommendations of the U.S. Pharmacopeia for drug dissolution studies in stomach and intestine, respectively. The SGF (pH 1.11) contained 0.03 M NaCl, 0.085 M HCl, and 0.32% (w/v) pepsin. The SIF contained 0.05 M KH2PO4; its pH was adjusted to 6.8 with 0.2 M NaOH. Additional simulated intestinal fluids were also utilized; including 0.2 M NaHCO3 (pH 8.30),31,32 Krebs buffer (pH 6.80) consisting of 118.0 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11.0 mM d-glucose, 2.5 mM CaCl2·H2O, and 25.0 mM NaHCO333,34 and 0.05 M KH2PO4 (pH 6.80) with and without 10 mg/mL pancreatin.29,30
Kd Measurements
The affinity of a sorbent for a target species is represented by a distribution coefficient (Kd) (mL/g). Kd is a mass-weighted partition coefficient between the solid phase and the liquid phase as follows
| 1 |
where Co and Cf are initial and final metal ion concentrations in solution determined by ICP-MS, V is solution volume in mL, and M is mass (g) of sorbent. For Kd measurements, the test solutions were spiked with multiple heavy metal ions (Cd(II), Hg(II), Pb(II)) and trivalent arsenic (As(III)) from chloride salts to obtain 50 μg/L each. A similar experiment was performed with only MeHg(I) from MeHgCl. A sorbent material was then added to the solution to obtain a solid per liquid ratio (S/L) of 0.2 g/L. The control was performed in the same fashion but without solid sorbent. The sample was then shaken for 2 h at 200 rpm on an orbital shaker. After 2 h, the solid was removed by centrifugation at 16 100g for 5 min and the supernatant was kept in 1 wt % HNO3 and 1 ppm Au to stabilize Hg species prior to the metal analysis. The metal concentrations in the control (no sorbent) and the test solutions (after being contacted with a sorbent material) were analyzed using an inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500x, Agilent Technologies, CA). Every experiment was conducted with appropriate controls (same metal solution with no sorbent), that underwent the same experimental conditions (including centrifuge) with the test group containing SH-SAMMS. This was to ensure that the difference in metal content between the control and the SH-SAMMS treated groups was due to the SH-SAMMS and not due to the metal oxide precipitation at high pH. It is also noted that based on the Ksp of metal hydroxides (e.g., Ksp ∼10–20 for Cu(OH)2 and Pb(OH)2),35,36 the precipitation is negligible when the metal content (50 ppb) and the hydroxide content (pH 6.8) are low as in our studies. All batch experiments were performed in triplicate and the averaged values and standard deviation were reported.
Hg Sorption Isotherms
The adsorption isotherm of Hg (as Hg(II) and MeHg(I)) is a measure of sorption capacity (in mg Hg/g SH-SAMMS) as a function of the equilibrium concentration of Hg in solution (in mg Hg/L). The adsorption isotherm was measured in deionized water spiked with mercury (final pH 4.0) at room temperature. The experiment was similar to Kd measurement but Hg concentrations were increased until the thiol binding sites were saturated (e.g., 0–2000 mg/L Hg conc; S/L of 0.2 g/L).
Competition by GSH
SH-SAMMS was incubated with an excess (7.5 mM) of either Hg(II) or MeHg(I) on an orbital shaker at 200 rpm and room temperature for 2 h to achieve loading of 350 mg Hg as Hg(II) or 250 mg Hg (as MeHg(I)) per gram of SH-SAMMS. The amount of Hg loaded on SH-SAMMS was determined by ICP-MS in the same fashion as the Kd measurement experiment. The Hg-loaded SH-SAMMS (Hg–S–SAMMS) were then washed three times with deionized water (500 mL/g of SH-SAMMS for each wash) to remove the unbound Hg species and finally resuspended at 5 mg/mL. The Hg–S–SAMMS was incubated by stirring at 200 rpm with 10 mM glutathione (GSH) for 4 h at 37 °C. The amount of Hg leached from the Hg–S–SAMMS was measured in the supernatant fluid by ICP-MS.
In Vitro Capture of Hg from Fish
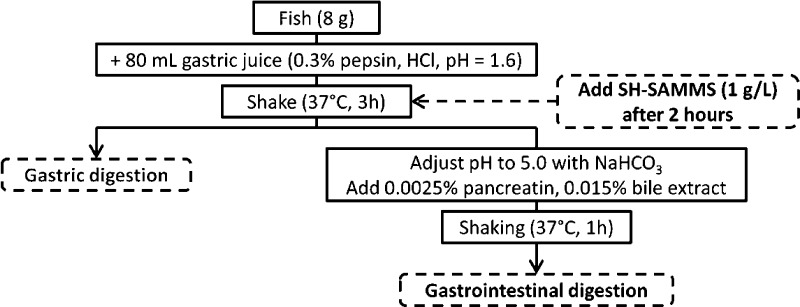
Various predator fish (kingfish, shark, Chilean seabass, red tuna, and white tuna) were purchased from a supermarket in Portland, OR, and quantified for total Hg content. Kingfish muscle containing 1.29 ± 0.03 mg total Hg/kg (wet weight), higher than FDA action level of 1.0 mg/kg, was selected for the study. Prior to mixing with SH-SAMMS, the fish was digested using a process shown in Figure 2. Briefly, gastric digestion was simulated by incubating 8 g of fish muscle on an orbital shaker at 150 rpm in 80 mL of 0.075 M HCl and 0.3% pepsin (pH 1.6) for 3 h at 37 °C. This was followed by 1 h of intestinal digestion simulation by adjusting the suspension’s pH to 5.0 with 1.0 M NaHCO3 and adding pancreatin (a hog pancreas extract containing amylase, lipase, and protease activities) and bile extract to 0.0025 and 0.015 wt %, respectively. SH-SAMMS were added to the fish tissue suspension at 0.5–2.5 g/L after 2 h of gastric digestion and remained in the suspension during the intestinal digestion. After the gastric and intestinal digestions, the suspensions were centrifuged at 16 100g for 15 min to pellet the SH-SAMMS and the mineral content of the supernatant was determined by ICP-MS. A control fish tissue was digested without SH-SAMMS. The wt % Hg removed and wt % change of other minerals by SH-SAMMS were compared with the control.
Figure 2.
Schematic of in vitro fish digestion and SH-SAMMS treatment processes.
Bacterial Conversion of Hg(II) Bound SH-SAMMS
The Escherichia coli MG1655 strains with or without the NR1 plasmid conferring mercury resistance via mercuric reductase expression37,38 were cultured in Luria–Bertani Broth (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl, and 1 mM NaOH) at 37 °C (with shaking at 200 rpm). Overnight cultures were diluted 100-fold into fresh broth and incubated at 200 rpm for 3.5 h to reach their exponential growth stage (absorbance at 600 nm, OD600, of 0.24), at which time HgCl2 (0, 10, or 50 μM), SH-SAMMS (100 μg/mL), or Hg–S–SAMMS (100 μg/mL SH-SAMMS with 1.2 mmol Hg(II)/g loaded) were added to replicate 10 mL aliquots of the Hg-sensitive and Hg-resistant bacterial cultures. Growth of the cultures was measured using OD600 at 0, 0.5, 1, 2, 4, 6, 10, 20, and 25 h after addition of HgCl2, SH-SAMMS or Hg–S–SAMMS.
Cell Culture and Cytotoxicity Studies
Immortal human colon epithelial cells, Caco-2, were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Corning/Cellgro, VA) supplemented with 10% fetal bovine serum (GIBCO, Life Technologies, NY) and 1X penicillin/streptomycin (Corning/Cellgro, VA). Cells were maintained at 37 °C in 5% CO2 air atmosphere and were passaged weekly by trypsinization (TrypLE, Life Technologies, NY). For cytotoxicity assays, cells were subcultured and seeded at 3000 cells/well in 96-well flat plates and grown to optimal confluency in 5 days. Cells were then directly exposed to SH-SAMMS, DMSA, DMPS (0 – 10,000 μg/mL), HgCl2 (0–0.32 mM), MeHgCl (0 – 0.04 mM) or Hg–S–SAMMS for 24 h. The Hg–S–SAMMS used had 0.16 mmol Hg(II) or MeHg(I) per gram of SH-SAMMS and were added to the Caco-2 culture media to obtain Hg concentrations in the well volume equivalent to those of soluble Hg counterparts above (e.g., Hg(II)-S-SAMMS was added at a dose of 2 mg/mL in cell culture well to achieve the equivalent dose of 0.32 mM Hg(II)). After 24 h, cell viability was quantified with the CellTiter-Glo Luminescent Cell Viability Assay and normalized to an untreated control (e.g., no mercury compounds or soluble chelators added).
Chemicals and Diet
Methylmercury chloride (CH3HgCl), cadmium chloride (CdCl2) and lead acetate (Pb(CH3COO)2) were purchased from Sigma Aldrich, USA. The rodent diet was Purina 5001 Rodent Chow (St. Louis, MO). The metal-rich diet was prepared by mixing powdered Purina 5001 with the aforementioned metal salts to achieve 0.01% by weight of each metal per weight of the food (inherently having insignificant amount of mercury (in ng/kg),39 compared to what was added). The SH-SAMMS-containing diet was prepared in the same manner but with 1.0% by weight of SH-SAMMS per weight of the food. All mixing was performed daily on dry material to avoid prebinding of metals to SH-SAMMS prior to administering to the rats. The diet was fed to rats as dried powder and consumption (in gram) was recorded daily.
Evaluation of Metals in Tissues and Blood Samples of Rats
Male Wistar rats weighing an average of 250 ± 10 g were purchased from Charles River Laboratory (Wilmington, MA). They were placed individually in metabolic cages (Tecniplast, Italy) during the entire period of the study. All animals were maintained on a 12 h light cycle (6 a.m. to 6 p.m.) and given water ad libitum. All animal experiments were approved by OHSU’s IACUC and were carried out under the auspices of the OHSU Department of Comparative Medicine.
Three groups of 8 week old rats (6/group) were fed rodent diets containing either: (1) metal-rich diet (prepared as above); (2) a diet with 1.0 wt % SH-SAMMS; or (3) a diet mixed with metals as stated above and 1.0 wt % SH-SAMMS. The rats were fed for 2 weeks and then the metal-rich diet was removed and the rats were regrouped (3/group) with half receiving normal diet and the other half receiving 1.0 wt % SH-SAMMS diet for an additional 2 weeks. Blood samples were collected from each animal twice weekly for monitoring heavy metal concentration in the blood. Rat body weight was measured daily except weekends. Then the rats were sacrificed, and blood, liver, kidneys, brain, bone, and muscles were collected. Blood and tissue samples were digested in concentrated nitric acid until fully digested. Then the samples were diluted 50-fold in deionized water prior to metal analysis by ICP-MS.
Results and Discussion
Performance of Various Sorbent Materials in Standard Simulated Gastrointestinal Fluids
Kd values are the solid phase analog to solution phase equilibrium constants and are widely used to quantify a material’s performance for trace collection. For low concentration levels typically encountered with heavy metal exposure, Kd values are preferable to parameters such as total number of binding sites or Langmuir isotherms, which assume higher concentrations and sorbent saturation. For complex biological matrices Kd values of 500 mL/g are acceptable, those >5000 mL/g are very good, and >50 000 mL/g are outstanding considering the high ionic strength and organic content typically found in these solutions.22
In simulated gastric fluid (SGF, pH 1.11), most cation chelators did not capture Cd(II), Hg(II), Pb(II), and As(III) (Table 1), whereas SH-SAMMS strongly captured As and both Hg(II) and MeHg(I) species as expected for a soft thiol ligand and soft metal ions according to Pearson’s hard–soft–acid–base (HSAB) principle that soft metal ions (Lewis acid) prefer to bind with soft ligands (Lewis base) and hard metal ions prefer to bind with hard ligands.40 The thiol group starts off protonated, and then loses the proton upon binding Hg. SH-SAMMS did not capture Cd(II) and Pb(II) in SGF (pH 1.11), likely due to proton competition with these borderline metal ions. IDAA-SAMMS (a variant of EDTA) and activated carbon (Darco KB-B) marginally captured Hg compared to SH-SAMMS. In simulated intestinal fluid (SIF, pH 6.8), SH-SAMMS was best for all four metal ions (Table 1). Higher Kd values of Hg(II) and MeHg(I) at pH 1.1 than at pH 6.8 may be attributed to increased wettability of adduct in acidic conditions since SH-SAMMS itself is mildly hydrophobic as a result of the propyl thiol coating.
Table 1. Affinities (Kd) of Four SAMMS and Commercial Sorbent Materials (Chelex-100 resin, GT-73 resin, and Darco KB-B Activated Carbon) for As(III), Cd(II), Hg(II), MeHg(I), and Pb(II).
|
Kda (mL/g) |
||||
|---|---|---|---|---|
| sorbent | As(III) | Cd(II) | Hg(II) | Pb(II) |
| in simulated gastric fluid (pH 1.11) | ||||
| SH-SAMMS | 1700 | 170 | 1 500 000 for Hg(II) | 0 |
| 170 000 for MeHg(I) | ||||
| IDAA-SAMMS | 0 | 44 | 690 000 | 0 |
| AcPhos-SAMMS | 340 | 120 | 1600 | 10 |
| Gly-Ur-SAMMS | 57 | 0 | 460 | 0 |
| Chelex-100 | 0 | 0 | 4500 | 0 |
| GT-73 | 0 | 0 | 1300 | 810 |
| Darco KB-B AC | 0 | 0 | 24000 | 0 |
| in simulated intestinal fluid (pH 6.80) | ||||
| SH-SAMMS | 27 000 | 770 000 | 270 000 for Hg(II) | 20000 |
| 88 000 for MeHg(I) | ||||
| IDAA-SAMMS | 0 | 200 000 | 11 000 | 18000 |
| AcPhos-SAMMS | 0 | 47 000 | 5700 | 13000 |
| Gly-Ur-SAMMS | 0 | 840 | 500 | 4500 |
| Chelex-100 | 0 | 38 000 | 47 000 | 12000 |
| GT-73 | 0 | 1400 | 3200 | 3700 |
| Darco KB-B AC | 0 | 1100 | 80 000 | 15000 |
Measured at metal ion conc. of 50 μg/L (each), solid per liquid ratio of 0.2 g/L.
The Kd values of the four metal ions for SH-SAMMS in four different simulated intestinal fluids showed that typical biotic anion electrolytes (chloride, phosphate, bicarbonate >0.05 M) did not impede sequestration of toxic metals by SH-SAMMS (Table 2). Table 2 also shows that SH-SAMMS has extremely high selectivity for heavy metals over both alkaline and alkaline earth metal ions, considered hard metal ions according to Pearson’s HSAB principle. This is evidenced by the extremely high Kd for the heavy metal ions in Krebs buffer (pH 6.80), which contained large excess (by mole over that of Hg) of Na(I) (6 × 105 fold), K(I) (2.4 × 104 fold), Mg(II) (4.8 × 103 fold), and Ca(II) (1 × 104 fold). SH-SAMMS performed much better than the commercial thiol resin sorbent, GT-73, likely due to the highly ordered and tightly packed thiol monolayers, which better allow for bis-coordination of metal ions. Amorphous GT-73 may rarely present two thiols in close proximity to establish the stronger multiligand S–Hg–S and S–Hg–O–Hg–S complexation that typically occurs in SH-SAMMS.13,41 We have previously reported SH-SAMMS to have much higher affinity (1 × 102 fold higher in Kd) and capacity (20-fold higher in capacity) for Hg(II) than GT-73, all measured in filtered groundwater.14 The IDAA-SAMMS was much better than EDTA-based Chelex-100, likely also owing to better ligand proximity affording higher order chelation. In both simulated gastrointestinal fluids, SH-SAMMS captured metal ions much better than the high surface area activated carbon (Darco KB-B), whose ligands (carboxylates, phenols, etc.) are also randomly ordered. Thus, SH-SAMMS was proven to be highly effective in vitro at sequestering Hg and other toxic, thiophilic metals in standard simulated gastrointestinal fluids.
Table 2. Affinity (Kd) of SH-SAMMS for As(III), Cd(II), Hg(II), and Pb(II) in Various Simulated Intestinal Fluids.
|
Kda (mL/g) in various simulated intestinal fluids |
||||
|---|---|---|---|---|
| matrix | As(III) | Cd(II) | Hg(II) | Pb(II) |
| 0.2 M NaHCO3 (pH 8.30) | 26 000 | 3 600 000 | 1 300 000 | 1 300 000 |
| Krebs buffer (pH 6.80) | 26 000 | 1 500 000 | 150 000 | 140 000 |
| 0.05 M H2KPO4 (pH 6.80) | 27 000 | 770 000 | 270 000 | 20 000 |
| 0.05 M H2KPO4 (pH 6.80), with 10 mg/mL pancreatin | 24 000 | 1 300 000 | 290 000 | 78 000 |
Measured at metal ion conc. of 50 μg/L (each), solid per liquid ratio of 0.2 g/L.
Hg Binding Capacity of SH-SAMMS
The adsorption isotherms of Hg(II) and MeHg(I) on SH-SAMMS, are well fitted by a Langmuir model (R2 >0.98) consistent with monolayer adsorption without precipitation of the metals (Figure 3). We previously reported this standard SH-SAMMS to contain 3.9 SH/nm2 or 2.8 mmol SH/g.12 Here we found (Figure 3) the maximum mercury binding capacities to be 385 mg/g for Hg(II) (1.9 mmol/g) and 250 mg for MeHg(II) (1.2 mmol/g). The molar ratio of S and Hg(II) of 1.47 is in agreement with our previous reports based on Extended X-ray Absorption Fine Structure (EXAFS) approach indicating that Hg(II) binds to SH-SAMMS as a mixture of S–Hg–O–Hg–-S (S/Hg = 1) and S–Hg–S (S/Hg = 2).41 The SH-SAMMS loading capacity for Hg(II) in water was the same as in SGF (380 mg/g).12 We also found the metal binding capacity of SH-SAMMS was not affected by temperature from 24 to 37 °C as expected for covalent bonding.12 Lastly, we have seen no evidence that binding Hg induces any agglomeration of the silica particles.
Figure 3.
Binding capacity of SH-SAMMS for inorganic Hg(II) and MeHg(I) in deionized water (pH 4.0); data modeled with Langmuir adsorption isotherm. All with SH-SAMMS at 0.2 g/L.
Impact of other Thiol Compounds and Proteins on SH-SAMMS Performance
Reduced glutathione (GSH) and cysteine (CysH) are abundant in the GI tract and may compete with SH-SAMMS for Hg. Since SH-SAMMS has a strong affinity for Hg even at low pH (Table 1), it should capture Hg in the stomach where levels of GSH and CysH are negligible,42 while the thiol concentrations measured in human small intestines were higher (e.g., from 3 to 8 mM).43 After SH-SAMMS loaded with Hg equivalent to 0.34 mM Hg(II) or 0.25 mM MeHg(I) in solution were incubated with 10 mM GSH for 4 h at 37 °C, we found that only 28 ± 0.1% of Hg(II) and 33 ± 1.7% of MeHg(I) were released into the solution. Thus, SH-SAMMS retained ∼70% of its Hg(II) or MeHg(I) load even when in equilibrium with small monothiols capable of entering its interior channels. In contrast, proteins (pepsin in SGF, Table 1 and pancreatin in SIF, Table 2), which are too large to enter the channels had no significant negative effect on the binding of Hg(II) on SH-SAMMS, consistent with the idea that the small pore size of SH-SAMMS excludes proteins that might foul metal binding sites inside SAMMS channels.
SH-SAMMS Captured Hg from Fish Digestate without Removing Most Essential Metals
SH-SAMMS effectively captured Hg(II) and MeHg(I) in simulated gastric and intestinal fluids as shown in Table 1, so we asked if they can capture Hg from digested fish. Kingfish contained 1.29 ± 0.03 mg total Hg/kg-wet weight and over 90% of total Hg in predator fish is methyl mercury.44,45 The fish was digested by simulating gastric and intestinal processes. After gastric digestion, soluble Hg was 85% of total Hg and remained the same after simulated intestinal digestion. We found 1.0 g/L of SH-SAMMS (added directly to the fish digestion without removing fish particulates) captured 62 ± 1.4% (n = 3) of soluble Hg (Table 3) after gastric digestion (pH 1.6) and 65 ± 0.7% (n = 3) after gastric + intestinal digestion (pH 5.0). Thus, Hg capture could largely occur in the stomach and the Hg would remain bound at higher pH with competing thiols in the large intestine (measured to be 1.5 mM by Ellman’s assay from the same digested fish). In contrast, 1.0 g/L of high surface area activated carbon (Darco KB-B) only removed ∼16 and 24% of Hg after gastric and gastric+intestinal digestion, respectively, consistent with lower Kd values (Table 1) than for SH-SAMMS. Hg capture by SH-SAMMS was dose-dependent with 25, 62, and 84% of fish Hg being captured by 0.5, 1.0, and 2.5 g/L of SH-SAMMS, respectively. The fish also contained a small amount of Pb, of which 49% was removed by SH-SAMMS in the simulated digestate. Importantly, SH-SAMMS did not deplete some Group I and II nutritional minerals from the fish tissue (i.e., Mg, K, Ca, Se, Rb, and Sr). In this in vitro test on the fish digestate, SH-SAMMS did remove some trace nutritional transition metals Fe, Zn, and Cu (Table 3), which could be soluble or bound to biomolecules smaller than the pore size of SH-SAMMS (to be accessible to thiol groups on the inner walls of SH-SAMMS). Removal of essential minerals is common with systemically administered metal chelators, DMSA, DMPS, or EDTA, but because SH-SAMMS acts only in the GI tract such mineral-depleting side effects on the host organism could be milder. We investigated this hypothesis in our animal studies described below.
Table 3. SH-SAMMS (1.0 g/L) Capture of Metals from in Vitro Fish Digestate.
| metal content (mg/kg fish-wet weight) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| digestion stage | system | Mg | K | Ca | Fe | Cu | Zn | Se | Rb | Sr | Hg | Pb |
| gastric | untreat I | 341 ± 3 | 4550 ± 31 | 57 ± 0 | 1.32 ± 0.32 | 0.31 ± 0.00 | 8.34 ± 0.09 | 3.74 ± 0.04 | 0.81 ± 0.01 | 0.32 ± 0.00 | 1.09 ± 0.02 | 0.005 ± 0.002 |
| SH-SAMMS | 340 ± 1 | 4540 ± 28 | 57 ± 0 | 1.12 ± 0.01 | 0.22 ± 0.01 | 8.34 ± 0.04 | 3.81 ± 0.04 | 0.81 ± 0.00 | 0.32 ± 0.00 | 0.41 ± 0.01 | 0.005 ± 0.000 | |
| % removal | 0 | 0 | 0 | 16 | 30 | 0 | 0 | 0 | 0 | 62 | 0 | |
| gastric + intestinal | untreat II | 378 ± 14 | 5520 ± 300 | 70 ± 1 | 1.93 ± 0.05 | 0.27 ± 0.01 | 8.31 ± 1.49 | 3.34 ± 0.02 | 0.90 ± 0.00 | 0.37 ± 0.01 | 1.01 ± 0.09 | 0.004 ± 0.003 |
| SH-SAMMS | 363 ± 13 | 5090 ± 330 | 64 ± 3 | 1.42 ± 0.03 | 0.02 ± 0.00 | 6.93 ± 0.31 | 3.47 ± 0.11 | 0.88 ± 0.05 | 0.36 ± 0.02 | 0.35 ± 0.01 | 0.002 ± 0.000 | |
| % removal | 4 | 8 | 9 | 26 | 94 | 17 | 0 | 2 | 2 | 65 | 49 | |
Bacterial Access to SH-SAMMS-Bound Hg
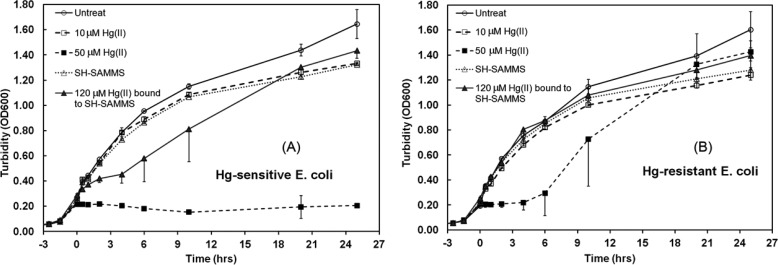
In the environment, bacterial methylation of Hg(II) is an established component of Hg cycling; e.g., Hg(II) is converted by bacteria in natural sediments to MeHg(I), which bioaccumulates in fish and the higher food chain.8,46 However, bacterial biotransformation of Hg compounds is not limited to the external environment. Summers et al. observed enrichment of Hg(II)-reducing bacteria in the primate GI tract in response to Hg exposure from dental mercury fillings.47 The ability to reduce Hg(II) makes the bacteria resistant to Hg(II) but the resulting monatomic Hg(0) vapor may be absorbed back into circulation instead of being excreted with feces.5,6,48 More recently, Summers’ group has also observed MeHg(I) formation in the feces of monkeys fitted with amalgam restorations (manuscript under review). Bacteria are typically spherical or rod-shape with the dimension between 0.5 and 3 micrometer.49 The small pores of SH-SAMMS, from 3.8 to 6.5 nm,12 would prevent bacterial access to Hg(II) bound in SH-SAMMS’ interior channels and hence could limit bacterial reduction and methylation of Hg(II) in the GI tract. We tested whether Hg bound to SAMMS is accessible to bacteria by exposing cultures of Hg(II)-reducing (aka “resistant”) E.coli and Hg(II)-nonreducing E.coli (aka “sensitive”)50 to soluble Hg(II) (as HgCl2) or to Hg–S–SAMMS. Two control cultures were those exposed to SH-SAMMS or untreated. The culture growth was measured by absorbance at 600 nm for Hg-sensitive E. coli (Figure 4A) and for Hg-resistant E. coli (Figure 4B). Addition of 10 μM HgCl2 to early exponential phase cultures had little effect on the growth of either E. coli strain but 50 μM HgCl2 initially inhibited growth of both strains. However, as expected the Hg-resistant strain recovered at 6 h after Hg addition and eventually reached a turbidity similar to its growth in medium without HgCl2; the lag period reflects the induction of the expression of the genes for mercury transformation. In contrast, the Hg-sensitive strain never recovered from 50 μM HgCl2 exposure since it cannot convert Hg(II) to less reactive, volatile Hg(0). The addition of SH-SAMMS alone to the E. coli cultures did not affect growth of either strain, indicating the material does not harm nor promote growth of this typical intestinal bacterium. Addition of Hg(II)-S-SAMMS (with the equivalent Hg(II) of 120 μM) initially slowed growth of the Hg-sensitive strain, but it fully recovered by 20 h. Addition of Hg–S–SAMMS to Hg-resistant E. coli had no effect on its growth. The brief dip in growth rate of the Hg-sensitive strain and the unchanged growth of the Hg-resistant strain indicated minimal Hg exposures, perhaps due to the release of surface bound Hg(II) by micromolar monothiols naturally secreted by bacteria during growth or possibly due to the presence of Hg bound to thiols external to the pores. Clearly most of the Hg(II) bound on SH-SAMMS was not accessible to the bacteria. SH-SAMMS’s effective sequestration of Hg(II) from intestinal bacteria will thus prevent enrichment of bacteria with genes for Hg(II) resistance and their genetically linked, transmissible antibiotic resistance genes47,51−53 and will also limit availability of Hg(II) for methylation by intestinal methanogens and sulfate reducing bacteria.
Figure 4.
Cell density (OD600) of (A) Hg-sensitive E. coli and (B) Hg-resistant E. coli after exposure to 10–50 μM HgCl2, 120 μM Hg bound to SH-SAMMS (0.1 g/L), SH-SAMMS (0.1 g/L), or untreated. Overnight E. coli cultures were diluted 100× into fresh Luria–Bertani Broth and treated 3.5 h later.
Low Cytotoxicity of SH-SAMMS and Hg-Bound SH-SAMMS to Intestinal Tissue Culture Cells
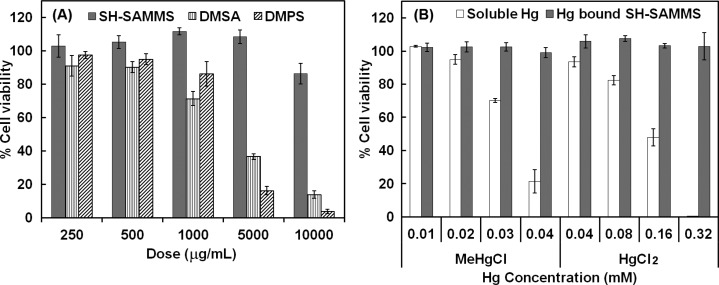
The Caco-2 cell line morphologically and functionally resembles the epithelial cells (enterocytes) lining the small intestine. We sought to establish a safety profile of the SAMMS material in this relevant in vitro cell model. We found SH-SAMMS to have low cytotoxicity for Caco-2 cells after 24-h exposure at doses from 0 to 10,000 μg/mL, whereas detectable toxicity of DMSA and DMPS was observed at 1,000 μg/mL and became severe at 5,000 μg/mL and above (Figure 5A). Although SH-SAMMS is not bioequivalent of soluble and absorbable DMSA and DMPS, they all are given orally and the cells of the GI tract will be exposed to the drugs. The Caco-2 cell represents the best qualitative prediction because it is closest to the site of action. The low cytotoxicity of SH-SAMMS is due to the fact that SH-SAMMS is not water-soluble and not taken up in the cells. It was demonstrated previously that SH-SAMMS >5 μm in particle size was not uptaken to Caco-2 cells and kept the cell monolayer integrity intact.12 It is worth noting that we compare the material safety on a per mass basis rather than molar basis of active components to mimic the prescribed dose of oral DMSA and DMPS (in mg/day). In addition, safety of SH-SAMMS should be considered as whole material (include the inactive silica substrate) and not just for active thiol groups. Nevertheless, when comparing active ingredients by molar basis, 10 000 μg/mL of SH-SAMMS contains 28 μmol/mL of thiol which is on par with 5000 μg/mL of DMSA (27 μmol/mL) or DMPS (22 μmol/mL). At these similar molar concentrations, SH-SAMMS is still the safest for Caco-2 cells.
Figure 5.
(A) Cell viability of Caco-2 cells after 24 h exposure to individual agents: SH-SAMMS (solid gray bar), DMSA (vertical striped gray bar), or DMPS (striped bar) at indicated dose range; and (B) the cell viability after 24 h exposure to MeHg(I) and Hg(II) as soluble species (white bar) and as SH-SAMMS bound (gray bar).
In Figure 5B, soluble Hg species, in the range of 0.04–0.32 mM for Hg(II) or 0.01–0.04 mM for MeHg(I), were highly toxic to Caco-2 cells exhibiting a Lethal Dose, 50% (LD50) of 0.17 mM for Hg(II) and 0.035 mM for MeHg(I). In contrast, equivalent doses of both Hg species (Hg(II) and MeHg(I)) were not toxic when bound to SH-SAMMS. This protection against mercury toxicity was maintained even when using 4-fold less SH-SAMMS for loading the maximum Hg studied, 0.32 mM Hg(II) or 0.04 mM MeHg(I) (data not shown).
SH-SAMMS for Oral Mercury Detoxification: In Vivo Studies
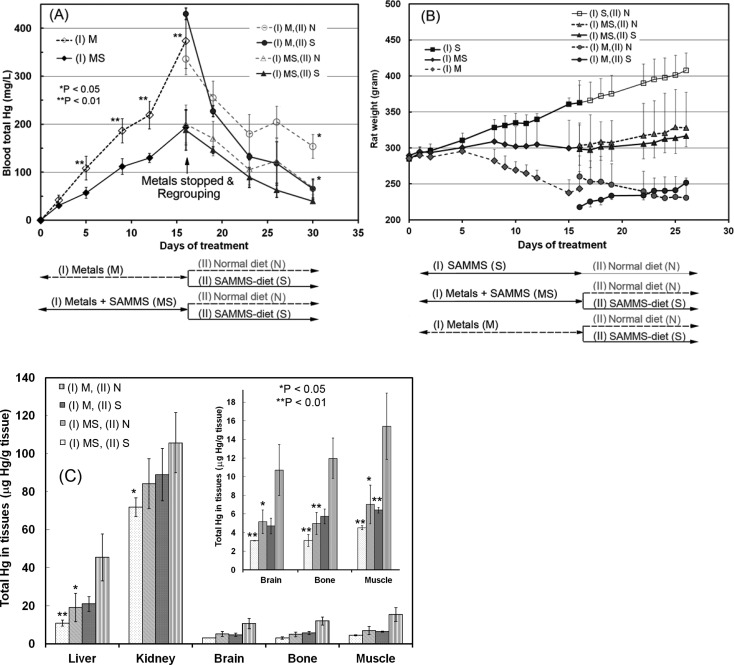
Because of its encouraging in vitro performance, SH-SAMMS was evaluated in rats for detoxification of MeHg(I), experiencing concurrent multi-metal stress. Rats on a metal-rich diet + SH-SAMMS had blood Hg levels much lower than rats fed the metal-rich diet alone (Figure 6A). When the metal-rich diet was stopped after 2 weeks, blood Hg levels in rats subsequently fed with the SH-SAMMS diet decreased faster and to a greater extent than in rats on the normal diet follow-on regime. SH-SAMMS use also prevented the weight loss typically associated with heavy metal toxicity (MS vs M, Figure 6B). Indeed, when the metal-rich diet was stopped, rats subsequently fed with SH-SAMMS gained weight, whereas rats switched to the normal diet continued to lose weight (see S vs N, Figure 6B). Thus, enhanced removal of previously accumulated mercury by SH-SAMMS assists recovery of the rats from prior toxic effects of heavy metals. Rats fed the SH-SAMMS diet alone gained weight normally (Figure 6B, (I) S), supporting the safety and biocompatibility of the material when administered orally.
Figure 6.
(A) Blood Hg content, (B) bodyweight, and (C) Hg contents in organs of rats after the following dietary treatment exposures (inset protocol flowchart): the initial phase (denoted I) consisted of 2 weeks of diet containing 0.01 wt % metals (Cd(II), Pb(II), and MeHg(I) (denoted M), 1.0 wt % SH-SAMMS (denoted S), or both (denoted MS) and then the second phase (denoted II) after regroupings to compare SH-SAMMS intervention (denoted S) to normal diet (denoted N) for another 2 weeks. p-values compared to no-SAMMS counterparts for (A) and “(I) M, (II) N” group for (C). Graphical inset used to expand low end scale for respective organs.
The Hg contents per gram of wet tissue at sacrifice (Figure 6C) revealed Hg accumulated in kidneys > liver > muscle ≈ bone ≈ brain. Rats fed the SH-SAMMS diet throughout the study had the lowest Hg accumulation, compared to those fed partially with SH-SAMMS, and those fed without SH-SAMMS. Thus, SH-SAMMS not only reduced the absorption of MeHg(I) when given concurrently, but also accelerated clearance of Hg deposited in the target organs.
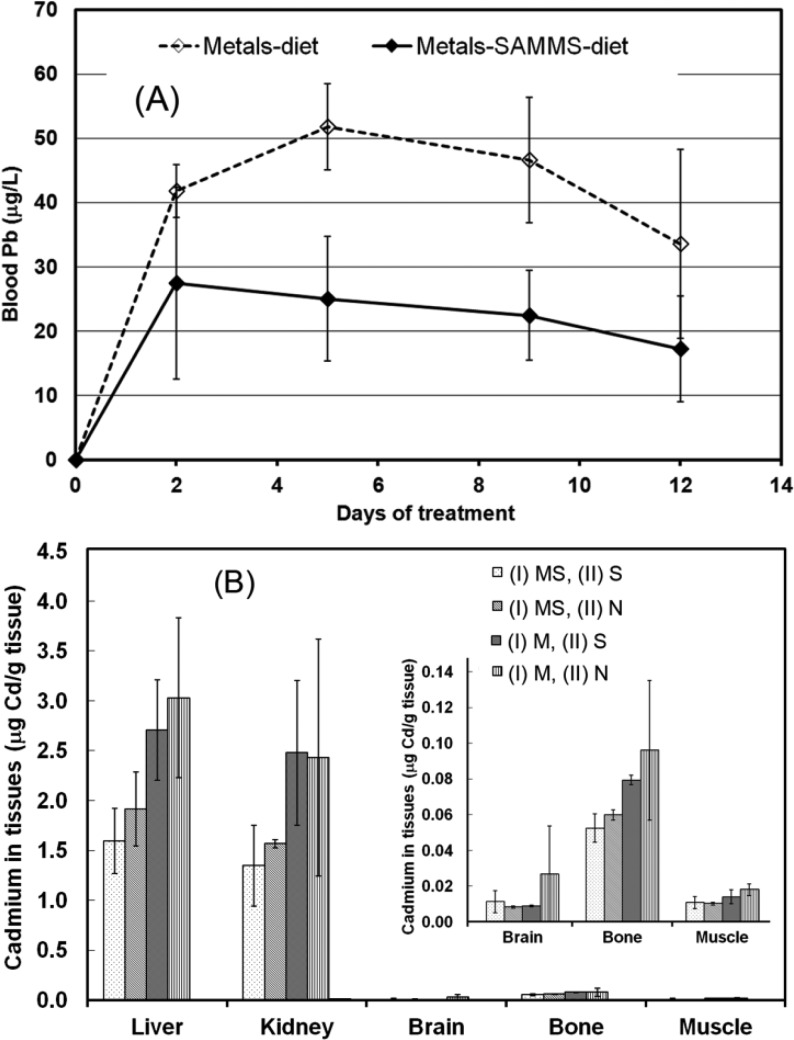
Capture of Dietary Cd(II) and Pb(II) in Rats
In addition to MeHg(I), SH-SAMMS effectively captured Cd(II) and Pb(II) from the diet as shown in Figure 7. Inorganic Cd(II) and Pb(II) are typically absorbed more poorly than mercurials and were detected in blood at much lower levels than MeHg(I). Pb was below detection in most tissues except bones (∼1–2 μg Pb/g of bone). However, the lower blood Pb with SH-SAMMS treatment compared to the no SH-SAMMS group was significant as shown in Figure 7A. Cd accumulated in liver ≈ kidney > bone > brain ≈ muscle, but the levels were 10- to 100-fold lower than Hg loading in the same organs (Figure 6C). SH-SAMMS consistently lowered Cd in these tissues as shown in Figure 7B.
Figure 7.
(A) Blood Pb in rats after first 2 weeks of treatment and (B) tissue Cd levels in rats after 4 weeks of treatment. Exposure intervals to the metal diet and SH-SAMMS are in Figure 6. Graphical inset used to expand low scale for respective organs.
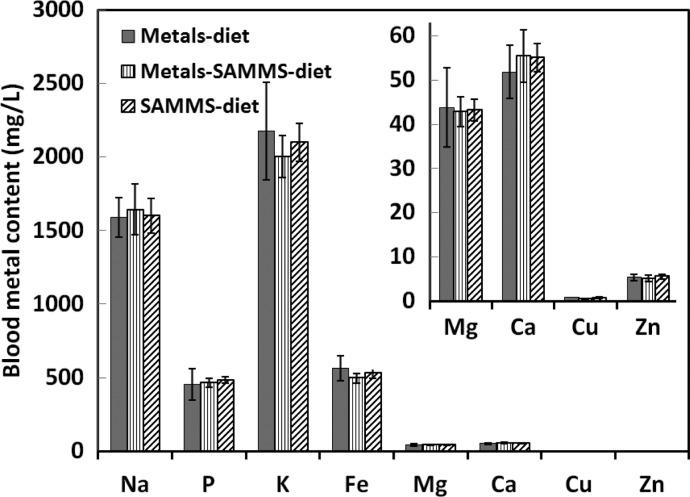
Capture of Essential Minerals
It is especially noteworthy that while largely preventing the accumulation of MeHg(I) by rat organs, SH-SAMMS did not diminish the blood contents of essential bulk and trace elements as shown in Figure 8. Unlike systemically administered chelating agents, SH-SAMMS exposure is restricted to the GI tract, and hence did not deplete most essential metal ions as measured in blood. Copper (Cu) could be captured by SH-SAMMS in vitro (Table 3), but its loss due to SH-SAMMS was much less dramatic when evaluated in animals. Blood Cu declined about 10% from prepretreatment levels after 2 days on SH-SAMMS, more importantly, levels did not decrease further after 5, 9, and 12 days of treatment. Interestingly, rats exposed to the heavy metals also had reduced blood Cu levels and the difference between the blood Cu levels of rats treated with SH-SAMMS or treated with heavy metals was not significant (p > 0.5) throughout the 12 days of treatment.
Figure 8.
Blood contents of essential metals of rats after the first 2 weeks of treatment as in Figure 6 (M, S, and MS). Graphical inset displays blood content of some biologically essential metals.
Conclusions
Our results show that SH-SAMMS has great potential for the detoxification of heavy metals. The treatment is a simple oral administration and the material properties can be engineered as therapeutic needs dictate. This novel application is enabled by the installation of high sorption affinity interfacial chemistry upon a nontoxic nanoporous silica material. The method was found to be viable for the treatment of Cd, Pb, and Hg exposure, and especially effective for the highly toxic methyl mercury as judged by: (a) its high affinity and selectivity for Hg and two other toxic metals in relevant simulated biological fluids and processes; (b) its rapid metal binding; (c) its large sorption capacity for Hg and toxic metals; and (d) its resistance to material degradation or to the release of captured metal ions; (e) even when challenged in the presence of soluble biological thiols. In terms of cellular interaction and safety; (f) SH-SAMMS does not damage or enter intestinal epithelial cells (Caco-2); (g) it has lower toxicity for Caco-2 cells than DMSA and DMPS on the same mass basis; and (h) the Hg- or MeHg-S-SAMMS complexes are completely innocuous for Caco-2 cells compared to equivalent doses of soluble Hg(II) or MeHg(I). We also found that (i) SH-SAMMS removed Hg from biologically digested fish tissue with minimal removal of several key nutritional metals; and (j) when orally administered to rodent animals, SH-SAMMS lowered blood and/or organ levels of MeHg(I), Cd(II), and Pb(II) but did not diminish essential minerals in blood. SH-SAMMS are effective in reducing organ load (vs the untreated group), despite possible competition with endogenous thiols such as H2S and methanethiol, products of human and microbial metabolism in the large bowel. Along the GI tract, the monolayer structure of SH-SAMMS should be largely unaffected and the Hg should be strongly bound via the thioalkoxide moiety (supported by high Kd values of Hg species going from pH 1.1 to 6.8 in Table 1). The efficacy of SH-SAMMS in the animals may in part be due to (k) its small pores that render metals bound in the internal channels which are inaccessible to bacteria. Bacterial inaccessibility of captured metals is especially important for Hg exposure since many intestinal bacteria can convert Hg(II) to membrane permeable Hg(0) or MeHg(I). SH-SAMMS that can immobilize mercury in the GI tract (l) would enhance the fecal excretion of mercury by disrupting its enterohepatic recirculation. Although SH-SAMMS does not absorb into the body, it may enhance the efficacy of current chelation therapies since SH-SAMMS can disrupt the recirculation of the toxic metals back from GI tract to the body. And, unlike systemically administered chelators, long-term use of SH-SAMMS is likely to be safe since it did not enter circulation, did not chemically burden the liver or the kidneys, did not impact the epithelial cells, and actually fostered regain of lost weight in previously intoxicated rats. Fumed silica has been FDA approved for food and pill additives for up to 2% by weight. We predict that SH-SAMMS will be as safe since the anticipated dose will likely be smaller and because SH-SAMMS has a much larger particle size, it will have less chance to absorb into the body. Longer term safety studies are underway and will be reported in due course. Disulfide bridges may form on SH-SAMMS when exposed to vigorous oxidizing conditions, potentially rendering the material ineffective for metal capture. However, this oxidation of the material does not happen spontaneously in air, as evidenced by the fact that SH-SAMMS have a shelf life of over 3 years in contact with air.
Acknowledgments
Research reported here was supported by awards to W.Y. from the National Institute of General Medical Sciences (NIGMS; R01GM089918), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R41DK094571), and the National Institute of Environmental Health Sciences (NIEHS; R21ES015620). We are grateful to Steward Advanced Materials (Chattanooga, TN) for providing a benchmarked SH-SAMMS (produced in large-scale). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
‡ Authors T.S. and J.M. contributed equally.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Richardson G. M.; Wilson R.; Allard D.; Purtill C.; Douma S.; Graviere J. Mercury Exposure and Risks from Dental Amalgam in the US Population, Post-2000. Sci. Total Environ. 2011, 409, 4257–4268. [DOI] [PubMed] [Google Scholar]
- Laks D. R. Assessment of Chronic Mercury Exposure within the U.S. Population, National Health and Nutrition Examination Survey, 1999–2006. BioMetals 2009, 22, 1103–1114. [DOI] [PubMed] [Google Scholar]
- Oskarsson A.; Schultz A.; Skerfving S.; Hallen I. P.; Ohlin B.; Lagerkvist B. J. Total and inorganic mercury in breast milk in relation to fish consumption and amalgam in lactating women. Arch. Environ. Health 1996, 51, 234–241. [DOI] [PubMed] [Google Scholar]
- Masur L. C. A Review of the Use of Mercury in Historic and Current Ritualistic and Spiritual Practices. Altern. Med. Rev. 2011, 16, 314–320. [PubMed] [Google Scholar]
- Clarkson T. W. The Three Modern Faces of Mercury. Environ. Health Perspect. 2002, 110Suppl 111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson T. W.; Vyas J. B.; Ballatori N. Mechanisms of Mercury Disposition in the Body. Am. J. Ind. Med. 2007, 50, 757–764. [DOI] [PubMed] [Google Scholar]
- Ballatori N.; Clarkson T. W. Biliary Secretion of Glutathione and of Glutathione-metal Complexes. Fundam. Appl. Toxicol. 1985, 5, 816–831. [DOI] [PubMed] [Google Scholar]
- Barkay T.; Miller S. M.; Summers A. O. Bacterial Mercury Resistance from Atoms to Ecosystems. FEMS Microbiol. Rev. 2003, 27, 355–384. [DOI] [PubMed] [Google Scholar]
- Dutczak W. J.; Ballatori N. Gamma-Glutamyltransferase-dependent Biliary-hepatic Recycling of Methyl Mercury in the Guinea Pig. J. Pharmacol. Exp. Ther. 1992, 262, 619–623. [PubMed] [Google Scholar]
- Leistevuo J.; Leistevuo T.; Helenius H.; Pyy L.; Osterblad M.; Huovinen P.; Tenovuo J. Dental Amalgam Fillings and the Amount of Organic Mercury in Human Saliva. Caries Res. 2001, 35, 163–166. [DOI] [PubMed] [Google Scholar]
- Fryxell G. E.; Mattigod S. V.; Lin Y.; Wu H.; Fiskum S.; Parker K.; Zheng F.; Yantasee W.; Zemanian T. S.; Addleman R. S.; Liu J.; Kemner K.; Kelly S.; Feng X. Design and Synthesis of Self-assembled Monolayers on Mesoporous Supports (SAMMS): The Importance of Ligand Posture in Functional Nanomaterials. J. Mater. Chem. 2007, 17, 2863–2874. [Google Scholar]
- Yantasee W.; Rutledge R. D.; Chouyyok W.; Sukwarotwat V.; Orr G.; Warner C. L.; Warner M. G.; Fryxell G. E.; Wiacek R. J.; Timchalk C.; Addleman R. S. Functionalized Nanoporous Silica for the Removal of Heavy Metals from Biological Systems: Adsorption and Application. ACS Appl. Mater. Interfaces. 2010, 2, 2749–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X.; Fryxell G. E.; Wang L. Q.; Kim A. Y.; Liu J.; Kemner K. M. Functionalized Monolayers on Ordered Mesoporous Supports. Science 1997, 276, 923–926. [Google Scholar]
- Yantasee W.; Warner C. L.; Sangvanich T.; Addleman R. S.; Carter T. G.; Wiacek R. J.; Fryxell G. E.; Timchalk C.; Warner M. G. Removal of Heavy Metals from Aqueous Systems with Thiol Functionalized Superparamagnetic Nanoparticles. Environ. Sci. Technol. 2007, 41, 5114–5119. [DOI] [PubMed] [Google Scholar]
- Chouyyok W.; Shin Y.; Davidson J.; Samuels W. D.; Lafemina N. H.; Rutledge R. D.; Fryxell G. E.; Sangvanich T.; Yantasee W. Selective Removal of Copper(II) from Natural Waters by Nanoporous Sorbents Functionalized with Chelating Diamines. Environ. Sci. Technol. 2010, 44, 6390–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche B.; Wiacek R.; Davidson J.; Koonsiripaiboon V.; Yantasee W.; Addleman R. S.; Fryxell G. E. Synthesis of Nanoporous Iminodiacetic Acid Sorbents for Binding Transition Metals. Inorg. Chem. Commun. 2009, 12, 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W.; Sangvanich T.; Creim J. A.; Pattamakomsan K.; Wiacek R. J.; Fryxell G. E.; Addleman R. S.; Timchalk C. Functional Sorbents for Selective Capture of Plutonium, Americium, Uranium, and Thorium in Blood. Health Phys. 2010, 99, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W.; Fryxell G. E.; Porter G. A.; Pattamakomsan K.; Sukwarotwat V.; Chouyyok W.; Koonsiripaiboon V.; Xu J.; Raymond K. N. Novel Sorbents for Removal of Gadolinium-based Contrast Agents in Sorbent Dialysis and Hemoperfusion: Preventive Approaches to Nephrogenic Systemic Fibrosis. Nanomedicine 2010, 6, e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W.; Fryxell G. E.; Addleman R. S.; Wiacek R. J.; Koonsiripaiboon V.; Pattamakomsan K.; Sukwarotwat V.; Xu J.; Raymond K. N. Selective Removal of Lanthanides from Natural Waters, Acidic Streams and Dialysate. J. Hazard. Mater. 2009, 168, 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W.; Fryxell G. E.; Lin Y.; Wu H.; Raymond K. N.; Xu J. Hydroxypyridinone Functionalized Self-assembled Monolayers on Nanoporous Silica for Sequestering Lanthanide Cations. J. Nanosci. Nanotechnol. 2005, 5, 527–529. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Fiskum S. K.; Yantasee W.; Wu H.; Mattigod S. V.; Vorpagel E.; Fryxell G. E.; Raymond K. N.; Xu J. Incorporation of Hydroxypyridinone Ligands into Self-assembled Monolayers on Mesoporous Supports for Selective Actinide Sequestration. Environ. Sci. Technol. 2005, 39, 1332–1337. [DOI] [PubMed] [Google Scholar]
- Fryxell G. E.; Lin Y.; Fiskum S.; Birnbaum J. C.; Wu H.; Kemner K.; Kelly S. Actinide Sequestration Using Self-assembled Monolayers on Mesoporous Supports. Environ. Sci. Technol. 2005, 39, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Chouyyok W.; Wiacek R. J.; Pattamakomsan K.; Sangvanich T.; Grudzien R. M.; Fryxell G. E.; Yantasee W. Phosphate Removal by Anion Binding on Functionalized Nanoporous Sorbents. Environ. Sci. Technol. 2010, 44, 3073–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryxell G. E.; Liu J.; Hauser T. A.; Nie Z.; Ferris K. F.; Mattigod S.; Gong M.; Hallen R. T. Design and Synthesis of Selective Mesoporous Anion Traps. Chem. Mater. 1999, 11, 2148–2154. [Google Scholar]
- Sangvanich T.; Sukwarotwat V.; Wiacek R. J.; Grudzien R. M.; Fryxell G. E.; Addleman R. S.; Timchalk C.; Yantasee W. Selective Capture of Cesium and Thallium from Natural Waters and Simulated Wastes with Copper Ferrocyanide Functionalized Mesoporous Silica. J. Hazard. Mater. 2010, 182, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchalk C.; Creim J. A.; Sukwarotwat V.; Wiacek R.; Addleman R. S.; Fryxell G. E.; Yantasee W. In Vitro and In Vivo Evaluation of a Novel Ferrocyanide Functionalized Nanopourous Silica Decorporation Agent for Cesium in Rats. Health Phys. 2010, 99, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Feng X.; Liu J.; Fryxell G. E.; Gong M. Mercury Separation and Immobilization Using Self-assembled Monolayers on Mesoporous Supports (SAMMS). Sep. Sci. Technol. 1999, 34, 1121–1132. [Google Scholar]
- Yantasee W.; Lin Y.; Fryxell G. E.; Busche B. J.; Birnbaum J. C. Removal of Heavy Metals from Aqueous Solution Using Novel Nanoengineered Sorbents: Self-assembled Carbamoylphosphonic Acids on Mesoporous Silica. Sep. Sci. Technol. 2003, 38, 3809–3825. [Google Scholar]
- USPXXII, 22nd ed.; United States Pharmacopeial Convention Inc.: Rockville, MD, 1990. [Google Scholar]
- USPXXVI, 26th ed.; United States Pharmacopeial Convention Inc.: Rockville, MD, 2003. [Google Scholar]
- Ellickson K. M.; Meeker R. J.; Gallo M. A.; Buckley B. T.; Lioy P. J. Oral Bioavailability of Lead and Arsenic from a NIST Standard Reference Soil Material. Arch. Environ. Contam. Toxicol. 2001, 40, 128–135. [DOI] [PubMed] [Google Scholar]
- Hamel S. C.; Ellickson K. M.; Lioy P. J. The Estimation of the Bioaccessibility of Heavy Metals in Soils using Artificial Biofluids by Two Novel Methods: Mass-balance and Soil Recapture. Sci. Total Environ. 1999, 243/244, 273–283. [DOI] [PubMed] [Google Scholar]
- Fadda H. M.; Basit A. W. Dissolution of pH Responsive Formulations in Media Resembling Intestinal Fluids: Bicarbonate Versus Phosphate Buffers. J. Drug Delivery Sci. Technol. 2005, 15, 273–279. [Google Scholar]
- Fadda H. M.; Merchant H. A.; Arafat B. T.; Basit A. W. Physiological Bicarbonate Buffers: Stabilisation and Use as Dissolution Media for Modified Release Systems. Int. J. Pharm. 2009, 382, 56–60. [DOI] [PubMed] [Google Scholar]
- Lide D. R.CRC Handbook of Chemistry and Physics, 88th ed; CRC Press: Boca Raton, FL, 2007. [Google Scholar]
- Olmsted J. A.; Williams G. M. In Chemistry, 4th ed; Wiley: New York; 2004. [Google Scholar]
- Summers A. O.; Silver S. Mercury Resistance in a Plasmid-Bearing Strain of Escherichia Coli. J. Bacteriol. 1972, 112, 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O.; Lewis E. Volatilization of mercuric chloride by mercury-resistant plasmid-bearing strains of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. J. Bacteriol. 1973, 113, 1070–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B.; Stern S.; Cernichiari E.; Gelein R. Methylmercury Contamination of Laboratory Animal Diets. Environ. Health Perspect. 2005, 113, 1120–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. G. Hard and Soft Acids and Bases, HSAB, Part 1: Fundamental Principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar]
- Kemner K. M.; Feng X.; Liu J.; Fryxell G. E.; Wang L. Q.; Kim A. Y.; Gong M.; Mattigod S. Investigation of the Local Chemical Interactions Between Hg and Self-assembled Monolayers on Mesoporous Supports. J. Synchrotron. Radiat. 1999, 6, 633–635. [DOI] [PubMed] [Google Scholar]
- Nalini S.; Balasubramanian K. A. Studies on Acid Soluble Thiols in the Human Gastric Juice. Biochem. Mol. Biol. Int. 1994, 32, 449–454. [PubMed] [Google Scholar]
- Busby R. W.; Kessler M. M.; Bartolini W. P.; Bryant A. P.; Hannig G.; Higgins C. S.; Solinga R. M.; Tobin J. V.; Wakefield J. D.; Kurtz C. B.; Currie M. G. Pharmacologic Properties, Metabolism, and Disposition of Linaclotide, a Novel Therapeutic Peptide Approved for the Treatment of Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation. J. Pharmacol. Exp. Ther. 2013, 344, 196–206. [DOI] [PubMed] [Google Scholar]
- Cabanero A. I.; Madrid Y.; Camara C. Mercury-selenium Species Ratio in Representative Fish Samples and Their Bioaccessibility by an In Vitro Digestion Method. Biol. Trace Elem. Res. 2007, 119, 195–211. [DOI] [PubMed] [Google Scholar]
- Torres-Escribano S.; Velez D.; Montoro R. Mercury and Methylmercury Bioaccessibility in Swordfish. Food Addit. Contam., Part A. 2010, 27, 327–337. [DOI] [PubMed] [Google Scholar]
- Barkay T.; Wagner-Dobler I. Microbial Transformations of Mercury: Potentials, Challenges, and Achievements in Controlling Mercury Toxicity in the Environment. Adv. Appl. Microbiol. 2005, 57, 1–52. [DOI] [PubMed] [Google Scholar]
- Summers A. O.; Wireman J.; Vimy M. J.; Lorscheider F. L.; Marshall B.; Levy S. B.; Bennett S.; Billard L. Mercury Released from Dental “Silver” Fillings Provokes an Increase in Mercury- and Antibiotic-resistant Bacteria in Oral and Intestinal Floras of Primates. Antimicrob. Agents Chemother. 1993, 37, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magos L.; Halbach S.; Clarkson T. W. Role of Catalase in the Oxidation of Mercury Vapor. Biochem. Pharmacol. 1978, 27, 1373–1377. [DOI] [PubMed] [Google Scholar]
- Freitas R. A. Microbivores: Artificial Mechanical Phagocytes using Digest and Discharge Protocol. J. Evol. Technol. 2005, 14, 55–106. [Google Scholar]
- Womble D. D.; Rownd R. H. Genetic and Physical Map of Plasmid NR1: Comparison with other IncFII Antibiotic Resistance Plasmids. Microbiol. Rev. 1988, 52, 433–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wireman J.; Liebert C. A.; Smith T.; Summers A. O. Association of Mercury Resistance with Antibiotic Resistance in the Gram-negative Fecal Bacteria of Primates. Appl. Environ. Microbiol. 1997, 63, 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert C. A.; Wireman J.; Smith T.; Summers A. O. Phylogeny of Mercury Resistance (mer) Operons of Gram-negative Bacteria Isolated from the Fecal Flora of Primates. Appl. Environ. Microbiol. 1997, 63, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert C. A.; Wireman J.; Smith T.; Summers A. O. The Impact of Mercury Released from Dental “Silver” Fillings on Antibiotic Resistances in the Primate Oral and Intestinal Bacterial Flora. Met. Ions Biol. Syst. 1997, 34, 441–460. [PubMed] [Google Scholar]